Introduction
In
vivo brains, the neuro signals depend on the molecular transmitters in the nerve systems. Here detection of the extracellular nerve current is related to the evaluation of sophisticated
in-vivo electro physiologic methods such as electro encephalogram [
1], magneto encephalography [
2], neurological disease [
3], brain computer interfacing and other analogous methods. The signal detection of these methods, however, requires complicated magnetic amplification [
5], spectrometric separation [
6], nuclear magnetic resonance [
7,
8], and photometric scanning. Moreover, these methods can be used only under laboratory conditions, are not applicable in
in-vivo direct implantation, and demand expensive instrumental systems. The electrochemical voltammetric method, however, is usable for
in-vivo implementable micro or macro probe techniques such as
in-vivo bladder assay [
9], live-brain application [
10], skin cell detection [
11], and
in-vivo vascular assay [
12]. Furthermore, electrochemical-circuit systems have long been recognized as simple [
13], fast-response, and powerful tools for signal amplification [
14], and accumulated stripping voltammetric methods are very sensitive for nano- or pico-range detection [
16,
17]. As such, in this study, highly sensitive [
18,
19] neuro detection was optimized by a handheld voltammetric circuit. Added to this, diagnostic application was performed to feeling-sense assay for human psychological neuro signals and
animum brain activity. So can be applicable for in vivo human plasma assay and in vitro [
22,
23,
24]
Experimental Design
Sensor Preparation A micro-carbon working electrode was developed by mixing 0.5-mL standard liquid metal mercury and 0.5 g carbon nanotube paste (Nanostructured & Amorphous Materials, Inc.). This was prepared overnight via catalyzed chemical vapor deposition (catalytic CVD) prior to use, magnetic stirring in a 2M nitric-acid solution, and cleansing with triplepurified water. The resulting material was then mixed with reagent-grade mineral oil (New Jersey USA, 1-800-01, Acro) and water in a 40:40:20% ratio, relatively. The resulting modified-paste was inserted into a 1-mm-diameter, 10 mm long needle-type plastic syringe with copper wire connecting the electrode to the voltammetric workstation. Counter and reference graphite pencil electrodes were prepared using 0.5 mm diameter Hipolymer HB pencil leads; the frontal part of the lead was connected with copper wire using parafilm (iNexus, South Korea, Inc.)
Carbon Nano Tube (CNT) paste probe
Sensor Preparation A micro-carbon working electrode was prepared by mixing 0.5-mL standard liquid metal mercury and 0.5 g carbon nanotube paste (Nanostructured & Amorphous Materials, Inc.). This was prepared overnight via catalyzed chemical vapor deposition (catalytic CVD) prior to use, magnetic stirring in a 2M nitric-acid solution, and cleansing with triplepurified water. The resulting material was then mixed with reagent-grade mineral oil (New Jersey USA, 1-800-01, Acro) and water glass in a 40:40:20% ratio, relatively. The resulting modified-paste was coated on the 0.1-mm-micro diameter copper wire and 0.1 mm thick copper paper with a 5-mm-diameter-circle electrode, then 0.3 mm copper wire connected to the voltammetric workstation. Counter and reference electrodes were prepared using 0.5 mm diameter Hipolymer HB pencil leads; the frontal part of the lead was connected with copper wire using parafilm (iNexus, South Korea, Inc.)
Preparation of electrode and detection systems
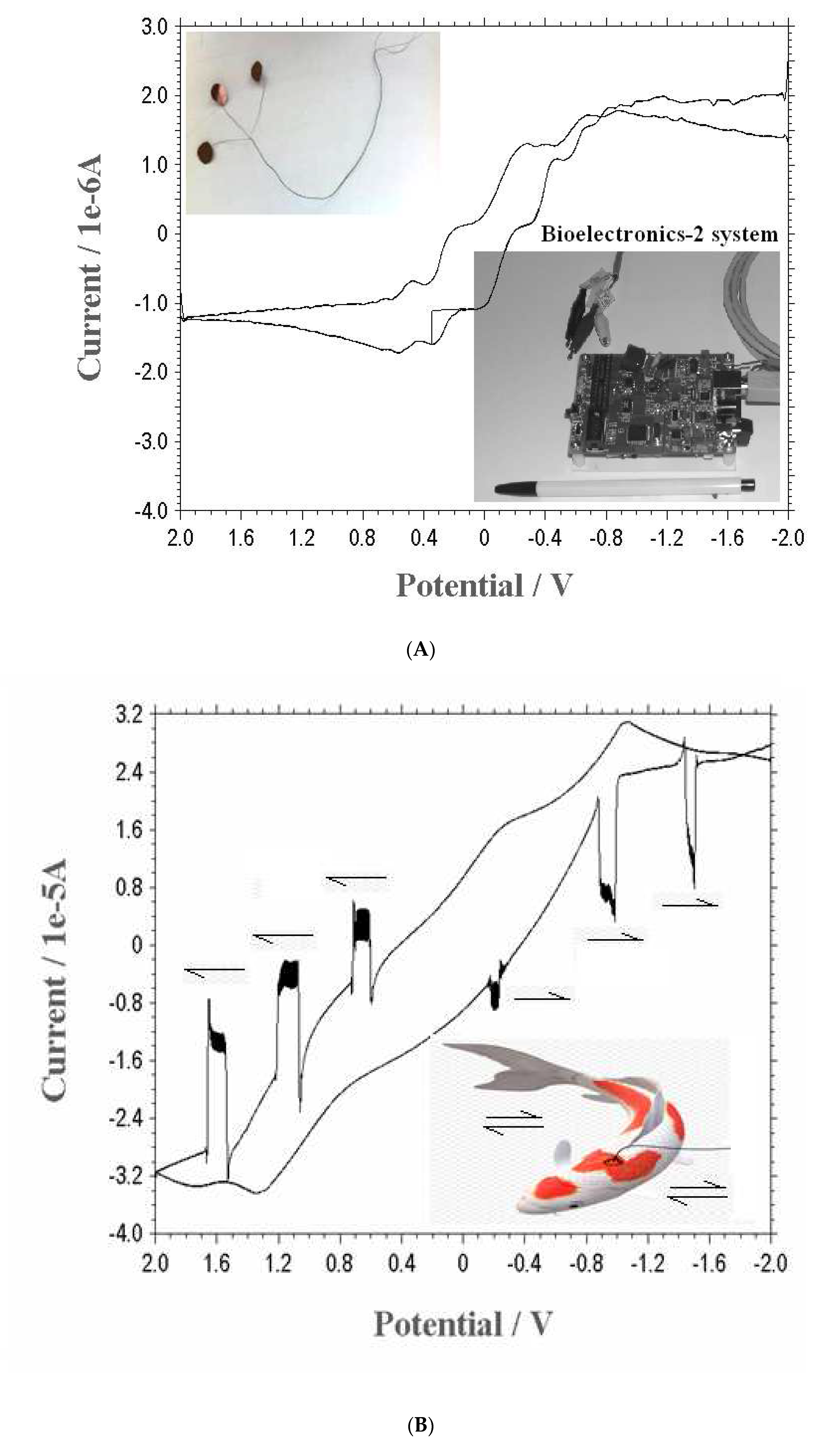
The three-electrode sensors of the counter, reference, and working probe prepared using 0.2-mm-thick copper paper with a 5-mm-diameter-circle electrode (CUE) were connected to voltammetric systems using a 0.3-mm Cu wire. (
Figure 1(A) insert photo shown), The voltammetric workstation was made in these authors’ institution. A method employing electrochemical neuro detection was employed using cyclic voltammetry and chronoamperometric circuits. It was carried out using the new bioelectronics-2 system, which was pioneered by these authors’ institution. The computer-controlled voltammetric system was developed with a +-2.0 V potential range, a 2 mA current range, a 10 pA measuring current, and a compact 3"×2"×1" size. The power input and data interface were connected by the USB port of a PC. The instrument is compact, as big as the usual cellular phone (
Figure 1(A) insert photo shown), and it can be used for biological, microorganism, and environmental trace assay.
In-vivo detection was performed using a 0.5-mm-diameter x 10-mm copper-wire-type implantable probe.
Optimization of the cyclic voltammetric potential
As
in-vivo currents depend on the applied input potentials and sensing probe amplifications, CUE was attached to the forehead cutis, under stable conditions, and CV scan was performed from -2.0 to 2.0 V under optimum conditions. The redox voltammogram shown in
Figure 1(A) is simple, and no signal was obtained from it. Thus, muscular-action signals were sought, and under optimum conditions, cyclic-potential variation was performed with 50 mv/sec movement, with three-time muscle strength, from positive to negative scan, which is related to the neuropotential windows. The results are shown in
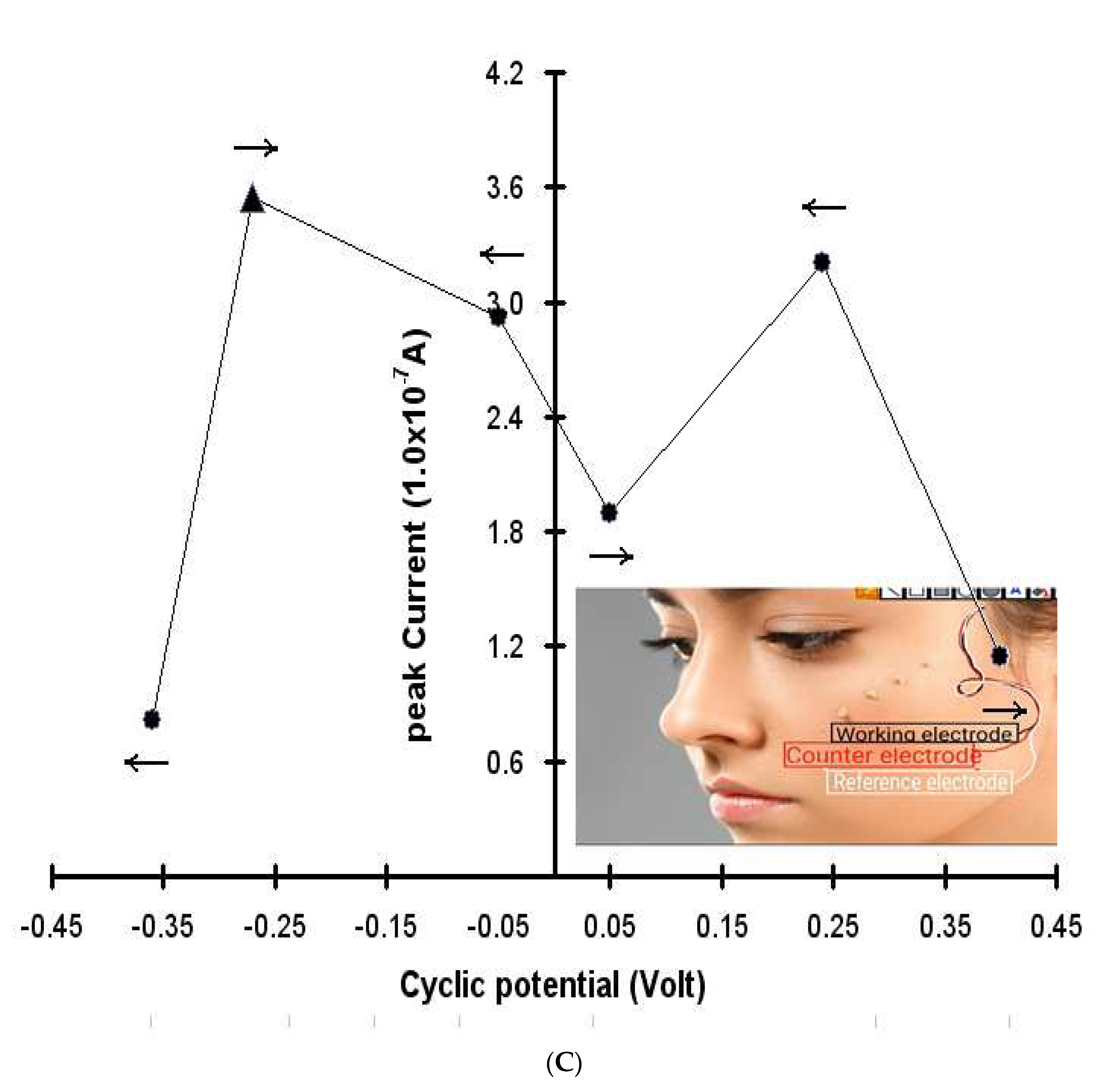
Figure 1(B). At the start, no action or peak current was obtained. Under these conditions, muscle actions were performed and were continued during the positive scan. In the -1.6, -1.0, and -0.3 negative direction, muscular actions were performed, then at 1.6, 1.1, and 0.6 V, same-strength muscular actions were performed, and a sensitive peak current appeared (i.e., sensitive during the positive then 0 potentials). The calculated peak results are shown in
Figure 1(C), where the X scale represents the scan potentials and the Y scale, the peak current. Under these conditions, a very sensitive outcurrent was obtained at -0.25 and +0.25 V. These results can be used for neurosensing detection and
in-vivo electrophysiological methods, such as electroencephalogram. Thus, more specific application was carried out.
Chronoamperometry and current amplification
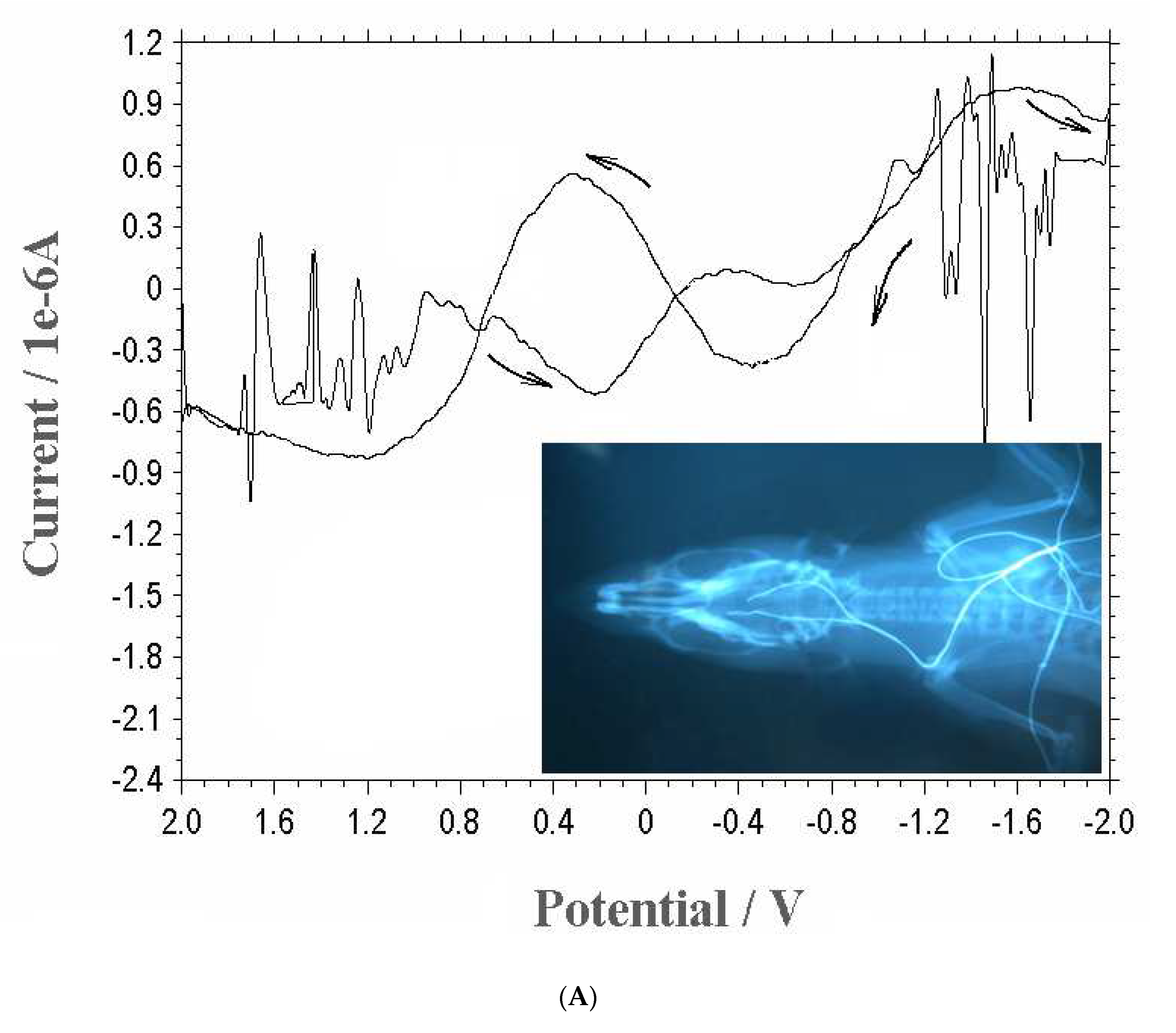
As neurocurrent detection depends on the applied redox potentials, amplitude current ranges, and detection skin surface, a three-electrode probe was attached to the back hand skins, and fish brain core, after which more specific cyclic-potential ranges were examined from -2.0 to 2.0 V.
Figure 2(A) shows the results of the cyclic-voltammetric neurocurrent of redox scan. During oxidation scan, muscle tension was performed and was repeated at three points, from -2.0 to 2.0 V. Here, however, only negative potentials were obtained from -2.0 to -1.4 V scan, and only the 1.42x10
-6, 17.8x10
-6, and 0.98x10
-6 A peak currents appeared. Thus, reduction scan was performed in the negative direction, but from 1.6 to 1.2 V, only the 0.92x10
-6, 0.78x10
-6, and 0.62x10
-6 A peak currents appeared. Here, both signals can be used for neurodetection, but more sensitive amperometric-current ranges are required. Thus, more specific experiments were performed.
Figure 2(B) shows the results from the 1.0x10
-1 to 1.0x10
-9 A exponential amplified variations, using a 50 mv/sec scan rate. Here, the linear curve represents the -1 to -9 amplifications, and the straight line is the sensing current for the backhand cutis through continuous muscular actions. A 1.0x10
-3 A maximum peak current is shown, and the other ranges are linearly varied. Thus, 1.0x10
-3 A was used for potentiometrics, and under this potential, the chronoamprometric-current sensitivity was examined, which can be applicable to direct neurodetection and sensing signals.
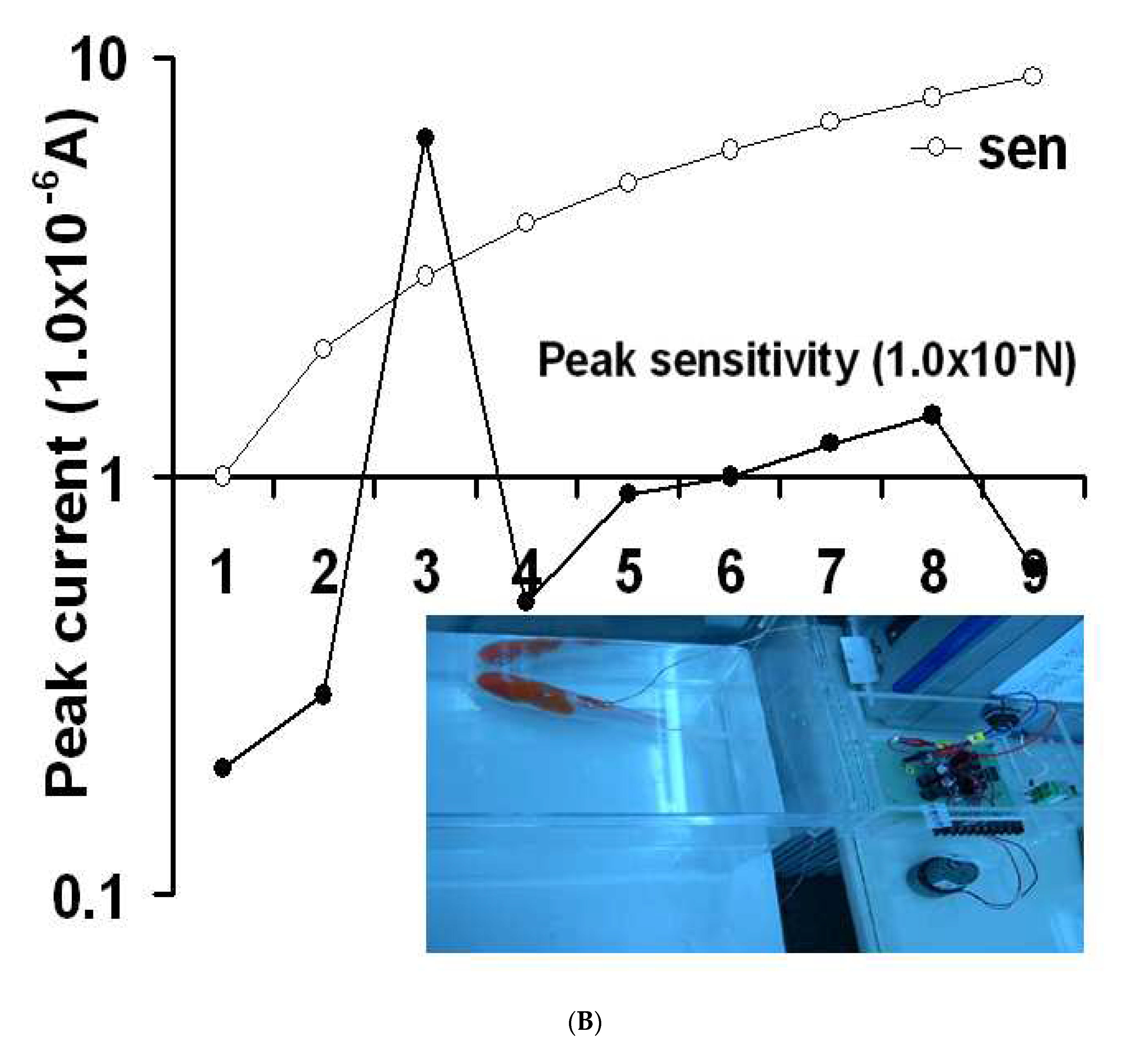
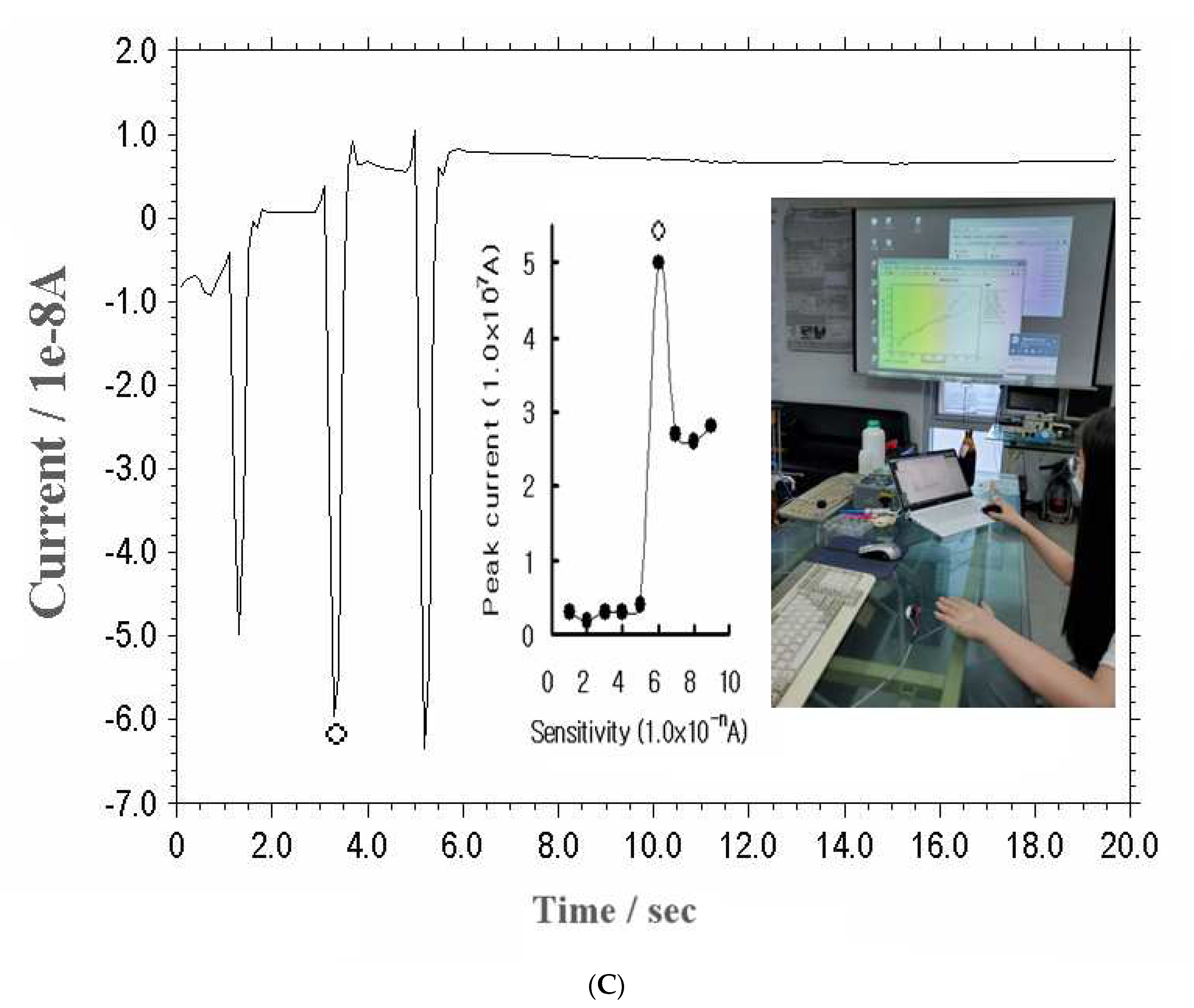
Figure 2(C) shows the results for the 10
-1-10
-9 A variations at the backhand cutis with the action current, and the inset curve shows the exact points. The first five points have no signal and are simple, but the 6
th point is very sensitive, and a 5.0x10
-7 A peak current was obtained, later decreasing to 3.0x10
-7 A. Thus, the 1.0x10
-6 amplification was fixed. The real current shown at this curve was from 10 to 50 sec, where the current was varied from 4.8 to 6.8x10
-8 A. These results are applicable to any sensing detection. Under these conditions, the advanced redox potential and brain signals were examined in humans and animals.
Application in the forehead cutis
For sensing detection, human skin and animal brain signals were analyzed. Under optimized conditions, a patch-type probe was attached to the forehead cutis and arms, after which the rat brain with action showed that the current in
Figure 3(A) is a real chronoamperometric peak. First, eye action was performed using the optimum parameters, and obtained with three points through three-time eye actions at 1.5, 3.5, and 5.5 sec/V were the 5.2x10
-11, 11.2x10
-11, and 7.5x10
-11 A sensitive peak currents, which responded to the muscle currents. Under these conditions, arm action was sought using the same method.
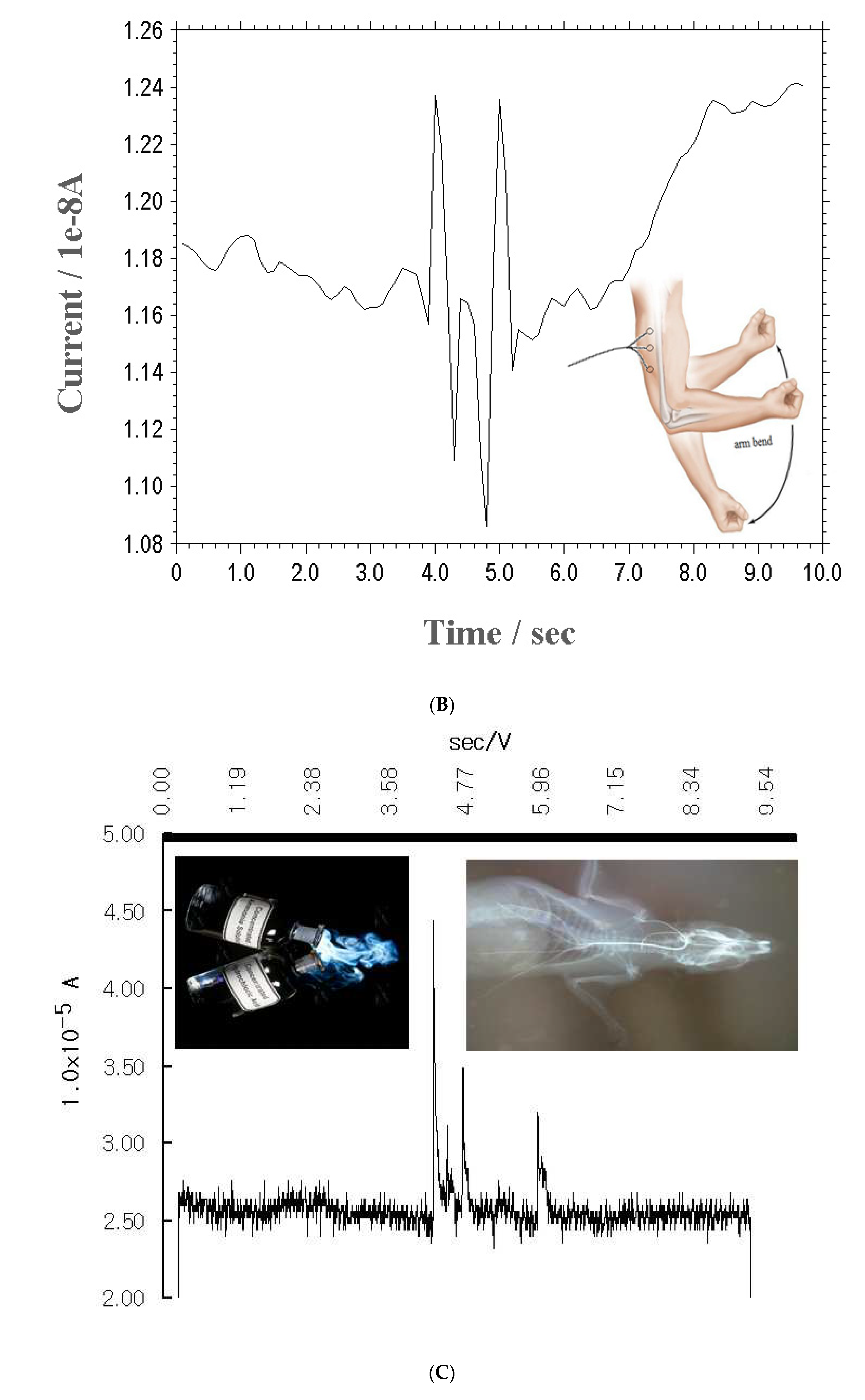
Figure 3(B) shows the results for the arm action for the chronoamperogram, for the three-point 4.0, 4.8, and 5.0 sec/V actions. Here, the peak currents were 0.09x10
-8, 0.12x10
-8, and 0.11x10
-8 A. They can thus be assayed for neurosignals. Then more specific experiments were performed using a rat brain in
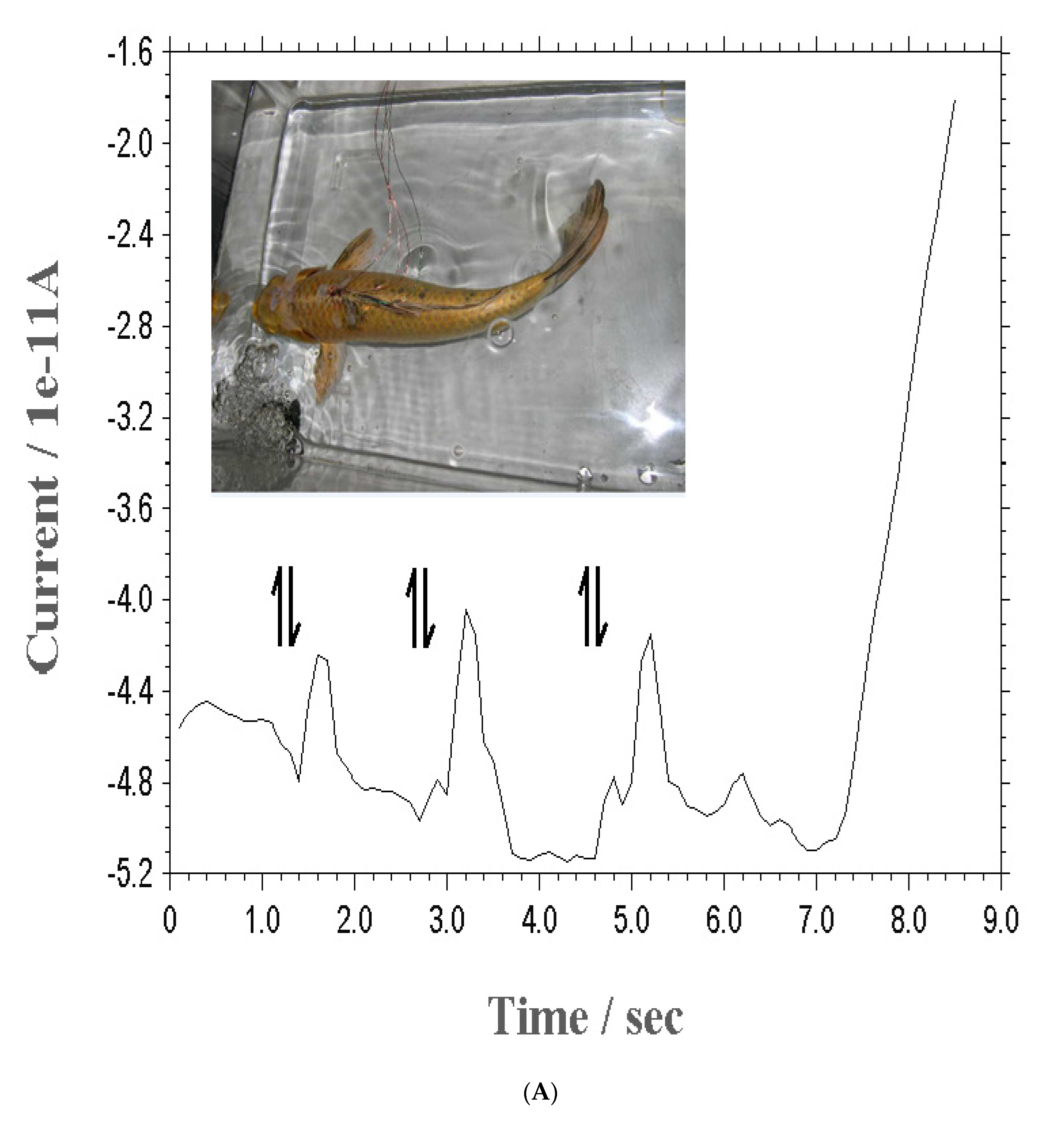
in-vivo implantation, which indicated greater sensitivity when applied to living brain tissue. A working electrode was inserted in the rat brain’s 0.5-mm core using a 0.3-mm-diameter needle-type micro hand drill, under anesthesia. A 10-mm-long, 0.1-mm-diameter Ag/AgCl Cl-coated Ag wire was used as the reference electrode. A counter electrode (0.1-mm-diameter Pt) was inserted 5 mm deep into the backbone tissue, and all the electrodes were cemented with a tooth binder and were connected to a 0.05-mm enamel-coated copper wire with a voltammetric system.
Figure 3(C) shows the chronoamperometric peak current results, under no-sensing conditions, with light, smell, noise, and any vibration. At the first peak, a simple, linear curve was obtained. Then a small test was performed three times, using ammonium solutions. Here, the chrono-range 24, 0.11, and 0.07x10
-5 A peak currents were obtained at 3.58-5.98 sec/V. As satisfactory sensing-signal results were obtained, the proposed method can be used for extracellular, neuropsychiatric, and other types of neurocontrol.
Conclusions
Electrochemical neuro sensing were sought using a voltammetric bio circuit, and a microprobe was directly implanted into the bran systems. The optimum diagnostic conditions were set at 30 s accumulation time, 2.0 V initial potential, -2.0 V switching potential on CV, and -0.3 or 0.3 V chronoamperometric potential, under the optimized potential can be attained to nano working current. Here, an eye action of muscular current was obtained, and the implanted in-vivo probe was activated to the five-sensing neuro assay. The results can be applied to brain sensing and to any other field requiring mammalian extracellular nerve analysis or real time in-vivo electro physiologic assay.
Author Contributions
This idea from Suw Young Ly, All authors read and approved the final manuscript.
Funding
This paper was supported by the academic research fund of Dr Myung Ki (Mike) Hong in 2021.
Declarations Ethics approval and consent to participate
All experiments were performed according to established guidelines for the ethical use.
Consent for publication
Not applicable.
Data Availability Statement
All materials are available by the corresponding author.
Conflicts of Interest
declare no conflict of interest.
References
- Ron-Angevin, R.; Díaz-Estrella, A. Brain–computer interface: Changes in performance using virtual reality techniques. Neurosci. Lett. 2009, 449, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Logar, V.; Škrjanc, I.; Belič, A.; Brežan, S.; Koritnik, B.; Zidar, J. Identification of the phase code in an EEG during gripping-force tasks: A possible alternative approach to the development of the brain-computer interfaces. Artif. Intell. Med. 2008, 44, 41–49. [Google Scholar] [CrossRef]
- Kübler, A.; Birbaumer, N. Brain–computer interfaces and communication in paralysis: Extinction of goal directed thinking in completely paralysed patients? Clin. Neurophysiol. 2008, 119, 2658–2666. [Google Scholar] [CrossRef]
- Valerie Morash, Ou Bai, Stephen Furlani, Peter Lin, Mark Hallett, Classifying EEG signals preceding right hand, left hand, tongue, and right foot movements and motor imageries, Clinical Neurophysiology 119 (2008) 2570–2578.
- Leocani, L.; Magnani, G.; Locatelli, T.; Martinelli, V.; Rovaris, M.; Filippi, M.; Falautano, M.; Santuccio, G.; Possa, F.; Comi, G. EEG correlates of cognitive impairment in MS. Neurol. Sci. 1998, 19, S413–S417. [Google Scholar] [CrossRef]
- Petsche, H.; Dimitrov, L.I.; Filz, O.; Wenger, E.; Holländer, I. The reflection of cognitive tasks in EEG and MRI and a method of its visualization. Brain Topogr. 1997, 9, 177–189. [Google Scholar] [CrossRef]
- Sijbers, J.; Van Audekerke, J.; Verhoye, M.; Van der Linden, A.; Van Dyck, D. Reduction of ECG and gradient related artifacts in simultaneously recorded human EEG/MRI data. Magn. Reson. Imaging 2000, 18, 881–886. [Google Scholar] [CrossRef]
- Roberts, K.; Papadaki, A.; Gonçalves, C.; Tighe, M.; Atherton, D.; Shenoy, R.; McRobbie, D.; Anand, P. Contact heat evoked potentials using simultaneous EEG and fMRI and their correlation with evoked pain. BMC Anesthesiol. 2008, 8, 8–8. [Google Scholar] [CrossRef] [PubMed]
- Ly, S.Y.; Lee, J.H. Human-Urine Diabetes Assay and In Vivo Rat Bladder Assay Using a Fluorine-Doped Carbon Nanotube Catheter Sensor. Ann. Biomed. Eng. 2009, 37, 2028–2033. [Google Scholar] [CrossRef] [PubMed]
- Ly, S.; Kim, D. Electrochemical Assay of Neurotransmitter Glycine in Brain Cells. Bull. Korean Chem. Soc. 2007, 28, 515–519. [Google Scholar] [CrossRef]
- Ly, S.Y.; Lee, C.H.; Jung, Y.S. Voltammetric Bioassay of Caffeine using Sensor Implant. NeuroMolecular Med. 2009, 11, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ly, S.Y. Diagnosis of copper ions in vascular tracts using a fluorine-doped carbon nanotube sensor. Talanta 2008, 74, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Ly, S.Y.; Choi, D.W. Implementation of a biocircuit implants for neurotransmitter release during neuro-stimulation. Curr. Neurovascular Res. 2013, 10, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Ly, S.Y.; Choa, S.H.; Shin, M.H.; Shin, H.J.; Kim, S.-R.; Yoo, H.-S.; Jung, Y.S.; Choi, J.B. Trace Uranium Assay Using Infrared Photo Diode Electrodes. Anal. Lett. 2010, 43, 1471–1480. [Google Scholar] [CrossRef]
- Ly, S.Y.; Kim, Y.K. Assay of glucose in urine and drinking water with voltammetric working sensors of infrared photo diode electrode. Sensors Actuators A: Phys. 2006, 127, 41–48. [Google Scholar] [CrossRef]
- Suw Young Ly․ Chang Hyun Lee, Clinical In Vivo Bio Assay of Glucose in Human Skin by a Tattoo Film Carbon Nano Tube Sensor, Journal of Oil & Applied Science Vol. 34, No. 3. September. 595~601,2017.
- Ly, S.-Y.; Yoo, H.-S.; Chun, S.-K. Detection of trace metal in distilled alcoholic drinks. Food Chem. 2013, 137, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Ly, S.Y.; Yoo, S.D.; Chun, S.K. Detection of Helicobacter pylori DNA in preliminary stage gastric cancer cells. Pathology 2012, 44, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Ly, S.Y.; Lee, J.-H.; Jung, D.H. Radioactive uranium measurement in vivo using a handheld interfaced analyzer. Environ. Toxicol. Chem. 2010, 29, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Ly, S.Y.; Heo, H.J.; Kim, M.J. Real Time Analysis of Neurotransmitters in the Brain Using a Micro-Electrode System. Curr. Neurovascular Res. 2010, 7, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Suw Young Ly; Yeoung Chan Kim; Huck Jun Hong; Jeongha Kim; Kyung Lee.
- Rapid Voltammetric Diagnosis of Escherichia Coli Contamination in Non-Treated Human Blood Plasma of Healthy and Sepsis-Infected Patients, IEEE Sensors 18, 2200-2205, 2018.
- Suw Young Ly; Kyung Lee; Hyung Tae Kang; Hai Soo Yoo Investigation of Trace Cobalt in Glass Beads From an Archaeological Tomb, IEEE Sensors Journal 6, 1325-1328, 2011.
- Cho, I.H.; Choi, K.J.; Choi, J.; Lee, K.; Ly, S.Y. Trace assay of insulin in a pharmacy drug with a paste electrode. Amino Acids 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ly, S.Y.; Choi, K.J.; Kim, J.H.; Lee, K. In Vivo Diagnostic Real-time Wireless Sensing of Glucose in Human Urine and Live Fish Deep Brain Cells. Int. J. Sensors, Wirel. Commun. Control. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).