Submitted:
16 August 2023
Posted:
17 August 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Grading of Meningiomas
3. Genomic Alterations and Epigenetic Modifications in Meningiomas
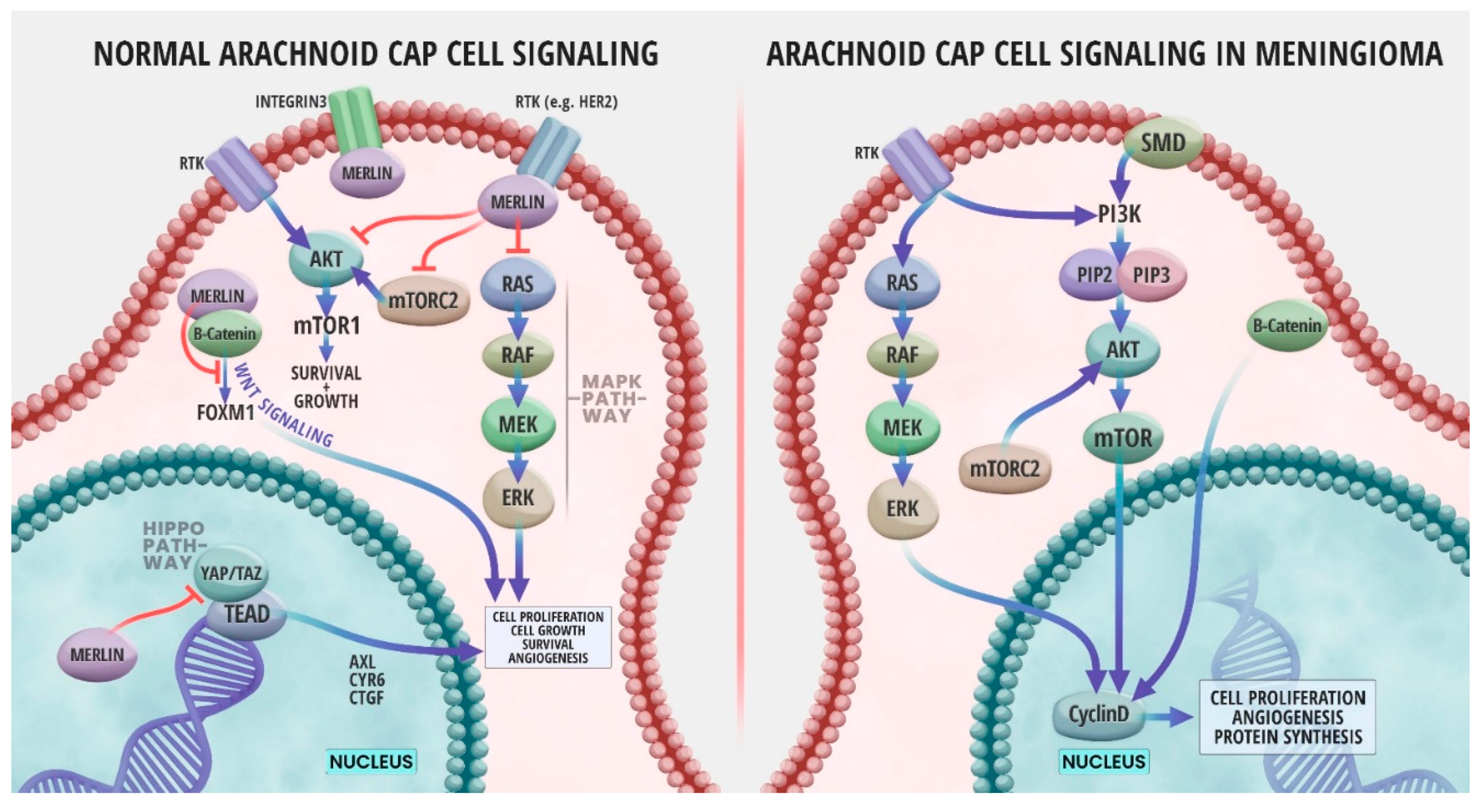
4. NF2/ Merlin Signaling Pathways in Meningioma
5. Biomarkers of Meningiomas
5.1. Current Diagnosis and Prognosis
5.2. The Need for a Profile of Biomarkers of Different Types
5.3. Exploring Protein Biomarkers as Meningioma Biomarkers
a. Serum Protein Biomarkers
b. Cerebrospinal Fluid Protein Biomarkers
5.4. LncRNA and miRNA in Diagnosis and Prognosis of Meningiomas
6. Animal Models for Discovery of Meningioma Biomarkers
7. Conclusions
Funding
Conflicts of Interest
References
- Gittleman, H.R.; Ostrom, Q.T.; Rouse, C.D.; Dowling, J.A.; de Blank, P.M.; Kruchko, C.A.; Elder, J.B.; Rosenfeld, S.S.; Selman, W.R.; Sloan, A.E.; et al. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer 2015, 121, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncology 2017, 19, v1–v88. [Google Scholar] [CrossRef] [PubMed]
- Flint-Richter, P.; Mandelzweig, L.; Oberman, B.; Sadetzki, S. Possible interaction between ionizing radiation, smoking, and gender in the causation of meningioma. Neuro-Oncology 2011, 13, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Pülhorn, H.; Röhrig, B.; Rainov, N.G. Predisposing conditions and risk factors for development of symptomatic meningioma in adults. Cancer Detection and Prevention 2005, 29, 440–447. [Google Scholar] [CrossRef]
- Achey, R.L.; Gittleman, H.; Schroer, J.; Khanna, V.; Kruchko, C.; Barnholtz-Sloan, J.S. Nonmalignant and malignant meningioma incidence and survival in the elderly, 2005–2015, using the Central Brain Tumor Registry of the United States. Neuro-Oncology 2019, 21, 380–391. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathologica 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Yang, S.Y.; Park, C.K.; Park, S.H.; Kim, D.G.; Chung, Y.S.; Jung, H.W. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry 2008, 79, 574–580. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Loewenstern, J.; Rutland, J.; Gill, C.; Arib, H.; Pain, M.; Umphlett, M.; Kinoshita, Y.; McBride, R.; Donovan, M.; Sebra, R.; et al. Comparative genomic analysis of driver mutations in matched primary and recurrent meningiomas. Oncotarget 2019, 10, 3506–3517. [Google Scholar] [CrossRef]
- Dumanski, J.P.; Carlbom, E.; Collins, V.P.; Nordenskjöld, M. Deletion mapping of a locus on human chromosome 22 involved in the oncogenesis of meningioma. Proceedings of the National Academy of Sciences 1987, 84, 9275–9279. [Google Scholar] [CrossRef]
- Schneider, G.; Lutz, S.; Henn, W.; Zang, K.D.; Blin, N. Search for putative suppressor genes in meningioma: significance of chromosome 22. Human Genetics 1992, 88, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Wellenreuther, R.; Kraus, J.A.; Lenartz, D.; Menon, A.G.; Schramm, J.; Louis, D.N.; Ramesh, V.; Gusella, J.F.; Wiestler, O.D.; von Deimling, A. Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. The American journal of pathology 1995, 146, 827–832. [Google Scholar] [PubMed]
- Gutmann, D.H.; Giordano, M.J.; Fishback, A.S.; Guha, A. Loss of merlin expression in sporadic meningiomas, ependymomas and schwannomas. Neurology 1997, 49, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Ruttledge, M.H.; Sarrazin, J.; Rangaratnam, S.; Phelan, C.M.; Twist, E.; Merel, P.; Delattre, O.; Thomas, G.; Nordenskjöld, M.; Collins, V.P.; et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet 1994, 6, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, M.D.; Espinosa, A.B.; Maíllo, A.; Sayagués, J.M.; Alguero, M.d.C.; Lumbreras, E.; Díaz, P.; Gonçalves, J.M.; Onzain, I.; Merino, M.; et al. Characterization of chromosome 14 abnormalities by interphase in situ hybridization and comparative genomic hybridization in 124 meningiomas: correlation with clinical, histopathologic, and prognostic features. American journal of clinical pathology 2005, 123, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Sayagués, J.M.; Tabernero, M.D.; Maíllo, A.; Espinosa, A.; Rasillo, A.; Díaz, P.; Ciudad, J.; López, A.; Merino, M.; Gonçalves, J.M.; et al. Intratumoral Patterns of Clonal Evolution in Meningiomas as Defined by Multicolor Interphase Fluorescence in Situ Hybridization (FISH). The Journal of Molecular Diagnostics 2004, 6, 316–325. [Google Scholar] [CrossRef]
- Strickland, M.R.; Gill, C.M.; Nayyar, N.; D'Andrea, M.R.; Thiede, C.; Juratli, T.A.; Schackert, G.; Borger, D.R.; Santagata, S.; Frosch, M.P.; et al. Targeted sequencing of SMO and AKT1 in anterior skull base meningiomas. Journal of Neurosurgery 2017, 127, 438–444. [Google Scholar] [CrossRef]
- Clark, V.E.; Harmancı, A.S.; Bai, H.; Youngblood, M.W.; Lee, T.I.; Baranoski, J.F.; Ercan-Sencicek, A.G.; Abraham, B.J.; Weintraub, A.S.; Hnisz, D.; et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nature Genetics 2016, 48, 1253–1259. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Horowitz, P.M.; Santagata, S.; Jones, R.T.; McKenna, A.; Getz, G.; Ligon, K.L.; Palescandolo, E.; Van Hummelen, P.; Ducar, M.D.; et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nature Genetics 2013, 45, 285–289. [Google Scholar] [CrossRef]
- Sahm, F.; Bissel, J.; Koelsche, C.; Schweizer, L.; Capper, D.; Reuss, D.; Böhmer, K.; Lass, U.; Göck, T.; Kalis, K.; et al. AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. Acta Neuropathologica 2013, 126, 757–762. [Google Scholar] [CrossRef]
- Reuss, D.E.; Piro, R.M.; Jones, D.T.W.; Simon, M.; Ketter, R.; Kool, M.; Becker, A.; Sahm, F.; Pusch, S.; Meyer, J.; et al. Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathologica 2013, 125, 351–358. [Google Scholar] [CrossRef]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Ozduman, K.; Avsar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef]
- Aldape, K.; Nejad, R.; Louis, D.N.; Zadeh, G. Integrating molecular markers into the World Health Organization classification of CNS tumors: a survey of the neuro-oncology community. Neuro Oncol 2017, 19, 336–344. [Google Scholar] [CrossRef]
- Papaioannou, M.-D.; Djuric, U.; Kao, J.; Karimi, S.; Zadeh, G.; Aldape, K.; Diamandis, P. Proteomic analysis of meningiomas reveals clinically distinct molecular patterns. Neuro-Oncology 2019, 21, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Wang, J.Z.; Singh, O.; Karimi, S.; Dalcourt, T.; Ijad, N.; Pirouzmand, N.; Ng, H.K.; Saladino, A.; Pollo, B.; et al. Loss of H3K27me3 in meningiomas. Neuro Oncol 2021, 23, 1282–1291. [Google Scholar] [CrossRef]
- Katz, L.M.; Hielscher, T.; Liechty, B.; Silverman, J.; Zagzag, D.; Sen, R.; Wu, P.; Golfinos, J.G.; Reuss, D.; Neidert, M.C.; et al. Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. Acta Neuropathologica 2018, 135, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.J.; Amiñoso, C.; Lopez-Marin, I.; Arjona, D.; Gonzalez-Gomez, P.; Alonso, M.E.; Lomas, J.; de Campos, J.M.; Kusak, M.E.; Vaquero, J.; et al. DNA methylation of multiple promoter-associated CpG islands in meningiomas: relationship with the allelic status at 1p and 22q. Acta Neuropathol 2004, 108, 413–421. [Google Scholar] [CrossRef]
- Barski, D.; Wolter, M.; Reifenberger, G.; Riemenschneider, M.J. Hypermethylation and transcriptional downregulation of the TIMP3 gene is associated with allelic loss on 22q12.3 and malignancy in meningiomas. Brain Pathol 2010, 20, 623–631. [Google Scholar] [CrossRef]
- Nazem, A.A.; Ruzevick, J.; Ferreira, M.J. Advances in meningioma genomics, proteomics, and epigenetics: insights into biomarker identification and targeted therapies. Oncotarget 2020, 11, 4544–4553. [Google Scholar] [CrossRef]
- Kshettry, V.R.; Ostrom, Q.T.; Kruchko, C.; Al-Mefty, O.; Barnett, G.H.; Barnholtz-Sloan, J.S. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro-Oncology 2015, 17, 1166–1173. [Google Scholar] [CrossRef]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. The Lancet Oncology 2016, 17, e383–e391. [Google Scholar] [CrossRef] [PubMed]
- Hashiba, T.; Hashimoto, N.; Izumoto, S.; Suzuki, T.; Kagawa, N.; Maruno, M.; Kato, A.; Yoshimine, T. Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas. Journal of Neurosurgery 2009, 110, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An overview of meningiomas. Future Oncology 2018, 14, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Chotai, S.; Schwartz, T.H. The Simpson Grading: Is It Still Valid? Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Gottschling, S.; Bolz, J.; Kornhuber, M.; Alfieri, A.; Holzhausen, H.-J.; Abbas, J.; Kösling, S. Distant metastases in meningioma: an underestimated problem. Journal of Neuro-Oncology 2013, 112, 323–327. [Google Scholar] [CrossRef]
- Enomoto, T.; Aoki, M.; Kouzaki, Y.; Abe, H.; Imamura, N.; Iwasaki, A.; Inoue, T.; Nabeshima, K. WHO Grade I Meningioma Metastasis to the Lung 26 Years after Initial Surgery: A Case Report and Literature Review. NMC Case Report Journal 2019, 6, 125–129. [Google Scholar] [CrossRef]
- Paix, A.; Waissi, W.; Antoni, D.; Adeduntan, R.; Noël, G. Visceral and bone metastases of a WHO grade 2 meningioma: A case report and review of the literature. Cancer/Radiothérapie 2017, 21, 55–59. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Duan, Y.; Ye, Y.; Xiao, L.; Mao, R. Subcutaneous Metastasis of Atypical Meningioma: Case Report and Literature Review. World Neurosurgery 2020, 138, 182–186. [Google Scholar] [CrossRef]
- Tsitsikov, E.N.; Hameed, S.; Tavakol, S.A.; Stephens, T.M.; Tsytsykova, A.V.; Garman, L.; Bi, W.L.; Dunn, I.F. Specific gene expression signatures of low grade meningiomas. Front Oncol 2023, 13, 1126550. [Google Scholar] [CrossRef]
- Harmancı, A.S.; Youngblood, M.W.; Clark, V.E.; Coşkun, S.; Henegariu, O.; Duran, D.; Erson-Omay, E.Z.; Kaulen, L.D.; Lee, T.I.; Abraham, B.J.; et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun 2017, 8, 14433. [Google Scholar] [CrossRef]
- Patel, A.J.; Wan, Y.W.; Al-Ouran, R.; Revelli, J.P.; Cardenas, M.F.; Oneissi, M.; Xi, L.; Jalali, A.; Magnotti, J.F.; Muzny, D.M.; et al. Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors. Proc Natl Acad Sci U S A 2019, 116, 21715–21726. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A clinically applicable integrative molecular classification of meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Bayley, J.C.t.; Hadley, C.C.; Harmanci, A.O.; Harmanci, A.S.; Klisch, T.J.; Patel, A.J. Multiple approaches converge on three biological subtypes of meningioma and extract new insights from published studies. Sci Adv 2022, 8, eabm6247. [Google Scholar] [CrossRef] [PubMed]
- Torp, S.H.; Solheim, O.; Skjulsvik, A.J. The WHO 2021 Classification of Central Nervous System tumours: a practical update on what neurosurgeons need to know-a minireview. Acta Neurochir (Wien) 2022, 164, 2453–2464. [Google Scholar] [CrossRef]
- Marastoni, E.; Barresi, V. Meningioma Grading beyond Histopathology: Relevance of Epigenetic and Genetic Features to Predict Clinical Outcome. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Pawloski, J.A.; Fadel, H.A.; Huang, Y.W.; Lee, I.Y. Genomic Biomarkers of Meningioma: A Focused Review. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Ruttledge, M.H.; Xie, Y.G.; Han, F.Y.; Giovannini, M.; Janson, M.; Fransson, I.; Werelius, B.; Delattre, O.; Thomas, G.; Evans, G.; et al. Physical mapping of the NF2/meningioma region on human chromosome 22q12. Genomics 1994, 19, 52–59. [Google Scholar] [CrossRef]
- Ichimura, K.; Yuasa, Y. [Molecular biological analysis of neurofibromatosis type 2 gene]. Nihon Rinsho 1993, 51, 2462–2466. [Google Scholar]
- Cui, Y.; Ma, L.; Schacke, S.; Yin, J.C.; Hsueh, Y.P.; Jin, H.; Morrison, H. Merlin cooperates with neurofibromin and Spred1 to suppress the Ras-Erk pathway. Hum Mol Genet 2021, 29, 3793–3806. [Google Scholar] [CrossRef]
- Maggio, I.; Franceschi, E.; Tosoni, A.; Nunno, V.D.; Gatto, L.; Lodi, R.; Brandes, A.A. Meningioma: not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol 2021, 10, Cns72. [Google Scholar] [CrossRef]
- Moussalem, C.; Massaad, E.; Minassian, G.B.; Ftouni, L.; Bsat, S.; Houshiemy, M.N.E.; Alomari, S.; Sarieddine, R.; Kobeissy, F.; Omeis, I. Meningioma genomics: a therapeutic challenge for clinicians. J Integr Neurosci 2021, 20, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Hua, L.; Han, T.; Tian, M.; Wang, D.; Tang, H.; Sun, S.; Chen, H.; Cheng, H.; Zhang, T.; et al. The CREB-binding protein inhibitor ICG-001: a promising therapeutic strategy in sporadic meningioma with NF2 mutations. Neurooncol Adv 2020, 2, vdz055. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, M.W.; Miyagishima, D.F.; Jin, L.; Gupte, T.; Li, C.; Duran, D.; Montejo, J.D.; Zhao, A.; Sheth, A.; Tyrtova, E.; et al. Associations of meningioma molecular subgroup and tumor recurrence. Neuro Oncol 2021, 23, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Abedalthagafi, M.; Bi, W.L.; Aizer, A.A.; Merrill, P.H.; Brewster, R.; Agarwalla, P.K.; Listewnik, M.L.; Dias-Santagata, D.; Thorner, A.R.; Van Hummelen, P.; et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro Oncol 2016, 18, 649–655. [Google Scholar] [CrossRef]
- Bi, W.L.; Zhang, M.; Wu, W.W.; Mei, Y.; Dunn, I.F. Meningioma Genomics: Diagnostic, Prognostic, and Therapeutic Applications. Front Surg 2016, 3, 40. [Google Scholar] [CrossRef]
- Zotti, T.; Scudiero, I.; Vito, P.; Stilo, R. The Emerging Role of TRAF7 in Tumor Development. J Cell Physiol 2017, 232, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yuan, J.; Deng, Y.; Fan, X.; Shen, J. Emerging role of SWI/SNF complex deficiency as a target of immune checkpoint blockade in human cancers. Oncogenesis 2021, 10, 3. [Google Scholar] [CrossRef]
- Ross, J.P.; Rand, K.N.; Molloy, P.L. Hypomethylation of repeated DNA sequences in cancer. Epigenomics 2010, 2, 245–269. [Google Scholar] [CrossRef]
- Robert, S.M.; Vetsa, S.; Nadar, A.; Vasandani, S.; Youngblood, M.W.; Gorelick, E.; Jin, L.; Marianayagam, N.; Erson-Omay, E.Z.; Günel, M.; et al. The integrated multiomic diagnosis of sporadic meningiomas: a review of its clinical implications. J Neurooncol 2022, 156, 205–214. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, K. Pan-cancer analysis of frequent DNA co-methylation patterns reveals consistent epigenetic landscape changes in multiple cancers. BMC Genomics 2017, 18, 1045. [Google Scholar] [CrossRef]
- Nassiri, F.; Mamatjan, Y.; Suppiah, S.; Badhiwala, J.H.; Mansouri, S.; Karimi, S.; Saarela, O.; Poisson, L.; Gepfner-Tuma, I.; Schittenhelm, J.; et al. DNA methylation profiling to predict recurrence risk in meningioma: development and validation of a nomogram to optimize clinical management. Neuro Oncol 2019, 21, 901–910. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, R.; Shukla, N.; Narwal, P.; Katiyar, A.; Mahajan, S.; Sahu, S.; Garg, A.; Sharma, M.C.; Suri, A.; et al. DNA methylation profiling of meningiomas highlights clinically distinct molecular subgroups. J Neurooncol 2023, 161, 339–356. [Google Scholar] [CrossRef]
- Choudhury, A.; Magill, S.T.; Eaton, C.D.; Prager, B.C.; Chen, W.C.; Cady, M.A.; Seo, K.; Lucas, C.G.; Casey-Clyde, T.J.; Vasudevan, H.N.; et al. Meningioma DNA methylation groups identify biological drivers and therapeutic vulnerabilities. Nat Genet 2022, 54, 649–659. [Google Scholar] [CrossRef]
- Jiang, C.; Song, T.; Li, J.; Ao, F.; Gong, X.; Lu, Y.; Zhang, C.; Chen, L.; Liu, Y.; He, H.; et al. RAS Promotes Proliferation and Resistances to Apoptosis in Meningioma. Mol Neurobiol 2017, 54, 779–787. [Google Scholar] [CrossRef]
- Pecina-Slaus, N. Merlin, the NF2 gene product. Pathol Oncol Res 2013, 19, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Mawrin, C.; Sasse, T.; Kirches, E.; Kropf, S.; Schneider, T.; Grimm, C.; Pambor, C.; Vorwerk, C.K.; Firsching, R.; Lendeckel, U.; et al. Different activation of mitogen-activated protein kinase and Akt signaling is associated with aggressive phenotype of human meningiomas. Clin Cancer Res 2005, 11, 4074–4082. [Google Scholar] [CrossRef] [PubMed]
- G Mougel, G.M. , R Appay, A Querdray, C Roche, A Jijon, I Konstantinova, A Soude, T Graillon, A Barlier. A Hippo signaling pathway is strongly involved in meningioma tumorigenesis Neuro-Oncology 2022, 24. [Google Scholar] [CrossRef]
- Hong, A.W.; Meng, Z.; Plouffe, S.W.; Lin, Z.; Zhang, M.; Guan, K.L. Critical roles of phosphoinositides and NF2 in Hippo pathway regulation. Genes Dev 2020, 34, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Baia, G.S.; Caballero, O.L.; Riggins, G.J. The Hippo signaling pathway and translational opportunities for brain cancers. CNS Oncol 2012, 1, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Sievers, P.; Chiang, J.; Schrimpf, D.; Stichel, D.; Paramasivam, N.; Sill, M.; Gayden, T.; Casalini, B.; Reuss, D.E.; Dalton, J.; et al. YAP1-fusions in pediatric NF2-wildtype meningioma. Acta Neuropathol 2020, 139, 215–218. [Google Scholar] [CrossRef]
- Laraba, L.; Hillson, L.; de Guibert, J.G.; Hewitt, A.; Jaques, M.R.; Tang, T.T.; Post, L.; Ercolano, E.; Rai, G.; Yang, S.M.; et al. Inhibition of YAP/TAZ-driven TEAD activity prevents growth of NF2-null schwannoma and meningioma. Brain 2022. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front Oncol 2022, 12, 819128. [Google Scholar] [CrossRef] [PubMed]
- Rong, R.; Tang, X.; Gutmann, D.H.; Ye, K. Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L. Proc Natl Acad Sci U S A 2004, 101, 18200–18205. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Karas, P.J.; Hadley, C.C.; Bayley, V.J.; Khan, A.B.; Jalali, A.; Sweeney, A.D.; Klisch, T.J.; Patel, A.J. The Role of Merlin/NF2 Loss in Meningioma Biology. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Barresi, V.; Lionti, S.; La Rocca, L.; Caliri, S.; Caffo, M. High p-mTOR expression is associated with recurrence and shorter disease-free survival in atypical meningiomas. Neuropathology 2019, 39, 22–29. [Google Scholar] [CrossRef]
- James, M.F.; Han, S.; Polizzano, C.; Plotkin, S.R.; Manning, B.D.; Stemmer-Rachamimov, A.O.; Gusella, J.F.; Ramesh, V. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol 2009, 29, 4250–4261. [Google Scholar] [CrossRef]
- Shih, K.C.; Chowdhary, S.; Rosenblatt, P.; Weir, A.B., 3rd; Shepard, G.C.; Williams, J.T.; Shastry, M.; Burris, H.A., 3rd; Hainsworth, J.D. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J Neurooncol 2016, 129, 281–288. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Kumthekar, P.; Wen, P.Y.; Barker, F.G.; Stemmer-Rachamimov, A.; Beauchamp, R.L.; Jordan, J.T.; Muzikansky, A.; Ramesh, V. Multi-center, single arm phase II study of the dual mTORC1/mTORC2 inhibitor vistusertib for patients with recurrent or progressive grade II-III meningiomas. Journal of Clinical Oncology 2021, 39, 2024–2024. [Google Scholar] [CrossRef]
- Pecina-Slaus, N.; Kafka, A.; Lechpammer, M. Molecular Genetics of Intracranial Meningiomas with Emphasis on Canonical Wnt Signalling. Cancers (Basel) 2016, 8. [Google Scholar] [CrossRef]
- Lau, Y.K.; Murray, L.B.; Houshmandi, S.S.; Xu, Y.; Gutmann, D.H.; Yu, Q. Merlin is a potent inhibitor of glioma growth. Cancer Res 2008, 68, 5733–5742. [Google Scholar] [CrossRef]
- Nowosielski, M.; Galldiks, N.; Iglseder, S.; Kickingereder, P.; von Deimling, A.; Bendszus, M.; Wick, W.; Sahm, F. Diagnostic challenges in meningioma. Neuro-Oncology 2017, 19, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Tagle, P.; Villanueva, P.; Torrealba, G.; Huete, I. Intracranial metastasis or meningioma? Surgical Neurology 2002, 58, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Bendszus, M.; Warmuth-Metz, M.; Burger, R.; Klein, R.; Tonn, J.C.; Solymosi, L. Diagnosing dural metastases: the value of 1 H magnetic resonance spectroscopy. Neuroradiology 2001, 43, 285–289. [Google Scholar] [CrossRef]
- Bourekas, E.C.; Wildenhain, P.; Lewin, J.S.; Tarr, R.W.; Dastur, K.J.; Raji, M.R.; Lanzieri, C.F. The dural tail sign revisited. AJNR. American journal of neuroradiology 1995, 16, 1514–1516. [Google Scholar]
- Wilms, G.; Lammens, M.; Marchal, G.; Demaerel, P.; Verplancke, J.; Van Calenbergh, F.; Goffin, J.; Plets, C.; Baert, A.L. Prominent dural enhancement adjacent to nonmeningiomatous malignant lesions on contrast-enhanced MR images. AJNR. American journal of neuroradiology 12.
- Huttner, H.B.; Bergmann, O.; Salehpour, M.; El Cheikh, R.; Nakamura, M.; Tortora, A.; Heinke, P.; Coras, R.; Englund, E.; Eyüpoglu, I.Y.; et al. Meningioma growth dynamics assessed by radiocarbon retrospective birth dating. EBioMedicine 2018, 27, 176–181. [Google Scholar] [CrossRef]
- Narai, A.; Hermann, P.; Auer, T.; Kemenczky, P.; Szalma, J.; Homolya, I.; Somogyi, E.; Vakli, P.; Weiss, B.; Vidnyanszky, Z. Movement-related artefacts (MR-ART) dataset of matched motion-corrupted and clean structural MRI brain scans. Sci Data 2022, 9, 630. [Google Scholar] [CrossRef]
- Usman, M.; Latif, S.; Asim, M.; Lee, B.-D.; Qadir, J. Retrospective Motion Correction in Multishot MRI using Generative Adversarial Network. Scientific Reports 2020, 10, 4786. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.N.; Braun, Y.; Plate, K.H. Classification of meningiomas—advances and controversies. Chinese Clinical Oncology 2017, 6, S2–S2. [Google Scholar] [CrossRef]
- Rogers, C.L.; Perry, A.; Pugh, S.; Vogelbaum, M.A.; Brachman, D.; McMillan, W.; Jenrette, J.; Barani, I.; Shrieve, D.; Sloan, A.; et al. Pathology concordance levels for meningioma classification and grading in NRG Oncology RTOG Trial 0539. Neuro-Oncology 2016, 18, 565–574. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, G.-J.; Zhang, G.-B.; Wang, L.; Wu, Z.; Jia, W.; Hao, S.-Y.; Ni, M.; Li, D.; Wang, K.; et al. A Logistic Regression Model for Detecting the Presence of Malignant Progression in Atypical Meningiomas. World Neurosurgery 2019, 126, e392–e401. [Google Scholar] [CrossRef]
- Parada, C.A.; Osbun, J.W.; Busald, T.; Karasozen, Y.; Kaur, S.; Shi, M.; Barber, J.; Adidharma, W.; Cimino, P.J.; Pan, C.; et al. Phosphoproteomic and Kinomic Signature of Clinically Aggressive Grade I (1.5) Meningiomas Reveals RB1 Signaling as a Novel Mediator and Biomarker. Clinical Cancer Research 2020, 26, 193–205. [Google Scholar] [CrossRef]
- Deng, J.; Hua, L.; Bian, L.; Chen, H.; Chen, L.; Cheng, H.; Dou, C.; Geng, D.; Hong, T.; Ji, H.; et al. Molecular diagnosis and treatment of meningiomas: an expert consensus (2022). Chinese Medical Journal 2022, 135, 1894–1912. [Google Scholar] [CrossRef]
- Gritsch, S.; Batchelor, T.T.; Gonzalez Castro, L.N. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 2022, 128, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashed, M.; Foshay, K.; Abedalthagafi, M. Recent Advances in Meningioma Immunogenetics. Front Oncol 2019, 9, 1472. [Google Scholar] [CrossRef]
- Sofela, A.A.; McGavin, L.; Whitfield, P.C.; Hanemann, C.O. Biomarkers for differentiating grade II meningiomas from grade I: a systematic review. Br J Neurosurg 2021, 35, 696–702. [Google Scholar] [CrossRef]
- Kishida, Y.; Natsume, A.; Kondo, Y.; Takeuchi, I.; An, B.; Okamoto, Y.; Shinjo, K.; Saito, K.; Ando, H.; Ohka, F.; et al. Epigenetic subclassification of meningiomas based on genome-wide DNA methylation analyses. Carcinogenesis 2012, 33, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Olar, A.; Wani, K.M.; Wilson, C.D.; Zadeh, G.; DeMonte, F.; Jones, D.T.; Pfister, S.M.; Sulman, E.P.; Aldape, K.D. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol 2017, 133, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Schrimpf, D.; Stichel, D.; Jones, D.T.W.; Hielscher, T.; Schefzyk, S.; Okonechnikov, K.; Koelsche, C.; Reuss, D.E.; Capper, D.; et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol 2017, 18, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Stichel, D.; Hielscher, T.; Sievers, P.; Berghoff, A.S.; Schrimpf, D.; Sill, M.; Euskirchen, P.; Blume, C.; Patel, A.; et al. Integrated Molecular-Morphologic Meningioma Classification: A Multicenter Retrospective Analysis, Retrospectively and Prospectively Validated. J Clin Oncol 2021, 39, 3839–3852. [Google Scholar] [CrossRef]
- Driver, J.; Hoffman, S.E.; Tavakol, S.; Woodward, E.; Maury, E.A.; Bhave, V.; Greenwald, N.F.; Nassiri, F.; Aldape, K.; Zadeh, G.; et al. A molecularly integrated grade for meningioma. Neuro Oncol 2022, 24, 796–808. [Google Scholar] [CrossRef]
- Liu, J.; Xia, C.; Wang, G. Multi-Omics Analysis in Initiation and Progression of Meningiomas: From Pathogenesis to Diagnosis. Frontiers in Oncology 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Suppiah, S.; Tonge, P.D.; Jalali, S.; Danesh, A.; Bruce, J.P.; Mamatjan, Y.; Klironomos, G.; Gonen, L.; Au, K.; et al. Therapeutic radiation for childhood cancer drives structural aberrations of NF2 in meningiomas. Nat Commun 2017, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Qian, J.; Ma, C. MPscore: A Novel Predictive and Prognostic Scoring for Progressive Meningioma. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Wang, A.Z.; Bowman-Kirigin, J.A.; Desai, R.; Kang, L.-I.; Patel, P.R.; Patel, B.; Khan, S.M.; Bender, D.; Marlin, M.C.; Liu, J.; et al. Single-cell profiling of human dura and meningioma reveals cellular meningeal landscape and insights into meningioma immune response. Genome Medicine 2022, 14, 49. [Google Scholar] [CrossRef]
- Shi, J. Machine learning and bioinformatics approaches for classification and clinical detection of bevacizumab responsive glioblastoma subtypes based on miRNA expression. Sci Rep 2022, 12, 8685. [Google Scholar] [CrossRef]
- Kitano, Y.; Aoki, K.; Ohka, F.; Yamazaki, S.; Motomura, K.; Tanahashi, K.; Hirano, M.; Naganawa, T.; Iida, M.; Shiraki, Y.; et al. Urinary MicroRNA-Based Diagnostic Model for Central Nervous System Tumors Using Nanowire Scaffolds. ACS Applied Materials & Interfaces 2021, 13, 17316–17329. [Google Scholar] [CrossRef]
- Bhawal, R.; Oberg, A.L.; Zhang, S.; Kohli, M. Challenges and Opportunities in Clinical Applications of Blood-Based Proteomics in Cancer. Cancers 2020, 12, 2428. [Google Scholar] [CrossRef] [PubMed]
- Gomes Marin, J.F.; Nunes, R.F.; Coutinho, A.M.; Zaniboni, E.C.; Costa, L.B.; Barbosa, F.G.; Queiroz, M.A.; Cerri, G.G.; Buchpiguel, C.A. Theranostics in Nuclear Medicine: Emerging and Re-emerging Integrated Imaging and Therapies in the Era of Precision Oncology. Radiographics 2020, 40, 1715–1740. [Google Scholar] [CrossRef]
- Salgues, B.; Graillon, T.; Horowitz, T.; Chinot, O.; Padovani, L.; Taïeb, D.; Guedj, E. Somatostatin Receptor Theranostics for Refractory Meningiomas. Curr Oncol 2022, 29, 5550–5565. [Google Scholar] [CrossRef]
- Tubre, T.; Hacking, S.; Alexander, A.; Brickman, A.; Delalle, I.; Elinzano, H.; Donahue, J.E. Prostate-Specific Membrane Antigen Expression in Meningioma: A Promising Theranostic Target. J Neuropathol Exp Neurol 2022. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, S.; Pan, T. The role of serum and urinary biomarkers in the diagnosis of early diabetic nephropathy in patients with type 2 diabetes. PeerJ 2019, 7, e7079. [Google Scholar] [CrossRef]
- Erkan, E.P.; Ströbel, T.; Dorfer, C.; Sonntagbauer, M.; Weinhäusel, A.; Saydam, N.; Saydam, O. Circulating Tumor Biomarkers in Meningiomas Reveal a Signature of Equilibrium Between Tumor Growth and Immune Modulation. Frontiers in Oncology 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, A.E.; Givalos, N.; Gakiopoulou, H.; Korkolopoulou, P.; Kotsiakis, X.; Boviatsis, E.; Agrogiannis, G.; Mahera, H.; Patsouris, E. Caspase-3 immunohistochemical expression is a marker of apoptosis, increased grade and early recurrence in intracranial meningiomas. Apoptosis 2007, 12, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Abbritti, R.V.; Polito, F.; Cucinotta, M.; Lo Giudice, C.; Caffo, M.; Tomasello, C.; Germanò, A.; Aguennouz, M. Meningiomas and Proteomics: Focus on New Potential Biomarkers and Molecular Pathways. Cancer Genomics Proteomics 2016, 13, 369–379. [Google Scholar] [PubMed]
- Ghantasala, S.; Pai, M.G.J.; Biswas, D.; Gahoi, N.; Mukherjee, S.; Kp, M.; Nissa, M.U.; Srivastava, A.; Epari, S.; Shetty, P.; et al. Multiple Reaction Monitoring-Based Targeted Assays for the Validation of Protein Biomarkers in Brain Tumors. Front Oncol 2021, 11, 548243. [Google Scholar] [CrossRef] [PubMed]
- Abou Khouzam, R.; Brodaczewska, K.; Filipiak, A.; Zeinelabdin, N.A.; Buart, S.; Szczylik, C.; Kieda, C.; Chouaib, S. Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies. Front Immunol 2020, 11, 613114. [Google Scholar] [CrossRef]
- El-Benhawy, S.A.; Sakr, O.A.; Fahmy, E.I.; Ali, R.A.; Hussein, M.S.; Nassar, E.M.; Salem, S.M.; Abu-Samra, N.; Elzawawy, S. Assessment of Serum Hypoxia Biomarkers Pre- and Post-radiotherapy in Patients with Brain Tumors. J Mol Neurosci 2022. [Google Scholar] [CrossRef]
- Valvona, C.J.; Fillmore, H.L.; Nunn, P.B.; Pilkington, G.J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol 2016, 26, 3–17. [Google Scholar] [CrossRef]
- Yoo, H.; Baia, G.S.; Smith, J.S.; McDermott, M.W.; Bollen, A.W.; VandenBerg, S.R.; Lamborn, K.R.; Lal, A. Expression of the Hypoxia Marker Carbonic Anhydrase 9 Is Associated with Anaplastic Phenotypes in Meningiomas. Clinical Cancer Research 2007, 13, 68–75. [Google Scholar] [CrossRef]
- Fattahi, M.J.; Sedaghat, F.; Malekzadeh, M.; Nejat, A.A.; Poostkar, M.; Saberi, Y.; Taghipour, M.; Ghaderi, A. Endocan serum levels in patients with low- and high-grade meningiomas: does this biomarker have an indicative role? The Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2021, 57, 92. [Google Scholar] [CrossRef]
- Atukeren, P.; Kunbaz, A.; Turk, O.; Kemerdere, R.; Ulu, M.O.; Turkmen Inanir, N.; Tanriverdi, T. Expressions of Endocan in Patients with Meningiomas and Gliomas. Dis Markers 2016, 2016, 7157039. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, T.; Achini, F.; Grotzer, M.A. Targeting cerebrospinal fluid for discovery of brain cancer biomarkers. Journal of Cancer Metastasis and Treatment 2016, 2, 176–187. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.K.; Yoo, Y.C.; Park, N.H.; Park, D.B.; Yoo, J.S.; An, H.J.; Park, Y.M.; Cho, K.G. Proteome analysis of human cerebrospinal fluid as a diagnostic biomarker in patients with meningioma. Med Sci Monit 2012, 18, Br450–Br460. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.; Elabbas, A.; Vyas, A.; Osborne, D.; Chigurupati, H.D.; Abbas, L.F.; Kampa, P.; M, H.F.; Sarwar, H.; Alfonso, M. Utilization of Cerebrospinal Fluid Proteome Analysis in the Diagnosis of Meningioma: A Systematic Review. Cureus 2021, 13, e20707. [Google Scholar] [CrossRef]
- Georgila, K.; Vyrla, D.; Drakos, E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers 2019, 11, 1097. [Google Scholar] [CrossRef]
- Rajagopal, M.U.; Hathout, Y.; MacDonald, T.J.; Kieran, M.W.; Gururangan, S.; Blaney, S.M.; Phillips, P.; Packer, R.; Gordish-Dressman, H.; Rood, B.R. Proteomic profiling of cerebrospinal fluid identifies prostaglandin D2 synthase as a putative biomarker for pediatric medulloblastoma: A pediatric brain tumor consortium study. Proteomics 2011, 11, 935–943. [Google Scholar] [CrossRef]
- Xiao, F.; Lv, S.; Zong, Z.; Wu, L.; Tang, X.; Kuang, W.; Zhang, P.; Li, X.; Fu, J.; Xiao, M.; et al. Cerebrospinal fluid biomarkers for brain tumor detection: clinical roles and current progress. Am J Transl Res 2020, 12, 1379–1396. [Google Scholar]
- Wang, P.; Piao, Y.; Zhang, X.; Li, W.; Hao, X. The concentration of CYFRA 21-1, NSE and CEA in cerebro-spinal fluid can be useful indicators for diagnosis of meningeal carcinomatosis of lung cancer. Cancer Biomark 2013, 13, 123–130. [Google Scholar] [CrossRef]
- O'Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne) 2018, 9, 402. [Google Scholar] [CrossRef]
- Kopkova, A.; Sana, J.; Fadrus, P.; Slaby, O. Cerebrospinal fluid microRNAs as diagnostic biomarkers in brain tumors. Clin Chem Lab Med 2018, 56, 869–879. [Google Scholar] [CrossRef]
- Zhi, F.; Zhou, G.; Wang, S.; Shi, Y.; Peng, Y.; Shao, N.; Guan, W.; Qu, H.; Zhang, Y.; Wang, Q.; et al. A microRNA expression signature predicts meningioma recurrence. Int J Cancer 2013, 132, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Slavik, H.; Balik, V.; Vrbkova, J.; Rehulkova, A.; Vaverka, M.; Hrabalek, L.; Ehrmann, J.; Vidlarova, M.; Gurska, S.; Hajduch, M.; et al. Identification of Meningioma Patients at High Risk of Tumor Recurrence Using MicroRNA Profiling. Neurosurgery 2020, 87, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Eraky, A.M. Non-coding RNAs as Genetic Biomarkers for the Diagnosis, Prognosis, Radiosensitivity, and Histopathologic Grade of Meningioma. Cureus 2023, 15, e34593. [Google Scholar] [CrossRef] [PubMed]
- Gareev, I.; Beylerli, O.; Liang, Y.; Xiang, H.; Liu, C.; Xu, X.; Yuan, C.; Ahmad, A.; Yang, G. The Role of MicroRNAs in Therapeutic Resistance of Malignant Primary Brain Tumors. Frontiers in Cell and Developmental Biology 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhi, F.; Shao, N.; Li, B.; Xue, L.; Deng, D.; Xu, Y.; Lan, Q.; Peng, Y.; Yang, Y. A serum 6-miRNA panel as a novel non-invasive biomarker for meningioma. Scientific Reports 2016, 6, 32067. [Google Scholar] [CrossRef]
- Carneiro, V.; Cirino, M.; Panepucci, R.; Peria, F.; Tirapelli, D.; Colli, B.; Carlotti Jr, C.G. The Role of MicroRNA 181d as a Possible Biomarker Associated With Tumor Progression in Meningiomas. Cureus 2021, 13, e19158. [Google Scholar] [CrossRef]
- Urbschat, S.; Landau, B.; Bewersdorf, N.C.; Schuster, C.; Wagenpfeil, G.; Schulz-Schaeffer, W.J.; Oertel, J.; Ketter, R. MicroRNA 200a as a histologically independent marker for meningioma recurrence: Results of a four microRNA panel analysis in meningiomas. Cancer Med 2022. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Kim, J.H. Diverse Effects of Exosomes on COVID-19: A Perspective of Progress From Transmission to Therapeutic Developments. Front Immunol 2021, 12, 716407. [Google Scholar] [CrossRef]
- Saugstad, J.A.; Lusardi, T.A.; Van Keuren-Jensen, K.R.; Phillips, J.I.; Lind, B.; Harrington, C.A.; McFarland, T.J.; Courtright, A.L.; Reiman, R.A.; Yeri, A.S.; et al. Analysis of extracellular RNA in cerebrospinal fluid. J Extracell Vesicles 2017, 6, 1317577. [Google Scholar] [CrossRef]
- Negroni, C.; Hilton, D.A.; Ercolano, E.; Adams, C.L.; Kurian, K.M.; Baiz, D.; Hanemann, C.O. GATA-4, a potential novel therapeutic target for high-grade meningioma, regulates miR-497, a potential novel circulating biomarker for high-grade meningioma. EBioMedicine 2020, 59, 102941. [Google Scholar] [CrossRef]
- Ricklefs, F.L.; Maire, C.L.; Wollmann, K.; Dührsen, L.; Fita, K.D.; Sahm, F.; Herold-Mende, C.; von Deimling, A.; Kolbe, K.; Holz, M.; et al. Diagnostic potential of extracellular vesicles in meningioma patients. Neuro Oncol 2022, 24, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nature Reviews Molecular Cell Biology 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lan, Y.; Xie, A.; Shi, J.; Zhao, H.; Xu, L.; Zhu, S.; Luo, T.; Zhao, T.; Xiao, Y.; et al. Comprehensive analysis of long noncoding RNA (lncRNA)-chromatin interactions reveals lncRNA functions dependent on binding diverse regulatory elements. J Biol Chem 2019, 294, 15613–15622. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ge, Y.; Wang, D.; Liu, Q.; Sun, S.; Hua, L.; Deng, J.; Luan, S.; Cheng, H.; Xie, Q.; et al. LncRNA-IMAT1 Promotes Invasion of Meningiomas by Suppressing KLF4/hsa-miR22-3p/Snai1 Pathway. Mol Cells 2022, 45, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Slavik, H.; Balik, V.; Kokas, F.Z.; Slavkovsky, R.; Vrbkova, J.; Rehulkova, A.; Lausova, T.; Ehrmann, J.; Gurska, S.; Uberall, I.; et al. Transcriptomic Profiling Revealed Lnc-GOLGA6A-1 as a Novel Prognostic Biomarker of Meningioma Recurrence. Neurosurgery 2022, 91, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ren, J.; Ma, J.; Wu, J.; Zhang, R.; Yuan, H.; Han, X. LINC00702/miR-4652-3p/ZEB1 axis promotes the progression of malignant meningioma through activating Wnt/beta-catenin pathway. Biomed Pharmacother 2019, 113, 108718. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wang, S.; Li, Q.; Ma, Y.; Sun, P. Long noncoding RNA LINC00460 targets miR-539/MMP-9 to promote meningioma progression and metastasis. Biomed Pharmacother 2018, 105, 677–682. [Google Scholar] [CrossRef]
- Ding, C.; Yi, X.; Xu, J.; Huang, Z.; Bu, X.; Wang, D.; Ge, H.; Zhang, G.; Gu, J.; Kang, D.; et al. Long Non-Coding RNA MEG3 Modifies Cell-Cycle, Migration, Invasion, and Proliferation Through AKAP12 by Sponging miR-29c in Meningioma Cells. Frontiers in Oncology 2020, 10. [Google Scholar] [CrossRef]
- Rana, M.W.; Pinkerton, H.; Thornton, H.; Nagy, D. Heterotransplantation of human glioblastoma multiforme and meningioma to nude mice. Proc Soc Exp Biol Med 1977, 155, 85–88. [Google Scholar] [CrossRef]
- Jensen, R.L.; Leppla, D.; Rokosz, N.; Wurster, R.D. Matrigel augments xenograft transplantation of meningioma cells into athymic mice. Neurosurgery 1998, 42, 130–135. [Google Scholar] [CrossRef]
- McCutcheon, I.E.; Friend, K.E.; Gerdes, T.M.; Zhang, B.M.; Wildrick, D.M.; Fuller, G.N. Intracranial injection of human meningioma cells in athymic mice: an orthotopic model for meningioma growth. J Neurosurg 2000, 92, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Boetto, J.; Peyre, M.; Kalamarides, M. Mouse Models in Meningioma Research: A Systematic Review. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Püttmann, S.; Senner, V.; Braune, S.; Hillmann, B.; Exeler, R.; Rickert, C.H.; Paulus, W. Establishment of a benign meningioma cell line by hTERT-mediated immortalization. Lab Invest 2005, 85, 1163–1171. [Google Scholar] [CrossRef]
- van Furth, W.R.; Laughlin, S.; Taylor, M.D.; Salhia, B.; Mainprize, T.; Henkelman, M.; Cusimano, M.D.; Ackerley, C.; Rutka, J.T. Imaging of murine brain tumors using a 1.5 Tesla clinical MRI system. Can J Neurol Sci 2003, 30, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Baia, G.S.; Dinca, E.B.; Ozawa, T.; Kimura, E.T.; McDermott, M.W.; James, C.D.; VandenBerg, S.R.; Lal, A. An orthotopic skull base model of malignant meningioma. Brain Pathol 2008, 18, 172–179. [Google Scholar] [CrossRef]
- Castle, K.D.; Chen, M.; Wisdom, A.J.; Kirsch, D.G. Genetically engineered mouse models for studying radiation biology. Transl Cancer Res 2017, 6, S900–s913. [Google Scholar] [CrossRef]
- Peyre, M.; Salaud, C.; Clermont-Taranchon, E.; Niwa-Kawakita, M.; Goutagny, S.; Mawrin, C.; Giovannini, M.; Kalamarides, M. PDGF activation in PGDS-positive arachnoid cells induces meningioma formation in mice promoting tumor progression in combination with Nf2 and Cdkn2ab loss. Oncotarget 2015, 6, 32713–32722. [Google Scholar] [CrossRef]
- Kawashima, M.; Suzuki, S.O.; Yamashima, T.; Fukui, M.; Iwaki, T. Prostaglandin D synthase (beta-trace) in meningeal hemangiopericytoma. Mod Pathol 2001, 14, 197–201. [Google Scholar] [CrossRef]
- Urade, Y.; Kitahama, K.; Ohishi, H.; Kaneko, T.; Mizuno, N.; Hayaishi, O. Dominant expression of mRNA for prostaglandin D synthase in leptomeninges, choroid plexus, and oligodendrocytes of the adult rat brain. Proc Natl Acad Sci U S A 1993, 90, 9070–9074. [Google Scholar] [CrossRef]
- Yamashima, T.; Sakuda, K.; Tohma, Y.; Yamashita, J.; Oda, H.; Irikura, D.; Eguchi, N.; Beuckmann, C.T.; Kanaoka, Y.; Urade, Y.; et al. Prostaglandin D synthase (beta-trace) in human arachnoid and meningioma cells: roles as a cell marker or in cerebrospinal fluid absorption, tumorigenesis, and calcification process. J Neurosci 1997, 17, 2376–2382. [Google Scholar] [CrossRef]
- von Werder, A.; Seidler, B.; Schmid, R.M.; Schneider, G.; Saur, D. Production of avian retroviruses and tissue-specific somatic retroviral gene transfer in vivo using the RCAS/TVA system. Nat Protoc 2012, 7, 1167–1183. [Google Scholar] [CrossRef] [PubMed]
- Kalamarides, M.; Niwa-Kawakita, M.; Leblois, H.; Abramowski, V.; Perricaudet, M.; Janin, A.; Thomas, G.; Gutmann, D.H.; Giovannini, M. Nf2 gene inactivation in arachnoidal cells is rate-limiting for meningioma development in the mouse. Genes Dev 2002, 16, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Kalamarides, M.; Stemmer-Rachamimov, A.O.; Takahashi, M.; Han, Z.Y.; Chareyre, F.; Niwa-Kawakita, M.; Black, P.M.; Carroll, R.S.; Giovannini, M. Natural history of meningioma development in mice reveals: a synergy of Nf2 and p16(Ink4a) mutations. Brain Pathol 2008, 18, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Peyre, M.; Stemmer-Rachamimov, A.; Clermont-Taranchon, E.; Quentin, S.; El-Taraya, N.; Walczak, C.; Volk, A.; Niwa-Kawakita, M.; Karboul, N.; Giovannini, M.; et al. Meningioma progression in mice triggered by Nf2 and Cdkn2ab inactivation. Oncogene 2013, 32, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Mandara, M.T.; Ricci, G.; Rinaldi, L.; Sarli, G.; Vitellozzi, G. Immunohistochemical identification and image analysis quantification of oestrogen and progesterone receptors in canine and feline meningioma. J Comp Pathol 2002, 127, 214–218. [Google Scholar] [CrossRef]
- Michelhaugh, S.K.; Guastella, A.R.; Varadarajan, K.; Klinger, N.V.; Parajuli, P.; Ahmad, A.; Sethi, S.; Aboukameel, A.; Kiousis, S.; Zitron, I.M.; et al. Development of patient-derived xenograft models from a spontaneously immortal low-grade meningioma cell line, KCI-MENG1. J Transl Med 2015, 13, 227. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.J.; Ryu, B.K.; Park, D.H.; Kong, D.S.; Chong, K.; Chae, Y.S.; Chung, Y.G.; Park, S.I.; Kang, S.H. Forkhead box M1 (FOXM1) transcription factor is a key oncogenic driver of aggressive human meningioma progression. Neuropathol Appl Neurobiol 2020, 46, 125–141. [Google Scholar] [CrossRef]
- Saydam, O.; Shen, Y.; Würdinger, T.; Senol, O.; Boke, E.; James, M.F.; Tannous, B.A.; Stemmer-Rachamimov, A.O.; Yi, M.; Stephens, R.M.; et al. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway. Mol Cell Biol 2009, 29, 5923–5940. [Google Scholar] [CrossRef]
- Tuchen, M.; Wilisch-Neumann, A.; Daniel, E.A.; Baldauf, L.; Pachow, D.; Scholz, J.; Angenstein, F.; Stork, O.; Kirches, E.; Mawrin, C. Receptor tyrosine kinase inhibition by regorafenib/sorafenib inhibits growth and invasion of meningioma cells. Eur J Cancer 2017, 73, 9–21. [Google Scholar] [CrossRef]
- Murnyák, B.; Bognár, L.; Klekner, Á.; Hortobágyi, T. Epigenetics of Meningiomas. Biomed Res Int 2015, 2015, 532451. [Google Scholar] [CrossRef]
- Di Bonaventura, R.; Martini, M.; Cenci, T.; Caccavella, V.M.; Barresi, V.; Gessi, M.; Albanese, A.; Lauretti, L.; Pallini, R.; D'Alessandris, Q.G.; et al. Dissecting Stemness in Aggressive Intracranial Meningiomas: Prognostic Role of SOX2 Expression. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Anis, S.E.; Lotfalla, M.; Zain, M.; Kamel, N.N.; Soliman, A.A. Value of SSTR2A and Claudin - 1 in Differentiating Meningioma from Schwannoma and Hemangiopericytoma. Open Access Maced J Med Sci 2018, 6, 248–253. [Google Scholar] [CrossRef] [PubMed]

| Biomarker Type | Known Biomarkers | Study design | Clinical Use | Reference | Publication Date |
|---|---|---|---|---|---|
| Genomic | NF2, TRAF7,AKT1, KLF4, SMO PIK3CA | Human studies (Review) | Diagnosis | [29] | 2020 |
| miRNA | miR-451, miR-711, miR-190a miR-17-5p, miR-199a, miR-190a, miR-186-5p, miR-155-5p, miR-22-3p, miR-24-3p, miR- 26b-5p, miR-27a-3p, miR-27b-3p, miR-96-5p,miR-146a-5p miR-106a-5p, miR-219-5p, miR-375, and miR-409-3p miR-4695-5p, miR-4286, miR-6732-5p, miR-6855-5p, miR-7977, miR-6765-3p, miR-6787-5p miR-181d |
Human clinical trial In-vivo human clinical trial Clinical Study In-vivo human Cohort study Clinical Human study Human clinical study on tissues and plasma |
Diagnosis/therapy response Prognosis Diagnosis,prognosis, histological grade & radiosensitivity Non-invasive diagnostic/prognostic Prognosis Diagnosis and prognosis |
[128] [132] [134] [136] [135] [137] |
2020 2013 2023 2016 2021 2021 |
| LncRNA | lnc-GOLGA6A-1 lncRNA-LINC00460 LncRNA- NUP210, LncRNA-SPIRE2, LncRNA-SLC7A1, and LncRNA-DMTN |
Human Clinical independent cohort study In-vitro human clinical study Clinical study |
Prognosis and pathogenesis Diagnosis Diagnosis,prognosis, histological grade & radiosensitivity |
[146] [149] [134] |
2022 2020 2023 |
| Epigenetic | TIMP3, HOXA7,HOXA9, HOXA10, RASSF1A SOX2 |
- Human clinical trial |
prognosis prognosis |
[171] [172] |
2015 2022 |
| Proteomic | APO-E, APO-J, PTGDS Caspase-3 EFEMP1 CEA |
Clinical study Screening Cohort Study Human clinical trial Review |
Diagnosis Non-invasive diagnosis and prognosis Diagnosis/therapy response Diagnosis |
[124] [113] [128] [123] |
2012 2019 2020 2016 |
| Histological | SSTR2A, Claudin-1 CA9 |
Human Clinical case Clinical study |
Diagnosis and sub-type determination Poor prognosis |
[173] [120] |
2018 2007 |
| Mouse Model | Advantages | Limitations |
|---|---|---|
| Heterotopic xenograft model | Very reliable in term of tumor take rates. | Lacks the key components of the meningiomas specific microenvironment. |
| Orthotopic xenograft model |
|
|
| Genetically Engineered Mouse Models (GEMM) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





