1. Introduction
Type 1 diabetes (T1D) represents one of the most prevalent endocrinological diseases among children and young adults, with a growing incidence rate reaching up to 2.9 new cases per year per 100 000 persons below 15 years of age [
1]. Although there is a continuous effort to improve insulin delivery systems and glycemia monitoring systems, less than a third of T1D patients are achieving optimal glycemic control [
2]. The major risk factors for developing retinal microvascular complications are the poor metabolic control and the duration of the disease. Diabetic retinopathy (DR) represents the most frequent diabetic microvascular complication, and it develops progressively with the duration of the disease, potentially leading to proliferative diabetic retinopathy (PDR) and irreversible loss of vision. The prevalence of PDR increases up to 25% at 15 years duration of the disease [
3]. Therefore, early diagnosis of DR has enormous therapeutic potential in preventing or delaying vision-threatening complications in young T1D patients.
High myopia is characterized by axial elongation, stretching of the posterior eye wall and retinal thinning, which can lead to multiple complications such as retinal detachment, macular holes, choroidal neovascularization, and retinoschisis [
4].
The presence of both diabetes and high myopia in the same patients is associated with higher retinal nerve fiber layer (RNFL) damage than either pathology alone [
5]. The mechanical stretching of the myopic eye wall and the diabetic neural degeneration accelerate thinning of the inner retinal layer [
6] acting as cumulated risk factors for the development of retinal complications.
Hemoglobin (Hb) is the main protein component of erythrocytes, mediating the transport of oxygen and carbon dioxide in the blood [
7]. The adult hemoglobin A (HbA) is present in 95 % of the adult population and is a tetrameric protein (α2β2) consisting of two α and two β subunits held together by non-covalent interactions [
8]. Hemoglobinopathies are highly heterogeneous, monogenic disorders related to hemoglobin synthesis [
9]. Hemoglobin D (HbD) is a β chain hemoglobin variant which was first described in 1951. It is also known as HbD Punjab or HbD Los Angeles and it is the fourth most frequently occurring hemoglobinopathy [
10]. HbD differs in structure from HbA, at position 121 on β chain where glutamine replaces glutamic acid [
11]. Specific hemoglobin variants such as HbD, may interfere with the accuracy of HbA1c values depending on the evaluation method used [
12]. We report a case of a T1D patient with HbD hemoglobinopathy and high myopia and discuss the retinal microvascular alterations of these conditions, visible on OCT angiography (angio-OCT) evaluation.
2. Materials and Methods
Case Presentation
We present the rare case of a 20-year-old female patient with T1D, HbD heterozygote variant, high myopia, and microvascular retinal alterations found on angio-OCT.
Her medical history revealed that the patient was an only child, had a birth weight of 2800g, and no history of prematurity. The patient was diagnosed with T1D at the age of two and has been on basal bolus insulin therapy since the diagnosis. While monitoring HbA1c, she was also diagnosed with HbD hemoglobinopathy. During childhood, severe stature deficit (less than 3 standard deviations) and delayed puberty were observed, which could not be explained only by suboptimal glycemic control. The clinical manifestations indicated a possible hypopituitarism, characterized by severe growth deficiency and pubertal delay, without characteristic signs of dysmorphic syndromes. The patient’s family did not consent for growth hormone investigations and possible treatment for hypostature. Her family history revealed that her parents were of relatively normal height, the mother’s height being 162 cm and her father’s height being 165 cm.
At the age of 7, the patient was also diagnosed with mild myopia of -2.00 spheric diopters (SD) in both eyes (BE) which progressed up to -10,00 SD currently, at the age of 20. Her mother was also diagnosed with myopia in adolescence and has a current refractive error of -2,50 SD BE.
The patient’s current height is 149 cm (less than 3 standard deviations) and her weight is 45 kg, with a body mass index (BMI) of 20.3 kg/m2. For the past 2 years she has been on a basal-bolus insulin regimen of three fixed doses of Aspart insulin (5 units before breakfast, 10 units before lunch, and 5 units before dinner) and 20 units of glargine insulin in the evening. With this therapy, the most recent HbA1c value was 6.5%, measured by high performance liquid chromatography (HPLC) (
Table 1). However, the average glycemic levels from her glucometer for the past 3 months varied between 160 - 184 mg%, which correspond to a HbA1c value between 7-8%. This discrepancy is due to the presence of the HbD identified in her blood. Her most recent hemoglobin electrophoresis (EDTA blood/ capillary electrophoresis) revealed a percentage of HbA of 56.6% and abnormal HbD fractions of 40.4%. Complete blood count, serum electrolytes, liver transaminases, kidney function tests, and lipid profile were within normal parameters. (
Table 1).
The ophthalmic examination revealed a best-corrected visual acuity (BCVA) of 0.7 LogMar BE with a refractive error of -10,00 SD BE. The intraocular pressure was 17 mmHg in the right eye (RE) and 18 mmHg in the left eye (LE). Slit lamp examination revealed normal anterior segment in BE, with no relative afferent pupillary defect.
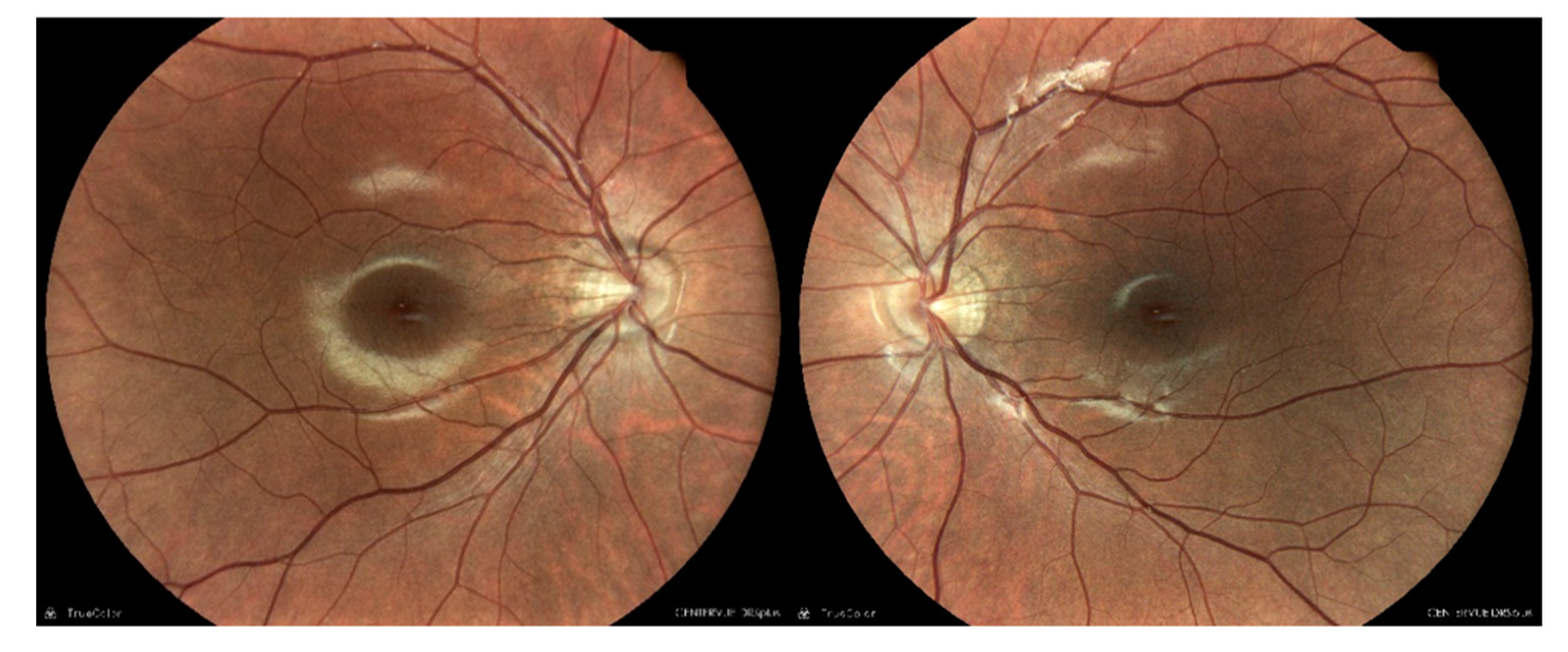
Dilated fundus examination of BE revealed mild peripapillary atrophy, a thin retina with visible choroidal vasculature and no clinical signs of DR.
Figure 1.
Dilated fundus photos showing BE mild peripapillary atrophy, thin retina with visible choroidal vasculature and no clinical signs of DR.
Figure 1.
Dilated fundus photos showing BE mild peripapillary atrophy, thin retina with visible choroidal vasculature and no clinical signs of DR.
The macular OCT examination revealed mild retinal thinning in the macular area: retinal thickness of RE 212 µm and LE 220 µm, without other alterations specific to high myopia or DR. The optic disc OCT examination showed a mean cup to disc (C/D) ratio of 0.3, with normal retinal nerve fiber layer thickness in BE.
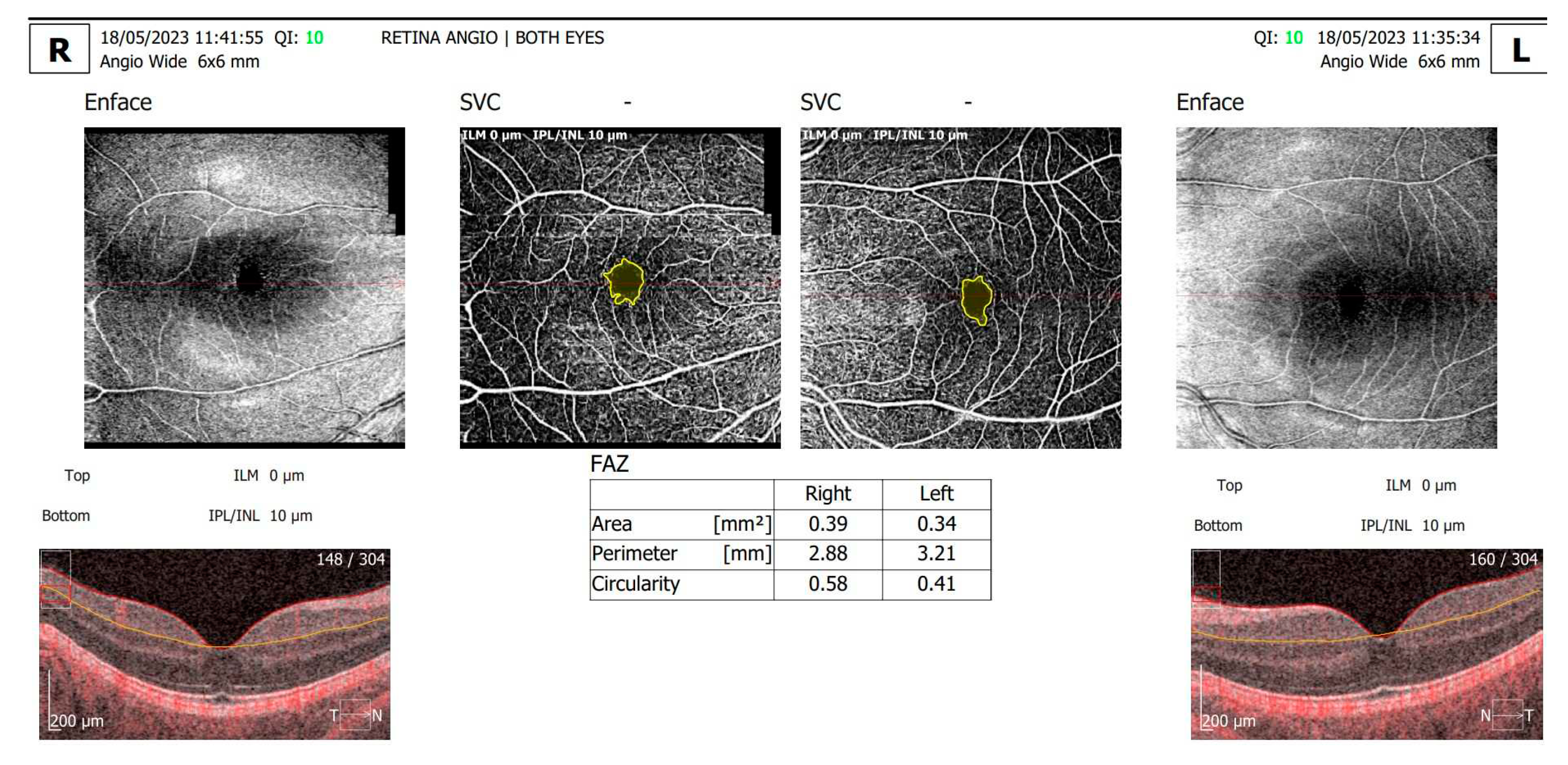
The angio-OCT scans (
Figure 2) revealed enlarged foveal avascular zone (FAZ) area and perimeter, with a decreased circularity index and decrease in capillary density the superficial and deep capillary plexus in BE. For the RE: the FAZ area vas 0.39 mm
2, the FAZ perimeter was 2.88 mm and the circularity index was 0.58; for the LE: the FAZ area vas 0.34 mm
2, the FAZ perimeter was 3.21 mm and the circularity index was 0.41. Although the patient attended previous regular ophthalmic examinations for DR screening, this was the patient’s first retinal angio-OCT evaluation.
3. Discussion
Review of Literature
Until now, only a few studies have focused on the co-existence of T1D and HbD hemoglobinopathy in the same patients, and the way that HbA1c evaluation methods can be influenced by the HbD variant. We have searched the PubMed database for the following keywords: Hemoglobin D, Diabetes Mellitus and HbA1c. We found a total of 5 scientific articles, 2 original research studies and 3 case reports (
Table 2) and full-text evaluation was performed.
Little RR and collaborators [
13] demonstrated that the presence of both HbD and Hemoglobin E variants interfered with ion-exchange HPLC methods of assessment of HbA1c, producing artificially low results for HbA1c. In their research regarding the interference of HbD on measurements of HbA1C, Lorenzo Medina and collaborators [
14] showed that in diabetic patients who were heterozygous for the HbD variant, HbA1c levels using the ADAMS HA-8160 HPLC method gave falsely low or unquantifiable results due to an abnormal separation of HbA1c. For HbD variant patients, they recommend measurement of HbA1c using other alternatives, such as a turbidimetric immunoassay which is less likely to be subject to interference. Pinés Corrales PJ and collaborators [
15] presented a case report on the interference of Hb D on the measurement of HbA1c indicating that the HPLC assay for HbA1c indicated lower values compared to those of serum glucose. Copplestone S and collaborators [
16] reported the case of a patient with poorly controlled diabetes, but apparently normal levels of HbA1C assessed by HPLC method. Shukla A and collaborators [
17] were the only ones reporting a case where the HbA1c values were falsely elevated using the HPLC method of assay.
HbA1c represents a minor fraction of adult hemoglobin, which is formed slowly and nonenzymatically from hemoglobin and glucose [
18]. The erythrocytes are freely permeable to glucose, therefore HbA1c is formed throughout their lifespan of approximately 120 days. The rate of HbA1c formation is directly proportional to the ambient glucose concentration. Monitoring HbA1c is indispensable for the treatment and follow up of T1D patients as it reflects the glycemic control of the previous 8–12 weeks [
19]. There are several factors that can interfere with HbA1C assay and may lead to less accurate results, and one of these factors is represented by hemoglobin variants, also called hemoglobinopathies. Multiple methods for measuring HbA1c levels are available, including cation-exchange HPLC, immunoassays, and enzyme-based assays. The most used method for measuring HbA1c levels is the cation-exchange HPLC due to its fast and reliable results, but this method can be vulnerable to the effect of hemoglobin variants [
20]. Currently, more than 1000 Hb variants have been identified with most of them being clinically silent.
The metabolic control and the duration of T1D are major factors influencing the risk of retinal microvascular complications leading to DR [
21]. Our patient was diagnosed with T1D at the early age of two, and shortly afterwards she was diagnosed with HbD heterozygote variant. She was also diagnosed with myopia of -2,00 SD at the age of seven, progressing to -10,00 SD at 20 years old. She has a current duration of T1D of 18 years, which increases the risk of DR complications. On ophthalmic examination she presented microvascular retinal alterations visible only on angio-OCT: enlarged FAZ area and perimeter, with decreased circularity index in BE. These retinal alterations can be attributed to both myopia and T1D and can accelerate the development of sight threatening complications. The normal FAZ in healthy subjects has a circular or slightly elliptical shape which becomes increasingly irregular in patients with DR, altering the FAZ perimeter and FAZ area as well [
22].
Myopia has a multifactorial etiology determined by a complex interaction of genetic and environmental factors such as higher socioeconomic status, excessive near-work activity, and lack of outdoor activity [
23]. Several research studies have investigated retinal parameters in patients with myopia and diabetes mellitus, with variable results. Some results suggest that hyperglycemia may predispose one to juvenile-onset myopia [
24] through higher levels of free insulin-like growth factor (IGF)-1 and lower levels of IGF-binding protein 3, leading to unregulated scleral growth and myopia. Ying Xiao and collaborators investigated whether the progression of myopia is accelerated in children with T1D, and they found evidence that myopia progression is accelerated in T1D children compared to non-diabetic children [
25]. Moreover, angio-OCT studies on patients with high myopia and diabetes type 2 (T2D) found that parafoveal vascular density of was decreased in myopic T2D patients compared to those without myopia [
26].
Although the technology used in the treatment of T1D is constantly advancing, aiming towards better glycemic control [
27], other sight threatening pathologies of the patient must be taken into consideration when monitoring the possible retinal complications. High-resolution OCT techniques which allow evaluation of retinal layers [
28] along with angio-OCT microvascular retinal evaluation are necessary screening tools for preventing sight-threatening complications in young diabetic patients with high myopia.
4. Conclusions
When monitoring the metabolic control for T1D patients with known haemoglobinopathies, such as HbD, the HbA1c levels measured by HPLC assay might fail to reflect the real mean values for glycemia. Awareness of Hb variants affecting HbA1c measurements is essential, to avoid poor management of diabetic patients, because haemoglobinopathies can produce an artifact whereby the hemoglobin variant is being measured instead of or in addition to HbA1c.
As Hb variants incidence is continuously increasing due to immigration, misleading HbA1C levels are becoming more frequent and should be tested with multiple methods such as turbidimetric immunoassay, or different tests should be considered such as glycated serum protein or glycated albumin.
For the rare association between T1D, HbD hemoglobinopathy and high myopia, advanced retinal screening methods such as angio-OCT are essential, in order to identify the early microvascular retinal alterations. Moreover, close monitorization of the patient’s metabolic control through multiple evaluation methods of the HbA1c is mandatory, as HPLC assay method underestimates the real HbA1c values in for patients with HbD hemoglobinopathy.
Author Contributions
Conceptualization, A.O.D. and V.S.; methodology, A.O.D., and A.E.T; software, A.C.T. and A.E.T.; validation, A.T.B., and V.S.; investigation, I.P. and A.E.T.; resources, I.P. and A.T.B.; data curation, A.O.D. and A.T.B.; writing—original draft preparation, A.O.D., and I.P.; writing—review and editing, V.S. and A.T.B.; visualization, A.C.T. and I.P.; supervision, V.S.; project administration, A.O.D. and V.S. All authors have read and agreed to the published version of the manuscript.
Funding
The Article Processing Charges were funded by the UNIVERSITY OF MEDICINE AND PHARMACY CRAIOVA, ROMANIA.
Institutional Review Board Statement
Ethics Committee of the University of Medicine and Pharmacy Craiova (Project Identification Code 861/07/06/2021).
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The authors declare that the data for this research are available from the correspondence authors upon reasonable request.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alassaf, A.; Mohamed, K.; Al Otaiby, A.; Al Wraidat, M.; Nashwan, A.J. Optic Neuritis in a Child With Poorly Controlled Type 1 Diabetes Mellitus: A Case Report. Cureus 2023, 15, e33474. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Hu, W.; Yan, Q.; Feng, B. Benefit or Risk in Patient with Type 1 Diabetes Based on Appropriated Dosage of Dapagliflozin: A Case Report. Medicina 2023, 59, 827. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Baccouche, B.; Del-Risco, N.; Park, J.; Song, A.; McAnany, J.J.; Kazlauskas, A. The Slow Progression of Diabetic Retinopathy Is Associated with Transient Protection of Retinal Vessels from Death. Int. J. Mol. Sci. 2023, 24, 10869. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-T.; Na, Y.-J.; Lee, S.-C.; Lee, M.-W. Impact of high myopia on inner retinal layer thickness in type 2 diabetes patients. Sci. Rep. 2023, 13, 1–7. [Google Scholar] [CrossRef]
- Bin Lim, H.; Shin, Y.-I.; Lee, M.W.; Lee, J.-U.; Lee, W.H.; Kim, J.-Y. Association of Myopia with Peripapillary Retinal Nerve Fiber Layer Thickness in Diabetic Patients Without Diabetic Retinopathy. Investig. Opthalmology Vis. Sci. 2020, 61, 30–30. [Google Scholar] [CrossRef]
- Seo, S.; Lee, C.E.; Jeong, J.H.; Park, K.H.; Kim, D.M.; Jeoung, J.W. Ganglion cell-inner plexiform layer and retinal nerve fiber layer thickness according to myopia and optic disc area: a quantitative and three-dimensional analysis. BMC Ophthalmol. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Harteveld, C.L.; Achour, A.; Arkesteijn, S.J.G.; ter Huurne, J.; Verschuren, M.; Bhagwandien-Bisoen, S.; Schaap, R.; Vijfhuizen, L.; el Idrissi, H.; Koopmann, T.T. The hemoglobinopathies, molecular disease mechanisms and diagnostics. Int. J. Lab. Hematol. 2022, 44, 28–36. [Google Scholar] [CrossRef]
- Russo, R.; Zucchelli, S.; Codrich, M.; Marcuzzi, F.; Verde, C.; Gustincich, S. Hemoglobin is present as a canonical α2β2 tetramer in dopaminergic neurons. Biochim. et Biophys. Acta (BBA) - Proteins Proteom. 2013, 1834, 1939–1943. [Google Scholar] [CrossRef]
- Klonoff, D.C. Hemoglobinopathies and Hemoglobin A1c in Diabetes Mellitus. J. Diabetes Sci. Technol. 2019, 14, 3–7. [Google Scholar] [CrossRef]
- Spandana, R.; Panneerselvam, K.; Mani, S.; Krishnamoorthy, N. An Interesting and Rare Case of Hemoglobin D-Punjab Variant in Tamil Nadu. Cureus 2022, 14, e22668. [Google Scholar] [CrossRef]
- Devi, A.M.S.; Rameshkumar, K.; Sitalakshmi, S. Hb D: A Not So Rare Hemoglobinopathy. Indian J. Hematol. Blood Transfus. 2014, 32, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Surchi, H.; Rea, R. A case of haemoglobinopathy and elevated HbA1c. Pr. Diabetes 2017, 34, 28–29. [Google Scholar] [CrossRef]
- Little, R.R.; Rohlfing, C.L.; Hanson, S.; Connolly, S.; Higgins, T.; Weykamp, C.W.; D'Costa, M.; Luzzi, V.; E Owen, W.; Roberts, W.L. Effects of Hemoglobin (Hb) E and HbD Traits on Measurements of Glycated Hb (HbA1c) by 23 Methods. Clin. Chem. 2008, 54, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Medina, M.; De-La-Iglesia, S.; Ropero, P.; Martin-Alfaro, R.; Quintana-Hidalgo, L. Interference of Hemoglobin D on Measurements of Hemoglobin A1c by the High-Performance Liquid Chromatography HA-8160 in 27 Patients. J. Diabetes Sci. Technol. 2012, 6, 1235–1237. [Google Scholar] [CrossRef]
- Pinés Corrales PJ, Martínez López R, González Cabrera A, Ibáñez Navarro P, Vicente Albiñana Á. Interference of Hb D-Los Angeles on the measurement of glycated hemoglobin. A case report. Endocrinol Diabetes Nutr. 2017 Jan;64(1):57-58.
- Copplestone, S.; Mackay, R.; Brennan, S. Normal glycated haemoglobin in a patient with poorly controlled diabetes mellitus and haemoglobin D Punjab: implications for assessment of control. New Zealand Med J. 2002, 115. [Google Scholar]
- Shukla, A.; Dabadghao, S.; Gupta, S.; Verma, S. Interference of hemoglobin D Punjab on measurements of glycated hemoglobin. Indian J. Pathol. Microbiol. 2015, 58, 572. [Google Scholar] [CrossRef]
- Joseph, I. Wolfsdorf, Katharine C. Garvey, Chapter 49 - Management of Diabetes in Children; Endocrinology: Adult and Pediatric (Seventh Edition), W.B. Saunders, 2016; ISBN 9780323189071. [Google Scholar]
- Hatayama, T.; Umeda, F.; Yamauchi, T.; Ideguchi, H. A diabetic patient in whom Hb Weesp was incidentally detected when her HbA1c level was measured. Diabetol. Int. 2019, 10, 300–302. [Google Scholar] [CrossRef]
- Georgia Kaiafa and others, Is HbA1c an ideal biomarker of well-controlled diabetes?, Postgraduate Medical Journal, Volume 97, Issue 1148, June 2021, Pages 380–383, https://doi.org/10.1136/postgradmedj-2020-138756. [CrossRef]
- Al Harbi, M.; Khandekar, R.; Kozak, I.; Schatz, P. Association between Sickle Cell Trait and the Prevalence and Severity of Diabetic Retinopathy. PLOS ONE 2016, 11, e0159215. [Google Scholar] [CrossRef]
- Dan, A.O.; Ștefănescu-Dima, A.; Bălășoiu, A.T.; Puiu, I.; Mocanu, C.L.; Ionescu, M.; Tănasie, A.C.; Târtea, A.E.; Sfredel, V. Early Retinal Microvascular Alterations in Young Type 1 Diabetic Patients without Clinical Retinopathy. Diagnostics 2023, 13, 1648. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.; Chia, A.; Htoon, H.; Lam, P.; Yap, F.; Ling, Y. Myopia in young patients with type 1 diabetes mellitus. Singap. Med J. 2015, 56, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Miller, J.B.; Lindeberg, S.; Jensen, C. An evolutionary analysis of the aetiology and pathogenesis of juvenile-onset myopia. Acta Ophthalmol. Scand. 2002, 80, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Qian, Y.; Yang, C.; Zou, H. Is myopia accelerated in type 1 diabetes mellitus children? Analyses from the ocular parameters. BMC Ophthalmol. 2023, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Jia, Z.; Fan, F.; Li, J.; Li, K. Clinical Observation of Macular Vessel Density in Type 2 Diabetics with High Myopia. Ophthalmic Res. 2022, 124–130. [Google Scholar] [CrossRef]
- Seget, S.; Włodarczyk, J.; Lutogniewska, W.; Rusak, E.; Dróżdż, M.; Jarosz-Chobot, P. The Use of a Hybrid Closed-Loop System for Glycemic Control in Two Pediatric Patients with Type 1 Diabetes Undergoing Minor Surgery. Healthcare 2023, 11, 587. [Google Scholar] [CrossRef]
- Ştefănescu-Dima, A. .; Corîci, C.A.; Mănescu, M.R.; Sas, T.N.; Iancău, M.; Mocanu, C.L. Posterior vitreous detachment and macular anatomical changes - a tomographic-electroretinographic study. Romanian J. Morphol. Embryol. 2016, 57, 751–758. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).