Submitted:
15 August 2023
Posted:
17 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethics statement
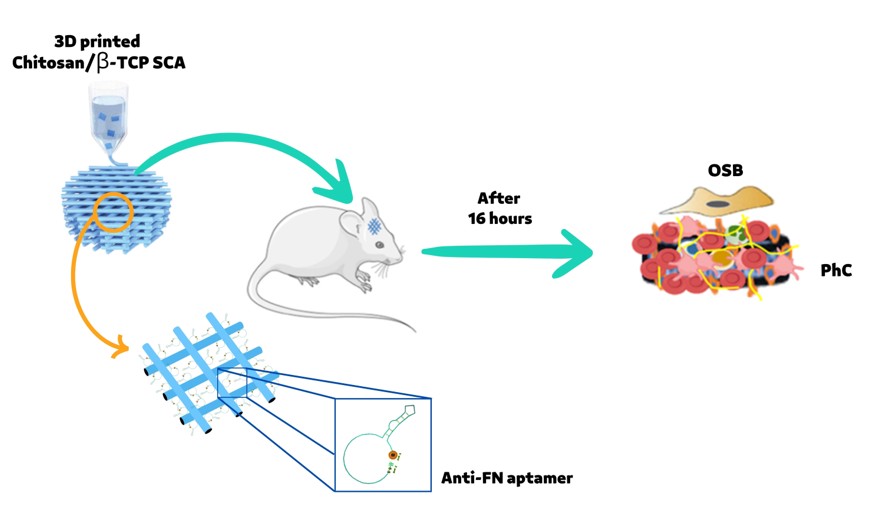
2.2. 3D chitosan/B-TCP scaffolds
2.3. Aptamer preparation
2.4. Scaffold functionalization: SCA and SCA+APT
2.5. Animal’s surgery and physiological blood clot formation
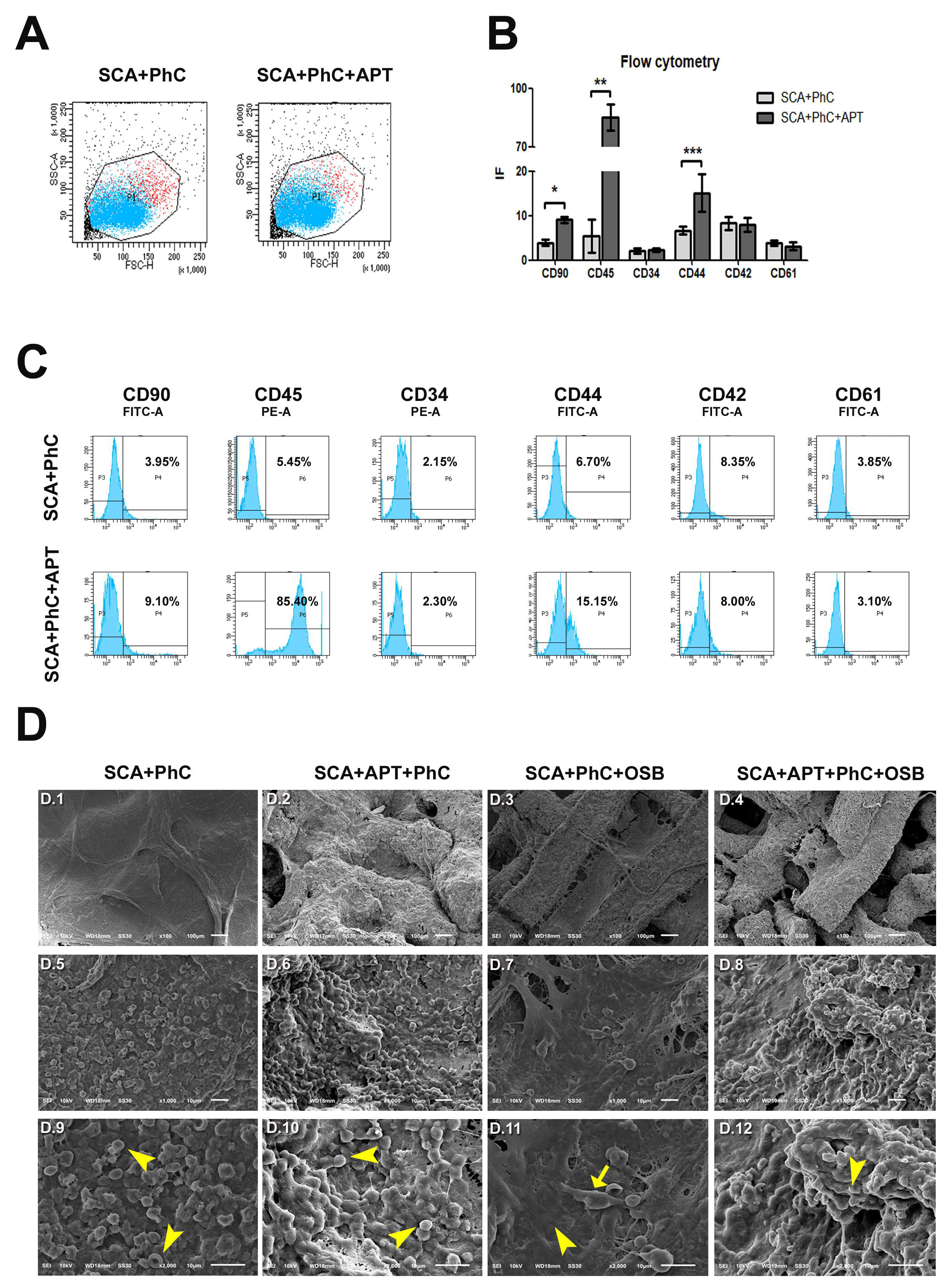
2.6. Flow cytometry (FACS) analysis
2.7. Cell culture
2.8. Scanning electron microscopy
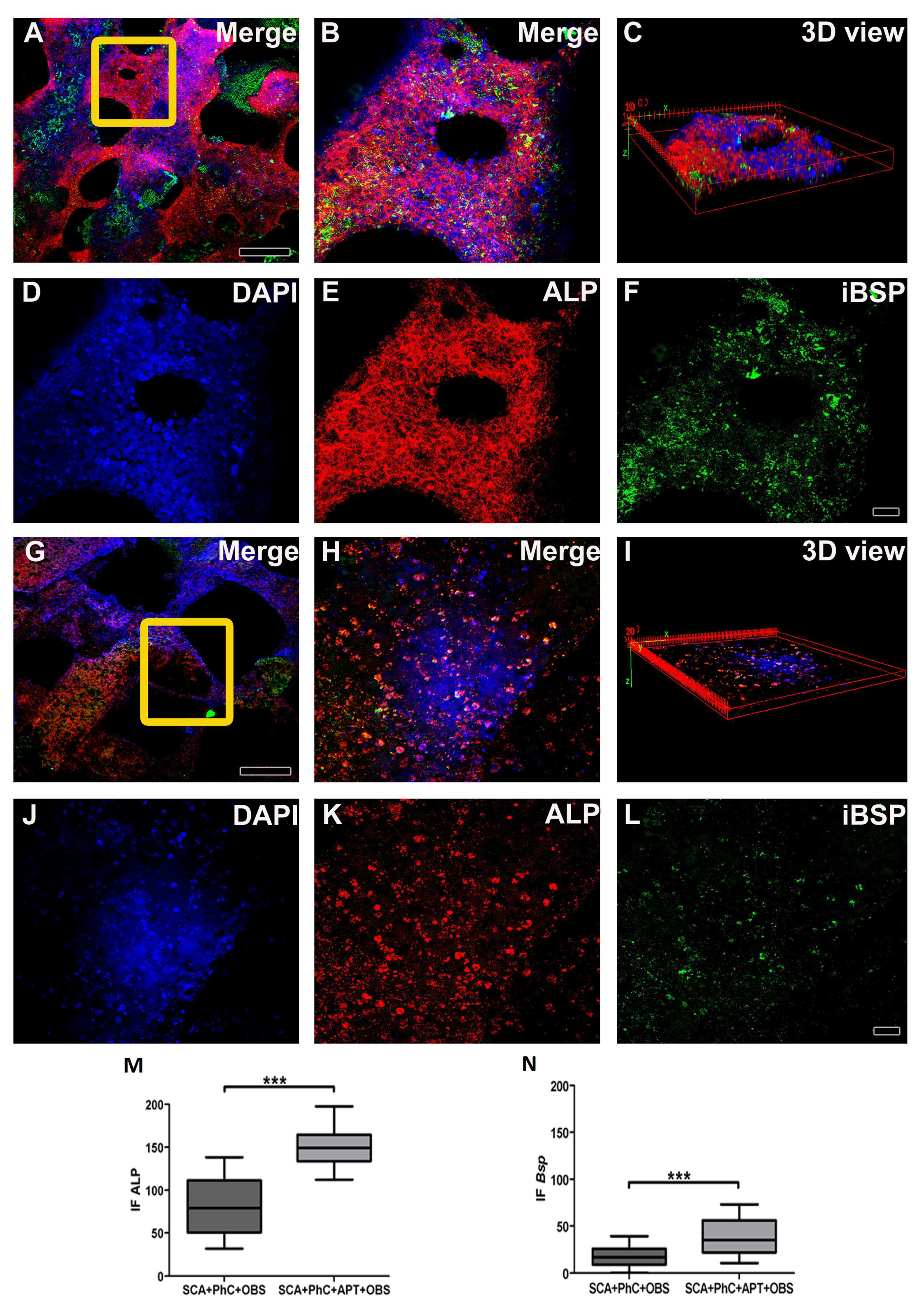
2.9. Immunofluorescence
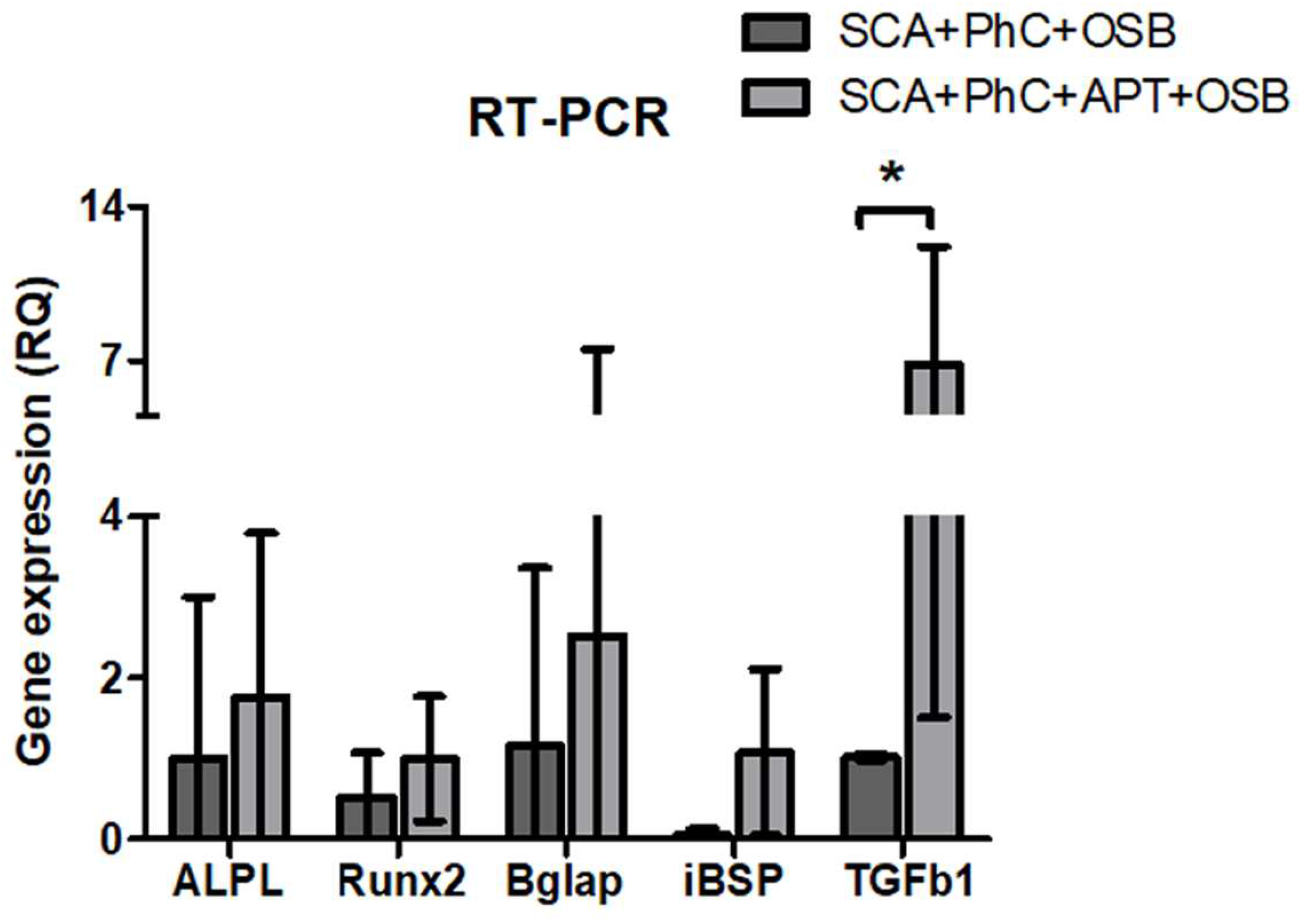
2.10. RNA extraction, cDNA synthesis and quantitative real time polymerase chain reaction (RT-PCR)
2.11. Statistical analysis
3. Results
3.1. Aptamers promote a faster recruitment of cells involved in healing processes
3.2. The physiological blood clot formed at interface of aptamer-enriched scaffold promote osteoblasts differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brouwer, K.M.; Lundvig, D.M.; Middelkoop, E.; Wagener, F.A.; Von den Hoff, J.W. Mechanical cues in orofacial tissue engineering and regenerative medicine. Wound repair and regeneration 2015, 23, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Progress in polymer science 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.; Xiao, Y. Formation of blood clot on biomaterial implants influences bone healing. Tissue Engineering Part B: Reviews 2014, 20, 697–712. [Google Scholar] [CrossRef]
- Davis, R.P.; Miller-Dorey, S.; Jenne, C.N. Platelets and coagulation in infection. Clinical & Translational Immunology 2016, 5, e89. [Google Scholar]
- Nurden, A.T. Platelet membrane glycoproteins: A look back into the past and a view to the future. Thrombosis and haemostasis 2007, 98, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A. Overview of the fracture healing cascade. Injury 2005, 36, S5–S7. [Google Scholar] [CrossRef]
- Ricardo, T.; Peggy, R.; Beat, K.; Eduardo, A.; Ilya, R. Time-of-Flight Secondary Ion Mass Spectrometry with Principal Component Analysis of Titania–Blood Plasma Interfaces 2013.
- Park, S.; Kim, G.; Jeon, Y.C.; Koh, Y.; Kim, W. 3D polycaprolactone scaffolds with controlled pore structure using a rapid prototyping system. Journal of Materials Science: Materials in Medicine 2009, 20, 229–234. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.; others. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab on a Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef]
- De Angelis, E.; Saleri, R.; Martelli, P.; Elviri, L.; Bianchera, A.; Bergonzi, C.; Pirola, M.; Romeo, R.; Andrani, M.; Cavalli, V.; others. Cultured Horse Articular Chondrocytes in 3D-Printed Chitosan Scaffold With Hyaluronic Acid and Platelet Lysate. Frontiers in Veterinary Science 2021, 8, 671776. [Google Scholar] [CrossRef]
- Parisi, L.; Galli, C.; Bianchera, A.; Lagonegro, P.; Elviri, L.; Smerieri, A.; Lumetti, S.; Manfredi, E.; Bettini, R.; Macaluso, G. Anti-fibronectin aptamers improve the colonization of chitosan films modified with D-(+) Raffinose by murine osteoblastic cells. Journal of Materials Science: Materials in Medicine 2017, 28, 1–12. [Google Scholar] [CrossRef]
- Parisi, L.; Rivara, F.; Costa, C.A.; Abuna, R.P.; Palioto, D.B.; Macaluso, G.M. Aptamers recognizing fibronectin confer improved bioactivity to biomaterials and promote new bone formation in a periodontal defect in rats. Biomedical materials 2020, 16, 015016. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Journal of Pharmacology and Pharmacotherapeutics 2010, 1, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Monroe, D.M.; Hoffman, M. The clotting system–a major player in wound healing. Haemophilia 2012, 18, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. European journal of pharmaceutics and biopharmaceutics 2000, 50, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Su, W.T.; Wu, P.S.; Ko, C.S.; Huang, T.Y. Osteogenic differentiation and mineralization of human exfoliated deciduous teeth stem cells on modified chitosan scaffold. Materials Science and Engineering: C 2014, 41, 152–160. [Google Scholar] [CrossRef]
- Maji, K.; Dasgupta, S.; Pramanik, K.; Bissoyi, A. Preparation and evaluation of gelatin-chitosan-nanobioglass 3D porous scaffold for bone tissue engineering. International journal of biomaterials 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. Journal of tissue engineering and regenerative medicine 2017, 11, 942–965. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, Y. Biomaterials regulating bone hematoma for osteogenesis. Advanced Healthcare Materials 2020, 9, 2000726. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, H. Fibronectin maintains the balance between hemostasis and thrombosis. Cellular and molecular life sciences 2016, 73, 3265–3277. [Google Scholar] [CrossRef]
- Xu, X.R.; Carrim, N.; Neves, M.A.D.; McKeown, T.; Stratton, T.W.; Coelho, R.M.P.; Lei, X.; Chen, P.; Xu, J.; Dai, X.; others. Platelets and platelet adhesion molecules: novel mechanisms of thrombosis and anti-thrombotic therapies. Thrombosis journal 2016, 14, 37–46. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound repair and regeneration 2009, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Chatakun, P.; Núñez-Toldrà, R.; Díaz López, E.; Gil-Recio, C.; Martínez-Sarrà, E.; Hernández-Alfaro, F.; Ferrés-Padró, E.; Giner-Tarrida, L.; Atari, M. The effect of five proteins on stem cells used for osteoblast differentiation and proliferation: a current review of the literature. Cellular and molecular life sciences 2014, 71, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Halper, J.; Kjaer, M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Progress in heritable soft connective tissue diseases 2014, pp. 31–47.
- Rahman, S.; Patel, Y.; Murray, J.; Patel, K.V.; Sumathipala, R.; Sobel, M.; Wijelath, E.S. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells. BMC cell biology 2005, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Faia-Torres, A.B.; Goren, T.; Ihalainen, T.O.; Guimond-Lischer, S.; Charnley, M.; Rottmar, M.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; others. Regulation of human mesenchymal stem cell osteogenesis by specific surface density of fibronectin: a gradient study. ACS Applied Materials & Interfaces 2015, 7, 2367–2375. [Google Scholar]
- Fan, Q.; Bai, J.; Shan, H.; Fei, Z.; Chen, H.; Xu, J.; Ma, Q.; Zhou, X.; Wang, C. Implantable blood clot loaded with BMP-2 for regulation of osteoimmunology and enhancement of bone repair. Bioactive Materials 2021, 6, 4014–4026. [Google Scholar] [CrossRef]
- Beeravolu, N.; McKee, C.; Alamri, A.; Mikhael, S.; Brown, C.; Perez-Cruet, M.; Chaudhry, G.R. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. JoVE (Journal of Visualized Experiments) 2017, p. e55224.
- Hocking, A.M.; Gibran, N.S. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Experimental cell research 2010, 316, 2213–2219. [Google Scholar] [CrossRef]
- Kim, I.; Lee, S.K.; Yoon, J.I.; Kim, D.E.; Kim, M.; Ha, H. Fibrin glue improves the therapeutic effect of MSCs by sustaining survival and paracrine function. Tissue engineering part a 2013, 19, 2373–2381. [Google Scholar] [CrossRef]
- Kim, J.H.; Jo, C.H.; Kim, H.R.; Hwang, Y.i. Comparison of immunological characteristics of mesenchymal stem cells from the periodontal ligament, umbilical cord, and adipose tissue. Stem Cells International 2018, 2018. [Google Scholar] [CrossRef]
- Rheinländer, A.; Schraven, B.; Bommhardt, U. CD45 in human physiology and clinical medicine. Immunology letters 2018, 196, 22–32. [Google Scholar] [CrossRef]
- Razinia, Z.; Castagnino, P.; Xu, T.; Vázquez-Salgado, A.; Puré, E.; Assoian, R.K. Stiffness-dependent motility and proliferation uncoupled by deletion of CD44. Scientific Reports 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: from adhesion molecules to signalling regulators. Nature reviews Molecular cell biology 2003, 4, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Seminars in reproductive medicine. © Thieme Medical Publishers, 2010, Vol. 28, pp. 180–203.
- Robertson, I.B.; Horiguchi, M.; Zilberberg, L.; Dabovic, B.; Hadjiolova, K.; Rifkin, D.B. Latent TGF-β-binding proteins. Matrix Biology 2015, 47, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone research 2016, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Elsafadi, M.; Shinwari, T.; Al-Malki, S.; Manikandan, M.; Mahmood, A.; Aldahmash, A.; Alfayez, M.; Kassem, M.; Alajez, N.M. Convergence of TGFβ and BMP signaling in regulating human bone marrow stromal cell differentiation. Scientific reports 2019, 9, 4977. [Google Scholar] [CrossRef]
- Grainger, D.J.; Mosedale, D.E.; Metcalfe, J.C. TGF-β in blood: a complex problem. Cytokine & growth factor reviews 2000, 11, 133–145. [Google Scholar]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Siller, A.F.; Whyte, M.P. Alkaline phosphatase: discovery and naming of our favorite enzyme. Journal of Bone and Mineral Research 2018, 33, 362–364. [Google Scholar] [CrossRef]
- Golub, E.; Harrison, G.; Taylor, A.; Camper, S.; Shapiro, I. The role of alkaline phosphatase in cartilage mineralization. Bone and mineral 1992, 17, 273–278. [Google Scholar] [CrossRef]
- Holm, E.; Aubin, J.E.; Hunter, G.K.; Beier, F.; Goldberg, H.A. Loss of bone sialoprotein leads to impaired endochondral bone development and mineralization. Bone 2015, 71, 145–154. [Google Scholar] [CrossRef]
| Gene’s symbol | Gene’s names | TaqMan assay |
|---|---|---|
| Runx2 | Runt related transcription factor 2 | Rn01512298_m1 |
| Alp | Alkaline phosphatase liver/bone/kidney | Rn01516028_m1 |
| Bsp | Bone sialoprotein | Rn00561414_m1 |
| Bglap | Bone Protein Containing Gamma-carboxyglutamic acid (Osteocalcin/oc) | Rn00566386_g1 |
| Tgfβ1 | Transforming growth factor beta 1 | Rn00572010_m1 |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | Rn99999916_s1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





