1. Introduction
Chronic kidney disease (CKD) is a progressive disease with high morbidity and mortality, affecting 8% to 16% of the world’s population [
1,
2,
3]. CKD is diagnosed when the estimated glomerular filtration rate (eGFR) falls below 60 mL/min/1.73 m
2, termed low eGFR, or albuminuria, defined as albumin to creatinine ratios (ACR) ≥ 30 mg/g creatinine in women and ≥ 20 mg/g creatinine in men that persists for at least 3 months [
1,
2]. Environmental exposure to the toxic metal pollutant cadmium (Cd) is linked to increases in risk of both low eGFR and albuminuria. Typically, studies of CKD associated with environmental Cd exposure employed levels of blood Cd ([Cd[
b) and excretion of Cd (E
Cd), normalized to creatine excretion (E
Cd/E
cr) as indicators of exposure [
3,
4]. Notably, however, these Cd exposure indicators have been found to be also associated with increased risks of diabetes [
5,
6,
7,
8,
9,
10] and hypertension [
11,
12,
13,
14,
15,
16] both of which are major risk factors for CKD. Hypertension is known to be a cause and the result of CKD [
17,
18]. Furthermore, in Dutch prospective cohort study, smoking, which is a source of Cd exposure, promoted kidney failure, evident from a reduction of eGFR to below 15 mL/min/1.73 m
2 [
19]. It is imperative that these covariates are adequately adjusted for in the estimation of risks of GFR loss and albuminuria due to renal Cd accumulation.
The dose-response relationship between Cd exposure and adverse kidney outcomes measured by a reduction in eGFR and albuminuria are limited. In comparison, numerous dose-effect studies employed tubule damage and tubulopathy reflected by tubular proteinuria have been employed as signs of the nephrotoxicity due to Cd accumulation. However, current evidence suggests that a sustained decrease in eGFR after Cd exposure is more suitable than the tubulopathy endpoint, especially for the purpose of determining protective exposure guidelines.
Also, current evidence suggests that excreted Cd (E
Cd) is related to tubular cell injury and death by a cumulative burden of Cd [
20]. The excreted Cd emanates from tubular epithelial cells in complexed with metallothionein (MT) as CdMT [
21], the storage form of Cd. Consequently, it is logical to normalize E
Cd to creatinine clearance (C
cr) rather than creatinine excretion (E
cr) because C
cr is a surrogate for GFR, which is the measurable analog of nephron number [
3,
20,
22].
The present study aims to explore if there is a potential toxicity threshold level of Cd accumulation in kidneys. To achieve this aim, changes in eGFR and albumin excretion (Ealb) together with risks of low eGFR and albuminuria were quantified in relation to both ECd and [Cd]b. The confounding impact of smoking, diabetes, and hypertension were also evaluated. We collected data from women and men who resided in Cd-polluted and non-polluted regions of Thailand to obtain a wide range of exposure level pivotal to dose-response analysis.
We normalized E
Cd and E
alb to C
cr as E
Cd/C
cr and E
alb/C
cr, respectively. This normalization method depicts an amount of Cd and albumin excreted per volume of filtrate, which approximates the amount of Cd and albumin excreted per nephron [
23]. The E
Cd/C
cr and E
alb/C
cr ratios are unaffected by creatinine excretion (E
cr), while the differences in urine flow rate (dilution) and the number of functioning nephrons among cohort participants are eliminated [
23]. E
alb/C
cr ≥ 20 mg/g creatinine indicate albuminuria in both men and women.
2. Materials and Methods
2.1. Cohort Participants
To establish a clear dose–response relationship, a population exposed to a wide range of Cd doses is required. Therefore, we selected subjects from two-population-based cross- sectional studies undertaken in a Cd-contaminated area of the Mae Sot District, Tak Province [
24], and a non-contaminated location in Nakhon-Si-Thammarat Province [
25] of Thailand. More females (
n = 354) were recruited to the present study than males (
n = 118), given that an increase in mortality from kidney disease was found, especially in women in a prospective cohort study of residents of a Cd -polluted area of Japan [
26].
The data from a nationwide survey of Cd levels in soils and food crops indicated that environmental exposure to Cd in Nakhon Si Thammarat was low [
27]. In comparison, the Cd content of the paddy soil samples from the Mae Sot district exceeded the standard of 0.15 mg/kg, and the rice samples collected from household storage contained four times the amount of the permissible Cd level of 0.1 mg/kg [
28]. An independent health survey reported the prevalence of low eGFR among those resided in the Cd-contaminated area of Mae Sot District to be 16.1% [
29].
The study protocol for the Mae Sot group was approved by the Institutional Ethical Committees of Chiang Mai University and the Mae Sot Hospital. The Office of the Human Research Ethics Committee, Walailak University of Thailand approved the study protocol for the Nakhon Si Thammarat group.
Prior to participation, all participants provided informed consent. They had lived at their current addresses for at least 30 years. Exclusion criteria were pregnancy, breast-feeding, a history of metal work, and a hospital record or physician’s diagnosis of an advanced chronic disease. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg [
30], a physician’s diagnosis, or prescription of anti-hypertensive medications. Diabetes was diagnosed, when fasting plasma glucose levels ≥ 126 mg/dL (
https://www.cdc.gov/diabetes/basics/getting-tested.html) (accessed on 12 August 2023) or a physician’s prescription of anti-diabetic medications.
2.2. Assessment of Cadmium Exposure and Adverse Effects
Assessment of long-term Cd exposure was based on ECd, while a recent exposure was indicated by [Cd]b. Kidney functional assessment was based on Ealb and eGFR. For these measurements of exposure and effects, samples of urine and whole blood were collected after overnight fast. Blood samples were collected within 3 h after urine sampling. Aliquots of blood and urine samples were stored at −80°C for later analysis.
Levels of Cd in urine and blood ([Cd]
u and [Cd]
b) were quantified by atomic absorption spectrophotometry. Blood control samples (ClinChek, Munich, Germany), urine standard reference material No. 2670 (National Institute of Standards, Washington, DC, USA), and the reference urine metal control levels 1, 2, and 3 (Lyphocheck, Bio-Rad, Hercules, CA, USA) were used for quality control, analytical accuracy, and precision assurance. The limit of detection (LOD) of Cd, defined as 3 times the standard deviation of blank measurements, was 0.3 µg/L for [Cd]
b and 0.1 µg/L for [Cd]
u. When a sample contained Cd below its LOD, the Cd concentration assigned was the LOD value divided by the square root of 2 [
31].
Urinary and plasma creatinine ([cr]u and [cr]p) assays were based on the Jaffe reaction. Urinary albumin assay was based on a turbidimetric method.
2.3. Estimated Glomerular Filtration Rate (eGFR)
In theory GFR is a measure of nephron function which is the product of the functioning nephron number and mean single nephron GFR [
32,
33,
34]. In practice, GFR is estimated from established chronic kidney disease epidemiology collaboration (CKD-EPI) equations, and is reported as estimated GFR (eGFR) [
34]. These CKD-EPI equations have been validated with inulin clearance [
35].
Male eGFR = 141 × [plasma creatinine/0.9]Y × 0.993age, where Y = −0.411 if [cr]p ≤ 0.9 mg/dL, and Y = −1.209 if [cr]p > 0.9 mg/dL. Female eGFR = 144 × [plasma creatinine/0.7]Y × 0.993age, where Y = −0.329 if [cr]p ≤ 0.7 mg/dL, and Y = −1.209 if [cr]p > 0.7 mg/dL. CKD stages 1, 2, 3a, 3b, 4, and 5 corresponded to eGFRs of 90–119, 60–89, 45–59, 30−44, 15–29, and < 15 mL/min/1.73 m2, respectively. For dichotomized comparison, CKD (abnormal eGFR) was defined eGFR ≤ 60 mL/min/1.73 m2.
2.4. Normalization of Excretion Rate
Ex was normalized to Ecr as [x]u/[cr]u, where x = Cd or alb; [x]u = urine concentration of x (mass/volume); and [cr]u = urine creatinine concentration (mg/dL). The ratio [x]u/[cr]u was expressed in μg/g of creatinine.
E
x was normalized to C
cr as E
x/C
cr = [x]
u[cr]
p/[cr]
u, where x = Cd or alb; [x]
u = urine concentration of x (mass/volume); [cr]
p = plasma creatinine concentration (mg/dL); and [cr]
u = urine creatinine concentration (mg/dL). E
x/C
cr was expressed as the excretion of x per volume of filtrate [
23].
Results obtained with C
cr-normalized data are shown herein. Results of analogous analyses with E
cr-normalized data are provide in
Supplemental Material (SM).
2.5. Statistical Analysis
Data were analyzed with IBM SPSS Statistics 21 (IBM Inc., New York, NY, USA). The Mann–Whitney U test was used to compared two groups, and Pearson’s chi-squared test was used to assess differences in percentages. Distribution of the variables was examined for skewness and those showing right skewing were subjected to logarithmic transformation before analysis, where required. Departure from normal distribution of variables was assessed by one sample Kolmogorov–Smirnov test.
A multiple linear regression model analysis was used to identify predictors of eGFR and Ealb/Ccr. The multivariable logistic regression analysis was used to determine the prevalence odds ratio (POR) for low eGFR and albuminuria. Univariate analysis of covariance via Bonferroni correction in multiple comparisons was used to obtain covariate-adjusted mean Ealb/Ccr and eta square (η2) values. For all tests, p-values ≤ 0.05 were considered to indicate statistical significance.
3. Results
3.1. Cohort Participants
Table 1 provides descriptive characteristics of this Thai cohort participants, recruited from a low- exposure locality, and a high-exposure region due to environmental Cd pollution.
A total of 482 persons (354 women and 118 men), mean age of 51.8 years, were included in this investigation. The respective percentages of smoking and diabetes were 29.7% and 18.3%, while about half (48.5%) had hypertension.
The overall % low eGFR and albuminuria (ACR criterion) were 8.1% and 15%. For Ccr-normalized data, the % (Ealb/Ccr) ×100 ≥ 20 mg/L filtrate was 16.9%. Of note the % abnormal Ealb was higher in the low eGFR group than normal eGFR group for both ACR and Ealb/Ccr criteria in both genders. Similarly, the % hypertension and diabetes were higher in the low eGFR than the normal eGFR group.
The mean [Cd]b (range) was 2.60 (0.03−20) µg/L. The mean (ECd/Ccr) ×100 (range) was 3.20 (0.02−25) µg/L filtrate. Corresponding mean ECd/Ecr (range) was 4.05 (0.03−31) µg/g creatinine.
3.2. Predictors of eGFR
Table 2 provides results of multiple linear regression analysis to define the independent variables that contributed to differences in eGFR.
In an inclusive model, all eight independent variables explained 27.8% of the total variation in eGFR. Age, ECd/Ccr and diabetes were the three main influential independent variables. All other five independent variables did not show a significant association with eGFR. In subgroup analysis, female eGFR was inversely associated with age (β = −0.511), ECd/Ccr (β = −0.126) and diabetes (β = −0.119). In comparison, male eGFR was associated with age only (β = −0.472).
In an analogous regression of eGFR with E
Cd normalized to creatinine excretion (E
Cd/E
cr) (
Table S1), eGFR was inversely associated with age only (β = −0.442). No significant association was seen between eGFR and all other six variables, including E
Cd/C
cr.
To investigate dose-response relationship of Cd exposure and changes in eGFR, we designated the low, middle and high ECd/Ccr tertiles to represent low, medium and high Cd burdens, respectively. In women, the cut-off values of (ECd/Ccr) ×100 for the low, medium and high Cd burdens were ≤ 1.44, 1.45−3.26, > 3.26 µg/L filtrate, respectively. Corresponding cut-off values of (ECd/Ccr) ×100 in men were ≤ 1.25, 1.26-3.25, > 3.25 µg/L filtrate.
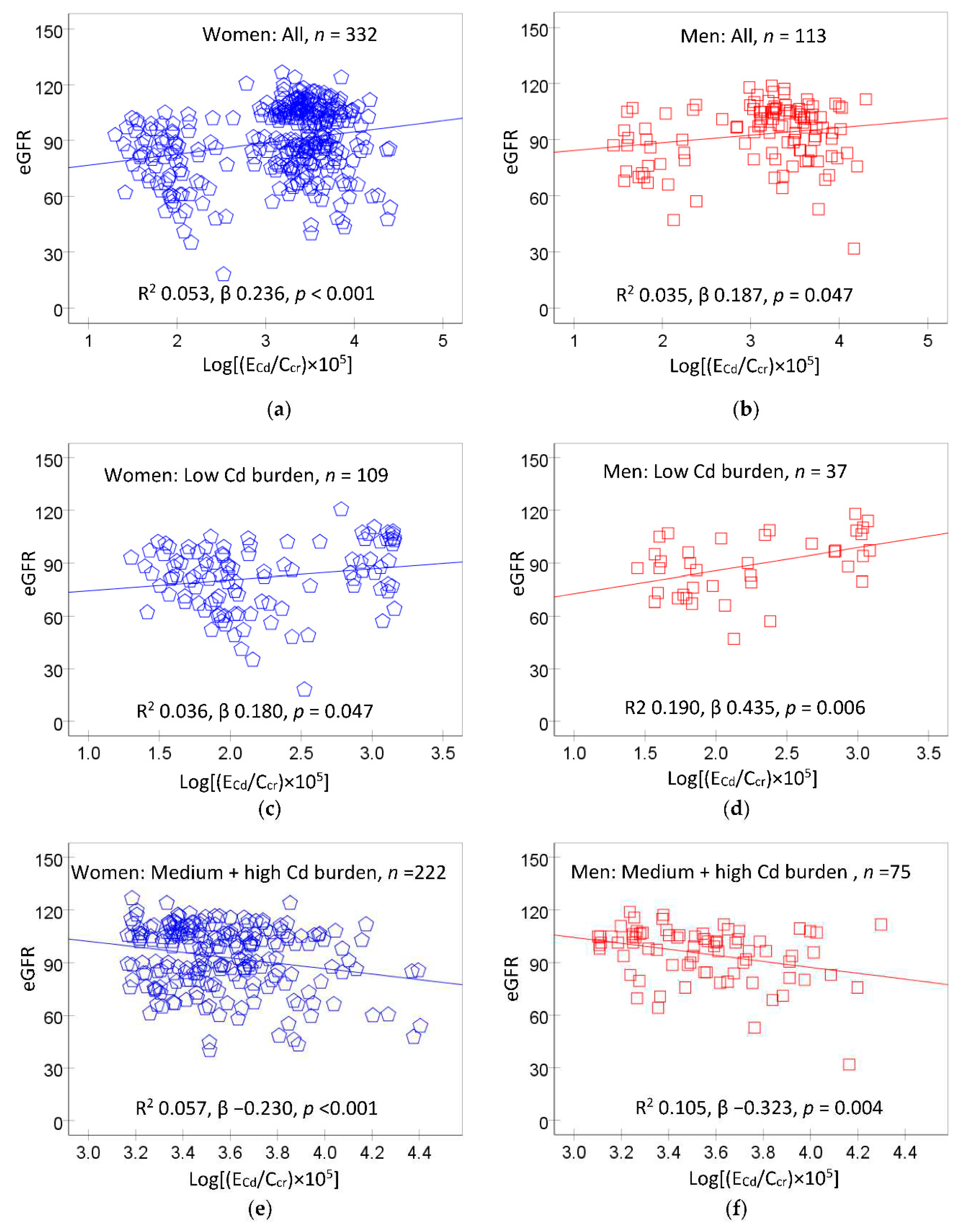
Figure 1 provides scatterplots relating eGFR to Cd excretion rate in women and men, and their subgroups according to Cd burden levels, described above.
In scatterplots including all women (
Figure 1a) and all men (
Figure 1b), a direct relationship between eGFR and E
Cd/C
cr was suggested. In subgroup analysis, a direct relationship of eGFR and E
Cd/C
cr was seen in women and men who had of the low Cd burden (
Figure 1c,d). In contrast, eGFR was inversely related to E
Cd/C
cr in women and men of the medium plus high Cd burden groups (
Figure 1e,f). These data indicate that E
Cd/C
cr of 1.44 µg/L filtrate may be a threshold level of Cd accumulation, above which eGFR began to drop.
To confirm or dispute the contrasting eGFR responses below or above E
Cd/C
cr of 1.44 µg/L filtrate, multiple regression analyses of eGFR across Cd burden groups were undertaken. Results are provided in
Table 3.
In distinction from a bivariate analysis (
Figure 1c,d), the association between eGFR and E
Cd/C
cr was statistically insignificant in the low Cd burden group after adjustment for confounding variables (
Table 3). In this low Cd burden group, eGFR was inversely associated with age only (β = −0.455), while its association of with E
Cd/C
cr became statistically insignificant (
Table 3).
Of interest, there was an inverse relationship between eGFR and ECd/Ccr in the medium and high Cd burden groups following similar adjustment for confounders. All eight independent variables explained, respectively 32.1% and 25.5% of total eGFR variation in the medium and high Cd burden groups. In the high Cd burden group, eGFR was more closely associated with ECd/Ccr (β = −0.349), compared with the medium Cd burden group (β = −0.284). Other predictors of eGFR in the medium Cd burden group, were age (β = −0.505) and systolic blood pressure (β = −0.230). Age was the other predictor of eGFR (β=−0.410) in the high Cd burden group.
3.3. Logistic Regression Analysis of Low eGFR
Table 4 provides results of logistic regression analysis that evaluated effects of Cd body burden and other six independent variables, on the prevalence odds ratios (POR) for low eGFR.
In all subjects, the POR values for low eGFR increased with age (POR 1.118) and in those with diabetes (POR 3.024), medium Cd burden (POR 8.265), and high Cd burden (POR 3.643). All other four independent variables did not significantly affect the POR for low eGFR.
Among women, the POR values for low eGFR increased with age (POR 1.114) and in the medium Cd burden group (POR 7.204). An increase in the POR for low eGFR in the high Cd burden group did not reach a statistically significant level (POR 3.218, p = 0.064).
An effects of Cd exposure on the POR for low eGFR in men could not be evaluated due a small number of men with low eGFR (n = 4). However, the POR for low eGFR among men was associated with hypertension (POR 2.478).
3.4. Multiple Regression Analysis of Albumin Excretion Rate
We used multiple regression analysis to identify if E
Cd/C
cr and other independent variables predicted rising E
alb/C
cr. We included eGFR in regression models to evaluate if functioning nephrons, indicated by eGFR values, had an independent effect on E
alb/C
cr. Results are provided in
Table 5.
In the low and medium Cd burden groups, neither ECd/Ccr nor eGFR showed a significant association with Ealb/Ccr. However, diabetes and hypertension predicted Ealb/Ccr in the low Cd burden group, while BMI was a sole predictor of Ealb/Ccr in the medium Cd burden group.
In the high Cd burden group, ECd/Ccr and eGFR both were associated with Ealb/Ccr. There was an inverse association between eGFR and Ealb/Ccr (β =−0.214), while ECd/Ccr showed a direct relationship with Ealb/Ccr (β = 0.173). All other six independent variables did not have a significant effect on Ealb/Ccr.
Of note, the relationship between albumin excretion and eGFR was obscure, when E
alb was normalized to E
cr (E
alb/E
cr). As data in
Table S2 indicate, E
alb/E
cr did not show a significant inverse association with eGFR (β = −0.092,
p = 0.078).
In subsequent analysis, scatterplots were used together with univariate analysis to quantify independent effects of E
Cd/C
cr, eGFR, diabetes and hypertension on E
alb/C
cr. Covariate adjusted mean E
alb/C
cr values were obtained for various subgroups as shown in
Figure 2.
In the low Cd burden group, a scatterplot indicated that the correlation of E
alb/C
cr and E
Cd/C
cr was statistically insignificant (
Figure 2a). After adjustment for covariates and interactions, those with diabetes, hypertension and low eGFR had higher E
alb/C
cr than non-diabetics, normotension and normal eGFR (
Figure 2b).
In the medium plus high Cd burden group, a scatterplot indicated a statistically significant relationship between E
alb/C
cr and E
Cd/C
cr (
Figure 2c). After adjustment for covariates and interactions, those with hypertension and low eGFR had higher E
alb/C
cr than those with normotension and normal eGFR (
Figure 2d).
3.5. Blood Cadmium and eGFR as Predictos of Albuminuria
Table 6 provides results of logistic regression analysis that evaluated if [Cd]
b, like E
Cd, predicted an increase in risk of abnormally high E
alb defined as (E
alb/Ccr)×100 ≥ 20 µg/L filtrate.
In all subjects, eGFR, diabetes, hypertension, and [Cd]b were independent variables showing associations with the POR for albuminuria. Comparing those with [Cd]b < 0.82 µg/L, POR values for albuminuria were increased by 2.4-fold and 2.7-fold in those with [Cd]b of 0.83−2.63 and ≥ 2.64 µg/L, respectively. For every 1 mL/min/1.73 m2 loss of eGFR, the POR for albuminuria rose by 4.3%. There was no effect of Cd on albuminuria in an analysis with Ecr-normalized data (Table 3S).
Among women, there was [Cd]b-related increment of the POR for albuminuria. The POR values for high albuminuria were increased by 3.4-fold and 3.8-fold in those with middle and high [Cd]b tertiles, respectively. In addition, eGFR, diabetes, and hypertension were independent variables showing associations with the POR for albumin in women. For every 1 mL/min/1.73 m2 loss of eGFR, the POR for albuminuria rose by 4.5%.
Among men, [Cd]b did not show an association with POR for albuminuria, eGFR did. For every 1 mL/min/1.73 m2 loss of eGFR, the POR for albuminuria rose by 4.5%, similar to the effect size found in women.
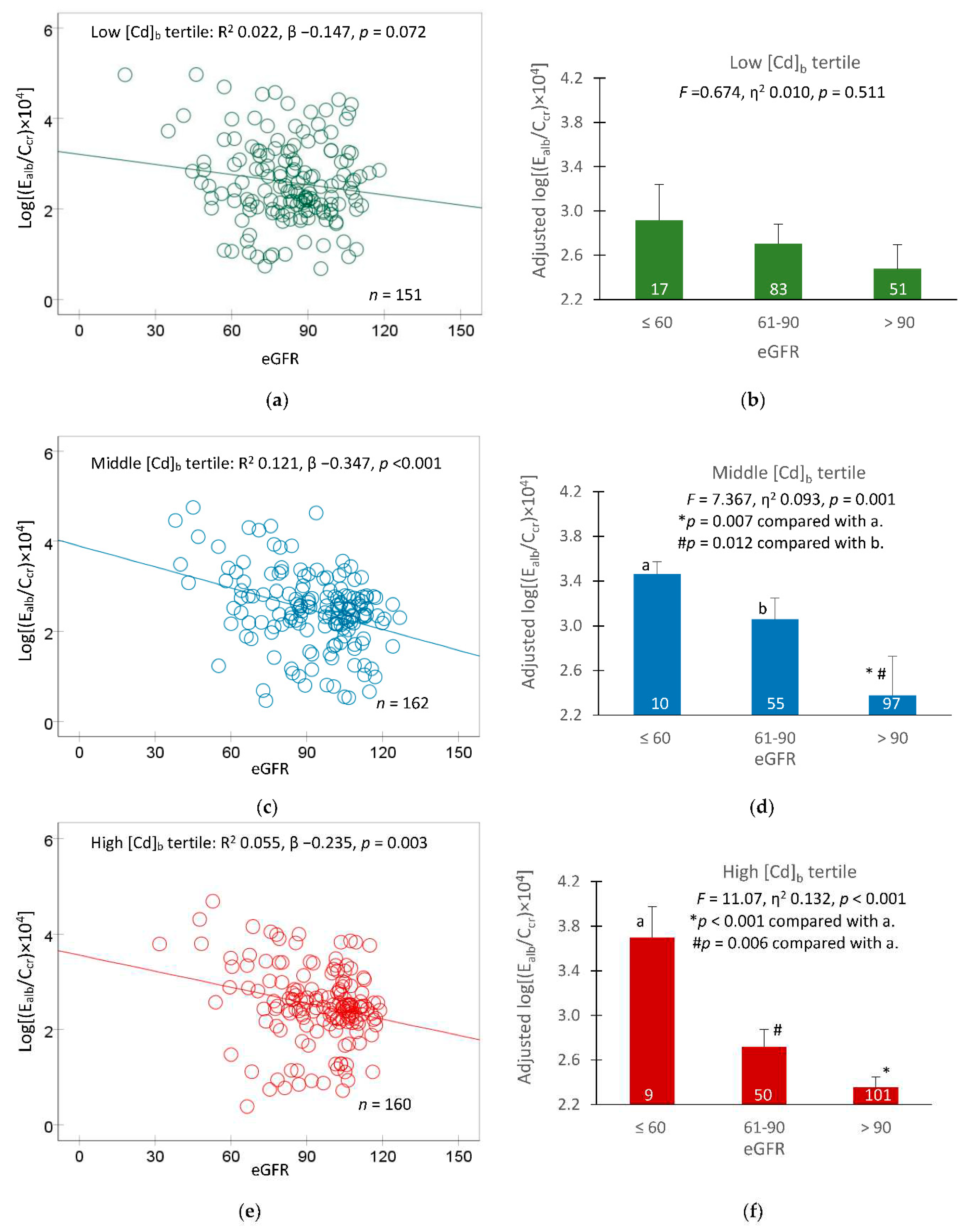
In a following analysis, covariate adjusted mean E
alb/C
cr values were obtained for groups of participants with different eGFR levels (≤ 60, 61−90 and > 90 mL/min/1.73 m
2) to reveal an effect of GFR loss, reflected by a reduction in eGFR. Results are shown in
Figure 3.
In the low [Cd]
b tertile group, the relationship between E
alb/C
cr and eGFR was statistically insignificant (
Figure 3a). Similarly, the covariate-adjusted mean E
alb/C
cr values did not show a significant variation across three eGFR subgroups.
In the middle and high [Cd]
b tertile groups, an inverse relationship was seen between E
alb/C
cr and eGFR (
Figure 3c,e), and an effect of GFR loss on E
alb/C
cr was apparent (
Figure 3d,f). In these two [Cd]
b tertile groups, mean E
alb/C
cr value was highest, middle and lowest in those with eGFR ≤ 60, 60−90, and > 90 mL/min/1.73 m
2, respectively.
4. Discussion
Herein, we used eGFR decline as a primary endpoint in our attempt to define a toxic accumulation level of Cd. We focused on this eGFR because attenuation of eGFR decline is employed in clinical trials to evaluate effects of treatment of CKD [
1,
2]. Albuminuria was concurrently examined, because it has also been associated with Cd exposure along with low eGFR [
4]. Previous studies show that the burden of Cd as µg/ g kidney tissue weight increases with age [
36,
37,
38]. They show also that sufficient tubule damage from the Cd accumulation disables GFR, leading to nephron atrophy, and a fall of GFR follows [
20,
22,
39,
40]. We hypothesized that this eGFR effect of Cd occurs at a very low kidney burden, producing E
Cd/E
cr below 5.24 µg/g creatinine. It is noteworthy that E
Cd itself is indicative of tubular cell injury and death [
3,
20]
We analyzed data were from 482 Thai nationals of which 8.1% had low eGFR and 15% had albuminuria (ACR criterion). Nearly half (48.3%) had hypertension, while 29.7% reported to be smokers, and 18.3% had diabetes. There was a 1250-fold difference in ECd/Ccr values, ranging from 0.0002 to 0.25 µg/L filtrate (mean 0.0032 µg/L filtrate). There was a 667-fold difference in [Cd]b, ranging between 0.03 and 20 µg/L (mean 2.60 µg/L). The wide ranges of these Cd burden and exposure matrices together with sufficiently high numbers of smokers and those with diabetes and hypertension provide an ideal scenario to define with certainty a toxic threshold level of Cd should it exist.
Like E
Cd/C
cr, E
Cd/E
cr showed a large variation (1033-fold), mean E
Cd/E
cr (range) was 4.05 (0.03−31) µg/g creatinine (
Table 1). However, in distinction from E
Cd/C
cr, there was no significant association between eGFR and E
Cd/E
cr (
Table S1). Consequently, a dose-response analysis was not possibly determined. Also, when E
alb was normalized to E
cr (E
alb/E
cr), an inverse relationship of albuminuria (ACR criterion) and eGFR was obscure (
Table S2) and there was no effect of Cd on POR for albuminuria (
Table S3).
4.1. Effects of Cadmium on eGFR
A direct relationship between eGFR and E
Cd/C
cr was seen in women and men with low-Cd burden (E
Cd/C
cr ≤ 0.014 in women and ≤ 0.012 µg/L in men) (
Figure 1c,d). However, such eGFR/E
Cd/C
cr relationship was weakened and became statistically insignificant, when covariates were adjusted. In comparison, eGFR showed an inverse association with E
Cd/C
cr, when E
Cd/C
cr values rose to levels > 0.014 µg/L filtrate in women and > 0.012 µg/L filtrate in men (
Figure 1e,f and
Table 3). In reflecting a dose response relationship, eGFR was more closely associated with in E
Cd/C
cr in the high burden group (β = −0.349), compared to medium Cd burden group (β = −0.284). An association of eGFR and E
Cd/C
cr was statistically insignificant.
Cd burden, diabetes and age were three of seven variables that affected the POR for low eGFR (
Table 4). In those with medium, high Cd burden, and diabetes, POR for low eGFR rose by 8.3-fold, 3.6-fold, and 3.0-fold, respectively. Per every 1-year rise in age, there was an 11.8% (95% CI, 6.2, 17.6) increase in the POR for low eGFR.
In summary, no effect of Cd on eGFR was found in those with E
Cd/C
cr values < 0.012 µg/L filtrate. This E
Cd/C
cr value of 0.012 µg/L filtrate is in ranges with the no-observed-adverse-effect level (NOAEL) equivalent of E
Cd/C
cr at 0.010-0.024 µg/L filtrate, obtained by benchmark dose method [
41]
4.2. Effects of Cadmium on Prevalence of Albuminuria
The bulk of albumin (~80%) in the glomerular ultrafiltrate is reabsorbed in the S1 sub-segment of the proximal tubule, where the receptor-mediated endocytosis involving the megalin/cubillin system is located [
41,
42,
43,
44]. Reabsorption of albumin also occurs in the distal tubule and collecting duct, where the process is mediated by the NGAL/lipocalin-2 receptor system [
45,
46,
47]. Experimental studies show that albuminuria was resulted from Cd selectively disabled albumin endocytosis which is mediated by the cubilin/megalin receptor system [
48] and that Cd may diminish expression of megalin and ClC5 channels [
49]. However, data in
Table 1 indicate that albuminuria, was more prevalent in the low eGFR, compared to normal eGFR groups., thereby suggesting that low eGFR could be a risk factor for albuminuria. We examined this phenomenon further, as described below.
In multiple regressions (
Table 5), both eGFR and ECd/Ccr appeared to have an effect on E
alb/C
cr. In logistic regression (
Table 6), POR for albuminuria rose by 4.3% (95% CI: 2.6, 6.1) for every 1 mL/min/1.73 m
2 loss of eGFR, and it was increased by 2.4-fold to 2.7-fold in those who had [Cd]
b ≥ 0.83 µg/L. A similar effect size of Cd on eGFR was seen in women and men although the causes of albuminuria differed (
Table 6). Additional evidence that Cd may affect eGFR and albumin reuptake simultaneously comes from a covariance analysis, where 9.3% to 13.2% of E
alb/C
cr variation could be attributable to eGFR levels in those with [Cd]b > 0.83 µg/L (
Figure 3d,f). eGFR levels did not explain the variability of E
alb/C
cr in the low [Cd]
b tertile group (
F = 0.674, η
2 0.010,
p = 0.511) (
Figure 3b).
Results described above could be interpreted to suggest that albuminuria could be a consequence of Cd-induced destruction of nephrons together with a direct effect on tubular reabsorption of albumin possibly through the cubilin/megalin receptor system of endocytosis. These Cd effects were not found in those who had [Cd]b < 0.83 µg/L.
4.3. Threshold-Based Exposure Guidelines
For most people, exposure to Cd is inevitable because the metal is present in nearly all food types. In Japan, for example, rice and its products, green vegetables, and cereals and seeds plus potatoes constituted, respectively to 38%, 17%, and 11% of total dietary Cd exposure [
50]. In response, health guidance such as a tolerable intake level of Cd and a reference dose (RfD) were determined [
51,
52,
53]. The Joint FAO/WHO Expert Committee on Food Additives and Contaminants (JECFA) suggested a tolerable monthly intake (TMI) of Cd to be 25 μg per kg body weight per month, equivalent to 0.83 μg per kg body weight per day (58 µg/day for a 70-kg person), and E
Cd/E
cr of 5.24 μg/g creatinine was adopted as a nephrotoxicity threshold value [
46]. Both figures were based on a risk assessment model that based solely on β
2-microglobulin excretion rate (E
β2M/E
cr) ≥ 300 μg/g creatinine, termed tubular proteinuria, as a toxic endpoint.
The European Food Safety Authority (EFSA) used also β
2M endpoint, but with inclusion of an uncertainty factor (safety margin). The EFSA suggested E
Cd/E
cr of 1 μg/g creatinine to be the toxicity threshold, and 0.36 μg/kg body weight per day (25 µg/day for a 70-kg person) as the RfD [
53]. The higher JECFA guidelines are adopted by most countries. These health guidelines values exceed the nephrotoxicity threshold identified in the present study. In theory, exposure guideline that provides sufficiently healthy protection should only be determined from the most sensitive endpoint with consideration given to subpopulations with increased susceptibility [
54] and the most recent scientific knowledge should be considered.
Of concern, eGFR decline due to Cd nephropathy has increasingly been reported in both children and adult populations. Lower eGFR values were associated with higher Cd excretion rates were observed in studies from Guatemala [
55] and Myanmar [
56]. In a prospective cohort study of Bangladeshi preschool children, an inverse relationship between E
Cd and kidney volume was seen in children at 5 years of age. This was in addition to a decrease in eGFR [
57]. E
Cd was inversely associated with eGFR, especially in girls. In another prospective cohort study, the reported mean for Cd intake among Mexican children was 4.4 µg/d at the baseline rose to 8.1 µg/d after nine years, when such Cd intake levels showed a marginally inverse association with eGFR [
58].
5. Conclusions
Environmental exposure to Cd was confirmed to be closely associated with a declining GFR and albuminuria. The current threshold for Cd exposure of 5.24 µg/g creatinine (ECd/Ecr), which is solely based on an increment of excretion of β2-microglobulin above 300 µg/g creatinine underestimates the level at which Cd induces kidney damage. Our results show that when a declining GFR is considered along with albuminuria the threshold equivalent is 0.01−0.02 µg/g creatinine. Now is the time to acknowledge there is no safe level of Cd exposure.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Predictors of eGFR based on cadmium excretion rate normalized to creatinine excretion (ECd/Ecr); Table S2: Predictors of albumin excretion rate normalized to creatinine excretion (Ealb/Ecr); Table S3: Prevalence odds ratios for low eGFR and albuminuria in relation to cadmium excretion rate normalized to creatine excretion (ECd/Ecr).
Author Contributions
Conceptualization, S.S.; methodology, S.S. and S.Y.; formal analysis, S.S.; investigation, S.Y., P.P., and T.K.; resources, G.C.G. and D.A.V.; writing—original draft preparation, S.S.; writing—review and editing, G.C.G. and D.A.V.; project administration, S.S. and S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This is not applicable for the present study, which used archived data [
24,
25].
Informed Consent Statement
Informed consent was obtained from all participants in the study prior to their participation.
Data Availability Statement
All data are contained within this article.
Acknowledgments
This work was supported with resources from the Centre for Kidney Disease Research, Translational Research Institute, and the Department of Kidney and Transplant Services, Princess Alexandra Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Phelps, K.R. Estimation of health risks associated with dietary cadmium exposure. Arch. Toxicol. 2023, 97, 329–358. [Google Scholar] [PubMed]
- Madrigal, J.M.; Ricardo, A.C.; Persky, V.; Turyk, M. Associations between blood cadmium concentration and kidney function in the U.S. population: Impact of sex, diabetes and hypertension. Environ. Res. 2018, 169, 180–188. [Google Scholar] [CrossRef]
- Xiao, L.; Li, W.; Zhu, C.; Yang, S.; Zhou, M.; Wang, B.; Wang, X.; Wang, D.; Ma, J.; Zhou, Y.; et al. Cadmium exposure, fasting blood glucose changes, and type 2 diabetes mellitus: A longitudinal prospective study in China. Environ. Res. 2021, 192, 110259. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Il’yasova, D.; Ivanova, A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care 2003, 26, 468–470. [Google Scholar] [CrossRef]
- Wallia, A.; Allen, N.B.; Badon, S.; El Muayed, M. Association between urinary cadmium levels and prediabetes in the NHANES 2005–2010 population. Int. J. Hyg. Environ. Health 2014, 217, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Little, B.B.; Reilly, R.; Walsh, B.; Vu, G.T. Cadmium is associated with type 2 diabetes in a Superfund Site Lead Smelter Community in Dallas, Texas. Int. J. Environ. Res. Public Health 2020, 17, 4558. [Google Scholar] [CrossRef]
- Guo, F.F.; Hu, Z.Y.; Li, B.Y.; Qin, L.Q.; Fu, C.; Yu, H.; Zhang, Z.L. Evaluation of the association between urinary cadmium levels below threshold limits and the risk of diabetes mellitus: A dose-response meta-analysis. Environ. Sci. Pollut. Res. Int. 2019, 26, 19272–19281. [Google Scholar] [CrossRef]
- Filippini, T.; Wise, L.A.; Vinceti, M. Cadmium exposure and risk of diabetes and prediabetes: A systematic review and dose-response meta-analysis. Environ. Int. 2022, 158, 106920. [Google Scholar] [CrossRef]
- Satarug, S.; Nishijo, M.; Ujjin, P.; Vanavanitkun, Y.; Moore, M.R. Cadmium-induced nephropathy in the development of high blood pressure. Toxicol. Lett. 2005, 157, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, N.; Fry, R.C.; Balakrishnan, P.; Navas-Acien, A.; Oliver-Williams, C.; Howard, A.G.; Cole, S.A.; Haack, K.; Lange, E.M.; Howard, B.V.; et al. Cadmium body burden and increased blood pressure in middle-aged American Indians: The Strong Heart Study. J. Hum. Hypertens. 2017, 31, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.A.; Park, E.; Kim, S.; Kim, B. Influence of serum ferritin combined with blood cadmium concentrations on blood pressure and hypertension: From the Korean National Health and Nutrition Examination Survey. Chemosphere 2022, 288, 132469. [Google Scholar] [CrossRef]
- Garner, R.E.; Levallois, P. Associations between cadmium levels in blood and urine, blood pressure and hypertension among Canadian adults. Environ. Res. 2017, 155, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.; Wai, K.M.; Kanda, A.; Ando, M.; Murashita, K.; Nakaji, S.; Ihara, K. Low Level of Serum Cadmium in Relation to Blood Pressures Among Japanese General Population. Biol. Trace Element Res. 2021, 200, 67–75. [Google Scholar] [CrossRef]
- Oliver-Williams, C.; Howard, A.G.; Navas-Acien, A.; Howard, B.V.; Tellez-Plaza, M.; Franceschini, N. Cadmium body burden, hypertension, and changes in blood pressure over time: Results from a prospective cohort study in American Indians. J. Am. Soc. Hypertens. 2018, 12, 426–437.e9. [Google Scholar] [CrossRef]
- Crowley, S.D.; Coffman, T.M. The inextricable role of the kidney in hypertension. J. Clin. Investig. 2014, 124, 2341–2347. [Google Scholar]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Kidney cadmium toxicity, diabetes and high blood pressure: The perfect storm. Tohoku J. Exp. Med. 2017, 241, 65–87. [Google Scholar] [CrossRef]
- Oosterwijk, M.M.; Hagedoorn, I.J.M.; Maatman, R.G.H.J.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Cadmium, active smoking and renal function deterioration in patients with type 2 diabetes. Nephrol. Dial. Transpl. 2023, 38, 876–883. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Ruangyuttikarn, W.; Nishijo, M.; Gobe, G.C.; Phelps, K.R. The source and pathophysiologic significance of excreted cadmium. Toxics 2019, 7, 55. [Google Scholar] [CrossRef]
- Wolf, C.; Strenziok, R.; Kyriakopoulos, A. Elevated metallothionein-bound cadmium concentrations in urine from bladder carcinoma patients, investigated by size exclusion chromatography-inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2009, 631, 218–222. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Nishijo, M.; Ruangyuttikarn, W.; Gobe, G.C.; Phelps, K.R. The effect of cadmium on GFR is clarified by normalization of excretion rates to creatinine clearance. Int. J. Mol. Sci. 2021, 22, 1762. [Google Scholar] [CrossRef] [PubMed]
- Phelps, K.R.; Gosmanova, E.O. A generic method for analysis of plasma concentrations. Clin. Nephrol. 2020, 94, 43–49. [Google Scholar] [CrossRef]
- Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013, 121, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Yimthiang, S.; Pouyfung, P.; Khamphaya, T.; Kuraeiad, S.; Wongrith, P.; Vesey, D.A.; Gobe, G.C.; Satarug, S. Effects of en-vironmental exposure to cadmium and lead on the risks of diabetes and kidney dysfunction. Int. J. Environ. Res. Public Health 2022, 19, 2259. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Nogawa, K.; Suwazono, Y.; Kido, T.; Sakurai, M.; Nakagawa, H. Lifetime cadmium exposure and mortality for renal diseases in residents of the cadmium-polluted Kakehashi River Basin in Japan. Toxics 2020, 8, 81. [Google Scholar] [CrossRef]
- Zarcinas, B.A.; Pongsakul, P.; McLaughlin, M.J.; Cozens, G. Heavy metals in soils and crops in Southeast Asia. 2. Thailand. Environ. Geochem. Health 2004, 26, 359–371. [Google Scholar] [CrossRef]
- Suwatvitayakorn, P.; Ko, M.S.; Kim, K.W.; Chanpiwat, P. Human health risk assessment of cadmium exposure through rice consumption in cadmium-contaminated areas of the Mae Tao sub-district, Tak, Thailand. Environ. Geochem. Health 2020, 42, 2331–2344. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Nguntra, P.; Kaewnate, Y.; Mahasakpan, P.; Limpatanachote, P.; Aunjai, T.; Jeekeeree, W.; Punta, B.; Funkhiew, T.; Phopueng, I. Human health effects from cadmium exposure: Comparison between persons living in cad-mium-contaminated and non-contaminated areas in northwestern Thailand. Southeast Asian J. Trop. Med. Publ. Health 2015, 46, 133–142. [Google Scholar]
- Bloch, M.J.; Basile, J.N. Review of Recent Literature in Hypertension: Updated Clinical Practice Guidelines for Chronic Kidney Disease Now Include Albuminuria in the Classification System. J. Clin. Hypertens. 2013, 15, 865–867. [Google Scholar] [CrossRef]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Soveri, I.; Berg, U.B.; Björk, J.; Elinder, C.G.; Grubb, A.; Mejare, I.; Sterner, G.; Bäck, S.E.; SBU GFR Review Group. Measuring GFR: A systematic review. Am. J. Kidney Dis. 2014, 64, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Smyth, B. From proteinuria to fibrosis: An update on pathophysiology and treatment options. Kidney Blood Press Res. 2021, 46, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Scmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- White, C.A.; Allen, C.M.; Akbari, A.; Collier, C.P.; Holland, D.C.; Day, A.G.; Knoll, G.A. Comparison of the new and traditional CKD-EPI GFR estimation equations with urinary inulin clearance: A study of equation performance. Clin. Chim. Acta 2019, 488, 189–195. [Google Scholar] [CrossRef]
- Satarug, S.; Baker, J.R.; Reilly, P.E.; Moore, M.R.; Williams, D.J. Cadmium levels in the lung, liver, kidney cortex, and urine samples from Australians without occupational exposure to metals. Arch. Environ. Health 2002, 57, 69–77. [Google Scholar] [CrossRef]
- Elinder, C.G.; Lind, B.; Kjellström, T.; Linnman, L.; Friberg, L. Cadmium in kidney cortex, liver, and pancreas from Swedish autopsies. Estimation of biological half time in kidney cortex, considering calorie intake and smoking habits. Arch. Environ. Health 1976, 31, 292–302. [Google Scholar] [CrossRef]
- Barregard, L.; Sallsten, G.; Lundh, T.; Mölne, J. Low-level exposure to lead, cadmium and mercury, and histopathological findings in kidney biopsies. Environ. Res. 2022, 211, 113119. [Google Scholar] [CrossRef]
- Schnaper, H.W. The tubulointerstitial pathophysiology of progressive kidney disease. Adv. Chronic Kidney Dis. 2017, 24, 107–116. [Google Scholar] [CrossRef]
- Chevalier, R.L. The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am. J. Physiol. Ren. Physiol. 2016, 311, F145–F161. [Google Scholar] [CrossRef]
- Satarug, S.; Đorđević, A.B.; Yimthiang, S.; Vesey, D.A.; Gobe, G.C. The NOAEL equivalent of environmental cadmium exposure associated with GFR reduction and chronic kidney disease. Toxics 2022, 10, 614. [Google Scholar] [CrossRef]
- Gburek, J.; Konopska, B.; Gołąb, K. Renal handling of albumin-from early findings to current concepts. Int. J. Mol. Sci. 2021, 22, 5809. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Sandoval, R.M.; Yadav, S.P.S.; Wagner, M.C. Albumin uptake and processing by the proximal tubule: Physiological, pathological, and therapeutic implications. Physiol. Rev. 2022, 102, 1625–1667. [Google Scholar] [CrossRef]
- Benzing, T.; Salant, D. Insights into glomerular filtration and albuminuria. N. Engl. J. Med. 2021, 384, 1437–1446. [Google Scholar] [CrossRef]
- Edwards, A.; Long, K.R.; Baty, C.J.; Shipman, K.E.; Weisz, O.A. Modelling normal and nephrotic axial uptake of albumin and other filtered proteins along the proximal tubule. J. Physiol. 2022, 600, 1933–1952. [Google Scholar] [CrossRef]
- Langelueddecke, C.; Roussa, E.; Fenton, R.A.; Wolff, N.A.; Lee, W.K.; Thévenod, F. Lipocalin-2 (24p3/neutrophil gelati-nase-associated lipocalin (NGAL) receptor is expressed in distal nephron and mediates protein endocytosis. J. Biol. Chem. 2012, 287, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Dizi, E.; Hasler, U.; Nlandu-Khodo, S.; Fila, M.; Roth, I.; Ernandez, T.; Doucet, A.; Martin, P.Y.; Feraille, E.; de Seigneux, S. Albuminuria induces a proinflammatory and profibrotic response in cortical collecting ducts via the 24p3 receptor. Am. J. Physiol. Renal Physiol. 2013, 305, F1053–F1063. [Google Scholar] [CrossRef] [PubMed]
- Santoyo-Sánchez, M.P.; Pedraza-Chaverri, J.; Molina-Jijón, E.; Arreola-Mendoza, L.; Rodríguez-Muñoz, R.; Barbier, O.C. Impaired endocytosis in proximal tubule from subchronic exposure to cadmium involves angiotensin II type 1 and cubilin receptors. BMC Nephrol. 2013, 14, 211. [Google Scholar] [CrossRef] [PubMed]
- Gena, P.; Calamita, G.; Guggino, W.B. Cadmium impairs albumin reabsorption by down-regulating megalin and ClC5 channels in renal proximal tubule cells. Environ. Health Perspect. 2010, 118, 1551–1556. [Google Scholar] [CrossRef]
- Watanabe, T.; Kataoka, Y.; Hayashi, K.; Matsuda, R.; Uneyama, C. Dietary exposure of the Japanese general population to elements: Total diet study 2013–2018. Food Saf. 2022, 10, 83–101. [Google Scholar] [CrossRef]
- Wong, C.; Roberts, S.M.; Saab, I.N. Review of regulatory reference values and background levels for heavy metals in the human diet. Regul. Toxicol. Pharmacol. 2022, 130, 105122. [Google Scholar] [CrossRef] [PubMed]
- JECFA. In Summary and Conclusions, Proceedings of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants, Seventy-Third Meeting, Geneva, Switzerland, 8–17 June 2010; JECFA/73/SC; Food and Agriculture Organization of the United Nations/World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Available online:. Available online: https://apps.who.int/iris/handle/10665/44521 (accessed on 12 August 2023).
- EFSA Scientific Committee. Update: Use of the benchmark dose approach in risk assessment. EFSA J. 2017, 15, 4658. [Google Scholar]
- Moffett, D.B.; Mumtaz, M.M.; Sullivan, D.W., Jr.; Whittaker, M.H. Chapter 13, General Considerations of Dose-Effect and Dose-Response Relationships, In Handbook on the Toxicology of Metals, 5th ed.; Volume I: General, Considerations, Nord-berg, G., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 299–317. [Google Scholar]
- Butler-Dawson, J.; James, K.A.; Krisher, L.; Jaramillo, D.; Dally, M.; Neumann, N.; Pilloni, D.; Cruz, A.; Asensio, C.; Johnson, R.J.; et al. Environmental metal exposures and kidney function of Guatemalan sugarcane workers. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Win-Thu, M.; Myint-Thein, O.; Win-Shwe, T.-T.; Mar, O. Environmental cadmium exposure induces kidney tubular and glomerular dysfunction in the Myanmar adults. J. Toxicol. Sci. 2021, 46, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Skröder, H.; Hawkesworth, S.; Kippler, M.; El Arifeen, S.; Wagatsuma, Y.; Moore, S.E.; Vahter, M. Kidney function and blood pressure in preschool-aged children exposed to cadmium and arsenic-potential alleviation by selenium. Environ. Res. 2015, 140, 205–213. [Google Scholar] [CrossRef]
- Rodríguez-López, E.; Tamayo-Ortiz, M.; Ariza, A.C.; Ortiz-Panozo, E.; Deierlein, A.L.; Pantic, I.; Tolentino, M.C.; Es-trada-Gutiérrez, G.; Parra-Hernández, S.; Espejel-Núñez, A.; et al. Early-life dietary cadmium exposure and kidney function in 9-year-old children from the PROGRESS cohort. Toxics 2020, 8, 83. [Google Scholar] [CrossRef]
Figure 1.
The relationship between eGFR and cadmium excretion rate. Scatterplots relate eGFR to log[(ECd/Ccr) ×105) in women (a) and men (b), women (c) and men (d) with low Cd burden and women (e) and men (f) with medium plus high Cd burden. Coefficients of determination (R2) and p-values are provided for all scatterplots together with numbers of participants in all subgroups. The cut-off values of (ECd/Ccr) ×100 for low, medium, and high Cd burden in women were ≤ 1.44, 1.45−3.26, > 3.26 µg/L filtrate, respectively. Corresponding cut-off values of (ECd/Ccr) ×100 in men were ≤ 1.25, 1.26-3.25, > 3.25 µg/L filtrate.
Figure 1.
The relationship between eGFR and cadmium excretion rate. Scatterplots relate eGFR to log[(ECd/Ccr) ×105) in women (a) and men (b), women (c) and men (d) with low Cd burden and women (e) and men (f) with medium plus high Cd burden. Coefficients of determination (R2) and p-values are provided for all scatterplots together with numbers of participants in all subgroups. The cut-off values of (ECd/Ccr) ×100 for low, medium, and high Cd burden in women were ≤ 1.44, 1.45−3.26, > 3.26 µg/L filtrate, respectively. Corresponding cut-off values of (ECd/Ccr) ×100 in men were ≤ 1.25, 1.26-3.25, > 3.25 µg/L filtrate.
Figure 2.
Albumin excretion rates among participants grouped by cadmium burden and other risk factors. Scatterplots relate log[(Ealb/Ccr) ×104] to log[ECd/Ccr) ×105] in participants with low Cd burden (a) and middle plus high Cd burdens (c). Coefficients of determination (R2) and p-values and numbers of participants are provided for all scatterplots. Bar graphs depict mean log[(Ealb/Ccr) ×104] in participants with low Cd burden (b) and middle plus high Cd burdens (d), grouped by gender, smoking status, blood pressure status, diabetes diagnosis and eGFR. Normal eGFR and low eGFR were defined, respectively, as eGFR > 60 and eGFR ≤ 60 mL/min/1.73 m2. Numbers of subjects are provided for all subgroups. All means were obtained via univariate covariance analysis with adjustment for covariates and interactions. The cut-off values of (ECd/Ccr) ×100 for the low, middle and high tertiles of Cd burden in women were ≤ 1.44, 1.45−3.26, > 3.26 µg/L filtrate, respectively. Corresponding cut-off values of (ECd/Ccr) ×100 in men were ≤ 1.25, 1.26-3.25, > 3.25 µg/L filtrate.
Figure 2.
Albumin excretion rates among participants grouped by cadmium burden and other risk factors. Scatterplots relate log[(Ealb/Ccr) ×104] to log[ECd/Ccr) ×105] in participants with low Cd burden (a) and middle plus high Cd burdens (c). Coefficients of determination (R2) and p-values and numbers of participants are provided for all scatterplots. Bar graphs depict mean log[(Ealb/Ccr) ×104] in participants with low Cd burden (b) and middle plus high Cd burdens (d), grouped by gender, smoking status, blood pressure status, diabetes diagnosis and eGFR. Normal eGFR and low eGFR were defined, respectively, as eGFR > 60 and eGFR ≤ 60 mL/min/1.73 m2. Numbers of subjects are provided for all subgroups. All means were obtained via univariate covariance analysis with adjustment for covariates and interactions. The cut-off values of (ECd/Ccr) ×100 for the low, middle and high tertiles of Cd burden in women were ≤ 1.44, 1.45−3.26, > 3.26 µg/L filtrate, respectively. Corresponding cut-off values of (ECd/Ccr) ×100 in men were ≤ 1.25, 1.26-3.25, > 3.25 µg/L filtrate.
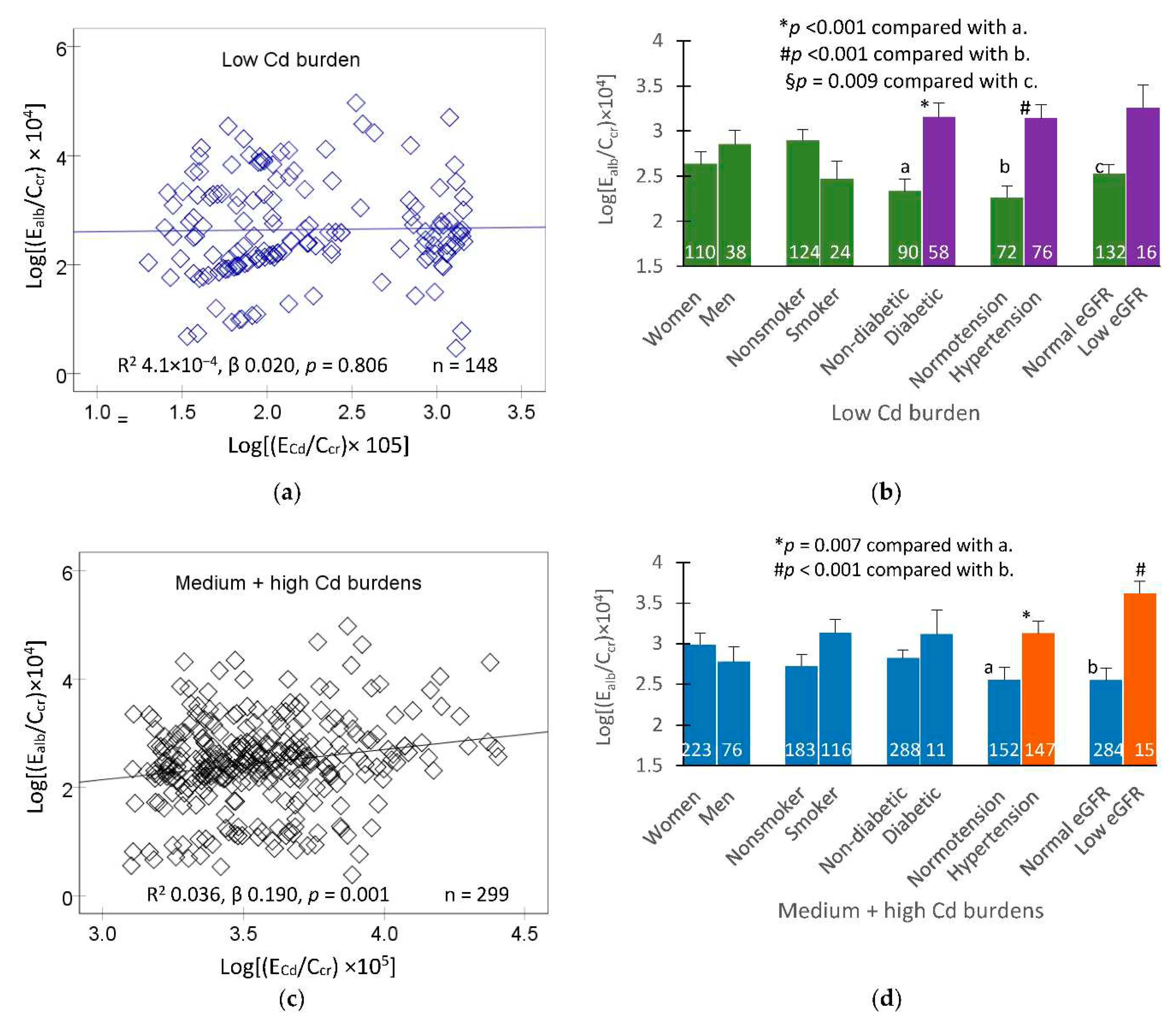
Figure 3.
Albumin excretion rates in participants grouped by blood cadmium and eGFR levels. Scatterplots relate log[(Ealb/Ccr) ×104] to eGFR in participants with low (a), middle (c) and high [Cd]b tertiles (e). Coefficients of determination (R2), p-values and numbers of subjects are provided for all scatterplots. Bar graphs depict mean log[(Ealb/Ccr) ×104] in participants of low (b), middle (d) and high [Cd]b tertiles (f) who had eGFR ≤ 60, 61−90 and > 90 mL/min/1.73 m2. All means were obtained via univariate covariance analysis with adjustment for covariates and interactions. The cut-off values of [Cd]b for low, middle, and high tertiles were < 0.82, 0.83−2.63, and ≥2.64 µg/L, respectively. Arithmetic means (SD) of [Cd]b in low, middle, and high tertiles were 0.31 (0.29), 1.86 (1.98), and 5.58 (3.28), respectively. For all tests, p-values ≤ 0.05 indicate statistically significant levels.
Figure 3.
Albumin excretion rates in participants grouped by blood cadmium and eGFR levels. Scatterplots relate log[(Ealb/Ccr) ×104] to eGFR in participants with low (a), middle (c) and high [Cd]b tertiles (e). Coefficients of determination (R2), p-values and numbers of subjects are provided for all scatterplots. Bar graphs depict mean log[(Ealb/Ccr) ×104] in participants of low (b), middle (d) and high [Cd]b tertiles (f) who had eGFR ≤ 60, 61−90 and > 90 mL/min/1.73 m2. All means were obtained via univariate covariance analysis with adjustment for covariates and interactions. The cut-off values of [Cd]b for low, middle, and high tertiles were < 0.82, 0.83−2.63, and ≥2.64 µg/L, respectively. Arithmetic means (SD) of [Cd]b in low, middle, and high tertiles were 0.31 (0.29), 1.86 (1.98), and 5.58 (3.28), respectively. For all tests, p-values ≤ 0.05 indicate statistically significant levels.
Table 1.
Characteristics of participants according to eGFR levels and gender.
Table 1.
Characteristics of participants according to eGFR levels and gender.
| Parameters |
All
n = 482 |
Normal eGFR a
|
Low eGFR |
Women
n = 329 |
Men
n = 114 |
Women
n = 35 |
Men
n = 4 |
| Age (years) |
51.8 ± 9.2 |
51.2 ± 8.7 |
49.8 ± 7.5 |
63.1 ± 11.3 |
56.0 ± 6.4 |
| BMI (kg/m2) |
24.8 ± 4.0 |
25.3 ± 4.0 |
23.5 ± 3.7 *** |
24.0 ± 3.6 |
26.7 ± 5.2 |
| eGFR b, mL/min/1.73 m2
|
90 ± 19 |
93 ± 15 |
94 ± 14 |
51 ± 9 |
47 ± 11 |
| Smoking (%) |
29.7 |
17.6 |
68.4 *** |
11.4 |
75.0 ## |
| Diabetes (%) |
18.3 |
16.7 |
13.2 |
45.7 |
50.0 |
| Hypertension (%) |
48.3 |
49.8 |
40.4 |
54.3 |
100 |
| SBP (480) |
129 ± 17 |
129 ± 16 |
127 ± 113 |
138 ± 18 |
147 ± 5 |
| DBP (480) |
81 ± 10 |
81 ± 10 |
81 ± 11 |
79 ± 8 |
86 ± 4 |
| Serum creatinine, mg/dL |
0.82 ± 0.22 |
0.74 ± 0.13 |
0.92 ± 0.14 *** |
1.19 ± 0.31 |
1.68 ± 0.43 ## |
| Urine creatinine, mg/dL |
113 ± 73 |
110 ± 74 |
134 ± 72 *** |
72 ± 33 |
73 ± 24 |
| Urine albumin, mg/L |
21 ± 57 |
13 ± 31 |
29 ± 77 |
60 ± 118 |
86 ± 106 |
| Blood Cd, µg/L |
2.60 ± 3.13 |
2.43 ± 2.96 |
3.13 ± 3.32 * |
2.19 ± 3.67 |
5.03 ± 5.03 |
| Urine Cd, µg/L |
4.21 ± 5.66 |
4.44 ± 6.15 |
3.84 ± 4.04 |
3.31 ± 5.71 |
2.26 ± 2.80 |
| Normalized to Ecr (Ex/Ecr) c
|
|
|
|
|
|
| ACR, mg/g creatinine |
24 ± 71 |
15 ± 41 |
27 ± 70 |
93 ± 181 |
112 ± 139 |
| Albuminuria (%) d
|
15.0 |
11.4 |
17.0 |
34.4 |
100 # |
| ECd/Ecr, µg/g creatinine |
4.05 ± 4.43 |
4.27 ± 4.46 |
3.32 ± 3.71 * |
4.63 ± 6.31 |
2.62 ± 3.04 |
| Normalized to Ccr, (Ex/Ccr) e
|
|
|
|
|
|
| (Ealb/Ccr) ×100, mg/L |
24 ± 85 |
12 ± 32 |
26 ± 67 |
125 ± 252 |
174 ± 205 |
| Abnormal Ealb/Ccr (%) f
|
16.9 |
12.9 |
17.0 |
46.9 |
100 # |
| (ECd/Ccr) ×100, µg/L |
3.20 ± 3.73 |
3.05 ± 3.25 |
3.05 ± 3.41 |
5.14 ± 7.44 |
5.20 ± 6.80 |
Table 2.
Predictors of eGFR in women and men.
Table 2.
Predictors of eGFR in women and men.
Independent
Variables/Factors |
eGFR, mL/min/1.73 m2
|
| All, n = 444 |
Women, n = 332 |
Men, n = 113 |
| β |
p |
β |
p |
β |
p |
| Age, years |
−0.503 |
<0.001 |
−0.511 |
<0.001 |
−0.472 |
<0.001 |
| BMI, kg/m2
|
−0.066 |
0.129 |
−0.050 |
0.310 |
−0.134 |
0.149 |
| Log[(ECd/Ccr) ×105], µg/L filtrate |
−0.121 |
0.022 |
−0.126 |
0.043 |
−0.097 |
0.367 |
| Systolic pressure, mmHg |
−0.087 |
0.134 |
−0.067 |
0.320 |
−0.147 |
0.230 |
| Diastolic pressure, mmHg |
−0.011 |
0.836 |
−0.020 |
0.745 |
0.022 |
0.856 |
| Gender |
−0.006 |
0.898 |
− |
− |
− |
− |
| Smoking |
0.033 |
0.492 |
0.031 |
0.533 |
0.034 |
0.710 |
| Diabetes |
−0.101 |
0.038 |
−0.119 |
0.036 |
−0.033 |
0.741 |
| Adjusted R2
|
0.278 |
<0.001 |
0.279 |
<0.001 |
0.216 |
<0.001 |
Table 3.
Comparing strength of association of eGFR with cadmium excretion rate across cadmium burden groups.
Table 3.
Comparing strength of association of eGFR with cadmium excretion rate across cadmium burden groups.
Independent
Variables/Factors |
eGFR, mL/min/1.73 m2
|
| Low Cd burden |
Medium burden |
High burden |
| |
β |
p |
β |
p |
β |
p |
| Age, years |
−0.455 |
<0.001 |
−0.505 |
<0.001 |
−0.410 |
<0.001 |
| BMI, kg/m2
|
−0.022 |
0.777 |
−0.136 |
0.064 |
−0.094 |
0.221 |
| Log[(ECd/Ccr) ×105], µg/L filtrate |
0.007 |
0.934 |
−0.230 |
0.002 |
−0.349 |
<0.001 |
| Systolic pressure, mmHg |
−0.038 |
0.722 |
−0.284 |
0.004 |
−0.050 |
0.611 |
| Diastolic pressure, mmHg |
0.087 |
0.372 |
0.113 |
0.226 |
−0.170 |
0.078 |
| Gender |
−0.024 |
0.798 |
−0.064 |
0.434 |
0.106 |
0.194 |
| Smoking |
0.094 |
0.324 |
−0.081 |
0.301 |
0.132 |
0.108 |
| Diabetes |
−0.128 |
0.104 |
0.089 |
0.260 |
0.022 |
0.775 |
| Adjusted R2
|
0.248 |
<0.001 |
0.321 |
<0.001 |
0.255 |
<0.001 |
Table 4.
Prevalence odds ratios for low eGFR in relation to cadmium body burden and other independent variables.
Table 4.
Prevalence odds ratios for low eGFR in relation to cadmium body burden and other independent variables.
| Independent variables/factors |
Low eGFR |
| All, n = 446 |
Women, n = 332 |
Men, n = 114 |
| POR (95% CI) |
p |
POR (95% CI) |
p |
POR (95% CI) |
p |
| Age, years |
1.118 (1.062, 1.176) |
<0.001 |
1.114 (1.057, 1.175) |
<0.001 |
1.291 (0.935, 1.783) |
0.120 |
| BMI, kg/m2
|
1.002 (0.908, 1.106) |
0.967 |
1.029 (0.923, 1.147) |
0.604 |
1.351 (0.876, 2.084) |
0.174 |
| Gender |
0.482 (0.133, 1.742) |
0.265 |
− |
− |
− |
− |
| Smoking |
1.388 (0.433, 4.450) |
0.582 |
1.123 (0.300, 4.206) |
0.863 |
0.495 (0.018, 13.92) |
0.679 |
| Diabetes |
3.042 (1.126, 8.213) |
0.028 |
2.709 (0.932, 7.878) |
0.067 |
3.713 (0.110, 125.6) |
0.465 |
| Hypertension |
1.175 (0.516, 2.679) |
0.701 |
1.228 (0.505, 2.990) |
0.651 |
2.478 (1.983, 3.098) |
0.030 a
|
| Cd body burden |
|
|
|
|
|
|
| Low |
Referent |
|
Referent |
|
Referent |
|
| Medium |
8.265 (1.711, 39.92) |
0.009 |
7.204 (1.438, 36.10) |
0.016 |
n/a |
n/a |
| High |
3.643 (1.150, 11.54) |
0.028 |
3.218 (0.934, 11.09) |
0.064 |
n/a |
n/a |
Table 5.
Comparing strength of association of albumin excretion rate with cadmium excretion rate across cadmium burden groups.
Table 5.
Comparing strength of association of albumin excretion rate with cadmium excretion rate across cadmium burden groups.
Independent
Variables/Factors |
Log [(Ealb/Ccr) ×104], µg/L filtrate |
| Low Cd burden |
Medium burden |
High burden |
| |
β |
p |
β |
p |
β |
p |
| Age, years |
0.049 |
0.609 |
−0.019 |
0.846 |
−0.082 |
0.381 |
| BMI, kg/m2
|
−0.007 |
0.932 |
0.248 |
0.004 |
−0.021 |
0.803 |
| Log[(ECd/Ccr ) × 105], µg/L filtrate |
0.177 |
0.050 |
0.144 |
0.098 |
0.173 |
0.044 |
| eGFR, mL/min/1.73 m2
|
−0.110 |
0.216 |
−0.147 |
0.130 |
−0.214 |
0.021 |
| Gender |
−0.150 |
0.122 |
−0.111 |
0.233 |
0.139 |
0.120 |
| Smoking |
−0.183 |
0.064 |
0.024 |
0.788 |
0.129 |
0.153 |
| Diabetes |
0.263 |
0.001 |
0.093 |
0.280 |
0.115 |
0.176 |
| Hypertension |
0.278 |
<0.001 |
0.121 |
0.148 |
0.104 |
0.204 |
| Adjusted R2
|
0.174 |
<0.001 |
0.103 |
0.003 |
0.082 |
0.009 |
Table 6.
Prevalence odds ratios for albuminuria in relation to blood cadmium levels and other independent variables.
Table 6.
Prevalence odds ratios for albuminuria in relation to blood cadmium levels and other independent variables.
Independent
Variables/factors
|
Albuminuria, (Ealb/Ccr) ×100 ≥ 20 µg/L filtrate |
| All, n = 473 |
Women, n = 357 |
Men, n = 116 |
| POR (95% CI) |
p |
POR (95% CI) |
p |
POR (95% CI) |
p |
| Age, years |
0.990 (0.955, 1.027) |
0.609 |
1.007 (0.965, 1.051) |
0.733 |
0.988 (0.904, 1.079) |
0.781 |
| BMI, kg/m2
|
1.028 (0.957, 1.104) |
0.448 |
1.033 (0.950, 1.123) |
0.450 |
1.011 (0.877, 1.166) |
0.881 |
| eGFR, mL/min/1.73 m2
|
1.043 (1.026, 1.061) |
<0.001 |
1.045 (1.025, 1.065) |
<0.001 |
1.045 (1.008, 1.084) |
0.017 |
| Gender |
0.559 (0.273, 1.146) |
0.112 |
− |
− |
− |
− |
| Smoking |
1.037 (0.494, 2.177) |
0.924 |
1.258 (0.496, 3.192) |
0.629 |
0.809 (0.254, 2.572) |
0.719 |
| Diabetes |
6.021 (2.813, 12.89) |
<0.001 |
5.996 (2.446, 14.69) |
<0.001 |
8.324 (1.642, 42.21) |
0.011 |
| Hypertension |
2.053 (1.167, 3.609) |
0.013 |
2.785 (1.397, 5.552) |
0.004 |
1.133 (0.371, 3.463) |
0.827 |
| Tertile of [Cd]b, µg/L |
|
|
|
|
|
|
| Low: < 0.82 |
Referent |
|
Referent |
|
Referent |
|
| Middle: 0.83−2.63 |
2.360 (1.097, 5.076) |
0.028 |
3.402 (1.324, 8.745) |
0.011 |
0.925 (0.237, 3.604) |
0.911 |
| High: ≥ 2.64 |
2.740 (1.174, 6.394) |
0.020 |
3.783 (1.369, 10.46) |
0.010 |
1.425 (0.263, 7.732) |
0.681 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).