1. Introduction
Evaporating liquid sessile drop is an important object both for theoretical investigations (evaporation dynamics, microfluidics, self-organization of solute, etc.) and wide variety of applications (printing technologies for functional coatings, medical diagnostics, food science, geophysics, etc.) [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10].
The model of diffusion-limited quasi-stationary evaporation of the spherical droplet in quiescent air environment was originally proposed by J.C. Maxwell [
11]. He calculated the diffusive drift of vapor from the surface of the evaporating droplet into the air, assuming that the vapor concentration at the surface of the droplet determines by saturated vapor density. This is valid for a drop radius much larger than the mean free path length of vapor molecules in the air. For example, this is not true for droplets smaller than 100 nm in the normal conditions.
Within the framework of the Maxwellian model (quasi-stationary evaporation), the diffusion equation practically turns into the Laplace equation for the vapor concentration,, with the following initial boundary conditions: on the drop surface . Outside the drop the vapor concentration is determined by the asymptotic value of the vapor concentration in the atmosphere (for aqueous solution, it is the relative humidity of the air, ).
It is considered that the liquid-gas transition layer is infinitely thin compared to the droplet size. Moreover, for correctness of the Maxwellian model it is necessary to assume that density of air is much greater than vapor density, so that diffusion of vapor is determined by the vapor diffusion coefficient in the air. In particular, this requirement means that we must consider liquid at a temperature much smaller than its boiling point at a given air pressure.
The smallness of the convective Stefan flux also has to be satisfied. Stephan showed for the first time [
12] that near the surface of an evaporating drop there is an air current directed away from the surface, because in order to maintain the constancy of the total pressure in the medium under conditions of vapor production by the liquid surface, along with the gradient of the vapor density there must exist an equal and opposite in direction gradient of the partial pressure of the other components of the air.
Fuchs showed [
13] that the relative contribution of the Stefan flux to the evaporation process is given by factor
, where
p is the pressure of the air,
and
are the saturated vapor pressure and the vapor pressure in the air far from the droplet, respectively. That factor for a drop of water under normal conditions does not exceed 1-2 percents (%). This is one of essential limitations of the Maxwellian model accuracy that indicates acceptable correctness of approximate solutions with respect to exact solutions of the diffusion problem. There are also other error factors: inaccurate determination of such parameters as drop surface temperature, vapor diffusion coefficient in air at a given temperature, saturated vapor pressure, etc. The capillary surface oscillations, air movement near the drop surface also are the factors that introduce uncertainties. Therefore, the approximate solutions whose accuracy is of the order of 5 percents can be considered acceptable.
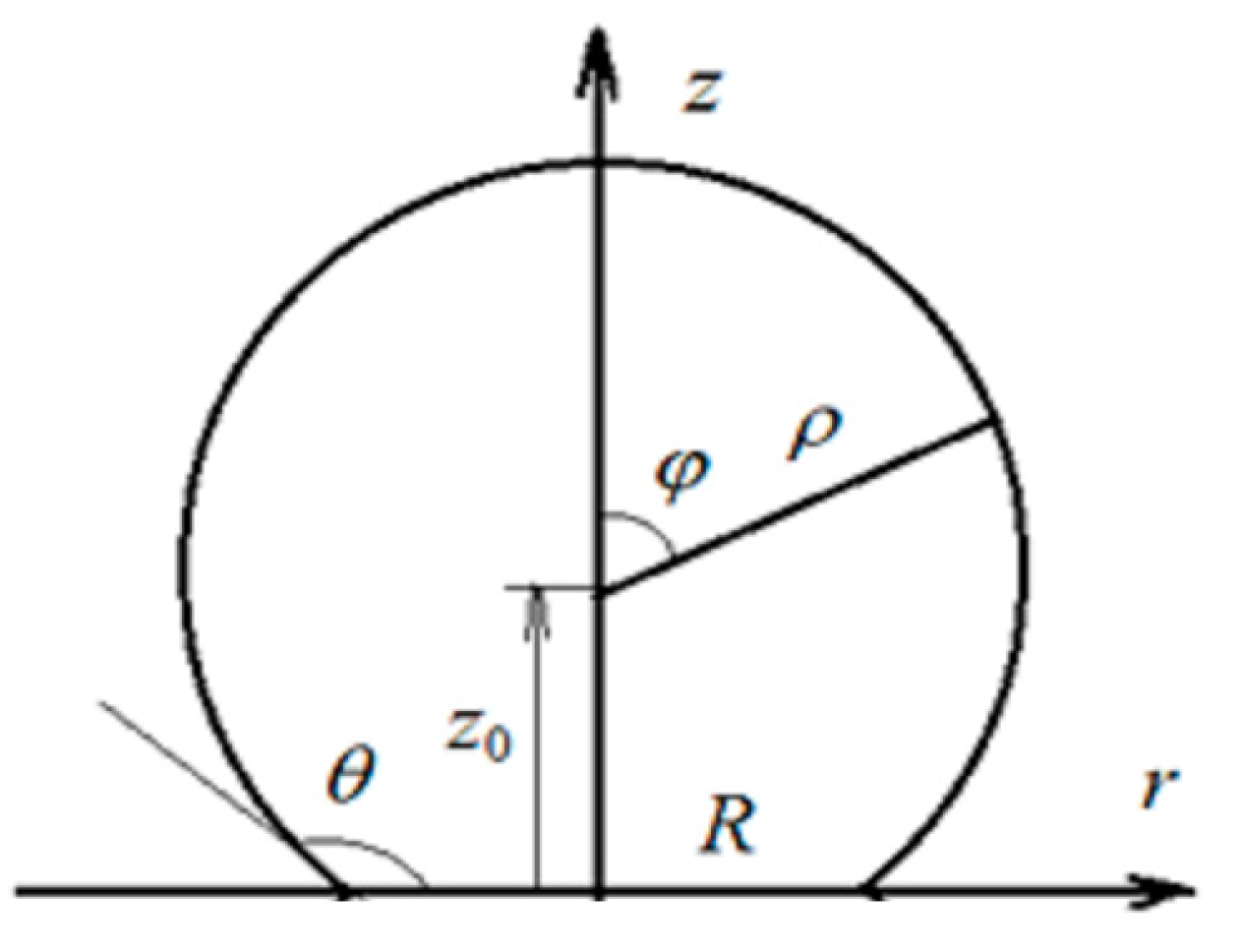
In the following, we consider the small drop deposited on a flat solid horizontal substrate (so-called sessile drop). It is easy to derive that the equilibrium shape of a sessile drop of a slowly evaporating liquid, the size of which is much smaller than the capillary constant (Bond number, Bo<<1), approximately corresponds to a spherical segment with the given contact (wetting) angle. Sessile water droplets with a height of less than 1 mm satisfy this criterion quite well.
The Maxwellian model was apparently first applied to such sessile drop in the paper of Picknett and Bexon [
14]. All solutions described below in this paper are given within the framework of this approach.
Exact analytical solutions have been obtained to describe the total evaporation rate (mass loss per unit time) and evaporation flux density (mass loss per unit surface area per unit time) of a small sessile liquid droplet having the shape of an axisymmetric spherical segment deposited on a horizontal substrate [
15,
16,
17,
18,
19,
20,
21,
22,
23,
24].
There are currently two alternative expressions for the droplet evaporation flux density that are mathematically equivalent. First, the following solution was proposed [
19,
20]
where
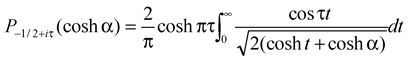
is the Legendre function of the first kind. Here, toroidal coordinate
ranges in the interval from 0 (top of the drop) to ∞ (contact line). So, this coordinate is related to the cylindrical coordinate
r by
where
is the contact angle of the droplet (
Figure 1). Equation (1) is a double integral, being an implicit function of cylindrical coordinate
r, which makes formula (1) extremely difficult to use in calculations. A simpler from a computational point of view, but mathematically equivalent expression in polar coordinates was also obtained [
21,
22]. It allows to calculate the flux density at the surface of the droplet as a function of the polar angle
φ explicitly (
Figure 1):
where
Unfortunately, unlike expression (1), equations (3)-(4) are not yet known to the wider community and are completely ignored in the latest topical review [
23].
For the total evaporation rate, the following expression was obtained [
20]:
where
D is a diffusion coefficient of the vapor in the air,
n is a vapor volume concentration outside the drop with the boundary conditions
n =
ns at the drop air-liquid surface and far from the drop,
R is the radius of contact line. However, as shown in [22], although the formula (5), correctly describes almost the entire dependence
, where
, it gives wrong result in the limit at
, which can be determined by the direct calculation.
From the point of view of application in computer simulations, this universal analytical expression that describes the evaporation flux density over the entire range of contact angles 0– π (0–180 degrees), is still quite complex. It requires significant computational resources. To accelerate the calculations of the evaporation flux density, it is reasonable to use simplified approximate expressions.
For example, there is a very good approximation for the integral evaporation flux proposed by Picknett and Bexon [
14]:
where
This expression has a maximum error of about 0.2% and looks much more preferable for simulation than the exact analytical solution (5). In
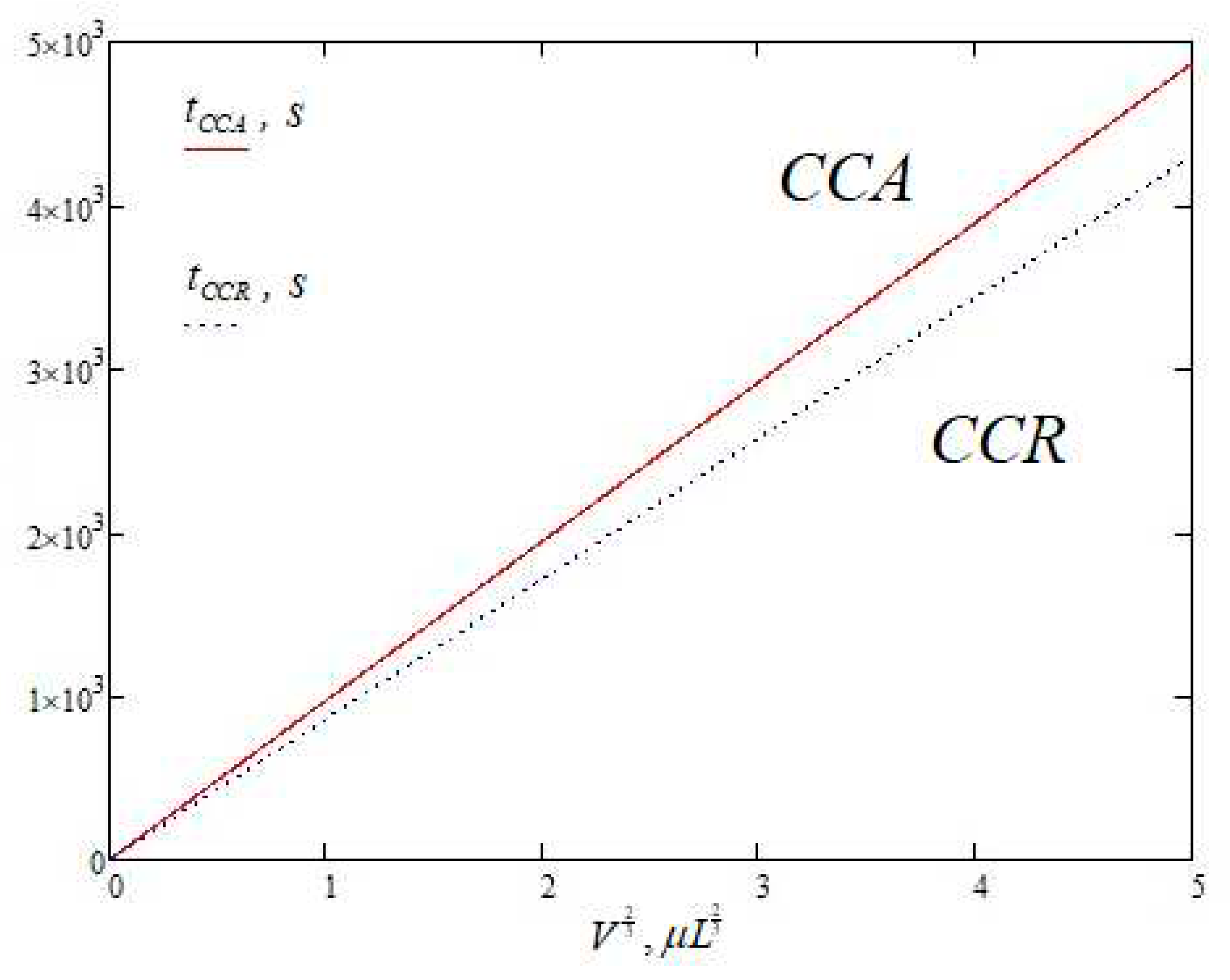
Section 3 of this paper, we propose a much simpler approximate solution in place of equations (6)-(7) and apply it to calculate the droplet evaporation time under different contact line motion scenarios.
Similarly, for the evaporation flux density, instead of the exact formulas (1) or (3), approximate expressions can be proposed for selected narrow ranges of droplet contact angles. So, earlier an expression for the evaporation flux density was proposed, applicable in the case of small contact angles [
24]:
where
.
Equation (8), being represented in the form of equations (3)-(4), can be rewritten as
where
This expression gives a quite good description for contact angles smaller than 30 degrees (π/6). The graph that is represented in
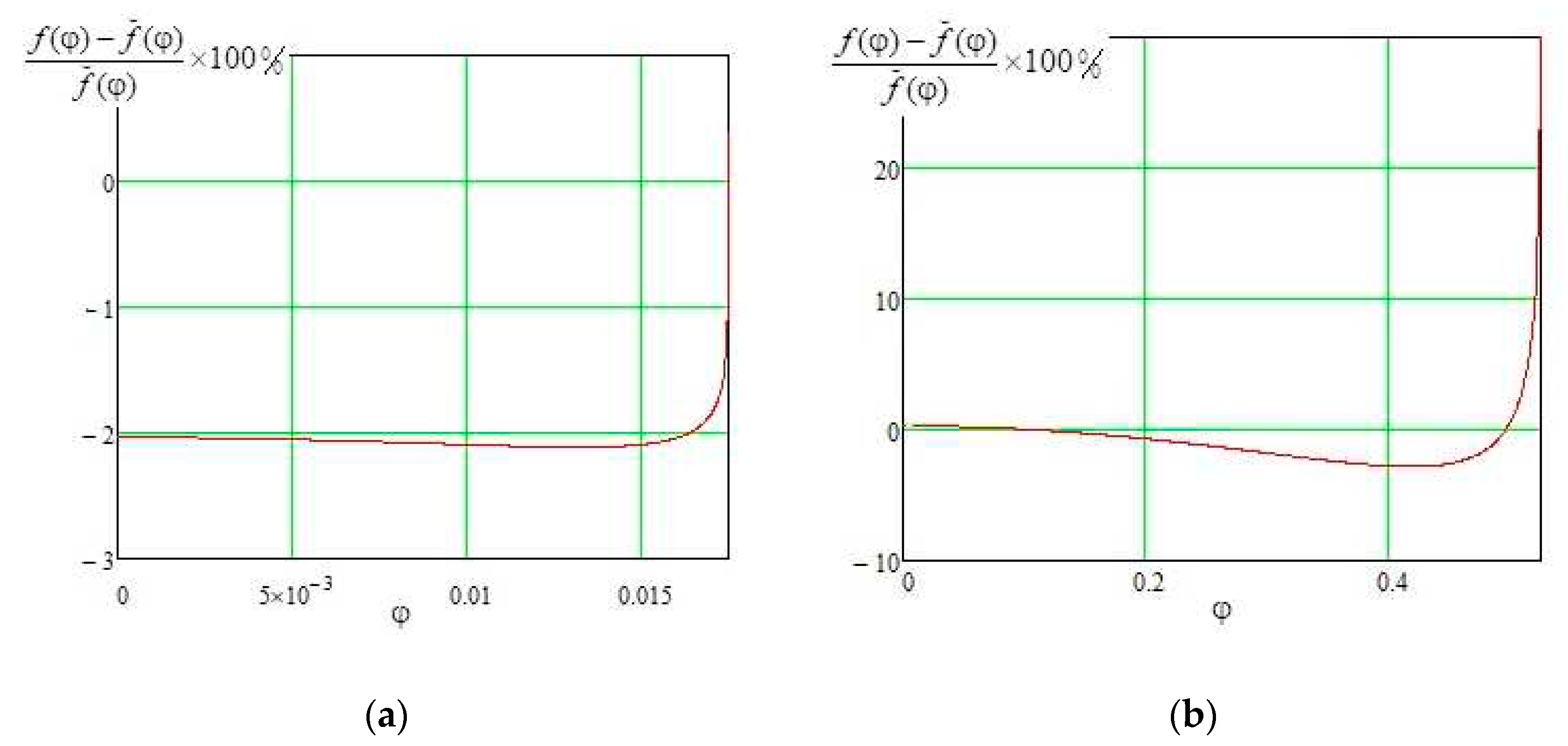
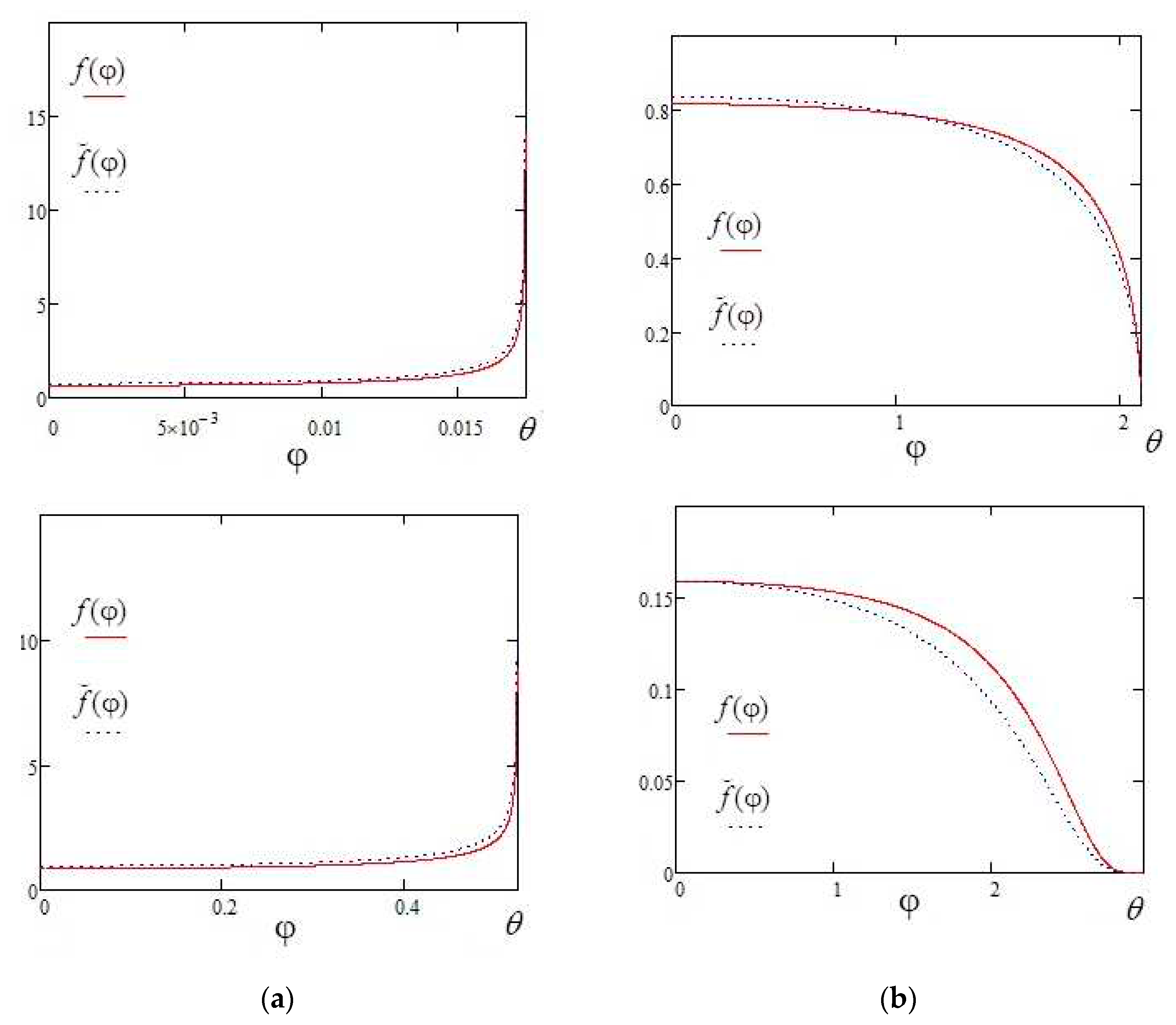
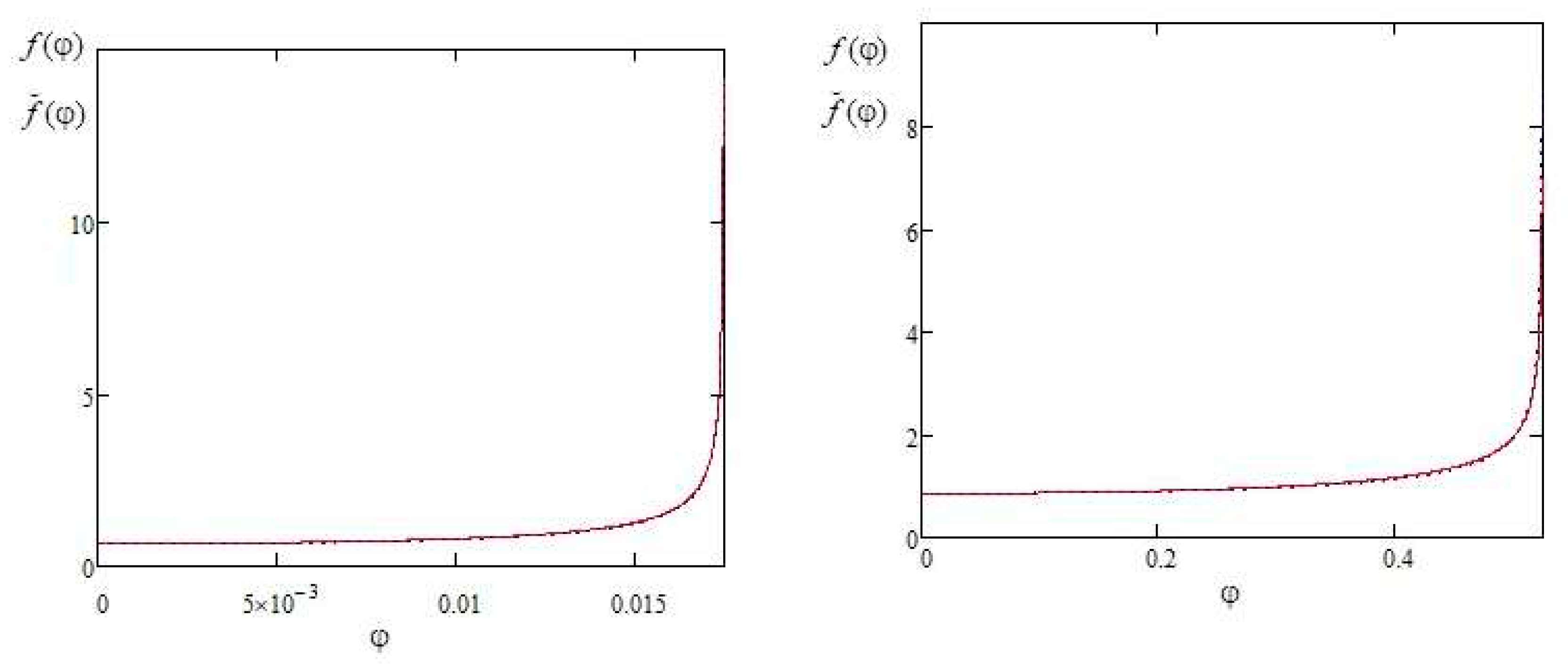
Figure 2 shows a comparison of the approximate expression (10) with the exact solution (3) for the contact angles of 1 degree and 30 degrees.
It can be seen from the comparison that the error of the approximate expression (10) increases as angle φ approaches θ. Except for the region of φ angles near θ, the deviation of the approximate formula (10) from the exact solution (4) practically does not exceed 2%, which makes formula (10) suitable for describing droplets with sharp contact angles in the range approximately 0-30 degrees.
However, the question of which simplified formulas would be appropriate to apply in other ranges of contact angles, for example, in the case of water drop deposited on hydrophobic substrates, still remains open. In
Section 3 of this paper, we propose an approximate variant of exact expressions (3)- (4) in the whole range of contact angles
) that answers this question.
2. New exact solutions for some values of contact angles
Previously [
21,
22], an expression was obtained that describes the vapor concentration near an evaporating drop:
This expression can also be represented as:
Here
. Integral (11a) can be represented as the sum of a finite number of terms for some specific contact angles [
25,
26]. It was shown that expression (11a) under the condition
can be rewritten as
Then, evaporation flux density is given by [
21,
22]
where
By differentiating in the formula (13), we get
where
It can be shown that the first sum in formula (16) gives identically zero (can be verified by direct calculation), and the terms in brackets in the second sum are equal in absolute value. With this in mind, the expression is greatly simplified
Taking into account (11), we get
Thus, for any
j, the evaporation flux density is given by [
25]
If
k=
j, the term under summation in (19) has the form
If
k=0, the term under summation in (19) has the same form
Taking into account (20) and (21), equation (19) can be transformed as
Expression (22) is the equivalent to (19).
To apply expressions (19) or (22) for calculations, it is necessary to take into account the formula (11) and following geometric relationship [
21,
22]:
To establish a relationship between the parameter
j and the corresponding contact angle
, one has to use the geometric relation
Table 1 shows the first two solutions corresponding to
j=1
, 2, 3. It's obvious that
First three solution of the expression (22) with
j=1,2,3 are placed into
Table 1. These mean that the general solution
given by equation (4) can be represented as:
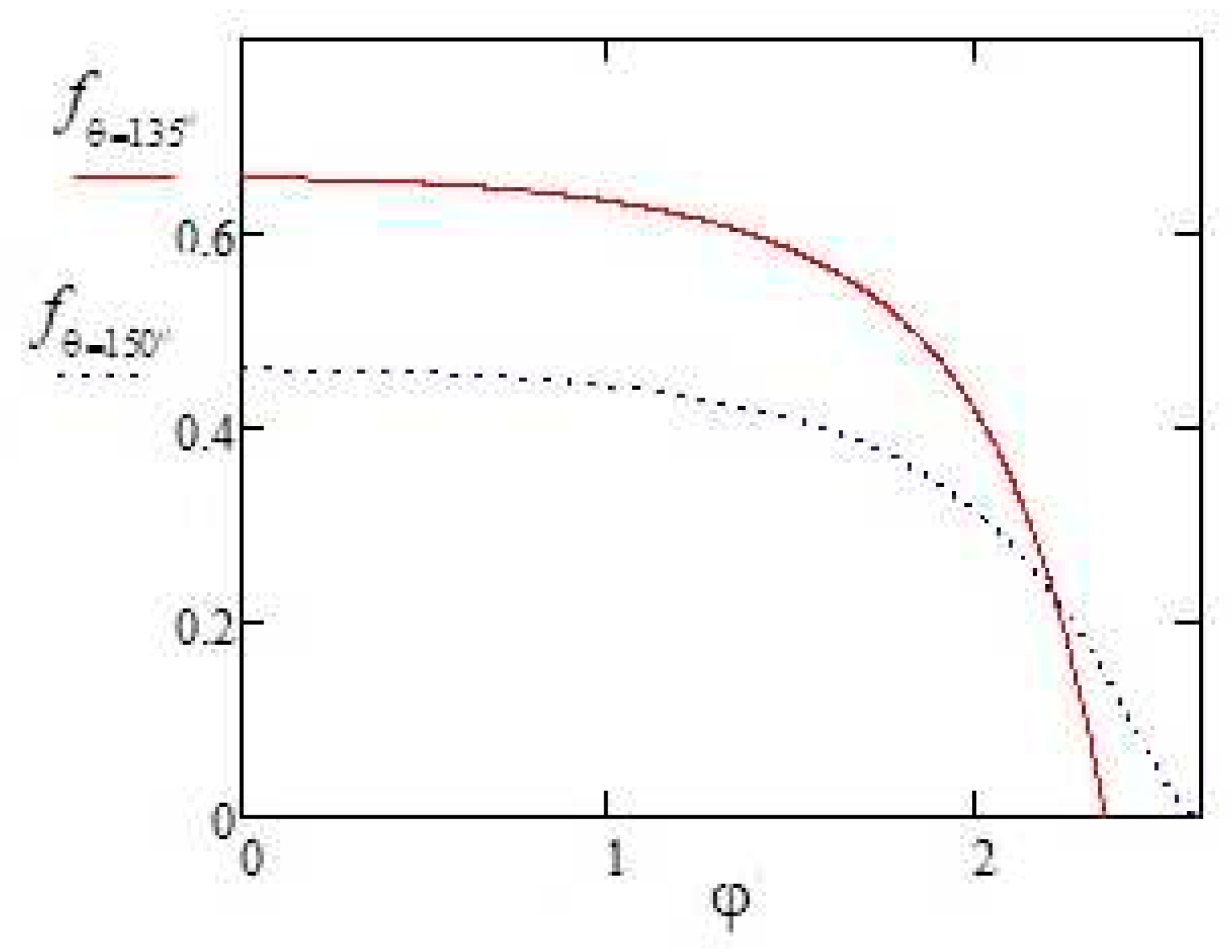
Figure 3 represents the dependences of the evaporation flux density (dimensionless) on the polar angle, which is given by formulas (28) and (29).
It is easy to verify by direct calculation that formula (4) gives the same curves, which confirms the correctness of the mathematical transformation that led to the formula (19) or the expression (22).
4. Discussion
The arsenal of formulas for calculating the slow evaporation of an axisymmetric drop of capillary dimensions deposited on a flat solid surface is reviewed. Such characteristics as vapor density, evaporation flux density, total evaporation rate are considered. Exact solutions obtained in the framework of the Maxwellian model, in which the evaporation process of the drop is limited by vapor diffusion from the drop surface to the surrounding air, are presented.
Along with the long-known solutions published by Popov et al. [
19,
20] during the last two decades, the existence of alternative expression to describe evaporation flux density is pointed out. This alternative equation depends explicitly on the polar angle and is a one-dimensional integral (3)-(4), while the corresponding mathematical equivalent expression of Popov et al. (1) is a double integral with implicit dependence on the cylindrical coordinate. We draw the attention of researchers to the paper [
22], which, apparently, remained unknown to the authors of the newest review of Wilson and D’Ambrosio [
23] on drop evaporation.
New complex solution (22) was derived for the evaporation flux density of a small liquid droplet having the shape of an axisymmetric spherical segment deposited on a horizontal substrate for the set of discrete contact angles , where j=1,2,3… As an example, very simple exact expressions (28) and (29) were obtained explicitly for the evaporation flux density for droplets with contact angles deg and deg that do not contain integral function. They can also be used as approximate expressions for a narrow range of contact angles around the specified values.
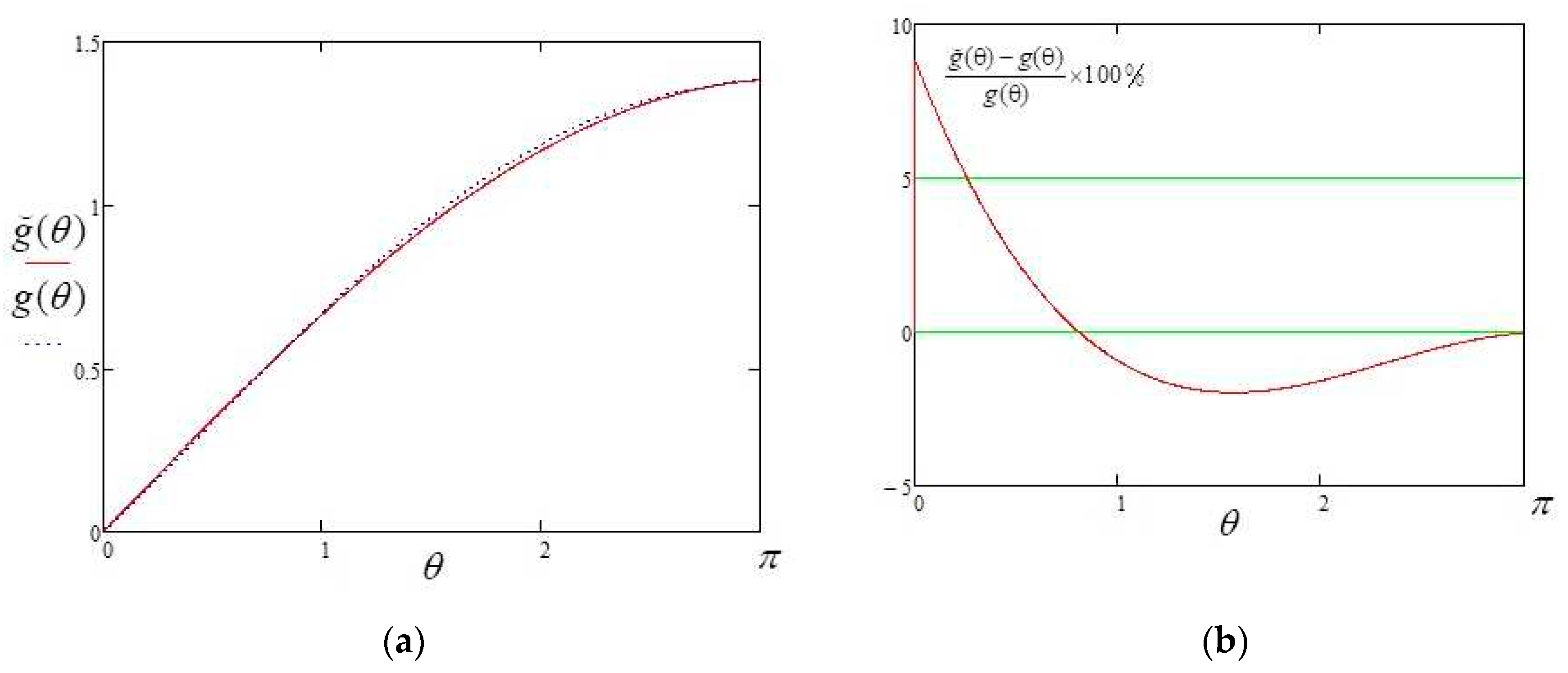
Also, new approximate solutions are presented for the first time: equation (30) - total evaporation rate and expression (50) - mass loss per unit surface area per unit time in the whole range of contact angles ). These expressions are described through elementary functions and do not contain integrals. Thus, they can be used in modeling without requiring significant computational resources.
Expression (50), taking into account (48), contains significant potential for successive improvements in accuracy through the breakdown of the contact angle determination domain into intervals and the introduction of individual correction factors. That may be a further task to advance work in this direction.
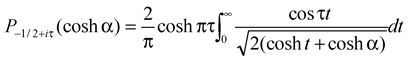 is the Legendre function of the first kind. Here, toroidal coordinate ranges in the interval from 0 (top of the drop) to ∞ (contact line). So, this coordinate is related to the cylindrical coordinate r by
is the Legendre function of the first kind. Here, toroidal coordinate ranges in the interval from 0 (top of the drop) to ∞ (contact line). So, this coordinate is related to the cylindrical coordinate r by