Submitted:
16 August 2023
Posted:
17 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Edge
2.1. Nanoribbon
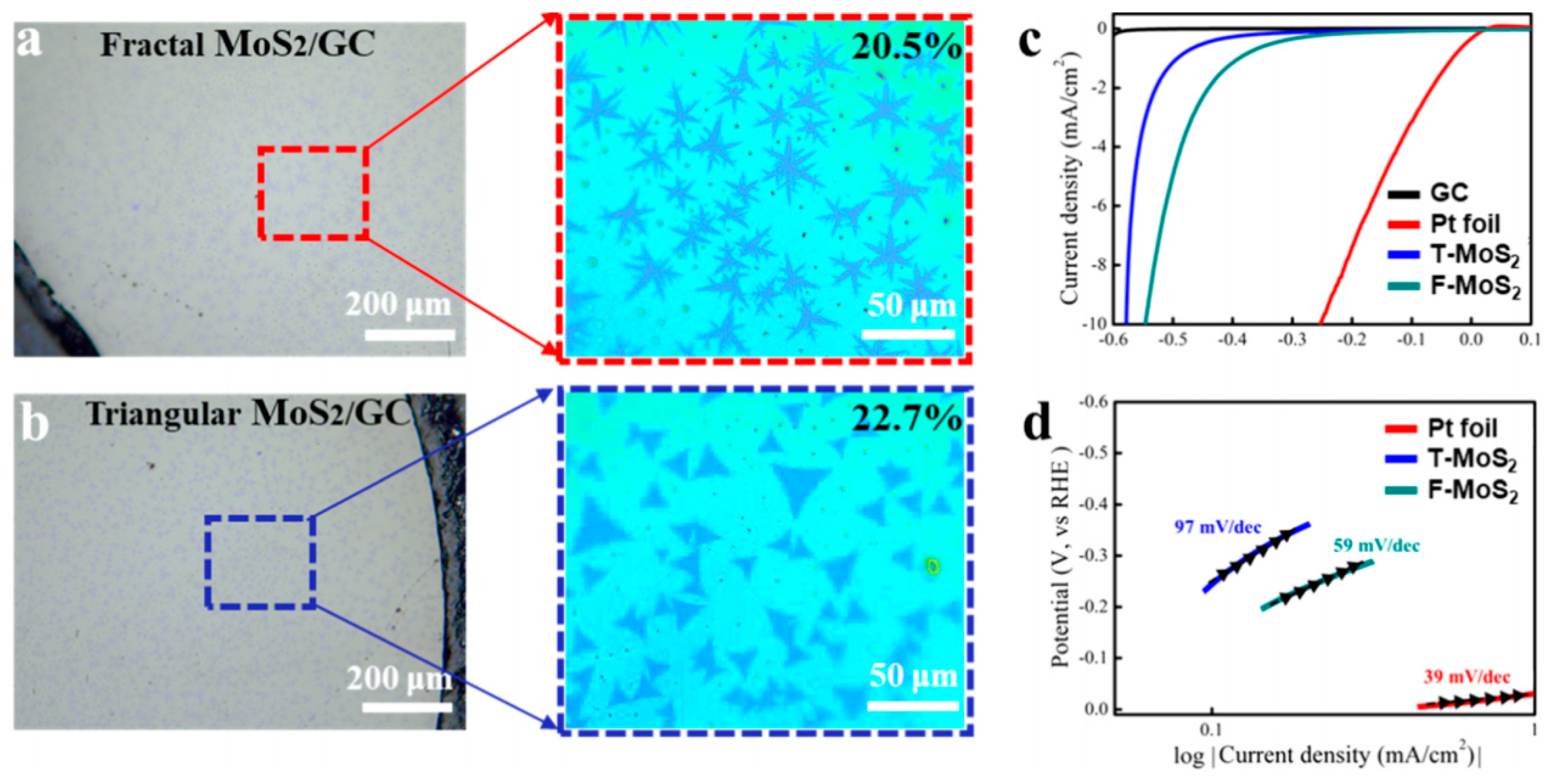
2.2. Fractal MoS2
3. Sulfur vacancies
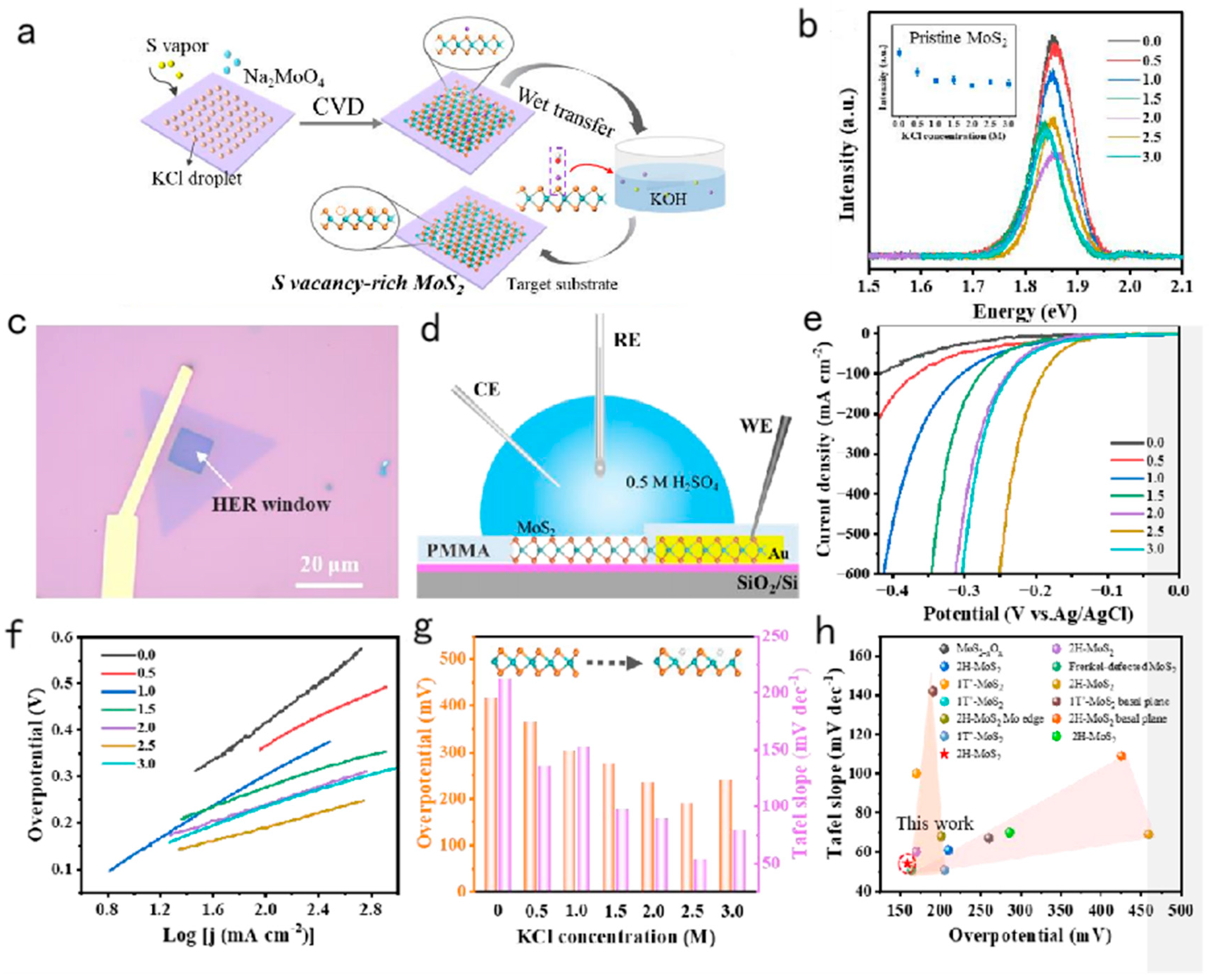
3.1. Salt-assisted chemical vapor deposition (CVD) method
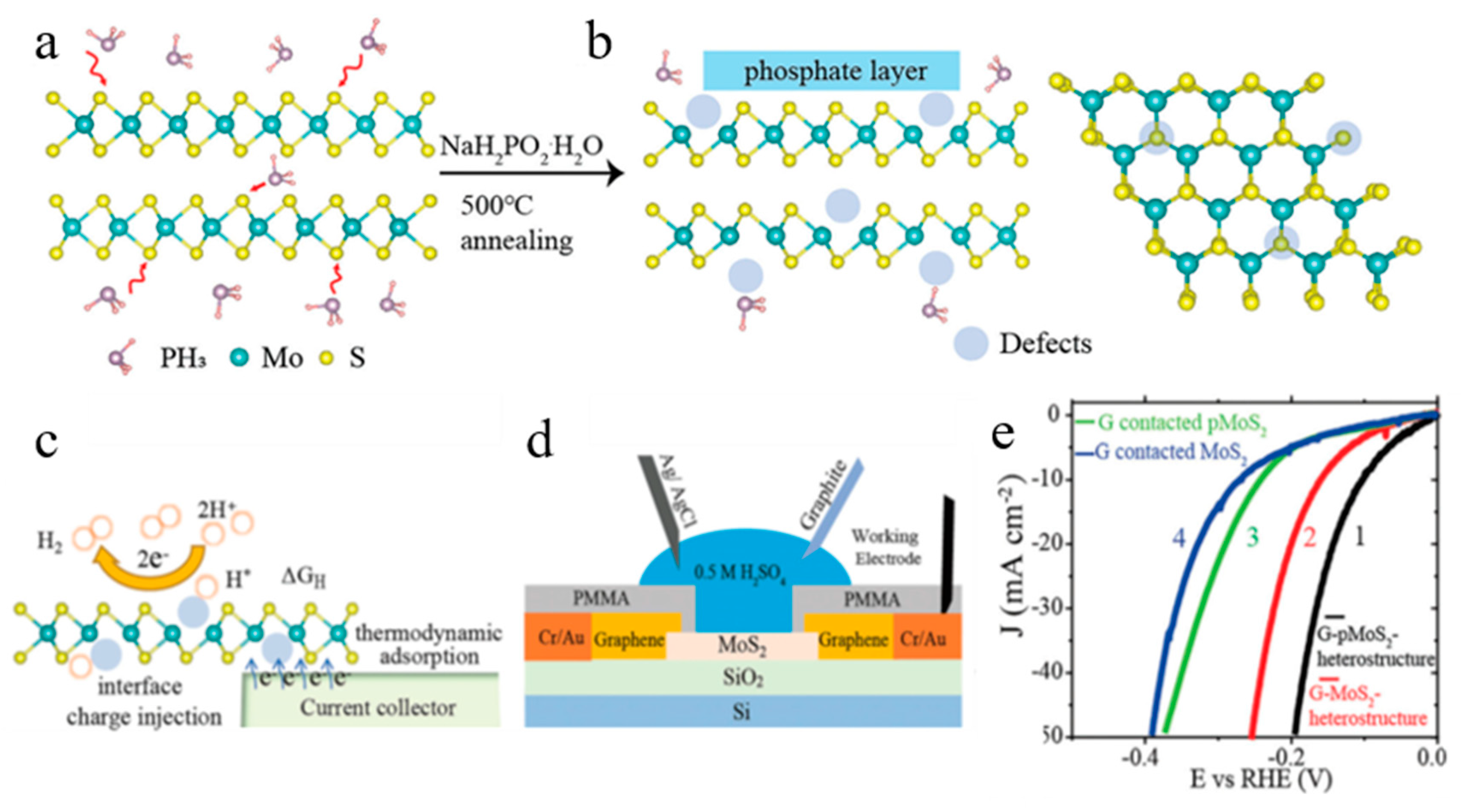
3.2. Controllable Thermochemical Generation of Active Defects
4. Phase
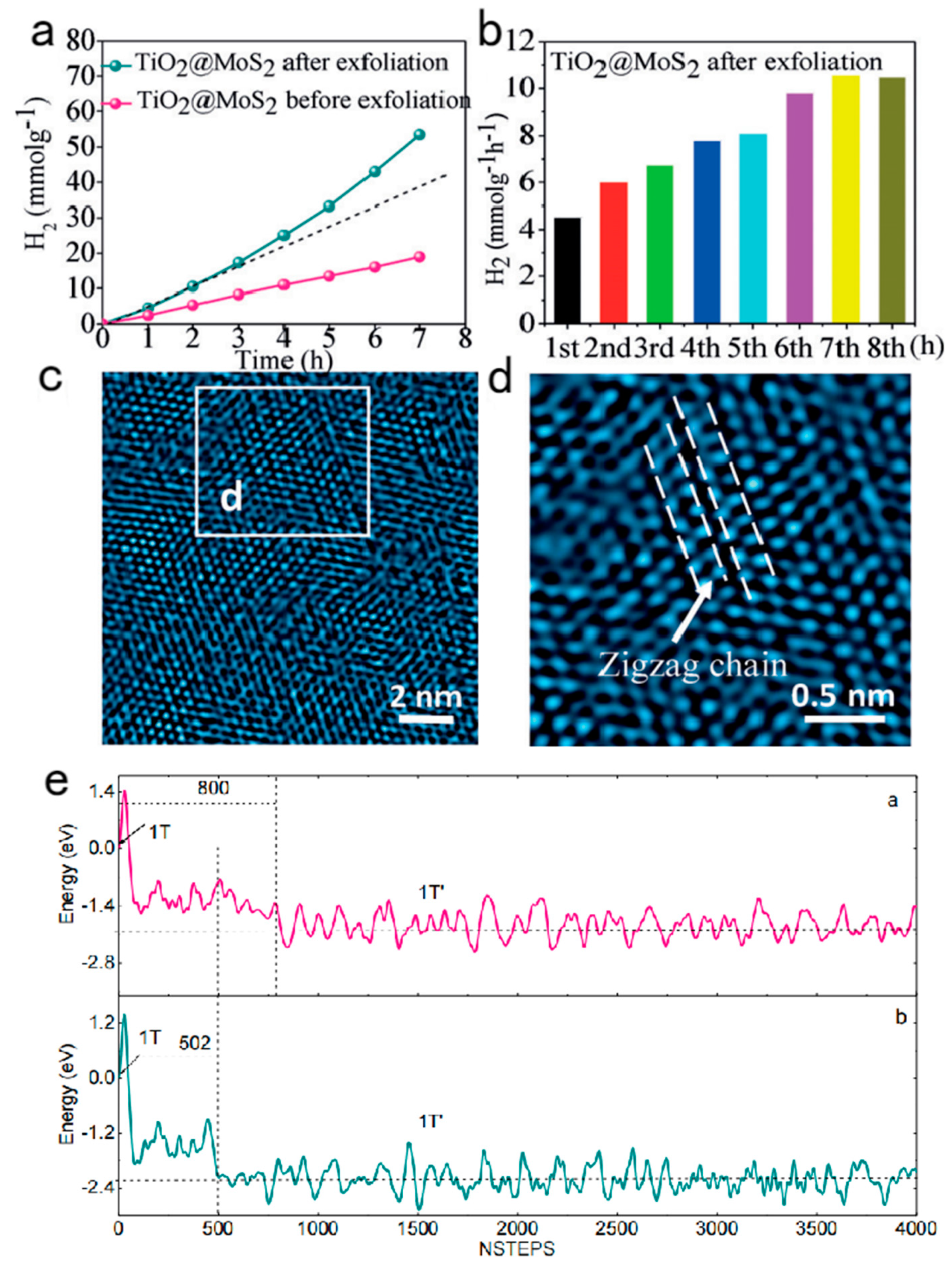
4.1. An Irreversible Phase Transition during Photocatalytic Hydrogen Evolution
4.2. Transient phase transition during the hydrogen evolution reaction
5. Conclusion and outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Yang, J.; Chhowalla, M. Recent strategies for improving the catalytic activity of 2D TMD nanosheets toward the hydrogen evolution reaction. Adv. Mater. 2016, 28, 6197–6206. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wang, L.; Xie, L.; Zhao, W.; Liu, S.; Zhuang, Z.; Liu, S.; Li, J.; Liu, X.; Zhao, Q. Amorphous molybdenum sulfide and its Mo-S motifs: Structural characteristics, synthetic strategies, and comprehensive applications. Nano Res. 2022, 15, 8613–8635. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T. F.; Bonde, J.; Chorkendorff, I.; Nørskov, J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Voiry, D.; Fullon, R.; Yang, J.; de Carvalho Castro e Silva, C.; Kappera, R.; Bozkurt, I.; Kaplan, D.; Lagos, M. J.; Batson, P. E.; Gupta, G. The role of electronic coupling between substrate and 2D MoS2 nanosheets in electrocatalytic production of hydrogen. Nat. Mater. 2016, 15, 1003–1009. [Google Scholar] [CrossRef]
- Li, H.; Tsai, C.; Koh, A. L.; Cai, L. L.; Contryman, A. W.; Fragapane, A. H.; Zhao, J. H.; Han, H. S.; Manoharan, H. C.; Abild-Pedersen, F. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2016, 15, 48–53. [Google Scholar] [CrossRef]
- Lin, L. X.; Sherrell, P.; Liu, Y. Q.; Lei, W.; Zhang, S. W.; Zhang, H. J.; Wallace, G. G.; Chen, J. Engineered 2D transition metal dichalcogenides—A vision of viable hydrogen evolution reaction catalysis. Adv. Energy Mater. 2020, 10, 1903870. [Google Scholar] [CrossRef]
- Sun, C.; Wang, L.; Zhao, W.; Xie, L.; Wang, J.; Li, J.; Li, B.; Liu, S.; Zhuang, Z.; Zhao, Q. Atomic-Level Design of Active Site on Two-Dimensional MoS2 toward Efficient Hydrogen Evolution: Experiment, Theory, and Artificial Intelligence Modelling. Adv. Funct. Mater. 2022, 2206163. [Google Scholar] [CrossRef]
- Lu, Q. P.; Yu, Y. F.; Ma, Q. L.; Chen, B.; Zhang, H. 2D transitionmetal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef]
- He, Q.; Wang, L.; Yin, K.; Luo, S. Vertically aligned ultrathin 1T-WS2 nanosheets enhanced the electrocatalytic hydrogen evolution. Nanoscale Res. Lett. 2018, 13, 167. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Y.; Wang, S.; Xie, L.; Chang, C.; Cheng, X.; Liu, M.; Wang, L.; Wang, L. Ingeniously designed Ni-Mo-S/ZnIn2S4 composite for multi-photocatalytic reaction systems. Chin. Chem. Lett. 2022, 33, 1468–1474. [Google Scholar] [CrossRef]
- Wang, H. T.; Lu, Z. Y.; Kong, D. S.; Sun, J.; Hymel, T. M.; Cui, Y. Electrochemical tuning of MoS2 nanoparticles on three-dimensional substrate for efficient hydrogen evolution. ACS Nano 2014, 8, 4940–4947. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Luo, J.; Duan, X.; Crittenden, J.; Liu, C.; Zhang, S.; Pei, Y.; Zeng, Y.; Duan, X. Self-optimization of the active site of molybdenum disulfide by an irreversible phase transition during photocatalytic hydrogen evolution. Angew. Chem. 2017, 129, 7718–7722. [Google Scholar] [CrossRef]
- Wang, H. T.; Lu, Z. Y.; Xu, S. C.; Kong, D. S.; Cha, J. J.; Zheng, G. Y.; Hsu, P. C.; Yan, K.; Bradshaw, D.; Prinz, F. B. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc. Natl. Acad. Sci. USA 2013, 110, 19701–19706. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S. Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Zou, X. X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Yu, Y. F.; Huang, S. Y.; Li, Y. P.; Steinmann, S. N.; Yang, W. T.; Cao, L. Y. Layer-dependent electrocatalysis of MoS2 for hydrogen evolution. Nano Lett. 2014, 14, 553–558. [Google Scholar] [CrossRef]
- Zhang, J.; Hong, H.; Lian, C.; Ma, W.; Xu, X. Z.; Zhou, X.; Fu, H. X.; Liu, K. H.; Meng, S. Interlayer-state-coupling dependent ultrafast charge transfer in MoS2 /WS2 bilayers. Adv. Sci. 2017, 4, 1700086. [Google Scholar] [CrossRef]
- Ji, Z. H.; Hong, H.; Zhang, J.; Zhang, Q.; Huang, W.; Cao, T.; Qiao, R. X.; Liu, C.; Liang, J.; Jin, C. H. Robust stacking-independent ultrafast charge transfer in MoS2 /WS2 bilayers. ACS Nano 2017, 11, 12020–12026. [Google Scholar] [CrossRef]
- Wang, L.; Shih, E. M.; Ghiotto, A.; Xian, L. D.; Rhodes, D. A.; Tan, C.; Claassen, M.; Kennes, D. M.; Bai, Y. S.; Kim, B. Correlated electronic phases in twisted bilayer transition metal dichalcogenides. Nat. Mater. 2020, 19, 861–866. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Cai, T.; Zhang, S.; Liu, Y.; Song, Y.; Dong, X.; Hu, L. Glucose-assisted synthesize 1D/2D nearly vertical CdS/MoS2 heterostructures for efficient photocatalytic hydrogen evolution. Chem. Eng. J. 2017, 321, 366–374. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Tang, Y.; Luo, S.; Liu, Y.; Zhang, S.; Zeng, Y.; Xu, Y. Vertical single or few-layer MoS2 nanosheets rooting into TiO2 nanofibers for highly efficient photocatalytic hydrogen evolution. Appl. Catal. B 2015, 164, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Yin, K.; Wang, L.; Lu, X.; Zhang, Y.; Liu, Y.; Yan, D.; Song, Y.; Luo, S. Engineering MoS2 nanomesh with holes and lattice defects for highly active hydrogen evolution reaction. Appl. Catal. B 2018, 239, 537–544. [Google Scholar] [CrossRef]
- Li, Y.; Yu, B.; Li, H.M.; Liu, B.; Yu, X.; Zhang, K.W.; Qin, G.; Lu, J.H.; Zhang, L.H.; Wang, L.L. Activation of hydrogen peroxide by molybdenum disulfide as Fenton-like catalyst and cocatalyst: Phase-dependent catalytic performance and degradation mechanism. Chin. Chem. Lett. 2023, 34, 107874. [Google Scholar] [CrossRef]
- Li, J.W.; Yin, W.N.; Pan, J.A.; Zhang, Y.B.; Wang, F.S.; Wang, L.L.; Zhao, Q. External field assisted hydrogen evolution reaction. Nano Res. 2023, 16, 8638–8654. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Zhang, Q.; Zhou, G.; Pei, Y.; Chen, S.; Wang, J.; Rao, A.; Yang, H.; Lu, B. Quasi-one-dimensional Mo chains for efficient hydrogen evolution reaction. Nano Energy 2019, 61, 194–200. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Xie, L.; Zhao, W.; Liu, X.; Zhuang, Z.; Zhuang, Y.; Chen, J.; Liu, S.; Zhao, Q. Dislocation-strained MoS2 nanosheets for high-efficiency hydrogen evolution reaction. Nano Res. 2022, 15, 4996–5003. [Google Scholar] [CrossRef]
- Xie, L.; Wang, L.; Zhao, W.; Liu, S.; Huang, W.; Zhao, Q. WS2 moire superlattices derived from mechanical flexibility for hydrogen evolution reaction. Nat. Commun. 2021, 12, 5070. [Google Scholar] [CrossRef]
- Liu, M.; Li, H.; Liu, S.; Wang, L.; Xie, L.; Zhuang, Z.; Sun, C.; Wang, J.; Tang, M.; Sun, S.; et al. Tailoring activation sites of metastable distorted 1T’ -phase MoS2 by Ni doping for enhanced hydrogen evolution. Nano Res. 2022, 15, 5946–5952. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Abroshan, H.; Zhang, L.; Kim, J. K.; Zhang, J. M.; Guo, J. H.; Siahrostami, S.; Zheng, X. L. Enhancing catalytic activity of MoS2 basal plane S-vacancy by Co cluster addition. ACS Energy Lett. 2018, 3, 2685–2693. [Google Scholar] [CrossRef]
- Mahler, B.; Hoepfner, V.; Liao, K.; Ozin, G. A. Colloidal synthesis of 1T-WS2 and 2H-WS2 nanosheets: Applications for photocatalytic hydrogen evolution. J. Am. Chem. Soc. 2014, 136, 14121–14127. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhang, Y. M.; Gao, T. L.; Yao, T.; Zhang, X. H.; Han, J. C.; Wang, X. J.; Zhang, Z. H.; Xu, P.; Zhang, P. Synergistic phase and disorder engineering in 1T-MoSe2 nanosheets for enhanced hydrogen-evolution reaction. Adv. Mater. 2017, 29, 1700311. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Yamaguchi, H.; Li, J. W.; Silva, R.; Alves, D. C. B.; Fujita, T.; Chen, M. W.; Asefa, T.; Shenoy, V. B.; Eda, G. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 2013, 12, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Tan, C. L.; Luo, Z. M.; Chaturvedi, A.; Cai, Y. Q.; Du, Y. H.; Gong, Y.; Huang, Y.; Lai, Z. C.; Zhang, X.; Zheng, L. R. Preparation of high-percentage 1T-phase transition metal dichalcogenide nanodots for electrochemical hydrogen evolution. Adv. Mater. 2018, 30, 1705509. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Zhao, W.; Liu, S.; Zhao, Q. Oxygen-facilitated dynamic active-site generation on strained MoS2 during photo-catalytic hydrogen evolution. Chem. Eng. J. 2021, 405, 127028. [Google Scholar] [CrossRef]
- Guo, Y. B.; Chen, Q.; Nie, A. M.; Yang, H.; Wang, W. B.; Su, J. W.; Wang, S. Z.; Liu, Y. W.; Wang, S.; Li, H. Q. 2D hybrid superlattice-based on-chip electrocatalytic microdevice for in situ revealing enhanced catalytic activity. ACS Nano 2020, 14, 1635–1644. [Google Scholar] [CrossRef]
- Chou, S. S.; Sai, N.; Lu, P.; Coker, E. N.; Liu, S.; Artyushkova, K.; Luk, T. S.; Kaehr, B.; Brinker, C. J. Understanding catalysis in a multiphasic two-dimensional transition metal dichalcogenide. Nat. Commun. 2015, 6, 8311. [Google Scholar] [CrossRef]
- Jin, H. Y.; Liu, X.; Chen, S. M.; Vasileff, A.; Li, L. Q.; Jiao, Y.; Song, L.; Zheng, Y.; Qiao, S. Z. Heteroatom-doped transition metal electrocatalysts for hydrogen evolution reaction. ACS Energy Lett. 2019, 4, 805–810. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, G.; Luo, H.; Zhang, Q.; Wang, J.; Zhao, C.; Rao, A.; Xu, B.; Lu, B. Enhancing catalytic activity of tungsten disulfide through topology. Appl. Catal. B 2019, 256, 117802. [Google Scholar] [CrossRef]
- Wang, L.; Duan, X.; Wang, G.; Liu, C.; Luo, S.; Zhang, S.; Zeng, Y.; Xu, Y.; Liu, Y.; Duan, X. Omnidirectional enhancement of photocatalytic hydrogen evolution over hierarchical “cauline leaf” nanoarchitectures. Appl. Catal. B 2016, 186, 88–96. [Google Scholar] [CrossRef]
- Li, M.Z.; Wang, L.L.; Zhang, X.Y.; Yin, W.A.; Zhang, Y.B.; Li, J.W.; Yin, Z.Y.; Cai, Y.T.; Liu, S.J.; Zhao, Q. Recent status and future perspectives of ZnIn2S4 for energy conversion and environmental remediation. Chin. Chem. Lett. 2023, 34, 107775. [Google Scholar] [CrossRef]
- Li, Y.; Hua, Y.Q.; Sun, N.; Liu, S.J.; Li, H.X.; Wang, C.; Yang, X.Y.; Zhuang, Z.C.; Wang, L.L. Moire superlattice engineering of two-dimensional materials for electrocatalytic hydrogen evolution reaction. Nano Res. 2023, 16, 8712–8728. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Liu, X.; Zhang, S.; Liu, C.; Yan, D.; Zeng, Y.; Pei, Y.; Liu, Y.; Luo, S. Monolayer MoS2 with S vacancies from interlayer spacing expanded counterparts for highly efficient electrochemical hydrogen production. J. Mater. Chem. A Mater. 2016, 4, 16524–16530. [Google Scholar] [CrossRef]
- Ye, G. L.; Gong, Y. J.; Lin, J. H.; Li, B.; He, Y. M.; Pantelides, S. T.; Zhou, W.; Vajtai, R.; Ajayan, P. M. Defects engineered monolayer MoS2 for improved hydrogen evolution reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef]
- Wang, L.; Shih, E. M.; Ghiotto, A.; Xian, L. D.; Rhodes, D. A.; Tan, C.; Claassen, M.; Kennes, D. M.; Bai, Y. S.; Kim, B. Correlated electronic phases in twisted bilayer transition metal dichalcogenides. Nat. Mater. 2020, 19, 861–866. [Google Scholar] [CrossRef]
- Yuan, Y.; Pan, J.; Yin, *!!! REPLACE !!!*; W., *!!! REPLACE !!!*; Yu, H.; Wang, F.; Hu, W.; Yan, D.; Wang, L. Effective strategies to promote Z (S)-scheme photocatalytic water splitting. Chin. Chem. Lett. 2023, 108724. [Google Scholar] [CrossRef]
- Yin, W.; Yuan, L.; Huang, H.; Cai, Y.; Pan, J.; Sun, N.; Zhang, Q.; Shu, Q.; Gu, C.; Zhuang, Z.; Wang, L. Strategies to accelerate bubble detachment for efficient hydrogen evolution. Chin. Chem. Lett. 2023, 108351. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, M.; Cai, Y., Zhuang Y.; Wang, L. The Advanced Progress of MoS2 and WS2 for Multi-Catalytic Hydrogen Evolution Reaction Systems. Catalysts. 2023, 13(8), 1148.
- Zhang, Y.; Pan, J.; Gong, G.; Song, R.; Yuan, Y.; Li, M.; Wang, L. In Situ Surface Reconstruction of Catalysts for Enhanced Hydrogen Evolution. Catalysts 2023, 13, 20. [Google Scholar] [CrossRef]
- Fan, P.; He, Y.; Pan, J.; Sun, N.; Zhang, Q.; Gu, C.; Chen, K.; Yin, W.; Wang, L. Recent advances in photothermal effects for hydrogen evolution. Chin. Chem. Lett. 2023, 108513. [Google Scholar] [CrossRef]
- Yin, Z.; Xie, L.; Yin, W.; Zhi, T.; Chen, K.; Pan, J.; Zhang, Y.; Li, J.; Wang, L. Advanced development of grain boundaries in TMDs from fundamentals to hydrogen evolution application. Chin. Chem. Lett. 2023, 108628. [Google Scholar] [CrossRef]
- Li, M.; Yin, W.; Pan, J.; Zhu, Y.; Sun, N.; Zhang, X.; Wan, Y.; Luo, Z.; Yi, L.; Wang, L. Hydrogen spillover as a promising strategy for boosting heterogeneous catalysis and hydrogen storage. Chem. Eng. J., 2023, 471, 144691. [Google Scholar] [CrossRef]
- Chen, D. R.; Muthu, J.; Guo, X. Y.; Chin, H. T.; Lin, Y. C.; Haider, G.; Ting, C.; Kalbáč f, M.; Hofmann, M.; Hsieh, Y. P. Edge-dominated hydrogen evolution reactions in ultra-narrow MoS2 nanoribbon arrays. J. Mater. Chem. A. 2023. [CrossRef]
- Wang, S.; Li, J.; Hu, S.; Kang, H.; Zhao, S.; Xiao, R.; Sui, Y; Chen, Z; Peng, S.; Jin, Z.; Liu, X; Zhang Y.; Yu, G. Morphology Regulation of MoS2 Nanosheet-Based Domain Boundaries for the Hydrogen Evolution Reaction. ACS Appl. Nano Mater., 2022, 5, 2273–2279. [CrossRef]
- Man, P.; Jiang, S.; Leung, K. H.; Lai, K. H.; Guang, Z.; Chen, H.; Huang, L.; Chen, T.; Gao, S.; Peng, Y.; Lee, C.; Deng, Q.; Zhao, J.; Ly, T. H. Salt-Induced High-Density Vacancy-Rich Two-Dimensional MoS2 for Efficient Hydrogen Evolution. Adv. Mater. 2023, 2304808. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, C.; Zhang, Y.; Wang, L.; Fan, X.; Zou, L.; Cai, Z. , Jiang J.; Zhou, S.; Zhang, B.; Li, W.; Chen, Z. Controllable Thermochemical Generation of Active Defects in the Horizontal/Vertical MoS2 for Enhanced Hydrogen Evolution. Adv. Funct. Mater. 2023, 2304302. [Google Scholar] [CrossRef]
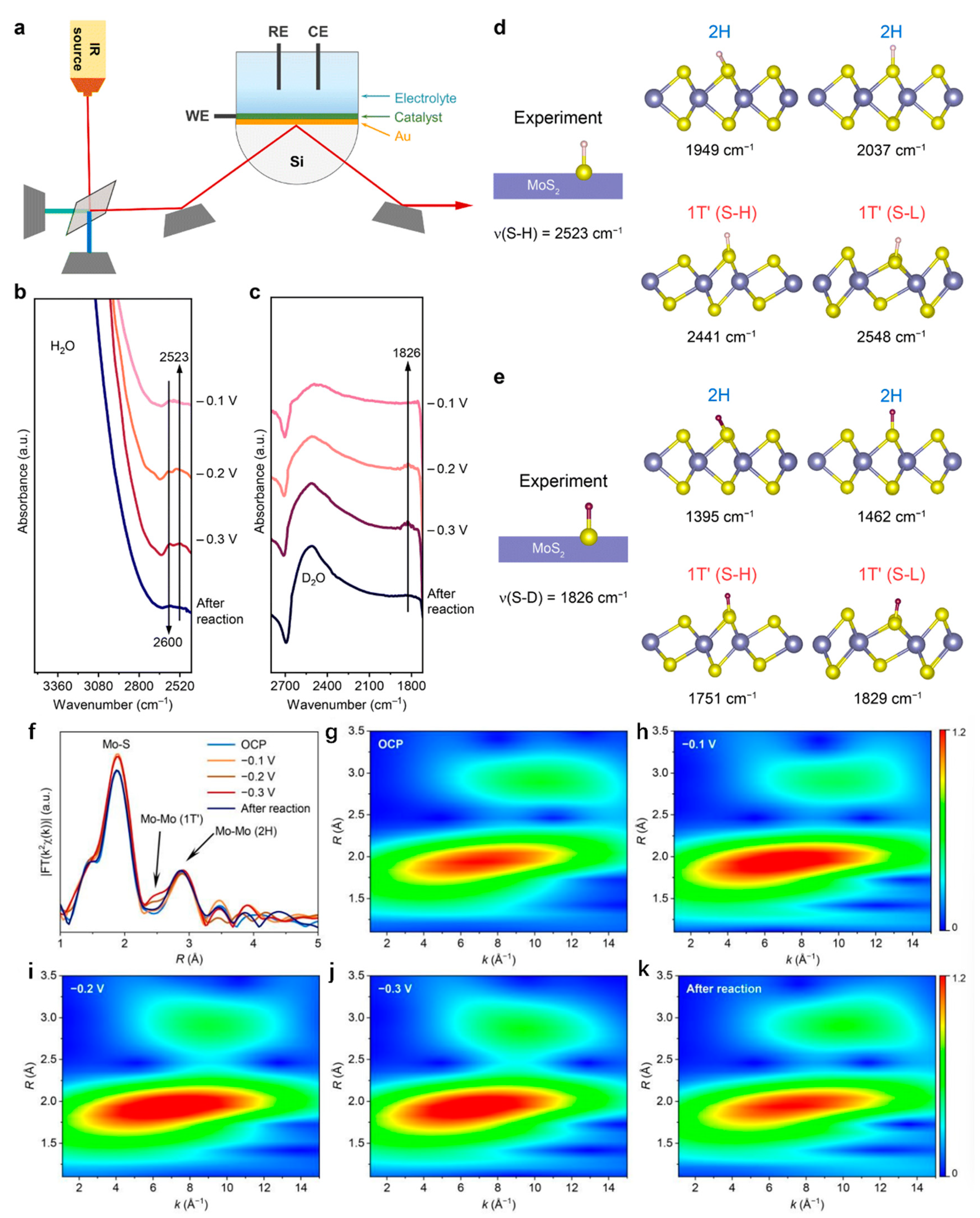
- Zhao, Y.; Li, H.; Yang, R.; Xie, S.; Liu, T.; Li, P.; Zhai, T. Transient phase transition during the hydrogen evolution reaction. Energy Environ. Sci., 2023. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




