1. Introduction
Plant hormones are biologically active compounds produced by their cells, which have a relatively complex structure and affect certain chemical molecules in the process of their metabolism. [
1]
In recent years, research on the role of representatives of a rather large class of steroid hormones, brassinosteroids, in the regulation of cell metabolism at the level of signaling systems has been developing at a rather rapid rate [
2,
3,
4,
5,
6,
7] .
BR, as a unique class of plant hormones, are involved in the regulation of plant growth and development [
3,
8], in particular under the influence of stress factors [
9,
10]. Changes in the concentration of endogenous BR [
11,
12,
13] play an important role in the adaptation of plant metabolism. Exogenous BR have been shown to increase growth [
14], respiration rate [
15], and plant resistance, in particular under drought conditions [
16] and pathogen attack [
17]. BR make a strong impact on photosynthesis and the membrane properties under acclimation to temperature stress [
14]. BR interact with the signaling pathways of other phytohormones [
18], in particular, that of abscisic acid (ABA) [
19], cytokinins [
20], auxins [
21,
22,
23], gibberellins (GCs) [
24,
25], jasmonic acid (JA) [
26], ethylene [
27], salicylic acid (SA) [
28] and other plant hormones [
29,
30,
31].
The experimental data also have shown that exogenous BR have a strong impact on endogenous BR biosynthesis. 24-epibrassinolide (EBL) at a low concentration 0.01-1 μM could increase the BR content in the leaves of barley plants [
9]. Also, EBL effectively ameliorated endogenous BR level and plant growth suppressed by a specific inhibitor of BR biosynthetic reactions – brassinazole [
9]. These studies point on complex interactions between different types of BR and other phytohormones important for a fine-tuning of plant metabolism.
To analyze the cross-interaction of brassinosteroids with other plant hormones, genetic approaches using transgenic plants and the exogenous effect of brassinosteroids on them have been widely applied [
3,
27,
32].
Considerably less attention is paid to determining changes in hormone content in vivo in plants under the exogenous action of brassinosteroids [
33].
The effects of BR on plant cell metabolism have been described in numerous publications, but the knowledge of its molecular mechanisms remains poorly studied. Further research opens up prospects for more efficient use of BR as an environmentally conscious regulator of plant growth and productivity [
34].
The goal of the present study is to analyze the effect of exogenous 24-epicastasterone (ECS) treatment on the endogenous content of key phytohormones, in particular auxins (indole-3-acetic acid (IAA)), abscisic, salicylic and jasmonic acids content in soybean leaves. ECS is a natural brassinosteroid phytohormone, that attracts our attention mainly because it is a direct biosynthetic precursor of EBL [
35], it is widely distributed in plants [
36]; and plays an important role in the regulation of shoots and leaf growth [
37].
Establishing the exogenous influence of the ECS on changes in the content of other hormones is another level of analysis that can provide information that will help shed more light on the complex network of cross-talk between BR and other hormones.
2. Results and Discussion
Indoleacetic [
38], abscisic [
39,
40], jasmonic [
41], and salicylic [
42], [
43] acids along with their metabolites play a key role in the regulation of plant cell metabolism as well as growth and development processes.
2.1. Interaction of Brassinosteroids with Auxins in Plant Cells
Auxins are strong inducers of plant cell metabolic reprogramming, providing rapid acceleration of their growth and development, acting locally or through distinct signaling. Yet, auxins have some inhibiting role in roots growth. IAA is one of the major and most common auxins produced by plants, which stimulates plant growth [
44,
45].
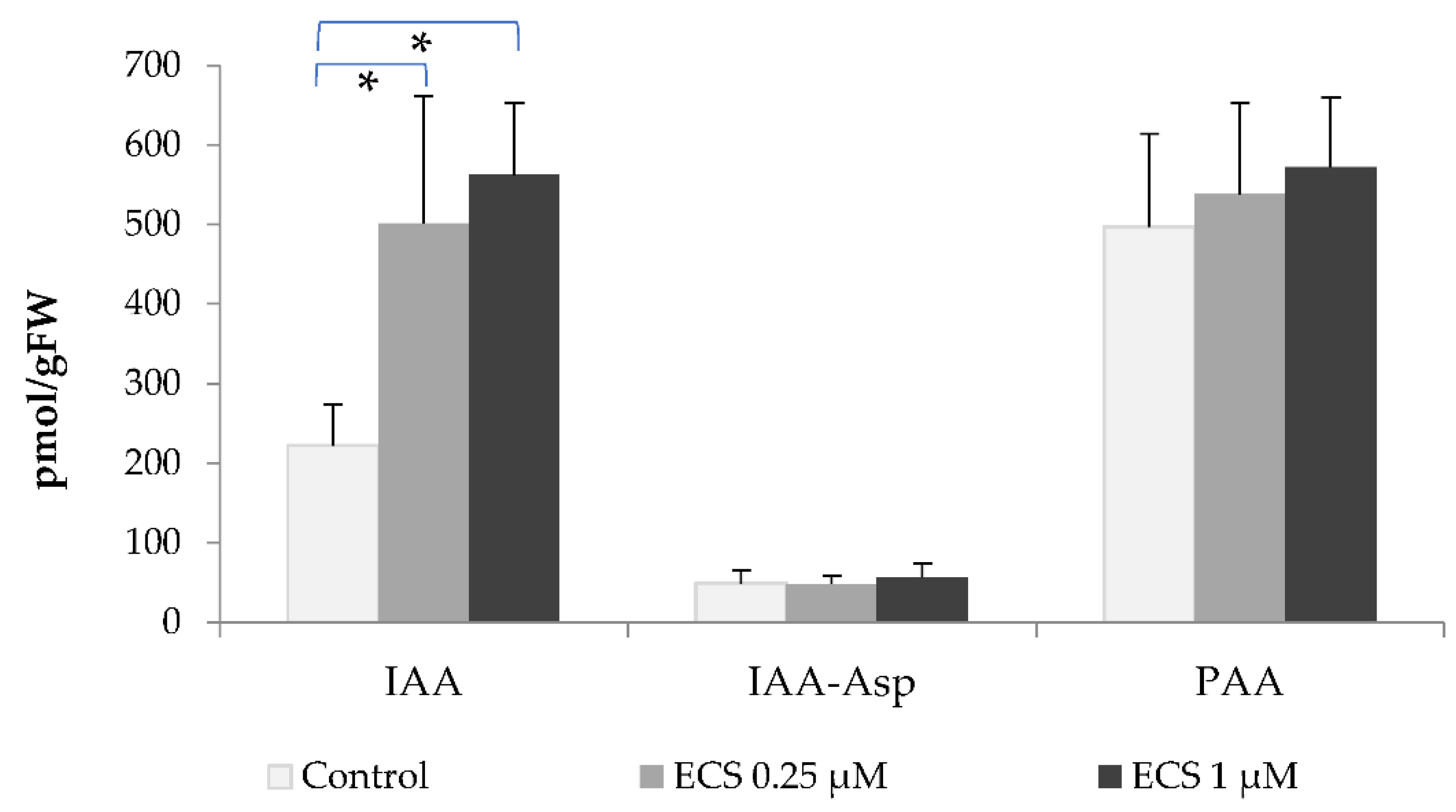
In our research, we detected the levels of IAA, phenylacetic acid (PAA), and IAA ester with aspartate (IAA-Asp) in soybean leaf tissues. The levels of auxins under control conditions was measured to be around 221.9 pmol/g FW for IAA, 48.5 pmol/g FW for IAA-Asp, and 497.1 pmol/g FW for PAA. Treatment of soybean plants with ECS solution (0.25 µM, or 1 µM) caused a strong increase in IAA content (
Figure 1). In contrast, no significant changes in IAA-Asp and PAA content have been observed in soybean leaf tissues treated with the indicated concentrations of ECS (
Figure 1).
PAA is a natural auxin [
46] found in a number of plants [
47], but to date, information on its distribution metabolism and function in plants is still limited, and our data on the response of PAA content to the effects of ECS on plants is a contribution to the study of its role in plants (
Figure 1). According to our results, the content of PAA in soybean leaves exceeded more than twice the content of IAA (
Figure 1), higher content of PAA compared to IAA was also found in a number of other plants among the possible explanations is higher activity and mobility in the tissues of IAA compared to PAA [
47].
A significant increase in the content of IAA was earlier shown in rice treated with EBL under salinity conditions. These changes correlated with enhanced plant growth under salt stress action [
48]. In contrast, no induction of IAA content by EBL was reported under optimal growth conditions [
49]. In another report, brassinolide (BL) – another type of BR – promoted auxin content in
Arabidopsis roots but inhibited auxin signaling [
49]. Moreover, inhibition of BR signaling in the outer tissues of roots results in a meristem insensitivity to both BR and auxin [
49]. In our study exogenous ECS under optimal conditions stimulated IAA content (
Figure 1). This might point to the importance of the particular BR species and concentrations used in evoking dissimilar response in different plant species. However, we observed no significant changes in IAA-Asp or PAA content; our data is the first observation of IAA-Asp and PAA levels in response to BR.
One of the key interactions between BR and auxins can be seen in their mutual effect on the regulation of gene expression. BR can regulate the expression of
YUC9 gene involved in auxin biosynthesis [
49], while auxins can modulate the expression of genes involved in BR biosynthesis [
50,
51].
BR and auxins also interact at the cellular level. BR can promote the transport of auxins by modulating the expression of auxin transporters
PINFORMED3 (PIN3), PIN4 [
52] and auxin responsive genes like
IAA18, IAA30, Auxin response factor12 [
53], while auxins can affect BR signaling by regulating the expression of BR receptors [
54].
Via the Genevestigator array data analysis we could see that the expression level of several genes involved in auxin biosynthesis and signaling is modified in response to ECS or BL treatment in soybean (
Glycine max) [
55] (
Table 1). Expression of soybean genes encoding FLAVIN-CONTAINING MONOOXYGENASE have been increased under ECS treatment (
Table 1). Treatment with ECS (12 hrs), propiconazole (an inhibitor of BR biosynthesis) or combined treatment has overall decreased an expression of genes coding auxin transporters or auxin signaling components in roots of soybean (
Table 1). In contrast, BL treatment in leaves strongly induced the same genes (
Table 1). This indicates that a hormone concentration, its type and a site of treatment have a strong impact on the outcome of genes expression.
Auxins are involved in the metabolism reprogramming of plant cells, providing acceleration of their growth and development [
56,
57]. Overexpression of PIN-LIKES auxin transporters in
imp mutants strongly influences BR signaling possibly by changing local auxin levels [
58]. Auxin and BR stimulate growth and affect the elongation of the hypocotyl, but the hormone crosstalk mechanism implicated in this process is not fully clear [
45,
59]. BR also stimulate auxin levels important for the induction of lateral roots grown under low nitrogen stress by inducing
YUC5,
YUC7, and
YUC8 auxin biosynthetic genes. In BR signaling mutants
bsk3,4,7,8 and
bzr1 the expression of the above-mentioned
YUC genes were not upregulated by low nitrogen conditions anymore. Interestingly, in
bzr1-1D mutant plants, which have a stabilized variant of BR-dependent transcription factor BRASSINAZOLE RESISTANT 1 (BZR1) auxin genes
YUC7 and
YUC8 were upregulated constantly with no respect to low nitrogen conditions [
22]. This clearly points to a close relation between BR and auxin biosynthesis on the genes level. Interactions between BR and auxins play a pivotal role in the regulation of several other key aspects of plant growth and development [
58,
60,
61,
62].
Our results on BR-dependent upregulation of IAA levels in soybean leaves are consistent with many published research articles that indicate that ECS might stimulate the growth of soybean plants via the regulation of auxin content.
2.2. Interaction of Brassinosteroids with Plant Cell Stress Hormones
2.2.1. Interaction of Brassinosteroids with Abscisic Acid
The results of microarray studies indicate that BR and ABA provide general regulation of the expression of hundreds of different genes involved in metabolic regulation, however not much of them have overlapped [
63,
64]. At the same time, the key molecular mechanisms and components of signaling systems involved in these phytohormone interactions require further studies.
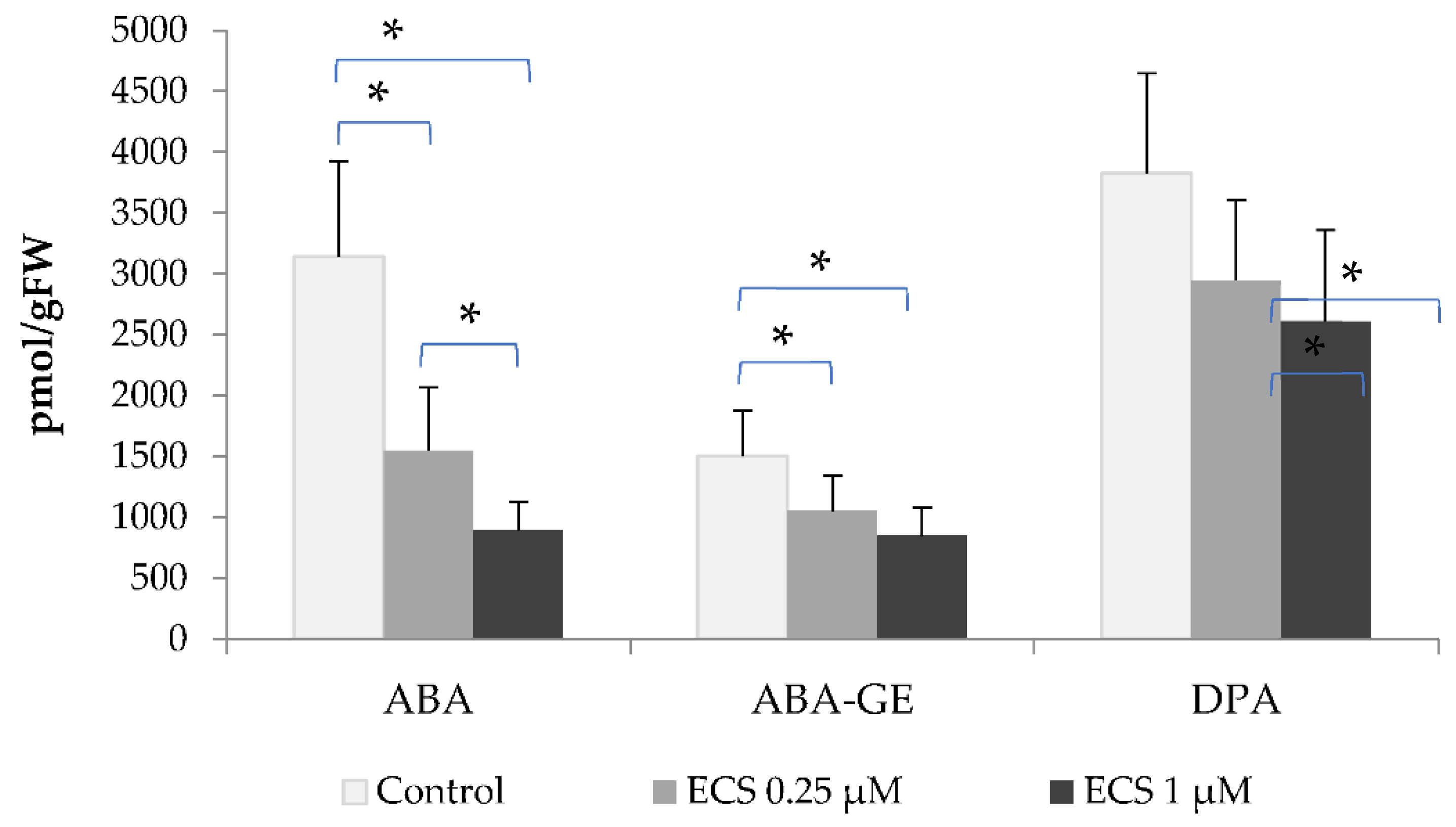
In the present study, we analyzed the contents of abscisic acid and its inactive metabolite dihydrophaseic acid (DPA) in soybean leaves. The ABA level of control plants was 3138.5 pmol/g FW, its glucose ester (ABA-GE) – 1501 pmol/g FW, and DPA – 3826.5 pmol/g FW (
Figure 2). Exogenous treatment with ECS decreased ABA and ABA-GE levels in soybean leaves (
Figure 2). We did not observe any significant changes in the DPA levels of the treated plants (
Figure 2). BR have previously been shown to reduce ABA accumulation, resulting in the inhibition of stomatal closure induced by ABA (Ha, Shang et al. 2018). Foliar application of EBL significantly reduced the level of abscisic acid in rice plants under stress or optimal conditions [
48]. Moreover, freezing conditions in tolerant barley lines itself promote the endogenous level of BR homocastaterone and decrease the ABA level [
13].
BR and ABA antagonistically regulate many key developmental processes such as germination and seed maturation [
65,
66]. BR biosynthetic mutant,
det2-1, and the BR signaling mutant,
bri1-1 are more sensitive to ABA inhibition of seed germination than the wild-type. These earlier observations also pointed out that the germination rate in mentioned mutants was not affected [
67]. However, further investigations with BR mutants
det2-1 and
bri1-301 have found a strong reduction of seed germination in mutant and wild-type lines by exogenous ABA [
64]. Overexpression of BR receptor BRI1 strongly increased the germination rate of seeds treated with ABA [
53]. The involvement of BRI1-associated receptor kinase 1 (BAK1) in the regulation of ABA signaling during seed germination and primary root growth has been shown [
68]. Moreover, inhibited germination of wild-type and
det2-1 lines, but not that of
bri1-301, by ABA can be rescued by BL treatment [
64]. It is known that the effects of ABA in seeds are connected mainly to the prevention of premature germination [
69]. In contrast, BR attenuate the effect of ABA and promote seed germination as well as vegetative growth and development of plants [
19,
70]. BR antagonize ABA-mediated responses in plants through a family of transcription factors BZR1 which can reduce expression of the main ABA-signaling component ABA INSENSITIVE 5 (ABI5) [
70]. Also, it has been found that BZR1 directly binds to the E-box sequences in the
ABA2 promoter region, decreasing the level of endogenous ABA in
A. thaliana [
19]. Treatment with brassinazole (Brz), a biosynthetic inhibitor of BR synthesis, increased
ABA2 expression, an effect that can be attenuated by the exogenous use of BL [
19]. The results of the studies performed by J. Moon with colleagues and our results on the use of ECS in soybean (Figure 4) indicate a key role of BR in the regulation of ABA level in plants [
19].
At the whole-plant level, BR and ABA can act also synergistically depending on the specific physiological process. For example, under salt stress, EBL positively regulates the expression of ABA biosynthesis genes (
OsNCED1, OsNCED2, OsNCED3, OsNCED4, OsNCED5, OsZEP1) and two catabolic genes
OsBG2 and
OsABAox3 in rice [
36]. Owing to these specific cell processes ABA regulates the response to environmental stresses (for complete review see [
71]). ABA through the transcriptional factor ABSCISIC ACID INSENSITIVE3 (ABI3) slightly induces the BR-biosynthesis regulatory gene
OsGSR1 that evidences ABA role in the stimulation of BR production [
72]. Moreover, ABA promotes the expression of
DWF4 and
CPD - key BR-biosynthesis genes, but for effective ABA-BR crosstalk BIN2 signaling component is required [
64]. Interestingly, while ABI3 promotes BR biosynthesis, BR-regulated transcription factor BES1 can directly bind another ABA transcription factor, ABSCISIC ACID INSENSITIVE5 (ABI5), which reduces ABI5 dependent genes and suppresses the ABA response [
73]. This suggests that BR-ABA antagonism and synergism on the level of hormones biosynthesis and signaling can be achieved by different transcription factors but conditions that impact these processes require further elucidation.
Seed germination is an essential stage of plant development, which is regulated by various endogenous signaling systems in interaction with environmental factors [
74].
2.2.2. Effect of 24-Epicastasterone on Salicylic and Benzoic Acids Levels
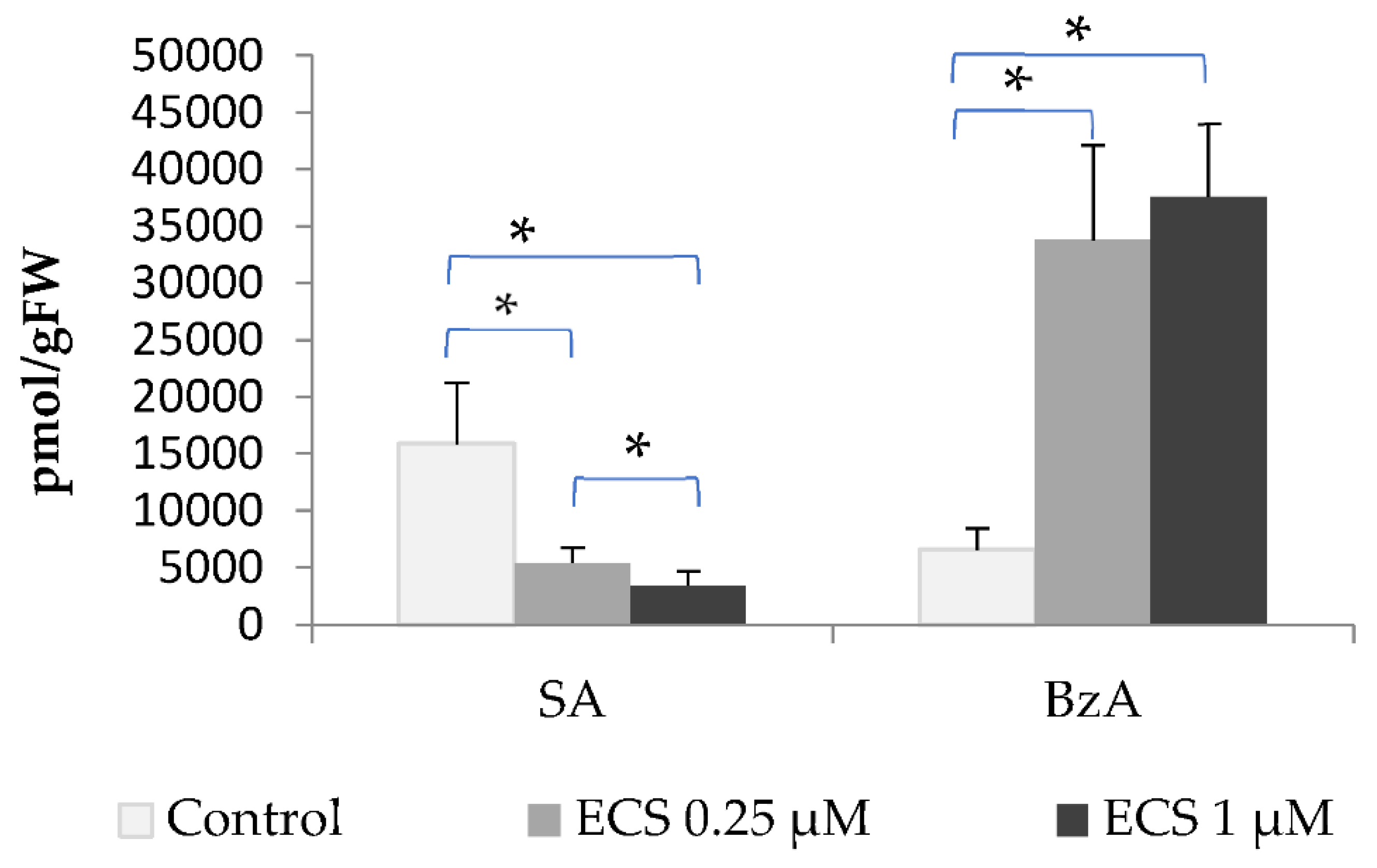
In soybean plants exposed to ECS, the content of SA decreased, while the content of BzA increased significantly, and in both cases, there wasn't a significant difference between studied ECS concentrations (
Figure 3).
Recent studies on hormone levels under optimal conditions observed that freezing-tolerant barley lines accumulate significant levels of SA but not BR (homocastasterone was analyzed in the paper). In contrast, under cold stress conditions tolerant lines accumulate more homocastasterone while SA content significantly drops [
13]. This data is consistent with our results (
Figure 3) and might point to BR and SA role in adaptation processes to abiotic stresses.
BR and SA signaling networks are known to be, at least in part, interconnected. BR can act synergistically and promote SA signaling by inactivating BR-INSENSITIVE 2 (BIN2). BIN2 was shown to phosphorylate TGA4 transcriptional factor (TF) which leads to its destabilization and prevents its functional interaction with NRP1 in mediating SA-regulated gene expression [
75]. Also, EBL have been shown recently to induce SALICYLIC ACID-BINDING PROTEIN2 in wheat [
53]. Moreover, because clade I TGAs (a group comprising TGA4) has a role in regulating SA biosynthesis by promoting the expression of SARD1 and CBP60g [
76], BR via the reported BIN2 phosphorylation of TGA4 can thus act in SA biosynthesis control. Besides, in
Arabidopsis lines overexpressing BRI1-associated receptor kinase 1 (BAK1) an increase in endogenous SA accumulation was reported suggesting that at least some components of the BR signaling cascade have a role in controlling SA accumulation [
77].
BR also can act antagonistically with SA. Based on published data the effect of BR in controlling SA accumulation differs in plant species. Rice plants treated with BL could accumulate less SA during brown planthopper infestation; this effect was attributed to the downregulation of SA biosynthesis genes - OsPAL and OsICS1 [
78]. However, latest data indicates that phenylalanine ammonia-lyase (PAL) pathway not directly leads to SA biosynthesis but have important role in regulation of SA biosynthesis [
79]. Also, BL was shown to reduce the SA content in tobacco [
80].
Bsk1 plants, deficient in BR-SIGNALING KINASE1 (BSK1), accumulated less SA following infection with
G. cichoracearum powdery mildew or
Pseudomonas syringae pv
tomato DC3000). Also, bsk1mutant resistance to pathogens was compromised [
81]. These data suggest that pathogen-induced SA accumulation relies on some components of the BR signaling cascade.
SA can be produced via the isochorismate synthase (ICS) pathway (for review see [
82]). In different plant species, the two pathways contribute unequally to SA biosynthesis. In
Arabidopsis, most SA is produced via the ICS pathway independently of BzA accumulation [
83]. In soybean both pathways, ICS and PAL, contribute equally to stress-induced SA accumulation [
84]. The fact that an increase in BzA following ECS treatment is not translated into SA accumulation may imply that BA2H is inactivated. More so, because the pool of active SA in plants depends not only on the activity of SA biosynthetic pathways but on the rate of SA conversion to inactive metabolites (for review see [
82]).
Recently, important results for understanding the pathways of SA synthesis in plants were obtained by Wu and colleagues [
79], who used stable isotopes (
13C
6-Phe and
13C
6-BzA) to investigate the pathways of SA biosynthesis in
Arabidopsis. They provided evidence that SA can be formed from benzoic acid, acting indepetetly the phenylalanine ammonia-lyase (PAL) pathway [
79].
Our data on the accumulation of BzA (
Figure 3) may evidence an important step in BR-SA crosstalk via regulation of SA/BzA levels. Our results suggest that ECS induces BzA levels in soybean, possibly via inactivation of BA2H. Later, BzA pool may enable rapid conversion to SA and activation of SA signaling. How SA biosynthesis is regulated by BR remains to be established.
2.2.3. Interaction of Brassinosteroids with Jasmonates (Jasmonic Acids and Jasmonic Acid-Isoleucine Levels)
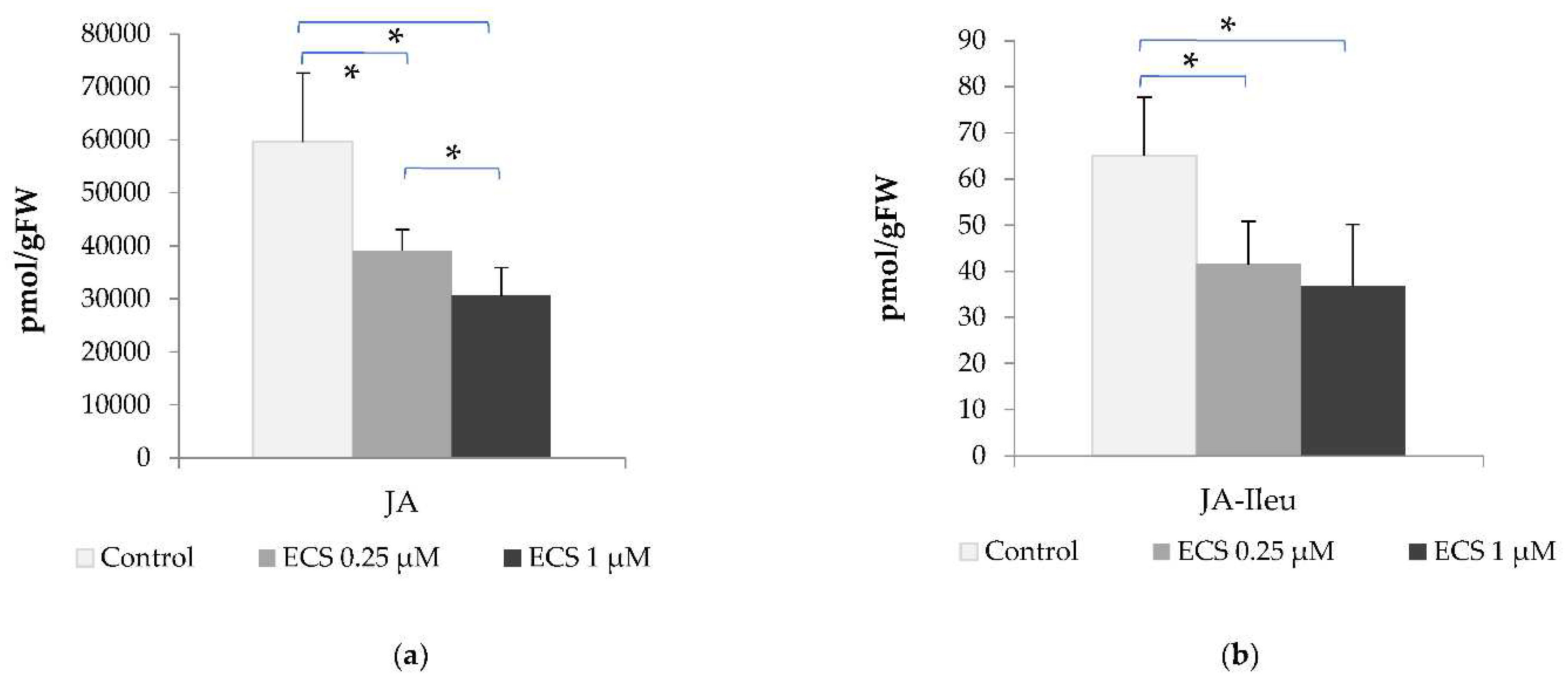
In the present study, we analyzed the content of JA and JA conjugate with isoleucine (JA-Ile). The amino acid conjugate of isoleucine with JA is one of the common forms of JA in plant cells [
85]. The content of JA in the control variant was determined in soybean leaves to be at the level of 65.03 pmol/g of FW and the JA-Ile content – 59636 pmol/g FW. Under exogenous treatment of plants with 0.25 µM ECS, a decrease in the JA and JA-Ile content was recorded. Higher concentrations of ECS (1 µM) did not lead to a further decrease in the level of JA and JA-Ile (
Figure 4).
Analysis of array data on genes involved in jasmonates biosynthesis also revealed genes with modified expression in response to ECS or BL treatment [
55] (
Table 1). Genes which encode JASMONIC ACID-AMIDO SYNTHETASE JAR1 are highly suppressed by treatment with exogenous ECS and BL (
Table 1). In contrast, propiconazole (a BR-biosynthesis inhibitor) has decreased their expression (
Table 1). JASMONIC ACID-AMIDO SYNTHETASE JAR1 catalyzes the formation of JA conjugates with amino acids. These results are consistent with our data on ECS influence on JA and JA-Ile levels (
Figure 4).
It has been shown previously, that mutants in BR biosynthesis or signaling pathway show higher accumulation of JA-precursor 12-oxo-phytodienoic acid [
86] pointing to BR-JA antagonism in the regulation of JA content. Also, JA inhibits root growth, and expression of BR biosynthetic gene
DWF4 and lowers endogenous BR content, while exogenously added BR can attenuate JA inhibitory effects on root growth [
87]. This data is also consistent with the results that we have obtained (
Figure 4).
Some studies point to concentration-dependent interconnection between BR and JA antagonism/synergism switches in different parts of the plant. For example, high levels of exogenously applied EBL in rice shoots have been demonstrated to induce the expression of the JA biosynthetic gene OsAOS2 and signaling gene OsJAmyb in plant roots. This also has been connected with the deterred activity of BR biosynthetic OsDwarf and signaling OsBRI1 genes. In contrast, low exogenous levels of EBL inhibited OsAOS2/OsJAmyb genes. These changes in JA and BR gene activities correlated with increased nematode resistance in plant roots, while the low exogenous concentration of EBL decreased plant resistance to nematode attack [
86].
The major portion of BR-JA antagonism depends on the common component of BR and JA pathways - BIN2 kinase. BIN2 kinase negatively regulates BR signaling by inactivating BR-dependent transcriptional factors BRI1-EMS-SUPPRESSOR1 (BES1) and BZR1. On the opposite, BIN2 positively regulates JA signaling by phosphorylating and promoting the degradation of JAZ proteins - JA signaling repressors. BR treatment represses BIN2 activity and JA signal pathway. Gain-of-function mutations of the BIN2 promote JA signaling and also stimulate JA accumulation [
88]. Rice BIN2 homolog OsGSK2 has been shown to interact directly with a JA repressor protein OsJAZ4 and positively regulate JA signaling and antiviral defense against rice black-streaked dwarf virus (RBSDV).
BIN2 kinase is also a part of BR-JA synergism. OsGSK2 kinase can not only stimulate JA-signaling but also act as a negative regulator through interaction with the JA transcription factor OsMYC2. OsMYC2 phosphorylation by OsGSK2 leads to OsMYC2 degradation and reduction of JA-mediated defense response against rice stripe virus (RSV). It has been shown that RSV suppresses BR endogenous levels to elevate the accumulation of OsGSK2 [
89]. So, at least in the case of plant response to RSV, BR and JA can act synergistically - inhibition of OsGSK2 kinase by BR promotes OsMYC2 activity and JA-dependent defense response.
All the discussions above evidence an important role of BR-JA crosstalk in the regulation of plant cell metabolism. Cross-interaction between JA and BR signaling pathways might be involved in the tight balancing between plant growth and resistance. Data we obtained suggest BR-JA antagonism in soybean plant leaves under optimal conditions in response to treatment with exogenous ECS (
Figure 4).
2.3. Effect of 24-Epicastasterone on Soybean Seed Yield
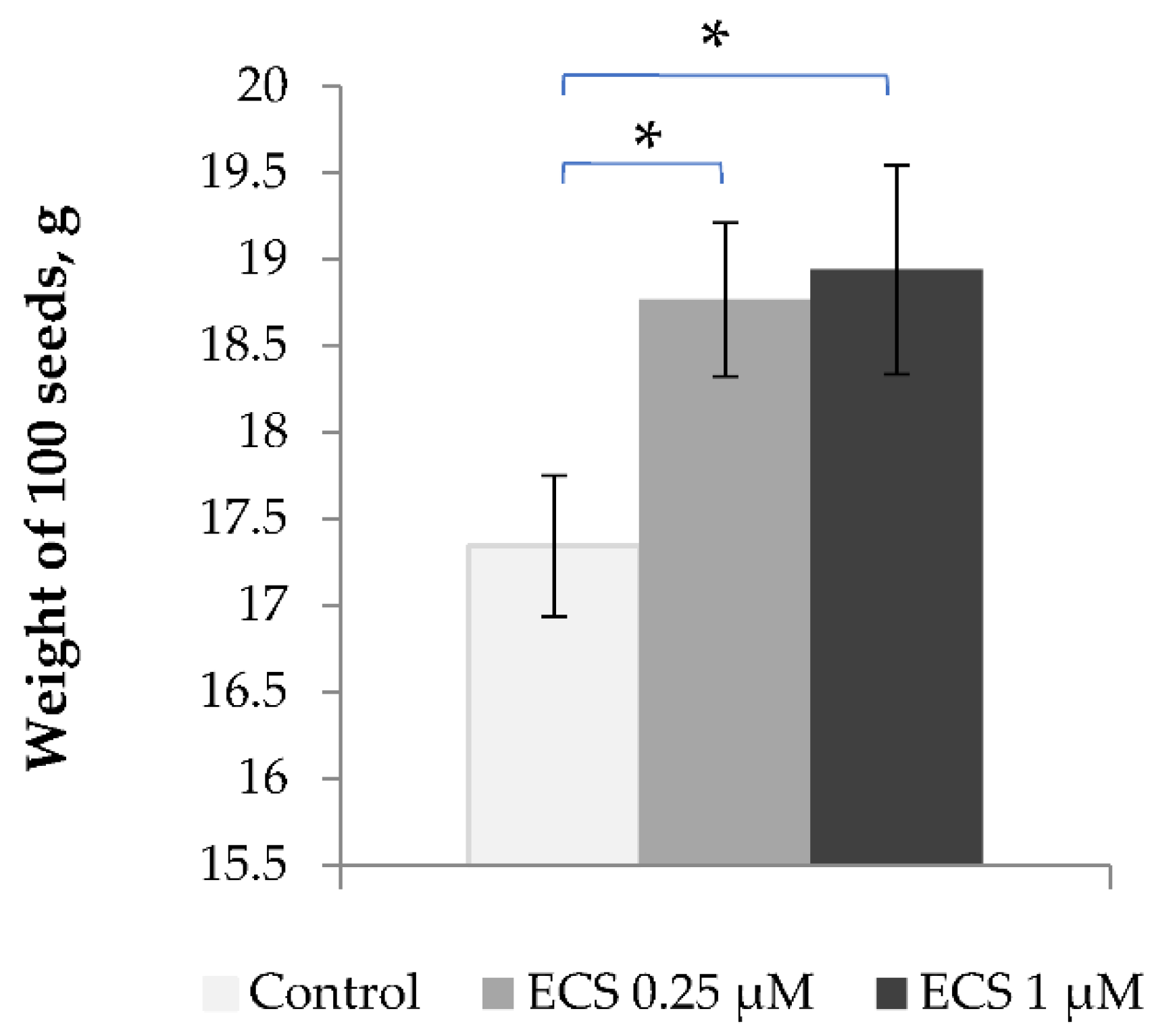
In our study we have evaluated the effect of exogenous ECS on soybean seed productivity as a long-term effect of ECS cross-hormonal interactions. We measured that the average weight of 100 seeds from control plants was corresponding to around 17.5 g, while the treatment of soybean plants with a ECS 0.25 or 1 µM led to an increase in the seed weight to almost 19 g for 100 seeds, an increase of about 8.5% (
Figure 5). Thus, the observed changes in the content of key soybean hormones induced by ECS treatment are linked with an increase in soybean seed productivity.
3. Materials and Methods
3.1. Plant Materials and Growth Conditions
Soybean seeds (Glycine max L. cv. Terek) were obtained from the experimental station of Poltava State Agricultural University (Ukraine). Seeds were sterilized by soaking in a 2.8% sodium hypochlorite (NaOCl) solution for 5 min and then rinsed three times with autoclaved distilled water for 2 min each, germinated on sterile filter paper, and the germinated seeds were placed in 3-liter plastic vessels filled with growth substrate mixture - Polissya soil (70%), washed sand (0.5-2.0 mm) (15%) and agroperlite (15%).
Plants were grown at a constant temperature of 25°C in an artificial climate chamber (14 h light: 10 h dark) under white light provided by a continuous wide-spectrum LED (Philips) at 250 μmol m−2 s −1 intensity. Leaves of soybean plants grown in a vegetation experiment were sprayed twice with ECS solutions (0.25 or 1 µM) - on 21st and 28th days after planting seedlings. On the 35th day after sowing, soybean leaves were sampled from one tier of ten different plant vessels, mixed within each variant, and four samples of 100 mg of leaf tissue were selected and frozen in liquid nitrogen for further determination of phytohormone content. Experiment have been repeated twice.
3.2. Treatment of Soybeans with ECS Solutions
ECS powder was synthetized in the laboratory of Volodymyr Khripach, an expert in the synthesis of different BR types and many of their derivatives. ECS dissolved in EtOH to obtain a 0.5 mM stock solution. The stock was diluted with distilled water to get 0.25 and 1 μM final solutions used for soybean treatments. ECS concentration 0.1 and 0.25 μM has been chosen based on preliminary experiments with BR effects on soybean and available published data on BR usage on different species.
Leaves of soybean plants grown in a vegetation experiment in plastic vessels were sprayed twice with ECS solutions (0.25 or 1 µM) - on the 21st and 28th days after planting seedlings using a hand sprayer; control plants were sprayed with a solvent EtOH diluted with water as ECS stock. EtOH concentration in final ECS solutions or control treatments didn’t exceed 0.01%.
3.3. Hormone Extraction and Quantification
Frozen samples (100 mg FW) were homogenized with liquid nitrogen in a mortar and pestle. The phytohormones were extracted with a cold (-20 °C) methanol/water/formic acid mixture (15/4/1, v/v) as described in [
90].
Internal isotope-labeled standards (10 pmol per sample) were added for hormone analysis): 13C6-IAA (Cambridge Isotope Laboratories);2 H4-SA (Sigma-Aldrich); 2H3-PA, 2H3-DPA, 2H5-ABA-GE (NRC-PBI); 2H6-ABA, 2H5-JA and others (Olchemim).
The extracts were passed through reversed-phase cation exchange SPE columns (Oasis-MCX, Waters) in a mixed mode (mixed phase-cation exchange). The hormone fraction containing ABA, IAA, SA, and JA was eluted with methanol. Hormone metabolites were analyzed using HPLC (Ultimate 3000, Dionex) coupled to a hybrid triple quadrupole/linear ion trap mass spectrometer (3200 Q TRAP, Applied Biosystems). Quantification of hormones was done using the isotope dilution method with multilevel calibration curves (R2 > 0.99). Data processing was carried out with Analyst 1.5 software (Applied Biosystems).
3.4. Estimation of Average Weight of Soybean Seeds
To evaluate the effect of the ECS treatment on an average weight of soybean seeds, plants were harvested by hand and pods were separated from each plant. The pods were dried in an oven at 35 °C for 24 hours to maintain homogeneous seed moisture as described by Poudel [
91].
3.5. Statistical Analysis
P values were calculated with a two-tailed Student’s t-test using Excel software. The sample size and number of independent biological repeats for each type of analysis are provided above.
4. Conclusions
The analysis of the mechanisms of interaction of BR with other plant phytohormones is a key process in elucidating the cooperation of hormonal signaling systems in the regulation of the growth and development of agricultural plants. Understanding the molecular mechanisms underlying this crosstalk is an important task for developing new strategies to improve plant growth and productivity. Our results showing exogenous ECS effect on the content of endogenous hormones in soybean leaves under optimal conditions point at the probable synergism of ECS with the growth-promoting hormones and its antagonism with stress hormones. However, BR synergism or antagonism with other phytohormones depends on specific conditions and might recruit different transcriptional factors; it might be inherently dependent on environmental stresses or pathogen attacks. We observed that BR can stimulate the accumulation of both growth-promoting IAA and BzA - inactive precursor of SA stress hormone - under optimal conditions that might point to efficient trading off between growth and possible stress resistance. Indeed, ECS stimulated seed yield of ECS-treated plants. We hope that the results obtained by our team will be useful to all researchers who analyze the interaction of brassinosteroids and salicylic acid, as well as its metabolites in plant cells.
Overall, the crosstalk between BR and other phytohormones is a complex and dynamic process that is still being actively studied by plant biologists.
Author Contributions
Conceptualization, J.M., V.K. (Vladimir Khripach) and V.K. (Volodymyr Kravets); methodology, J.M., P.D., and V.K. (Volodymyr Kravets); formal analysis, P.D., R.F. and S.K.; data curation, P.D., S.K., and V.S. (Volodymyr Kravets); compound biosynthesis, V.K. (Vladimir Khripach); writing—original draft preparation, V.K. (Volodymyr Kravets), M.D., I.P., Y.B.; writing—review and editing, M.D., I.P., Y.B., P.D., J.M., E.R., V.K. (Vladimir Khripach), and V.K. (Volodymyr Kravets); project administration, P.D., J.M.,E.R., and V.K. (Volodymyr Kravets); funding acquisition, P.D., V.K. (Vladimir Khripach) and V.K. (Volodymyr Kravets). All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by grants of NAS Ukraine (№ 13-03-20/21, 2.1.10.32-20-24), The Belarusian Republican Foundation for Fundamental Research (BRFFR X20УKA-023), The European Regional Development Fund-Project “Centre for Experimental Plant Biology” (No. CZ.02.1.01/ 0.0/0.0/16_019/0000738).
Data Availability Statement
Data is available on the request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kinoshita, T.; Caño-Delgado, A.; Seto, H.; Hiranuma, S.; Fujioka, S.; Yoshida, S.; Chory, J. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 2005, 433, 167–171. [Google Scholar] [CrossRef]
- Fujiyama, K.; Hino, T.; Kanadani, M.; Watanabe, B.; Jae Lee, H.; Mizutani, M.; Nagano, S. Structural insights into a key step of brassinosteroid biosynthesis and its inhibition. Nature Plants 2019, 5, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. The Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Chmur, M.; Gruszka, D. Comprehensive Overview of the Brassinosteroid Biosynthesis Pathways: Substrates, Products, Inhibitors, and Connections. Frontiers in Plant Science 2020, 11. [Google Scholar] [CrossRef]
- Gruszka, D.; Bajguz, A.; Li, Q.-F.; Hayat, S.; Hansson, M.; Wang, X.; Li, J. Editorial: An Update on Brassinosteroids: Homeostasis, Crosstalk, and Adaptation to Environmental Stress. Frontiers in Plant Science 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Neubus Claus, L.A.; Liu, D.; Hohmann, U.; Vukašinović, N.; Pleskot, R.; Liu, J.; Schiffner, A.; Jaillais, Y.; Wu, G.; Wolf, S.; et al. BRASSINOSTEROID INSENSITIVE1 internalization can occur independent of ligand binding. Plant Physiology 2023. [Google Scholar] [CrossRef]
- Park, C.-H.; Park, Y.J.; Youn, J.-H.; Roh, J.; Kim, S.-K. Brassinosteroids and Salicylic Acid Mutually Enhance Endogenous Content and Signaling to Show a Synergistic Effect on Pathogen Resistance in Arabidopsis thaliana. Journal of Plant Biology 2023, 66, 181–192. [Google Scholar] [CrossRef]
- Vukašinović, N.; Wang, Y.; Vanhoutte, I.; Fendrych, M.; Guo, B.; Kvasnica, M.; Jiroutová, P.; Oklestkova, J.; Strnad, M.; Russinova, E. Local brassinosteroid biosynthesis enables optimal root growth. Nature Plants 2021, 7, 619–632. [Google Scholar] [CrossRef]
- Bajguz, A.; Orczyk, W.; Gołębiewska, A.; Chmur, M.; Piotrowska-Niczyporuk, A. Occurrence of brassinosteroids and influence of 24-epibrassinolide with brassinazole on their content in the leaves and roots of Hordeum vulgare L. cv. Golden Promise. Planta 2019, 249, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Sadura, I.; Libik-Konieczny, M.; Jurczyk, B.; Gruszka, D.; Janeczko, A. HSP Transcript and Protein Accumulation in Brassinosteroid Barley Mutants Acclimated to Low and High Temperatures. International Journal of Molecular Sciences 2020, 21, 1889. [Google Scholar] [CrossRef]
- Janeczko, A.; Pociecha, E.; Dziurka, M.; Jurczyk, B.; Libik-Konieczny, M.; Oklestkova, J.; Novák, O.; Pilarska, M.; Filek, M.; Rudolphi-Skórska, E.; et al. Changes in content of steroid regulators during cold hardening of winter wheat - Steroid physiological/biochemical activity and impact on frost tolerance. Plant Physiology and Biochemistry 2019, 139, 215–228. [Google Scholar] [CrossRef]
- Gruszka, D. Exploring the Brassinosteroid Signaling in Monocots Reveals Novel Components of the Pathway and Implications for Plant Breeding. International journal of molecular sciences 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Malaga, S.; Janeczko, A.; Janowiak, F.; Waligórski, P.; Oklestkova, J.; Dubas, E.; Krzewska, M.; Nowicka, A.; Surówka, E.; Rapacz, M.; et al. Involvement of homocastasterone, salicylic and abscisic acids in the regulation of drought and freezing tolerance in doubled haploid lines of winter barley. Plant Growth Regulation 2020, 90, 173–188. [Google Scholar] [CrossRef]
- Sadura, I.; Janeczko, A. Brassinosteroids and the Tolerance of Cereals to Low and High Temperature Stress: Photosynthesis and the Physicochemical Properties of Cell Membranes. International Journal of Molecular Sciences 2022, 23, 342. [Google Scholar] [CrossRef] [PubMed]
- Derevyanchuk, M.; Kretynin, S.; Iakovenko, O.; Litvinovskaya, R.; Zhabinskii, V.; Martinec, J.; Blume, Y.; Khripach, V.; Kravets, V. Effect of 24-epibrassinolide on Brassica napus alternative respiratory pathway, guard cells movements and phospholipid signaling under salt stress. Steroids 2017, 117, 16–24. [Google Scholar] [CrossRef]
- Janeczko, A.; Gruszka, D.; Pociecha, E.; Dziurka, M.; Filek, M.; Jurczyk, B.; Kalaji, H.M.; Kocurek, M.; Waligórski, P. Physiological and biochemical characterisation of watered and drought-stressed barley mutants in the HvDWARF gene encoding C6-oxidase involved in brassinosteroid biosynthesis. Plant Physiology and Biochemistry 2016, 99, 126–141. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, L.; Liu, X.; Zhang, Q.; Zhuansun, X.; Fahima, T.; Krugman, T.; Sun, Q.; Xie, C. Glycerol-Induced Powdery Mildew Resistance in Wheat by Regulating Plant Fatty Acid Metabolism, Plant Hormones Cross-Talk, and Pathogenesis-Related Genes. International Journal of Molecular Sciences 2020, 21, 673. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-Y.; Li, Y.; Cao, D.-M.; Yang, H.; Oh, E.; Bi, Y.; Zhu, S.; Wang, Z.-Y. The F-box Protein KIB1 Mediates Brassinosteroid-Induced Inactivation and Degradation of GSK3-like Kinases in Arabidopsis. Molecular Cell 2017, 66, 648–657. [Google Scholar] [CrossRef]
- Moon, J.; Park, C.-H.; Son, S.-H.; Youn, J.-H.; Kim, S.-K. Endogenous level of abscisic acid down-regulated by brassinosteroids signaling via BZR1 to control the growth of Arabidopsis thaliana. Plant Signaling & Behavior 2021, 16, 1926130. [Google Scholar] [CrossRef]
- Mouchel, C.F.; Osmont, K.S.; Hardtke, C.S. BRX mediates feedback between brassinosteroid levels and auxin signalling in root growth. Nature 2006, 443, 458–461. [Google Scholar] [CrossRef]
- Youn, J.-H.; Kim, M.K.; Kim, E.-J.; Son, S.-H.; Lee, J.E.; Jang, M.-S.; Kim, T.-W.; Kim, S.-K. ARF7 increases the endogenous contents of castasterone through suppression of BAS1 expression in Arabidopsis thaliana. Phytochemistry 2016, 122, 34–44. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; von Wirén, N. Local auxin biosynthesis acts downstream of brassinosteroids to trigger root foraging for nitrogen. Nat Commun 2021, 12, 5437–5437. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.L.; Pandey, A.; Gupta, S.; Singh, A.P. The interplay of auxin and brassinosteroid signaling tunes root growth under low and different nitrogen forms. Plant Physiology 2022. [Google Scholar] [CrossRef]
- Hu, S.; Wang, C.; Sanchez, D.L.; Lipka, A.E.; Liu, P.; Yin, Y.; Blanco, M.; Lübberstedt, T. Gibberellins Promote Brassinosteroids Action and Both Increase Heterosis for Plant Height in Maize (Zea mays L.). Frontiers in Plant Science 2017, 8, 1039. [Google Scholar] [CrossRef]
- Unterholzner, S.J.; Rozhon, W.; Papacek, M.; Ciomas, J.; Lange, T.; Kugler, K.G.; Mayer, K.F.; Sieberer, T.; Poppenberger, B. Brassinosteroids Are Master Regulators of Gibberellin Biosynthesis in Arabidopsis. The Plant Cell 2015, 27, 2261–2272. [Google Scholar] [CrossRef]
- Liao, K.; Peng, Y.-J.; Yuan, L.-B.; Dai, Y.-S.; Chen, Q.-F.; Yu, L.-J.; Bai, M.-Y.; Zhang, W.-Q.; Xie, L.-J.; Xiao, S. Brassinosteroids Antagonize Jasmonate-Activated Plant Defense Responses through BRI1-EMS-SUPPRESSOR1 (BES1). Plant Physiology 2020, 182, 1066–1082. [Google Scholar] [CrossRef]
- Zhao, N.; Zhao, M.; Tian, Y.; Wang, Y.; Han, C.; Fan, M.; Guo, H.; Bai, M.-Y. Interaction between BZR1 and EIN3 mediates signalling crosstalk between brassinosteroids and ethylene. New Phytologist 2021, 232, 2308–2323. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Ruelland, E. Magical mystery tour: Salicylic acid signalling. Environmental and Experimental Botany 2014. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Yu, J.-Q.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Benefits of brassinosteroid crosstalk. Trends in Plant Science 2012, 17, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, D. The Brassinosteroid Signaling Pathway—New Key Players and Interconnections with Other Signaling Networks Crucial for Plant Development and Stress Tolerance. International Journal of Molecular Sciences 2013, 14, 8740–8774. [Google Scholar] [CrossRef]
- Li, C.; Xu, M.; Cai, X.; Han, Z.; Si, J.; Chen, D. Jasmonate Signaling Pathway Modulates Plant Defense, Growth, and Their Trade-Offs. International Journal of Molecular Sciences 2022, 23, 3945. [Google Scholar] [CrossRef] [PubMed]
- Graeff, M.; Rana, S.; Marhava, P.; Moret, B.; Hardtke, C.S. Local and Systemic Effects of Brassinosteroid Perception in Developing Phloem. Current Biology 2020, 30, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Bai, L.; Miao, L.; Liu, Y.; Li, S.; Yu, X.; Li, Y. 24-Epibrassinolide Ameliorates Endogenous Hormone Levels to Enhance Low-Temperature Stress Tolerance in Cucumber Seedlings. International Journal of Molecular Sciences 2018, 19, 2497. [Google Scholar] [CrossRef] [PubMed]
- Ackerman-Lavert, M.; Savaldi-Goldstein, S. Growth models from a brassinosteroid perspective. Current Opinion in Plant Biology 2020, 53, 90–97. [Google Scholar] [CrossRef]
- Fujioka, S.; Yokota, T. Biosynthesis and metabolism of brassinosteroids. Annual review of plant biology 2003, 54, 137–164. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Tretyn, A. The Chemical Structures and Occurrence of Brassinosteroids in Plants. In Brassinosteroids: Bioactivity and Crop Productivity, Hayat, S., Ahmad, A., Eds.; Springer Netherlands: Dordrecht, 2003; pp. 1–44. [Google Scholar]
- Cheng, L.; Li, M.; Min, W.; Wang, M.; Chen, R.; Wang, W. Optimal Brassinosteroid Levels Are Required for Soybean Growth and Mineral Nutrient Homeostasis. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bhardwaj, M.; Tran, L.-S.P. Integration of Auxin, Brassinosteroid and Cytokinin in the Regulation of Rice Yield. Plant and Cell Physiology 2022, 63, 1848–1856. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.-J.; Bressan, R.A.; Song, C.-P.; Zhu, J.-K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. Journal of Integrative Plant Biology 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Sano, N.; Marion-Poll, A. ABA Metabolism and Homeostasis in Seed Dormancy and Germination. International Journal of Molecular Sciences 2021, 22, 5069. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G. Jasmonates: biosynthesis, perception and signal transduction. Essays in Biochemistry 2020, 64, 501–512. [Google Scholar] [CrossRef]
- Pokotylo, I.; Hodges, M.; Kravets, V.; Ruelland, E. A ménage à trois: salicylic acid, growth inhibition, and immunity. Trends in Plant Science 2022, 27, 460–471. [Google Scholar] [CrossRef]
- Ullah, C.; Chen, Y.-H.; Ortega, M.A.; Tsai, C.-J. The diversity of salicylic acid biosynthesis and defense signaling in plants: Knowledge gaps and future opportunities. Current Opinion in Plant Biology 2023, 72, 102349. [Google Scholar] [CrossRef]
- Tan, S.; Luschnig, C.; Friml, J. Pho-view of Auxin: Reversible Protein Phosphorylation in Auxin Biosynthesis, Transport and Signaling. Molecular Plant 2021, 14, 151–165. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, J.; Zhang, M.; Li, X.; Sun, Y.; Zhang, M.; Ding, Z. Auxin promotes hypocotyl elongation by enhancing BZR1 nuclear accumulation in Arabidopsis. Science Advances 2023, 9, eade2493. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Mashiguchi, K.; Tanaka, K.; Hishiyama, S.; Sakai, T.; Hanada, K.; Kinoshita-Tsujimura, K.; Yu, H.; Dai, X.; Takebayashi, Y.; et al. Distinct Characteristics of Indole-3-Acetic Acid and Phenylacetic Acid, Two Common Auxins in Plants. Plant and Cell Physiology 2015, 56, 1641–1654. [Google Scholar] [CrossRef]
- Perez, V.C.; Zhao, H.; Lin, M.; Kim, J. Occurrence, Function, and Biosynthesis of the Natural Auxin Phenylacetic Acid (PAA) in Plants. Plants 2023, 12, 266. [Google Scholar] [CrossRef]
- Shahzad, R.; Harlina, P.W.; Ewas, M.; Zhenyuan, P.; Nie, X.; Gallego, P.P.; Ullah Khan, S.; Nishawy, E.; Khan, A.H.; Jia, H. Foliar applied 24-epibrassinolide alleviates salt stress in rice (Oryza sativa L.) by suppression of ABA levels and upregulation of secondary metabolites. Journal of Plant Interactions 2021, 16, 533–549. [Google Scholar] [CrossRef]
- Ackerman-Lavert, M.; Fridman, Y.; Matosevich, R.; Khandal, H.; Friedlander-Shani, L.; Vragović, K.; Ben El, R.; Horev, G.; Tarkowská, D.; Efroni, I.; et al. Auxin requirements for a meristematic state in roots depend on a dual brassinosteroid function. Current Biology 2021, 31, 4462–4472. [Google Scholar] [CrossRef]
- Chung, Y.; Maharjan, P.M.; Lee, O.; Fujioka, S.; Jang, S.; Kim, B.; Takatsuto, S.; Tsujimoto, M.; Kim, H.; Cho, S.; et al. Auxin stimulates DWARF4 expression and brassinosteroid biosynthesis in Arabidopsis. 2011, 66, 564-578. 66. [CrossRef]
- Xiong, Y.; Wu, B.; Du, F.; Guo, X.; Tian, C.; Hu, J.; Lü, S.; Long, M.; Zhang, L.; Wang, Y.; et al. A crosstalk between auxin and brassinosteroid regulates leaf shape by modulating growth anisotropy. Molecular Plant 2021, 14, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Hacham, Y.; Sela, A.; Friedlander, L.; Savaldi-Goldstein, S. BRI1 activity in the root meristem involves post-transcriptional regulation of PIN auxin efflux carriers. Plant Signaling & Behavior 2012, 7, 68–70. [Google Scholar] [CrossRef]
- Sharma, N.; Chaudhary, C.; Khurana, P. Transcriptome profiling of somatic embryogenesis in wheat (Triticum aestivum L.) influenced by auxin, calcium and brassinosteroid. Plant Growth Regulation 2022, 98, 599–612. [Google Scholar] [CrossRef]
- Sakamoto, T.; Fujioka, S. Auxins increase expression of the brassinosteroid receptor and brassinosteroid-responsive genes in Arabidopsis. Plant Signal Behav 2013, 8, e23509. [Google Scholar] [CrossRef] [PubMed]
- Hruz, T.; Laule, O.; Szabo, G.; Wessendorp, F.; Bleuler, S.; Oertle, L.; Widmayer, P.; Gruissem, W.; Zimmermann, P. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Advances in bioinformatics 2008, 2008, 420747. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Pérez, M.; Steinbrecher, T.; Gawthrop, F.; Pavlović, I.; Novák, O.; Tarkowská, D.; Strnad, M.; Marone, F.; Nakabayashi, K.; et al. Molecular mechanisms and hormonal regulation underpinning morphological dormancy: a case study using Apium graveolens (Apiaceae). The Plant Journal 2021, 108, 1020–1036. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Smalle, J.A. Auxin/Cytokinin Antagonistic Control of the Shoot/Root Growth Ratio and Its Relevance for Adaptation to Drought and Nutrient Deficiency Stresses. 2022, 23, 1933.
- Sun, L.; Feraru, E.; Feraru, M.I.; Waidmann, S.; Wang, W.; Passaia, G.; Wang, Z.-Y.; Wabnik, K.; Kleine-Vehn, J. PIN-LIKES Coordinate Brassinosteroid Signaling with Nuclear Auxin Input in Arabidopsis thaliana. Current Biology 2020, 30, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, Y.; Hellner, J.; Giannini, C.; Xu, X.; Pauwels, J.; Ma, Q.; Dejonghe, W.; Han, H.; Cotte, B.V.d.; et al. Proteome-wide cellular thermal shift assay reveals unexpected cross-talk between brassinosteroid and auxin signaling. Proceedings of the National Academy of Sciences 2022, 119, e2118220119. [Google Scholar] [CrossRef]
- Vert, G.; Walcher, C.L.; Chory, J.; Nemhauser, J.L. Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proceedings of the National Academy of Sciences 2008, 105, 9829–9834. [Google Scholar] [CrossRef]
- Chaiwanon, J.; Wang, Z.-Y. Spatiotemporal Brassinosteroid Signaling and Antagonism with Auxin Pattern Stem Cell Dynamics in Arabidopsis Roots. Current Biology 2015, 25, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Sharma, I.; Pati, P.K. Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Frontiers in Plant Science 2015, 6. [Google Scholar] [CrossRef]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different Plant Hormones Regulate Similar Processes through Largely Nonoverlapping Transcriptional Responses. Cell 2006, 126, 467–475. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, Z.; Wang, X. The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc Natl Acad Sci USA 2009, 106. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, D. BRASSINOSTEROID INSENSITIVE2 Interacts with ABSCISIC ACID INSENSITIVE5 to Mediate the Antagonism of Brassinosteroids to Abscisic Acid during Seed Germination in Arabidopsis. The Plant Cell 2014, 26, 4394–4408. [Google Scholar] [CrossRef] [PubMed]
- Clouse, Steven D. Brassinosteroid/Abscisic Acid Antagonism in Balancing Growth and Stress. Developmental Cell 2016, 38, 118–120. [Google Scholar] [CrossRef]
- Steber, C.M.; McCourt, P. A Role for Brassinosteroids in Germination in Arabidopsis. Plant Physiology 2001, 125, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Kong, L.; Zhu, Y.; Pei, D.; Chen, X.; Wang, Y.; Qi, J.; Song, C.; Yang, S.; Gong, Z. BAK1 plays contrasting roles in regulating abscisic acid-induced stomatal closure and abscisic acid-inhibited primary root growth in Arabidopsis. Journal of Integrative Plant Biology 2022, 64, 1264–1280. [Google Scholar] [CrossRef]
- Seo, M.; Nambara, E.; Choi, G.; Yamaguchi, S. Interaction of light and hormone signals in germinating seeds. Plant Molecular Biology 2008, 69, 463. [Google Scholar] [CrossRef]
- Yang, X.; Bai, Y.; Shang, J.; Xin, R.; Tang, W. The antagonistic regulation of abscisic acid-inhibited root growth by brassinosteroids is partially mediated via direct suppression of ABSCISIC ACID INSENSITIVE 5 expression by BRASSINAZOLE RESISTANT 1. Plant, Cell & Environment 2016, 39, 1994–2003. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nature Reviews Molecular Cell Biology 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Li, Q.; Xu, F.; Chen, Z.; Teng, Z.; Sun, K.; Li, X.; Yu, J.; Zhang, G.; Liang, Y.; Huang, X.; et al. Synergistic interplay of ABA and BR signal in regulating plant growth and adaptation. Nature Plants 2021, 7, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dou, L.; Gong, Z.; Wang, X.; Mao, T. BES1 hinders ABSCISIC ACID INSENSITIVE5 and promotes seed germination in Arabidopsis. New Phytologist 2019, 221, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-W.; Youn, J.-H.; Roh, J.; Kim, J.-M.; Kim, S.-K.; Kim, T.-W. Brassinosteroids enhance salicylic acid-mediated immune responses by inhibiting BIN2 phosphorylation of clade I TGA transcription factors in Arabidopsis. Molecular Plant 2022, 15, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Busta, L.; Zhang, Q.; Ding, P.; Jetter, R.; Zhang, Y. TGACG-BINDING FACTOR 1 (TGA1) and TGA4 regulate salicylic acid and pipecolic acid biosynthesis by modulating the expression of SYSTEMIC ACQUIRED RESISTANCE DEFICIENT 1 (SARD1) and CALMODULIN-BINDING PROTEIN 60g (CBP60g). New Phytologist 2018, 217, 344–354. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shang, Y.; Joo, S.-H.; Kim, S.-K.; Nam, K.H. Overexpression of BAK1 causes salicylic acid accumulation and deregulation of cell death control genes. Biochemical and Biophysical Research Communications 2017, 484, 781–786. [Google Scholar] [CrossRef]
- Pan, G.; Liu, Y.; Ji, L.; Zhang, X.; He, J.; Huang, J.; Qiu, Z.; Liu, D.; Sun, Z.; Xu, T.; et al. Brassinosteroids mediate susceptibility to brown planthopper by integrating with the salicylic acid and jasmonic acid pathways in rice. Journal of experimental botany 2018, 69, 4433–4442. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, W.; Zhao, Q. Salicylic acid biosynthesis is not from phenylalanine in Arabidopsis. Journal of Integrative Plant Biology 2023, 65, 881–887. [Google Scholar] [CrossRef]
- Nakashita, H.; Yasuda, M.; Nitta, T.; Asami, T.; Fujioka, S.; Arai, Y.; Sekimata, K.; Takatsuto, S.; Yamaguchi, I.; Yoshida, S. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. The Plant journal : for cell and molecular biology 2003, 33, 887–898. [Google Scholar] [CrossRef]
- Shi, H.; Shen, Q.; Qi, Y.; Yan, H.; Nie, H.; Chen, Y.; Zhao, T.; Katagiri, F.; Tang, D. BR-SIGNALING KINASE1 Physically Associates with FLAGELLIN SENSING2 and Regulates Plant Innate Immunity in Arabidopsis The Plant Cell 2013, 25, 1143-1157. [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. 2020, 11. 11. [CrossRef]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Shine, M.B.; Yang, J.W.; El-Habbak, M.; Nagyabhyru, P.; Fu, D.Q.; Navarre, D.; Ghabrial, S.; Kachroo, P.; Kachroo, A. Cooperative functioning between phenylalanine ammonia lyase and isochorismate synthase activities contributes to salicylic acid biosynthesis in soybean. The New phytologist 2016, 212, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Action of jasmonates in plant stress responses and development — Applied aspects. Biotechnology Advances 2014, 32, 31–39. [Google Scholar] [CrossRef]
- Nahar, K.; Kyndt, T.; Hause, B.; Höfte, M.; Gheysen, G. Brassinosteroids Suppress Rice Defense Against Root-Knot Nematodes Through Antagonism With the Jasmonate Pathway. Molecular Plant-Microbe Interactions® 2012, 26, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Han, C.; Peng, W.; Huang, Y.; Peng, Z.; Xiong, X.; Zhu, Q.; Gao, B.; Xie, D. A Leaky Mutation in DWARF4 Reveals an Antagonistic Role of Brassinosteroid in the Inhibition of Root Growth by Jasmonate in Arabidopsis. Plant Physiology 2009, 151, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhai, Y.; Li, L.; Yang, Z.; Ge, X.; Yang, Z.; Zhang, C.; Li, F.; Ren, M. BIN2 negatively regulates plant defence against Verticillium dahliae in Arabidopsis and cotton. 2021, 19, 2097-2112. 19. [CrossRef]
- Hu, J.; Huang, J.; Xu, H.; Wang, Y.; Li, C.; Wen, P.; You, X.; Zhang, X.; Pan, G.; Li, Q.; et al. Rice stripe virus suppresses jasmonic acid-mediated resistance by hijacking brassinosteroid signaling pathway in rice. PLOS Pathogens 2020, 16, e1008801. [Google Scholar] [CrossRef]
- Dobrev, P.I.; Vankova, R. Quantification of Abscisic Acid, Cytokinin, and Auxin Content in Salt-Stressed Plant Tissues. In Plant Salt Tolerance: Methods and Protocols, Shabala, S., Cuin, T.A., Eds.; Humana Press: Totowa, NJ, 2012; pp. 251–261. [Google Scholar]
- Poudel, S.; Vennam, R.R.; Shrestha, A.; Reddy, K.R.; Wijewardane, N.K.; Reddy, K.N.; Bheemanahalli, R. Resilience of soybean cultivars to drought stress during flowering and early-seed setting stages. Scientific Reports 2023, 13, 1277. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).