Submitted:
16 August 2023
Posted:
18 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials
2.1. Contaminants
2.2. Sludge
2.3. Membrane
2.4. Acetic Acid (AA)
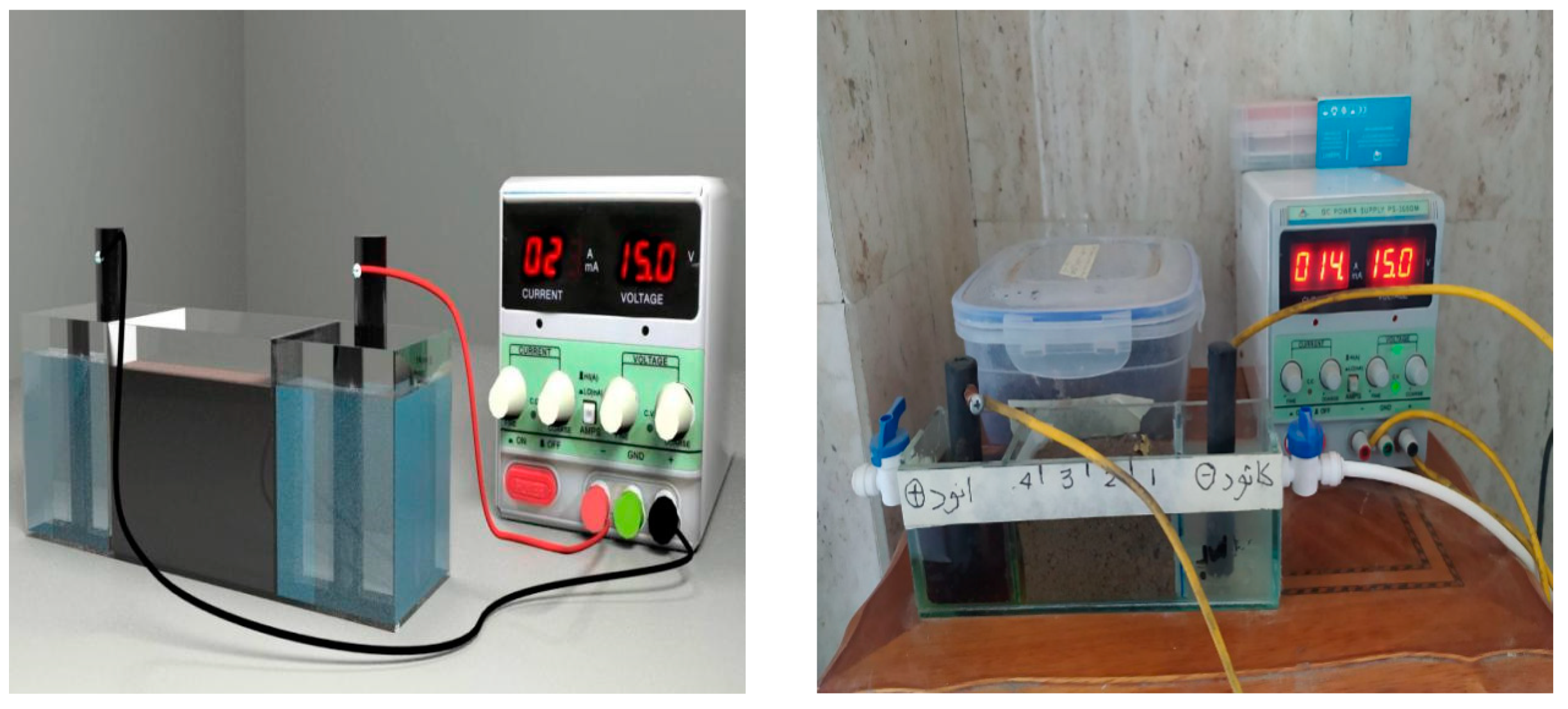
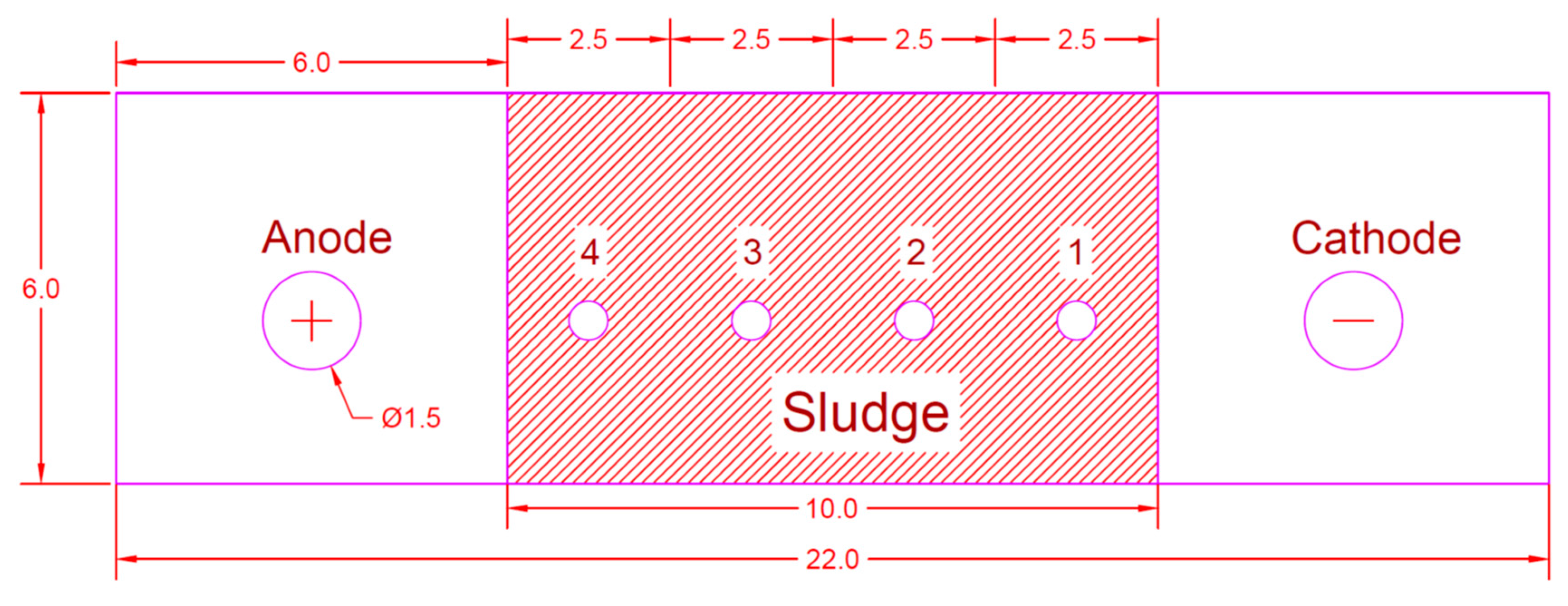
3. Experimental Setup
Analysis of Samples
4. Results and Discussion
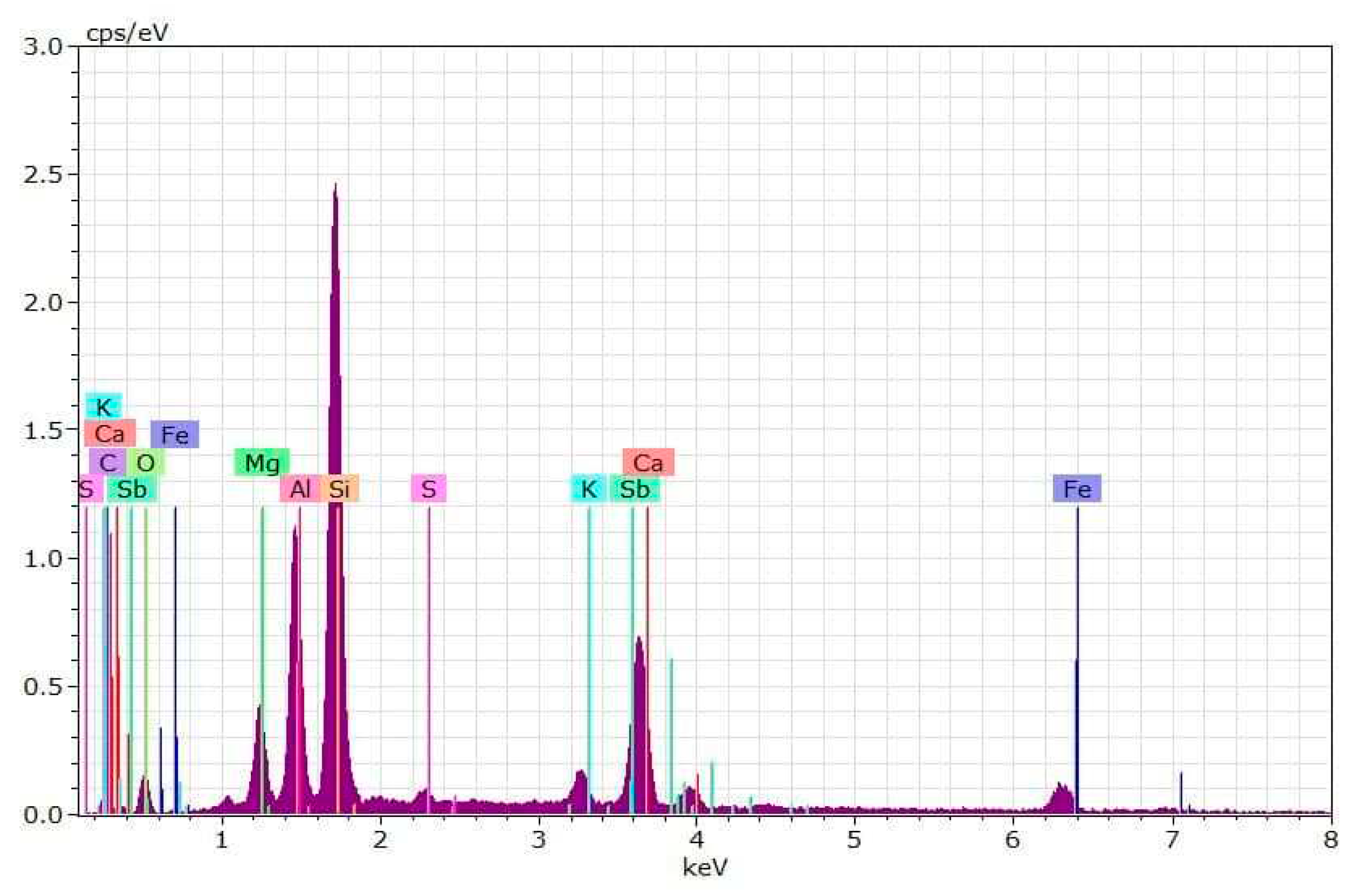
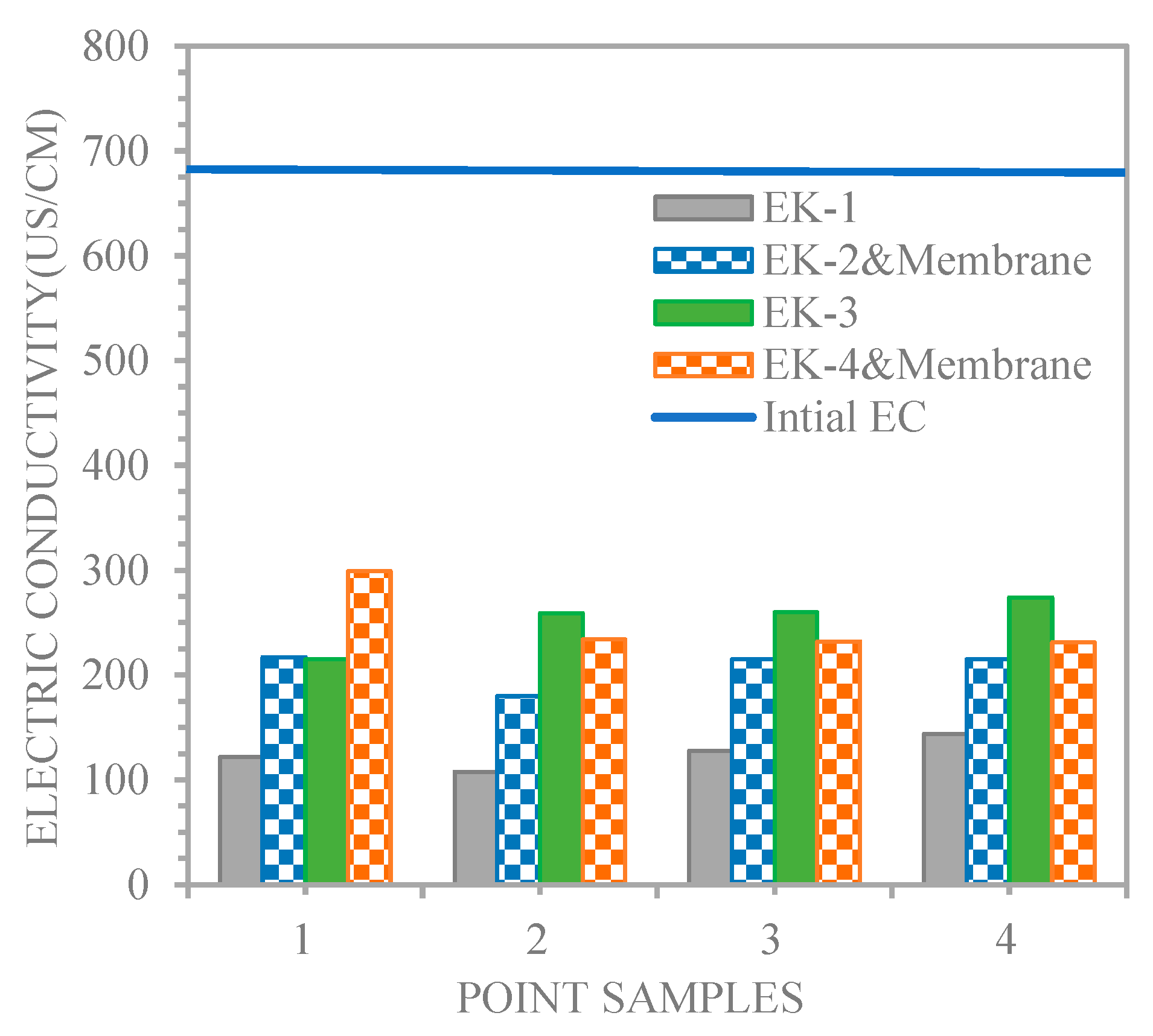
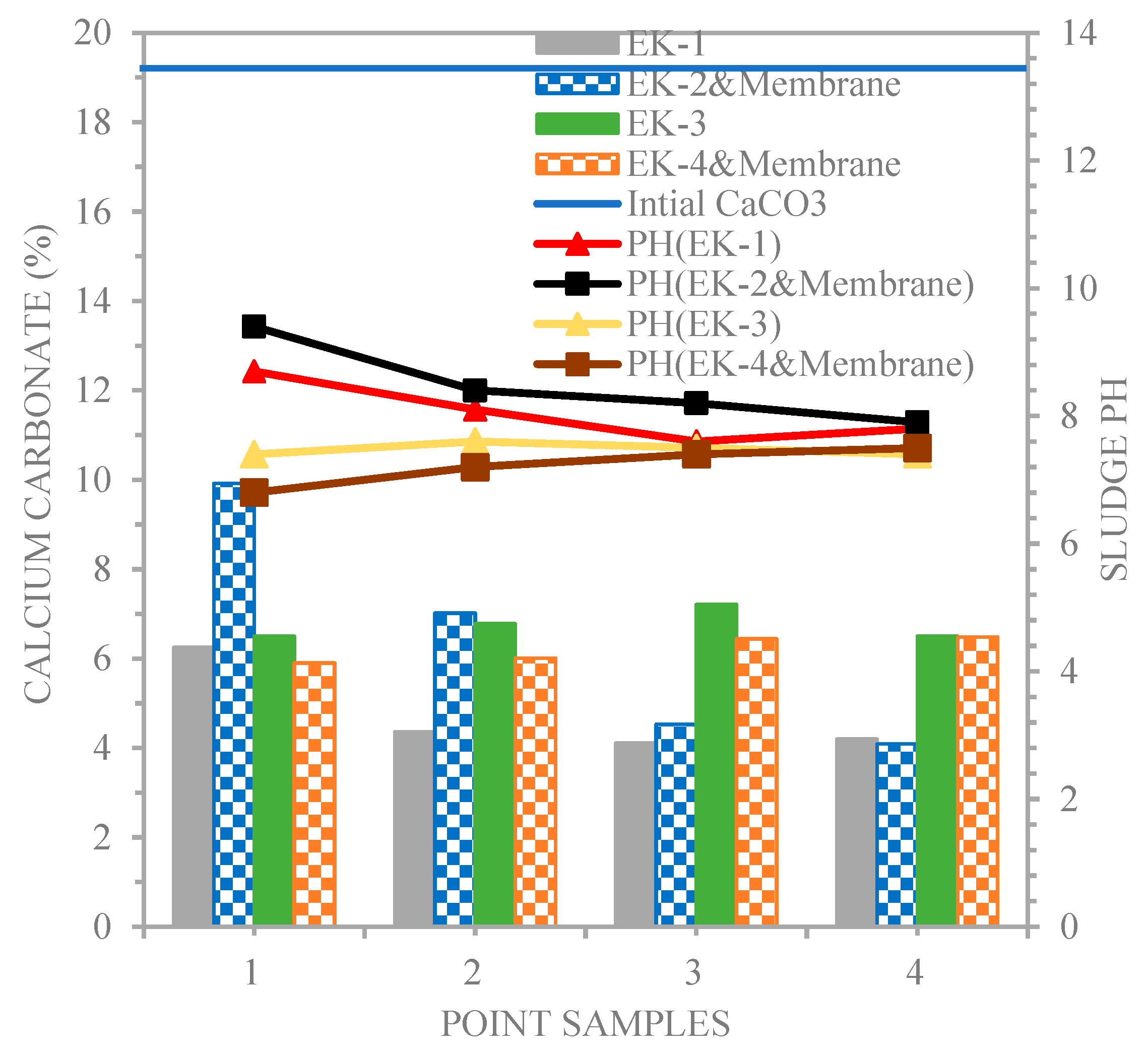
4.1. Results of Sludge Analyses
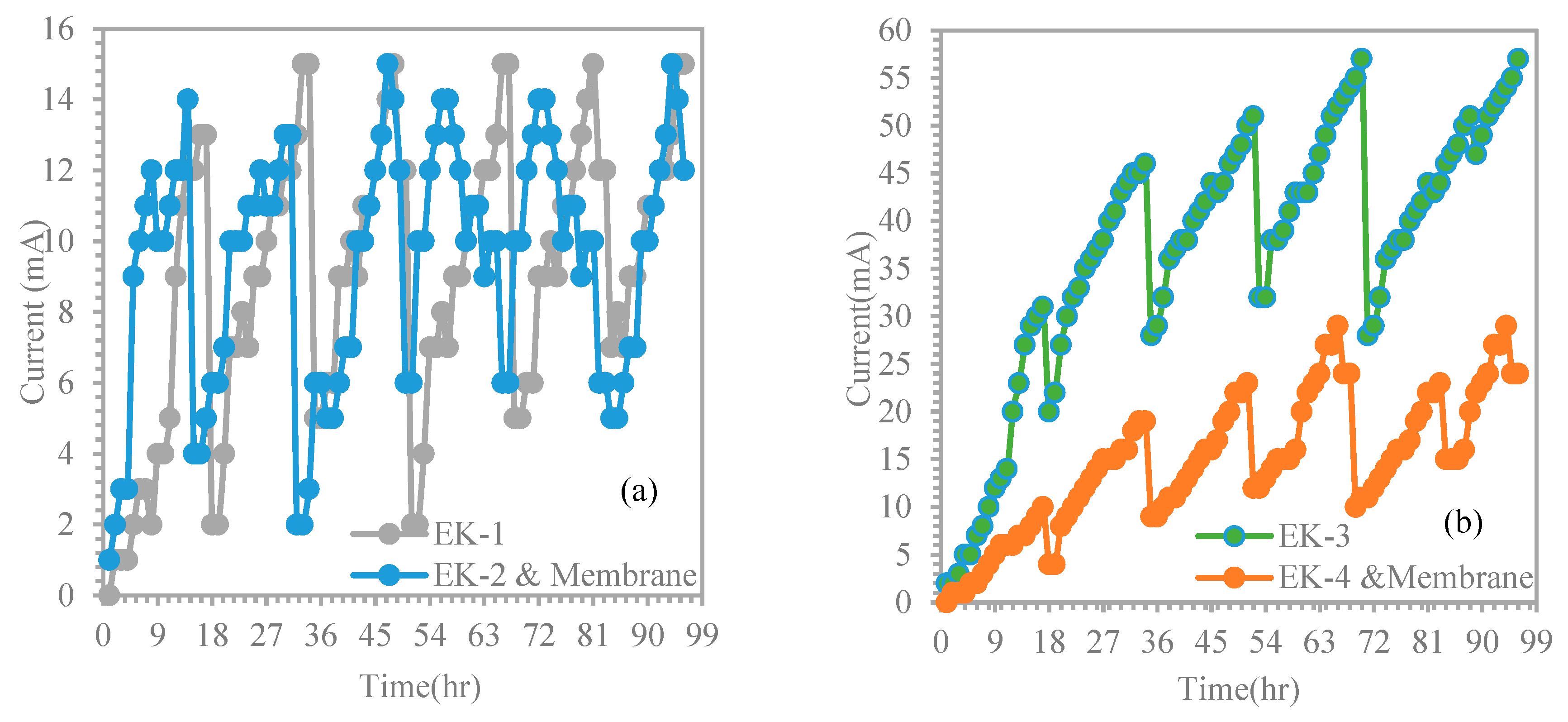
4.2. Unenhanced Condition Experiment
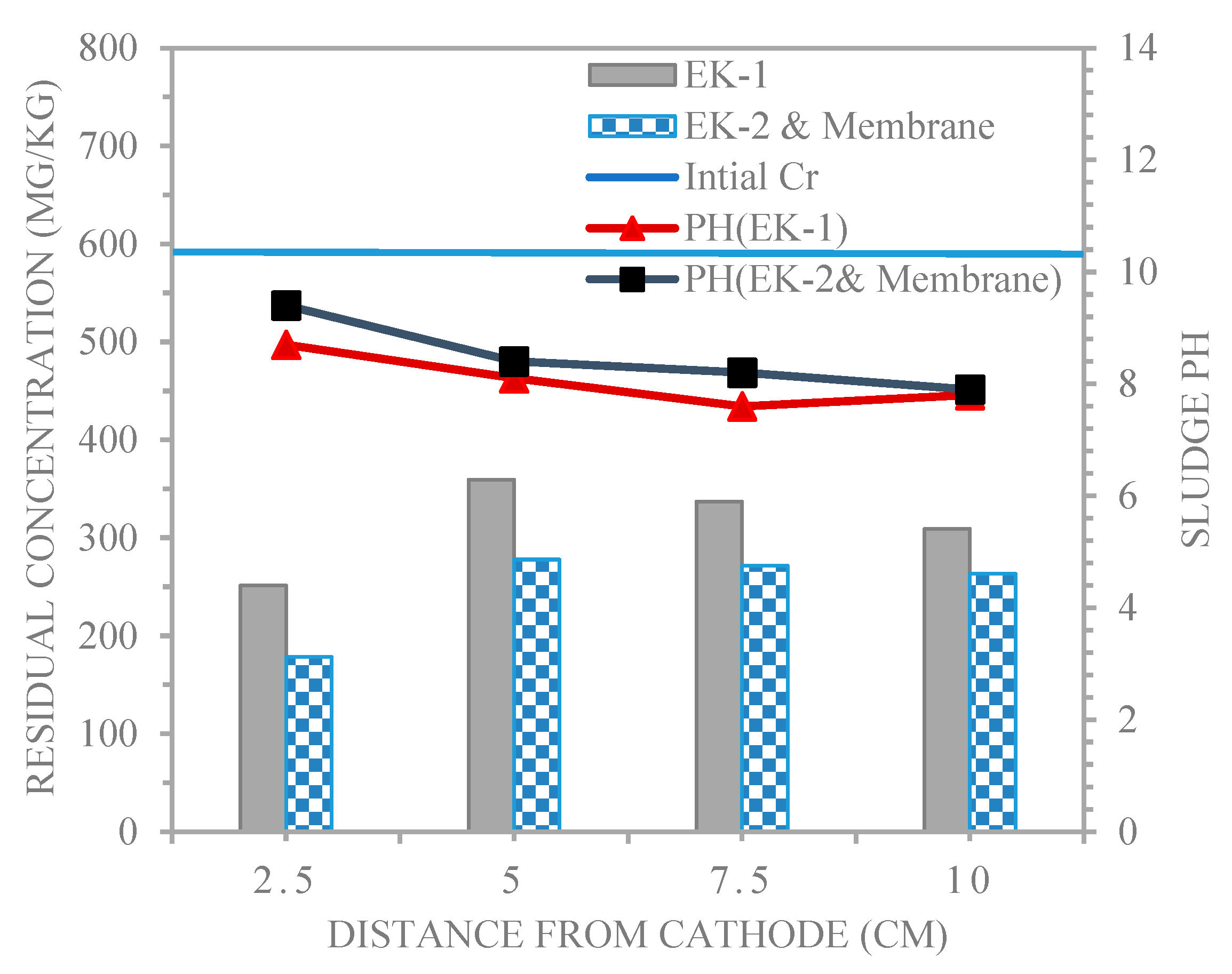
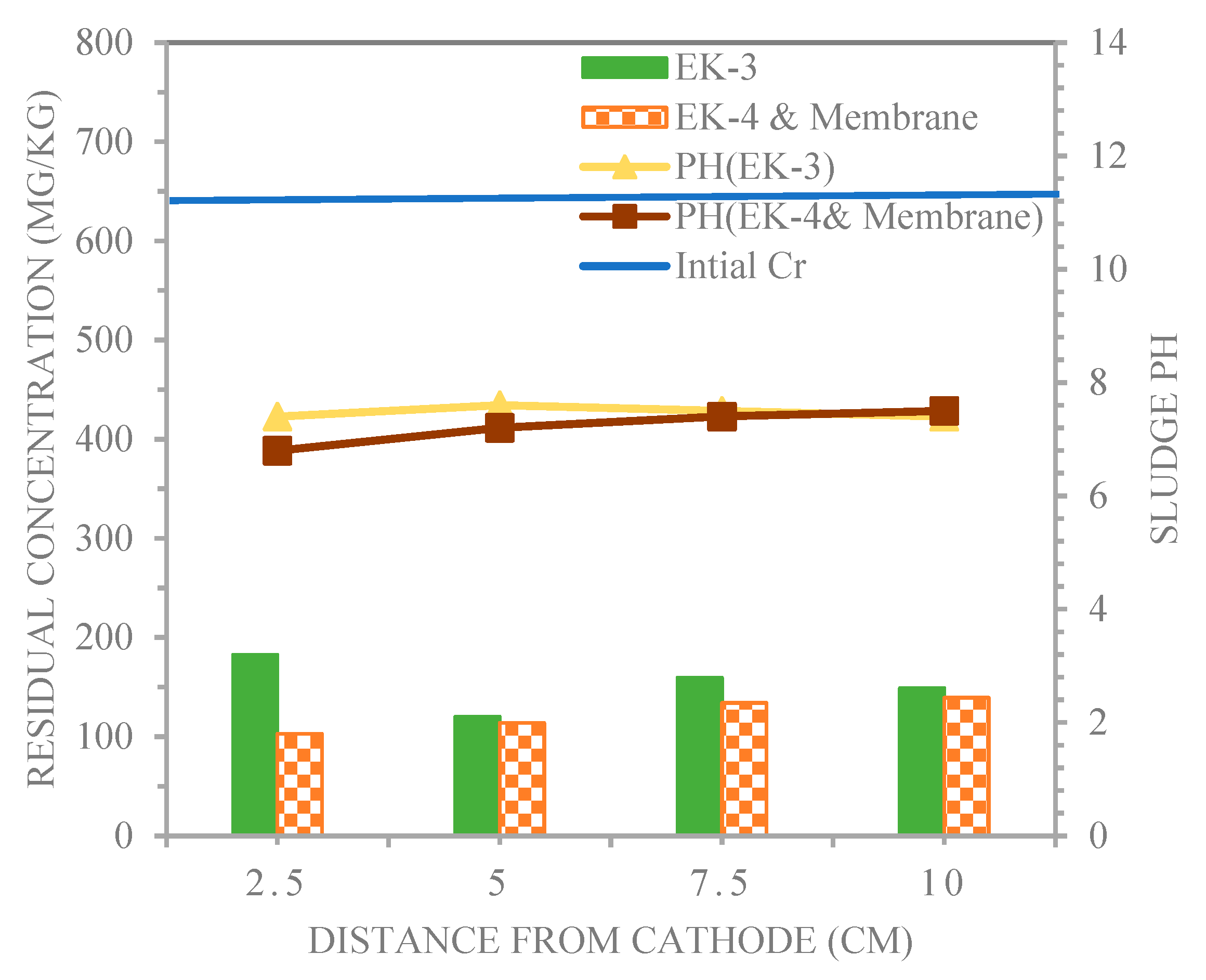
4.3. Distributions for pH and Chromium in the Electro kinetic Experiments.
5. Conclusions
- The Cr+3 removal efficiencies in the electro kinetic and membrane techniques were higher than those in the electro kinetic techniques conducted under the same purging solution conditions.
- The electro kinetic process offers an advantage by using the membrane technique, in which there is no accumulation of chromium at all the sampling points of all the experiments, and this is a success in itself.
- The membrane technique for acetic acid as a catholyte witnessed an excessively low pH of 6.8 in the EK-4 & Membrane system at point 1 sampling points in the remediation of chromium contaminated sludge. In addition to providing a higher removal efficiency by using the same acetic acid, the average removal efficiencies for the EK-3 and EK-4 &Membrane methods were 74.4% and 79.6% at the 1, 2, 3, and 4 sampling points, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acar, Y.B.; and Alshawabkeh, A.N. (1996). Electro-kinetic remediation. I: pilot-scale tests with lead-spiked kaolinite. Journal of Geotechnical Engineering, 122, 173-185. [CrossRef]
- Almeira, J.; Peng, C.; Wang, Z. Effect of different electrode configurations on the migration of copper ions during the electrokinetic remediation process. Asia-Pac. J. Chem. Eng. 2009, 4, 581–585. [Google Scholar] [CrossRef]
- Al-Khafaji, Z.S.; Jafer, H.; Dulaimi, A.; Atherton, W.; and Al Masoodi, Z. (2017). The Soft Soil Stabilisation Using Binary Blending of Ordinary Portland Cement And High Alumina Silica Waste Material, in The 3rd BUiD Doctoral Research Conference, the British University in Dubai, 13th May 2017, UAE. Dubai, 13th May 2017, UAE.
- Al-Khafaji, Z. S.; AL-Naely, H. K.; Al-Najar, A. E. A Review Applying Industrial Waste Materials in Stabilisation of Soft Soil. Electronic Journal of Structural Engineering 2018, 18, 2. [Google Scholar] [CrossRef]
- Delil, A. D.; and Köleli, N. (2018). The Removal of Pb and Cd from Heavily Contaminated Soil in Kayseri, Turkey by a Combined Process of Soil Washing and Electrodeposition. Soil and Sediment Contamination. 27(6): 469-484. [CrossRef]
- Faisal, A.A.H.; Hussein, A.A. An Acidic Injection Well Technique for Enhancement of the Removal of Copper from Contaminated Soil by Electro kinetic Remediation Process. Separation Science and Technology 2015, 50, 2578–2586. [Google Scholar]
- Faisal, A.A.H.; Rashid, I.T. Enhancement Solution to Improve Remediation of Soil Contaminated with Lead by Electrical Field. Journal of Engineering 2015, 21, 11. [Google Scholar] [CrossRef]
- Hayder, M. R.; Ayad, A. H. F. Removal of dissolved cadmium ions from contaminated wastewater using raw scrap zero-valent iron and zero valent aluminum as locally available and inexpensive sorbent wastes. Iraqi Journal of Chemical and Petroleum Engineering 2018, 19, 39–45. [Google Scholar]
- Hussain, A. J.; and Al-Khafaji, Z. S. (2020). Reduction of environmental pollution and improving the (Mechanical, physical and chemical characteristics) of contaminated clay soil by using of recycled oil. Journal of Advanced Research in Dynamical and Control Systems, 12(4 Special Issue), 1276–1286. [CrossRef]
- Kim, W.S.; Jeon, E.K.; Jung, J.M.; Jung, H.B.; Ko, S.H.; Seo, C.I.; and Baek, K. ( 2014). Field application of electro-kinetic remediation for multi-metal contaminated paddy soil using two-dimensional electrode configuration. Environmental Science Pollution Research, 21, 4482–4491. [CrossRef]
- Matloub, F. K.; Sulaiman, M. M. Investigating the effect of PH and salt Concentration on Cathodic Protection of Pipe- Lines. Technology 2018, 9, 474–480. [Google Scholar]
- Ottosen, L.M.; Larsen, T.H.; Jensen, P.E.; Kirkelund, G.M.; Kerrn-Jespersen, H.; Tuxen, N.; Hyldegaard, B.H. Electrokinetics applied in remediation of subsurface soil contaminated with chlorinated ethenese A review. Chemosphere 2019, 235, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.; Graves, A.R.; Burgess, P.J.; Van der werf, W.; and Herzog, F. (2007). Integrating environmental and economic performance to assess modern silvoarable agroforestry in Europe. EcologicalEconomics,Volume63,Issue4,Pages759-767. [CrossRef]
- Rashid, I.T. (2015). Improve Remediation of Soil Contaminated with Lead and Chromium by Electrical Field Technique. Ph.D., Thesis, Baghdad University, College of Engineering.
- RisingSun Membrane Technology (Beijing) Co., Ltd. Available online: www.risingsunmem.com.
- Rosul, N.; Amal, H. K. Resolving the accumulation effect of lead ions in the contaminated soil by electro-kinetic remediation. Solid State Technology 2020, 63, 1–16. [Google Scholar]
- Saeedi, M.; Li, L.Y.; and Gharehtapeh, M. (2013). Effect of alternative electrolytes on enhanced electrokinetic remediation of hexavalent chromium in clayey soil. Int. J. Environ. Res., 7(1):39-50. [CrossRef]
- Shen, Z.; Chen, X.; Jia, J.; Qu, L.; Wang, W. Comparison of electrokinetic soil remediation methods using one fixed anode and approaching anodes. Environmental Pollution 2007, 150, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Shucai, L.; Tingting, L.; Gang, L.; Fengmei, L.; Shuhai, G. Enhanced electrokinetic remediation of chromium contaminated soil using approaching anodes. Front. Environ. Sci. Eng. 2012, 6, 869–874. [Google Scholar]
- Sulaiman, M. M.; Matloub, F. K.; and Shareef, Z. N. (2018). Simulation and optimization of natural gas sweetening process: A case study of Ng sweeting unit designed by CHEN group in the Gulf of Mexico. AIP Conference Proceedings. American Institute of Physics, 2030(1), p. 20075. [CrossRef]
- Sun; Z.; Wu, B.; Guo, P.; Wang, S.; and Guo, S. (2019). Enhanced electrokinetic remediation and simulation of cadmium contaminated Lead-Contaminated Kaolinite and Natural Soils. CSAWAC 47 (4) · Vol. 47 · No. 4.
- Talib, A.N.A.; Tajudin, S.A.A.; Sunar, N.M. Copper Removal from Soil using EKR Technique. Journal of Applied Geoscience and Built Environmen 2019, 1, 1–6. [Google Scholar]
- Turer, D.; and Genc, A. (2005). Assessing effect of electrode configuration on the efficiency of electrokinetic remediation by sequential extraction analysis. Journal of Hazardous Materials B119 167–174. /: https. [CrossRef]
- Wan, Y.; Wang, A.; Shen, M. Restoration of Cadmium Contaminated Soil Using Approaching Anode Method of Polygonal Electrode. Ekoloji 2019, 28, 1041–1047. [Google Scholar]
- Zainab, O. Al Masoodi.; Zainab, Al Khafaji.; Hassnen, M. Jafer.; and Anmar Dulaimi, W. A. (2017) ‘The effect of a high alumina silica waste material on the engineering properties of a cement-stabilised soft soil’, in The 3rd BUiD Doctoral Research Conference. Dubai, AUE.
- Zhang, Z.; Ren, W.; Zhang, J.; and Zhu, F. (2019). Electrokinetic remediation of Pb near the e-waste dismantle site with Fe(NO3)3 as cathode electrolyte. Environmental Technology. [CrossRef]



| Property | Value |
|---|---|
| Particle size distribution Sand (%) Silt (%) Clay (%) |
1.1 63.5 35.3 |
| Cation exchange capacity (cmol.kg-1) |
32.87 |
| Porosity (%) | 51.69 |
| pH value | 8.26 |
| Electric conductivity, EC (μs/cm) | 1500 |
| Organic Matter (OMC %) | 5.34 |
| Calcium carbonate CaCO3 (%) | 25.18455 |
| Sulphate ions, SO4 (mg/l) | 0.00514 |
| Chloride ions, Cl-1 (mg/l) | 0.5998 |
| Total suspended solids, TSS (mg/l) | 600 |
| Model: CE2 | |||||
|---|---|---|---|---|---|
| Physical and Chemical Properties | U.S. Units | Metric Units | |||
| Functional group | Sulfonic acid | Sulfonic acid | |||
| Exchange capacity | min. | meq/g | 1.4 | ||
| Current density | max. | Ampere/ft2 | 50 | Ampere/m2 | 538 |
| Area resistance | ohm/cm | 0.1 N NaCl | 25 | 0.1 N NaCl | 25 |
| 1.0 N NaCl | 10 | 1.0 N NaCl | 10 | ||
| Permeslectivity | 0.5 N NaCl /1.0 N NaCl | 96 | 96 | ||
| Water permeability @5psi | max. | ml/hr/ft2 | 50 | ml/hr/ft2 | 538 |
| Mullen burst test | min. | psi | 150 | bar | 10.3 |
| Stability | pH range | 1-10 | 1-10 | ||
| Stability | max.temp | 176 | 80 | ||
| Dimensions | max.width | inches | 43 | meters | 1.09 |
| Dimensions | max.length | inches | 122 | meters | 3.1 |
| Dimensions | approx.thickness | mils | 20 | mm | 0.51 |
| Ionic form as shipped | Na+ | Na+ | |||
| Storability | of products | max.years | 2 | max.years | 2 |
| Storability | temp range | 40-75 | 4-24 | ||
| Series | Experiment Designation |
Conc. (mg/kg) |
Time (hr) |
PS (pH) | Electrodes Arrangement | Membrane | |
|---|---|---|---|---|---|---|---|
| Cathode | Anode | ||||||
| Series I | EK-1 | 599.8 | 96 | DW | DW | One Cathode + One Anode |
---- |
| Series II | EK-2 & Membrane | 599.8 | 96 | DW | DW | One Cathode + One Anode |
Membrane |
| Series III | EK-3 | 599.8 | 96 | AA | DW | One Cathode + One Anode |
---- |
| Series IIII | EK-4 & Membrane | 599.8 | 96 | AA | DW | One Cathode + One Anode |
Membrane |
| Sludge Samples | Chloride Ions (mg/l) | |||||||
|---|---|---|---|---|---|---|---|---|
| EK-1 | Ek-2&Membrane | EK-3 | Ek-4&Membrane | |||||
| Native Sludge | 0.5998 | Reduction (%) | 0.5998 | Reduction (%) | 0.5998 | Reduction (%) | 0.5998 | Reduction (%) |
| 1 | 0.0999 | 83.3 | 0.1999 | 66.7 | 1.1996 | -100 | 1.16 | -93.4 |
| 2 | 0.0999 | 83.3 | 0.4998 | 16.7 | 1.299 | -116.6 | 1.099 | -83.2 |
| 3 | 0.1599 | 73.3 | 0.4998 | 16.7 | 1.299 | -116.6 | 1.099 | -83.2 |
| 4 | 0.199 | 66.8 | 0.5990 | 0.13 | 1.399 | -133.2 | 1.1996 | -100 |
| Parameters | Points of samples(EK-1) | Points of samples (EK-2) & Membrane | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Initial Con. of Cr(II) | 599.8 mg/kg | |||||||
| Con. of Cr(II) mg/kg | 251.3 | 359.2 | 337 | 309.2 | 178.4 | 277.8 | 271.6 | 263.4 |
| Reduction (%) | 58.1 | 40.1 | 43.8 | 48.4 | 70.2 | 53.6 | 54.7 | 56.1 |
| Average (%) | 47.6 | 58.6 | ||||||
| Parameters | Points of Samples(EK-3) | Points of Samples (EK-4) & Membrane | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Initial Con. of Cr(II) | 599.8 mg/kg | |||||||
| Con. of Cr(II) mg/kg | 182.6 | 120.2 | 159.5 | 149.1 | 102.8 | 113.8 | 134.1 | 139.3 |
| Reduction (%) | 69.5 | 79.9 | 73.4 | 75.1 | 82.8 | 81 | 77.6 | 76.8 |
| Average (%) | 74.4 | 79.6 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




