1. Introduction
Heterocycles containing the 1,2,3-triazole fragment are active ingredients in a variety of drugs, including antibiotics (eg, cefatrizine and tazobactam). 1,2,3-triazole derivatives exhibit a variety of promising biological properties, including antibacterial, antituberculosis, antiviral, and anticancer properties [
1,
2,
3,
4,
5].
The diverse pharmacological activities of 1,2,4-triazoles as fungicides, antivirals, herbicides, and catalase inhibitors have led to deep interest in discovering new entities for their broader applications. There are many drugs based on 1,2,4-triazole in clinical use for the treatment of various diseases such as antifungals (fluconazole, itraconazole and voriconazole), antivirals (rifavirin)[
6,
7,
8,
9].
Therefore, the current work reports the synthesis and crystal structure of new heterocycle containing both 1,2,3-triazole and 1,2,4-triazole moieties using a simple procedure. Recently, the synthesis and structure elucidation of new heterocycles have been reported [
10,
11,
12,
13].
2. Results and Discussion
2.1. Synthesis
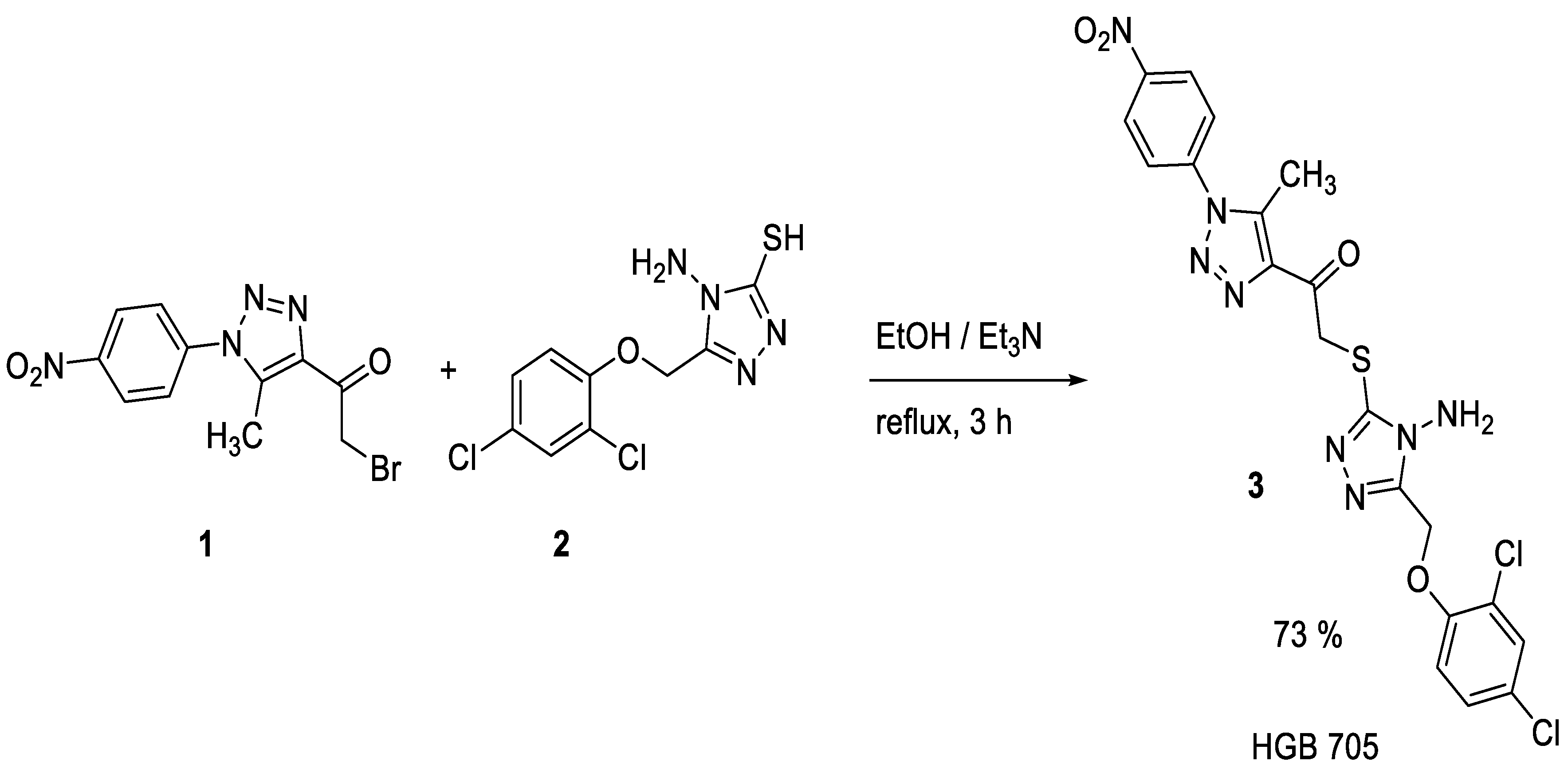
The heating under reflux conditions of 2-bromo-1-(5-methyl-1-(4-nitrophenyl)-1
H-1,2,3-triazol-4-yl)ethan-1-one (
1) with 4-amino-5-((2,4-dichlorophenoxy)methyl)-4
H-1,2,4-triazole-3-thiol (
2) in absolute ethanol in the presence of triethyl amine as catalyst gave 2-((4-amino-5-((2,4-dichlorophenoxy)methyl)-4
H-1,2,4-triazol-3-yl)thio)-1-(5-methyl-1-(4-nitrophenyl)-1
H-1,2,3-triazol-4-yl)ethan-1-one (
3) in 73% yield (
Scheme 1). Crystallization of the solid obtained from dimethylformamide (DMF) gave
3 in crystal form.
2.2. NMR Spectroscopy
The 1H NMR spectrum of 3 showed the both methylene protons (CH2) as a singlet signal at 4.95 and 6.10 ppm and the presence of a singlet signal that appeared at 5.30 ppm due to the NH2 protons. We cannot do the 13C NMR due to the poor solubility of product 3.
2.3. Crystal Structure Analysis
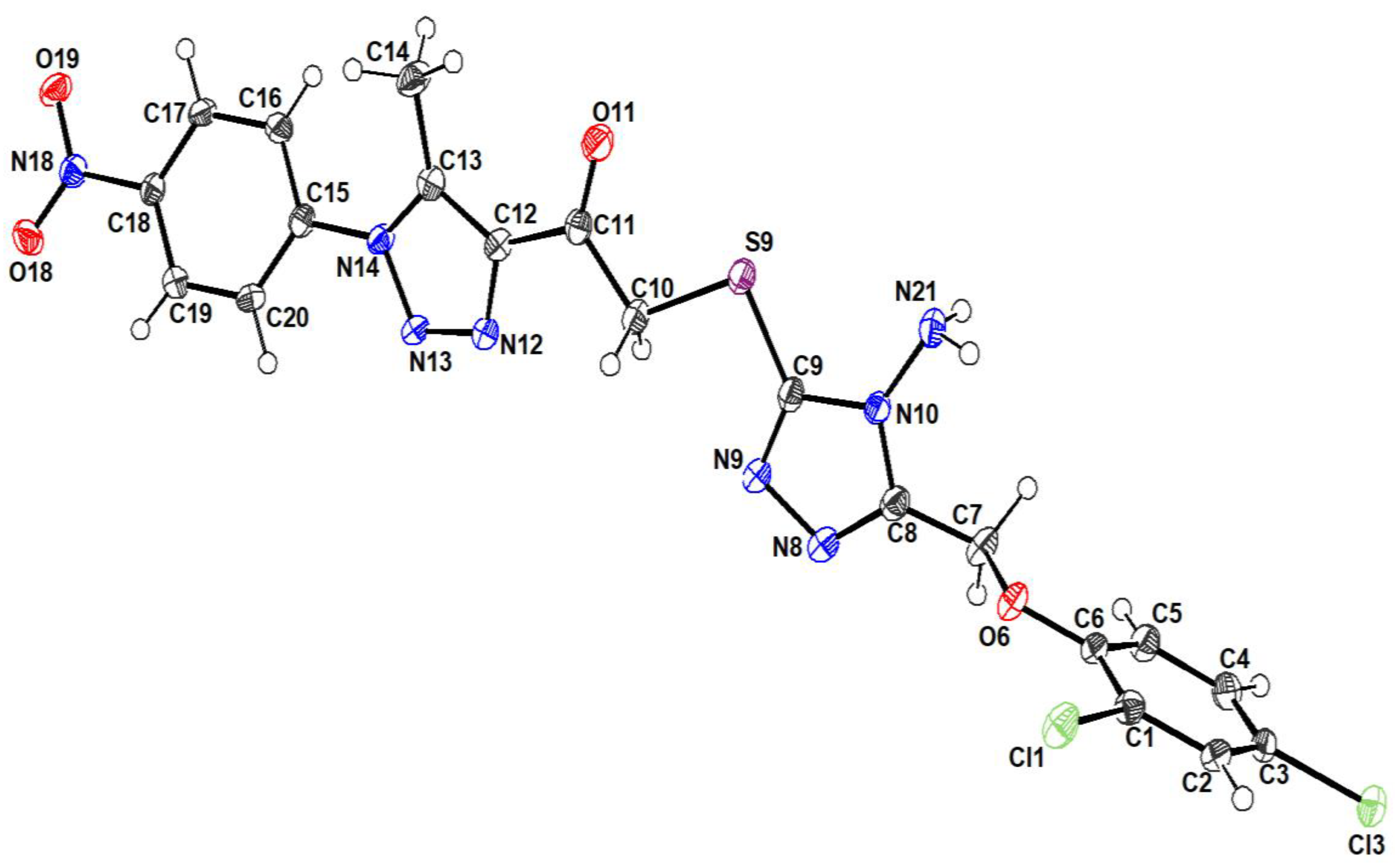
An ortep view of the geometry of the title structure can be seen in
Figure 1. The structure typically comprises two formulae in the unit cell and a monoclinic crystal system with the
P21/c space group. The molecule geometry is in good agreement with the reported standard bond distance [
14].
The constituents of the structure showed a planar appearance for each component separately. However, torsion angles between the consisting moieties give a non-planar appearance to the compound, such as C6/O6/C7/C8 (178.6°), N13/N14/C15/C20 (25.50°), and N9/C9/S9/C10 (5.76°). Also, the angle between the planes of the rings (C9 N9 N8 C8 N10) and (C5 C4 C3 C2 C1 C6) is 85°.
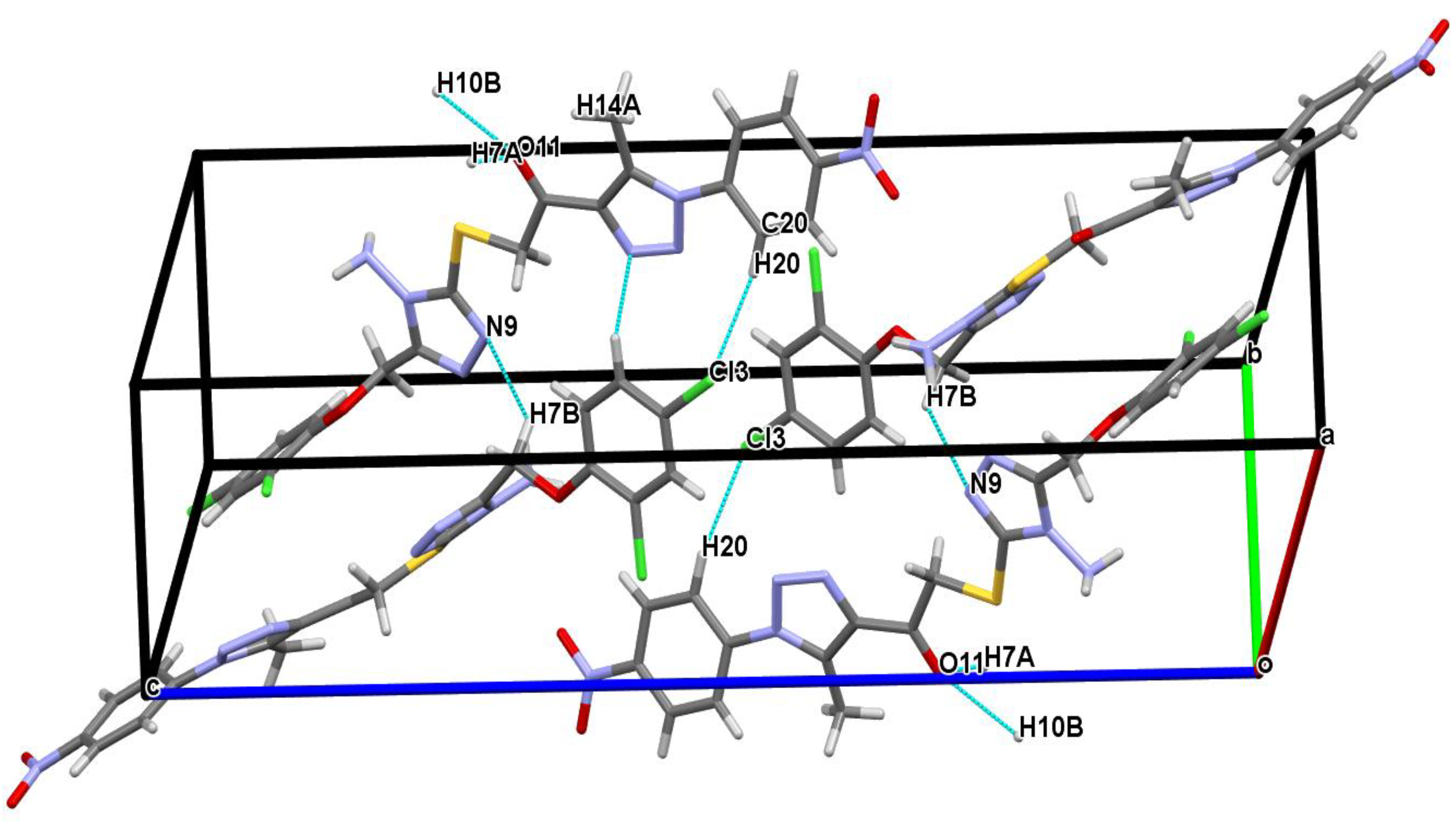
Figure 2 shows that the structure is stabilized by hydrogen bonds between the carbon atom and the nitrogen, oxygen, and chlorine atoms, as follows: C7—H7B---N9, C7—H7A--O11, C10—H10B---O11 and C20—H20---Cl3 together. Beautiful parallel networks with a wavy pattern could be seen in the crystal packing (
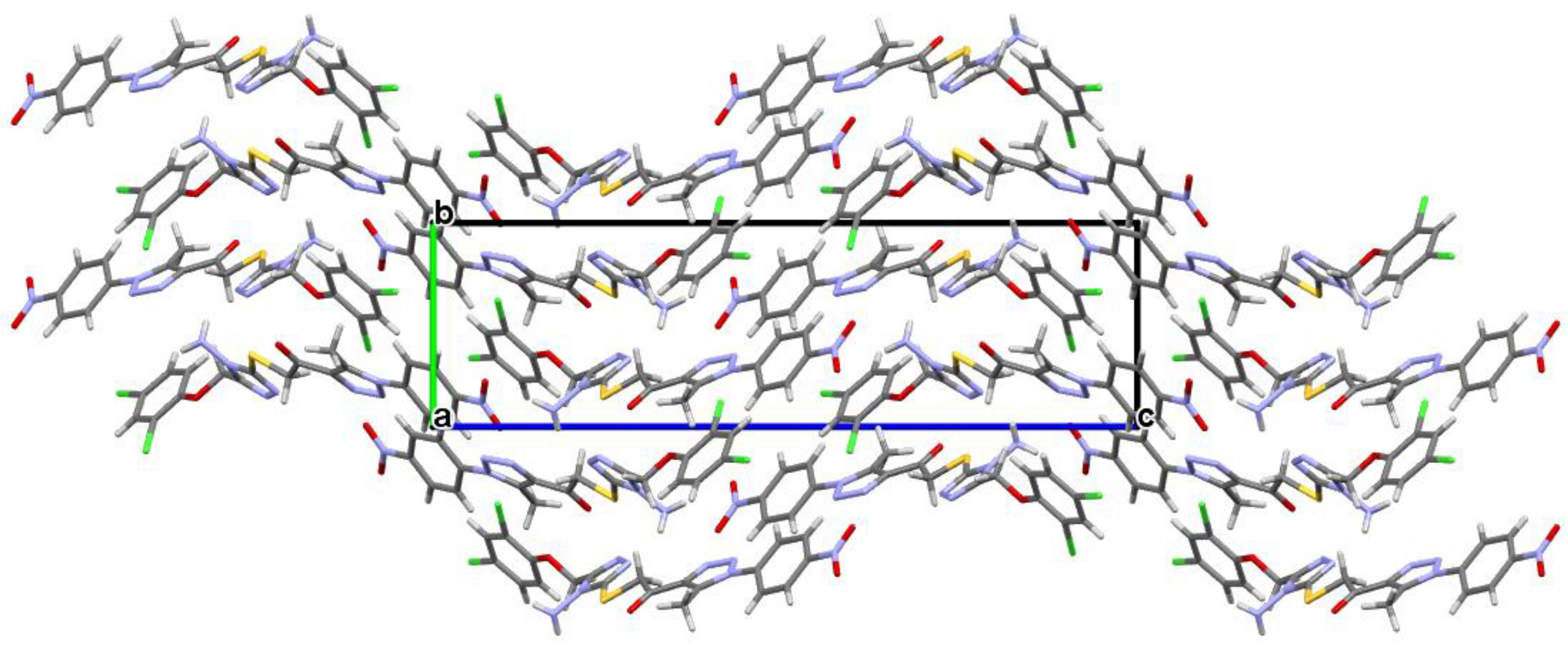
Figure 3).
3. Materials and Methods
3.1. General
Chemicals and solvents were obtained from Merck. The IR was recorded on Bruker Tensor 27 FTIR spectroscope. The NMR spectra (600 MHz) of
3 were performed on a Bruker NMR spectrometer. The chemical shift (δ) was reported in ppm. The NMR spectra were recorded in DMSO-d
6. Literature procedures were used to prepare
1 [
15] and
2 [
16].
3.2. Synthesis of 3
A mixture of 1 (0.65 g, 2.0 mmol), 2 (0.58 g, 2.0 mmol), triethyl amine (0.2 ml) in absolute ethanol (15 mL) was heated under reflux conditions for 3 h. Left to cool to room temperature, the colorless solid produced was collected by filtration. The product was washed with EtOH, dried, and recrystallized from DMF to give 3 in 73% yield, mp 230–23℃. IR: 3345 (NH2), 3171-2915 (CH), 1681 (C=O),1613-1596(C=N) cm–1. 1H NMR: 2.55 (s, 3H, Me), 4.95 (s, 2H, CH2), 5.30 (s, 2H, NH2), 6.10 (s, 2H, CH2), 7.40 (s, 2H, Ar), 7.69 (s, 1H, Ar), 8.04 (d, 2H, Ar), 8.50 (d, 2H, Ar). Anal. Calcd. for C20H16Cl2N8O4S (535.36): C, 44.87; H, 3.01; N, 20.93. Found: C, 44.93; H, 3.19; N, 20.99%.
3.3. X-ray Crystallography
A Bruker APEX-II CCD X-ray diffractometer (Mo X-ray tube) was used to collect data from a colorless plate crystal that had approximate dimensions of 0.286 x 0.274 x 0.046 mm3 [
17]. The structure was solved and refined using SHELXT [
18] and SHELXL [
19]. The structure was analyzed and graphically demonstrated by the crystallographic computer programs PLATON [
20], Mercury [
21], and ORTEP-3 for Windows [
22].
The full crystallographic details are included in the
Supplementary Materials (CIF and
Tables S1–S3). The data have been deposited in the Cambridge Crystallographic Data Centre (CSD) with the number CCDC 2288217.
4. Conclusions
A new heterocycle containing both 1,2,3-triazole and 1,2,4-triazole moieties has been synthesized with good yield using a simple procedure. The structure of the title heterocycle was established using X-ray single crystal diffraction and nuclear magnetic resonance spectroscopy.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. 1H NMR spectra, CIFs, and checkcif reports for heterocycle 3.
Author Contributions
Conceptualization: B.F.A-W. and A.A.F.; methodology: J.C.F. and A.F.M.; X-ray crystal structure. investigation: B.F.A.-W., A.F.M.. J.C.F., and A.A.F..; writing—original draft preparation: B.F.A.-W., A.F.M,. J.C.F., and A.A.F.; writing—review and editing: B.F.A.-W., A.F.M., J.C.F., and A.A.F.. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and the supplementary material.
Acknowledgments
We thank the National Research Centre and Cardiff University for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
A sample of the title compound is available from the authors.
References
- Liang, T.; Sun, X.; Li, W.; Hou, G.; Gao, F. 1,2,3-Triazole-Containing Compounds as Anti–Lung Cancer Agents: Current Developments, Mechanisms of Action, and Structure–Activity Relationship. Front. Pharmacol. 2021, 12, 661173. [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg Med Chem. 2019, 27, 3511-3531. [CrossRef]
- Khan, S.A.; Akhtar, M.J.; Gogoi, U.; Meenakshi, D.U.; Das, A. An Overview of 1,2,3-triazole-Containing Hybrids and Their Potential Anticholinesterase Activities. Pharmaceuticals 2023, 16, 179. [CrossRef]
- Kumar, S.; Khokra, S.L.; Yadav, A. Triazole analogues as potential pharmacological agents: a brief review. Futur J. Pharm. Sci. 2021, 7, 106. [CrossRef]
- H. A. Mohamed, M. S. Bekheit, E. F. Ewies, H. M. Awad, R. Betz, E. C. Hosten, B. F. Abdel-Wahab,Design of new hybrids indole/phthalimide/oxadiazole-1,2,3 triazole agents and their anticancer properties,Journal of Molecular Structure, Volume 1274, 2023, 134415. [CrossRef]
- Abdelli, S. Azzouni, R. Plais, A. Gaucher, M. L. Efrit, D. Prim, Recent advances in the chemistry of 1,2,4-triazoles: Synthesis, reactivity and biological activities, Tetrahedron Letters, Volume 86, 2021, 153518. [CrossRef]
- Yi-Nan Cheng, Zhen-Hua Jiang, Lian-Sheng Sun, Zi-Yang Su, Meng-Meng Zhang, Hong-Lian Li, Synthesis of 1,2,4-triazole benzoyl arylamine derivatives and their high antifungal activities, European Journal of Medicinal Chemistry, Volume 200, 2020, 112463. [CrossRef]
- Dai, J.; Tian, S.; Yang, X.; Liu, Z. Synthesis methods of 1,2,3-/1,2,4-triazoles: A review. Front. Chem., 2022, 10. [CrossRef]
- Kumari, M.; Tahlan, S.; Narasimhan, B. et al. Synthesis and biological evaluation of heterocyclic 1,2,4-triazole scaffolds as promising pharmacological agents. BMC Chemistry 2022, 15, 5. [CrossRef]
- Abdel-Wahab, B. F.; Mohamed, H. A.; Farahat, A. A.; Kariuki, B. M.; El-Hiti, G. A. Reactivity of 4-bromoacetyl-1,2,3-triazoles towards amines and phenols: synthesis and antimicrobial activity of novel heterocycles. Heterocycles 2022, 104, 1601–1613. [CrossRef]
- Abdel-Wahab, B.F.; Farahat, A.A.; Kariuki, B.M.; El-Hiti, G.A. (E)-1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethan-1-one Oxime. Molbank 2023, 2023, M1593. [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Farahat, A.A.; El-Hiti, G.A. Synthesis and structure determination of 1-(4-methoxyphenyl)-5-methyl-N’-(2-oxoindolin-3-ylidene)-1H-1,2,3-triazole-4-carbohydrazide. Molbank 2022, 2022, M1374. [CrossRef]
- Abdel-Wahab, B.F.; Mabied, A.F.; Fettinger, J.C.; Hassan, A.H.E.; Farahat, A.A. 2-((5-(5-Methyl-2-phenyl-1H-imidazol-4-yl)-1,3,4-oxadiazol-2-yl)thio)-1-phenylethan-1-one. Molbank 2023, 2023, M1666. [CrossRef]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Supplement. Tables of bond lengths determined by X-ray and neutron diffraction. Part 2. Organometallic compounds and co-ordination complexes of the d- and f-block metals. J. Chem. Soc. Dalton Trans. 1989, 12, S1–S83. [CrossRef]
- Bunev, A. S.; Trushkova, Yu. O.; Ostapenko, G. I.; Statsyuk, V. E.; Peregudov, A. S. Synthesis of 4-(1H-1,2,3-triazol-4-yl)-1,3-thiazole-2-amine derivatives. Chem. Heterocycl. Cpds. 2014, 50, 1027-1031. [CrossRef]
- Bano, Q.; Tiwari, N.; Giri, S.; Nizamuddin; Synthesis and fungicidal activities of 3-(aryloxymethyl)-6-substistuted 1,2,4- triazolo[3,4-b]-1,3,4- thiadiazoles. Indian J. Chem. 1992, 31B, 714-18.
- Bruker. APEX III; Bruker AXS Inc.: Madison, WI, USA, 2019.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [CrossRef]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Cryst. 2009, D65, 148–155. [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235.
- Farrugia, L.J.WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).