1. Introduction
Tuberculosis (TB) remains a problem of concern for public health worldwide, with drug resistance tuberculosis (DR-TB) adding to the problem. The initial treatment for TB is based on Isoniazid (INH) and rifampin (RIF)medications [
1,
2,
3,
4]. The largest concern for TB control worldwide is now DR-TB which is defined as TB that is resistant to the two most effective and commonly used first-line drugs, INH and RIF [
5,
6,
7,
8]. Resistance to first-line drugs for TB has been linked to various gene mutations, including
rpoB, katG, and inhA [
6]. The approach involving sharing knowledge between healthcare professionals (HCP) in healthcare facilities (HCF), diagnostic laboratories (DL), and research institutes (RI) can help to enhance a better understanding of the epidemiology of DR-TB.
According to other studies [
9,
10], there is increased interest in considering information sharing as a valuable resource for healthcare organizations, and TB management is at the center of this knowledge-sharing among health institutions. Knowledge management is defined by the World Health Organization (WHO) and other sources of evidence as "a set of principles, tools, and practices that enable people to create, share, translate, and apply knowledge to create value and improve effectiveness [
11,
12].” Knowledge exchange for improved management and the transmission of up-to-date healthcare management information to staff, patients, decision-makers, and other sectors have been the defining characteristics of collaboration across various health stakeholders, including HCP, HCF, and DL, for the effectiveness in TB. Additionally, it is essential to provide health information and counseling to the community so that HCP who interact with patients will have a better awareness of the condition and be able to provide high-quality medical treatment [
9,
10]. Studies conducted elsewhere have shown that fostering a culture of information sharing inside companies is crucial for the success of health institutions and the people who use them by boosting intellectual capital, cutting costs, and boosting competitiveness [
9,
10,
11,
12]. To advance their expertise and deliver evidence-based healthcare, health professionals require current health information from reliable sources. Medical personnel in resource-constrained nations have a history of not sharing their information and experiences with one another, which has led to a variety of medical blunders, hence the idea of mapping this model between these health stakeholders (HCF, HCP and DL). Based on information from HCF, DL, and RI an information system should be established to facilitate data storage as part of the strategy to build TB epidemiological information in rural areas of Eastern Cape. When HCF, DL, and RI work collaboratively there will be a storage of data quality which will be comprised of data completeness, which is the overlooked gap due to not knowing the importance of how completeness of data entry by collaborating organizations in the information system is important. The insufficient completeness of data makes it difficult to evaluate the actions to be taken and the epidemiological profile of affected areas, making it challenging to make decisions and develop disease control plans. A comprehensive strategy that incorporates healthcare facilities, diagnostic services, and research services is required to increase the inadequate understanding of TB transmission in rural parts of the Eastern Cape. The multisectoral cooperation between these parties will also be economically advantageous in terms of resource allocation, staff motivation, and readily available information sources on interventions to enhance the issue of TB management in the research region.
2. Materials and Methods
We set out to map a model for management of drug resistant tuberculosis in rural areas of Eastern Cape using information of gene mutation, genotypes of DR-TB isolates, distribution of M. tuberculosis mutations and spoligotypes and factors associated with treatment outcomes of M. tuberculosis. Model information is based on three categories:
2.1. Category 1
Gene mutations and the genotypes of DR-TB (mono DR-TB, MDR-TB and XDR-TB) in the rural Eastern Cape Province that were identified by using different assays. From patients who were suspected to be having TB, their 1157 sputum samples were collected and analyzed. These analytic methods identified regions of DR-TB mutations, heteroresistance, and genetic diversity [
13].
2.2. Category 2
Distribution of M. tuberculosis mutations and spoligotypes was investigated to identify transmission hotspots of DR-TB. The identification of geographical areas with a high incidence of disease was done by analyzing Line probe assay (LPA) and spoligotype results of M. tuberculosis isolates using the QGIS 3.14 software. LPA score and banding patterns were used to determine the type of DR-TB, gene mutations and heteroresistance. Clinics within hospitals with the same coordinates were merged in the analysis [
14].
2.3. Category 3
The investigation of treatment outcomes and associated factors among tuberculosis patients was done using the ambidirection method where clinic records from 457 patients with DR-TB were examined for data collection while 101 patients were followed up prospectively until the end of treatment [
15]. The data collected included socio-demographics, clinical data, and treatment outcomes data were analyzed using Stata version 17.0. The odds ratio and 95% confidence interval were calculated to check the association between variables where p ≤ 0.05 was considered statistically significant [
15].
2.4. Categories Key Points for Model Mapping
The key points used to map the model for spread of DR-TB are:
2.4.1. Gene Mutation and Regions of Mutations
Analysis of the RIF-associated mutations revealed a prevalence of the rpoB gene which is known to be a hot spot of resistance in M. tuberculosis. The region of rpoB S531L was the most prevalent with mutations and is associated with major MDR-TB outbreaks in many parts of the world. Most study samples exhibited a pronounced dominance of mutations within the rpoB codon 531 that is known as a marker of RIF resistance [
16]. The katG gene's codon 315 mutations are primarily responsible for INH resistance. Numerous investigations conducted all around the world revealed the same pattern of relationship between the S315T mutation and high levels of INH resistance. One of the main mechanisms of INH resistance in MTB is the katG gene mutation [
16].
2.4.2. Heteroresistance
Heteroresistance occurs when resistant and susceptible strains of an infection infect a patient at the same time [
17,
18], and is known as a precursor to full resistance or low levels of drug-resistant TB. Studies by Faye et al., [
13] it was observed as it is increasing yearly in rural areas of Eastern Cape. The results of treatment could be negatively impacted by heteroresistance as it can develop when drug-resistant individuals spread susceptible and resistant M. tuberculosis strains to untreated cases or newly infected patients. Given that MDR isolates are known to be more likely to carry katG S315T mutation than non-MDR strains [
19,
20], it was discovered that katG gene had more heteroresistant strains than rpoB and InhA genes, which can result from mixed infection or clonal heterogeneity and is thought to be the first step toward total resistance, shows that INH in this region is beginning to exhibit full resistance.
2.4.3. Type of Lineage
The Beijing family is associated with drug resistance in some parts of the world [
27] and is also known to be more transmissible than other families and more prevalent in Eastern Cape and Western Cape [
22,
23]. In addition, Faye et al., study findings have confirmed this high prevalence in the rural Eastern Cape as well [
13,
14]. Other studies elsewhere have also confirmed this high prevalence in other provinces of South Africa such as Western Cape, Limpopo, and Mpumalanga [
35,
36]. Studies by Ameeruddin and Luke [
34], Ano et al. [
25], and Gagneux et al. [
33], the katG-S315T mutation has a low-fitness cost, spread to Beijing strains and other strains, and is more likely to be clustered. Treatment failure is positively correlated with katG mutations [
27].
2.4.4. HIV-TB Coinfection
According to Faye et al., [
15] the rate of co-infection of TB and HIV was high with men being mostly infected and resulting in undesirable treatment outcomes in rural areas of Eastern Cape. Tuberculosis and HIV/AIDS are the major public health problems in many parts of the world particularly in resource-limited countries like South Africa. It has been a long time ago having proved that the double burden of TB and HIV is one of the major global health challenges [
28,
29,
30]. TB is the leading immune-suppressing infection and the commonest cause of death among HIV-infected patients [
22,
23,
31] WHO estimated that patients living with HIV are at 20 times higher risk of acquiring TB than their counterparts [
1,
2,
3,
30,
31].
2.4.5. Treatment Outcomes
The treatment success rate (65.8%) was lower than the WHO threshold standard with a high proportion of patients being lost to the follow-up. The co-infection of TB and HIV resulted in undesirable treatment outcomes in rural areas of the Eastern Cape [
15].
One of the most efficient and targeted medications for the treatment of the disease brought on by M. tuberculosis is INH. It is a key component of current short-course chemotherapy for tuberculosis and is frequently used to treat latent MTB infection (LTBI) to halt the progression of the infection to active disease and the consequent spread of TB. A typical first stage in the transition to MDR is the development of INH resistance [
16]. Treatment success rate (TSR) is a critical factor in the global End TB strategy. With 76% national TSR in South Africa, the country still falls short of the standard set by WHO, the global health body [
30].
The model key points recommended technical approach and justification are detailed in
Table 1
3. Discussion
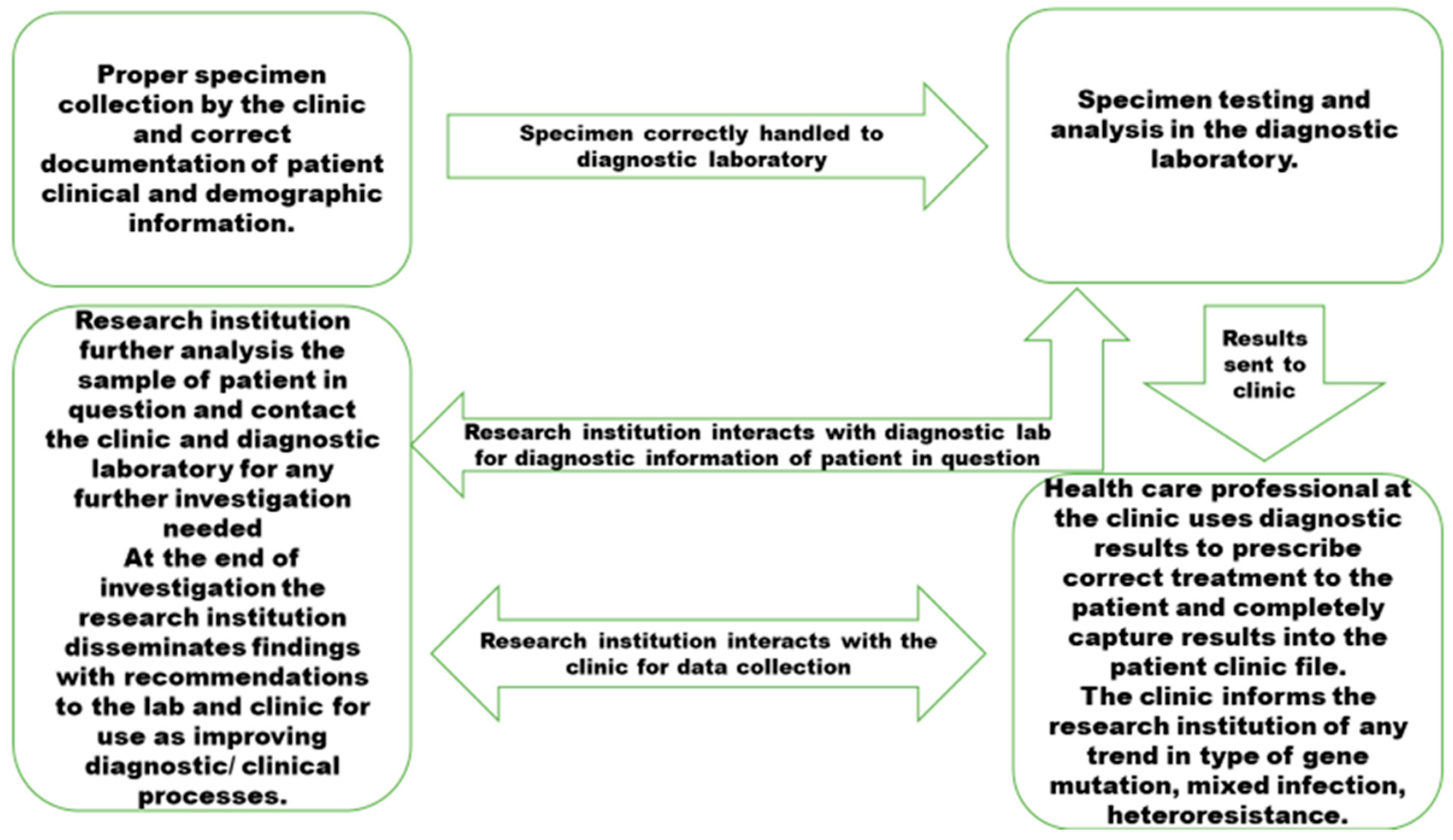
Model mapping for management and spread of DR-TB gene mutations and lineages in rural Eastern Cape needs quality pre analytical and post analytical processes which are well communicated among institutions responsible for TB management,
Figure 1.
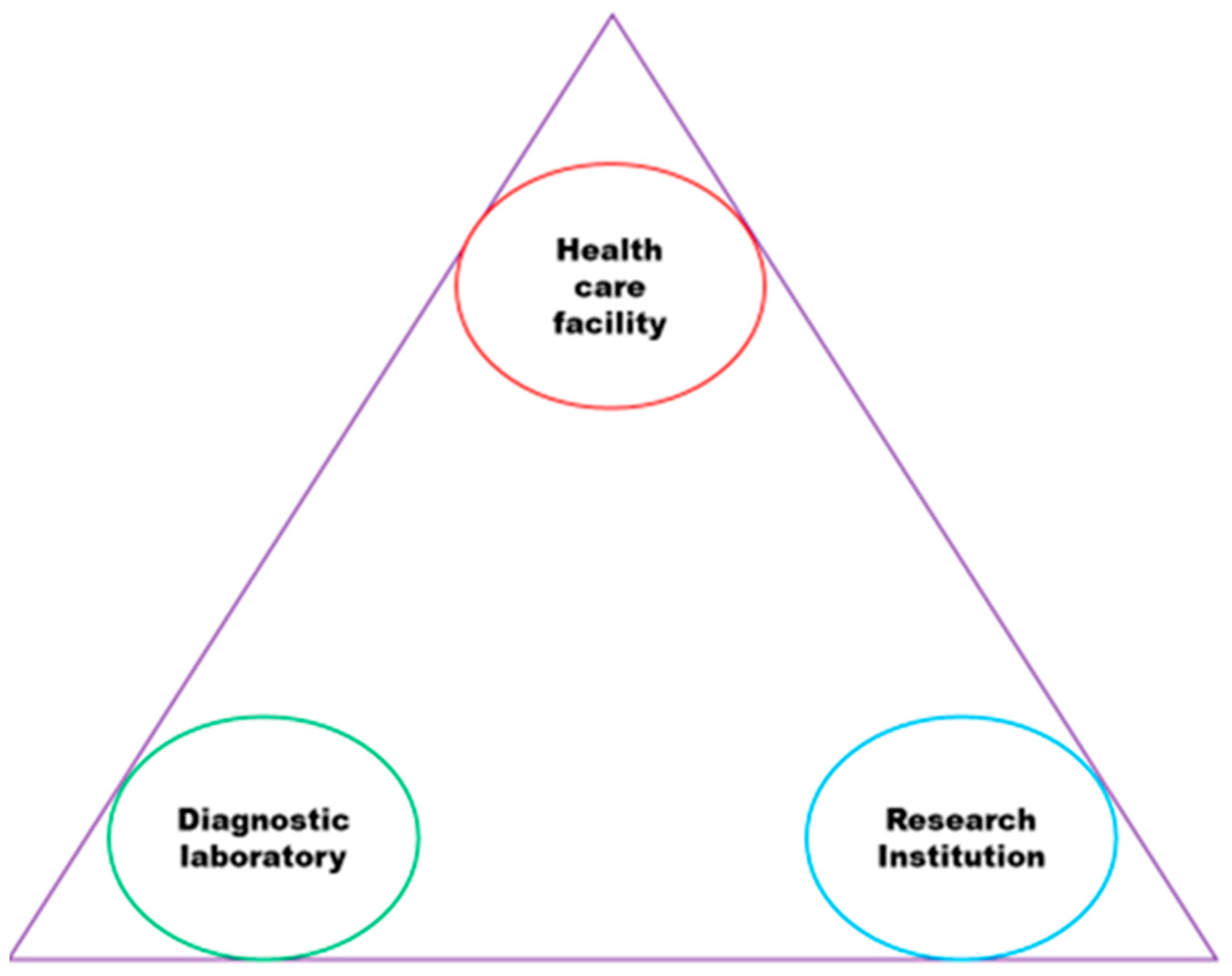
The institutions shown in
Figure 2 were involved in the three categories of this study and have been observed to have a weak connection, which negatively impacts health programs intended to strengthen TB control and reduce drug resistance. Effective sharing of TB information between DL, RI, and HCF through collaboration can enhance the information that HCP provide to patients in a way that patients can understand.
The increasing number of drug-resistant TB cases, heteroresistance, and treatment failures in the study area is a major concern. This is likely to be due to the lack of a collaborative approach to tackling TB among HCF, DL, and RI. Therefore, it is crucial to establish cooperation between HCF, DL, and RI to address these issues as it requires multidisciplinary professionals to work together with the institutions depicted in
Figure 2 to effectively analyze DR-TB mutations and strains for TB control. To achieve comprehensive TB control, all healthcare organizations (HCF, DL, and RI) must be included in the effort, as outlined by the World Health Organization's (WHO) Global Report [
30]. The collaborators to ensure that disease updates are precise must establish a strict system for entering quality data as the failure to provide complete and quality data may result in challenges in accurately characterizing the true epidemiological state of TB [
32,
33,
34,
35,
36,
37]. To effectively monitor and manage the spread of DR-TB gene mutations and strains, it is necessary to do spatial analysis at least every three months using data entered by the collaborators in the system. Failure to do so can lead to misinterpretation of the disease's epidemiological situation and case follow-up due to incomplete disease notification instruments and follow-up procedures [
32,
33,
34,
35,
36,
37]. Lesson learnt from Covid-19 pandemic is that there is a need of collaborative TB management preparedness for any pandemic that can be experienced so that interruption on TB control and management is not compromised. Before the emergence of SARS-CoV-2, tuberculosis (TB) was the biggest infectious killer of humans. An projected 1.6 million persons died from TB worldwide in 2021, with the majority of deaths taking place in low- and middle-income nations. (LMICs) [
38]. An estimated 10 million people contracted TB before the COVID-19 epidemic in 2019. Among them, 7.1 million people received diagnoses and notifications, leaving 2.9 million people without diagnoses. The pandemic had a terrible impact on TB services, increasing the diagnosis gap to 4.2 million cases [
39]. Only 6.4 million of the estimated 10.6 million TB cases were diagnosed and notified to national TB programs globally in 2021.
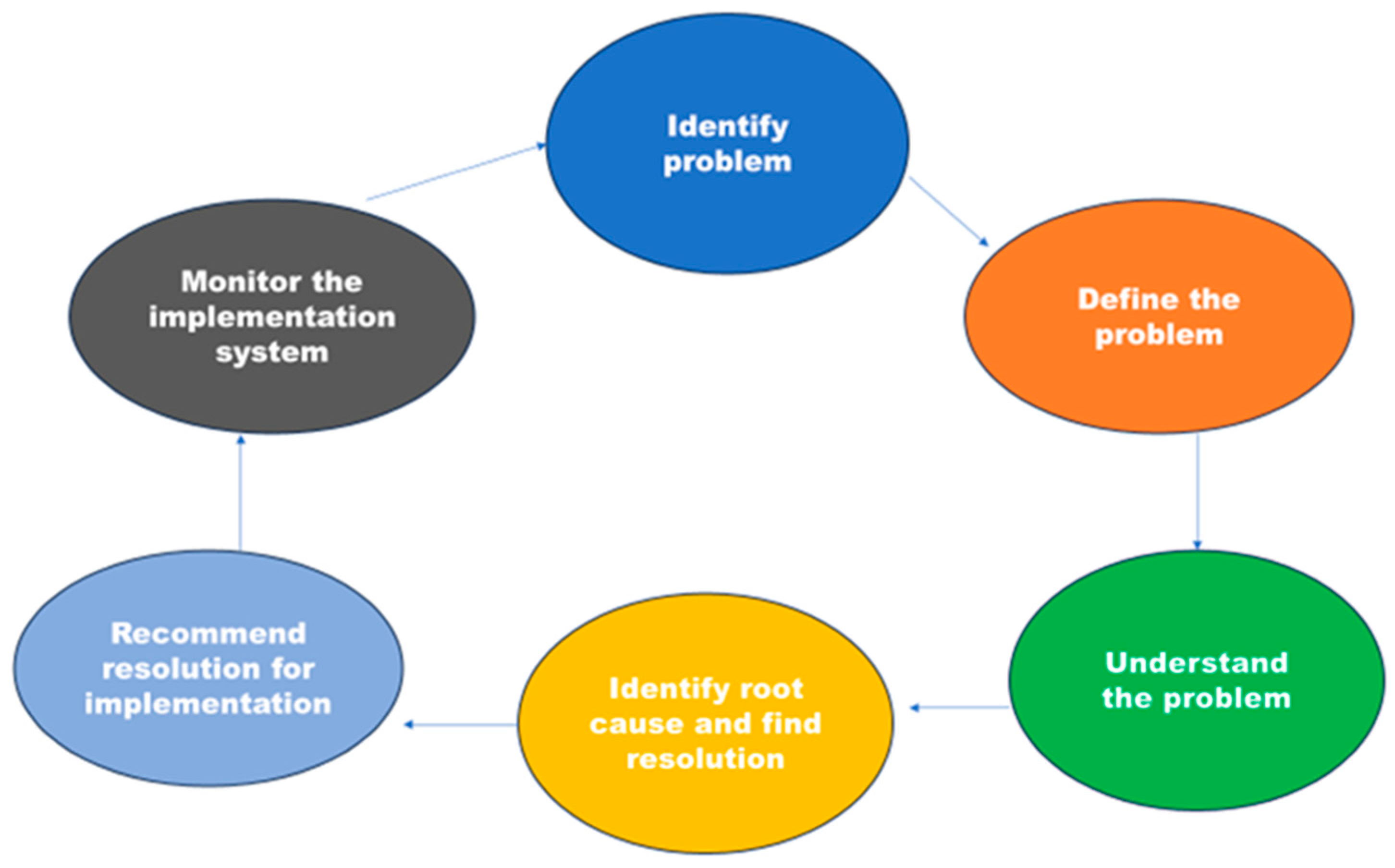
The focus is on tackling the risks posed by DR-TB genetic mutations and strains in rural regions. These areas have been pinpointed as the primary sources of the TB epidemic in rural Eastern Cape [
13,
14,
15]. It is important to regularly evaluate existing systems and note trends in DR-TB using key points, as shown in
Figure 3. This allows opportunities to refine and adapt to the changing epidemiology of DR-TB, as well as incorporate new advances in DR-TB control. However, collaboration for enhancing DR-TB control has been less emphasized in rural areas. Thus, this model illustrates how collaboration practices can influence the implementation of standardized TB control programs in different locations.
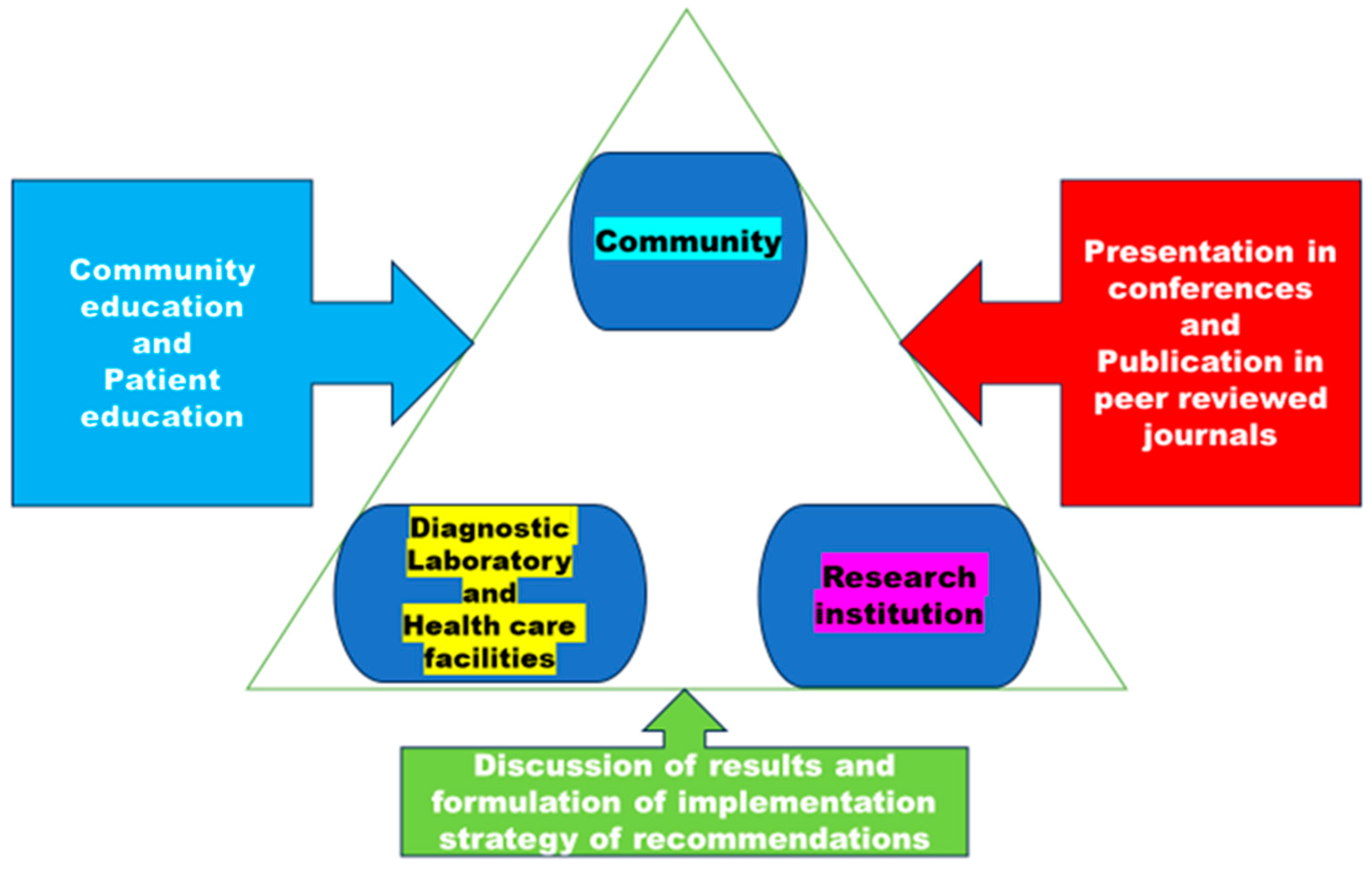
Despite the availability of effective treatments since the 1980s, tuberculosis remains a significant global public health concern which requires proper management of information regarding DR-TB. Proper dissemination of information from laboratories and healthcare professionals will assist HCP to gain a better understanding of the disease's epidemiology through deep learning and data analysis, while patients will receive a more comprehensive education. In
Figure 4, there is a communication plan that emphasizes the importance of managing knowledge between three collaborators.
Policymakers can utilize this model as a concept demonstrator to aid in making decisions based on analytical evidence when managing the spread of DR-TB, gene mutations, lineages and treatment outcomes in rural areas of the Eastern Cape. Mapping a customized models is crucial for representing individual communities due to unique factors that influence disease spread within them, this emphasizes the significance of having tailored models for the effective management of TB in rural areas of Eastern Cape. The HCF provides clinical care for a large number of tuberculosis patients, while research facilities continue to investigate the provide information on
M. tuberculosis received from DL. While all three collaborators will participate in the same intervention, their ability to exchange knowledge and implement improvements to integrate services will vary. Research conducted in different locations has shown that incorporating a collaborative knowledge translation framework, along with providing resources like facilitation and distributed leadership, within a transdisciplinary team consisting of research institutions, healthcare workers, and laboratories, can enhance collaboration and aid in achieving transdisciplinary research goals [
35]. TB is an important disease to model for identifying intervention strategies, especially in vulnerable communities like rural areas of Eastern Cape where the disease is a burden [
30]. It is important to note that models on TB transmission are specific to certain geographic areas and should not be generalized. Therefore, this model is developed for this particular study setting with the idea that it will allow policymakers to determine which aspects to focus on and which intervention strategies are most likely to be successful in a specific area. As there have been unacceptable levels of
M. tuberculosis spread in this study setting, renewed attention must be given to reducing its spread. To provide healthcare services tailored to each community, various service delivery models have been created, including one that ensures ongoing collaboration among healthcare organizations to understand the epidemiology of TB in rural areas of the Eastern Cape. The objective is to have a good model on DR-TB.
4. Study Limitation
The fact that the outcomes of this study are all dependent on those that have been published in manuscript parts 1, 2, and 3 is one of its limitations. These conclusions also have limited generalizability because they are exclusively based on data from the rural areas of Eastern Cape research. The fact that the study's findings may be used to create and enhance intervention methods in the rural areas of Eastern Cape Province and other contexts is one of its strengths.
5. Conclusions
Collaboration among clinicians, researchers, and laboratories is crucial to benefit patients. The model for collaboration between HCF, DL and RI will enhance educational health programs which are to be tailored to the specific needs of the community, taking into account the latest information on DR-TB gene mutations and strains.
Author Contributions
L.M.F.; Conceptualization, methodology, writing original draft; C.B.; writing—review and editing, S.V. and T.A.; supervision, N.D.; writing—review, editing, methodology. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: Financial support for this study was obtained from the South African Medical Research Council (SAMRC) Research development grant (Pilot grant).
Institutional Review Board Statement
This study was conducted by the Declaration of Helsinki. Approved granted Research Ethics and Biosafety Committee of the Faculty of Health Sciences of Walter Sisulu University (Ref. No. 026/2019) and Eastern Cape Department of Health (Reference Number EC_201904_011).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be requested from the corresponding author.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Notoatmodjo, S. ; Promosi Kesehatan dan Ilmu Perilaku; Rineka Cipta: Jakarta, Indonesia, 2010. [Google Scholar]
- Bam, T.S.; Aditama, T.Y.; Chiang, C. Y.; Rubaeah, R.; Suhaemi, A. Smoking cessation and smoke-free environments for tuberculosis patients in Indonesia A cohort study. BMC Public Health 2015, 15, 604. [Google Scholar] [CrossRef]
- Vera Rahardjo, S.S.; Murti, B. Health Belief Model and Precede Proceed on the Risk Factors of Multidrug-Resistant Tuberculosis in Surakarta, Central Java. J. Epidemiol. Public Health 2017, 2, 241–254. [Google Scholar]
- Louwagie, G.M.; Ayo-Yusuf, O.A. Tobacco use patterns in tuberculosis patients with high rates of human immunodeficiency virus co-infection in South Africa. BMC Public Health 2013, 13, 1031. [Google Scholar] [CrossRef]
- Gupta, H.; Mahajan, S.; Lal, M.; Toor AK, Deepti, S. S.; Chawla, N. Prevalence of tobacco consumption and smoking and its effect on outcome among microbiologically confirmed new pulmonary tuberculosis patients on daily regimen of DOTS in Amritsar city. J Family Med Prim Care 2022, 11, 2150–2154. [Google Scholar]
- Kant, S.; Maurya, A.K.; Kushwaha, R.A.S.; Nag, V.L. Multi Drug-resistant tuberculosis; an i6trogenic problem. Biosciences Trends, 2010, 4, 48–53. [Google Scholar]
- Santha, T.; Garg, R.; Frieden, T.; Chandrasekaran, V.; Subramani, R.; Gopi, P.; Selvakumar, N.; Ganapathy, S.; Charles, N.; Rajamma, J. Risk Factors associated with default, failure, and death among tuberculosis patients treated in a DOTS program in Tiruvallur District, South India, 2000. Int J Tuberc Lung Dis. 2002, 6, 780–788. [Google Scholar] [PubMed]
- Lavigne, M.; Rocher, I.; Steensma, C.; Brassard, P. The impact of smoking on adherence to treatment for latent tuberculosis infection. BMC Public Health. 2006, 6, 66. [Google Scholar] [CrossRef]
- WHO: Technical paper on regional strategy for knowledge management to support public health. WHO Regional Office Publisher; 2006. Available online: http://www.who.int/kms/about/en/.
- Veronique L: Integrated knowledge translation for globally oriented public health practitioners and scientists: Framing together sustainable transferontier knowledge translation vision. J Multidisciplinary Health care 2010, 3, 33–47.
- Ipe, M. : Knowledge sharing in organizations: A conceptual framework. Human Resource Dev Rev 2003, 2, 337–359. [Google Scholar] [CrossRef]
- Pan, S.; Scarborough, H. : A sociotechnical view of knowledge sharing at Buckman Laboratories’. J Knowl Manag 1998, 2, 55–66. [Google Scholar] [CrossRef]
- Faye, L.M.; Hosu, M.C.; Oostvogels, S.; Dippenaar, A.; Warren, R.M.; Sineke, N.; Vasaikar, S.; Apalata, T. The Detection of Mutations and Genotyping of Drug-Resistant Mycobacterium Tuberculosis Strains Isolated from Patients in the Rural Eastern Cape Province. Infect. Dis. Rep. 2023, 15, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Faye, L.M.; Hosu, M.C.; Vasaikar, S.; Dippenaar, A.; Oostvogels, S.; Warren, R.M.; Apalata, T. Spatial Distribution of Drug-Resistant Mycobacterium tuberculosis Infections in Rural Eastern Cape Province of South Africa. Pathogens 2023, 12, 475. [Google Scholar] [CrossRef] [PubMed]
- Faye, L.M.; Hosu, M.C.; Iruedo, J.; Vasaikar, S.; Nokoyo, K.A.; Tsuro, U.; Apalata, T. Treatment Outcomes and Associated Factors among Tuberculosis Patients from Selected Rural Eastern Cape Hospitals: An Ambidirectional Study. Trop. Med. Infect. Dis. 2023, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Lipin, M.Y.; Stepanshina, V.N.; Shemyakin, I.G.; Shinnick, T.M. Association of specific mutations in katG, rpoB, rpsL, and rrs genes with spoligotypes of multidrug-resistant Mycobacterium tuberculosis isolates in Russia. Clin. Microbiol. Infect. 2007, 13, 620–626. [Google Scholar] [CrossRef]
- Andersson, D.I.; Nicoloff, H.; Hjort, K. Mechanisms and clinical relevance of bacterial heteroresistance. Nat Rev Microbiol. 2019, 17, 479–496. [Google Scholar] [CrossRef]
- Zheng, Y.; Xia, H.; Bao, X.; Zhao, B.; He, P.; Zhao, Y. Highly Sensitive Detection of Isoniazid Heteroresistance in Mycobacterium Tuberculosis by Droplet Digital PCR. Infection and Drug Resistance. 2022, 6245. [Google Scholar] [CrossRef]
- Khan, A.H.; Sulaiman, S.A.S.; Hassali, M.A.; Khan, K.U.; Ming, L.C.; Mateen, O.; Ullah, M.O. Effect of smoking on treatment outcome among tuberculosis patients in Malaysia; a multicenter study. BMC Public Health 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Hazbon, M.H. et al. Population genetics study of isoniazid resistance mutations and evolution of multidrug-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother, 2006, 50, 2640–264. [Google Scholar] [CrossRef]
- van Doorn, H.R.; de Haas, P.E.W.; Kremer, K.; Vandenbroucke-Grauls, C.M.J.E.; Borgdorff, M.W.; van Soolingen, D. Public health impact of isoniazid-resistant Mycobacterium tuberculosis strains with a mutation at amino acid position 315 of katG: A decade of experience in The Netherlands. Clin Microbiol Infect 2006, 12, 769–775. [Google Scholar] [CrossRef]
- Karmakar, M.; Trauer, J.M.; Ascher, D.B.; Denholm, J.T. Hyper transmission of Beijing lineage Mycobacterium tuberculosis: Systematic review and meta-analysis. J. Infect. 2019, 79, 572–581. [Google Scholar] [CrossRef]
- Said, H.; Ratabane, J.; Erasmus, L.; Gardee, Y.; Omar, S.; Dreyer, A.; Ismail, F.; Bhyat, Z.; Lebaka, T.; van der Meulen, M.; et al. Distribution and Clonality of drug-resistant tuberculosis in South Africa. BMC Microbiol. 2021, 21, 157. [Google Scholar] [CrossRef] [PubMed]
- Shangase, Z.; Tsoka-Gwegweni, J. M.; Okem, A. Smoking prevalence among inpatients with drug-resistant tuberculosis in KwaZulu-Natal, South Africa. Tobacco-Induced Diseases. 2018, 16, 276. [Google Scholar] [CrossRef]
- Ano, H. et al. Relationship between the isoniazid-resistant mutation katGS315T and the prevalence of MDR-/XDR-TB in Osaka, Japan. Int J Tuberc Lung Dis 2008, 12, 1300–1305. [Google Scholar]
- Swaminathan, S.; Narendran, G. HIV and tuberculosis in India. Journal of Biosciences. 2008, 33, 527. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Mathema, B.; Zhao, Q.; Zheng, X.; Li, D.; Jiang, W.; Wang, W.; Xu, B. Comparison of the sociodemographic and clinical features of pulmonary TB patients infected with sub-lineages within the W-Beijing and non-Beijing Mycobacterium tuberculosis. Tuberculosis. 2016, 97, 18–25, 11(4) 2016: e0153563. [Google Scholar] [CrossRef]
- Kementerian Kesehatan, Republik Indonesia. Information Tuberculosis; Ministry of Health: Jakarta, Indonesia, 2018; Available online: https://pusdatin.kemkes.go.id/article/view/18101500001/infodatin-tuberkulosis-2018.html (accessed on 27 July 2023).
- Holmes, E.A.F.; Hughes, D.A.; Morrison, V.L. Predicting adherence to medications using health psychology theories: A systematic review of 20 years of empirical research. Value Health 2014, 17, 863–876. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report; WHO/HTM/TB/2017.23: Geneva, 2017. [Google Scholar]
- van Doorn, H.R.; de Haas, P.E.W.; Kremer, K.; Vandenbroucke-Grauls, C.M.J.E.; Borgdorff, M.W.; van Soolingen, D. Public health impact of isoniazid-resistant Mycobacterium tuberculosis strains with a mutation at amino acid position 315 of katG: A decade of experience in The Netherlands. Clin Microbiol Infect 2006, 12, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.; Magula, N.P. Treatment outcomes of Gene Xpert positive tuberculosis patients in KwaMashu Community Health Centre, KwaZulu-Natal, South Africa: A retrospective review. S. Afr. J. Infect. Dis. 2021, 36, a217. Systematic review and meta-analysis. J. Infect. 2019, 79, 572–581. [CrossRef] [PubMed]
- Gagneux, S. et al. Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis. PLoSPathog 2006, 2, e61. [Google Scholar] [CrossRef]
- Ameeruddin, N. U.; Luke Elizabeth, H. Impact of isoniazid resistance on the virulence of global and south Indian clinical isolates of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2014, 94, 557–563. [Google Scholar] [CrossRef]
- Ahmad, S.; Mokaddas, E.; Al-Mutairi, N.; Eldeen, H.S.; Mohammadi, S. Discordance across Phenotypic and Molecular Methods.
- for Drug Susceptibility Testing of Drug-Resistant Mycobacterium tuberculosis Isolates in a Low TB Incidence Country. PLoS ONE 2016, 11, e0153563. [CrossRef]
- Tolani; et al. BMC Infectious Diseases 2012, 12, 9. Available online: http://www.biomedcentral.com/1471-2334/12/9.
- Richardson, E.T.; Lin, S.Y.G.; Pinsky, B.A.; Desmond, E.; Banaei, N. First documentation of isoniazid reversion in Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2009, 13, 1347–13. [Google Scholar] [PubMed]
- Global Tuberculosis Report 2022 (World Health Organization, 27 January 2023). Available online: https://go.nature.com/3FW2RVs.
- Pai, M. , Kasaeva, T. & Swaminathan, S. N. Eng. J. Med. 2022, 386, 1490–1493. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).