1. Introduction
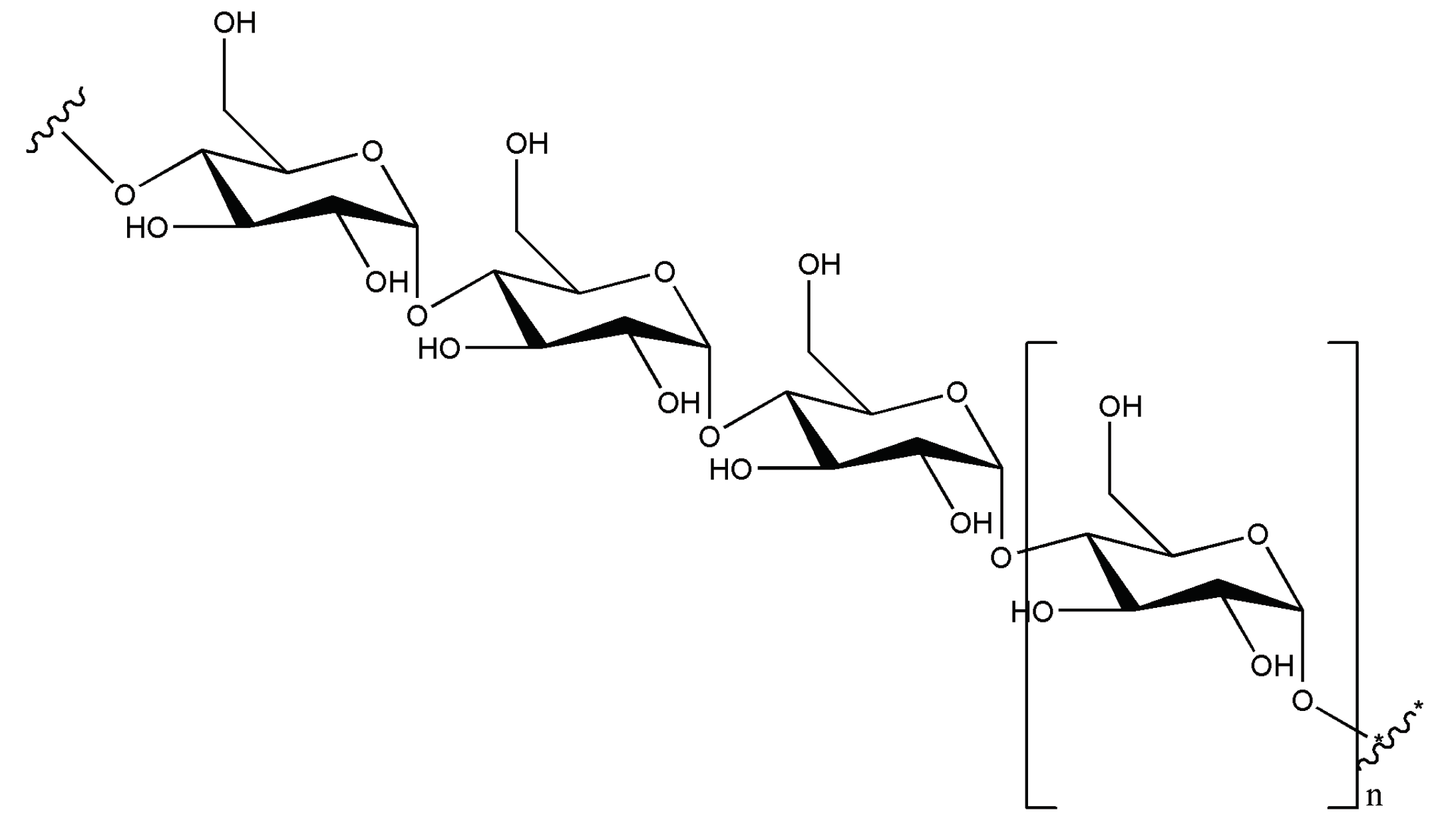
Starch is a mixture of amylose and amylopectin in a ratio of ~20-30% to 70-80%, both of which are polymers of glucopyranose. Amylose is a linear polymer typically containing 300-3000 (or sometimes much more) monomeric units, always bound via α(1→4) glycosidic bonds (cf.
Figure 1).
1 Amylopectin has a similar structure, additionally features ramifications via α(1→6) bonds. Both polymers are sparsely soluble in water – with amylose more so than amylopectin. Inside living organisms, the enzyme amylase is mainly responsible for hydrolyzing starch. Dextrins, which are polysaccharides with a low degree of polymerization, are produced by partial hydrolysis of starch; with complete hydrolysis, glucose is formed.
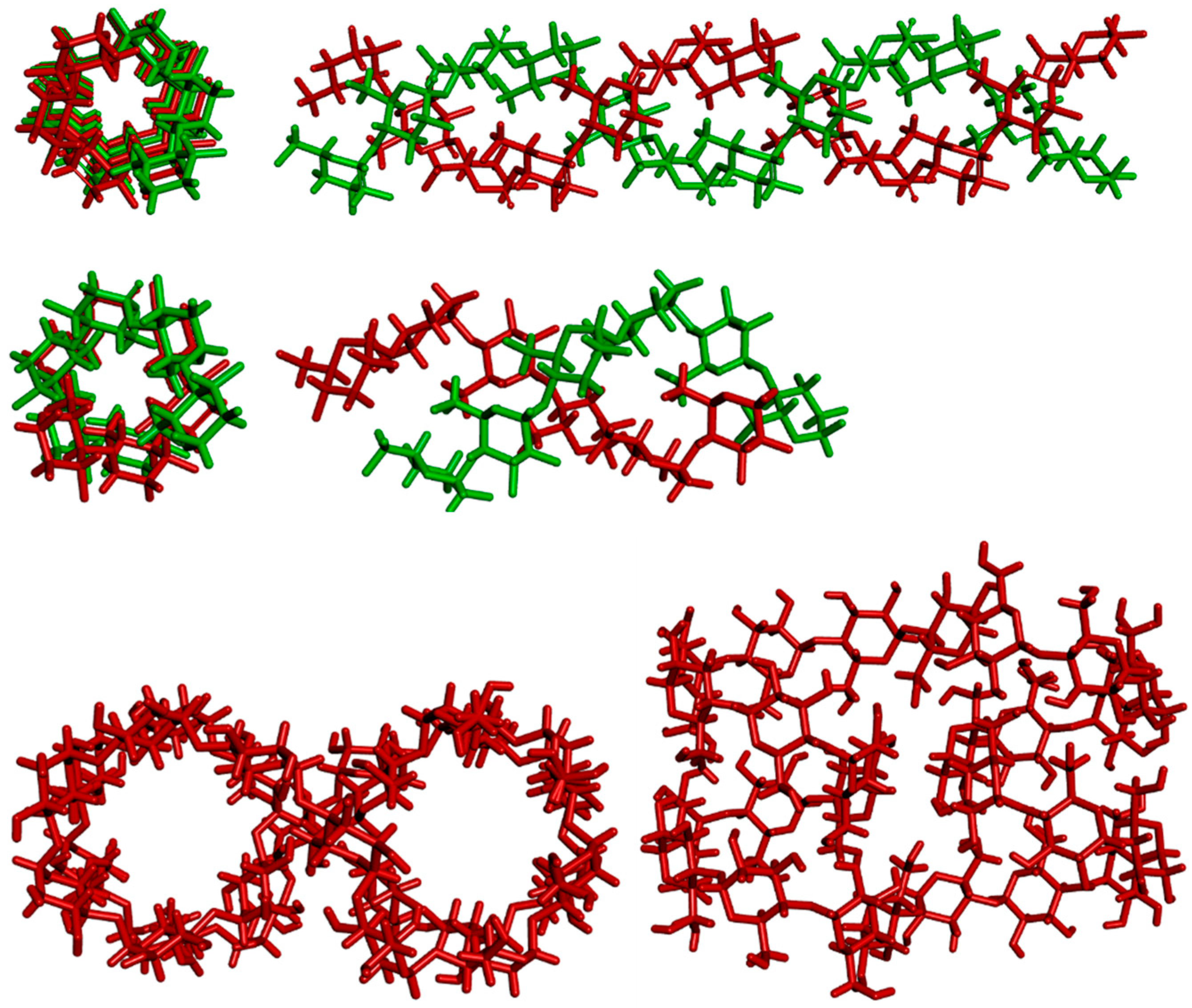
2 Amylose can exist in a disordered amorphous conformation or in two types of helical forms (
Figure 2). The first type is a double helix with itself (including forms A and B). The second type is the V form, which consists of a single helix and is the one typically discussed in biochemistry textbooks. The V form features an internal cavity large enough to accommodate hydrophobic guest molecules such as iodine, fatty acids or small aromatic compounds. In 1930, Katz studied the aging of bread and cooking with bread, and with the help of X-ray diffraction, in the analyzed powder he found, in addition to the type A and B models known until then from native starch, another crystalline form of starch, which he called model V. His idea was the German word "Verkleitsterung", which means gelatinization
3. He found the same V-type pattern when he prepared pasta, which was precipitated with alcohols.
4 Bear identified other different V-type patterns depending on the precipitation agent.
5 Native starch was fractionated by Meyer et al., separating amylose from amylopectin using hot water.
6
The A- and B-amylose both form parallel-stranded double helices of 6 × 2 glucoses per turn and right-handed7–12 or left-handed13 turns. Structurally, these two structures differ only in packing arrangement and water content. They also differ in biological locations - with A preferentially in grains and B in tubers7. A polymorph C of amylose has also been described, consisting of a mixture of A- and B- unit cells.18
The V form is also found naturally, and is structured as a single left-handed helix with 6 glucose units per turn and a step height of 7.91 to 8.17 Å.7–9 The V form can be isolated by precipitation from aqueous solution using alcohols, ketones, fatty acids, iodine, or salts that form inclusion complexes (thus behaving similarly cyclodextrins, α-cyclodextrin or cyclohexaamylose).14,15 The glucose units in V-amylose (as in cyclodextrins) are all in the syn orientation. This entails hydrogen bonds between the secondary hydroxyl groups O(3)n⋅⋅⋅O(2)n+l, alongside O(6)n⋅⋅⋅O(2)n+6 hydrogen bonds between turns7–9.
Variants of the V-type structure of amylose have been described, each marked with a subscript typically indicating the number of glucose units per shift. The most common variant is V6 form,16 though V8 and V7 have also been described. The latter would provide even more space for the guest molecule to bind.17
Amylose V is the allomorph known for its deep blue complexes with iodine. The iodine molecules are caught in the channels within the helices, where also molecules of the solvent can be present. Depending on the solvent, slightly different crystallographic structures are formed. The structure presented in
Figure 2 is from crystals are grown with water as solvent, dubbed amylose V
h. This crystalline form features 6 glucose units in the unit cell, aligned as a single strand. Within the helix, there are three water molecules, statistically positioned on 6 positions. In the spaces between helices, there is one water molecule each, on one of three possible positions. The helices point upwards and downwards in a random fashion.
16
Cyclodextrins are glucose oligomers featuring the same type of glycosidic bonds as starch, and can be obtained enzymatically. Depending on size, some cyclodextrins can arrange themselves in circular fashion appearing similar to single turns of the amylose helix, and then forming dimers which generate large cylinders, similar to the amylose helix in its overall structure.
2. General Considerations of Reaction Starch with Iodine
Colin and Claubry discovered in 1814 the reaction between starch and iodine (or, more specifically, with iodide-iodine solutions, as molecular I2 is otherwise very insoluble in water in the absence of I- ions). Since then, the reaction is found both in organic chemistry classes in school and in qualitative and quantitative analysis courses. Over time, many experiments have shown that the starch-iodine complex shows absorption at ~600 nm, a strong dark blue color.19 A more detailed description of the reaction was provided by H. H. Landolt in 188620. The color is mainly due to the complex of iodine with amylose complex that absorbs at ~ 620 nm. The affinity of iodine for amylopectin is distinctly smaller ( ~20 times), and the resulting complex is reddish-violet, with a maximum at ~540 nm. In 1948, Gilbert and Marriott21 showed that at higher concentrations of iodide, the ratio of iodide ions to iodine molecules increases to at least one, thus a purple tint appears in the blue complex.
The nature of the amylose-iodine complex has been debated for many decades,22 especially in terms of stoichiometry and charge of the poly-iodine substructures within the helix. It is now generally accepted that iodide ions are also required in the process. Thus, the iodine atoms would align inside the amylose helix as a mixture/combination of I2 and I- 22,23,25–28 with an unusual metallic-like structure.24 This mixture would entail not isolated I- ions, but rather In¯ polyiodides (n=3, 5, 9…).29–33 The wavelength of the absorbance maximum in the amylose-iodine complex is known to depend on the chain length. Thus, glucose chains of 4 to 6 units yield no color, those 8 to 12 yield a red color with a peak at 520 nm reminiscent of amylopectin. Longer chains progressively show a bathochromic shift until a length of 30 to at 35 units, when the blue color is reached with a peak at 600-620 nm. Very similar spectra are also obtained by the synthetic action of potato phosphorylase on starch, when chains of 50-150 units are obtained. This relationship between chain length and iodine color has also been applied to branched polysaccharides. Comparisons of the iodine/iodide spectra with dextrin, amylopectin, glycogen or various synthetic oligosaccharides, estimations of the extent of helical portions available for iodine binding in amylopectin and glycogen were formulated, at 8-18 glucose residues. In line with these observations, hydrolysis of amylose by α-amylase or under acid catalysis gradually changes the absorption maximum from blue to red. On the other hand, hydrolysis with β-amylase leads to a hypochromic but no hypsochromic shift. This can be explained by the fact that β-amylase remains bound to its substrate until it completely degrades it, rather than gradually degrading all polymer chains at the same time. In this way, little or no red-colored intermediate dextrins remain in the mixture.34 Variations in color for iodine-amylopectin complexes can be observed due to the difference in structures35, branched chain length36 and branching points in amylopectin37. If the degree of polymerization in amylopectin entails 15 glucose units, complexation of iodine is not observed at any temperature; if it exceeds 30, then iodine binds to amylopectin at either 1.5°C or 20°C36. Mold and Synge38 used potentiometric and spectrophotometric titrations for complexation of iodine/iodide with the products of enzymatic hydrolysis of amylose, i.e. dextrins with different molecular masses. Dextrins of less than 10 glucose units were essentially unreactive, those of 10–25 units were orange, those 25–40 were red, and those of 40–130 were blue. Ono39 showed that the λmax of the amylose-iodine complex shifted to shorter wavelengths with increasing iodide concentration; this was interpreted as evidence of breaking the polyiodine chains by the permeated iodide ions.
3. Dependence on the Nature of the Organic (Bio)polymer
Besides starch, there is a long list of natural polymers that afford colored complexes with iodine. These include chitosan, glycogen, silk, wool, albumin, cellulose, xylan, and natural rubber. A large number of synthetic polymers have also been described to react with iodine. Examples include poly(vinyl alcohol) (PVA), poly(vinyl pyrrolidone) (PVP), nylons, poly(Schiff base)s, polyaniline, unsaturated polyhydrocarbons (carbon nanotubes, fullerenes C60/C70, and polyacetylene.40 It is important to note that most of these polymers do not feature helical structures of the type seen in amylose; binding of the iodine on the outside of the organic polymer, or between polymer chains, is likely occurring in such cases.
Differences in the blue color were reported depending on the size (and, implicitly, biological source) of the polymer – be it amylose or related poly and oligo saccharides, or other organic polymers.35,37,40–54 Yu and co-workers55 showed that when complexation occurs between I2/KI and potato amylose, speciation of iodide varies. Data from Raman and UV-visible spectroscopy were interpreted as evidence that the primary forms of iodine were the monoanions I3¯ and I5¯, but larger units were also present such as I93¯, I113¯, I133¯ and I153¯ (with bands at 460-480, 560-590, 660-700 and 710-740 nm, respectively) - with higher iodide concentrations expectedly favoring shorter polyiodide chains. By adding an iodine - potassium iodide solution to a solution of cellulose, Abe56 obtained an intense blue solution. At 80°C the color disappeared, but on cooling it reappeared. Takahashi57 reported a dark purple adduct upon treating chitin with I2/KI solution for 24 hours at room temperature. Depending on the amount reactant ratios, the iodine content of the adduct was in the range of 9-20%, with one molecular iodine per 6.4 chitin residues. Yajima et al.58 prepared an purple complex (maximum at 550 nm) by freezing a mixture of chitosan and I2/KI solution at -20°C and then thawing it at 4°C.
Glycogen, as also discussed above, yields a reddish-brown complex with iodine. The largest maximum is at 395 nm,59 but as shown by Kumari et al.60,61 and Lecker et al.62 a series of UV absorption bands is also present at 408, 453, 496, 560, 650, and 698 nm. The bands at 408, 453, 560, and 650 nm were comparable to those of amylopectin (412, 458, 550, and 640 nm), and the bands at 496 and 698 nm were assigned to an I4 species. Bands at 453 and 560 nm were more visible at higher iodine concentrations.
Stromeyer in 1815 mentions that wool and silk (i.e., the protein structure therein) give yellow colors on exposure to iodine.40 Amyloid peptides also yield a blue color with molecular iodine. In the presence of sulfuric acid, this shifts to blue-violet, cf. Aterman.63 Dzwolak64 described the formation of an insulin-amyloid complex in the presence of I2/KI stable up to 90°C, interpreted as an inclusion complex between the amyloid fibrils and iodine.
Pritchard and Serra65 reported that reaction of poly(vinyl acetate) (PVAc) with molecular iodine in methanol in the presence of aqueous KI yields a deep red precipitate (darker at higher iodine concentrations) interpreted as a I2-PVAc adduct. Two UV-vis absorbance bands were reported for the complex, at 520 and 510 nm. Hughes et al.66 noted that the red color of the I2-PVAc complex is independent of the method by which the polymer was prepared. Solutions of the PVA-iodine complex (I2-PVA) feature intense 600–620 nm, 650–680 nm, 480–500 nm, and 350 nm67 bands depending on the conditions. The complex of I2 with poly(vinylpyrrolidone) (povidone or PVP) has a maximum at 361 nm, with the iodine molecule reported to be attached to the PVP matrix through non-covalent interactions with the carbonyl groups. A poly(N-methyl-4-vinyl pyridinium) triiodide was reported to produce a dark brown powder with molecular iodine in a water-alcohol solution, with bands at 295 nm, 367 nm and 460 nm. 68
Charge transfer complexes of iodine with ferrocenyl-bearing Schiff bases have also been described.69 Liu et al.70 showed that the brown poly(ferrocenyl-Schiff) bases trun black after tretament with iodine in acetone. In the infrared (IR) spectra, iodine binding resulted in a decrease of the ~ 1610 cm-1 signal of the Schiff base, accompanied by the appearance of a new wide absorption band at 450-480 nm with a long tail up to ~900 nm in the UV-vis spectra.
Shirakawa71 examined the reaction polyacetylene (PAC) with iodine. Strong IR bands were noted at 870 cm-1 and 1390 cm-1. In the UV-vis spectrum, analysis, bands at 365 nm and 502 nm were noted to be suggestive for the presence of I3¯ and I2 respectively; a band at 280 nm characteristic for unreacted PAC was noted to decrease in intensity upon doping.
Treatment of poly(p-phenylene vinylene) or phen(p-phenyvinylene) (PPV) with iodine vapor showed a significant effect on its luminescence.72 Three bands in the UV-visible-NIR spectra, at 688 nm, 1724 nm, and 2292 nm interpreted as evidence for a PPV-iodine complex. Polybutadiene, poly(cis-isoprene) and their copolymers were reported to display an intense absorption band at 305 nm in the UV spectrum (with some variations depending on solvent) upon treatment with iodine.73 Sreeja et al.74 found that acrylonitrile butadiene rubber developed two new broad bands appeared between 300 nm and 500 nm with, while the 262 nm band attributed to the C=C bonds decreased. Poly(β-pinene) treated with iodine vapor was shown by Vippa et al.75 to bands at 310 nm and 400 nm in the UV-vis spectrum, with the latter interpreted as evidence for a charge transfer between the double bond and I2.
4. Geometrical Data
Crystalline units in starch and in its partial degradation products have long been explored76-78. Transmission electron microscopy79, light microscopy80, the use of atomic force microscopy (AFM)81–88 and X ray and Neutron Small Angle Scattering89 have allowed for some partial visualization of repeating units in amylose. Microstructures were observed, that were called nodules, initially with diameter variation between 150 and 300 nm81, but then also smaller particles of 20–50 nm82,84,85,89 (e.g., 130-250 nm for pea starch78, 20-50 nm for potato starch87 or 10-30 nm for corn granules81). Using combined methods of X-ray diffraction and stereochemical packing analysis of the amylose-iodine complex, Bluhm and Zugenmaier32 reported that the iodine atoms align almost linearly in the center of the amylose chain. Eight hydration water molecules were found per unit cell, located in the amylose helices. The amylose left-handed helix was reported to have an outer diameter of~13 Å and a pitch of 8 Å (six 1,4-glucose units per pitch) hosting an internal cavity of ~ 5 Å.24,25,27,28,30,32,92-95 Some statistical disorder was noted within the polyiodide chain, with an average iodine-iodine distance of ~3.1 Å. This value is larger than 2.67 Å in molecular I2 as well as than the 2.90 Å in ionic I3-, but distinctly shorter than the 4.3 Å sum of van der Waals radii for two iodine atoms. With the help of the electron-gas theory, the 620 nm maximum in the UV-vis spectrum was suggested to be due to 14 iodine atoms when assuming an equidistant distance of 3.1 Å.90,91 However, the x-ray diffraction data so far cannot distinguish between a structure where the iodine atoms are placed equidistantly at 3.1 Å vs. a structure consisting of I2 and/or In- units (with internal I-I distances lower than 3.1 Å) placed in non-covalent contact with each other. For instance, In- units with n=5-7 (and assuming the same internal I-I distance as in I3-) placed at 4.3 Å from each other would allow an average distance of 3.1 Å across the crystal structure in line with experiment. If assuming the intermolecular distance to be shorter than the sum of van der Waals radii (as expected in complexes displaying charge-transfer bands), the experimentally-observed iodine-iodine distance of 3.1 Å could be reached with, e.g., I3- units placed at 3.7 Å from each other, or I5- units placed at 4.1 Å. The same average distance could be obtained by a chain of I2 molecules placed within 3.6 Å from each other.
5. The I2-Only Hypothesis
Many textbooks still list the amylose structure as featuring I2 units aligned inside the helical channel. The driving force for this arrangement would be the hydrophobic character of the I2 molecules, which thus escape solvent to align in a more hydrophobic environment inside the helix.96 The non-covalent interactions between I2 would then facilitate charge-transfer bands to appear, and hence the intense blue color. NMR and UV-vis studies have shown that I- ions are not involved in the iodine-amylose helix, but help to dissolve iodine in water.53 Simulations of the UV-vis spectra using semiempirical INDO configuration interaction have been reported to support a (C6H10O5)16.5 · (I2)3 stoichiometry for the amylose-iodine complex, refuting instead In- (n=3, 5, or 7) as possible candidates.97
Water molecules were reported to modulate the structure of the iodine-amylose complex.98 It was also noted that neither the dimensions of the amylose helix nor the rigidity or the helical vs. random coil secondary structure within the amylose polymer were affected by iodine binding – all of which suggest no strong specific inter-molecular bonding between amylose and iodine. This is also consistent with a simple inclusion complex involving neutral molecules; by contrast, the charge in In- may have been expected to induce local changes in the neighboring polyscachharide units.37,99–105
According to semiempirical calculations, binding of water or of interspersed water and iodine molecules inside the amylose helix results in slight steric distortions of the polymer.106 The water molecules were found to adhere closely to the walls of the internal channel of amylose and to exclude I2 from interactions, suggesting that I2 molecules alone would be unable to dislocate water molecules from inside the amylose helix. In fact, I2 solutions (in alcohol, so as to not require iodide for solubilization) were only found effective in binding to starch at high temperatures – while at lower temperatures vapor I2 easily adsorbs/binds to solid/dry amylose in the absence of water.107
6. Poly-Iodine Anions as Candidates
It is in fact now generally accepted that formation of the intense blude color in the amylose-iodine reaction requires iodide ions not only simply as an accessory that allows solubilization of I2 in water, but rather because a combination of I2 and I- chains is present inside the amylose helix, most likely involving In- units.23–28 The (poly) anionic character of these guest ligands may be taken, as discussed above, to be at odds with the hydrophobic nature of the interior cavity of the helix, especially as no counterions have been discussed or are presumed to be present throughout the cavity. This issue has been addressed by proposing a structure consisting of alternating tri-iodide units and I2 molecules occurs, and where the more hydrophilic ends/entrances of the helix would remain unoccupied by iodine.108 To explain the fact that at room temperature molecular iodine can bind to amylose in solid state but not in solution, it was noted that the secondary structure of amylose, including the internal diameter of the helix. varies depending on the environment (e.g., solvent, ionic strength, pH, temperature, surfactants).49,50,54,109–112 Moreover, the solid-state amylose-I2 complex is not stable in water.113 To complicate matters, it has also been reported that in the iodine-amylose complex (somewhat similarly to the above-discussed cases of other organic polymers), large amounts of iodine can associate outside the helical cavity, with inter- and intra-chain associations also important.26 Theoretical studies of the complex have been interpreted as evidence that at low temperatures a I6 structure dominates, while at higher temperatures nonlinear geometries also appear. However, the experimental data has led to descriptions of the amylose-bound iodine chains as featuring 3-4 to 14-15 and as high as 160 atoms.24–28 Potentiometric titrations at low iodide concentrations have been interpreted as evidence for a 3/2 I2/I- ratio (hence, formally I82-). However, as the structure appears consistently affected by the concentrations of the reactants (especially the iodine-iodide ratios, as followed e.g. by UV-vis and circular dichroism titrations), I4-, I7-, I9-, I62-, I82-, I102-, I42-, I6-, and I242- structures have also been proposed.24,26,27,50,52,53,114–117 Rawlings and Schneider119 using the statistics of binding isotherms of I2/I3- and the large variation of the value of the term of the ratio (R), determined the intrinsic binding constant of I3- to amylose, whose value was much higher than that of I2. Likewise, the mixed binding energy between I2–I3- exceeded those between I2-I2 and I3-–I3- species26. Statements95 which assume that I- is not required sometimes do not take into account that it is formed by hydrolysis of I2 itself. Also, it is known that by adding an acid to an aqueous solution of iodine, the blue color is suppressed.23,119
In addition to classical short units such as I3- ions and iodine molecules, the presence of I5-120 and I7-121 ions was also suggested. These proposals can be based on the fact that the composition of the unit is variable and is influenced by the degree of polymerization of the amylose and the concentration of the iodide ion. At the end of the amylose chain are 7-8 glucose residues, which do not participate in iodine binding113.
Stopped-flow UV-vis and circular dichroism (CD) kinetics have revealed that shorter chains of iodine enter the amylose helix very fast (less than 1 millisecond), and then rearrange rapidly inside the helix without further contributions from the excess iodine/iodide in solution.25,122 The optical rotatory dispersion (ORD) spectrum of the amylose-iodine complex was noted to change even when no changes in the UV-vis spectrum are observe; this was interpreted as evidence for complex dynamics of the helix during which the length of the poly-iodine chains remains unaffected.123 In the structure of of the synthetic complex (benzamide)z H+I3-, a poly-I3 structure was reported – and a range of kinetic, spectroscopic (UV-vis, CD, Raman, X-ray absorption) and thermodynamic data have been interpreted to support such a structure in the amylose-iodine complex, too.117,124–127 On the other hand, the X-ray diffraction data data on the amylose-iodine complex has been interpreted as inconsistent with arrangements consisting of single only I2 or only I3-.31,128,129 A I5- structure was proposed for the amylose-iodine complex based on the similarities of the Raman and Mössbauer spectra with those of polycrystalline (trimesic acid · H2O)10H+I5-,.120,130 Of the main bands in the Raman spectra of starch–iodine complexes, at 27, 55, 109, and 160 cm-1, three were assigned to I5- as the dominant species while the 109 cm-1 was assigned to I3- present as a minor species or impurity.131–136 Further resonance Raman, 129I Mössbauer spectroscopy120,137 and X-ray diffraction128, 138, 139 data has supported I5- or I2·I3- as the dominant species in the amylose-iodine complex. Equilibrium studies in solution have confirmed that I5- is present as a free species, with I42- and I62- also present at higher I2 or I- concentrations.140–142 Teitelbaum et al.120 presented evidence that the I5- ion is present in the helix. Part of the explanation may be that the formation of the I5- ion in the solid complex is produced by the hydrolysis or alcoholysis of iodine, and the amylose studied in this case was freed from water and alcohols.113,120 Analyzing the absorption spectra of aqueous solution of iodine at pH=4.8, in acetate buffer, three peaks were found at 286, 350 and 460 nm.144 The peaks at 286 and 350 nm were attributed to the presence of I3- ions. If amylose was added to this solution, the peak at 286 nm disappeared, the peak at 350 nm had a slight enhancement, and the peak at 460 nm shifted to 620-650 nm. By adding iodic acid to amylose, the blue color does not appear; this indicates the necessity of the presence of anions for the formation of the colored complex.23,119,145
7. Structural Role of the Solvent
Benesi and Hildebrand146 in 1949 showed that iodine is pink-red in benzene, purple in CCl4 (similarly to vapor iodine), and reddish-brown in alcohol – all of which was interpreted as evidence for charge transfer complexes. Bernal-Uruchurta et al.147 provided a microscopic explanation of the interaction of iodine with water. Kereev and Shnyrev studied iodine, iodide148, and triiodide in water and noted a band at 203 nm, which they attributed to iodine, and a band at 461 nm which was then assigned to a H2O-I2 charge-transfer complex. To complicate such studies, I3- is formed by the oxidation of water with iodine. Analyzing iodine solution in water, the absorption bands of I3- are at 284 nm and 351 nm. Density functional theory (DFT) calculations showed that two I2 molecules can bond to two O lone pairs in H2O in nearly tetrahedral geometry with stabilization energy - 8.3 kcal/mol. The complex was described as involving charge-transfer character, with a band in the UV-vis spectrum due to the excitation of electrons from the lone pair of oxygen of the molecular complex at 202 nm, where the molecular orbitals of iodine are destabilized and those of water are stabilized. When iodine is bound to water, the lone pairs on oxygen become equivalent due to hybridization.149 An small effect of water molecules on the structure of the iodine-amylose complex was described as previously discussed.98
8. The I5--I2 Hypothesis
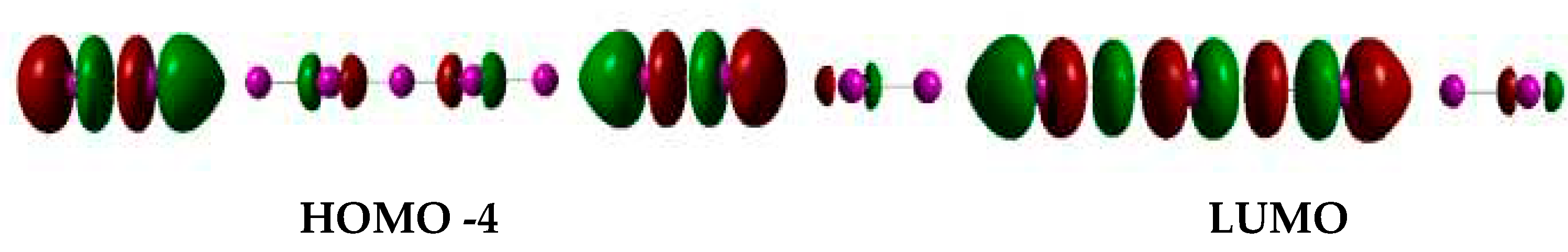
Recent DFT calculations on iodine/iodide chains have been interpreted as evidence that without iodide the blue color cannot be formed in the starch-iodine system. These simulations propose that the nature of the complex consists of alternating sets of I
2 and I
x- units where the nature of the charge transfer bands responsible for the blue color involves transfer from the I
x- σ* orbitals (HOMO) to the I
2 σ* LUMO orbitals (cf.
Figure 3). By analyzing the TD-DFT-computed (time dependent density functional) UV-vis spectra of various candidates (I
2 chains vs. mixtures of I
2 and I
x- with various values of x), and cross-checking with DFT geometry optimizations, a unit of I
2-I
5--I
2, in a repetitive manner within the amylose helix was the only structure that would fit the experimental data.
106 Poly-I
2 structures were shown to be responsible for enhanced blue color under certain conditions (e.g., consistent with the experimental observations on dry/solid amylose). From semiempirical calculations, poly-I
n- structures were found unlikely to exist inside the amylose helix (as no distinct local energy minima were identified for such arrangements). Moreover, TD-DFT simulations of the UV-vis spectra of such chains were found less consistent with experiment, compared to I
2/I
n- pairs. Charge transfer bands from the occupied I
n- (n>3) σ* to the empty I
2 σ * orbital were instead found to be reasonably responsible for the blue color. Of these, the I
2-I
5-I
2 trimeric assemblies (i.e., n=5) were the smallest units that represent local minima in DFT geometry optimizations. These DFT-optimized units remarkably showed average iodine-iodine distances essentially identical to the 3.1 Å value seen experimentally in the iodine-amylose complex. The distinct charge-transfer character of the UV-vis bands (cf.
Figure 3) also brings about a strong dependence on the dielectric constant in the region ε ~ 1 - 30, which in turn was proposed to explain at least part of the dependence of the UV-vis properties of amylose-iodine/iodine complexes on various external factors that may subtly affect amylose architecture and hence exposure of the interior cavity to solvent (e.g., temperature, other solutes, solvents, chain length).
106
9. Conclusions
The iodine-starch (or, more specifically, iodine-amylose) reaction has a two-century history and a wide range of practical applications. Similar reactions occur with other organic polymers. The nature of the reaction is generally accepted to entail alignment of iodine atoms inside the amylose helix. However, the structural details are still a source of confusion in many current reference sources, with alternative explanations given such as poly-I2 (chain of neutral iodine molecules), poly-I3- (chain of I3 anions), poly-Ix (chains of anionic structures of various lengths), or mixtures of I2 and Ix-. The most recent data suggests that the best explanation is a (probably repetitive) I2-I5-I2 unit. .
Author Contributions
Conceptualization, R.S.D.; formal analysis, S/P., R.S.D.; resources, R.S.D.; writing—original draft preparation, S.P.; writing—review and editing, R.S.D. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
Funding from the Romanian Ministry of Education and Research (projects PN-III-P4-ID-PCCF-2016-0142 and PN-III-P1-1.1-PD-2021-0279) is gratefully acknowledged.
Conflicts of Interest
“The authors declare no conflict of interest.”
References
- Nelson, D.; MIchael, M. Cox. Principles of Biochemistry. Principles of Biochemistry, 5th ed.; W.H. Freeman and Company: New York, NY, USA, 2008. [Google Scholar]
- Green, M.M.; Blankenhorn, G.; Hart, H. Which Starch Fraction Is Water-Soluble, Amylose or Amylopectin? J. Chem. Educ. 1975, 52, 729. [Google Scholar] [CrossRef]
- Katz, J.R. Abhandlungen Zur Physikalischen Chemie Der Stärke Und Der Brotbereitung. Z. Für Phys. Chem. 1930, 150A, 37–59. [Google Scholar] [CrossRef]
- Katz, J.R.; Derksen, J.C. Abhandlungen Zur Physikalischen Chemie Der Stärke Und Der Brotbereitung. Z. Für Phys. Chem. 1933, 167A, 129–136. [Google Scholar] [CrossRef]
- Bear, R.S. The Significance of the “V” X-ray Diffraction Patterns of Starches 1. J. Am. Chem. Soc. 1942, 64, 1388–1392. [Google Scholar] [CrossRef]
- Meyer, K.H.; Bernfeld, P.; Wolf, E. Recherches Sur l’amidon III. Fractionnement et Purification de l’amylose de Maïs Naturel. Helv. Chim. Acta 1940, 23, 854–864. [Google Scholar] [CrossRef]
- Sarko, A.; Zugenmaier, P. Fiber Diffraction Methods. Am. Chem. Soc. Wash. DC 1980, 141, 459–482. [Google Scholar]
- Rappenecker, G.; Zugenmaier, P. Carbohydr. Res. 1981, 89, 11–19.
- Murphy, V.G.; Zaslow, B.; French, A.D. Biopolymers 1975, 14, 1487–1501. 14.
- Brisson, J.; Chanzy, H.; Winter, W.T. T. Int. J. Biol. Macromol. 1991, 13, 31–39. [Google Scholar] [CrossRef]
- Veregin, R.P.; Fyfe, C.A.; Marchessault, R.H. Macromolecules 1987, 20, 3007–3012. 20.
- Gidley, M.J.; Bociek, S.M. J. Am. J. Am. Chem. Soc. 1988, 110, 3820–3829. [CrossRef]
- Imberty, A.; Chanzy, H.; Pérez, S.; Bulèon, A.; Tran, V. The Double-Helical Nature of the Crystalline Part of A-Starch. J. Mol. Biol. 1988, 201, 365–378. [Google Scholar] [CrossRef]
- Saenger, W. Inclusion Compounds; Atwood, J.L. , Davies, J.E.D., MacNicol, D.D., Eds.; Academic: London, UK, 1984; Volume 2. [Google Scholar]
- Harata, K. Comprehensive Supramolecular Chemistry; Atwood, J.L. , Davies, J.E.D., MacNicol, D.D., Eds.; Pergamon: Oxford, UK, 1996. [Google Scholar]
- Brisson, J.; Chanzy, H.; Winter, W.T. . The Crystal and Molecular Structure of Vh Amylose by Electron Diffraction Analysis. Int. J. Biol. Macromol. 1991, 13, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Orlova, Y.; Kovalev, V.; Ungar, Y.; Shimoni, E. Structural and Functional Properties of Amylose Complexes with Genistein. J Agric Food Chem 2008, 56, 4212–4218. [Google Scholar] [CrossRef] [PubMed]
- Sarko, A.; Wu, H.-C. H. The Crystal Structures of A-, B- and C-Polymorphs of Amylose and Starch. Starch-Starke 1978, 30, 73–78. [Google Scholar] [CrossRef]
- Rani, A.; Ali, U. Degree-Based Topological Indices of Polysaccharides: Amylose and Blue Starch-Iodine Complex. J. Chem. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Landolt, H. Uber Die Zeitdauer Der Reaction Zwischen Jodsaure Und Schwefliger Saure. Ber. Dtsch. Chem. Ges. 1886, 19, 1317–1365. [Google Scholar] [CrossRef]
- Gilbert, G.A.; Marriott, J.V.R. Starch-Iodine Complexes. Part I. Trans. Faraday Soc. 1948, 44, 84–93. [Google Scholar] [CrossRef]
- Stein, R.S.; Rundle, R.E. On the Nature of the Interaction between Starch and Iodine. J. Chem. Phys. 1948, 16, 195–207. [Google Scholar] [CrossRef]
- Thoma, J.A.; French, D. The Starch-Iodine-Iodide Interaction. Part I. Spectrophotometric Investigations 1. J. Am. Chem. Soc. 1960, 82, 4144–4147. [Google Scholar] [CrossRef]
- Bersohn, R.; Isenberg, I. Metallic Nature of the Starch-Iodine Complex. J. Chem. Phys. 1961, 35, 1640–1643. [Google Scholar] [CrossRef]
- Hiromi, K.; Shibaoka, T.; Ono, S. Kinetic Studies of Amylose-Iodine-Iodide Reaction by Stopped-Flow Method. J. Biochem. 1970, 68, 205–214. [Google Scholar] [CrossRef]
- Yajima, H.; Nishimura, T.; Ishii, T.; Handa, T. Effect of Concentration of Iodide on the Bound Species of I2/I−3 in the Amylose-Iodine Complex. Carbohydr. Res. 1987, 163, 155–167. [Google Scholar] [CrossRef]
- Cronan, C.L.; Schneider, F.W. Cooperativity and Composition of the Linear Amylose-Iodine-Iodide Complex. J. Phys. Chem. 1969, 73, 3990–4004. [Google Scholar] [CrossRef]
- Cramer, F.; Herbst, W. Die Lichtabsorption von Jodkettenmolekeln. Naturwissenschaften 1952, 39, 256–256. [Google Scholar] [CrossRef]
- Rundle, R.E. The Configuration of Starch and Starch-Iodine Complex. I. The Dichroism of Flow of Starch-Iodine Solutions. J. Am. Chem. Soc. 1943, 65, 554–558. [Google Scholar] [CrossRef]
- Rundle, R.E. The Configuration of Starch in the Starch-Iodine Complex. V. Fourier Projections from X-Ray Diagrams. J. Am. Chem. Soc. 1947, 69, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
- Noltemeyer, M.; Saenger, W. X-Ray Studies of Linear Polyiodide Chains in α-Cyclodextrin Channels and a Model for the Starch-Iodine Complex. Nature 1976, 259, 629–632. [Google Scholar] [CrossRef]
- Bluhm, T.L.; Zugenmaier, P. Detailed Structure of the Vh-Amylose-Iodine Complex: A Liner Polyiodine Chain. Carbohydr. Res. 1981, 89, 1–10. [Google Scholar] [CrossRef]
- Handa, T.; Yajima, H. Conformation of Amylose-Iodine-Iodide Complex in Aqueous Solution. Biopolymers 1981, 20, 2051–2072. [Google Scholar] [CrossRef]
- Swanson, A.M. IV. Relation of the Iodine Color to the Structure. Stud. Struct. Polysacch. 1947, 825–837. [Google Scholar]
- Swanson, M.A. Studies on the Structure of Polysaccharides: IV. Relation of the Iodine Color to the Structure. J. Biol. Chem. 1948, 172, 825–837. [Google Scholar] [CrossRef]
- Banks, W.; Greenwood, C.T.; Khan, K.M. The Properties of Synthetic Amylopectin with Long External-Chains. Starch - Stärke 1970, 22, 292–296. [Google Scholar] [CrossRef]
- Hirai, M.; Hirai, T.; Ueki, T. Effect of Branching of Amylopectin on Complexation with Iodine as Steric Hindrance. Polym. (Guildf) 1994, 35, 2222–2225. [Google Scholar] [CrossRef]
- Mould, D.L.; Synge, R.M. Separations of Polysaccharides Related to Starch by Electrokinetic Ultrafiltration in Collodion Membranes. Biochem. J. 1954, 58.4, 571–600. [Google Scholar] [CrossRef]
- Ono, S.; Tsuchihashi, S.; Kuge, T. On the Starch-Iodine Complex. J. Am. Chem. Soc. 1953, 75, 3601–3602. [Google Scholar] [CrossRef]
- Moulay, S. Molecular Iodine/Polymer Complexes. J. Polym. Eng. 2013, 33, 389–443. [Google Scholar] [CrossRef]
- Séne, M.; Thévenot, C.; Prioul, J.L. Simultaneous Spectrophotometric Determination of Amylose and Amylopectin in Starch from Maize Kernel by Multi-Wavelength Analysis. J. Cereal Sci. 1997, 26, 211–221. [Google Scholar] [CrossRef]
- Sashio, M.; Tanaka, M. Thermal Reaction of Poly(Vinyl Alcohol)-Iodine Complex Membranes. J. Polym. Sci. Polym. Chem. Ed. 1985, 23, 905–909. [Google Scholar] [CrossRef]
- Dintzis, F.R. Instability of Solutions of Amylose-Iodine Complex in Concentrated Calcium Chloride. Starch - Stärke 1974, 26, 56–58. [Google Scholar] [CrossRef]
- Tashiro, K.; Gakhutishvili, M. Crystal Structure of Cellulose-Iodine Complex. Polym. (Guildf) 2019, 171, 140–148. [Google Scholar] [CrossRef]
- Konishi, T.; Tanaka, W.; Kawai, T.; Fujikawa, T. Iodine L -Edge XAFS Study of Linear Polyiodide Chains in Amylose and α-Cyclodextrin. J. Synchrotron Radiat. 2001, 8, 737–739. [Google Scholar] [CrossRef]
- Knutson, C.A. Evaluation of Variations in Amylose–Iodine Absorbance Spectra. Carbohydr. Polym. 2000, 42, 65–72. [Google Scholar] [CrossRef]
- Nishimura, T.; Yajima, H.; Ishii, T.; Endo, R. Effect of Molecular Weight of Amylose on the Iodine Coloring Species Responsible for the Optical Properties of Amylose-Iodine Complexes. Kobunshi Ronbunshu 1989, 46, 537–544. [Google Scholar] [CrossRef]
- SenGupta, U.K.; MuKherjee, A.K.; SenGupta, K.K. Spectrophotometric Studies on Amylose-Iodine and Amylopectin-Iodine Complexes. Kolloid-Z. Und Z. Für Polym. 1966, 208, 32–34. [Google Scholar] [CrossRef]
- Sakajiri, T.; Kikuchi, T.; Simon, I.; Uchida, K.; Yamamura, T.; Ishii, T.; Yajima, H. Molecular Dynamics Approach to Study the Discrepancies in the Thermal Behavior of Amylose and Chitosan Conformations. J. Mol. Struct. Theochem. 2006, 764, 133–140. [Google Scholar] [CrossRef]
- Szejtli, J.; Augustat, S.; Richter, M. Molecular Configuration of Amylose and Its Complexes in Aqueous Solutions. Part III. Investigation of the DP Distribution of Helical Segments in Amylose-Iodine Complexes. Biopolymers 1967, 5, 17–26. [Google Scholar] [CrossRef]
- McMullan, R.K.; Saenger, W.; Fayos, J.; Mootz, D. Topography of Cyclodextrin Inclusion Complexes. Carbohydr. Res. 1973, 31, 211–227. [Google Scholar] [CrossRef]
- Baldwin, R.R.; Bear, R.S.; Rundle, R.E. The Relation of Starch—Iodine Absorption Spectra to the Structure of Starch and Starch Components 1. J. Am. Chem. Soc. 1944, 66, 111–115. [Google Scholar] [CrossRef]
- Davis, H.; Khan, A. Determining the Chromophore in the Amylopectin–Iodine Complex by Theoretical and Experimental Studies. J. Polym. Sci. A Polym. Chem. 1994, 32, 2257–2265. [Google Scholar] [CrossRef]
- Rendleman, J.A. The Reaction of Starch with Iodine Vapor. Determination of Iodide-Ion Content of Starch–Iodine Complexes. Carbohydr. Polym. 2003, 51, 191–202. [Google Scholar] [CrossRef]
- Yu, X.; Houtman, C.; Atalla, R.H. The Complex of Amylose and Iodine. Carbohydr. Res. 1996, 292, 129–141. [Google Scholar] [CrossRef]
- Abe, T. The Visible and Ultraviolet Absorption Spectra of Cellulose- and Amylose-Iodine Complexes. Bull. Chem. Soc. Jpn. 1958, 31, 661–662. [Google Scholar] [CrossRef]
- Takahashi, Y.J. Binding Properties of Alginic Acid and Chitin. Inclus. Phenom. 1978, 5, 525–534. [Google Scholar] [CrossRef]
- Yajima, H.; Morita, M.; Hashimoto, M.; Sashiwa, H.; Kikuchi, T.; Ishii, T. Complex Formation of Chitosan with Iodine and Its Strucutre and Spectroscopic Properties-MOlecular Assembly and Thermal Hysteresis Behavior. Int. J. Thermophys. 2001, 22, 1265–1283. [Google Scholar] [CrossRef]
- Gunasekaran, M. Physiological Studies on Phymatotrichum Omnivorum II. Physiocochemical Properties of Glycogen. Arch. Mikrobiol. 1972, 84, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Roman, A.; Khan, A. Chromophore and Spectrum of the Glycogen-Iodine Complex. J. Polym. Sci. Part A. Polym. Chem. 1996, 34, 2975–2980. [Google Scholar] [CrossRef]
- Kumari, S.; Lecker, D.N.; Khan, A. Iodine Binding Capacity and Iodine Binding Energy of Glycogen. J. Polym. Sci. Part. A. Polym. Chem. 1997, 35, 927–931. [Google Scholar] [CrossRef]
- Lecker, D.N.; Kumari, S.; Khan, A. Iodine Binding Capacity and Iodine Binding Energy of Glycogen. J. Polym. Sci. Part. A. Polym. Chem. 1997, 35, 1409–1412. [Google Scholar] [CrossRef]
- Aterman, K. A Historical Note on the Iodine-Sulphuric Acid Reaction of Amyloid. Histochemistry 1976, 49, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Dzwolak, W. Insulin Amyloid Fibrils Form an Inclusion Complex with Molecular Iodine: A Misfolded Protein as a Nanoscale Scaffold. Biochemistry 2007, 46, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.G.; Serra, F.T. Complexation of Polyvinyl Acetate with Iodine. Talanta 1973, 20, 541–546. [Google Scholar] [CrossRef]
- Hughes, J. Analytical Behaviour of Poly(Vinyl Acetate) and Its Hydrolysis Products with Iodine. Talanta 1979, 26, 1161–1163. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.C.; Fleischer, D.; Henglein, A.; Bössler, H.M.; Trisnadi, J.; Tanaka, H. Addition Compounds and Complexes with Polymers and Models. Pure Appl. Chem. 1974, 38, 227–247. [Google Scholar] [CrossRef]
- Chernov’yants, M.S.; Burykin, I.V.; Pisanov, R.V.; Shalu, O.A. Synthesis and Antimicrobial Activity of Poly(N-Methyl-4-Vinylpyridinium Triiodide). Pharm. Chem. J. 2010, 44, 61–63. [Google Scholar] [CrossRef]
- Pal, S.K.; Krishnan, A.; Das, P.K.; Samuelson, A.G. Schiff Base Linked Ferrocenyl Complexes for Second-Order Nonlinear Optics. J. Organomet. Chem. 2000, 604, 248–259. [Google Scholar] [CrossRef]
- Liu, W.-J.; Xiong, G.-X.; Zeng, D.-H. Synthesis and Electrical Properties of Three Novel Poly (Ferrocenyl-Schiff Bases) and Their Charge Transfer Complexes with Iodine. J. Inorg. Organomet. Polym. 2010, 20, 97–103. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.F.; Heeger, A.J. Synthesis of Electrically Conducting Organic Polymers: Halogen Derivatives of Polyacetylene, (CH)x. J. Chem. Soc. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Bakueva, L.; Matheson, D.; Musikhin, S.; Sargent, E.H. Luminescence of Pure and Iodine Doped PPV: Internal Energetic Structure Revealed through Spectral Signatures. Synth. Met. 2002, 126, 207–211. [Google Scholar] [CrossRef]
- Tutorskii, I.A.; Sokolova, L.V. Mechanism of the Reaction of Polybutadiene with Molecular Iodine. Polym. Sci. USSR 1977, 19, 176–183. [Google Scholar] [CrossRef]
- Sreeja, R.; Najidha, S.; Remya Jayan, S.; Predeep, P.; Mazur, M.; Sharma, P.D. Electro-Optic Materials from Co-Polymeric Elastomer–Acrylonitrile Butadiene Rubber (NBR). Polym. (Guildf) 2006, 47, 617–623. [Google Scholar] [CrossRef]
- Vippa, P.; Rajagopalan, H.; Thakur, M. Electrical and Optical Properties of a Novel Nonconjugated Conductive Polymer, Poly (Β-pinene). J. Polym. Sci. Part. B. Polym. Phys. 2005, 43, 3695–3698. [Google Scholar] [CrossRef]
- Nagelli, W. Beitrage Zur Naheren Kenntniss Der Starkegruppe. Ann. Der Chem. 1874, 173, 218–227. [Google Scholar] [CrossRef]
- Badenhuizen, N.P. Die Struktur Des Starkekorns. Protoplasma 1937, 28, 293–326. [Google Scholar] [CrossRef]
- Gallant, D.J.; Bouchet, B.; Baldwin, P.M. Microscopy of Starch: Evidence of a New Level of Granule Organization. Carbohyd. Polym. 1997, 32, 177–191. [Google Scholar] [CrossRef]
- Helbert, W.; Chanzy, H. The Ultrastructure of Starch from Ultrathin Sectioning in Melamine Resin. Starch/Starke 1996, 48, 185–188. [Google Scholar] [CrossRef]
- Atkin, N.J.; Abeysekera, R.M.; Cheng, S.L.; Robards, A.W. An Experimentally-Based Predictive Model for the Separation of Amylopectin Subunits during Starch Gelatinization. Carbohyd. Polym. 1998, 36, 173–192. [Google Scholar] [CrossRef]
- Baker, A.A.; Miles, M.J.; Helbert, W. Internal Structure of the Starch Resistant Granule Revealed by AFM. Carbohyd. Res. 2001, 330, 249–256. [Google Scholar] [CrossRef]
- Baldwin, P.M.; Adler, J.; Davies, M.; Melia, D. High Resolution Imaging of Starch Granule Surfaces by Atomic Force Microscopy. J. Cereal Sci. 1998, 27, 255–265. [Google Scholar] [CrossRef]
- Dang, J.M.C.; Copeland, L. Imaging Rice Grains Using Atomic Force Microscopy. J. Cereal Sci. 2003, 37, 165–170. [Google Scholar] [CrossRef]
- Ohtani, T.; Yoshimo, T.; Hagiwara, S.; Maekawa, T. &T. High-Resolution Imaging of Starch Granule Structure Using Atomic Force Microscopy. Starch/Starke 2000, 52, 150–153. [Google Scholar]
- Park, H.; Xu, S.; Seetharaman, S. A Novel in Situ Atomic Force Microscopy Imaging Technique to Probe Surface Morphological Features of Starch Granules. Carbohyd. Res. 2011, 346, 847–853. [Google Scholar] [CrossRef]
- Ridout, M.J.; Gunning, A.P.; Parker, M.L.; Wilson, R.H.; Morris, V.J. Using AFM to Image the Internal Structure of Starch Grsnules. Carbohyd. Polym. 2002, 50, 123–132. [Google Scholar] [CrossRef]
- Szymonska, J.; Krok, F. Potato Starch Granule Nanostructure Studied by Highresolution Non-Contact AFM. Int. J. Biol. Macromol. 2003, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Waduge, R.N.; Xu, S.; Seetharaman, S. Iodine Absorption Properties and Its Effect on the Crystallinity of Developing Wheat Starch Granules. Carbohydr. Polym. 2010, 82, 786–794. [Google Scholar] [CrossRef]
- Doutch, J.; Gilbert, E.P. Characterisation of Large Scale Structures in Starch Granules via Small-Angle Neutron and X-Ray Scattering Techniques. Carbohydr. Polym. 2013, 91, 444–451. [Google Scholar] [CrossRef]
- Cramer, F.; Windel, H. Über Einschlußverbindungen, X. Mitteil.: Die Blauen Jodverbindungen Der Cumarine Und Anderer Verwandter Verbindungen. Chem. Ber. 1956, 89, 354–365. [Google Scholar] [CrossRef]
- Saenger, W. The Structure of the Blue Starch-Iodine Complex. Naturwissenschaften 1984, 71, 31–36. [Google Scholar] [CrossRef]
- Barrett, A.J.; Barrett, K.L.; Khan, A. Effects of Acetone, Ethanol, Isopropanol, and Dimethyl Sulfoxide on Amylose-Iodine Complex. J. Macromol. Sci. Part A 1998, 35, 711–722. [Google Scholar] [CrossRef]
- Fonslick, J.; Khan, A. Thermal Stability and Composition of the Amylose–Iodine Complex. J. Polym. Sci. A Polym. Chem. 1989, 27, 4161–4167. [Google Scholar] [CrossRef]
- Rundle, R.E.; French, D. The Configuration of Starch and the Starch—Iodine Complex. II. Optical Properties of Crystalline Starch Fractions 1. J. Am. Chem. Soc. 1943, 65, 558–561. [Google Scholar] [CrossRef]
- Rundle, R.E.; French, D. The Configuration of Starch in the Starch—Iodine Complex. III. X-Ray Diffraction Studies of the Starch—Iodine Complex 1. J. Am. Chem. Soc. 1943, 65, 1707–1710. [Google Scholar] [CrossRef]
- Immel, S.; Lichtenthaler, F.W. The Hydrophobic Topographies of Amylose and Its Blue Iodine Complex. Starch - Stärke 2000, 52, 1–8. [Google Scholar] [CrossRef]
- Minick, M.; Fotta, K.; Khan, A. Polyiodine Units in Starch-Iodine Complex: INDO CI Study of Spectra and Comparison with Experiments. Biopolymers 1991, 31, 57–63. [Google Scholar] [CrossRef]
- Zaslow, B.; Miller, R.L. Hydration of the “V” Amylose Helix 1. J. Am. Chem. Soc. 1961, 83, 4378–4381. [Google Scholar] [CrossRef]
- Dintzis, F.R.; Beckwith, A.C.; Babcock, G.E.; Tobin, R. Amylose-Iodine Complex. I. Sedimentation Behavior. Macromolecules 1976, 9, 471–478. [Google Scholar] [CrossRef]
- Moulik, S.P.; Gupta, S. Effects of Solvents on the Spectrophotometric and Hydrodynamic Behavior of Amylose and Its Iodine Complex. Carbohydr. Res. 1980, 81, 131–143. [Google Scholar] [CrossRef]
- Senior, M.B.; Hamori, E. Investigation of the Effect of Amylose/Iodine Complexation on the Conformation of Amylose in Aqueous Solution. Biopolymers 1973, 12, 65–78. [Google Scholar] [CrossRef]
- Vladimirov, A.V.; Volkova, T.V.; Agafonov, A.V. Temperature Dependence of Stability Constants of the Iodine-Iodide-Amylose Complexes. Russ. J. Phys. Chem. A 2003, 77, 612–615. [Google Scholar]
- Zhang, Q.; Lu, Z.; Hu, H.; Yang, W.; Marszalek, P.E. Direct Detection of the Formation of V-Amylose Helix by Single Molecule Force Spectroscopy. J. Am. Chem. Soc. 2006, 128, 9387–9393. [Google Scholar] [CrossRef]
- Dintzis, F.R.; Tobin, R.; Beckwith, A.C. Amylose-Iodine Complex. II. Molecular Weight Estimates. Macromolecules 1976, 9, 478–482. [Google Scholar] [CrossRef]
- Mikus, F.F.; Hixon, R.M.; Rundle, R.E. The Complexes of Fatty Acids with Amylose 1. J. Am. Chem. Soc. 1946, 68, 1115–1123. [Google Scholar] [CrossRef]
- Pesek, S.; Lehene, M.; Brânzanic, A.M.V.; Silaghi-Dumitrescu, R. On the Origin of the Blue Color in The Iodine/Iodide/Starch Supramolecular Complex. Molecules 2022, 27, 8974. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.T.; Khan, A. Amylose-Iodine Complex Formation without KI: Evidence for Absence of Iodide Ions within the Complex. J. Polym. Sci. A Polym. Chem. 1999, 37, 2711–2717. [Google Scholar] [CrossRef]
- Cesaro, A.; Benegas, J.C.; Ripoll, D.R. Molecular Model of the Cooperative Amylose-Iodine-Triiodide Complex. J. Phys. Chem. 1986, 90, 2787–2791. [Google Scholar] [CrossRef]
- Kuge, T.; Ono, S. Amylose-Iodine Complex. III. Potentiometric and Spectrophotometric Studies. Bull. Chem. Soc. Jpn. 1960, 33, 1273–1278. [Google Scholar] [CrossRef]
- Schulz, W.; Sklenar, H.; Hinrichs, W.; Saenger, W. The Structure of the Left-Handed Antiparallel Amylose Double Helix: Theoretical Studies. Biopolymers 1993, 33, 363–375. [Google Scholar] [CrossRef]
- Moulik, S.P.; Gupta, S. Environment-Induced, Physicochemical Behavior of Amylose-Iodine Complexes. Carbohydr. Res. 1979, 71, 251–264. [Google Scholar] [CrossRef]
- Peng, Q.-J.; Perlin, A.S. Observations on N.M.R. Spectra of Starches in Dimethyl Sulfoxide, Iodine-Complexing, and Solvation in Water-Di-Methyl Sulfoxide. Carbohydr. Res. 1987, 160, 57–72. [Google Scholar] [CrossRef]
- Murdoch, K.A. The Amylose-Iodine Complex. Carbohydr. Res. 1992, 233, 161–174. [Google Scholar] [CrossRef]
- Knutson, C.A.; Cluskey, J.E.; Dintzis, F.R. Properties of Amylose-Iodine Complexes Prepared in the Presence of Excess Iodine. Carbohydr. Res. 1982, 101, 117–128. [Google Scholar] [CrossRef]
- Murakami, H. Electronic Structure of the Amylose-Iodine Complex. J. Chem. Phys. 1954, 22, 367–374. [Google Scholar] [CrossRef]
- Nishimura, T.; Yajima, H.; Kubota, S.; Ishii, T.; Endo, R. Polymer Effect on the Iodine Coloring Species Responsible for the Spectroscopic Properties of Amylose-Iodine Complexes. Kobunshi Ronbunshu 1990, 47, 717–725. [Google Scholar] [CrossRef]
- Nishimura, T.; Yajima, H.; Kubota, S.; Ishii, T.; Endo, R. Effect of I- Concentration on the Optical Properties of Amylose-Iodine Complexes. Kobunshi Ronbunshu 1988, 45, 945–952. [Google Scholar] [CrossRef]
- Rawlings, P.K.; Schneider, F.W. Models for Competitive Cooperative Linear Adsorption. The Amylose–Iodine–Iodide Complex. J. Chem. Phys. 1970, 52, 946–952. [Google Scholar] [CrossRef]
- Kuge, T.; Ono, S. Advances in Carbohydrate Chemistry and Biochemistry. Bull. Chem. Sot. Jpn., 1960, 33, 1269–1272. [Google Scholar] [CrossRef]
- Teitelbaum, R.C.; Ruby, S.L.; Marks, T.J. A Resonance Raman/Iodine Moessbauer Investigation of the Starch-Iodine Structure. Aqueous Solution and Iodine Vapor Preparations. J. Am. Chem. Soc. 1980, 102, 3322–3328. [Google Scholar] [CrossRef]
- Cesàro, A.; Jerian, E.; Saule, S. Physicochemical Studies of Amylose and Its Derivatives in Aqueous Solutions: Thermodynamics of the Iodine-Triiodide Complex. Biopolymers 1980, 19, 1491–1506. [Google Scholar] [CrossRef]
- Nishimura, T.; Yajima, H.; Ishii, T.; Endo, R. Study of the Bluing Mechanism of Amylose-Iodine Complexes by CD Stopped-Flow Method. Kobunshi Ronbunshu 1991, 48, 525–528. [Google Scholar] [CrossRef]
- Wolf, R.; Schulz, R.C. Optical Rotatory Dispersion of the Starch Iodine Complex. Part 2. J. Macromol. Sci. Part A - Chem. 1968, 2, 821–832. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Vladimirov, A.V.; Volkova, T.V. The Concentration Dependences of the Stability Constants of Iodine-Iodide-Amylose Complexes in Aqueous Solutions of Electrolytes. Russ. J. Phys. Chem. A 2004, 78, 1584–1587. [Google Scholar]
- Yamamoto, M.; Sano, T.; Harada, S.; Yasunaga, T. Interaction of Amylose with Iodine. II. Kinetic Studies of the Complex Formation by the Temperature-Jump Method. Bull. Chem. Soc. Jpn. 1982, 55, 3702–3706. [Google Scholar] [CrossRef]
- Foster, J.F.; Zucker, D. Length of the Amylose–Iodine Complex as Determined by Streaming Dichroism. J. Phys. Chem. 1952, 56, 170–173. [Google Scholar] [CrossRef]
- Nishimura, T.; Yajima, H.; Ishii, T.; Endo, R. Effect of Molecular Weight of Amylose on the Iodine Coloring Species Responsible for the Optical Properties of Amylose-Iodine Complexes. Kobunshi Ronbunshu 1989, 46, 537–544. [Google Scholar] [CrossRef]
- Noltemeyer, M.; Saenger, W. Topography of Cyclodextrin Inclusion Complexes. 12. Structural Chemistry of Linear.Alpha.-Cyclodextrin-Polyiodide Complexes. X-Ray Crystal Structures of (Alpha.-Cyclodextrin)2.LiI3.I2.8H2O and (.Alpha.-Cyclodextrin)2.Cd0.5).I5.27H2O. Models for the Blue. J. Am. Chem. Soc. 1980, 102, 2710–2722. [Google Scholar] [CrossRef]
- Betzel, C.; Hingerty, B.; Noltemeyer, M.; Weber, G.; Saenger, W.; Hamilton, J.A. (?-Cyclodextrin)2 KI7 9 H2O. Spatial Fitting of a Polyiodide Chain to a given Matrix. J. Incl. Phenom. 1983, 1, 181–191. [Google Scholar] [CrossRef]
- Bowmaker, G. Bonding and Nuclear Quadrupole Coupling in Linear Pentaiodide Ions. Aust. J. Chem. 1978, 31, 2713. [Google Scholar] [CrossRef]
- Nimz, O.; Geßler, K.; Usón, I.; Laettig, S.; Welfle, H.; Sheldrick, G.M.; Saenger, W. X-Ray Structure of the Cyclomaltohexaicosaose Triiodide Inclusion Complex Provides a Model for Amylose–Iodine at Atomic Resolution. Carbohydr. Res. 2003, 338, 977–986. [Google Scholar] [CrossRef]
- Ziegast, G.; Pfannemüller, B. Resonance Raman Studies of Amaylose—Iodine Complexes. Int. J. Biol. Macromol. 1982, 4, 419–424. [Google Scholar] [CrossRef]
- Heyde, M.E.; Rimai, L.; Kilponen, R.G.; Gill, D. Resonance-Enhanced Raman Spectra of Iodine Complexes with Amylose and Poly(Vinyl Alcohol), and of Some Iodine-Containing Trihalides. J. Am. Chem. Soc. 1972, 94, 5222–5227. [Google Scholar] [CrossRef]
- Yu, X. The Complex of Amylose and Iodine. Carbohydr. Res. 1996, 292, 129–141. [Google Scholar] [CrossRef]
- Okuda, M.; Hiramatsu, T.; Yasuda, M.; Ishigaki, M.; Ozaki, Y.; Hayashi, M.; Tominaga, K.; Chatani, E. Theoretical Modeling of Electronic Structures of Polyiodide Species Included in α-Cyclodextrin. J. Phys. Chem. B 2020, 124, 4089–4096. [Google Scholar] [CrossRef]
- Mizuno, M.; Tanaka, J.; Harada, I. Electronic Spectra and Structures of Polyiodide Chain Complexes. J. Phys. Chem. 1981, 85, 1789–1794. [Google Scholar] [CrossRef]
- Teitelbaum, R.C.; Ruby, S.L.; Marks, T.J. On the Structure of Starch-Iodine. J. Am. Chem. Soc. 1978, 100, 3215–3217. [Google Scholar] [CrossRef]
- Hach, R.J.; Rundle, R.E. The Structure of Tetramethylammonium Pentaiodide 1,1a. J. Am. Chem. Soc. 1951, 73, 4321–4324. [Google Scholar] [CrossRef]
- Herbstein, F.H.; Kapon, M. Zigzag Chains of Alternating Molecules and Triiodide Ions in Crystalline (Phenacetin)2.HI5. Nat. Phys. Sci. 1972, 239, 153–154. [Google Scholar] [CrossRef]
- Haddock, A.; Steidemann, M.; Readnour, M. Polyiodide Equilibria in Aqueous Solutions of Iodine and Iodide. Synth. React. Inorg. Met. -Org. Chem. 1979, 9, 39–56. [Google Scholar] [CrossRef]
- Ramette, R.W.; Sandford, R.W. Thermodynamics of Iodine Solubility and Triiodide Ion Formation in Water and in Deuterium Oxide. J. Am. Chem. Soc. 1965, 87, 5001–5005. [Google Scholar] [CrossRef]
- Sekine, T. Abstracts. Nippon. Kagaku Zassi 1969, 90, 951–983. [Google Scholar] [CrossRef]
- Reddy, J.M.; Knox, K.; Robin, M.B. Crystal Structure of HI 3 ·2C 6 H 5 CONH 2 : A Model of the Starch—Iodine Complex. J. Chem. Phys. 1964, 40, 1082–1089. [Google Scholar] [CrossRef]
- Mould, D.L. Potentiometric and Spectrophotometric Studies of Complexes of Hydrolysis Products of Amylose with Iodine and Potassium Iodide. Biochem. J. 1954, 58, 593–600. [Google Scholar] [CrossRef]
- Bhide, S.V.; Kale, N.R. Ligand-Induced Structural Changes in Amylose Partially Complexed with Iodine. Biochim. Biophys. Acta (BBA) - General. Subj. 1976, 444, 719–726. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Bernal-Uruchurtu, M.I.; Kerenskaya, G.; Janda, K.C. Structure, Spectroscopy and Dynamics of Halogen Molecules Interacting with Water. Int. Rev. Phys. Chem. 2009, 28, 223–265. [Google Scholar] [CrossRef]
- Kireev, S.V.; Shnyrev, S.L. Study of Molecular Iodine, Iodate Ions, Iodide Ions, and Triiodide Ions Solutions Absorption in the UV and Visible Light Spectral Bands. Laser Phys. 2015, 25, 075602. [Google Scholar] [CrossRef]
- Prasanna; Shrikanth, B. K.; Hegde, M.S. Formation and Structure of Iodine: Water (H2O-I2) Charge-Transfer Complex. J. Chem. Sci. 2021, 133, 51. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).