Submitted:
17 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
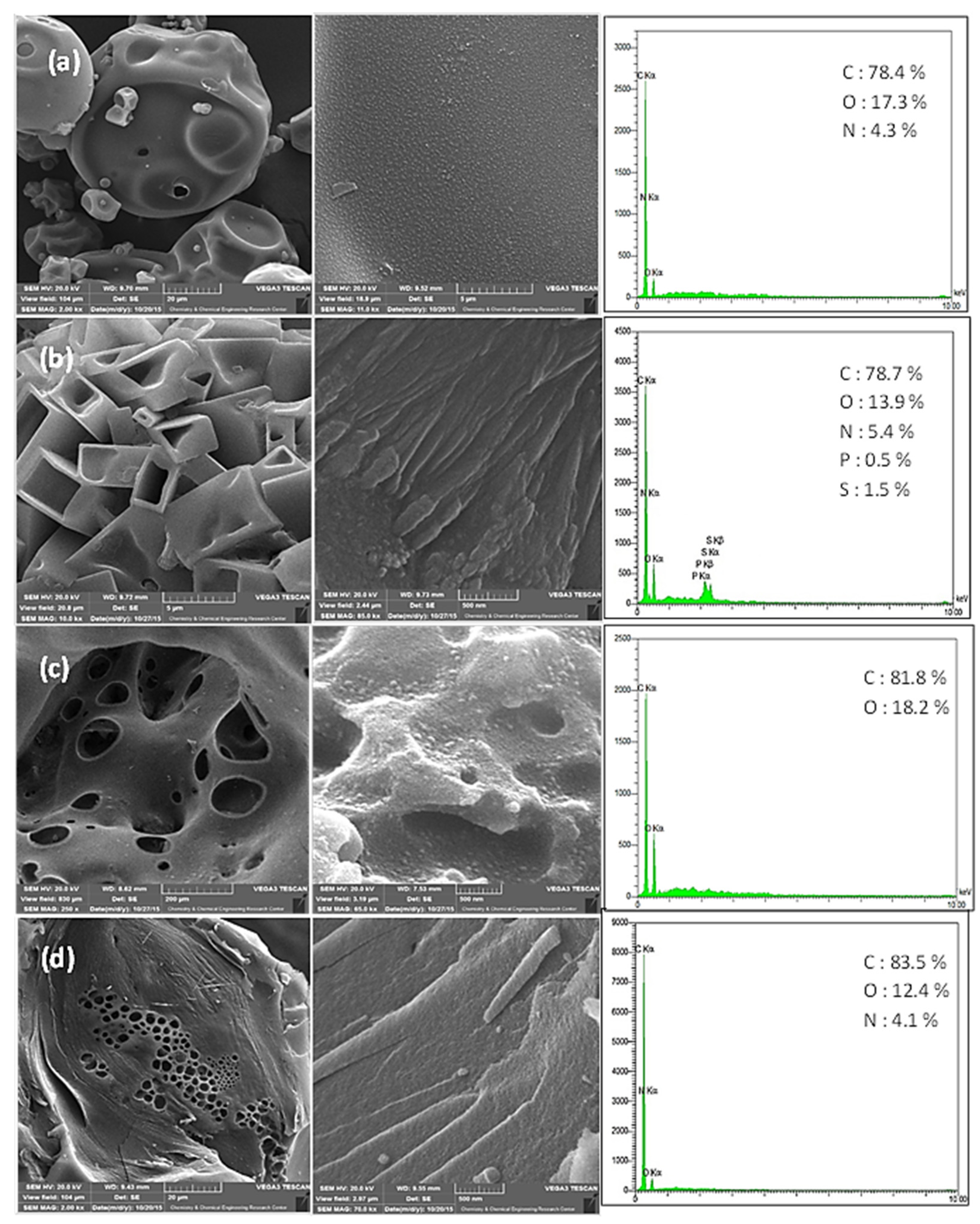
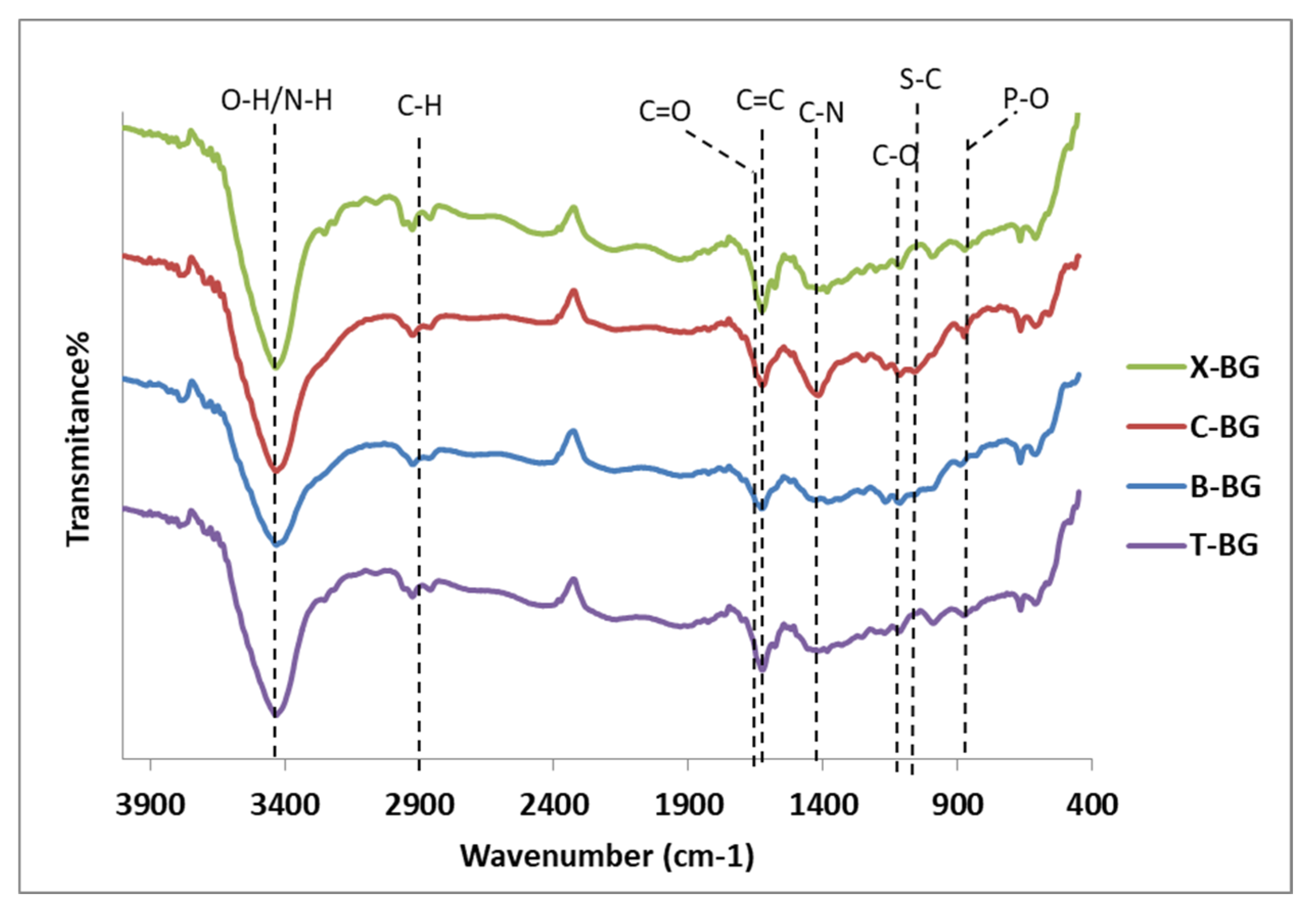
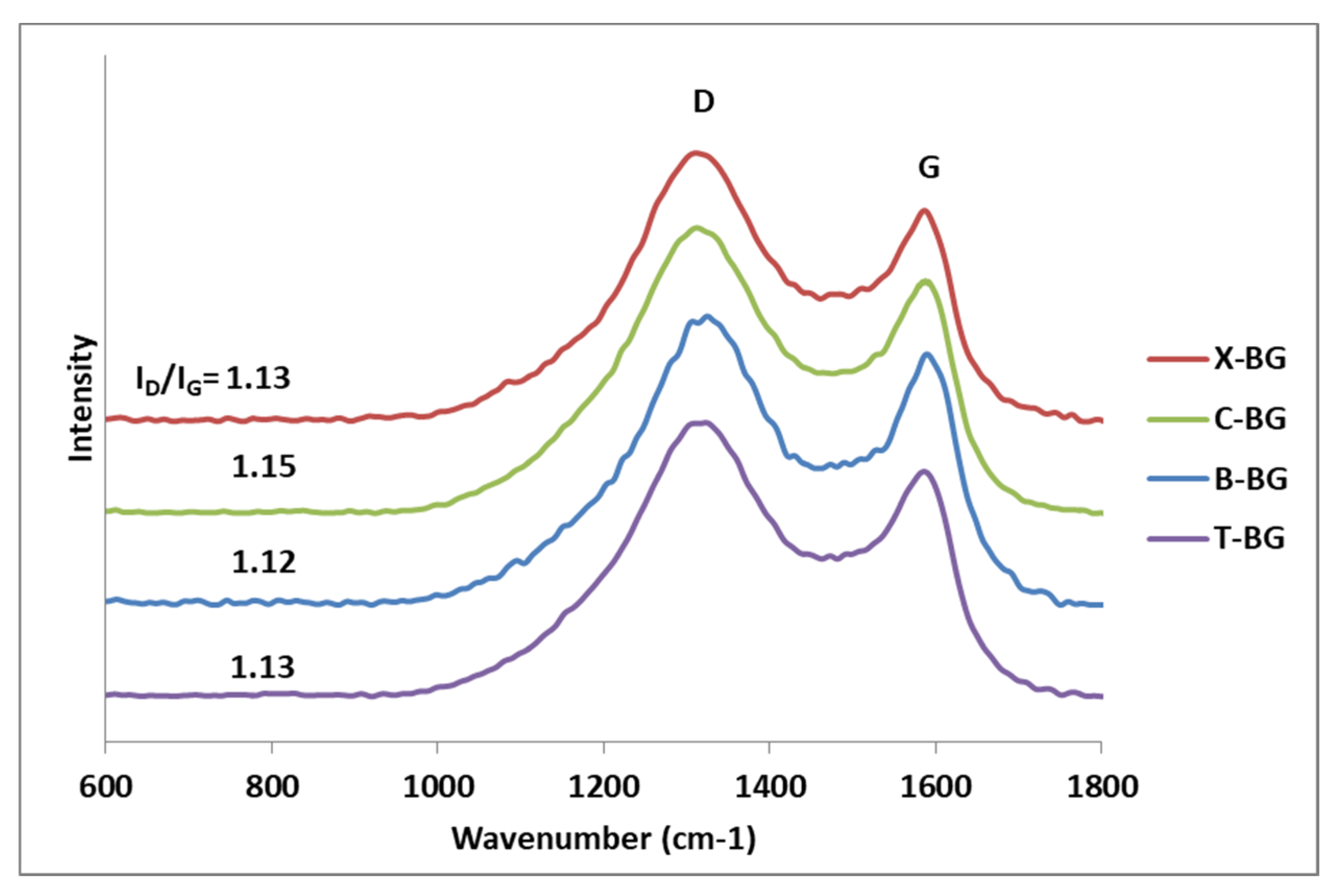
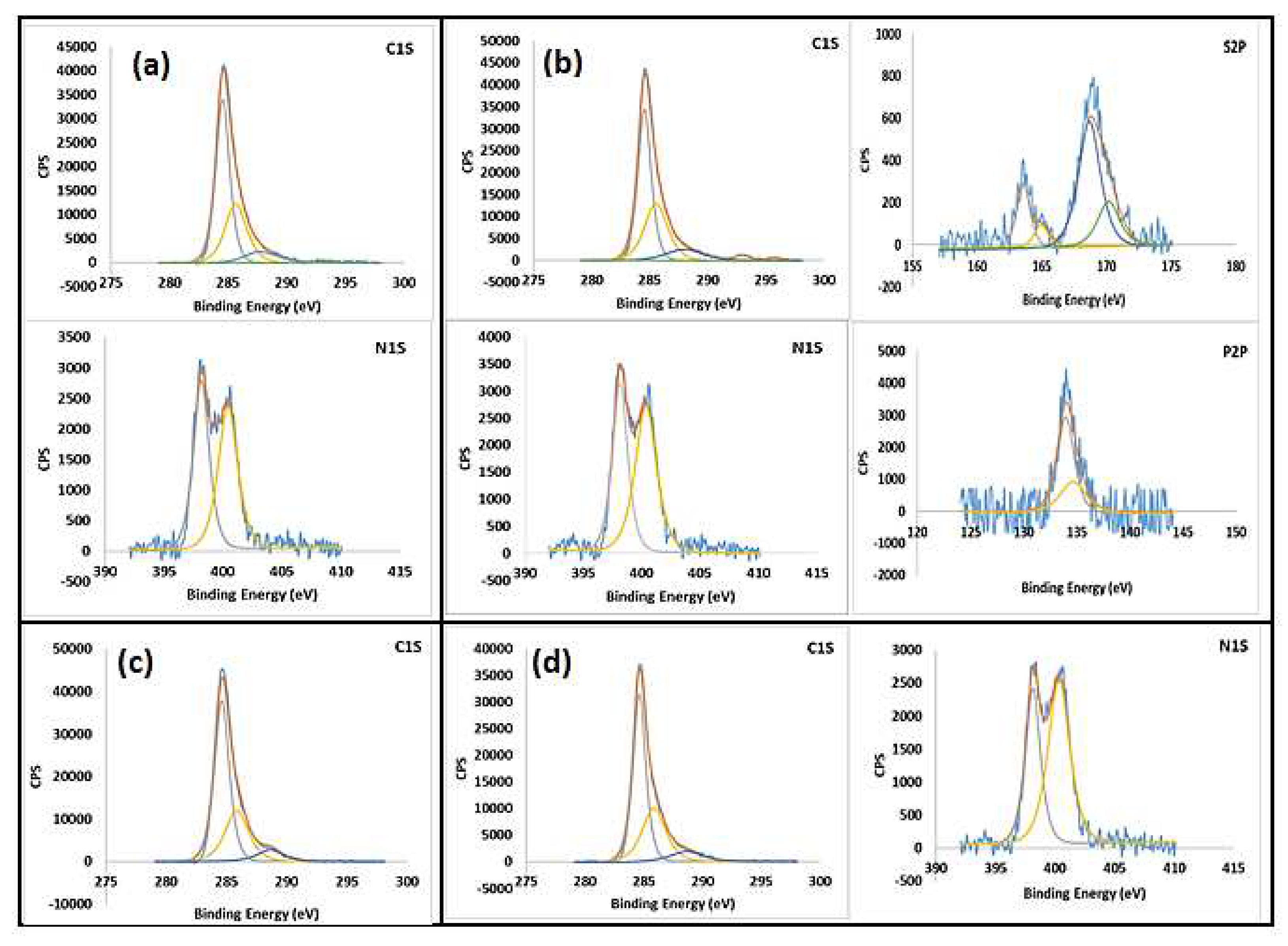
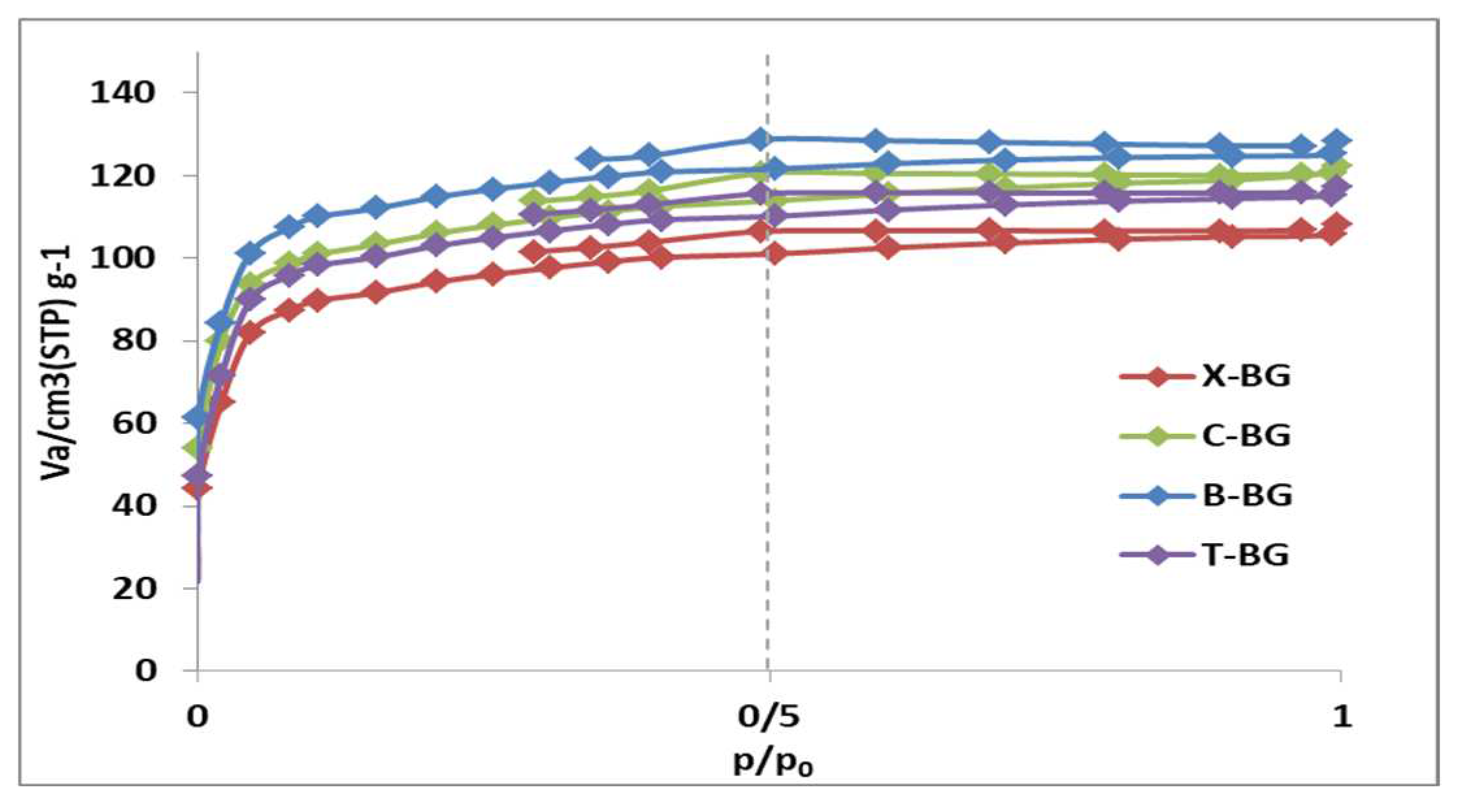
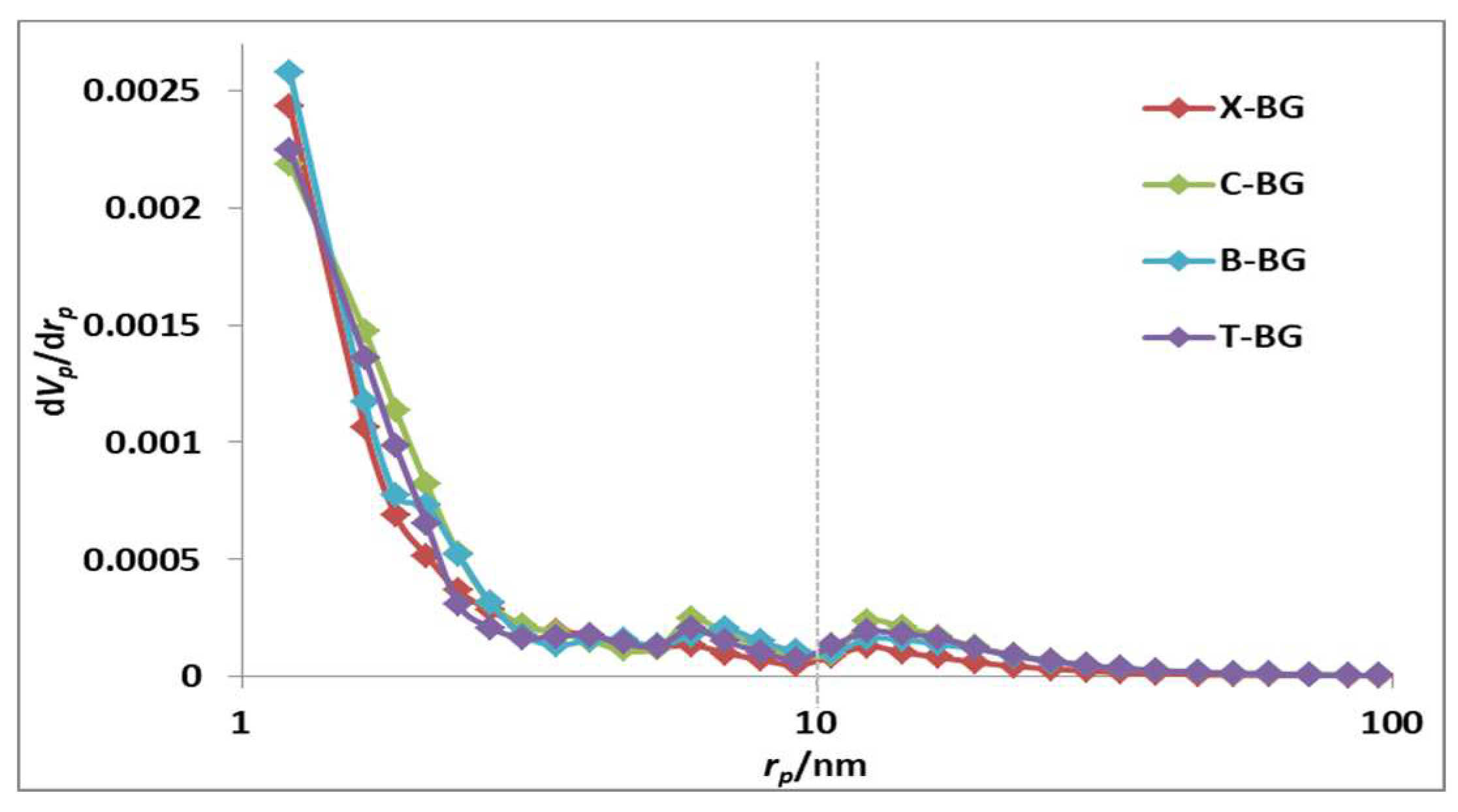
2.1. Characterization of synthesize bio-graphenes
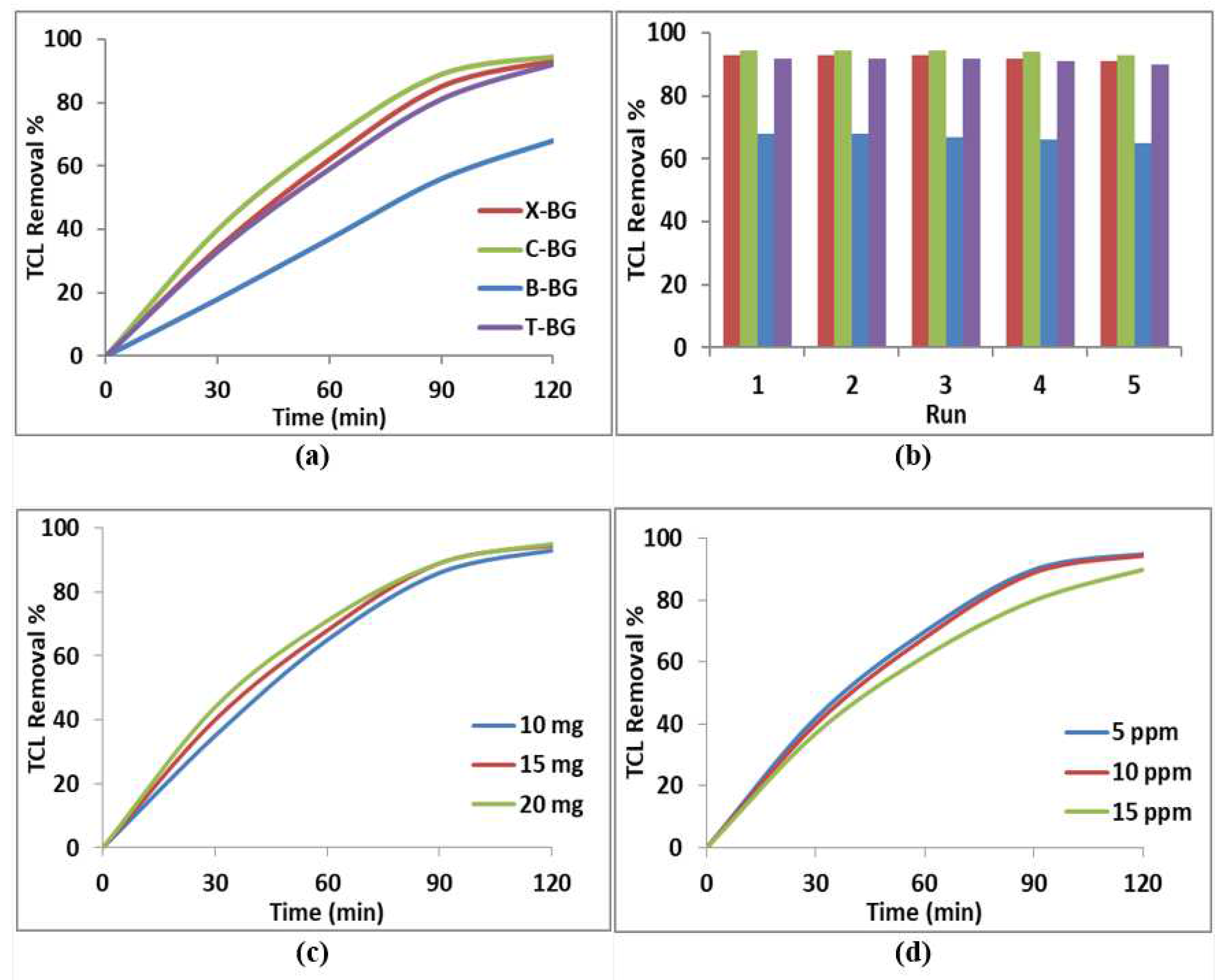
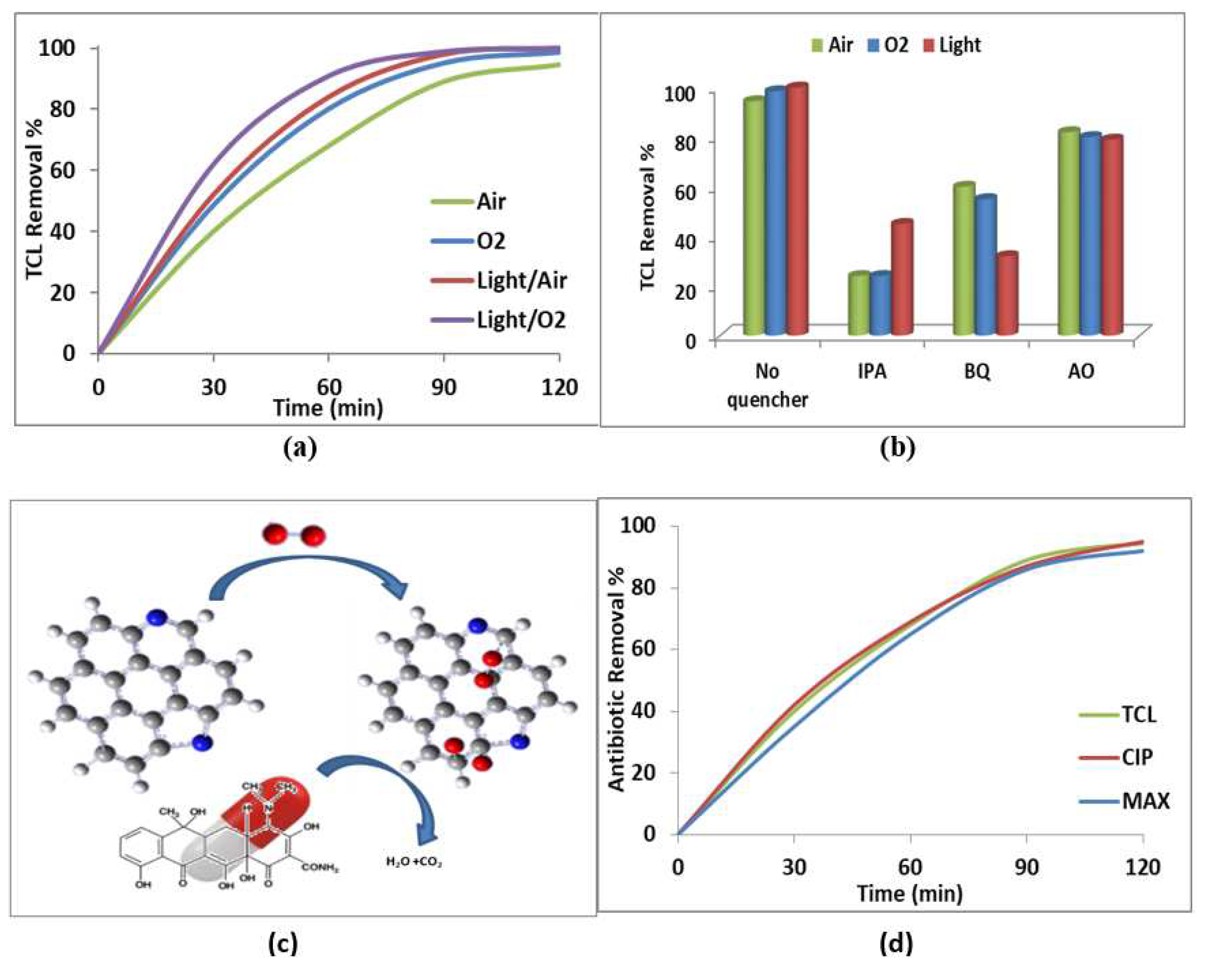
2.2. Catalytic Study
3. Experimental
3.1. Materials and Methods
1.2. Synthesis of bio-graphenes
1.2. Catalytic study
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Larsson, D.J.; de Pedro, C.; Paxeus, N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals, J. hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.L.; Chang, J.S; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater, J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef]
- Ghasemzadeh-mohammadi, V.; Zamani, B.; Afsharpour, M.; Mohammadi, A. Extraction of caffeine and catechins using microwave-assisted and ultrasonic extraction from green tea leaves: an optimization study by the IV-optimal design. Food Sci. Biotech. 2017, 26, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices–a review, J. environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yan, Z.; Zhang, Y.; Xu, W.; Kong, D.; Shan, Z.; Wang, N. Behavior of antibiotic resistance genes under extremely high-level antibiotic selection pressures in pharmaceutical wastewater treatment plants, Sci. Total Environ., 2018, 612, 119–128. [Google Scholar] [CrossRef]
- Shimizu, Y.; Okuno, Y.-i.; Uryu, K.; Ohtsubo, S.; Watanabe, A. Filtration characteristics of hollow fiber microfiltration membranes used in membrane bioreactor for domestic wastewater treatment, Water Res. 1996, 30, 2385–2392. 30.
- Afsharpour, M.; Khomad, E. Synthesis of bio-inspired porous silicon carbides using Cortaderia selloana and Equisetum arvense grasses as remarkable sulfur adsorbents. Intern. J. Environ. Sci Technol. 2019, 16, 3125–3134. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the Fate and Removal of Antibiotics in Engineered Biological Treatment Systems: A Critical Review, Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Zhu, G.; Lu, Y. ; AL-Falahi,A. H.; Lu, Y.; Huang, Sh.; Wan, Z. Removal of antibiotics from wastewater and its problematic effects on microbial communities by bioelectrochemical Technology: Current knowledge and future perspectives, Environ. Eng. Res. 2021, 26, 190405. [Google Scholar]
- Jiang, F.; Qiu, B.; Sun, D. Advanced degradation of refractory pollutants in incineration leachate by UV/Peroxymonosulfate, Chem. Eng. J. 2018, 349, 338–346. [Google Scholar]
- Gomi, L.S.; Afsharpour, M. Porous MoO3@SiC hallow nanosphere composite as an efficient oxidative desulfurization catalyst. Appl. Organometal. Chem. 2019, 33, e4830. [Google Scholar] [CrossRef]
- Foroughi, M.; Khiadani, M.; Kakhki, S.; Kholghi, V.; Naderi, Kh.; Yektay, S. Effect of ozonation-based disinfection methods on the removal of antibiotic resistant bacteria and resistance genes (ARB/ARGs) in water and wastewater treatment: a systematic review, Sci. Total Environ. 2022, 811, 151404. [Google Scholar] [CrossRef] [PubMed]
- Gomi, L.S.; Afsharpour, M.; Lianos, P. Novel Porous SiO2@SiC Core-Shell Nanospheres Functionalized with an Amino Hybrid of WO3 as an Oxidative Desulfurization Catalyst. J. Indust. Eng. Chem. 2020, 89, 448–457. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Z.; Gao, J.; Xie, Y.; Li, L.; Qin, S.; Wang, Q.; Mao, D.; Luo, Y. Simultaneous removal of antibiotics and antibiotic resistance genes from pharmaceutical wastewater using the combinations of up-flow anaerobic sludge bed, anoxic-oxic tank, and advanced oxidation technologies, Water Res. 2019, 159, 511–520. 159.
- Afsharpour, M.; Darvishi-Farash, S. Novel synthesis of siligraphene/tungstates (g-SiC/AWO) with promoted transportation of photogenerated charge carriers via direct Z-scheme heterojunctions, Sci. Report. 2023, 13, 10022. [Google Scholar] [CrossRef] [PubMed]
- Afsharpour, M.; Elyasi, M.; Javadiaan, H.R. A Novel N-Doped Nanoporous Bio-Graphene Synthesized from Pistacia lentiscus Gum and Its Nanocomposite with WO3 Nanoparticles: Visible-Light-Driven Photocatalytic Activity. Molecule. 2021, 26, 6569. [Google Scholar] [CrossRef] [PubMed]
- Gomi, L.S.; Afsharpour, M.; Ghasemzadeh, M.; Lianos, P. Bio-inspired N, S-doped siligraphenes as novel metal-free catalysts for removal of dyes in the dark. J. Mol. Liquid. 2019, 295, 111657. [Google Scholar] [CrossRef]
- Afsharpour, M.; Amooee, S. Porous biomorphic silica@ZnO nanohybrids as the effective photocatalysts under visible light, Environ. Sci. Pollut. Res. 2022, 29, 49784–49795. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Sun, J.; Yao, F.; Wang, S.; Wang, Y.; Wang, X.; Li, X.; Niu, C.; Wang, D. Enhanced photocatalytic degradation of tetracycline by AgI/BiVO4 heterojunction under visible-light irradiation: mineralization efficiency and mechanism, ACS appl. Mater.Interface. 2016, 8, 32887–32900. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Y.; Yang, X.; Li, F.; Li, A.; Liu, Y.; Zhang, J.; Zhou, Z.; Ni, L. Fabricating carbon quantum dots doped ZnIn2S4 nanoflower composites with broad spectrum and enhanced photocatalytic Tetracycline hydrochloride degradation, Mater. Res. Bull. 2018, 97, 158–168. [Google Scholar] [CrossRef]
- Wang, H.; Yao, H.; Pei, J.; Liu, F.; Li, D. Photodegradation of tetracycline antibiotics in aqueous solution by UV/ZnO, Desalin. Water Treatment, 2016, 57, 19981–19987. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Z.; Wang, Y. Visible active N-doped TiO2/reduced graphene oxide for the degradation of tetracycline hydrochloride, Chem. Phys. Lett. 2018, 691, 408–414. [Google Scholar]
- Wang, H.; Ye, Z.; Liu, C.; Li, J.; Zhou, M.; Guan, Q.; Lv, P.; Huo, P.; Yan, Y. Visible light driven Ag/Ag3PO4/AC photocatalyst with highly enhanced photodegradation of tetracycline antibiotics, Appl. Surface Sci. 2015, 353, 391–399. [Google Scholar] [CrossRef]
- Ma, N.; Xu, J.; Bian, Z.; Yang, Y.; Zhang, L.; Wang, H. BiVO4 plate with Fe and Ni oxyhydroxide cocatalysts for the photodegradation of sulfadimethoxine antibiotics under visible-light irradiation, Chem. Eng. J. 2020, 389, 123426. [Google Scholar]
- Rana, A.; Kumar, A.; Sharma, G.; Naushad, M.; Bathula, Ch.; Stadler, F.J. Pharmaceutical pollutant as sacrificial agent for sustainable synergistic water treatment and hydrogen production via novel Z- scheme Bi7O9I3/B4C heterojunction photocatalysts, J. Mol. Liquid. 2021, 343, 17652. [Google Scholar] [CrossRef]
- Ivan, R.; Popescu, C.; Antohe, V.A.; Antohe, S.; Negrila, C.; Logofatu, C.; del Pino, A.P.; György, E. Iron oxide/hydroxide–nitrogen doped graphene-like visible-light active photocatalytic layers for antibiotics removal from wastewater, Sci. Report. 2023, 13, 2740. [Google Scholar]
- Song, Y.; Tian, J.; Gao, S.; Shao, P.; Qi, J.; Cui, F. Photodegradation of sulfonamides by gC3N4 under visible light irradiation: effectiveness, mechanism and pathways, Appl. Catal. B: Environ. 2017, 210, 88–96. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Feng, Y.; Xie, Z.; Zhang, Q.; Jin, X.; Liu, H.; Liu, Y.; Lv, W.; Liu, G. Facile synthesis of carbon quantum dots loaded with mesoporous gC3N4 for synergistic absorption and visible light photodegradation of fluoroquinolone antibiotics, Dalton Trans. 2018, 47, 1284–1293. 47.
- Li, C.; Sun, Z.; Huang, W.; Zheng, S. Facile synthesis of g-C3N4/montmorillonite composite with enhanced visible light photodegradation of rhodamine B and tetracycline, J. Taiwan Inst. Chem. Eng. 2016, 66, 363–371. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, B.; Yang, j.; Yuan, Y.; Zhang, J. Persulfate-enhanced degradation of ciprofloxacin with SiC/g-C3N4 photocatalyst under visible light irradiation, Chemosphere. 2021, 276, 130217. 276.
- Afsharpour, M.; Behtooei, H.R.; Shakiba, M.; Martí, V. Novel N,P,S co-doped graphenic SiC layers (g-SiC) in visible-light photodegradation of antibiotics and inactivating the bacteria. Process Saf. Environ. Protect. 2022, 166, 704–717. [Google Scholar] [CrossRef]
- Darvishi-Farash, S.; Afsharpour, M.; Heidarian, J. Novel siligraphene/g-C3N4 composites with enhanced visible light photocatalytic degradations of dyes and drugs. Environ. Sci. Pollut. Res. 2021, 28, 5938–5952. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Wang, Sh. Catalytic degradation of antibiotics by metal-free catalysis over nitrogen-doped grapheme, Catal. Today. 2020, 357, 341–349. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, X.; Zhang, X.; Yang, K.; Li, Y. Simultaneous degradation of trace antibiotics in water by adsorption and catalytic oxidation induced by N-doped reduced graphene oxide (N-rGO): synergistic mechanism, Mater. Res. Express 2022, 9, 065601. [Google Scholar] [CrossRef]
- Siddiqui, A.S.; Ahmad, M.A.; Nawaz, M.H.; Hayat, A.; Nasir, M. Nitrogen-doped graphene oxide as a catalyst for the oxidation of Rhodamine B by hydrogen peroxide: application to a sensitive fluorometric assay for hydrogen peroxide, Microchim. Acta. 2020, 187, 47. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; O’Donnell, K.; Sun, H.; Wang, Y.; Wang, Sh. Sulfur and Nitrogen Co-Doped Graphene for Metal-Free Catalytic Oxidation Reactions, Small. 2015, 11, 3036–3044. 11.
- Long, J.; Xie, X.; Xu, J.; Gu, Q. ; Chen, L; Wang, X. Nitrogen-Doped Graphene Nanosheets as Metal-Free Catalysts for Aerobic Selective Oxidation of Benzylic Alcohols, ACS Catal. 2012, 2, 622−631. , 2.
- Luo, E.; Xiao, M.; Ge, J.; Liu, Ch.; Xing, W. Selectively doping pyridinic and pyrrolic nitrogen into a 3D porous carbon matrix through template-induced edge engineering: enhanced catalytic activity towards the oxygen reduction reaction, J. Mater. Chem. A. 2017, 5, 21709–21714. [Google Scholar] [CrossRef]
- Wang, X.X.; Zou, B.; Du, X.X.; Wang, J.N. N-doped carbon nanocages with high catalytic activity and durability for oxygen reduction, J. Mater. Chem. A, 2015, 3, 12427–12435. 3.
- Afsharpour, M.; Gomi, L.S.; Elyasi, M. Novel metal-free N-doped bio-graphenes and their MoO3 bifunctional catalysts for ultra-deep oxidative desulfurization of heavy fuel, Separ. Purif. Technol. 2021, 274, 119014. [Google Scholar] [CrossRef]
- Miao, H.; Li, Sh.; Wang, Zh.; Sun, Sh.; Kuang, M.; Liu, Zh.; Yuan, J. Enhancing the pyridinic N content of Nitrogen-doped graphene and improving its catalytic activity for oxygen reduction reaction, Intern. J. Hydrogen Energy. 2017, 42, 28298–28308. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




