1. Introduction
Post-hypoxic disorders of the cardiovascular system occupy one of the leading places in the structure of morbidity in newborns, occurring, according to various sources, in 40–70% infants who have experienced prenatal hypoxia. These disorders contribute significantly to a multitude of often severe ailments in both children and adults [
1,
2]. The mechanisms underlying the emergence of post-hypoxic cardiac disorders have received limited investigation, rendering them a pressing concern within pediatric cardiology. The clinical symptoms of this pathology in the acute period are polymorphic, often disguised as other diseases, it is often necessary to carry out differential diagnostics with congenital heart defects, congenital carditis, cardiomyopathies [
3,
4].
Experimental and clinical research has unveiled a connection between prenatal hypoxia and an increased risk of coronary disease in later adulthood. Moreover, this delayed effect of perinatal hypoxia lies in the expression of a number of factors leading to a change in the tolerance of the heart to acute oxygen deficiency at a later age. To date, there is no consensus on the phased complex therapy of post-hypoxic heart disease. Therefore, the identification of new structural, molecular and biochemical features of post-hypoxic cardiovascular disorders in newborns and the development of pharmacotherapy on this basis is of scientific interest.
In contemporary viewpoints, endothelial dysfunction and associated disorders in the NO system underlie the development of many cardiovascular diseases [
5,
6]. Under the influence of hypoxia, infection and other damaging factors, the functioning of the nitroxidergic system is disrupted, accompanied by the development of pathology on the part of organs and systems, including the cardiovascular system. Nonetheless, scant published data exist elucidating the role of the NO system in the onset of cardiovascular pathologies in newborns and the potential cardioprotective effects offered by its modulators.
Several studies have established the cardio- and endothelioprotective properties of drugs that can both increase the synthesis of NO and the bioavailability of this messenger [
7]. In this regard, we are considering L-arginine, Thiotriazoline, Angiolin, and Mildronate. L-arginine is a substrate for the formation of NO in vascular endothelial cells and exhibits antioxidant, cytoprotective, antihypoxic, and membrane-stabilizing properties [
8,
9]. Thiotriazoline (morpholinium 3-methyl-1,2,4-triazolyl-5-thioacet) is a specific scavenger of NO and its cytotoxic forms, increases the bioavailability of NO, protecting it from ROS. Thiotriazoline under conditions of ischemia enhances the compensatory activation of the malate-aspartate shuttle mechanism, reduces the inhibition of oxidation processes in the Krebs cycle while maintaining the intracellular ATP pool, exhibits hepato-, cardioprotective, anti-ischemic and antioxidant properties [
10,
11,
12].
Angiolin ([S]-2,6-diaminohexane acid 3-methyl-1,2,4-triazolyl-5-thioacecate) increases the expression of VEGF and the density of proliferating endotheliocytes of muscle-type vessels and microvasculature, increases the bioavailability of NO, preserves the ultrastructure of mitochondria at ischemia. It also enhances the production of ATP during ischemia due to the activation of compensatory activation of the malate-aspartate shuttle mechanism, increases the expression of eNOS, and exhibits endothelial, cardio-, neuroprotective, antioxidant and anti-ischemic properties [
13,
14].
Mildronate reduces the formation of carnitine from its precursor - gamma-butyrobetaine, the accumulation of the latter stimulates the synthesis of NO. Mildronate reduces carnitine-mediated transport of long-chain fatty acids across mitochondrial membranes without affecting the metabolism of short-chain fatty acids and activates an alternative energy production system - glucose oxidation [
15]. Mildronate exhibits anti-ischemic and cardioprotective properties [
16]. Thus, the search and development of approaches to the pharmacotherapy of prenatal myocardial damage based on the modulation of the NO system is an urgent task of modern pharmacology. The foregoing theoretically substantiates the prospects of studying the modulators of the NO system with different mechanisms of action - L-arginine, Thiotriazoline, Angiolin and Mildronate as a means of cardioprotection of posthypoxic disorders of the cardiovascular system in newborns.
For the first time, the proposed scheme for the use of drugs of the NO system (L-arginine, Thiotriazoline, Angiolin and Mildronate) in the complex therapy of posthypoxic cases of the cardiovascular system in newborns, which is often interrupted by NO-dependent mechanisms of damage, will be experimentally confirmed. for the first time, a protective effect of modulators of the NO system (L-arginine, Thiotriazoline, Angiolin and Mildronate) was discovered, aimed at inhibiting apoptosis, nitrosative stress, normalizing energy metabolism, the NO system of the heart and restoring its electrophysiological activity. On the basis of the conducted studies, the possibility of using L-arginine, thiotriazoline, angiolin and mildronate in the complex therapy of disorders of the cardiovascular system of newborns after prenatal hypoxia will be theoretically substantiated and experimentally proven.
The aim of the research: to conduct a primary assessment of the cardioprotective effect of L-arginine, Thiotriazoline, Angioline and Mildronate on the effect on molecular markers of the cardiovascular system of rats after prenatal hypoxia.
2. Materials and Methods
2.1. PH experimental model and laboratory animal characteristics.
Zaporizhzhia State Medical University Commission on Bioethics approved the experimental study (protocol No. 33 of June 26, 2021).
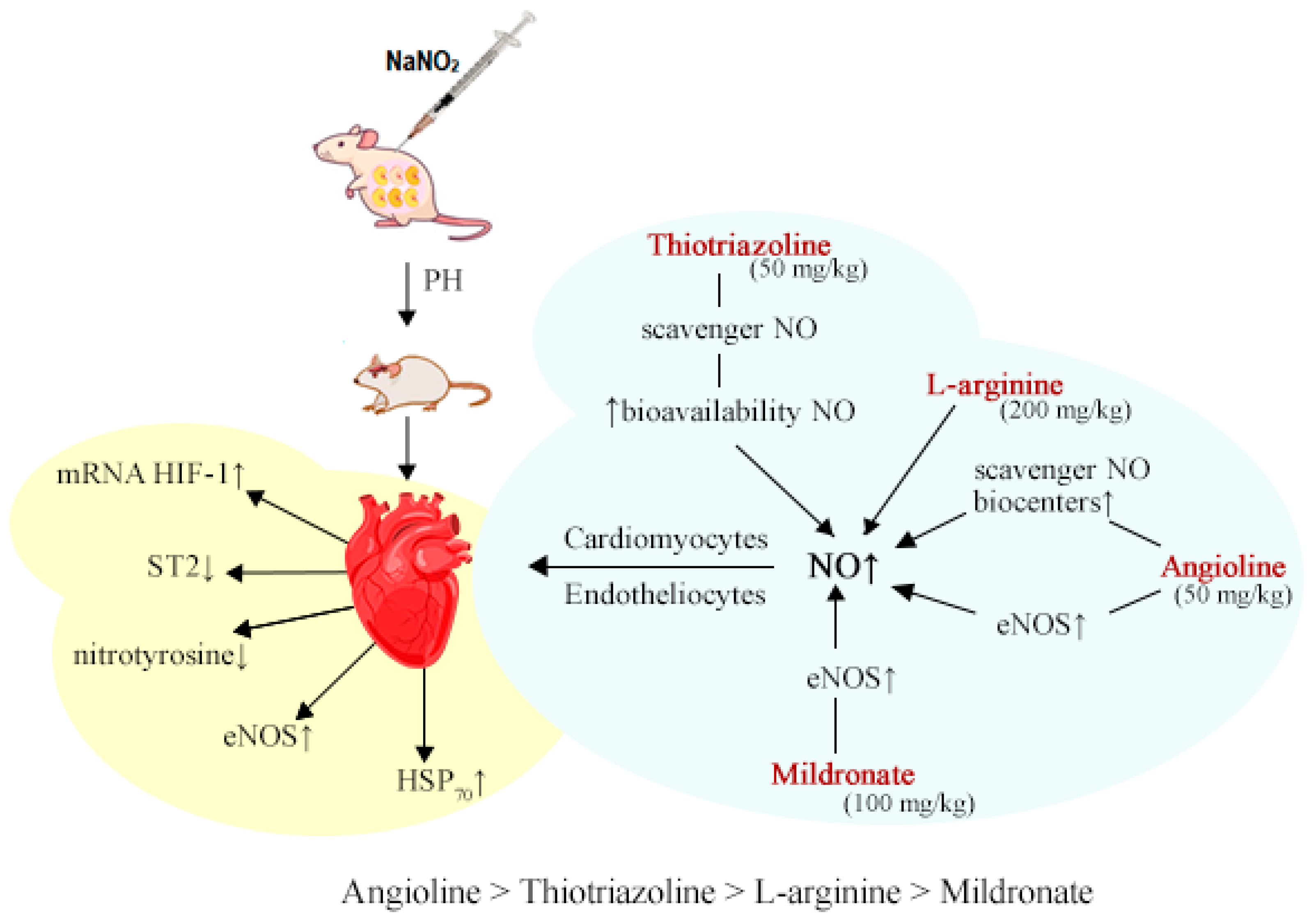
Fifty outbred white rat females and ten males, each weighing 220-240 g and aged 4.5 months, were used in the studies. They were taken from the vivarium of the Institute of Pharmacology and Toxicology of the National Medical Academy of Ukraine. The rats lived in typical vivarium settings, which included 20–25°C, 50–55% humidity, daylight, a feed that was appropriate for this species of laboratory animals, and unlimited access to water. The chronic hematic nitrite-induced PH model was employed in this study [
17,
18]. For a fixed term of pregnancy, mature male rats were placed with virgin female rats with a ratio of 2 males per 4 females. The Pregnancy period was counted starting from the discovery of spermatozoids in the vaginal smear (day 1 of the pregnancy). Modelling hematic hypoxia was performed in the prenatal period of development by daily intraperitoneal administration of sodium nitrite solution to pregnant female rats from day 16 to day 21 of the pregnancy at 50 mg/kg (the dose causing moderate hypoxia) [
17]. Control pregnant rats received physiological solution in the same regime. The progeny was divided into groups: 1, healthy pups from females with physiologically normal pregnancy which received physiological solution; 2, control group of pups after PH which received physiological solution daily; 3– 6 groups of pups after PH that received drugs daily from postnatal day 1 to day 30.
2.2. Justification for the selected drugs and their characteristics.
We selected medicines that have been shown in experiments to affect the NO system):
1 Thiotriazoline (Morpholinium-3-methyl-1,2,4- triazolyl-5-thioacetic acid) (2.5% solution for injections, “Arterium”, Ukraine), metabolitotropic cardioprotector and antioxidant, 50 mg/kg, i/p [
19].
2. Angiolin ([S]-2,6-diaminohexane acid 3-methyl-1,2,4-triazolyl-5-thioacecate) (substance, RPA “Farmatron”, Ukraine), anti-ischemic, endothelium protective drug, 50 mg/kg, i/p [
13,
20].
3. L-arginine (42% solution for injection in vial, Tivortin, Yuria-pharm, Ukraine), an NO precursor; it mitigates disruptions in the nitroxidergic system in ischemia, 200 mg/kg, i/p [
21,
22].
4. Mildronate (2-(2-carboxyethyl)-1,1,1-trimethylhydrazinium) (10% injection solution in ampoules, Grindex (Latvia), metabolitotropic agent, 100 mg/kg, i/p [
15].
Rats were withdrawn from the experiment on day 30 and 60 under thiopental anesthesia (40 mg/kg). The blood from the celiac artery were harvested for studies.
2.3. Preparation of biological material
Blood was taken from the abdominal aorta by syringe, serum was separated by centrifugation at +4°C at 1500 rpm for 20 min on an Ependorff 5804R centrifuge. The apical part of the heart was placed in Bouin's fixative for 24 hours. After standard procedure of tissue dehydration and its impregnation with chloroform and paraffin, the myocardium was cast in paraplast (MkCormick, USA). Serial histological sections 5 μm thick were prepared on a Microm-325 rotational microscope (Microm Corp., Germany), which were then used for real-time PCR studies after treatment with 0-xylene and ethanol
2.4. Enzyme-linked immunoassay.
The technique is based on solid-phase enzyme-linked sandwich immunosorbent assay. The level of the heat-shock protein HSP70, was measured in the mitochondrial and cyto-plasmatic fractions of the brain by enzyme-linked immunoassay using AMP'DR HSP70 high sensitivity ELISA kit # ENZ-KIT-101-0001, Enzo (Sweden). The concentration of HSP70 was expressed in ng/mL. Also, the molecular marker of myocardial damage ST2 protein was determined in the blood serum by the solid-phase immunosorbent sandwich ELISA method using the Critical Diagnostics Presage® ST2 Assay kit (REF# BC-1065, USA). The concentration of ST2 was expressed in ng/mL. The activity of endothelial NO synthase (eNOS) was determined in blood serum by enzyme immunoassay (Cloud-Clone Corporation kit, #PAA868Ra01, USA). The con-centration of eNOS, was expressed in pg/mL. Nitrotyrosine was determined by sol-id-phase immunosorbent sandwich ELISA, ELISA Kit (Cat. No. HK 501-02, Netherlands) from Hycult Biotech and expressed in nM/mL.
2.5. Polymerase Chain Reaction in Real-Time
Real-time PCR (Polymerase Chain Reaction) amplification was performed to quantify the expression levels of HIF-1 mRNA , using Maxima SYBR Green/ROX qPCR Master Mix (2x) (ThermoScientific, USA) with gene-specific primers on a Biorad CFX 96 Real-Time PCR Detection System. The reaction mixture contained 10 µL of 2x Maxima SYBR Green/ROX qPCR Master Mix, 0.5 µL of each gene-specific primer, 2 µL of cDNA template, and nuclease-free water to a final volume of 20 µL. The PCR cycling conditions involved initial denaturation at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 15 sec, primer annealing at 60°C for 40 sec, and elongation at 72°C for 40 sec. The registration of fluorescence intensity occurred automatically at the end of the elongation stage of each cycle through the automatic SybrGreen channel.
The actin beta (Actb) gene was used as the reference gene to normalize the expression levels of the target genes. The expression levels of the target genes were quantified relative to the expression of the housekeeping gene using the comparative Ct (2^-ΔΔCt) method. The Ct values were converted to relative expression values using the formula 2^-ΔCt, where ΔCt = (Ct target gene - Ct housekeeping gene). The relative expression values were then converted to Log2 values using the formula Log2 (relative expression).
2.6. Statistical analysis.
Experimental data were statistically analyzed using “StatisticaR for Windows 6.0” (StatSoft Inc., № AXXR712D833214FAN5), “SPSS16.0”, and “Microsoft Office Excel 2010” software. Prior to statistical tests, we checked the results for normality (Shapiro-Wilk and Kolmogorov-Smirnov tests). In normal distribution, intergroup differences were considered statistically significant based on the parametric Student’s t-test. If the distribution was notnormal the comparative analysis was conducted using the non-parametric Mann-Whitney U-test. To compare independent variables in more than two selections. We applied ANOVA dispersion analysis for normal distribution and the Kruskal-Wallis test for no-normal distribution. To analyze correlations between parameters, we used correlation analysis based on the Pearson or Spearman correlation coefficient. For all types of analysis, the differences were considered statistically significant at р <0.05 (95%).
3. Results
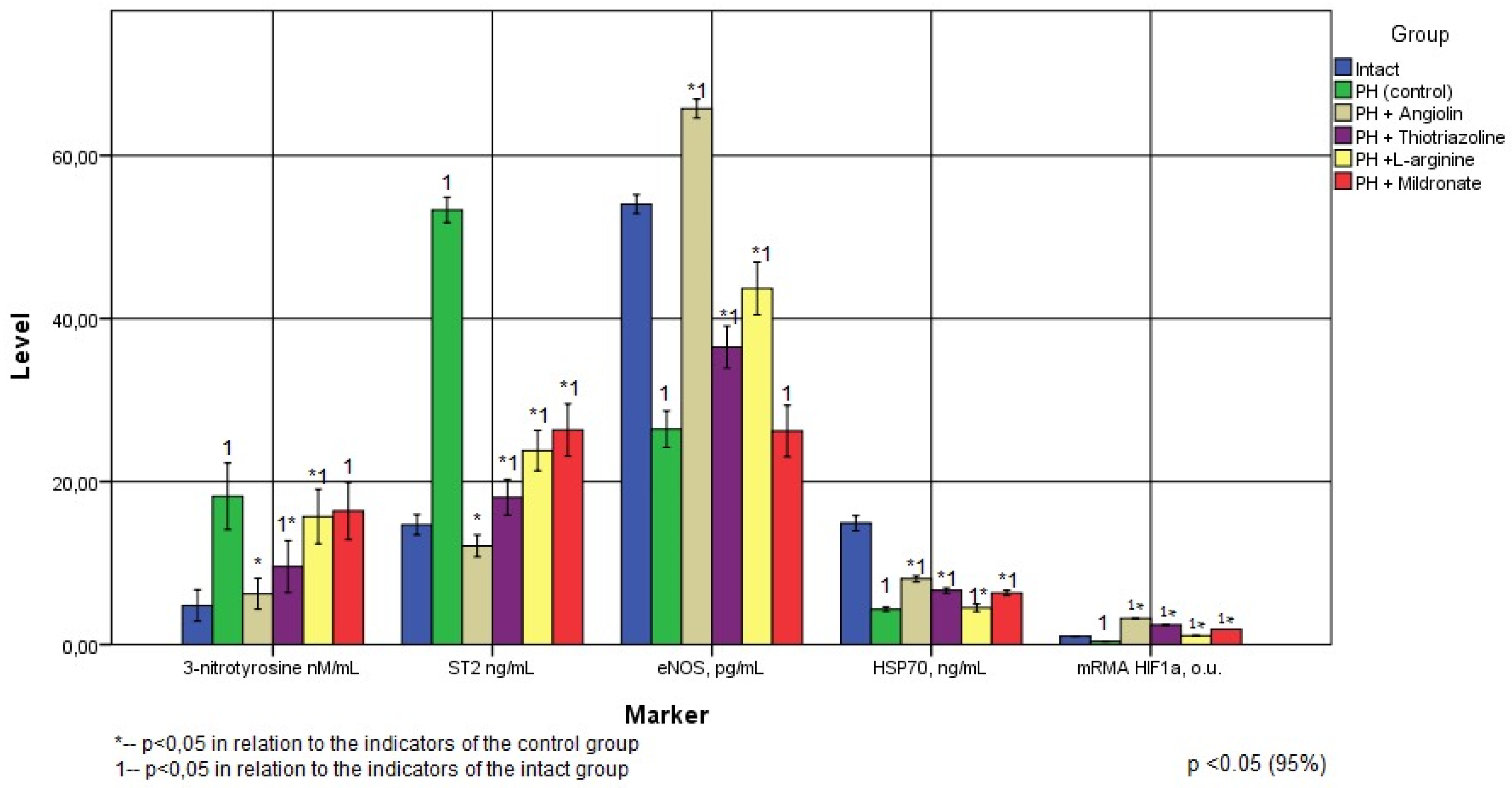
As a result of the research, it was found that in rats that underwent intrauterine hypoxia, 1 month (6.28 times) after birth and 2 months (3.63 times) after birth, a significant increase in the specific cardiomarker ST2 was observed in the blood. ST2 (Suppression of tumorogenicity 2, Growth Stimulation expressed gene 2, stimulating growth factor expressed by gene 2, aka IL1RL1) is a member of the IL-1 receptor superfamily. ST2 is the IL-33 receptor. ST2 is a marker of fibrosis and remodeling of cardiac tissue, released by cardiomyocytes and fibroblasts [
23] An increase in ST2 concentration may indicate the formation of ischemic cardiomyopathy, myocardial remodeling, and impaired contractile function of the heart. As can be seen from
Table 1 and
Table 2 (
Figure 1 and
Figure 2), even at the 2nd month of life, the concentration of this marker remains quite high.
Our investigations revealed a persistent elevation in nitrotyrosine levels within the blood of rats following intrauterine hypoxia exposure. Specifically, in 1-month-old rats, the increase was approximately 5.5 times, while in 2-month-old rats, it reached 3.8 times (as detailed in
Table 1 and
Table 2). These findings align with the outcomes of previous research indicating heightened cardiac oxidative stress in both male and female rats subjected to intrauterine hypoxia [
24]. Increased oxidative stress is closely associated with cardiovascular diseases such as hypertension and coronary heart disease and causes hypertrophy, fibrosis, and apoptosis leading to impaired cardiac function [
25].
One hallmark of myocardial injury post-prenatal hypoxia, emphasized by various researchers, is hypoxia-induced damage to myocardial mitochondria during the antenatal phase. This damage renders mitochondria a source of reactive oxygen species and pro-apoptotic proteins, simultaneously leading to energy production deterioration (indicated by decreased ATP levels). Consequently, this deterioration triggers notable oxidative stress activation and subsequent apoptosis [
26]. The data obtained by a number of researchers demonstrate that in rats after intrauterine hypoxia, there is an increase in the mitochondrial cytochrome-C protein in the blood, during a decrease in the average density of mitochondria and the density of cristae, as well as a decrease in the expression of mitochondrial Mn-SOD [
27,
28].
Also, several works have established that intrauterine hypoxia changes the expression profile of 48 genes associated with metabolic and oxidative stress, such as the subunit of glutathione-S-transferase and cytochrome-C-oxidase. We also found a persistent decrease in eNOS expression after intrauterine hypoxia - 2.5 times in 1-month-old animals, and 2-fold in 2-month-old animals (
Figure 1 and
Figure 2). The effect of intrauterine hypoxia on eNOS expression depends on the duration of hypoxia, since an increase in ROS production can impair NO bioavailability and suppress eNOS expression [
1,
20,
29] The dynamics of the relationship between ROS and NO determines vascular tone, since the hypoxia-induced increase in ROS and, consequently, the ROS/NO ratio in the fetus enhances peripheral vasoconstriction and increased myocardial ischemia. An excess of NADPH during prenatal hypoxia is the cause of the formation of ROS, which can react with NO to form a stable peroxynitrite anion, reducing the bioavailability of NO [
12]. The revealed decrease in eNOS expression during a significant increase in the level of nitrotyrosine in both 1 and 2 month old rats after intrauterine hypoxia may indicate in favor of the formation of endothelial dysfunction in these animals. At an older age, endothelial dysfunction can be a predictor of such formidable diseases of the cardiovascular system as myocardial infarction, chronic heart failure, ischemic and hemorrhagic strokes [
30].
Our research unveiled a consistent decrease in the expression of the 70 kDa heat shock protein (HSP
70) post-intrauterine hypoxia. This decrease was approximately 5.6 times in 1-month-old animals and 3.44 times in 2-month-old animals (as outlined in
Figure 1 and
Figure 2). We concluded that intrauterine hypoxia causes intrauterine programming of the hsp70 gene, which leads to inhibition of its response to warm stress and loss of endogenous cardioprotection at a later age. As a result of the study, we found suppression of HIF-1 mRNA expression - in the heart in 1-month-old animals by 79% and in 2-month-old animals by 61.1% (
Figure 1 and
Figure 2). Hypoxia-induced factors - HIF play the role of transcription factors and regulate the expression of genes encoding the synthesis of proteins involved in the physiological response to hypoxia/ischaemia [
16]. HIFs exhibit cytoprotective properties under hypoxia, stimulate reparative processes, increase the concentration of free radical traps (haem-hydroxylase-1, haem-oxygenase-1), VEGF, angiopoietin). [
18]. HIF-1 in conditions of hypoxia affect energy metabolism, regulating compensatory shunts of ATP synthesis, increases glutathione synthesis and increases cell resistance to oxidative stress [
25]. HSP70 is known to prologue the "life time" of HIF-1. We found that suppression of HIF-1 mRNA expression after intrauterine hypoxia occurs against the background of HSP70 deficiency. A sufficient number of studies have shown multidirectional changes in the concentration of HIF-1 its forms, at different types of hypoxia of its duration and in different organs. [
22]. Under conditions of nitrosative stress and increase in the level of cytotoxic products of NO and ATP deficiency in tissues, there is a decrease in HIF, associated with activation of ubiquitin-independent pathway of degradation of oxidatively modified HIF-1α and suppression of its synthesis at the stage of ATP deficiency. The regulatory role of NO in the regulation of HIF-1a mRNA expression is known [
8].
Thus, one of the therapeutic strategies to reduce cardiac dysfunction that develops after prenatal hypoxia may be the normalization of the NO system and the reduction of oxidative stress. Thus, the administration of drugs to animals after prenatal hypoxia for 30 days had a therapeutic effect in varying degrees of severity, both immediately and a month after the end of their administration.
Angiolin showed the most significant therapeutic effect. Thus, in groups of animals, when it was used, a decrease in ST2 by 77% was found immediately after the end of its administration compared with the control, and a month after the cessation of the administration of Angiolin, the ST2 values in this group did not differ statistically from the values of the intact group. All this indicates a significant cardioprotective effect of Angiolin. The introduction of Angiolin led to a significant increase in eNOS expression by 3.74 times immediately and 2.5 times a month after withdrawal compared with control and by 58.8% and 153.8% compared with intact during a decrease in the level of nitrotyrosine (a decrease by 56.5% immediately and the normalization of this indicator a month after administration compared with the control). Angiolin increased the expression of HSP70 (an increase of 2 times immediately and an increase of 1.84 times a month after administration in relation to the control). Angiolin increases HIF-1 mRNA expression 23-fold in the heart of 1-month-old animals subjected to intrauterine hypoxia and 8.2-fold in 2-month-old animals A positive effect on the NO system and a decrease in oxidative stress during an increase in mRNA HIF-1 and HSP70 seem to provide Angiolin with its cardioprotective effect after intrauterine hypoxia. It should be noted that the cardioprotective effect of Angiolin persisted even one month after discontinuation of the drug.
Similar in direction, but less pronounced effect was observed with the introduction of Thiotriazoline and L-arginine. Thus, in the groups receiving Thiotriazoline, there was a significant decrease in ST2 by 68.2% and 66.2% in accordance with the observation period, and in the groups receiving L-arginine, this indicator decreased by 73% and 55.3%, respectively.
The introduction of Thiotriazoline and L-arginine had an antioxidant effect and led to a decrease in oxidative stress. Thus, in the groups receiving Thiotriazoline, there was a significant decrease in nitrotyrosine by 41% and 48% in accordance with the terms of observation, and in the groups receiving L-arginine, this indicator significantly decreased by 22% only immediately after a 30-day administration of the drug. Thiotriazoline and L-arginine had a positive effect on eNOS expression. Thus, in the group treated with L-arginine, the eNOS index returned to normal immediately after discontinuation of the drug, and its expression increased by 65.5% compared with the control one month after discontinuation of the drug. The introduction of Thiotriazoline provided a significant increase in eNOS expression by 75.3% and 38% in accordance with the observation period. The administration of Thiotriazoline and L-arginine significantly increased the concentration of HSP70 in the blood of rats after intrauterine hypoxia for different periods of observation - L-arginine immediately after the cessation of its administration (by 124% compared with the control), and Thiotriazoline 30 days after the cessation of its administration (by 53% compared to control). Thiotriazolin significantly increased HIF-1 mRNA expression both in 1-month-old ani-mals and most significantly (6.2-fold compared to control) in 2-month-old animals. L-arginine administration significantly increased L-arginine mRNA expression immedi-ately after drug withdrawal (9-fold compared with control). Apparently, this is due to the effect on different parts of the NO/SH-mechanism of triggering HIF-1 mRNA expression.
Mildronate had a significant cardioprotective effect during its course administration to rats after intrauterine hypoxia - a decrease in ST2 by 69.5% and 50.6% for various periods of observation. According to the degree of influence on this indicator, Mildronate did not differ from L-arginine and Thiotriazoline, but was inferior to Angiolin. Mildronate showed no antioxidant effect and did not reduce the concentration of nitrotyrosine in the blood of rats after intrauterine hypoxia. Mildronate increased the expression of eNOS in the blood of experimental rats only immediately after the course administration, and a month after the drug was discontinued, the effect was not significant. Mildronate also significantly increased the concentration of HSP70 and the expression of HIF-1 mRNA within a month after the end of the course. According to the degree of influence on this indicator, Mildronate was inferior to L-arginine and Angiolin, remaining a competitor to Thiotriazoline (
Figure 3).
In the blood and heart of both 1-month-old and 2-month-old rats subjected to intrauterine hypoxia, a noteworthy reduction in the expression of modulators within the NO system was observed. Specifically, a decrease in eNOS expression coincided with the suppression of HIF-1 mRNA and a reduction in HIF-1 mRNA concentration. This cascade of events also saw a decrease in HSP70 concentration, thereby initiating oxidative stress (manifested by elevated nitrotyrosine levels) and resultant myocardial damage (highlighted by increased ST2 levels). The NO system was identified as a pivotal element in this context. Pharmacological agents (Angiolin, Thiotriazoline, L-Arginine, Mildronate) that operate at various junctures within this system (influencing synthesis, bioavailability, and NO protection) displayed a notable cardioprotective effect post-pharmacological intervention. Importantly, these agents positively impacted the quantified parameters, underscoring their potential as beneficial interventions.
4. Discussion
The revealed primary cardioprotective effect of Angiolin when administered after intrauterine hypoxia, with the preservation of the effect even after a monthly withdrawal, are explained by its following properties. Angiolin under conditions of acute cerebral ischemia exhibits pronounced endothelioprotective properties - it preserves the density of endotheliocytes, increases the concentration in the nuclei of RNA, increases the density of proliferating endotheliocytes (BrdY-test), increases the efficiency of utilization of endogenous L-arginine, and increases the expression of vascular endothelial factor (VEGF), as well as eNOS, and the presence of divalent sulfur in its structure determines the property of the NO scavenger [
25]. Angiolin is able, together with vitamin C, to form L-carnitine and normalize the functioning of mitochondria [
15].
In our work, it was found that Angiolin improved the ultrastructure of hippocampal CA1-zone neurons under conditions of chronic cerebral ischemia (reduced the destruction of cristae, uneven electron density of the matrix, increased the density of mitochondria), and also reduced the concentration of intramitochondrial iNOS and increased the concentration of cytological and intramitochondrial HSP70. It is known that 70 kDa heat shock proteins act as endogenous cytoprotectors during ischemia, hypoxia, exposure to toxins, and a sharp increase in temperature [
31]. To date, it is known that the mechanisms of the protective action of HSP70 are realized due to the restoration of the correct tertiary structure of damaged proteins, as well as in the formation and dissociation of protein complexes. Our studies revealed the positive role of HSP70 aimed at reducing the formation of mitochondrial dysfunction in neurons of the sensorimotor cortex and hippocampus in cerebral ischemia [
28].
In vitro experiments in a suspension of neurons in rat pups found that the introduction of HSP70 reduces the degree of damage to key enzymes of energy metabolism and enzymes of antioxidant protection of neuron enzymes. Also, it was found that HSP70 takes part in the regulation of the functioning of compensatory energy shunts in acute ischemia. We found that HSP70 "prolongs" the action of HIF-1a, and also independently maintains the expression of NAD-MDH-mx, thereby maintaining the activity of the compensatory mechanism of ATP production - the malate-aspartate shuttle mechanism for a long time [
10]. Our works have shown that Angiolin can activate the malate-aspartate shuttle mechanism in the myocardium during ischemia [
32]. Thiotriazoline exhibits the properties of a scavenger of cytotoxic forms of NO, has a protective effect on NO transport, due to a positive effect on the thiol-disulfide balance and an increase in the level of reduced thiols and glutathione. In addition, we suggest that Thiotriazoline itself can be an NO carrier, forming stable S-nitrosyl complexes with it. Thiotriazoline exhibits a cardioprotective effect, positively affecting energy metabolism in ischemic myocardium - increases ATP during ischemia and hypoxia due to the normalization of the Krebs cycle, increases the utilization of glucose, free fatty acids, activates the conversion of lactate to pyruvate. Thiotriazoline is also known to exhibit a cardioprotective effect and to increase the endurance of animals under working hypoxia by increasing HIF-1 and preserving mitochondrial ultrastructure. Due to the antioxidant action, Thiotriazoline maintains the threshold sensitivity of receptors, maintains membrane fluidity, and protects phospholipids from oxidation [
22].
L-Arginine is a substrate for the formation of NO in vascular endothelial cells, a peripheral vascular dilatation factor. Formed from arginine NO, reduces the total peripheral vascular resistance and blood pressure, reduces oxygen starvation, especially in the tissues of the heart [
32]. The role of NO in the mechanisms of endotheliocyte proliferation, regulation of expression of vascular endothelial growth factor (VEGF), placental growth factor (PGF), angiopoietins (ANG-1, ANG-2) and receptor proteins (SFLT-1, STIE-2) is known. It has been shown that VEGF plays an important role in physiological pregnancy by regulating placental angiogenesis, reducing the incidence of placental insufficiency [
5,
19]. Physiological NO concentrations are known to regulate the expression of pro-angiogenic VEGF-A and PGF in vitro in human trophoblasts. Administration of iNOS inhibitors and a decrease in NO levels in pregnant mice resulted in an increase in blood pressure. NO at physiological concentrations can reduce the expression of pro-inflammatory cytokines and endothelial adhesion receptors and is capable of urgent expression of HIF-1a.
The properties of mildronate have been studied for a long time. It is known that milronate through organic carnitine cation transporter 2 can reduce the level of L-carnitine and inhibit the transfer of fatty acids through mitochondrial membranes during acute ischemia or hypoxia. Mildronate prevents the accumulation of toxic metabolic intermediates acylcarnitine and acyl-CoA, which damage cell membranes and block the delivery of ATP from mitochondria to cell organelles [
28]. Treatment with Mildronate is accompanied by a compensatory increase in the expression in the myocardium of a number of genes encoding lipid metabolism enzymes - lipoprotein lipase, fatty acid translocase, carnitine palmitoyltransferase I and triacylglycerol synthesis enzymes. Mildronate is able to improve myocardial contractility, hexokinase activity, and the ratio of ATP / ADP / AMP by activating AMP-activated protein kinase, which restores ATP levels [
16].
Mildronate can increase NO production in the ischemic myocardium and brain. by modifying γ-butyrobetaine ester pools. Mildronate administration inhibits the hydroxylation of γ-butyrobetaine and increases the intracellular pool of γ-butyrobetaine, which esterification exhibits cholinomimetic properties. Esters of γ-butyrobetaine through acetylcholine receptors on endothelial cells can activate eNOS [
16]. However, in a number of studies, the effect of mildronate on NO production was not confirmed.16
Thus, the results obtained established the primary cardioprotective effect of modulators of the NO system with different mechanisms of action - Thiotriazoline, Mildronate, L-arginine and, especially, Angiolin after prenatal hypoxia. The results obtained substantiate the prospects for further research.
Author Contributions
Conceptualization, O.P., I.B., O.Y.; methodology, I.B., A.K.; software, O.P., I.B., A.K; validation, O.P., I.B.; formal analysis, A.K.; investigation, O.P., I.B; data curation, O.P., I.B., O.Y.; writing—original draft preparation, O.P., O.Y.; writing—review and editing, I.B., A.K., V.O.; visualization, O.P.; supervision, I.B., V.O., A.K. All authors have read and agreed to the published version of the manuscript.