1. Introduction
Hepatitis E is a widespread disease worldwide and is a serious public health concern. Acute hepatitis E can cause febrile jaundice, asthenia, anorexia, and nausea-vomiting but regresses spontaneously within 4-6 weeks1. The disease can progress to fulminant and acute hepatitis infection therefore causing death. Every year there are an estimated 20 million HEV infections worldwide, leading to an estimated 3.3 million symptomatic cases of hepatitis E. The mortality rate of hepatitis E in humans ranges from 0.5 to 4%2. Pregnant women are more susceptible to complicated forms of the disease resulting in a 20% mortality rate in the last trimester3. Indeed, there is strong evidence that HEV is a major contributor to maternal morbidity and mortality in Southeast Asia, particularly in pregnant women in the third trimester. This makes HEV infection the most serious of all viral hepatitis in pregnancy4–7.
The causative agent, Hepatitis E virus (HEV), is a non-enveloped, single-stranded, positive RNA virus with a 7.5 kilobases (kb) genome size of, belonging to the Hepeviridae family, Hepevirus genus8. HEV presents a large diversity with four distinct HEV genotypes (1-4) circulating worldwide with specific geographical distribution of each genotypes9–11. Both HEV genotypes 3 and 4 infect several infect several mammalian animals, with occasional transmission to humans 12.
Serological diagnosis of HEV infection rely on both IgG and IgM antibodies detection by ELISA. IgM antibodies, which indicate a recent disease, are detectable from the early symptomatic stages with a maximum peak after a month following the primary infection. This is followed by substantial IgM decrease of IgM from 2 to 6 months at most following the infections13. However, IgG antibodies known to indicate an o infection, persist from 18 months to more than 10 years in the body, (depending on the sensitivity of the used assay14. However, serological cross-reactivity has been described between the four HEV genotypes15–17.
Rapid proliferation of traditional gold mining sites in the Kédougou region, Southeastern Senegal, led to an increasing demography in the area with many people originating from the neighbouring West African countries. The elevated number of newly established mining villages is unfortunately associated with poor hygiene, unsafe water usage and thus bad sanitation conditions triggering disease’s occurrence. In 2014, a hepatitis E outbreak has been reported in the Kédougou region including several cases of febrile jaundice. Investigations exhibited the epidemic’s beginning from March 201218. Herein, we investigated the HEV seroprevalence in population of the Kédougou region using serum samples collected from patients with jaundice from 2012 to 2014.
2. Methods
Ethical Statement
The Senegalese National Ethical Committee at the Ministry of Health and Social Action (MoHSA) has approved our current surveillance protocol. Consent forms were obtained from all adults or parents for minors included in this study. Field investigations were regularly supervised by the authorities of the MoHSA in Senegal. Additional samples used in this study were collected in the frame of the national integrated surveillance program of fevers in Senegal and were available from the collection of the WHO Collaborating Centre for Arboviruses and Hemorrhagic Fevers at Institut Pasteur de Dakar. All methods including the use of human samples, were performed in accordance with the Declaration of Helsinki.
Study area
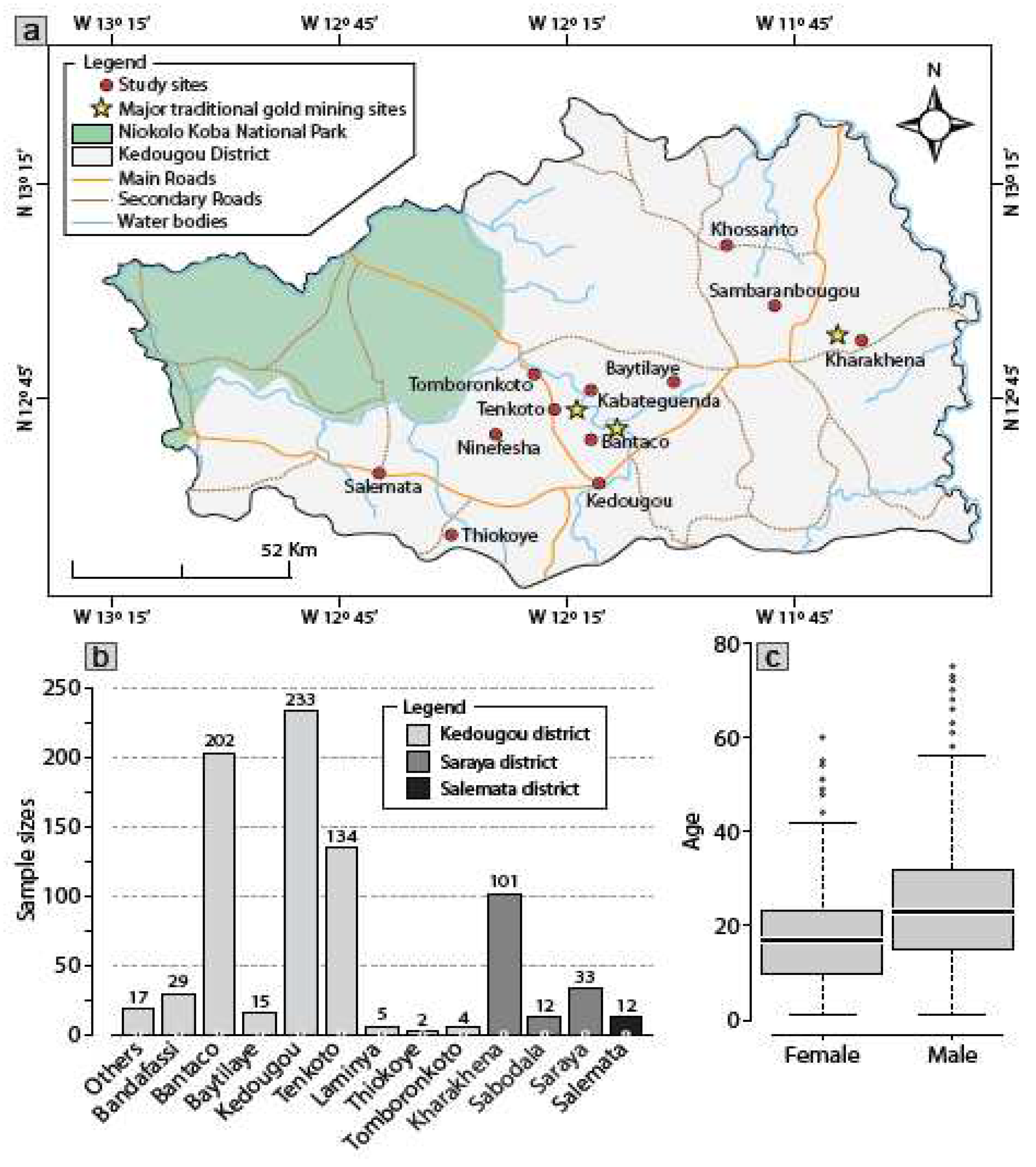
The study was conducted in Kédougou, a region located in Southeastern Senegal (12°32′ N,12°11′ W). Insets are obtained from ArcGIS Desktop V10.8.1 (ESRI 2021, Redlands, CA: Environmental Systems Research Institute; https:// desktop. arcgis. com/ en/) and sampling sites are placed on base map using their respective XY coordinates and ArcGIS Desktop (
Figure 1a). The population of Kédougou is of 184,276, 5.1% of whom are under 18 years old, and the average population density is of 8 persons per km2
19. Kédougou shares borders with the Republic of Guinea and Mali. The climate is Sudano-Guinean with a single rainy season from May to October. Temperatures are generally high with an annual average of 28.4 °C. Agriculture is the main economic activity in the region, but hunting and logging are also source of contact between humans and wildlife
19. The Gambia River flows through the region and creates during the dry season temporary pools with dense vegetation. Recently, gold mining has become one of the most important economic activities in the region.
Data collection
Following 2014 HEV outbreak in Kédougou, a retrospective sero-surveillance study was carried out from February 2012 to November 2014. All patients included in this study were working in the three main traditional mining gold villages (Kharakhena, Bantaco, Tenkoto) which are supervised by the health districts of Kédougou and Saraya, respectively. Twelve samples from suspected individuals from the health district of Salemata, a non-gold mining area were included in our study. Clinical and epidemiological data retrieved from health care registration books and the database of the virology department of the Institut Pasteur de Dakar in Senegal, were analyzed in addition to both anti-HEV IgM and anti-HEV IgG serological data.
Serological testing
A total of 799 archived sera collected from PCR-negative suspected cases and jaundiced contacts in the Kédougou region were tested using the IgM ELISA 3.0 (ref: 2P35-01(23160-096) and MP Diagnostics IgG ELISA (ref: 2F09-02(21150-096T) kits according to the manufacturer’s procedures (MP DIAGNOSTICS), for the detection of IgM HEV and IgG HEV antibodies. All samples were processed in duplicates.
Statistical analysis
Statistical analyses were performed using R version 4.0.3 (R Corporation). The age distribution by sex was represented by a boxplot showing the age distribution in the two groups, as well as the geographical distribution of the patients. The 95% confidence intervals (CI) were calculated using analysis of variance. Univariate comparison of proportions between groups (age, sex, status, and geographic location) was performed using analysis of variance. To estimate the strength of association, odds ratios (ORs) and associated 95% CIs were used. Variables with significant univariate associations at a P value < 0.05 were entered into a multivariate binomial regression model. P<0.05 or a 95% CI not spanning 1.0 was considered statistically significant for all analyses.
3. Results
Study population
Senegal is a West African country where mining activities have emerged over the last years particularly in Kédougou. The large number of undeclared migrants working in the traditional gold mining villages continue to increase (
Figure 1-a). The majority of the included patients lived in the Kédougou district (n= 641), followed by the Saraya district (n= 146) and the non-mining gold sanitary district of Salemata (n= 12) (
Figure 1-b). Out of the 799 patients included in our study, we identified 470 contacts and 290 suspected cases. The median age was 19 years (1-88 years) with a male/female sex ratio of 1.9 (
Figure 1-c). Of the 799 sera analyzed, laboratory tests showed a total of 404 samples positive for anti-HEV, including 332 IgM, 251 IgG and 179 IgM+IgG.
Sero-epidemiological analysis
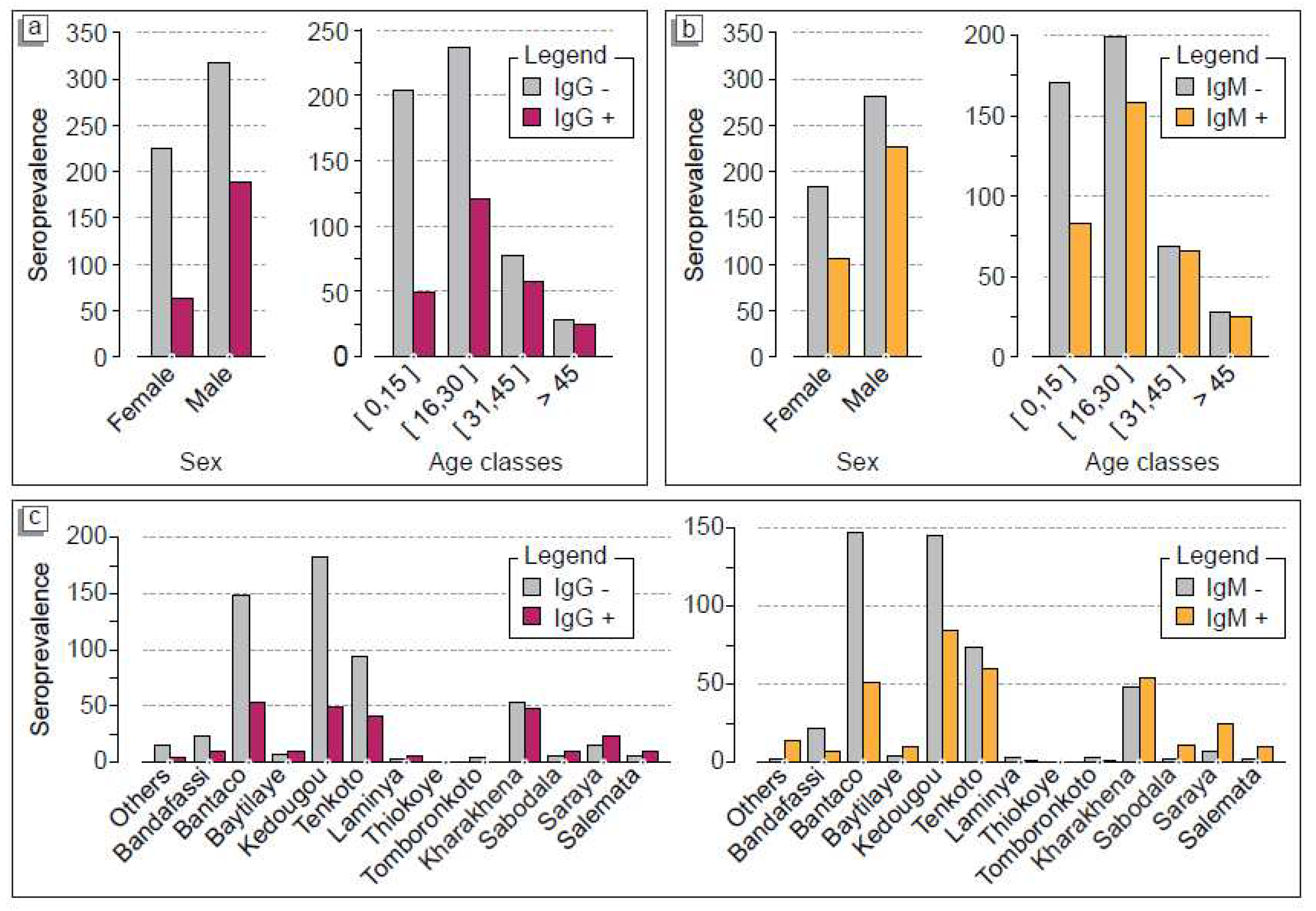
Statistical analysis showed that in Kédougou district, the villages of Thiokoye (100%, n= 1), Laminya (60%, n= 3), Baytillaye (53.3%, n= 8) and Tenkoto (29.8%, n= 40) had the highest seroprevalences for IgG (p-value= 1.88x10
-6) (
Table 1). In Saraya district, the highest seroprevalences were observed in Sabadola village (81.8%), Saraya town (60.6%) and Kharakhena village (47.5%) (p-value= 0.043). In Saraya district and Kédougou district, there was a positivity rate of 20/145 (53.1%) and 163/619 (26.3%), respectively. However, in Salemata district, we found that 8 out of 12 suspected patients (66.6%) tested positive for anti-HEV IgG. For IgM seroprevalence, the statistical analysis showed a highest seroprevalence in Kédougou district (220/620). This overall 35,5% recent HEV infections were distributed in Baytilaye 11/15, Thiokoye 1/2, Tenkoto 60/134, Laminya 2/5 and the city of Kédougou 86/233 (36.9%) (p-value= 1.88x10
-6). In Saraya district, the highest seroprevalence rate were observed in the villages of Sabadola 11/12 (91.7%), Saraya 25/33 (75.8%) and Kharakhena 53/101 (52.5%) (p-value= 0.043). As for IgG, we found a high rate of IgM seroprevalence in the Salemata district 10/12 (83.3%). Overall, Saraya was associated with 70% old HEV infections while Kédougou showed 35% of anti-HEV IgM. Of the 8 deaths recorded among the included patients with positive IgM serology, 3 were from the Kédougou district and 5 from the Saraya district while 50% of these patients included pregnant women (n= 4). These results indicate that both old and recent HEV infections were circulating since 2012 two years before the declared 2014 outbreak. More importantly, our data showed that HEV infections spread from a traditional mining gold village also reported to be the epicenter of HEV outbreak (Kharakhena) to the surrounding villages and city. This impact of human movement on virus circulation was also observed in Salemata district.
Gender and age distribution of IgM and IgG HEV positive cases
Out of the of 332 IgM-positive cases, the active population aged from 16-30 years-old group show the (47.9%) highest recent HEV infections rate, followed by the 15 years old population, the 31-45 years-old group and the age group above 45 years with 25%, 19.6% and 7.5%, respectively (p-value = 0.05592).
Table 2 displays the same pattern for old HEV infections rates. Indeed, out of the of 251 IgG-positive cases, the highest rate was recorded in the 16-30 years-old group with 48.2%, followed by the 31-45 years-old group, the age group under 15 years and the and the age group above 45 years with 22.7%, 19.5% and 9.6%, respectively (p-value = 0.05592) (
Table 2). Moreover, if we consider gender balance, we found that HEV infections were affecting most likely men than women also see that men with 68.6% vs. 31.4% of IgM-positive and 75.3% vs. 24.7% of IgG positive cases p-value = 4.496x10
.-6 (
Table 2). Next, we sought to map out the overall distribution of HEV infections.
Figures 2a and
2-b showed the risk factors of HEV infection including both suspected and confirmed cases. For both IgM and IgG seroprevalence, we found a similar pattern with patients over 45 years-old, showing a higher risk of developing the disease of 88.8% (n= 24) and 96.15% (n= 25), respectively. However, the 31-45 years-old, 16-30 years-old and under 15 years groups exhibited prevalence of (n= 57) 74.02%, (n= 121) (51.05%) and (n= 49) (24.13%), respectively (
Figure 2-a, b). In terms of geographical distribution, the villages of Kharkhena, the 2014 HEV epidemic epicentre, Bantaco and Tenkoto, which are all villages hosting the largest traditional gold panning sites, had more cases developing both IgG and IgM antibodies indicating that the virus was present in the population from 2012 to 2014. This is also the case for Kédougou, which hosts the reference center for the entire Kédougou region (
Figure 2-c).
Table 3 indicates that of the 290 suspected cases included in our study, 35.7% (n= 123) tested positive for IgG antibodies. However, the rate of IgM-positive cases was significantly higher (63.8% (n= 185)), (p-value = 2.2x10
-16). The overall prevalence of suspected cases developing both IgM and IgG antibodies at the same time was 37.2% (n= 108). In addition, the same trend was observed among contacts. Among the 470 contacts, 25.3% (n=119) were positive for IgG antibodies (p-value = 2.2x10
-16) while 30% (n= 141) of them were positive for IgM (p-value = 2.2x10
-16) (
Table 3). The overall prevalence of contacts developing both IgM and IgG antibodies at the same time had an antibody rate of 14.25% (n= 67).
Overlapping IgM and IgG distribution and evolution pattern from 2012 to 2014.
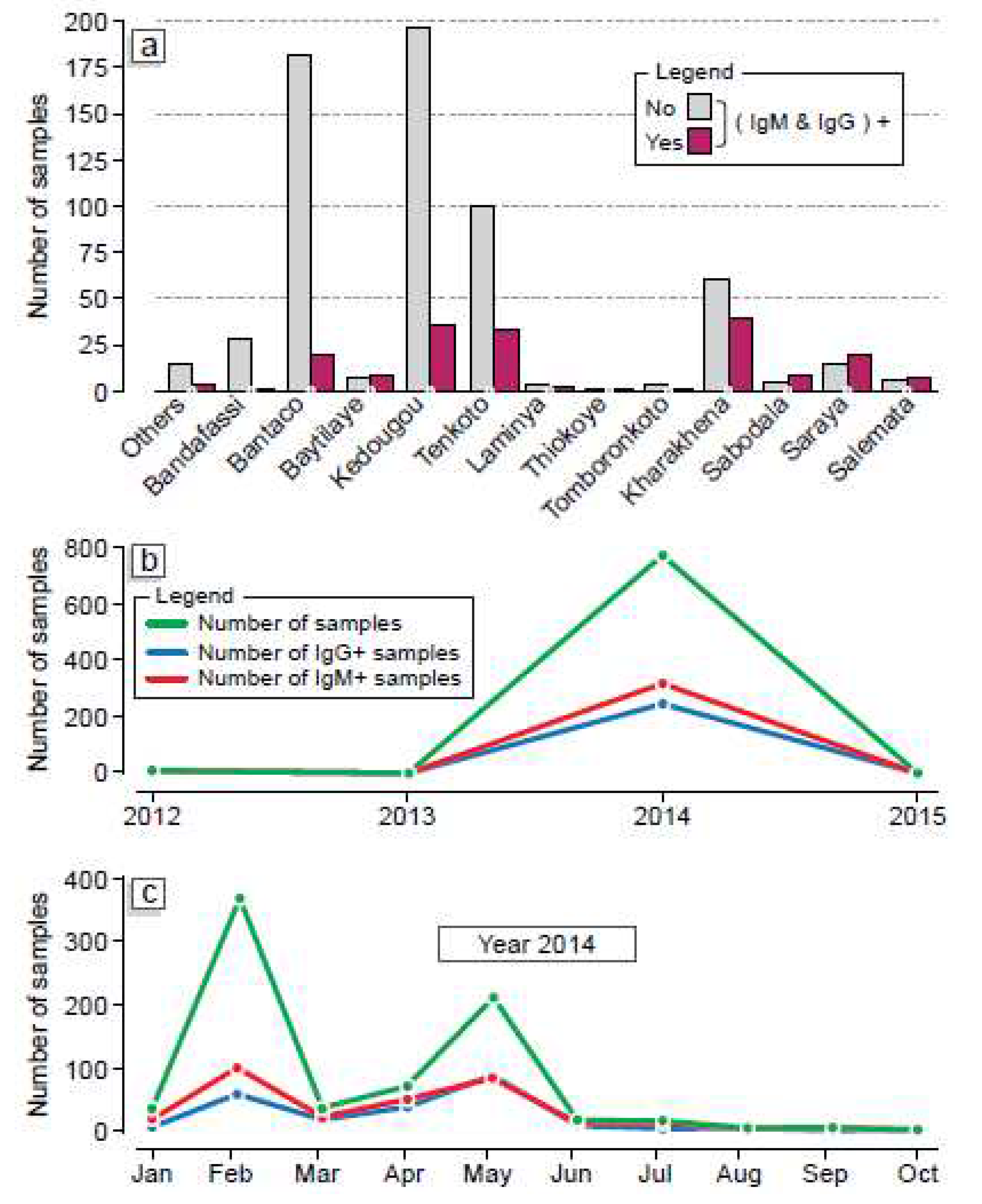
The comparative analysis of individuals developing IgM type antibodies as well as those developing IgG type antibodies came from Kharahena (considered the epicenter of the epidemic), Tenkoto and Bantako which are villages that host the largest traditional gold panning sites in the region (
Figure 3-a). The commune of Kédougou recorded a significant number of patients with both HEV IgM and IgG antibodies (
Figure 3-a). The comparative study of individuals developing both IgM and IgG antibodies over the years showed there was a discrete/silent HEV evolution loci from 2012 to 2013. However, in 2013 there was a rapid expansion of HEV infections in the Kégoudou region leading to the outbreak previously declared from January 2014
20.
Figure 3-b showed that both old and recent HEV infections increased from 2013 with two peaks of infection recorded in February and May 2014, respectively.
4. Discussion
In our study, 799 HEV RNA-negative samples were tested for IgM and IgG antibodies which are indicating recent and old infections. With 332 IgM-positive cases versus 251 IgG-positive cases we found despite negative PCR detection a significant IgM/IgG ratio in Kédougou from 2012 to December 2014 as previously reported18. Our IgG serological data shows that HEV was circulating in the region since 2012. This shows that the classical PCR detection method alone is not sufficient to assess the HEV surveillance. ELISA must be coupled with routine PCR detection methods to prevent HEV outbreaks. Our work has shown the impact of human movements from traditional mining gold villages where sanitation, precarious hygienic conditions and access to drinking water are often challenges on HEV expansion. In fact, the highest seroprevalence recorded in the Salemata district could be related to the return of patients from gold mining sites such as Kharakhena, Tenkoto and Bantaco. Seroprevalence and HEV risk associated were found to directly impact males than females with 68.6% versus 31.4% IgM+ and 75.3% versus 24.7% IgG+. These data are similar to those previously reported from Asia21. However, we found that pregnant women are highly susceptible to HEV infection as previously reported4 hus, training of clinicians and healthcare workers in the Kédougou region in the appropriate management of this disease whose signs in terms of jaundice are reminiscent of yellow fever, could be needed to avoid high mortality rate in this vulnerable group in the future. In addition, clinical monitoring of Senegalese women for HEV during pregnancy and postpartum, especially in high-risk settings as Kédougou, is critical during the last trimester of pregnancy22,23. The fact that the 16-30 years old group was significantly affected by the disease could be due to the routine activities as workers in the traditional gold panning mines as previously reported in Nepal24. In addition, the risk of contracting HEV in people over the age of 45 years may be related to their fragile immunity, which can rapidly deteriorate over the years20. Given the seriousness of hepatitis E, particularly in pregnant women, in Senegal, and more particularly in Kédougou, the hepatitis E virus must be suspected in the presence of any unexplained acute hepatitis, even if it does not involve travel to an endemic zone and especially if it occurs in a patient over 40 years of age. Although there is no etiological treatment for this disease, vulnerable populations such as pregnant women and the elderly should be particularly vigilant regarding detection and early management in order to avoid high mortality. Although a low seroprevalence was found in children under 15 years in our study (25% IgM+ and 19% IgG+), more seroprevalence studies could be promoted in children across Sub-saharan Africa, particularly those under 2 years as an unusual high mortality (13%) has been previously reported in Uganda25. However, their possible involvement as disease vector to pregnant women could be also assessed. The non-negligible rate of IgM antibodies in contacts could be explained by the asymptomatic nature of the majority of hepatitis E cases in an outbreak26,27, symptomatic cases could have also participated to the progressive spread of the disease in Kédougou from 2012 to 201420, in contrast to the brutal and massive circulation generally reported in HEV epidemics28,29. To evaluate the real burden of future hepatitis E epidemics, dual testing of suspected cases using both serology testing and real-time PCR could be considered.
Given the similarity of HEV to yellow fever in terms of signs, HEV testing should be used as differential diagnosis. In addition, it is necessary to sensitize the population on the modes of HEV transmission and the usual hygiene measures such as systematic hands washing after leaving the toilets, before preparing the meal, after contact with live animals or products of animal origin, using clean latrines, covering wells23 Next, it is necessary to supply the population with drinking water in the gold mining sites by installing new boreholes and water purification systems at the household level. Finally, improvement of sanitation and wastewater management systems in gold mining sites could be prioritized.
Author Contributions
B.D.S., M.F., A.S., Ous. F., and A.A.S., designed and conducted the study; B.D.S performed the laboratory analyses; B.D.S., A.S., and A.A.S. performed the field investigations; A.G., A.M., B.D.S., M.F., C.T.D., A.S and B.S analyzed and interpreted the data, all authors reviewed the manuscript and accepted the latest version. First authors: B.D.S. and MF. Third authors A.G and C.T.D. Last authors: AM, N.D, A.A.S, AS.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors and was only supported by the Institut Pasteur de Dakar. The information hasn’t been previously presented in any meeting or conference.
Acknowledgments
We acknowledge colleagues of virology department at Institut Pasteur de Dakar, Senegal for sharing supportive information necessary for establishment and accomplishment of this study
References
- WHO. Epidemiological update: increasing mortality calls for action 03. Global hepatitis report, 2017 7–20 (2017).
- Hepatitis E. https://www.who.int/news-room/fact-sheets/detail/hepatitis-e.
- Ranger-Rogez, S., Alain, S. & Denis, F. Virus des hépatites : transmission mère-enfant. Pathologie Biologie 50, 568–575 (2002).
- Chandra, V., Taneja, S., Kalia, M. & Jameel, S. Molecular biology and pathogenesis of hepatitis E virus. J Biosci 33, 451–464 (2008).
- Devi, S. G., Kumar, A., Kar, P., Husain, S. A. & Sharma, S. Association of pregnancy outcome with cytokine gene polymorphisms in HEV infection during pregnancy. J Med Virol 86, 1366–1376 (2014).
- Fiore, S. & Savasi, V. Treatment of viral hepatitis in pregnancy. 10, 2801–2809 (2009). [CrossRef]
- Ornoy, A. & Tenenbaum, A. Pregnancy outcome following infections by coxsackie, echo, measles, mumps, hepatitis, polio and encephalitis viruses. Reproductive Toxicology 21, 446–457 (2006).
- Reyes, G. R. et al. Isolation of a cDNA from the Virus Responsible for Enterically Transmitted Non-A, Non-B Hepatitis. Science (1979) 247, 1335–1339 (1990).
- Schlauder, G. G. & Mushahwar, I. K. Genetic heterogeneity of hepatitis E virus*. J Med Virol 65, 282–292 (2001).
- Mushahwar, I. K. Hepatitis E virus: molecular virology, clinical features, diagnosis, transmission, epidemiology, and prevention. J Med Virol 80, 646–658 (2008).
- Aggarwal, R. & Naik, S. Epidemiology of hepatitis E: Current status. J Gastroenterol Hepatol 24, 1484–1493 (2009).
- Kamar, N., Dalton, H. R., Abravanel, F. & Izopet, J. Hepatitis E Virus Infection. (2014) doi:10.1128/CMR.00057-13.
- Huang, S., Zhang, X., Jiang, H., Yan, Q. & Ai, X. Profile of Acute Infectious Markers in Sporadic Hepatitis E. PLoS One 5, 13560 (2010).
- Grandadam, M. et al. Evidence for hepatitis E virus quasispecies. Journal of General Virology 85, 3189–3194 (2004).
- Huang, F. F. et al. Detection by Reverse Transcription-PCR and Genetic Characterization of Field Isolates of Swine Hepatitis E Virus from Pigs in Different Geographic Regions of the United States. J Clin Microbiol 40, 1326 (2002).
- Kamar, N., Dalton, H. R., Abravanel, F. & Izopet, J. Hepatitis E virus infection. Clin Microbiol Rev 27, 116–138 (2014).
- Li, T. C. et al. Hepatitis E Virus Transmission from Wild Boar Meat. Emerg Infect Dis 11, 1958 (2005).
- Djilocalisse Sadio, B. et al. First hepatitis E outbreak in Southeastern Senegal The Rapid proliferation of traditional gold mining sites in the. 11 & Amadou Alpha Sall 11, 11.
- Perez, F. APPLICATION OF A DISTRIBUTED HYDROLOGIC MODEL FOR THE APPLICATION OF A DISTRIBUTED HYDROLOGIC MODEL FOR THE ANALYSIS OF LAND USE CHANGE IN KEDOUGOU, SENEGAL ANALYSIS OF LAND USE CHANGE IN KEDOUGOU, SENEGAL. (2019) doi:10.37099/mtu.dc.etdr/901.
- Benjelloun, S. et al. Seroepidemiological study of an acute hepatitis E outbreak in Morocco. Res Virol 148, 279–287 (1997).
- Kamar, N. et al. Hepatitis E. The Lancet 379, 2477–2488 (2012).
- Elduma, A. H. & Osman, W. M. Dengue and hepatitis E virus infection in pregnant women in Eastern Sudan, a challenge for diagnosis in an endemic area. Pan African Medical Journal-ISSN 19, 1937–8688 (2014).
- Diouara, A. A. M. et al. Hepatitis E Virus Seroprevalence and Associated Risk Factors in Pregnant Women Attending Antenatal Consultations in Senegal. Viruses 2022, Vol. 14, Page 1742 14, 1742 (2022).
- Joon, A., Rao, P., Shenoy, S. M. & Baliga, S. Prevalence of Hepatitis A virus (HAV) and Hepatitis E virus (HEV) in the patients presenting with acute viral hepatitis. Indian J Med Microbiol 33 Suppl, S102–S105 (2015).
- Patel, R. C., Kamili, S. & Teshale, E. Hepatitis E virus infections in children age 0-15, Uganda outbreak, 2007. J Clin Virol 73, 112–114 (2015).
- Hughes, J. M., Wilson, M. E., Teshale, E. H., Hu, D. J. & Holmberg, S. D. The Two Faces of Hepatitis E Virus. doi:10.1086/653943.
- Purcell, R. H. & Emerson, S. U. Hepatitis E: An emerging awareness of an old disease. J Hepatol 48, 494–503 (2008).
- Tsarev, S. A. et al. Experimental Hepatitis E in Pregnant Rhesus Monkeys: Failure to Transmit Hepatitis E Virus (HEV) to Offspring and Evidence of Naturally Acquired Antibodies to HEV. J Infect Dis 172, 31–37 (1995).
- Nimgaonkar, I., Ding, Q., Schwartz, R. E. & Ploss, A. Hepatitis E virus: advances and challenges. Nat Rev Gastroenterol Hepatol 15, 96–110 (2018).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).