1. Introduction
The brain endothelial cell (BEC) glycocalyx (ecGCx) is a BEC surface apical coating consisting of a complex polysacchraride (sweet husk) gel, mesh-like network of membrane-bound proteoglycans, glycoproteins, and glycosaminoglycans (GAGs) covering the apical luminal surface of the BECs. Importantly, the ecGCx may be considered as a gatekeeper of BECs since it is important in maintaining physiological and homeostasis functions, which includes: prevention of vascular permeability, inflammation, blood coagulation, and promotes the synthesis of the vasculoprotective nitric oxide (NO) via its mechano-sensing and transduction capabilities [
1,
2,
3].
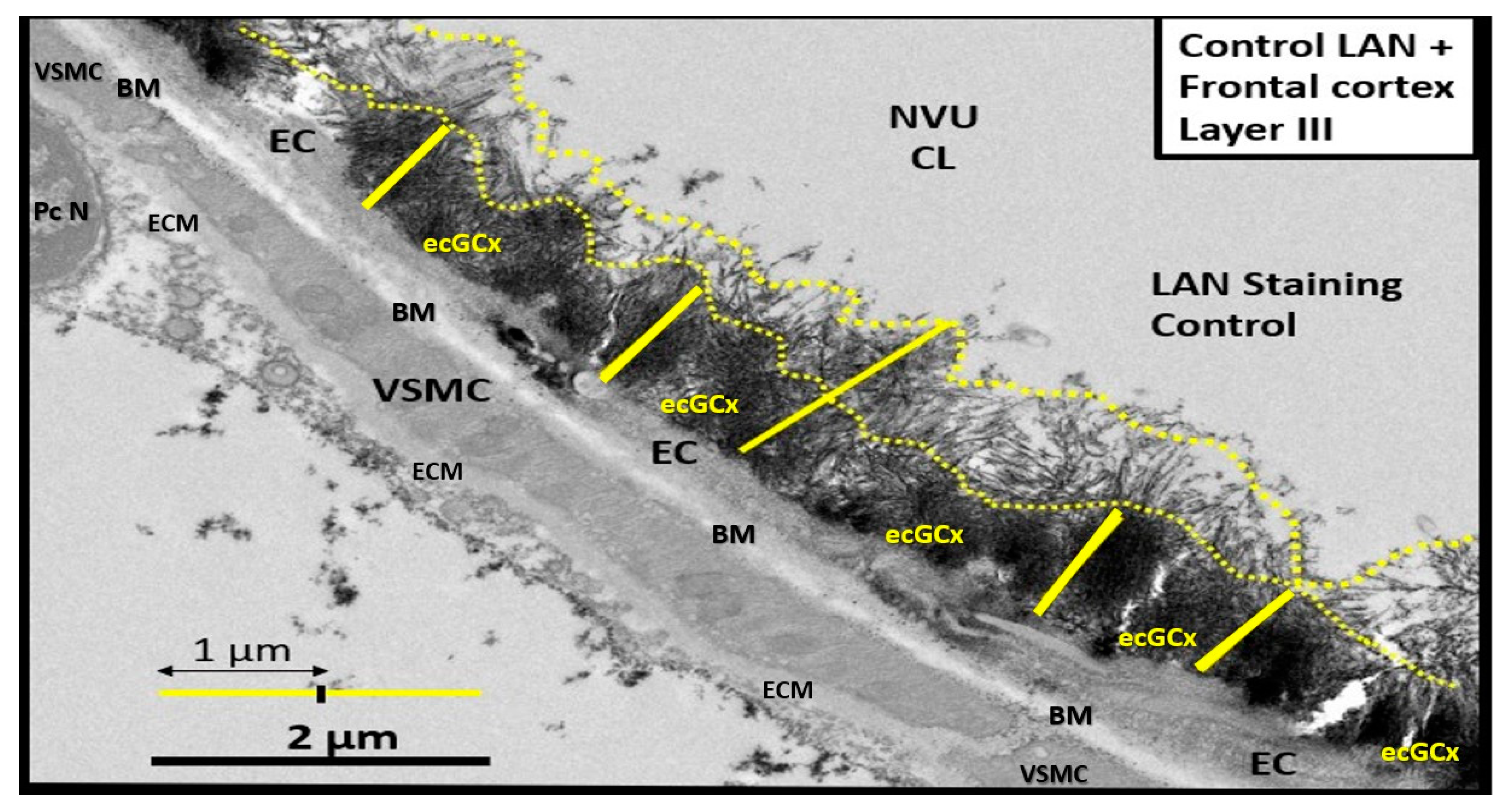
The ecGCx was first identified by transmission electron microscopy (TEM) by Luft utilizing ruthenium red staining to visualize the ecGCx in 1966 [
4]. Recently, the ecGCx has been able to be identified with lanthanum (La(3+) nitrate (LAN) staining with perfusion fixation by transmission electron microscopy (TEM) specifically in the brain microvasculature neurovascular unit (NVU) blood-brain barrier (BBB) arterioles, precapillary arterioles, true capillaries, and postcapillary venules that has been both reliable and reproducible by author and others (
Figure 1) [
5,
6,
7,
8,
9,
10].
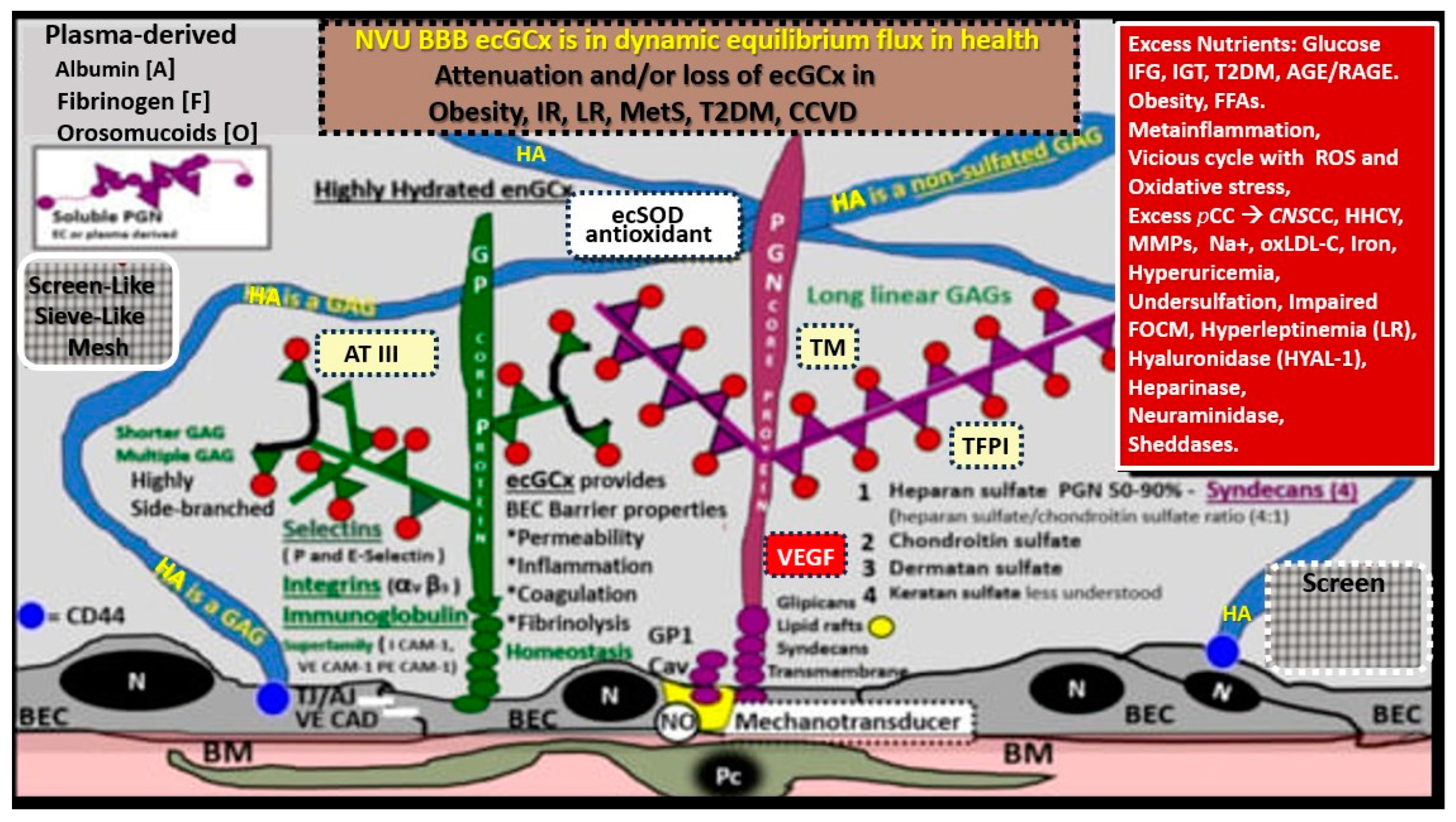
Even at the ultra, nano structure level the net negatively charged ecGCx stains so intensively electron-dense due the ecGCx attracting the highly positively-charged lanthanum La(3+ nitrate LAN of electron-dense staining lanthanum nitrite that the proteins cannot be seen. Therefore, the following illustration is utilized in order to better understand the proteoglycan, glycoproteins and GAGs including hyaluronan (HA) and the covalently bonded GAGs to proteoglycans and glycoproteins side chains with their large amount of negatively charged sulfate residues (
Figure 2) [
6,
7,
11,
12,
13,
14].
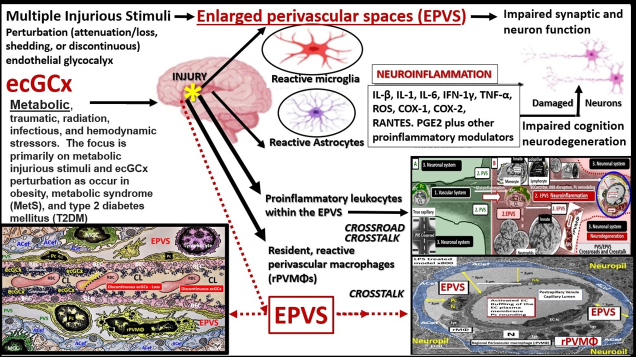
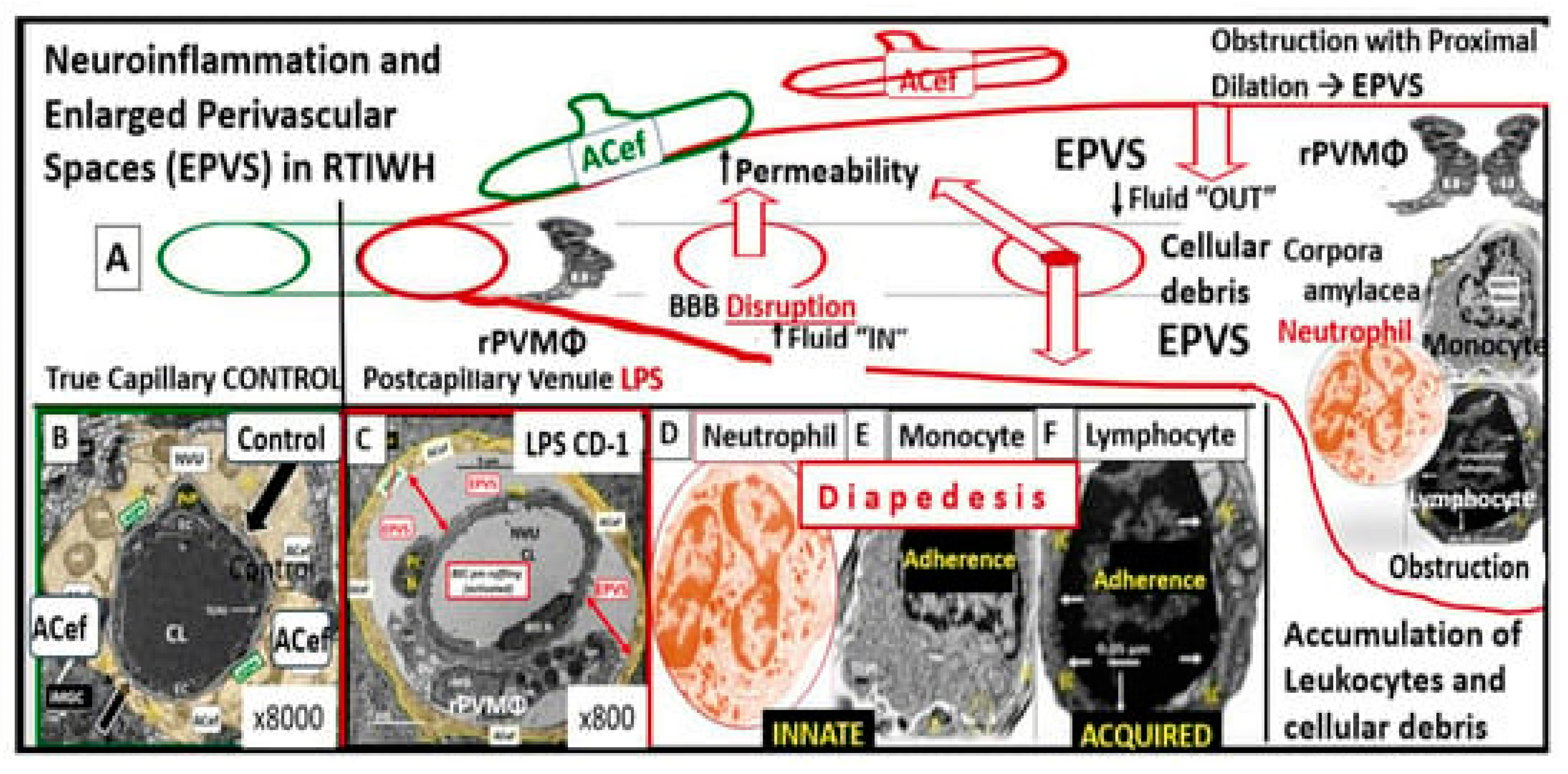
Previously, the brain endothelial cells have been examined as central players in the development of EPVS and as a result, this also places the ecGCx at a critical point in the development of EPVS [
15]. The ecGCx is known to have multiple actions/functions: including its physical and charge barrier, mechanical transduction, regulation of vascular permeability, modulation of inflammatory response, anticoagulation functions [
16,
17]. The ecGCx plays a critical role and is also a critical component of the extended neurovascular unit that not only influences structure and function of the BBB but also performs multiple physiological functions including normal neuronal homeostasis [
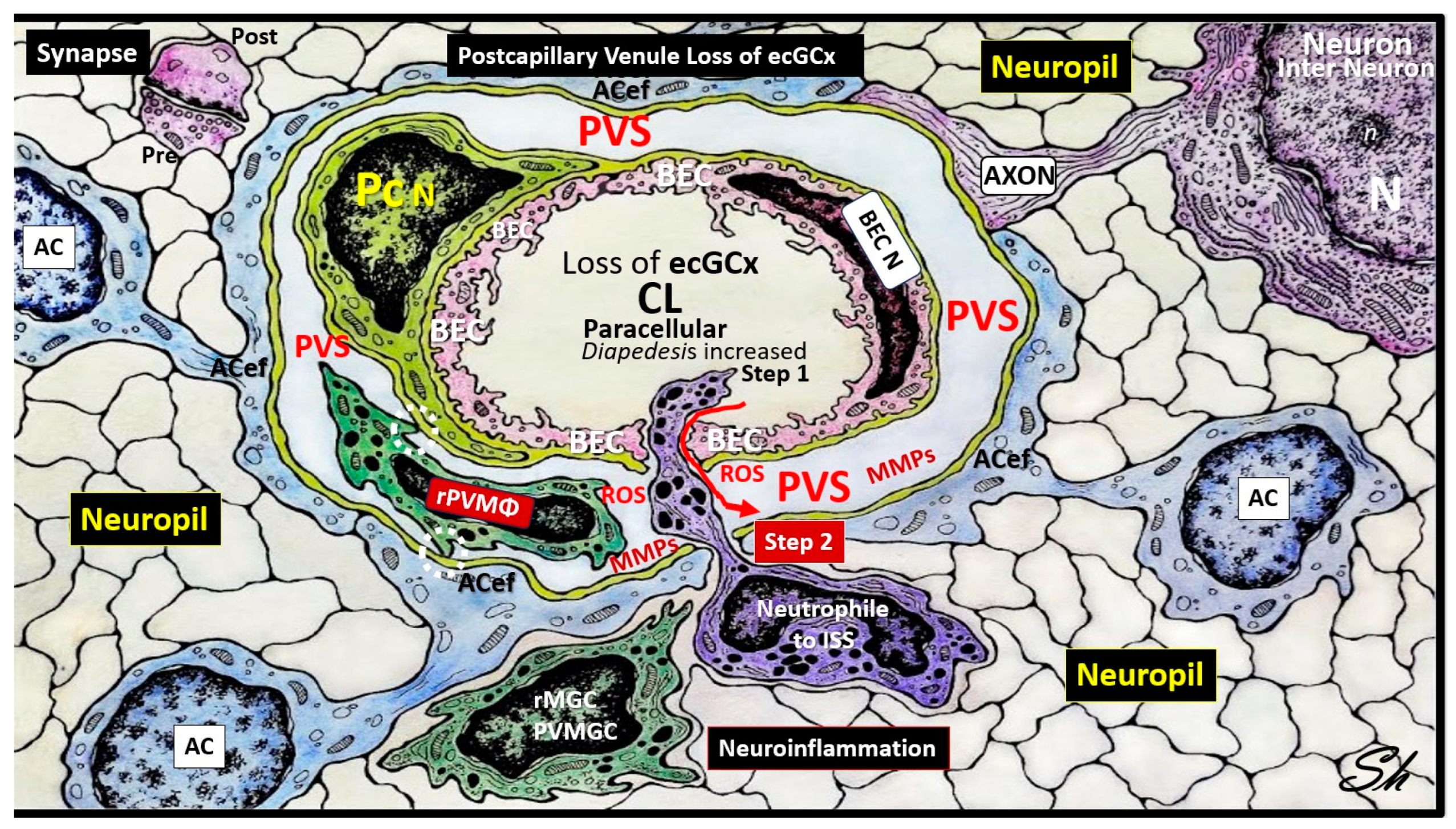
18]. The first line of defense or barrier property against BEC injury is the ecGCx that performs a vital and critical role in maintaining brain homeostasis against BEC injury. When there is an attenuation, loss, or a discontinuous ecGCx of the NVU, the BBB will undergo disruption and allow increased NVU permeability. Interruption of the first barrier of the tripartite barrier (consisting of (1) ecGCx; (2) BECs; (3) extravascular compartment will allow for increased permeability in the postcapillary venule that will be permissive to both fluids and proinflammatory peripherally-derived leukocytes into the perivascular space (PVS) to result in enlargement as well as increased neuroinflammation (
Figure 3) [
6,
7,
18,
19].
EPVS are thought to have numerous causes by multiple mechanisms including: (1) increased fluid and neurotoxic proteins that enter the PVS due to BBB dysfunction
/disruption as a result of increased permeability; (2) increased fluid inflow into the PVS due to increased BBB permeability as a result of BBB disruption and concurrent astrocyte endfeet (ACef) dysfunction, detachment, separation and aquaporin-4 dysfunction with decreased water uptake allowing for the accumulation of water in the PVS; (3) stalling or obstruction of impaired glymphatic efflux of the PVS conduit due to inflammation as a result of the accumulation of excess leukocytes with phagocytosis and accumulation of excessive phagocytic debris, oxidative stress, in addition to the activation of increased matrix metalloproteinases (MMPs) that result in stagnation, stalling, and/or varying degrees of PVS conduit glymphatic system obstruction of the waste removal mechanisms; (4) microvascular arteriole or venule vascular stiffening and/or aberrant spiraling of arterioles that are associated with decreased vascular pulsatility, which result in decreased fluid flow within the PVS, which contribute to PVS enlargement (mechanisms [
1,
2,
3,
4] that were discussed in
Figure 3 and it’s legend previously); (5) neuronal atrophy or apoptotic surrounding neurons or their axons [
19,
20,
21,
22,
23,
24,
25,
26,
27,
28]. Notably, EPVS do not develop all at once, but are thought to be associated with a sequence of events and exist as in an evolutionary spectrum such that they develop gradually to result in SVD, neuroinflammation, impaired cognition and neurodegeneration (
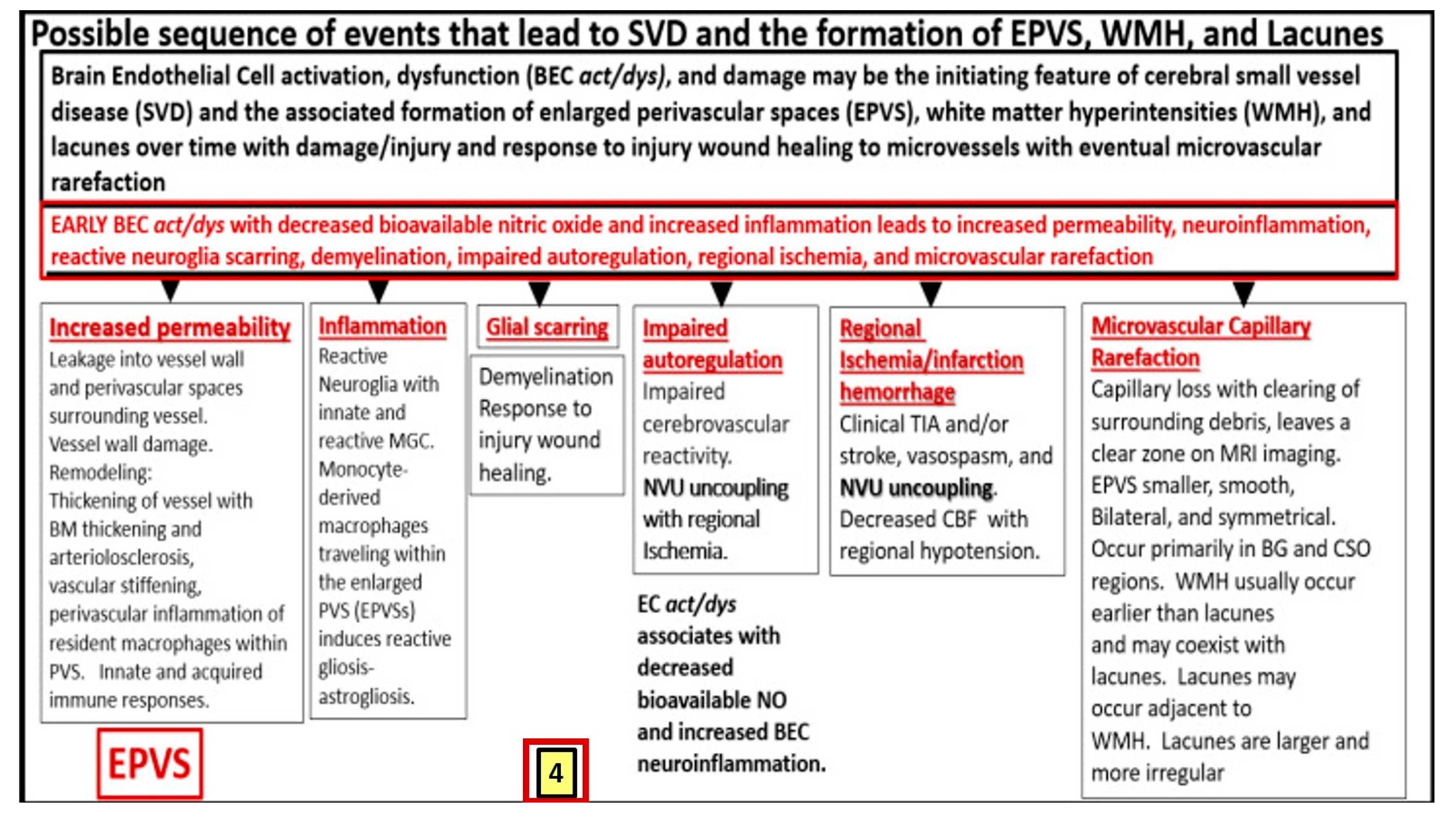
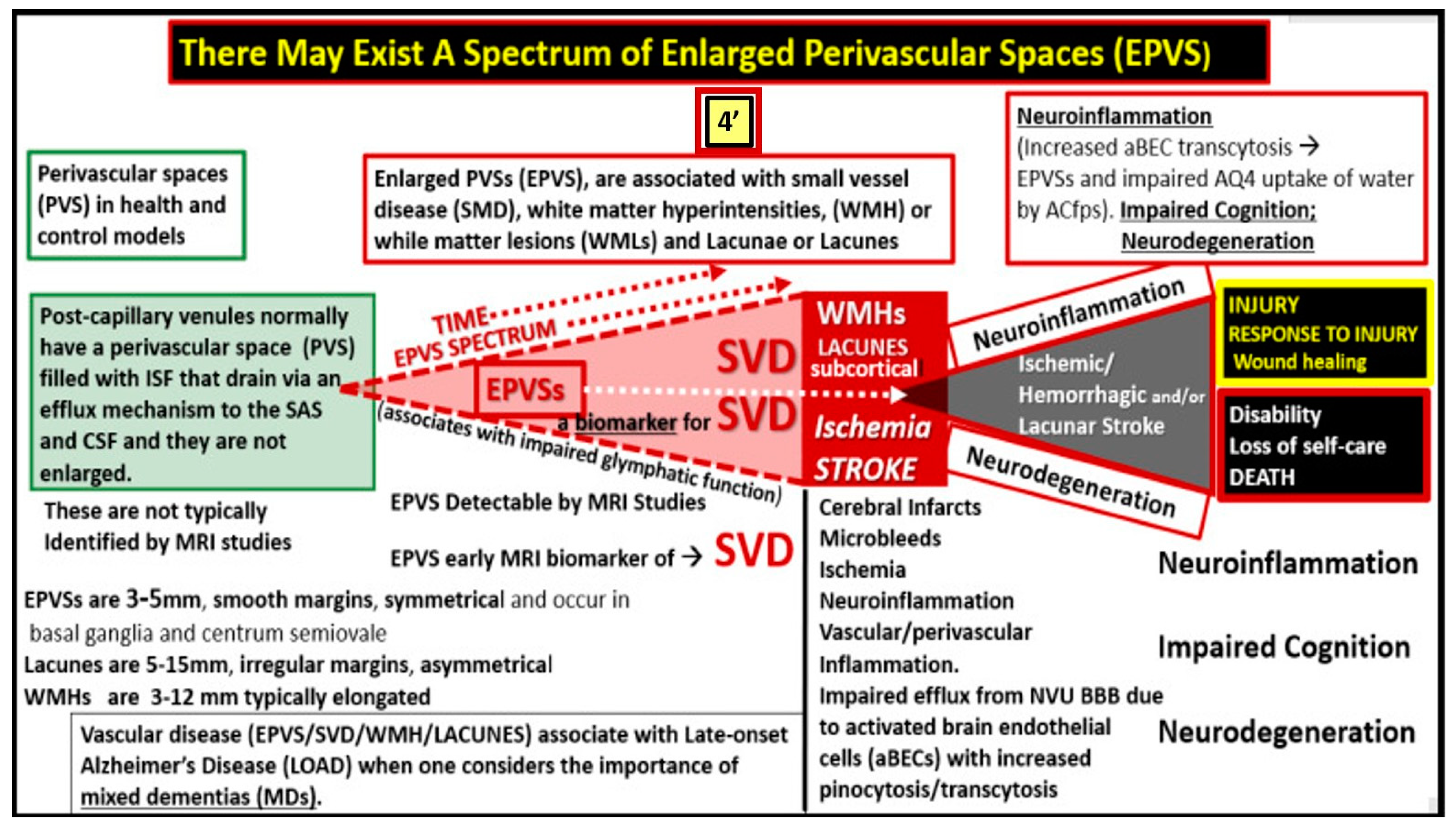
Figure 4,4’) [
19,
20,
28]
EPVS are recognized as important aberrant structural remodeling changes in various neuropathologies and are currently known as biomarkers for cerebral small vessel disease (SVD) in addition to vascular dementia (VaD), which are known to associate with lacunar stroke and white matter hyperintensities (WMH) [
20,
22,
23,
24,
25,
26,
29]. Notably, EPVS are associated with hypertension, advancing age, intracerebral hemorrhages, microbleeds, lacunes, cerebrocardiovascular diseases, transient ischemic episodes and stroke, SVD, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADISIL), cerebral amyloid angiopathy (CAA), metabolic syndrome (MetS), obesity, type 2 diabetes mellitus (T2DM), WMH, sporadic Alzheimer’s and Parkinson’s disease, and non-age-related multiple sclerosis [
20,
21,
22,
23,
24,
26,
27,
29,
30,
31,
32,
33,
34,
35]. Further, our global population is already one of the oldest in history and it is only expected to continue to increase [
5]. Since it is known that EPVS associate with aging it is felt that EPVS will continue to be more prevalent and are expected to grow in the coming years [
20,
36]. Additionally, EPVS are related to extracranial atherosclerosis, cerebromacrovascular, and cerebromicrovascular disease [
36] in addition to age-related neurodegenerative diseases such as sporadic Alzheimer’s and Parkinson’s disease.
The ecGCx is known to maintain normal neuronal homeostasis. It acts specifically as a physical and charge barrier, a mechanical transducer, a regulator of vascular permeability, a modulator of inflammatory response, and anticoagulation. An intact glycocalyx is necessary for the maintenance, stability, and integrity of the internal environment of brain homeostasis. Thus, damage to the ecGCx is capable of resulting in dysfunction of these barriers as a result of this disruption [
16].
2. The ecGCx Structure
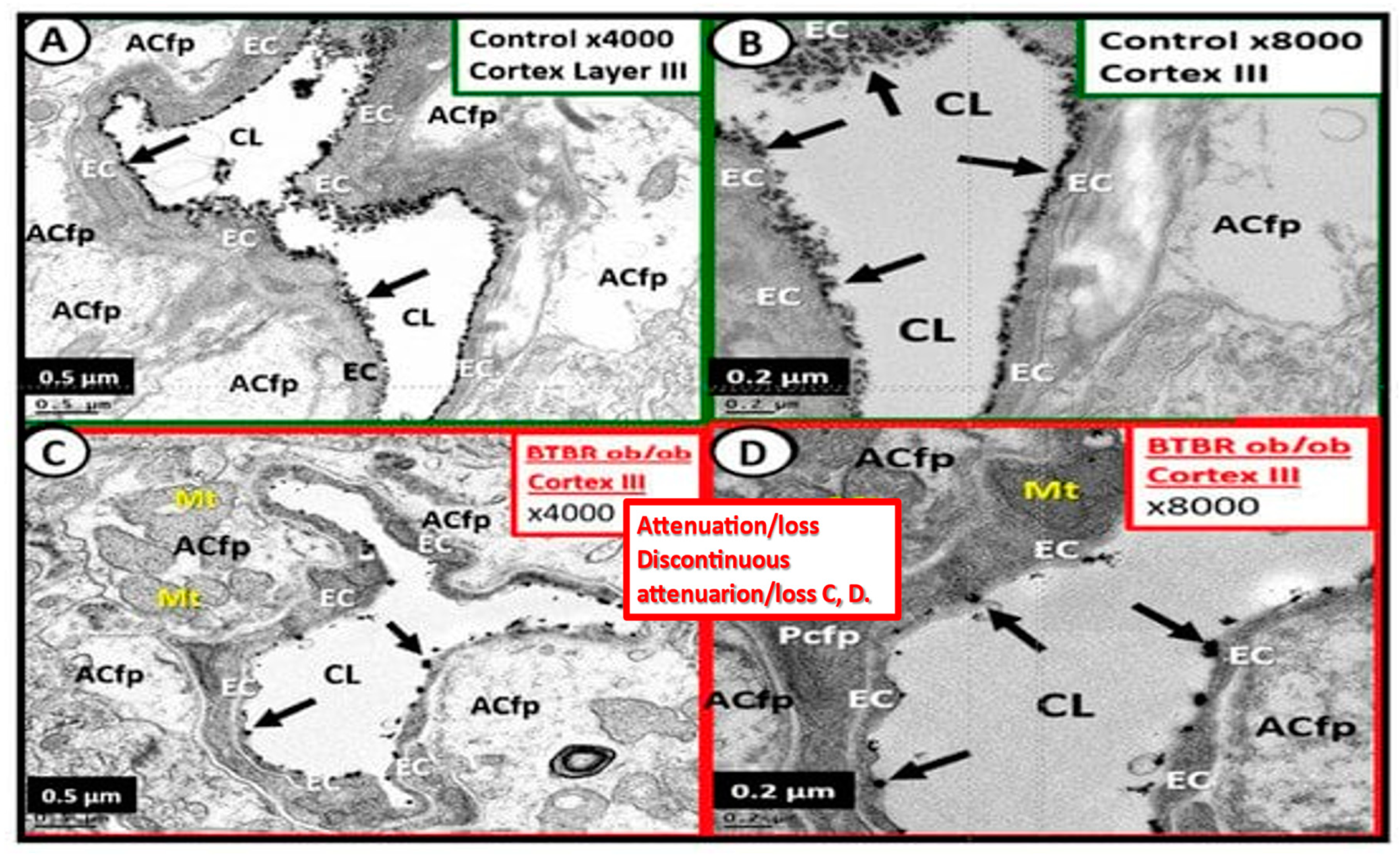
The ecGCx is a nanosized structure making it difficult to study with the light microscope and only recently has lanthanum nitrite become more widely available for transmission electron microscopy TEM studies to supplement Lufts’ earlier work with ruthenium red to stain and capture the endothelial glycocalyx utilizing TEM in 1966 [
4] and most recently author and Ando et al. [
10]. Even at the ultra, nano structure level the ecGCx stains so intensively electron negative due the net negative electrostatic charge of the ecGCx, which attracts lanthanum La3+ nitrate LAN staining specifically in the brain by perfusion fixation and imaged with transmission electron microscopy presented and discussed in
Figure 2 [
6,
8]. Notably, the obese, insulin resistant, and diabetic DTBR
ob/ob female mouse model at 20-weeks of age revealed that the NVU BECs had an attenuation, loss, and/or became discontinuous with lanthanum nitrate perfusion fixation and staining [
6].
Figure 5.
These electron micrographs depict the attenuation, loss, or discontinuous brain endothelial cell glycocalyx (ecGCx) in lanthanum nitrate stained, obese, insulin resistant diabetic BTBR
ob/ob at 20 weeks of age in female mouse models.
Panels A and B are from the normal control model (heterozygous BTBR
ob/ob) and demonstrated the normal lanthanum staining of the ecGCx and note the continuous electron dense nature of this surface coating layer with lanthanum.
Panels C and D depict the small black discontinuous dots (clumping) in contrast to the continuous ecGCx in control models. These remodeling changes would contribute to increased permeability at the NVU BBB to allow the passage of fluid and inflammatory leukocytes into the perivascular spaces to result in enlargement. Thus, these findings rendered the BBB disrupted due to perturbations of its ecGCx. Reproduction of this modified image is permitted by CC 4.0 [
6]
.
Figure 5.
These electron micrographs depict the attenuation, loss, or discontinuous brain endothelial cell glycocalyx (ecGCx) in lanthanum nitrate stained, obese, insulin resistant diabetic BTBR
ob/ob at 20 weeks of age in female mouse models.
Panels A and B are from the normal control model (heterozygous BTBR
ob/ob) and demonstrated the normal lanthanum staining of the ecGCx and note the continuous electron dense nature of this surface coating layer with lanthanum.
Panels C and D depict the small black discontinuous dots (clumping) in contrast to the continuous ecGCx in control models. These remodeling changes would contribute to increased permeability at the NVU BBB to allow the passage of fluid and inflammatory leukocytes into the perivascular spaces to result in enlargement. Thus, these findings rendered the BBB disrupted due to perturbations of its ecGCx. Reproduction of this modified image is permitted by CC 4.0 [
6]
.
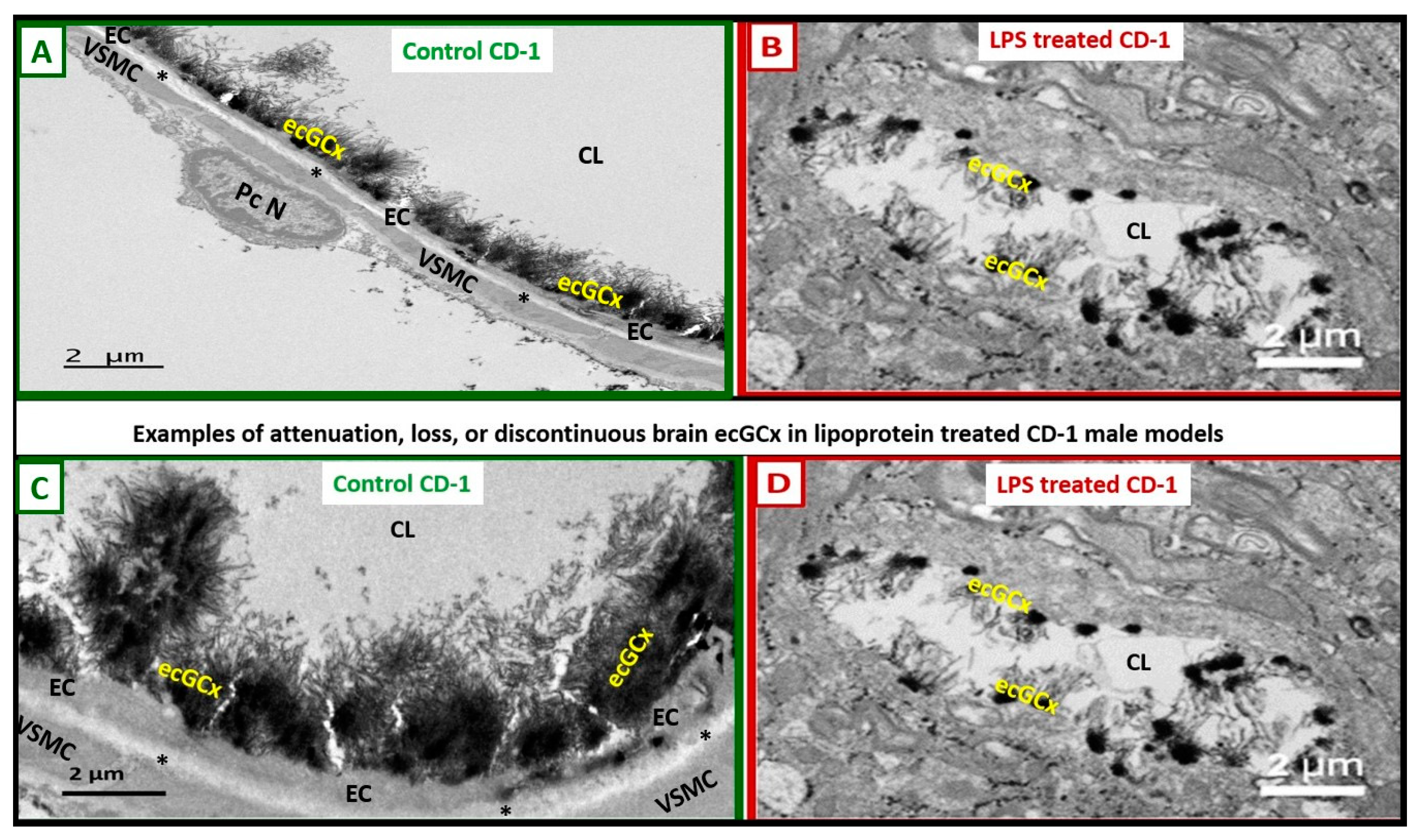
The next representative image is from an LPS-treated male CD-1 mouse model at seven-weeks of age (
Figure 6) [
8].
3. ecGCx Acts as a Physical Barrier
The ecGCx is a molecular sieve with extensive interlacing of its constituent molecules (
Figure 2), which limits the exposure of multiple proteins including: adhesion molecules such as intercellular adhesion molecule-1/
vascular cell adhesion molecule-1 (ICAM-1/VCAM-1), von Willebrand factor, antithrombin, tissue factor pathway inhibitors, NO synthase and extracellular superoxide dismutase [
11]. In addition, the ecGCx excludes leukocytes, erythrocytes, and platelets such that they cannot easily penetrate the ecGCx [
11,
16,
17]. Subsequently, a complete and stable ecGCx inhibits the interaction not only between molecules and cells such as leukocytes, plasma cell and BECs.
4. The ecGCx Acts as an Electrostatic Charge Barrier
The high sulfation of the GAGs within the ecGCx, orosomucoids, and albumin interact to create a net negative electrostatic charge and allows for the electrostatic repulsion of negatively charged molecules, while promoting the facilitation of positively charged molecules due to electrostatic charge absorption [
11,
37,
38,
39].
5. The ecGCx as a Mechanosensor
Blood pressure and shear stress affect the ecGCx and are capable of inducing changes within the BECs to affect its cytoskeleton and result in increased eNOS and bioavailable NO synthesis to result in vasodilation and increase flow commensurate with increased pressure and shear stress [
40]. This is thought to be largely due the interaction between glycosylphosphatidylinositol (GPI), which anchors glypican-1 to allow for the activation of endothelial nitric oxide synthase (eNOS) to synthesize bioavailable nitric NO via the calcium-calmodulin-dependent caveolin-1(CAV-1) protein [
6,
7,
16,
38,
40]. Notably, vascular permeability is modified by the molecular size, structure and especially the electrostatic charge of the ecGCx [
16,
38,
41].
6. The ecGCx as a Regulator of Permeability
Paracellular tight and adherens junctions (TJ/AJs) remain the main proteins involved in the control of NVU BEC permeability; however, in the past decade the ecGCx has also been recognized as a key regulator of permeability [
18]. Previous observations have suggested that the main osmotic pressure gradient opposite to fluid filtration begins with ecGCx, but the effect of interstitial protein concentration was small [
42]. Importantly, the ecGCx is now considered to be the first barrier of a three-part BBB with the BEC as the 2
nd barrier and the abluminal BEC BM and ACef (considered the extravascular compartment) as its 3
rd barrier [
18]. Thus, the ecGCx is a major permeability barrier of BECs [
41,
42]
7. The ecGCx as a Regulator of Inflammation
The ecGCx heparan sulfate proteoglycans (HSPGs) are known to serve as a ligand for certain selectins and chemokines and play an important role in the turnover of leukocytes at the postcapillary venules [
43]
. Chronically excessive peripherally-derived cytokines and chemokines and dysregulation are a central feature in the development of neuroinflammation, neurodegeneration, and impaired cognition [
44]. Since excessive cytokines/chemokines eventually result in ecGCx attenuation and/or shedding this protective effect will be lost such that inflammation begets inflammation and a vicious cycle will ensure and herein lies the problem in obesity, MetS and T2DM. Additionally, the ecGCx is able to (1) shield BECs from integrins and other molecules that stimulate the binding of proinflammatory leukocytes to the endothelial plasma membrane surface coat as well as interfering with antigen presentation and T cell activation; (2) the ecGCx is able to create a regional chemokine gradient that favors leukocyte activation; (3) it also regulates the activity of chemokines, cytokines, and growth factors by protecting them from enzymatic degradation [
16]. Notably, a healthy ecGCx provides steric hindrance of receptor/ligand binding during leukocyte adhesion to the endothelium and is known to regulate inflammation due to various causes by different mechanisms [
45], which includes: it’s shielding effects from integrins and co-stimulating molecules that are important in inhibiting the binding of proinflammatory leukocytes to endothelial surfaces. For example,
glycosaminoglycans interact selectively with chemokines (such as RANTES, CxCL1, MCP-1, IL-8, MIP1alpha) and modulate receptor binding and cellular responses [
46]; it’s mechanisms of inhibiting antigen presentation and T cell activation; it’s protective mechanisms of cytokines and growth factors from enzymatic degradation; it’s mechanisms of binding regulatory proteins and aiding in complement inhibition [
47].
In healthy homeostatic conditions, true capillaries without pia mater and PVS are important for the uptake of water, oxygen, nutrients, and metabolic efflux while the post-capillary venule and its PVS are responsible for the transmigration of immune cells (innate and adaptive leukocytes) into the CNS parenchyma as previosly described by Owens et al [
48]. Importantly, Owens et.al have shared the concept of the 2 steps to neuroinflammation wherein step-one consists of the proinflammatory leukocytes breeching the BBB (due to BBB disruption via perturbation of the ecGCx and dysfunctional or damaged TJ/AJ) and entering into the PVS and step-two consists of PVS proinflammatory leukocytes breaching the outer basement lamina of the perivascular space (via perivascular space proinflammatory-derived oxidative stress and MMP damage to the astrocyte basal lamina) to allow proinflammatory leukocytes to enter the ISS of the neuronal parachyma upon transmigration from the PVS (
Figure 7) [
48].
Notably, PVS serve as a conduit for the efflux of waste to the SAS and CSF to the dural venous sinus and the systemic circulation, which is now termed the glymphatic system initially described by Iliff and others [
49].
8. The ecGCx as an Anticoagulant
The ecGCx physical barrier prevents the blood cells and endothelium from coming into direct contact and thus avoid coagulation [
11]
. The ecGCx is a significant physiological binding site for anticoagulant factors, tissue factor pathway inhibitor (TFPI) and antithrombin III (AT III) [
11,
51]. Further, antithrombin III combines with HSPGNs to enhance its anticoagulant effect and thrombomodulin can bind to chondroitin sulfate that converts thrombin into an activator of protein C pathway to strengthen the anticoagulant pathway [
11]. The tissue pathway inhibits FVIIa (Hageman factor) and FXa (xabans) and interacts with HSPGs and the ecGCx [
11,
43]. Additionally, TFPI is a Kunitz-type protease inhibitor that inhibits the initial reactions of blood coagulation that resides in the ecGCx and forms a major pool of TFPI [
52]. Additionally, thrombomodulin (TH) resides within the ecGCx and provides natural anticoagulant properties to the endothelium by inhibiting thrombin [
53,
54]. These previous actions of the ecGCx summarily contribute to the natural inhibition the coagulation process as in (
Figure 2).
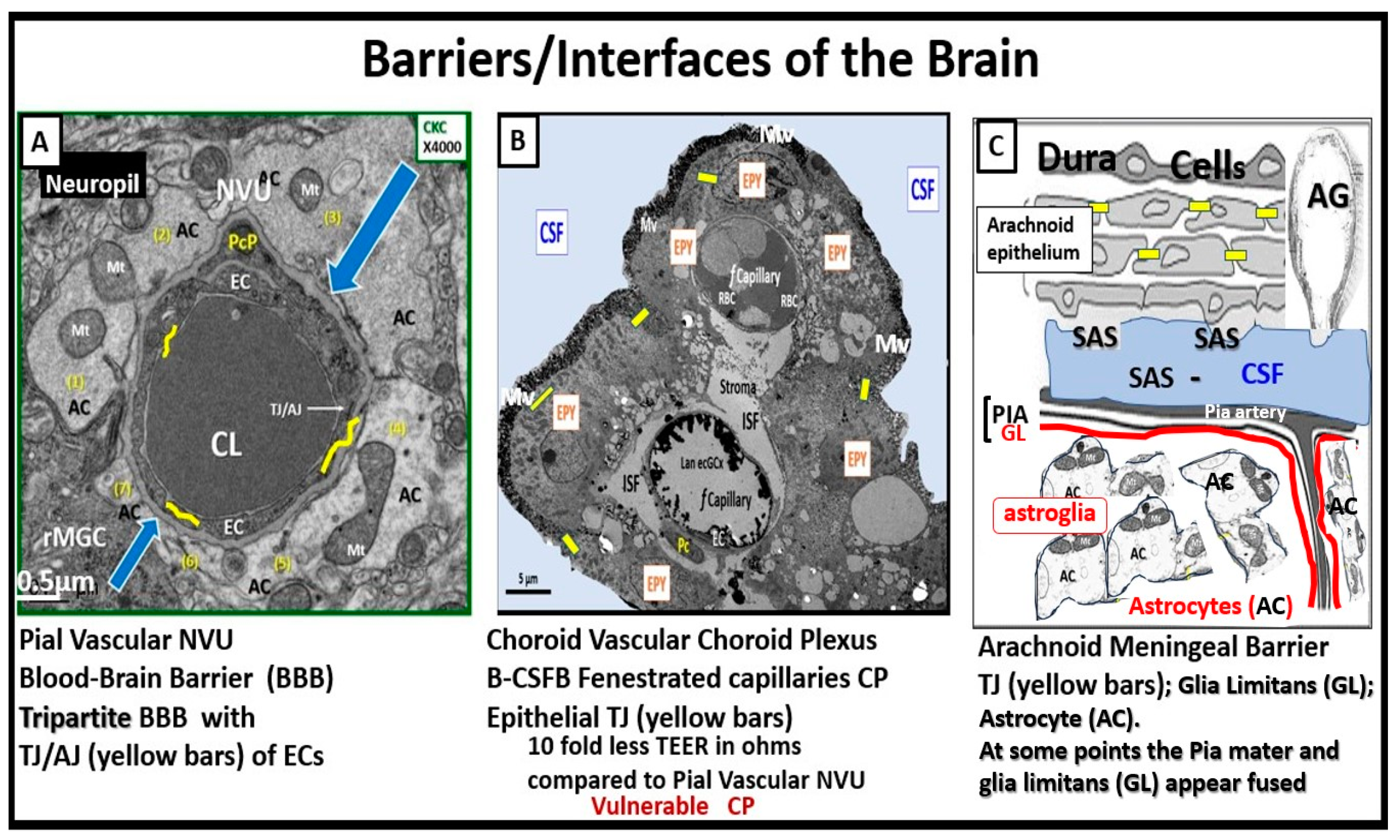
9. The Importance of the Brain Barriers/Interfaces
While this review has focused heavily on the BBB with tight junctions (Claudins and Occludins and junctional adherens molecules (JAMs) and adherens junctions (VE-cadherins) (TJ/AH), there are actually three main barriers/interfaces in the CNS [
55,
56,
57]. In addition to the BBB there are two other important barriers that should discussed, namely the blood-cerebrospinal fluid barrier (B-CSFB) and the meningeal arachnoid barrier, which is also a B-CSFB (
Figure 8) [
56,
58,
59,
60].
Notably, the B-CSFB fenestrated capillaries stain heavily with lanthanum nitrite (LAN) in control models perfused fixed staining in contrast to LPS treated models (
Figure 8B). Additionally, the B-CSFB is also present at the median eminence of the third ventricle lined by the epithelial tanycytes and the circumventricular organs of the hypothalamic nuclei [
56]. Also, the arachnoid barrier, which is a component of the B-CSFB located in the meninges and is composed of arachnoid epithelial cells with tight junctions (Claudin 11) and adherens junctions (E-Cadherins) (
Figure 8C) [
61].
The choroid plexus (CP) and its B-CSFB are critical for maintaining brain homeostasis. It’s aberrant function and increased permeability has been recently associated with different diseases of the CNS including neurodegenerative diseases such as Alzheimer’s disease, autoimmune disease such as multiple sclerosis. Also, the CP/B-CSFB as been shown to be important in restoring homeostasis after stroke and brain injury [
62,
63].
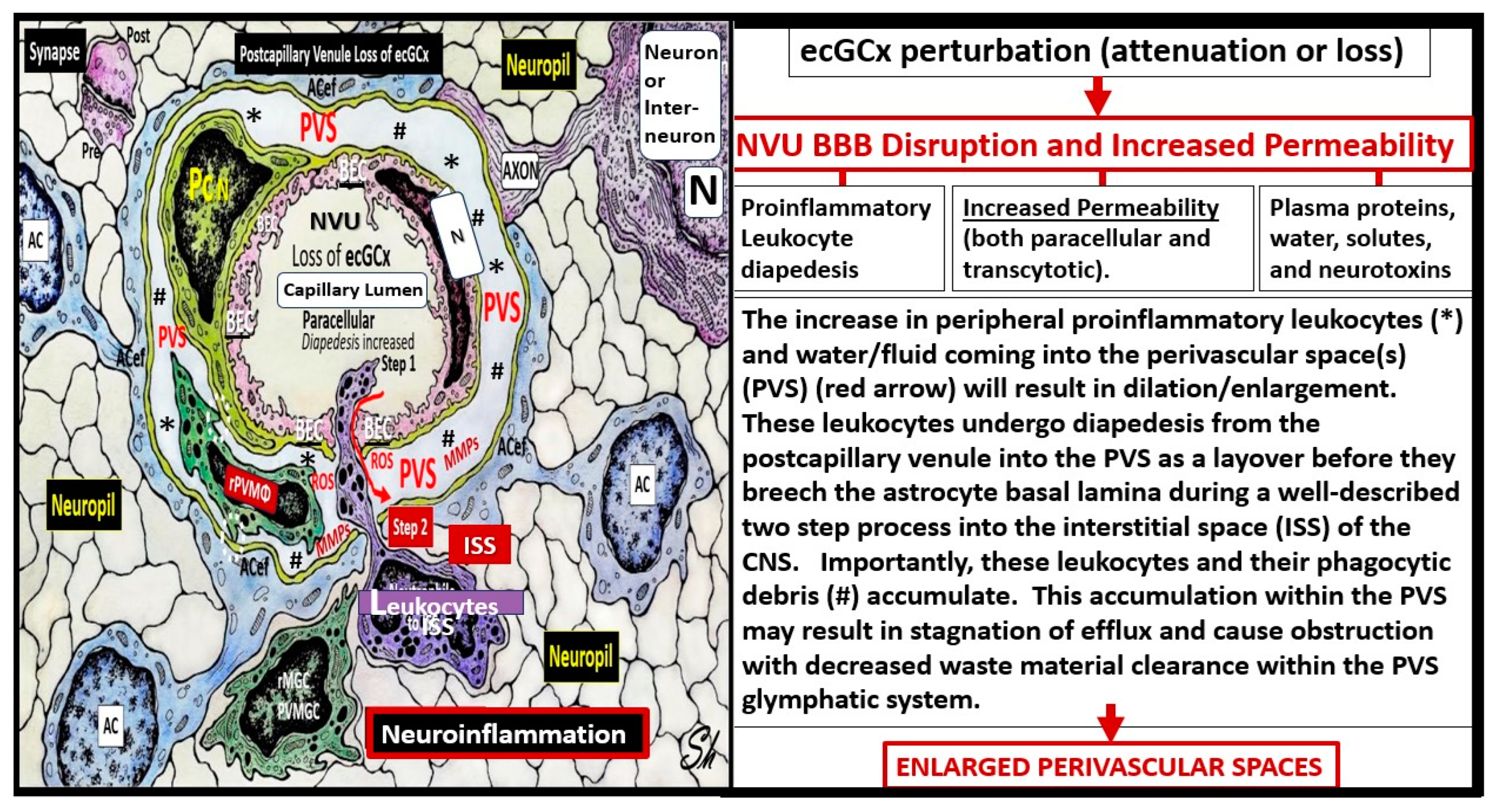
10. Possible Mechanisms of Action Regarding How Attenuation and/or Loss of ecGCx Contributes to the Development of Enlarged Perivascular Spaces.
When the ecGCx becomes attenuated and/or lost the NVU BBB is known to become disrupted and develops increased permeability with leakage of water, solutes, plasma proteins, and proinflammatory leukocytes, peripheral cytokines/chemokines, ROS, and MMPs (
Figure 7) [
64]. The Loss of the ecGCx also leads to a destabilization of adjacent BEC actin cortical web, which in turn is known disrupt junctional (tight and adherens junction) protein alignment and prevent intercellular communication between adjacent ECs. Upon this destabilization of the tight and adherens junctions this paracellular junction will develop increased permeability to proinflammatory leukocytes, plasma, and plasma proteins and other neurotoxicities [
65].
EPVS are critical conduits for interstitial fluid and metabolic waste into the CSF [
66], and EPVS efflux could be negatively affected by compromised BBB integrity and associated BEC inflammation [
67]. As noted in previous figures (
Figure 7 and
Figure 8A), the NVU BBB is a selective barrier composed of true capillary, postcapillary venule BEC, TJ/AJ, pericytes, and astrocytes [
68]. The excess accumulation of the peripheral proinflammatory leukocytes including increased peripherally-derived monocyte-derived macrophages that become perivascular macrophages, antigen presentation by pericytes, BECs, and rPVMΦ protease’s (MMPs), cytokines/chemokines and oxidative stress will result in excessive phagocytic debris within the PVS. Thus, this accumulation of excessive debris, stagnation of flow within the EPVS will result in impaired efflux within the EPVS or glymphatic system allowing neurotoxins to accumulate and not be cleared [
20,
23]. In addition to affecting the integrity of the paracellular TJ/AJs between BECs, increased BBB permeability will also affect endothelial function that also relates to cSVD. As a result of these remodeling changes, EPVS are not only considered as a biomarker for cerebral small vessel disease but also a possible marker for BBB disruption or dysfunction, and impaired glymphatic efflux [
20,
23].
11. Conclusion
The ecGCx is a key and critical regulator of permeability, cerebral blood flow, capillary perfusion, and cell adhesion all of which are necessary for maintenace of CNS homeostasis. Therefore, we should do all we can to maintain the integrity of ecGCx to decrease the development of EPVS as a result of NVU BBB disruption with increased permeability as a result of attenuation and/or loss of the ecGCx. For this reason, the possible mechanisms of how ecGCx perturbations with attenuation and/or loss might result in EPVS are shared (
Figure 9).
The concept that the glia limitans (the basal lamina of perivascular ACef) serve as a second barrier for the passage of proinflammatory leukocytes is substantiated by Horng et al [
69].
We now live in one of the oldest global populations ever recorded in history that will continue to increase over at least the next two decades until the so-called global baby boomer generation has passed [
5,
20]. Additionally, it is currently known that along with aging comes increasing age-related diseases such as cerebrocardiovascular diseases, stroke, impaired cognition, and dementias (especially sporadic Alzheimer’s and Parkinson’s disease) and frailty. Further, these age-related diseases are major causes of disability and dependence in elderly individuals that causes a great financial and societal burden [
70].
When discussing health with older individuals over the past 30 plus years of my clinical practice in family medicine, three of the most feared diseases are the development of stroke, dementia, and cancer because they so often result in the inability for older people to care for themselves. However, cognitive impairment and dementia are primarily the result of a combination of vascular brain injury and neurodegeneration. Also, vascular injury manifest as a highly prevalent pathologic disease (SVD) over a long period time instead of acute onset transient ischemic episodes and strokes [
71]. Importantly, these SVD abnormalities are manifest on studies utilizing non-invasive magnetic resonance imaging (MRI) and consist of MRI abnormalities termed white matter hyperintensities, lacunes, microbleeds, microthrombi, and most recently EPVS [
72]. Notably, EPVS have been determined and accepted as biomarkers for SVD [
20,
31]. In addition to EPVS being biomarkers for SVD, they have also been identified as markers for increased risk of cognitive decline and dementia, independent of other SVD markers [
73]. Thus, this is why author wished to share this emerging accumulation of knowledge regarding the importance of these PVS/EPVS in the growing scientific field of neuroaging. It is estimated that ~ two billion of the global population will be greater than 60 years of age by 2050 or 1/5 of the world’s population [
74].
Intriguingly, enlarged perivascular spaces, the glymphatic system, and the ecGCx are in their early stages of exponential growth in understanding how they relate to various injurious processes resulting in neuroinflammation and multiple diseases that affect the brain through neurovascular and neurodegenerative diseases with many future questions that are needed to be asked and answered. It is hoped that this review will provide the stimulus for the future search to ask these questions in order to provide the necessary answers.
Author Contributions
Author is totally responsible for the Conceptualization, Methodology, Software, Validation, Formal Analysis, Investigation, Resources, Data Curation, Writing—Original Draft Preparation, Writing—Review and Editing, Visualization, Supervision, Project Administration, and Funding Acquisition. Author has read and agreed to the published version of the manuscript.
Funding
Author has not received grants from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The tissues provided for the representative electron microscopic images utilized in this manuscript were all approved in advance by the University of Missouri Institutional Animal Care and Use Committee (No. 190), and animals were cared for in accordance with National Institutes of Health guidelines and by the Institutional Animal Care and Use Committees at the Harry S. Truman Memorial Veterans Hospital and University of Missouri, Columbia, MO, USA, and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data and materials can be provided upon reasonable request.
Acknowledgments
The author would like to acknowledge Tatyana Shulyatnikova for the contributions of many artistic illustrations and editing of this manuscript. The author would also like to acknowledge DeAna Grant Research Specialist of the Electron Microscopy Core Facility at the Roy Blunt NextGen Precision Health Research Center, University of Missouri, Columbia, Missouri. The author also acknowledges the kind support of the William A. Banks Lab at the VA Medical Center-Seattle, Washington. .
Conflicts of Interest
Author declares no conflicts of interest.
Abbreviations
AC: astrocyte; ACef, astrocyte end-feet; AGE/RAGE, advanced glycation end products/receptor for advanced glycation end products; AQP4, aquaporin-4; ATIII, antithrombin three; BBB, blood–brain barrier; BEC(s), brain endothelial cell(s); BECact/dys, brain endothelial cell activation/dysfunction; BM, basement membrane; CAV-1, calcium-calmodulin-dependent caveolin-1; CL, capillary lumen; EPVS, enlarged perivascular spaces; GS, glymphatic space; GPI, glycosylphosphatidylinositol HA, hyaluronan; HSPGs, heparan sulfate proteoglycans; ICAM-1, intercellular adhesion molecule-1; ISF, interstitial fluid; ISS, interstitial space; LAN, lanthanum nitrate; La3+, lanthanum plus 3; LOAD, late-onset Alzheimer’s disease; LPS, lipopolysaccharide; MetS, metabolic syndrome; MGCs, microglia cells; MMP-2,-9, matrix metalloproteinase-2,-9; NVU, neurovascular unit; Pc, pericyte; Pcfp, pericyte foot process; PVS, perivascular spaces; PVS/EPVS, perivascular space/enlarged perivascular space; rPVMΦ, resident perivascular macrophages; SAS, subarachnoid space; rPVMΦ, reactive perivascular macrophage; SVD, cerebral small vessel disease; T2DM, type 2 diabetes mellitus; TEM, transmission electron microscopy; TH, thrombomodulin; TIPI, tissue factor pathway inhibitor; TI/AJs, tight and adherens junctions; VAD, vascular artery disease; VCAM-1, vascular cell adhesion molecule-1; WMH; white matter hyperintensities.
References
- Reed MJ, Damodarasamy M, Banks WA. The extracellular matrix of the blood–brain barrier: structural and functional roles in health, aging, and Alzheimer’s disease. Tissue Barriers. 2019; 7(4): 1651157. [CrossRef]
- Jin J, Fang F, Gao W, Chen H, Wen J, Wen X, Chen J. The Structure and Function of the Glycocalyx and Its Connection With Blood-Brain Barrier. Front Cell Neurosci. 2021;15:739699. [CrossRef]
- Fu BM, Tarbell JM. Mechanosensing and transduction by endothelial surface glycocalyx: composition, structure, and function. Wiley Interdiscip Rev Syst Biol Med. 2013;5(3):381–390. [CrossRef]
- Luft, JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966 Nov-Dec;25(6):1773-1783.
- Hayden, MR. Type 2 Diabetes Mellitus Increases The Risk of Late-Onset Alzheimer's Disease: Ultrastructural Remodeling of the Neurovascular Unit and Diabetic Gliopathy. Brain Sci. 2019;9(10):262. [CrossRef]
- Hayden MR, Banks WA. Deficient Leptin Cellular Signaling Plays a Key Role in Brain Ultrastructural Remodeling in Obesity and Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021;22, 5427 2021;22(11):5427. [CrossRef]
- Hayden, MR. The Mighty Mitochondria Are Unifying Organelles and Metabolic Hubs in Multiple Organs of Obesity, Insulin Resistance, Metabolic Syndrome, and Type 2 Diabetes: An Observational Ultrastructure Study. Int J Mol Sci. 2022;23(9):4820. [CrossRef]
- Erickson MA, Shulyatnikova T, Banks WA, Hayden MR. Ultrastructural Remodeling of the Blood-Brain Barrier and Neurovascular Unit by Lipopolysaccharide-Induced Neuroinflammation. Int J Mol Sci. 2023;24(2):1640. [CrossRef]
- Zhu J, Li Z, Ji Z,Wu Y, He Y, ChangY, Peng Y, Zimmerman Z, Wang S, Wang D, Kaibin Huang K, Pan S. Glycocalyx is critical for blood-brain barrier integrity by suppressing caveolin1-dependent endothelial transcytosis following ischemic stroke. Brain Pathology. 2022;32(1):e13006. [CrossRef]
- Ando Y, Okada H, Takemura G, Suzuki K, Takada C, Tomita H, et al. Brain-Specific Ultrastructure of Capillary Endothelial Glycocalyx and Its Possible Contribution for Blood Brain Barrier. Sci Rep. 2018;8(1):17523. [CrossRef]
- Reitsma S, Slaaf DW, Vink H, van Zandvoort MAMJ, Egbrink MGA. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;45(3):345-359. [CrossRef]
- Berdiaki A, Neagu M, Spyridaki I, Kuskov A, Serge Perez S, Nikitovic D. Hyaluronan and Reactive Oxygen Species Signaling-Novel Cues from the Matrix? Antioxidants (Basel). 2023;12(4):824. [CrossRef]
- Huxley VH, Curry FE. Differential actions of albumin and plasma on capillary solute permeability. Am J Physiol. 1991;260(5 Pt 2):H1645-654. [CrossRef]
- duPreez HN, Aldous C, Hayden MR, Kruger HG, Lin J. Pathogenesis of COVID-19 described through the lens of an undersulfated and degraded epithelial and endothelial glycocalyx. FASEB. 2022;36(1):e22052. [CrossRef]
- Hayden, MR. Brain Endothelial Cells Play a Central Role in the Development of Enlarged Perivascular Spaces in the Metabolic Syndrome. Medicina (Kaunas). 2023 Jun 11;59(6):1124. [CrossRef]
- Zhao F, Zhong L, Luo Y. Endothelial glycocalyx as an important factor in composition of blood-brain barrier. CNS Neurosci Ther. 2021;27:26–35. [CrossRef]
- Foote CA, Soares RN, Ramirez-Perez FI, Ghiarone T, Aroor A, Manrique-Acevedo C, Jaume Padilla J, Martinez-Lemus L. Endothelial Glycocalyx. Compr Physiol. 2022;12(4):3781-3811. [CrossRef]
- Kutuzov N, Flyvbjerg H, Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood–brain barrier. Proc Natl Acad Sci U S A. 2018;115(40):E9429-E9438. [CrossRef]
- Hayden, MR. Brain Injury: Response to Injury Wound Healing Mechanisms and Enlarged Perivascular Spaces in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Medicina (Kaunas). 2023;59(7):1337. [CrossRef]
- Shulyatnikova T, Hayden MR. Why ARE Perivascular Spaces Important? Medicina (Kaunas). 2023;59(5):917. [CrossRef]
- Bown CW, Carare RO, Schrag MS, Jefferson AL. Physiology and Clinical Relevance of Enlarged Perivascular Spaces. Neurology. 2022;98;107–117. 98. [CrossRef]
- Brown R, Benveniste H, Black SE, Charpak S, Dichgans M, Joutel A, Nedergaard M, Smith KJ, Zlokovic BV, Wardlaw JM. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc. Res. 2018;114:1462–1473. [CrossRef]
- Doubal FN, Maclullich AMJ, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged Perivascular Spaces on MRI Are a Feature of Cerebral Small Vessel Disease. Stroke. 2010;41(3):450-454. [CrossRef]
- Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. Vascular dysfunction-The disregarded partner of Alzheimer's Disease. Alzheimer’s Dement. 2019;15(1):158-167. [CrossRef]
- Loos CMJ, Klarenbeek P, van Oostenbrugge RJ, Staals J. Association between Perivascular Spaces and Progression of White Matter Hyperintensities in Lacunar Stroke Patients. PLoS One. 2015; 10(9): e0137323. [CrossRef]
- Heier LA, Bauer CJ, Schwartz L, Zimmerman RD, Morgello S, Deck MD. Large Virchow-Robin spaces: MR-clinical correlation. Am J Neuroradiol. 1989;10(5):929-936.
- Trolli F, Cipollini V, Moci M, Morena E, Palotai M, Rinaldi V, Romano C. Ristori G, Giubilei F, Salvetti M, Orzi F, Guttmann CRG, Cavallari M. Perivascular Unit: This Must Be the Place. The Anatomical Crossroad Between the Immune Vascular and Nervous System. Front Neuroanat. 2020;14: 17. [CrossRef]
- Hayden, MR. Pericytes and Resident Perivascular Macrophages Play a Key Role in the Development of Enlarged Perivascular Spaces in Obesity, Metabolic Syndrome and Type 2 Diabetes Mellitus. Preprints 2023, 2023070461, [doubt will be accepted prior to uploading this new paper]. [Google Scholar] [CrossRef]
- Okar SV, Hu F, Shinohara RT, Beck ES, Reich DS, Ineichen BV. The etiology and evolution of magnetic resonance imaging-visible perivascular spaces: Systematic review and meta-analysis. Front in Neuroscience. 2023;17:1038011. [CrossRef]
- Benjamin P, Trippier S, Lawrence AJ, Lambert C, Zeestraten E, Williams OA, Patel B, Morris RG, Barrick TR, MacKinnon AD, Markus HS. Lacunar Infarcts, but Not Perivascular Spaces, Are Predictors of Cognitive Decline in Cerebral Small-Vessel Disease. Stroke. 2018 Mar;49(3):586-593. [CrossRef]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12: 822–838. [CrossRef]
- Francis F, Ballerini L, Wardlaw JM. Perivascular spaces and their associations with risk factors, clinical disorders and neuroimaging features: A systematic review and meta-analysis. Int J Stroke. 2019;14(4):174749301983032. [CrossRef]
- Arba F, Quinn TJ, Hankey GJ, Lees KR, Wardlaw JM, Ali M, et al. Enlarged perivascular spaces and cognitive impairment after stroke and transient ischemic attack. Int J Stroke. 2016;13(1). [CrossRef]
- Bokura H, Kobayashi S, Yamaguchi S. Distinguishing silent lacunar infarction from enlarged Virchow-Robin spaces: a magnetic resonance imaging and pathological study. J Neurol. 1998;245(2):116-122. [CrossRef]
- Zhu YC, Tzourio C, Soumaré A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. 2010;41(11):2483-2490. [CrossRef]
- Gutierrez J, Rundek T, Ekind MSV, Sacco RL, Wright CB. Perivascular Spaces Are Associated with Atherosclerosis: An Insight from the Northern Manhattan Study. AJNR Am J Neuroradiol. 2013; 34(9): 1711–1716. [CrossRef]
- Lieleg O, Baumgarter RM, Bausch AR. Selective filtering of particles by the extracellular matrix: an electrostatic bandpass. Biophys J. 2009;97(6):1569-1577. [CrossRef]
- Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol. 2000;278(1):H285-H289. [CrossRef]
- Jiang XZ, Ventikos Y, Luo KH. Microvascular ion transport through endothelial glycocalyx layer: new mechanism and improved Starling principle. Am J Physiol Heart Circ Physiol. 2019;317:H104-H113. [CrossRef]
- Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272(30):18522-18525. [CrossRef]
- Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann Biomed Eng. 2012;40(4):828-839. [CrossRef]
- Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol. 2004;557(Pt 3):889-907. [CrossRef]
- O’Callaghan P, Zhang X, JP. Heparan Sulfate Proteoglycans as Relays of Neuroinflammation. J Histochem Cytochem. 2018; 66(4): 305–319. [CrossRef]
- Ramesh G, MacLean AG, Philipp MT. Cytokines and Chemokines at the Crossroads of Neuroinflammation, Neurodegeneration, and Neuropathic Pain. Mediators Inflamm. 2013;2013: 480739. [CrossRef]
- Mulivor AW, Lipowsky HH. Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286:H1672-H1680. [CrossRef]
- Kuschert GSV, Coulin F, Power CA, Proudfoot AEI, Hubbard RE, Hoogewerf AJ, Wells TNC. Glycosaminoglycans Interact Selectively with Chemokines and Modulate Receptor Binding and Cellular Responses. Biochemistry. 1999; 38, 39, 12959–12968. [CrossRef]
- Milusev A, Rieben R, Sorvillo N. The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders. Front Cardiovasc Med. 2022;9:897087. [CrossRef]
- Owens T, Bechmann I, Engelhardt B. Perivascular Spaces and the Two Steps to Neuroinflammation. Journal of Neuropathology & Experimental Neurology. 2008;67(12);1113–1121. [CrossRef]
- Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R, Nedergaard M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014; 34:16180–16193. [CrossRef]
- Bohr T, Hjorth PG, Holst SC, Hrabětová S, Kiviniemi V, Lilius T, et, al. The glymphatic system: Current understanding and modeling. iScience. 2022;25(9):104987. [CrossRef]
- Gao L, Lipowsky HH. Composition of the Endothelial Glycocalyx and its Relation to its Thickness and Diffusion of Small Solutes. Microvasc Res. 2010;80(3):394-401. [CrossRef]
- Kato, H. Regulation of functions of vascular wall cells by tissue factor pathway inhibitor: basic and clinical aspects. Arterioscler Thromb Vasc Biol. 2002;22:539-548. [CrossRef]
- Boffa MC, Karmochkine M. Thrombomodulin: an overview and potential implications in vascular disorders. Lupus. 1998;(7 Suppl 2):S120-S125. [CrossRef]
- Martin FA, Murphy RP, Cummins PM. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am. J. Physiol. Heart Circ. Physiol. 2013;304(12):H1585-H1597. [CrossRef]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev. 2019;99(1):21-78. [CrossRef]
- Erickson MA, Banks WA. Neuroimmune Axes of the Blood–Brain Barriers and Blood–Brain Interfaces: Bases for Physiological Regulation, Disease States, and Pharmacological Interventions. Pharmacol Rev. 2018;70(2):278–314. [CrossRef]
- Iliff, J. , Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates E, Deane R, Goldman SA, et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012;4:147ra111. [CrossRef]
- Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. 2010;37(1):13-25. [CrossRef]
- Liddelow, SA. Development of the choroid plexus and blood-CSF barrier. Front Neurosci. 2015;9:32. [CrossRef]
- Johanson CE, Stopa EG, McMillan PN. The blood-cerebrospinal fluid barrier: structure and functional significance. Methods Mol Biol. 2011;686:101-31. [CrossRef]
- Derk J, Como CN, Jones HE, Joyce LR, Kim S, Spencer BL, et. al. Formation and Function of the meningeal arachnoid barrier and development around the developing mouse brain. Dev Cell. 2023;58(8):635-644.e4. [CrossRef]
- Solár P, Zamani A, Kubíčková L, Dubový P, Joukal M. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. 2020;17(1):35. [CrossRef]
- Johanson CE, Johanson NL. Choroid Plexus Blood-CSF Barrier: Major Player in Brain Disease Modeling and Neuromedicine. J. Neurol. Neuromed. 2018;3:39–58. [CrossRef]
- Li Y, Li M, Yang L, Qin W, Yang S, Yuan J, Jiang T, Hu W. The relationship between blood–brain barrier permeability and enlarged perivascular spaces: a cross-sectional study. Clin Interv Aging. 2019;14:871–878. [CrossRef]
- Mensah SA, Nersesyan AA, Ebong EE. Endothelial Glycocalyx-Mediated Intercellular Interactions: Mechanisms and Implications for Atherosclerosis and Cancer Metastasis. Cardiovasc Eng Tech. 2021;12:72-90. [CrossRef]
- Abbott, NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45:545–552. [CrossRef]
- Satizabal CL, Zhu YC, Dufouil C, Tzourio C. Inflammatory proteins and the severity of dilated Virchow-Robin Spaces in the elderly. J Alzheimers Dis. 2013;33:323–328. [CrossRef]
- Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: a review. J Cereb Blood Flow Metab. 2016;36:72–94. [CrossRef]
- Horng S, Therattil A, Moyon S, Gordon A, Kim K, Arqaw AT. Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J Clin Invest. 2017;127(8):3136–3151. [CrossRef]
- Seshadri S, Wolf P.A. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6:1106-1114. [CrossRef]
- Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: three-year follow-up of the Austrian Stroke Prevention Study. Neurology. 1999;53(1):132-139. [CrossRef]
- Boutinaud P, Tsuchida A, Laurent A, Adonias F, Hanifehlou Z, Nozais V. 3D Segmentation of Perivascular Spaces on T1-Weighted 3 Tesla MR Images With a Convolutional Autoencoder and a U-Shaped Neural Network. Front Neuroinform. 2021;8;15:641600. [CrossRef]
- Paradise M, Crawford JD, Lam BCP, Wen W, Kochan NA, Makkar S, Dawes L, Trollor J, Draper B, Brodaty H, Sachdev PS. Association of Dilated Perivascular Spaces With Cognitive Decline and Incident Dementia. Neurology. 2021;96(11):e1501-e1511. [CrossRef]
- Huentelman MJ, Talboom JS, Lewis CR, Chen Z, Barnes CA. Reinventing Neuroaging Research in the Digital Age. Trends Neurosci. 2020;43(1):17-23. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).