Submitted:
21 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study sample
2.2. Data Collection
2.3. Statistical tests
2.3.1. Descriptive data
2.3.2. Regression and Tree Analysis
3. Results
3.1. Sample descriptives
3.2. Associations: Generalised Linear Models
3.2.1. Univariate analysis
3.2.2. Multivariate analysis
3.3. Relationships between variables: Classification and Regression Tree (CART) analysis
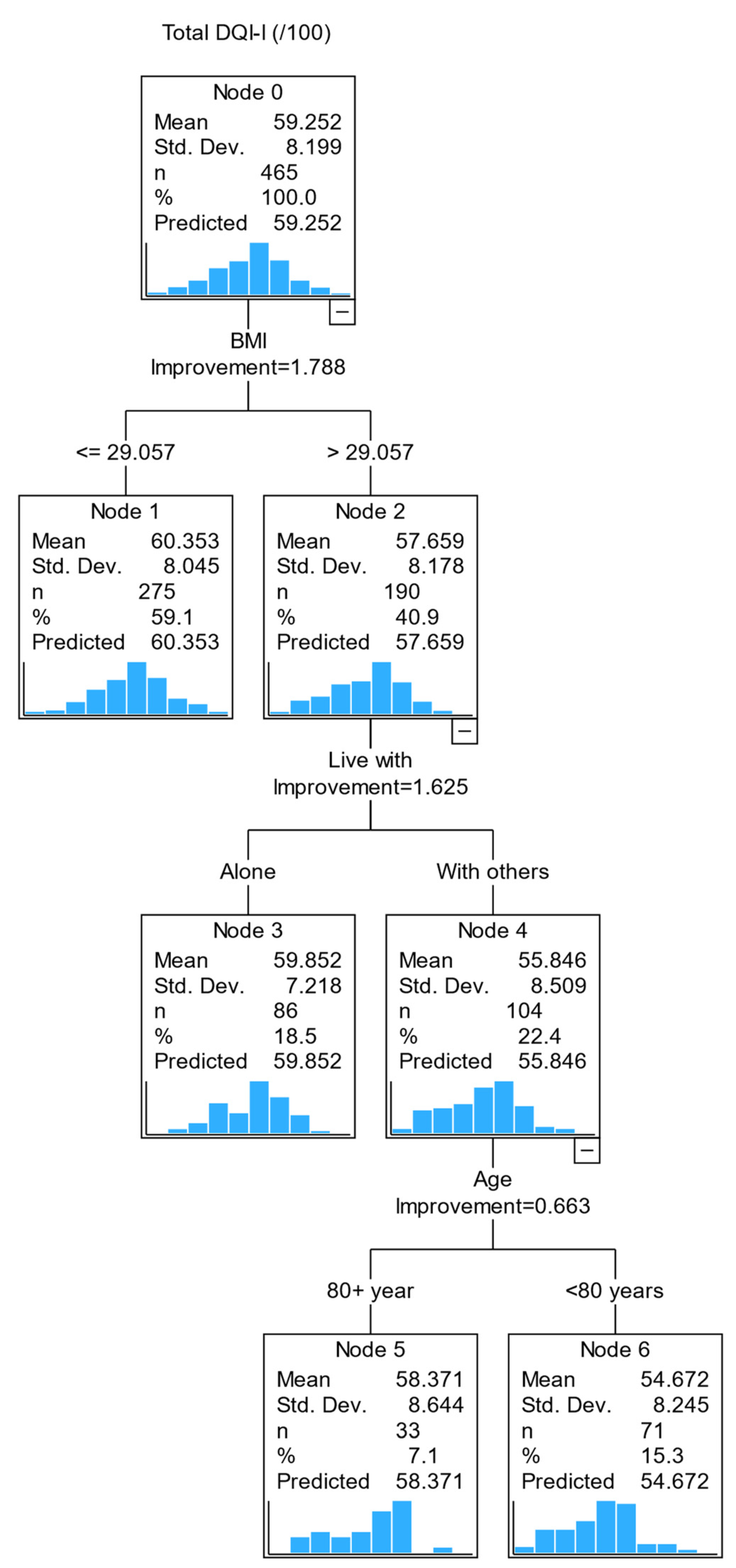
4. Discussion
Strengths and limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Demographic and health variables | DQI-I | Variety | Adequacy | Moderation | Balance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Std. error | p-value | B | Std. error | p-value | B | Std. error | p-value | B | Std. error | p-value | B | Std. error | p-value | |
| Age, (<80 years; reference: 80+ years) | -1.474 | 0.7572 | 0.052 | -0.247 | 0.3085 | 0.423 | -0.426 | 0.4101 | 0.299 | -0.622 | 0.3931 | 0.114 | -0.178 | 0.1395 | 0.202 |
| Sex (male; reference: female) | -0.776 | 0.7718 | 0.314 | 0.375 | 0.3132 | 0.231 | 0.083 | 0.4173 | 0.843 | -1.394 | 0.3953 | <0.001 | 0.159 | 0.1418 | 0.261 |
| Ethnic group (European; reference: non-European) | 2.260 | 1.2934 | 0.081 | 0.458 | 0.5264 | 0.384 | 1.381 | 0.6979 | 0.048 | 0.117 | 0.6727 | 0.862 | 0.305 | 0.2381 | 0.200 |
| Education level (primary; reference: tertiary) | 0.041 | 2.2660 | 0.985 | -0.108 | 0.9196 | 0.907 | -1.280 | 1.2224 | 0.295 | 1.201 | 1.1728 | 0.306 | 0.227 | 0.4164 | 0.585 |
| (Secondary; reference: tertiary) | -0.079 | 0.7784 | 0.919 | -0.225 | 0.3159 | 0.477 | -0.205 | 0.4199 | 0.625 | 0.365 | 0.4029 | 0.365 | -0.014 | 0.1430 | 0.924 |
| Live with (alone; reference: others) | 1.578 | 0.7619 | 0.038 | 0.356 | 0.3103 | 0.251 | 0.341 | 0.4131 | 0.409 | 0.999 | 0.3941 | 0.011 | -0.118 | 0.1406 | 0.399 |
| Deprivation (reference: high) Low | 1.959 | 0.9405 | 0.037 | 0.604 | 0.3827 | 0.114 | 1.137 | 0.5072 | 0.025 | -0.082 | 0.4900 | 0.867 | 0.300 | 0.1731 | 0.083 |
| Medium | 1.621 | 0.9595 | 0.091 | 0.474 | 0.3904 | 0.225 | 1.112 | 0.5174 | 0.032 | -0.244 | 0.4998 | 0.626 | 0.279 | 0.1766 | 0.115 |
| Medical conditions (1; reference: 2+) | 0.120 | 0.8882 | 0.892 | -0.004 | 0.3606 | 0.992 | -0.244 | 0.4796 | 0.611 | 0.129 | 0.4604 | 0.779 | 0.238 | 0.1629 | 0.143 |
| Vision (impaired; reference: non impaired) | -0.569 | 1.5001 | 0.705 | -0.546 | 0.6086 | 0.370 | 0.513 | 0.8100 | 0.527 | -0.108 | 0.7778 | 0.890 | -0.428 | 0.2751 | 0.120 |
| Hearing (impaired; reference: non impaired) | 1.966 | 1.4367 | 0.171 | -0.008 | 0.5845 | 0.989 | 1.046 | 0.7760 | 0.178 | 0.950 | 0.7450 | 0.202 | -0.022 | 0.2646 | 0.934 |
| BMI, kg/m2 | -0.194 | 0.0769 | 0.012 | -0.062 | 0.0312 | 0.047 | -0.093 | 0.0416 | 0.025 | -0.023 | 0.0401 | 0.574 | -0.016 | 0.0141 | 0.258 |
| Waist circumference, cm | -0.055 | 0.0298 | 0.064 | -0.009 | 0.0121 | 0.461 | -0.022 | 0.0160 | 0.165 | -0.022 | 0.0155 | 0.150 | -0.002 | 0.0055 | 0.753 |
| Medications (1; reference: 2+) | -1.160 | 1.2710 | 0.361 | 0.403 | 0.5162 | 0.435 | -1.236 | 0.6847 | 0.071 | -0.248 | 0.6594 | 0.707 | -0.079 | 0.2338 | 0.735 |
| Supplements (0; reference: 1+) | -0.133 | 0.7653 | 0.862 | -0.274 | 0.3105 | 0.377 | -0.278 | 0.4132 | 0.501 | 0.106 | 0.3968 | 0.790 | 0.314 | 0.1399 | 0.025 |
| Alcohol consumption (never; reference: regular) | -0.369 | 1.0012 | 0.713 | -0.806 | 0.4055 | 0.047 | -0.311 | 0.5409 | 0.565 | 0.428 | 0.5192 | 0.410 | 0.321 | 0.1835 | 0.081 |
| (Occasional; reference: regular) | 0.806 | 0.8704 | 0.355 | -0.289 | 0.3525 | 0.412 | 0.316 | 0.4702 | 0.501 | 0.501 | 0.4514 | 0.267 | 0.278 | 0.1596 | 0.082 |
| Smoking (non-smoker; reference: smoker) | 4.936 | 3.6754 | 0.179 | 2.202 | 1.4917 | 0.140 | 3.936 | 1.9806 | 0.047 | -2.404 | 1.9059 | 0.207 | 1.202 | 0.6746 | 0.075 |
| NEADL score | 0.219 | 0.1662 | 0.188 | 0.052 | 0.0676 | 0.444 | 0.143 | 0.0897 | 0.112 | 0.042 | 0.0863 | 0.623 | -0.018 | 0.0306 | 0.562 |
| SPPB score | 0.187 | 0.1785 | 0.295 | 0.172 | 0.0721 | 0.017 | 0.123 | 0.0963 | 0.202 | -0.099 | 0.0925 | 0.286 | -0.009 | 0.0328 | 0.781 |
| MoCA score (not impaired; reference: impaired) | -0.507 | 0.9252 | 0.583 | -0.307 | 0.3752 | 0.414 | -0.413 | 0.4986 | 0.408 | 0.700 | 0.4779 | 0.143 | -0.488 | 0.1686 | 0.004 |
| GDS score (no depression; reference: depression) | -0.228 | 1.0013 | 0.820 | -0.124 | 0.4065 | 0.760 | 0.028 | 0.5409 | 0.959 | -0.313 | 0.5190 | 0.546 | 0.181 | 0.1839 | 0.324 |
| Demographic and health variables | DQI-I | Variety | Adequacy | Moderation | Balance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Std. error | p-value | B | Std. error | p-value | B | Std. error | p-value | B | Std. error | p-value | B | Std. error | p-value | |
| Age, (<80 years; reference: 80+ years) | -1.838 | 1.3889 | 0.186 | -0.344 | 0.5442 | 0.527 | -0.754 | 0.7696 | 0.327 | -0.600 | 0.6749 | 0.374 | -1.404 | 2.4930 | 0.573 |
| Sex (male; reference: female) | -0.732 | 1.4186 | 0.606 | -0.112 | 0.5537 | 0.840 | 0.697 | 0.7826 | 0.373 | -1.763 | 0.6726 | 0.009 | 4.461 | 2.5103 | 0.076 |
| Ethnic group (European; reference: non-European) | -0.733 | 2.4075 | 0.761 | 0.019 | 0.9393 | 0.983 | 0.098 | 1.3309 | 0.941 | -1.502 | 1.1601 | 0.195 | 6.520 | 4.2694 | 0.127 |
| Education level (primary; reference: tertiary) | 0.734 | 4.4166 | 0.868 | 1.427 | 1.7175 | 0.406 | 0.094 | 2.4257 | 0.969 | 0.542 | 2.1298 | 0.799 | -13.295 | 7.8215 | 0.089 |
| (Secondary; reference: tertiary) | -0.736 | 1.4238 | 0.605 | -0.325 | 0.5537 | 0.557 | -1.146 | 0.7820 | 0.143 | 0.896 | 0.6866 | 0.192 | -1.615 | 2.5214 | 0.522 |
| Live with (alone; reference: others) | 2.731 | 1.4060 | 0.052 | 1.038 | 0.5488 | 0.058 | 1.034 | 0.7822 | 0.186 | 0.956 | 0.6851 | 0.163 | -2.985 | 2.5313 | 0.238 |
| Deprivation (low; reference: high) | 2.422 | 1.7735 | 0.172 | 0.049 | 0.6975 | 0.943 | 1.910 | 0.9638 | 0.047 | 0.030 | 0.8638 | 0.972 | 4.318 | 3.1674 | 0.173 |
| (Medium; reference: high) | 2.507 | 1.7245 | 0.146 | 0.106 | 0.6782 | 0.876 | 2.570 | 0.9372 | 0.006 | -0.630 | 0.8400 | 0.453 | 4.612 | 3.0799 | 0.134 |
| Medical conditions (1; reference: 2+) | -1.263 | 1.5795 | 0.424 | -0.183 | 0.6172 | 0.767 | -0.657 | 0.8731 | 0.452 | -0.709 | 0.7646 | 0.354 | 2.858 | 2.8181 | 0.311 |
| Vision (impaired; reference: non impaired) | -4.390 | 2.7860 | 0.115 | -1.730 | 1.0865 | 0.111 | -0.623 | 1.5515 | 0.688 | -1.651 | 1.3539 | 0.223 | -3.856 | 5.0079 | 0.441 |
| Hearing (impaired; reference: non impaired) | 1.092 | 2.4885 | 0.661 | -0.430 | 0.9706 | 0.658 | 0.514 | 1.3755 | 0.709 | 1.280 | 1.2017 | 0.287 | -2.720 | 4.4429 | 0.540 |
| BMI, kg/m2 | -0.001 | 0.1381 | 0.994 | 0.066 | 0.0535 | 0.220 | 0.125 | 0.0757 | 0.098 | -0.153 | 0.0657 | 0.020 | -0.388 | 0.2447 | 0.113 |
| Waist circumference, cm | 0.046 | 0.0558 | 0.406 | 0.028 | 0.0217 | 0.199 | 0.082 | 0.0298 | 0.006 | -0.059 | 0.0268 | 0.029 | -0.050 | 0.1007 | 0.618 |
| Medications (1; reference: 2+) | -2.636 | 2.0071 | 0.189 | 0.090 | 0.7873 | 0.909 | -1.769 | 1.1062 | 0.110 | -0.823 | 0.9755 | 0.399 | -1.339 | 3.6044 | 0.710 |
| Supplements (0; reference: 1+) | -0.095 | 1.4198 | 0.947 | -0.367 | 0.5530 | 0.507 | -0.393 | 0.7840 | 0.616 | 0.121 | 0.6877 | 0.860 | 5.436 | 2.4976 | 0.030 |
| Alcohol consumption (never; reference: regular) | -2.726 | 1.8244 | 0.135 | -0.643 | 0.7194 | 0.371 | -1.759 | 1.0080 | 0.081 | 0.045 | 0.8956 | 0.960 | -3.688 | 3.2937 | 0.263 |
| (Occasional; reference: regular) | 1.112 | 1.5913 | 0.485 | 0.185 | 0.6275 | 0.768 | 0.318 | 0.8793 | 0.718 | 0.614 | 0.7812 | 0.432 | -0.049 | 2.8729 | 0.986 |
| Smoking (non-smoker; reference: smoker) | 5.788 | 8.5987 | 0.501 | 1.192 | 3.3576 | 0.723 | 5.772 | 4.7360 | 0.223 | -0.797 | 4.1710 | 0.849 | -3.793 | 15.3810 | 0.805 |
| NEADL score | 0.057 | 0.2766 | 0.836 | 0.009 | 0.1079 | 0.935 | 0.006 | 0.1529 | 0.969 | 0.063 | 0.1339 | 0.638 | -0.207 | 0.4940 | 0.676 |
| SPPB score | -0.080 | 0.3031 | 0.791 | 0.049 | 0.1182 | 0.681 | -0.035 | 0.1675 | 0.836 | -0.112 | 0.1466 | 0.444 | 0.179 | 0.5414 | 0.740 |
| MoCA score (not impaired; reference: impaired) | 1.281 | 1.7996 | 0.477 | 0.604 | 0.6973 | 0.386 | 0.868 | 0.9930 | 0.382 | 0.050 | 0.8717 | 0.954 | -2.413 | 3.2173 | 0.453 |
| GDS score (no depression; reference: depression) | 0.079 | 1.8228 | 0.965 | 0.909 | 0.7071 | 0.199 | -0.094 | 1.0073 | 0.926 | -0.783 | 0.8807 | 0.374 | 0.474 | 3.2561 | 0.884 |
| Demographic and health variables | DQI-I | Variety | Adequacy | Moderation | Balance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Std. error | p-value | B | Std. error | p-value | B | Std. error | p-value | B | Std. error | p-value | B | Std. error | p-value | |
| Age, (<80 years; reference: 80+ years) | -1.252 | 0.8823 | 0.156 | -0.108 | 0.3431 | 0.753 | -0.157 | 0.4212 | 0.709 | -0.761 | 0.4480 | 0.090 | -2.258 | 1.6636 | 0.175 |
| Sex (male; reference: female) | -0.730 | 0.8991 | 0.417 | 0.603 | 0.3472 | 0.083 | -0.188 | 0.4282 | 0.661 | -1.190 | 0.4527 | 0.009 | 0.450 | 1.6962 | 0.791 |
| Ethnic group (European; reference: non-European) | 3.720 | 1.4864 | 0.012 | 0.680 | 0.5807 | 0.242 | 2.026 | 0.7053 | 0.004 | 0.864 | 0.7618 | 0.257 | 1.508 | 2.8284 | 0.594 |
| Education level (primary; reference: tertiary) | -0.399 | 2.5654 | 0.876 | -0.879 | 0.9930 | 0.376 | -2.029 | 1.2146 | 0.095 | 1.627 | 1.3014 | 0.211 | 8.825 | 4.8104 | 0.067 |
| (Secondary; reference: tertiary) | 0.228 | 0.9074 | 0.802 | -0.238 | 0.3512 | 0.499 | 0.190 | 0.4296 | 0.659 | 0.192 | 0.4603 | 0.676 | 0.836 | 1.7014 | 0.623 |
| Live with (alone; reference: others) | 0.805 | 0.8860 | 0.363 | -0.106 | 0.3439 | 0.757 | -0.237 | 0.4221 | 0.574 | 1.182 | 0.4461 | 0.008 | -0.335 | 1.6721 | 0.841 |
| Deprivation (low; reference: high) | 1.705 | 1.0818 | 0.115 | 0.750 | 0.4183 | 0.073 | 0.658 | 0.5155 | 0.202 | 0.020 | 0.5522 | 0.970 | 2.769 | 2.0418 | 0.175 |
| (Medium; reference: high) | 1.378 | 1.1286 | 0.222 | 0.821 | 0.4363 | 0.060 | 0.630 | 0.5378 | 0.241 | -0.238 | 0.5760 | 0.680 | 1.646 | 2.1300 | 0.440 |
| Medical conditions (1; reference: 2+) | 1.014 | 1.0485 | 0.333 | 0.256 | 0.4068 | 0.529 | 0.208 | 0.4997 | 0.677 | 0.369 | 0.5336 | 0.489 | 1.815 | 1.9767 | 0.359 |
| Vision (impaired; reference: non impaired) | 1.257 | 1.7273 | 0.467 | 0.031 | 0.6702 | 0.963 | 1.107 | 0.8205 | 0.177 | 0.562 | 0.8785 | 0.523 | -4.422 | 3.2490 | 0.173 |
| Hearing (impaired; reference: non impaired) | 2.267 | 1.7240 | 0.188 | 0.040 | 0.6702 | 0.953 | 1.069 | 0.8206 | 0.193 | 1.002 | 0.8772 | 0.254 | 1.581 | 3.2574 | 0.627 |
| BMI, kg/m2 | -0.171 | 0.1028 | 0.096 | 0.000 | 0.0399 | 0.991 | -0.011 | 0.0491 | 0.817 | -0.134 | 0.0520 | 0.010 | -0.249 | 0.1936 | 0.198 |
| Waist circumference, cm | -0.064 | 0.0367 | 0.083 | 0.013 | 0.0143 | 0.345 | -0.015 | 0.0175 | 0.383 | -0.056 | 0.0185 | 0.002 | -0.055 | 0.0695 | 0.428 |
| Medications (1; reference: 2+) | 0.726 | 1.6297 | 0.656 | 1.286 | 0.6278 | 0.041 | 0.122 | 0.7759 | 0.875 | -0.556 | 0.8283 | 0.502 | -1.265 | 3.0719 | 0.681 |
| Supplements (0; reference: 1+) | 0.053 | 0.8877 | 0.953 | -0.118 | 0.3441 | 0.733 | -0.017 | 0.4225 | 0.969 | -0.023 | 0.4514 | 0.960 | 2.096 | 1.6690 | 0.209 |
| Alcohol consumption (never; reference: regular) | 1.012 | 1.1664 | 0.386 | -0.817 | 0.4504 | 0.070 | 0.564 | 0.5548 | 0.309 | 0.581 | 0.5930 | 0.327 | 6.840 | 2.1656 | 0.002 |
| (Occasional; reference: regular) | 0.755 | 1.0085 | 0.454 | -0.405 | 0.3894 | 0.298 | 0.461 | 0.4797 | 0.337 | 0.311 | 0.5127 | 0.544 | 3.883 | 1.8724 | 0.038 |
| Smoking (non-smoker; reference: smoker) | 5.214 | 3.9235 | 0.184 | 2.823 | 1.5169 | 0.063 | 4.061 | 1.8586 | 0.029 | -3.235 | 1.9923 | 0.104 | 15.647 | 7.3633 | 0.034 |
| NEADL score | 0.266 | 0.2050 | 0.195 | 0.038 | 0.0796 | 0.632 | 0.164 | 0.0974 | 0.092 | 0.077 | 0.1044 | 0.462 | -0.132 | 0.3873 | 0.733 |
| SPPB score | 0.195 | 0.2209 | 0.377 | 0.135 | 0.0854 | 0.114 | 0.029 | 0.1052 | 0.784 | 0.047 | 0.1124 | 0.674 | -0.162 | 0.4167 | 0.697 |
| MoCA score (not impaired; reference: impaired) | -0.982 | 1.0486 | 0.349 | -0.505 | 0.4061 | 0.213 | -0.650 | 0.4985 | 0.192 | 0.777 | 0.5322 | 0.144 | -6.024 | 1.9498 | 0.002 |
| GDS score (no depression; reference: depression) | -0.397 | 1.1694 | 0.734 | -0.683 | 0.4518 | 0.131 | 0.037 | 0.5567 | 0.947 | -0.016 | 0.5948 | 0.979 | 2.647 | 2.1996 | 0.229 |
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495, . [CrossRef]
- O’caoimh, R.; Sezgin, D.; O’donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing 2020, 50, 96–104, . [CrossRef]
- Barrett P, Twitchin S, Kletchko S, Ryan F. The living environments of community-dwelling older people who become frail: another look at the living standards of older New Zealanders survey. Social Policy Journal of New Zealand. 2006;28:133-57.
- Verlaan, S.; Ligthart-Melis, G.C.; Wijers, S.L.; Cederholm, T.; Maier, A.B.; de van der Schueren, M.A.E. High Prevalence of Physical Frailty Among Community-Dwelling Malnourished Older Adults–A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2017, 18, 374–382. [CrossRef]
- Hirani, V.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Seibel, M.J.; Waite, L.M.; Handelsman, D.J.; Cumming, R.G. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. Age Ageing 2016, 46, 413–420, . [CrossRef]
- Crow, R.S.; Lohman, M.C.; Titus, A.J.; Cook, S.B.; Bruce, M.L.; Mackenzie, T.A.; Bartels, S.J.; Batsis, J.A. Association of Obesity and Frailty in Older Adults: NHANES 1999–2004. J. Nutr. Heal. Aging 2018, 23, 138–144, . [CrossRef]
- Villareal, D.T.; Banks, M.; Siener, C.; Sinacore, D.R.; Klein, S. Physical Frailty and Body Composition in Obese Elderly Men and Women. Obes. Res. 2004, 12, 913–920, . [CrossRef]
- Woo J, Leung J, Kwok T. BMI, Body Composition, and Physical Functioning in Older Adults. 2007;15(7):1886-94.
- Lang, P.-O.; Michel, J.-P.; Zekry, D. Frailty Syndrome: A Transitional State in a Dynamic Process. Gerontology 2009, 55, 539–549, . [CrossRef]
- Wirt A, Collins CE. Diet quality - what is it and does it matter? Public Health Nutrition. 2009;12(12):2473-92.
- Payette H, Shatenstein B. Determinants of Healthy Eating in Community-dwelling Elderly People. Canadian Journal of Public Health. 2005;96(3):S30-S5.
- Thiele, S.; Mensink, G.B.; Beitz, R. Determinants of diet quality. Public Heal. Nutr. 2004, 7, 29–37, . [CrossRef]
- Thorpe, M.G.; Milte, C.M.; Crawford, D.; McNaughton, S.A. A Revised Australian Dietary Guideline Index and Its Association with Key Sociodemographic Factors, Health Behaviors and Body Mass Index in Peri-Retirement Aged Adults. Nutrients 2016, 8, 160, . [CrossRef]
- Huang, C.H.; Okada, K.; Matsushita, E.; Uno, C.; Satake, S.; Martins, B.A.; Kuzuya, M. Sex-Specific Association between Social Frailty and Diet Quality, Diet Quantity, and Nutrition in Community-Dwelling Elderly. Nutrients 2020, 12, 2845, . [CrossRef]
- Host, A.; McMahon, A.-T.; Walton, K.; Charlton, K. Factors Influencing Food Choice for Independently Living Older People—A Systematic Literature Review. J. Nutr. Gerontol. Geriatr. 2016, 35, 67–94, . [CrossRef]
- Bloom, I.; Edwards, M.; Jameson, K.A.; Syddall, H.E.; Dennison, E.; Gale, C.R.; Baird, J.; Cooper, C.; Sayer, A.A.; Robinson, S. Influences on diet quality in older age: the importance of social factors. Age Ageing 2016, 46, 277–283, . [CrossRef]
- Atkins, J.L.; Ramsay, S.E.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Wannamethee, S.G. Diet quality in older age: the influence of childhood and adult socio-economic circumstances. Br. J. Nutr. 2015, 113, 1441–1452, . [CrossRef]
- Bailey, R.L.; Ledikwe, J.H.; Smiciklas-Wright, H.; Mitchell, D.C.; Jensen, G.L. Persistent oral health problems associated with comorbidity and impaired diet quality in older adults. J. Am. Diet. Assoc. 2004, 104, 1273–1276, . [CrossRef]
- Deierlein, A.L.; Morland, K.B.; Scanlin, K.; Wong, S.; Spark, A. Diet Quality of Urban Older Adults Age 60 to 99 Years: The Cardiovascular Health of Seniors and Built Environment Study. J. Acad. Nutr. Diet. 2013, 114, 279–287, . [CrossRef]
- Schoufour, J.D.; de Jonge, E.A.; Jong, J.C.K.-D.; van Lenthe, F.J.; Hofman, A.; Nunn, S.P.; Franco, O.H. Socio-economic indicators and diet quality in an older population. Maturitas 2018, 107, 71–77, . [CrossRef]
- Pinto de Souza Fernandes D, Duarte MSL, Pessoa MC, Franceschini SdCC, Ribeiro AQ. Evaluation of diet quality of the elderly and associated factors. Archives of Gerontology and Geriatrics. 2017;72:174-80.
- de Freitas, T.I.; Previdelli, .N.; Ferreira, M.P.D.N.; Marques, K.M.; Goulart, R.M.M.; Aquino, R.d.C.d. Factors associated with diet quality of older adults. 2017, 30, 297–306, . [CrossRef]
- Park, S.; Kim, H.J.; Kim, K. Do Where The Elderly Live Matter? Factors Associated with Diet Quality among Korean Elderly Population Living in Urban Versus Rural Areas. Nutrients 2020, 12, 1314, . [CrossRef]
- Bloom, I.; Lawrence, W.; Barker, M.; Baird, J.; Dennison, E.; Sayer, A.A.; Cooper, C.; Robinson, S. What influences diet quality in older people? A qualitative study among community-dwelling older adults from the Hertfordshire Cohort Study, UK. Public Health Nutr. 2017, 20, 2685–2693, . [CrossRef]
- Nohan AF, Adznam SNA, Jamaluddin R, Norazman CW. Diet quality and its associated factors among community dwelling older adults in urban district in Kuala Lumpur, Malaysia. Malays J Med Health Sci. 2020;16:153-62.
- Wong, J.E.; Haszard, J.J.; Howe, A.S.; Parnell, W.R.; Skidmore, P.M.L. Development of a Healthy Dietary Habits Index for New Zealand Adults. Nutrients 2017, 9, 454, . [CrossRef]
- Kim, C.-O. Food choice patterns among frail older adults: The associations between social network, food choice values, and diet quality. Appetite 2016, 96, 116–121, . [CrossRef]
- Tay, E.; Barnett, D.; Leilua, E.; Kerse, N.; Rowland, M.; Rolleston, A.; Waters, D.L.; Edlin, R.; Connolly, M.; Hale, L.; et al. The Diet Quality and Nutrition Inadequacy of Pre-Frail Older Adults in New Zealand. Nutrients 2021, 13, 2384, . [CrossRef]
- Teh, R.; Kerse, N.; Waters, D.L.; Hale, L.; Pillai, A.; Leilua, E.; Tay, E.; Rolleston, A.; Edlin, R.; Maxted, E.; et al. Study protocol of a randomised controlled trial to examine the impact of a complex intervention in pre-frail older adults. Aging Clin. Exp. Res. 2019, 31, 1407–1417, . [CrossRef]
- Statistics New Zealand. National and subnational period life tables: 2017–2019. Wellington, New Zealand: New Zealand Government; 2021.
- Simpson, E.; Bradley, J.; Poliakov, I.; Jackson, D.; Olivier, P.; Adamson, A.J.; Foster, E. Iterative Development of an Online Dietary Recall Tool: INTAKE24. Nutrients 2017, 9, 118, . [CrossRef]
- Dietary intake data were collected using Intake24.org (NZ 2018): an open source dietary assessment research tool, freely available to researchers, maintained and developed by the Nutrition Measurement Platform, MRC Epidemiology Unit, University of Cambridge, in collaboration with Open Lab, Newcastle University.
- Adamson, A.; Davies, K.; Wham, C.; Kepa, M.; Foster, E.; Jones, A.; Mathers, J.; Granic, A.; Teh, R.; Moyes, S.; et al. Assessment of Dietary Intake in Three Cohorts of Advanced Age in Two Countries: Methodology Challenges. J. Nutr. Heal. Aging 2023, 27, 59–66, . [CrossRef]
- Sivakumaran S, Huffman L, Gilmore Z, Sivakumaran S. New Zealand FOODfiles 2016 manual. The New Zealand Institute for Plant & Food Research Limited and Ministry of Health. 2017.
- Kim, S.; Haines, P.S.; Siega-Riz, A.M.; Popkin, B.M. The Diet Quality Index-International (DQI-I) Provides an Effective Tool for Cross-National Comparison of Diet Quality as Illustrated by China and the United States. J. Nutr. 2003, 133, 3476–3484, . [CrossRef]
- Chan, R.; Leung, J.; Woo, J. Dietary Patterns and Risk of Frailty in Chinese Community-Dwelling Older People in Hong Kong: A Prospective Cohort Study. Nutrients 2015, 7, 7070–7084, . [CrossRef]
- Lemon, S.C.; Roy, J.; Clark, M.A.; Friedmann, P.D.; Rakowski, W. Classification and regression tree analysis in public health: Methodological review and comparison with logistic regression. Ann. Behav. Med. 2003, 26, 172–181, . [CrossRef]
- Wham, C.A.; Teh, R.; Moyes, S.; Dyall, L.; Kepa, M.; Hayman, K.; Kerse, N. Health and social factors associated with nutrition risk: Results from life and living in advanced age: A cohort study in New Zealand (LILACS NZ). J. Nutr. Health Aging 2015, 19, 637–645, . [CrossRef]
- O'Keeffe M, Kelly M, O'Herlihy E, O'Toole PW, Kearney PM, Timmons S, et al. Potentially modifiable determinants of malnutrition in older adults: A systematic review. Clinical nutrition: official journal of the European Society of Parenteral and Enteral Nutrition. 2019;38(6):2477-98.
- Choi, Y.J.; A Ailshire, J.; Crimmins, E.M. Living alone, social networks in neighbourhoods, and daily fruit and vegetable consumption among middle-aged and older adults in the USA. Public Heal. Nutr. 2020, 23, 3315–3323, . [CrossRef]
- Herman, C.P. The social facilitation of eating. A review. Appetite 2015, 86, 61–73, . [CrossRef]
- Lane K, Poland F, Fleming S, Lambert N, Macdonald H, Potter J, et al. Older women's reduced contact with food in the Changes Around Food Experience (CAFE) study: choices, adaptations and dynamism. Ageing & Society. 2014;34(4):645-69.
- Zhao, H.; Andreyeva, T. Diet Quality and Health in Older Americans. Nutrients 2022, 14, 1198, . [CrossRef]
- Ward, R.E.; Orkaby, A.R.; Chen, J.; Hshieh, T.T.; Driver, J.A.; Gaziano, J.M.; Djousse, L. Association between Diet Quality and Frailty Prevalence in the Physicians’ Health Study. J. Am. Geriatr. Soc. 2019, 68, 770–776, . [CrossRef]
- Feart C. Nutrition and frailty: Current knowledge. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2019;95:109703.
- Wray, C.M.; Byers, A.L. Methodological Progress Note: Classification and Regression Tree Analysis.. J. Hosp. Med. 2020, 15, 549–551.
- Aguayo, G.A.; Donneau, A.-F.; Vaillant, M.T.; Schritz, A.; Franco, O.H.; Stranges, S.; Malisoux, L.; Guillaume, M.; Witte, D.R. Agreement Between 35 Published Frailty Scores in the General Population. Am. J. Epidemiology 2017, 186, 420–434, . [CrossRef]
- University of Otago and Ministry of Health. A Focus on Nutrition: Key findings of the 2008/09 New Zealand Adult Nutrition Survey. Wellington: Ministry of Health; 2011.
- Adamson AJ, Collerton J, Davies K, Foster E, Jagger C, Stamp E, et al. Nutrition in advanced age: dietary assessment in the Newcastle 85+ study. Eur J Clin Nutr. 2009;63(S1):S6-S18.
Adamson, A.J.; Collerton, J.; Davies, K.; Foster, E.; Jagger, C.; Stamp, E.; Mathers, J.C.; Kirkwood, T.; The Newcastle 85+ Study Core Team Nutrition in advanced age: dietary assessment in the Newcastle 85+ study. Eur. J. Clin. Nutr. 2009, 63, S6–S18, https://doi.org/10.1038/ejcn.2008.60.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
| Characteristics | All (n = 465) |
|---|---|
| Age, n (% ≥ 80 years) | 242 (52) |
| Sex, n (% female) | 275 (59.1) |
| Ethnic group a, n (% European/ Pākehā) | 421 (90.5) |
| Education level, n (%) | |
| Primary | 14 (3.0) |
| Secondary | 256 (55.1) |
| Tertiary | 195 (41.9) |
| Living arrangement, n (%) | |
| Alone | 204 (43.9) |
| With others | 261 (56.1) |
| NZ Dep Index 2018, n (%) | |
| Low, 1 – 4 | 174 (37.4) |
| Medium, 5 – 7 | 159 (34.2) |
| High, 8 – 10 | 132 (28.4) |
| Medical conditions, n (%) | |
| 0-1 | 112 (24.1) |
| ≥ 2 | 353 (75.9) |
| Vision, n (% impaired) | 32 (6.9) |
| Hearing, n (% impaired) | 35 (7.5) |
| BMI (kg/m2) median (IQR) | 28.3 (25.3 – 31.4) |
| Waist circumference (cm) median (IQR) Male Female |
102.5 (95.8 – 109.5) 93.8 (85.4 – 101.9) |
| Medications, n (%) | |
| 1 | 46 (9.9) |
| ≥ 2 | 419 (90.1) |
| Supplements, n (%) | |
| 0 | 261 (56.1) |
| ≥ 1 | 204 (43.9) |
| Alcohol consumption, n (%) | |
| Never | 111 (23.9) |
| Occasional | 187 (40.2) |
| Regular | 167 (35.9) |
| Smoking, n (% smoker) | 5 (1.1) |
| NEADL score, median (IQR) | 20 (18 – 21) |
| SPPB score, median (IQR) | 9 (8 – 10) |
| MoCA, median (IQR) Cognitive impairment b, n (%) |
25 (23 - 27) 100 (21.5) |
| Total DQI-I scores, median (IQR) Variety, median (IQR) Adequacy, median (IQR) Moderation, median (IQR) Balance, median (IQR) |
60 (11) 14 (5) 30 (7) 12 (6) 7 (2) |
| Demographic and health variables | B | 95% CI | p-value |
|---|---|---|---|
| DQI-I total score | |||
| (Intercept) | 52.36 | 42.28, 62.43 | <0.001 |
| Age (<80 year; reference: 80+ years) | -0.99 | -2.55, 0.57 | 0.213 |
| NZ Dep Index (low; reference: high) | 2.14 | 0.29, 3.99 | 0.024 |
| (Medium; reference: high) | 1.49 | -0.39, 3.37 | 0.121 |
| Live alone (reference: with others) | 1.60 | 0.06, 3.15 | 0.042 |
| BMI, kg/m2 | -0.17 | -0.33, -0.02 | 0.026 |
| Smoking (reference: smoker) | 5.91 | -1.22, 13.03 | 0.104 |
| NEADL | 64.32 | 56.53, 73.20 | 0.164 |
| Variety score | |||
| (Intercept) | 11.174 | 7.52, 14.82 | <0.001 |
| Deprivation (low; reference: high) | 0.38 | -0.37, 1.14 | 0.320 |
| (Medium; reference: high) | 0.28 | -0.49, 1.04 | 0.478 |
| BMI, kg/m2 | -0.06 | -0.12, 0.01 | 0.068 |
| Alcohol consumption (never; reference: regular) | -0.84 | -1.65, -0.04 | 0.040 |
| (Occasional; reference: regular) | -0.23 | -0.93, 0.47 | 0.526 |
| Smoking (reference: smoker) | 2.71 | -0.20, 5.63 | 0.068 |
| SPPB score | 0.13 | -0.02, 0.27 | 0.086 |
| Adequacy score | |||
| (Intercept) | 26.378 | 21.657, 31.099 | <0.001 |
| Deprivation (low; reference: high) | 1.08 | 0.09, 2.07 | 0.033 |
| (Medium; reference: high) | 0.97 | -0.04, 1.97 | 0.060 |
| BMI, kg/m2 | -0.10 | -0.181, -0.018 | 0.016 |
| Smoking (non-smoker; reference: smoker) | 3.92 | 0.08, 7.75 | 0.045 |
| Medications (1; reference: 2+) | -1.54 | -2.87, -0.20 | 0.025 |
| NEADL | 0.15 | -0.03, 0.32 | 0.093 |
| Moderation score | |||
| (Intercept) | 12.882 | 12.047, 13.716 | <0.001 |
| Age, (<80 years; reference: 80+ years) | -0.65 | -1.41, 0.11 | 0.095 |
| Sex (male; reference: female) | -1.41 | -2.18, -0.63 | <0.001 |
| Balance score | |||
| (Intercept) | 4.39 | 3.96, 4.82 | <0.001 |
| Deprivation (low; reference: high) | 0.28 | -0.06, 0.62 | 0.104 |
| (Medium; reference: high) | 0.29 | -0.05, 0.64 | 0.092 |
| Supplement (no, reference: yes) | 0.31 | 0.04, 0.59 | 0.023 |
| Alcohol consumption (never; reference: regular) | 0.38 | 0.02, 0.73 | 0.039 |
| (Occasional; reference: regular) | 0.28 | -0.02, 0.59 | 0.072 |
| MoCA score (not impaired; reference: impaired) | -0.49 | -0.82, -0.16 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





