1. Introduction
Optimization and predictions of plant growth responses to present and future environments require an enhanced understanding of below-ground processes. Aboveground growth and development of woody perennials can be influenced by timing of root growth, root distribution and root interactions with their surrounding environment [
1,
2,
3]. For example, shallow rooting has negative consequences on fruit tree performance in dry years but a positive influence in a wet spring [
2]. In addition, the manipulation of root growth through rootstocks [
4] can reduce excessive shoot growth [
5], carbohydrate metabolism and whole plant growth [
6]. Tree performance and productivity may be related to constraints in fine root growth and root functioning. In addition, perennial fruit tree root growth may be genetically controlled by rootstocks or the interactions between rootstocks and scions [
7,
8,
9]. For example, commonly used rootstocks in grapevines are derived from diverse climatic backgrounds and have genotypic differences [
8,
10]. Generally, these variations may impact both root and shoot growth, and eventually the sustainable productivity of fruit trees [
11]. Because of their genetic diversity, rootstocks can effect scion vigor [
12], flower induction [
9], and may even alter mineral and water uptake [
13] due to their different root anatomy. Therefore, a better understanding of root dynamics within fruit crops, such as root growth dynamics and root lifespan, has implications for fertilizer and irrigation management.
Unlike the aboveground tissues, root growth can continue in mature trees all through the year [
14,
15,
16,
17]. There is little information about root growth dynamics in woody plants, including grapevines. Root growth studies are scarce as methods are complex, time consuming and tedious either through non-destructive direct monitoring or by destructively observation. Long-term studies on larger woody plants are particularly uncommon. However, it has been observed that root growth dynamics of grapevines vary between cultivars, vine age, management practices and environmental stress. Freeman and Smart [
18] found that Shiraz vines had rapid root growth 10 weeks after bud break with maximum root development when shoot growth had ceased and also after harvest. In a study with five woody cultivars, grape root activity occurred in early spring prior to bud break and during bud break, and in mid to late summer when shoot growth had ceased [
19]. More recently, Callejas et al. [
15] reported that grapevine roots displayed several growth peaks; they were most pronounced at flowering, veraison and harvest and these dynamics were different at three soil depths.
The varying root growth dynamics at different soil depths results in different root distribution dynamics. Bassoi et al. [
13] found that grapevine rootstocks had a similar rooting dynamics under micro-sprinkler irrigation conditions. Roots were present to a 100 cm depth and approximately 90 % of the roots were distributed down to a 60 cm depth, with a larger root occurrence in the first 40 cm. Some studies have reported that the distribution of roots in the soil profile can be related to genetic factors which also regulate the density of roots [
20,
21]. In addition, the distinct distribution of grapevine roots is a result of different soil environments [
22,
23], particularly soil temperature and soil moisture [
15,
16,
24,
25]. When soil temperature is not a limiting factor, soil water content may regulate root development [
26]. However, when all the factors that impact on root growth are optimal, soil temperature is the main impacting factor of root development. The ideal range of soil temperature for maximum root growth of walnut was between 21 and 24 °C [
27]. In citrus, maximum root growth was observed around 29 °C and declined below 22 °C [
28]. Woodham and Alexander [
29] and Kliewer [
30] indicated that the optimal soil temperature for grapevine root growth is close to 30 °C. The warm soils can stimulate root growth and nutrient uptake of grapevines [
31,
32].
In Australia, South Burnett, Riverina and Hunter Valley are the warmest grape growing regions with median growing season temperatures of 22.9, 21.5 and 20.7 °C, respectively [
33]. Wagga Wagga, in the Riverina region of southern NSW, is the second warmest grape growing region in Australia. However, our study would be the longest hot climate study that has been attempted since studies on root lifespan in Merlot vineyard at Oakville, California, USA [
26]. Therefore, in hot climates, it would be informative to understand seasonal root growth and distribution in hot soils [
34]. In addition, in terms of vineyard management, potential genotypic differences between rootstocks in seasonal fine root growth dynamics and root distribution are another important aspect. Therefore, we selected two of the most widely planted rootstocks (Ramsey and Schwarzmann) and another important grapevine rootstock (140 Ruggeri) as they are believed to be the most favourable rootstocks for Shiraz vineyards in warm and hot climates [
35] and relevant to the Australian industry. Finally, a two-stage seasonal root growth study was conducted in a four-year comparison of rootstocks, and temperature and soil moisture were monitored in the last three years.
2. Materials and Methods
2.1. Location and vines
The field experiment was carried out in a Shiraz rootstock field trial within a commercial vineyard located at Charles Sturt University, Wagga Wagga, NSW Australia (35º05’S 147º 35’E) over five growing seasons within a seven-year period from 2007 to 2014. The trial was established in 1998 with
Vitis vinifera (cv. Shiraz, clone PT23) and nine different rootstocks planted as own-rooted vines in a Latin square design across 10 vine rows and then field-grafted to the same Shiraz clone in the following year. The vines were planted at 2 m spacing along the row with 3 m between rows, trained to a bi-lateral permanent cordon, spur pruned and drip irrigated. There were six adjacent vines of each graft combination in each of the 10 replicate rows. For this study, we selected four replicates of the self-grafted Shiraz, and four replicates of the rootstocks 140 Ruggeri (V. berlandieri × V. rupestris), Ramsey (V. champinii), Schwarzmann (V. riparia × V. rupestris). Herbicides were used to maintain bare ground in the undervine area as part of the standard management for the vineyard, but during the root observation period any weeds that appeared between herbicide applications were removed manually. Nutrition, pest management, and other vineyard operations were consistent with common commercial vineyard practices. The major phenology stages were recorded using the modified E-L system [
36].
2.2. Installation of minirhizotron tubes
Two clear polycarbonate (minirhizotron) tubes of 100 cm length with an external diameter of 55 mm were installed in each replicate in autumn 2007, three months prior to the start of the study. The tubes were installed 20 cm from the trunk of the two centre vines in each replicate, aligned with the vine row to avoid damage from vineyard machinery, and angled under the vines at 30 degrees from vertical (
Figure 1). This resulted in 8 observation tubes per genotype and 32 tubes in total in the trial. The bottom of each tube was permanently sealed with a clear acrylic plastic end cap glued in position to prevent water ingress. The top of the minirhizotron tube that extended above the soil surface were painted black and enclosed within removable opaque covers made from heavy duty white PVC pipe with the top end sealed with a matching PVC end-cap to exclude light, water and insects. These covers were held in place with a tight rubber seal and the base of the tube cut at an angle to sit flush with the soil surface.
2.3. Soil water and temperature monitoring
Soil moisture was recorded during the 2012/2013 and 2013/2014 seasons using 16 MEA Gbugs (Measurement Engineering Australia Pty. Ltd, SA) installed in two of the four replicates of each rootstock. Each logger was connected to three gypsum blocks (measurement range of -50 to -500 kPa) at depths of 10 cm, 30 cm and 60 cm under an emitter and logged every 2 hours. In one replicate, soil temperature was recorded at the same depths along the irrigation line either directly under an emitter or in the non-wetter area between two emitters using T-type thermocouples connected to a 12-channel digital thermometer (BTM-4208SD, Lutron Instruments, Taiwan). Subsequent statistical analysis used the average temperature of the two readings at each depth.
2.4. Weather data
We used analysis of variance (ANOVA) to analyse the mean light interception in both experiments. All significance testing was performed at the 0.05 level and where a significant effect was found, the pairwise 95% least significant difference (lsd) was used to make comparisons. Linear and non-linear models were used to investigate relationships between variables measured within the light interception experiments. We used a log10 transformation to improve the assumptions underlying the ANOVA performed on data from the planting systems experiment.
2.5. Weather data
Weather data from 2007 to 2014 were collected at a weather station located at the New South Wales Department of Primary Industries in Wagga Wagga. This weather station was located approximately 1.5 km from the experiment. Rainfall, maximum, average and minimum air temperature were recorded daily.
2.6. Root observation and digital image collection
A minirhizotron camera system was used for non-destructive root observation (BTC-100X, Bartz Technology LLC, CA, USA) of the four genotypes, from the soil surface to an equivalent vertical depth of 60 cm. Digital images were captured fortnightly from 50 consecutive 1.2 cm fixed windows in each tube over the period of August 2007 to October 2010 and June 2012 to June 2014. Throughout these five seasons, 169600 digital images were collected.
For root image analysis, we used a simplified visual assessment procedure where the number of new white roots appearing at each date were counted rather than traced. Due to the very large number of images tracing all individual roots manually was not possible, and the definition of roots in the images was not always clear enough to rely on automated detection. Partly due to a slow accumulation of a fine layer of clay particles covering some windows which progressively obscured the view of roots with time, and partly due to the use of extruded polycarbonate tubes which had a slightly textured surface that reduced the optical clarity. Information from the images was therefore extracted manually, with the images for each tube viewed by depth and date using Rootfly software (Wells and Birchfield, Clemson University, SC, USA), and then the number of new roots that had appeared in the image since the last date entered into a spreadsheet manually.
2.7. Statistical analysis
2.7.1. Analysis across all observation dates
Based on the objectives of this research, we classified a root as growing or not growing to enable the investigation of growth dynamics across the soil profile and seasons. Roots that were ‘not growing’ were those that were not visibly growing as assessed by the minirhizotron images. We used a generalised linear mixed model, in the form of a binomial logistic regression to analyse this binomial data. This model was fitted in the statistical software R (version 3.2.0) using the ASReml-R (asreml-3.0) package. The model used for this analysis (Model 1) can be symbolically written as:
Binomial growth ~ average air temperature + genotype + season + phenological stages + year + observation date + replicate + tube + soil depth (and all the interactions of these variables)
To match the root observation data to the daily air temperature data, averages for the period until the observation date were calculated.
An alternative assessment of the data was achieved by concentrating on only the growing roots and newly appearing roots. The number of new roots at each observation date was recorded and used as the response variable in a linear mixed model fitted in the statistical software R (version 3.2.0) using the ASReml-R (asreml-3.0) package. It was necessary to log transform this data, so the model assumption of homogeneity was met. The model used for this analysis (Model 2) can be symbolically written as:
ln (Number of new roots) ~ average air temperature + genotype + season + phenological stages + year + observation date + replicate + tube+ soil depth (and all the interactions of these variables)
A log-likelihood ratio test was used to determine whether the random terms were significant in both Models 1 and 2. Year refers to the calendar year while season refers to spring, summer, winter or autumn. Phenological stage refers to the developmental stage of the vine, and observation date refers to the day the image was taken. Soil temperature was recorded at the depth of 10, 30 and 60 cm. It was categorical. However, root images are collected from the top of the soil to 60 cm and it was a continuous variable. Soil depth is included as a random factor in our Model because the root image locations were a continuous variable in this experiment.
2.7.2. Analysis across last three years of observation dates
Soil temperature and moisture in the last three years of data collection were recorded at hourly intervals and air temperature was recorded on a daily basis. To match the root observation data to the environmental factors, averages for the period until the observation date were calculated. Before deciding which environmental factors to use in the analysis, we calculated the correlation between the factors as one of the assumptions of the analysis methods used is that the independent variables need to be uncorrelated with each other.
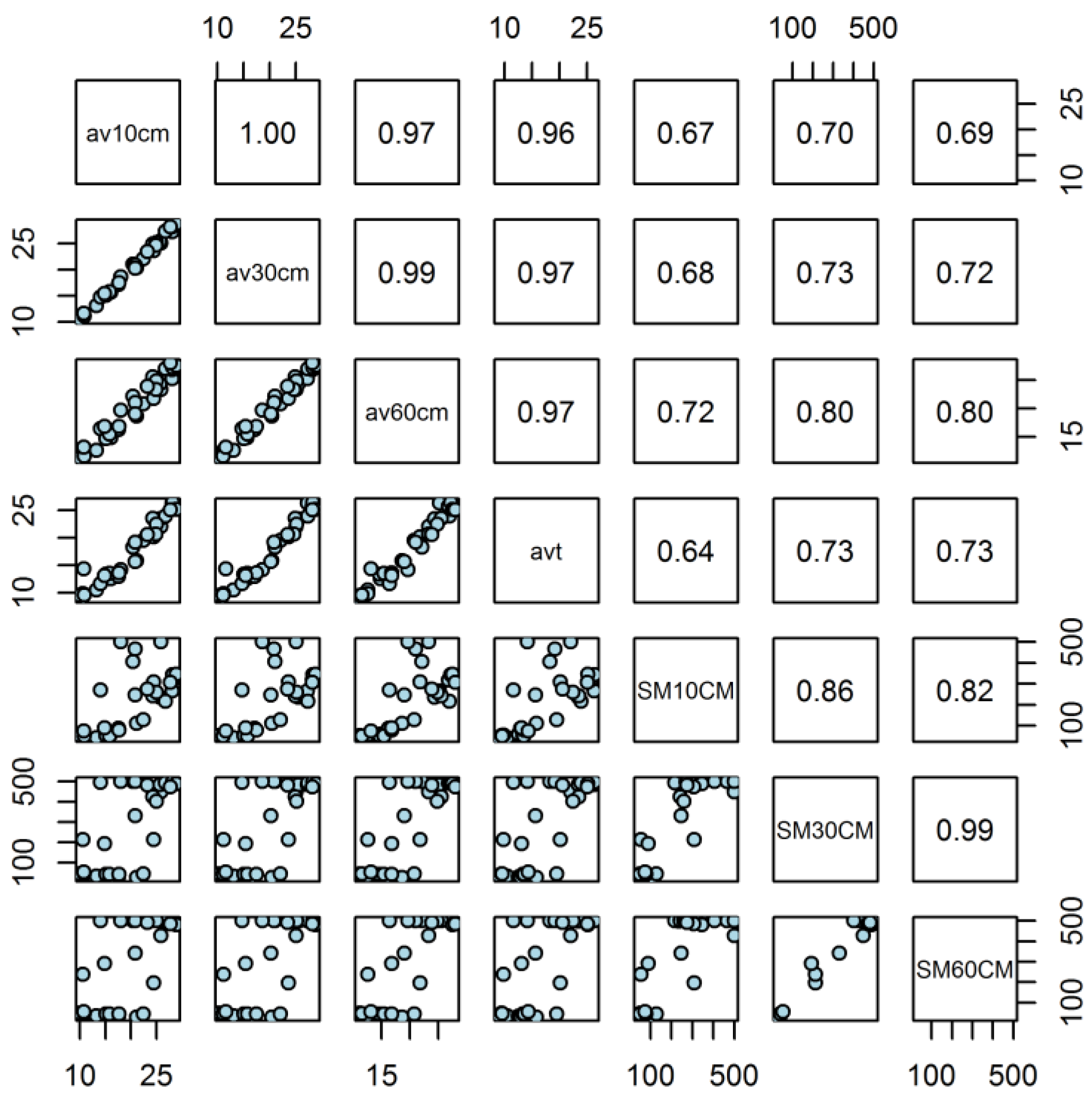
Soil moisture at 30 and 60 cm were correlated with each other (
Figure 2), and therefore 10 and 30 cm were used in our model. Air temperature and soil temperature were strongly correlated, therefore, only one of these measurements was used in the model. Air temperature was retained for this analysis to maintain consistency with the analysis across all observation dates. The model we used for this analysis (Model 3) can be symbolically written as:
ln (Number of new roots) ~ average air temperature + genotype + season + phenological stages + year + observation date + soil moisture at depth 10 cm + soil moisture at depth 30 cm + replicate + tube + depth (and all the interactions of these variables)
We used the 5% significance level as statistically significant in this analysis. The model assumptions were that the residuals were normally distributed; they had a constant variance and were independent.
3. Results
3.1. The model outcomes
The root parameters related to genotypes and other abiotic factors computed for the ASReml described in the Methods section are shown in
Table 1 and
Table 2. The outcomes of each model are explained in detail in the subsequent sections.
3.2. Soil moisture, soil and air temperature, phenological stages
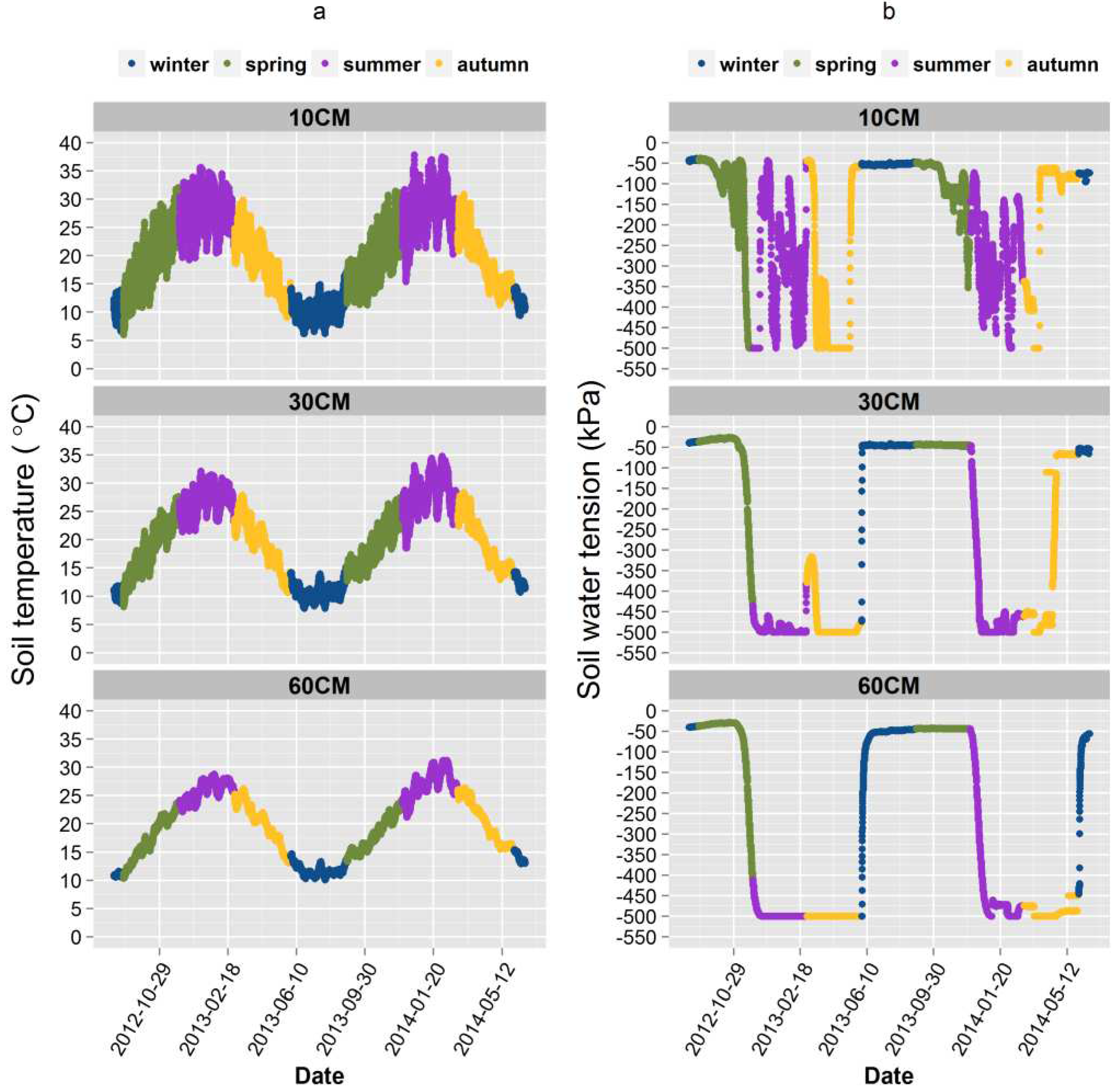
Soil water tension was higher at the depth of 10 cm compared to the other two depths, 30 cm and 60 cm. The soil became very dry once summer started with the driest period occurring during summer and autumn with some fluctuations at the depths of 30 cm and 60 cm (Figure 6-2 b). Notably, the soil water content at 30 cm was highly correlated with soil moisture at 60 cm (
Figure 2). Soil water at 10 cm was higher due to its proximity to the point of irrigation and rainfall.
Maximum summer soil temperatures in the 2013/2014 season were between 35-37 °C and greater by approximately 5 °C relative to the 2012/2013 season at the 10 cm and 30 cm depths. Soil temperatures in winter, spring and autumn were similar, in the range of 5-15 °C, 10- 30 °C and 30-10 °C respectively (
Figure 3 a). In addition, the soil temperature at the depth of 30 cm was highly correlated to the temperature at the 60 cm depth (
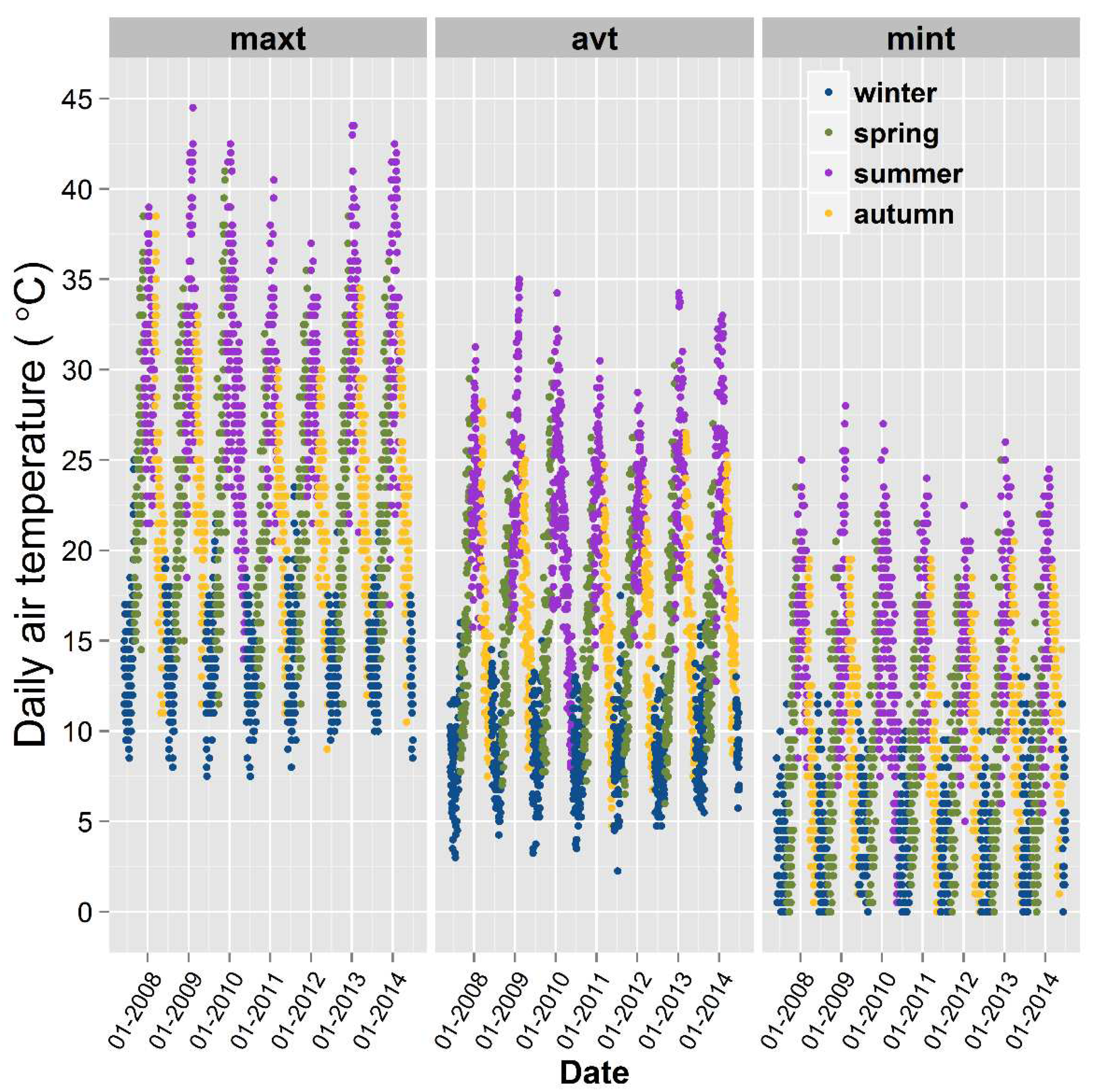
Figure 2). The maximum air temperature in 2009 reached 44 °C and are compared to other six years in
Figure 4. The maximum air temperature was lower than 40 °C in year one and year five. However, we found no year-to-year difference in average air temperatures which was calculated based on the values between each two observation dates (data not shown in the table as those not significant terms were removed).
The timing of the major phenological stages varied between years, fluctuating between 4 to 10 days. However, the harvest date of 2009 was around 20 days earlier than the other years (
Table 3) in this study and this could be related to the higher air temperature in that season and/or management factors.
3.3. Total new root production in different seasons and phenological stages
Total new root numbers varied between genotype, with 140 Ruggeri having the greatest total root numbers compared to Ramsey, followed by Schwarzmann and Shiraz. Maximum new root production was observed in spring at bloom in each species while it reached a minimum in autumn at harvest. Interestingly, Ramsey had more new root production in winter and at bud-break compared to the other genotypes (
Table 4). Notably, the new root population in winter was higher than autumn growth.
3.4. New root production, vertical root distribution dynamics and seasonal root growth dynamics of grapevines
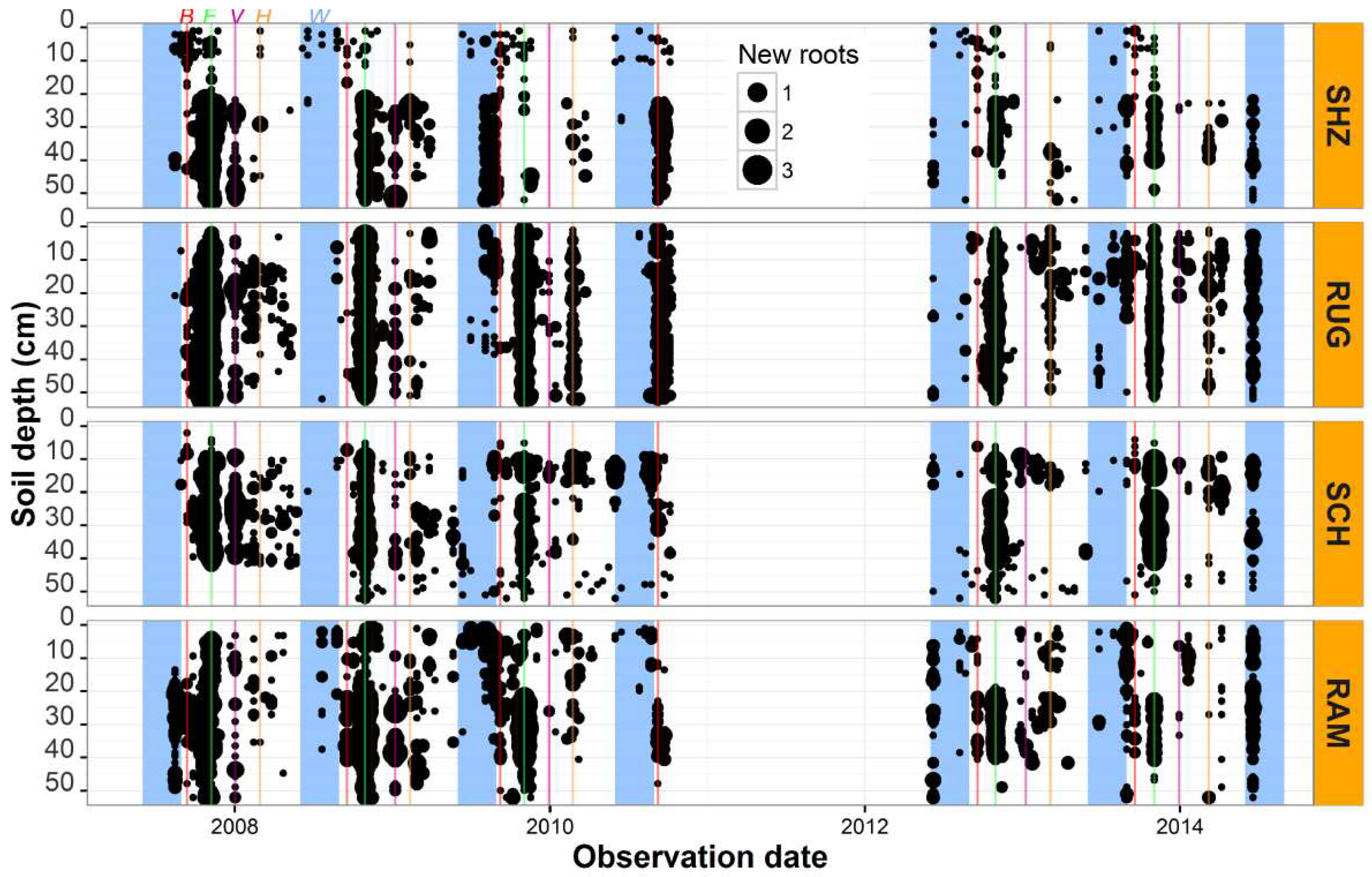
We non-destructively observed substantial new root production through the visible sides of the minirhizotrons. Year to year variability was found in new root growth among the genotypes and new root populations also varied through the soil profile (
Figure 5). New root numbers were significantly different between rootstocks (p < 0.001) (
Table 2). In this vineyard, vertical root distribution to the depth studied was unique for each genotype over the period of seven years. The depth of roots was one of the influencing factors in this new production (p < 0.05) (
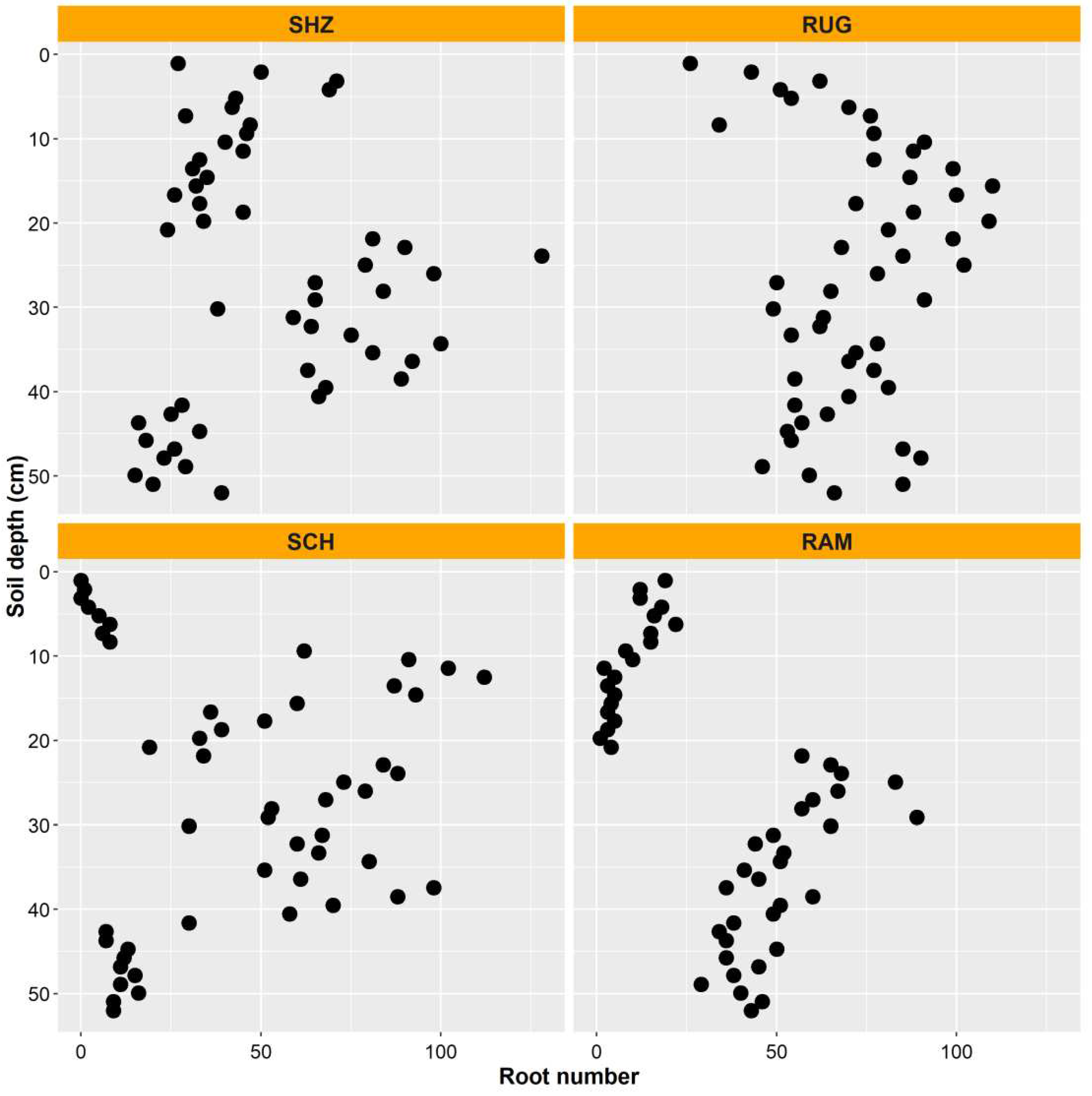
Table 1). Roots of 140 Ruggeri were distributed most evenly with depth, while most new Shiraz roots were found below 20 cm. Higher root numbers were present in Schwarzmann between the 10 cm and 40 cm soil layers, while there was a tendency for greater root numbers in Ramsey rootstocks below the 20 cm soil depth with only a few new roots in the upper part of the soil (
Figure 6).
Distinctive seasonal root growth activity occurred over the seven years. Root growth was most pronounced around flowering, followed by veraison, bud-burst and harvest (
Figure 5). New roots formed in spring were greater than those produced later in the season. The seasonal dynamics in root growth were similar in the different rootstocks, but the amount of root growth varied between the rootstocks. Overall, when considering the significant interactions between new root production with direct or indirect related factors, we found that new root growth was significantly affected by genotype (p < 0.01), air temperature (p < 0.001), season (p < 0.001), development stage (p < 0.001), year (p < 0.001) and observation date (p < 0.001) (
Table 2). In the last three years, we found that new root production also was significantly related to soil temperature (p < 0.01) and soil moisture at the depths of 30 cm or 60 cm (p < 0.01). Additionally, the combination of genotype with soil temperature (p < 0.001) or with soil moisture at the depth of 10 cm (p < 0.05) influenced new root growth (
Table 1).
4. Discussion
4.1. New root production, vertical root distribution dynamics and seasonal root growth dynamics of grapevines
New root production in grapevines was a function of biotic and abiotic factors such as genotype, phenological stage, temperature, moisture, season and year. We found that the seasonal dynamics in root production were consistent over the five seasons. New root production began in late winter and peaked at flowering in spring when soil temperature reached between 15 and 30 °C throughout the soil profile. The active root population declined in mid or late summer and after harvest. Based on these periodic changes in new root growth over the seven years, we suggest that the initiation of new root production in grapevines could be partially influenced by soil temperature and/or plant demand for nutrient/water resources. Year to year difference were found in timing of root growth (
Table 1) over the seven years. However, we analysed new root growth presence of soil temperature and moisture in the model from the last three years of this study, and we found that new root production had no significant difference in observation date (
Table 1).
Unimodal dynamics of root production, with one clear flush of new roots through the season, have been found in several fruit crops including grapevines. This unimodal curve may relate to a temperate environment where dormancy starts soon after crop harvest [
2,
10,
24]. In contrast, a multimodal distribution in new root production was evident here with a major flush occurring at flowering and minor modes at other phenological stages. The amplitude between the major and minor modes varied between rootstocks. Unlike in other cooler grape growing regions, where dormancy begins shortly after harvest, the vine leaves in our warm climate vineyard remained functional for two to three months prior to leaf senescence. This may provide vines with enough carbohydrates that go beyond root reserve replenishment requirements and allow more root flushes during the post-harvest period and even after leaf fall.
According to the previous study, maximum root growth in grapevines occurs before flowering and after harvest [
18]. Van Zyl [
37] found that root production of grapevines began after bud-break and maximum growth rates occurred at the bloom stage, after which the growth rate decreased. However, in that study, a new root flush period started after harvest. Alternatively,, the main root flushes of Merlot and Concord were observed in summer between bloom and veraison and little root production occurred before harvest and during dormancy when conditions were favourable [
3]. We found that the main period of new root production occurred in spring, followed by summer, winter and autumn. This variability in when root production occurs may be related to varying soil temperatures and root carbon availability during the major phenological stages. The presence of new root production during dormancy can be explained by Bhar [
14]. He showed that some grapevine roots can grow continuously during dormancy where the ambient soil temperature was 3 °C or higher. Furthermore, Eissenstat et al. [
3] found year to year differences were evident in the specific timing of root production and dynamics appeared to be influenced by cultural practices such as irrigation [
38] and pruning [
39,
40].
4.2. Influence of soil temperature on grapevine root growth
When other abiotic factors are not limiting, soil temperature initiates root growth and influences distribution [
15,
24,
31,
41]. We found that the soil temperature at 10, 30 and 60 cm was highly correlated to the air temperature (
Figure 2). Soil temperature and air temperature were also highly correlated therefore only one of these could be used in our model. We recorded soil temperature data in the last three years. Thus, air temperature was used as this data was collected over the full 7 years. Generally, the soil temperature was lower than the air temperature and the seasonal fluctuation occurred with depth depending on the changes of soil moisture and aboveground plant growth [
25]. In grapevines, the optimal soil temperature for root growth is close to 30 °C [
29,
30]. The average annual root growth of table grapes (Vitis Vinifera L.) cv. Thompson Seedless was not correlated with the annual soil temperature and higher thermal diffusivity in soil profile favoured root production [
15]. Based on our last three years of results, the maximum root growth occured in spring at the flowering stage when the soil temperature was in the range of 15 to 30 °C and then new root production decreased over summer until autumn. However, the roots started to grow during early dormancy while the soil temperature was 10 to 15 °C. Interestingly, in autumn, there was little new root growth after harvest when soil temperature was around 10-30 °C, a similar temperature range during spring when root production was prolific.
4.3. Influence of soil moisture on grapevine root growth
Efficiency of water-use affects grapevine performance and lack of water will cause limitations to plant growth and yield [
42]. We found that there was no apparent influence of soil moisture at 10 cm on the production of new roots, however there was an effect at 30 cm. Irrigation was successful at consistently wetting only the top 10 cm while most of the roots were further down the profile where the presence of irrigation water was more sporadic. The soils at 30 cm may have been sufficiently dry to limit root production at this depth. Irrigation methods in vineyard management have a significant effect on moisture diffusion within the soil profile, resulting in different root growth dynamics and water use efficiency of the grapevine [
37,
43,
44]. The data of the more recent years indicate that there are fewer new roots forming relative to the previous years and this might be the consequence of the irrigations not reaching the 30 cm depth due to less applied water. Unfortunately, no irrigation records are available to verify this. Further studies wetting the entire soil profile would give a better indication of new root production in response to soil moisture.
4.4. Influence of genotype on grapevine root distribution
We found that the four different rootstocks had different root production behaviours. Maximum root production peaks appeared at flowering in each rootstock. The rootstocks behaved differently at the other stages. For example, Schwarzmann and Shiraz had greater root populations at veraison than harvest and this was followed by bud-break. Conversely, 140 Ruggeri had a contrary result. Ramsey had greater new root production at bud-break, followed by veraison and harvest (
Figure 5). Other fruit crops such as peach grafted on five different rootstocks had similar seasonal dynamics of new root production. Fine root populations reached a minimum in winter and declined during the final stages of fruit development [
7]. In the same study, spring some rootstocks produced greater root length compared to those produced later in the season.
We found that the genotypes had different amounts of new roots through the soil profile (
Figure 6) and total new root production was significantly different at the different soil depths (
Table 1). Information on these differing root growth behaviours can potentially be used to plan time of fertiliser application and irrigation amounts. A peach rootstock with a different genetic background (K119-50) formed a large amount of new roots below 69 cm, unlike four other rootstocks [
7]. Consistent with our study, different grapevine species had different root numbers. For example, the greatest root numbers were observed in Dogridge followed by Barbera and Concord (intermediate), then Noble with the smallest amount of roots [
45]. In addition, the root distribution dynamics varied between cultivars. Noble had shallow roots, with approximately 35 % of the total roots in the 0-15 cm depth whereas most of the roots of Dogridge occurred in the 90- 105 cm soil profile. We found that new root production by 140 Ruggeri occurred in the 0 - 52 cm layer with the greatest root numbers between 10 - 30 cm. Shiraz had a maximum number of new roots in the 20- 40 cm depth, whereas Schwarzmann had the greatest root populations between 10-40 cm. Ramsey had a very unique root distribution dynamic and most of the new roots occurred in the soil zone at 20 – 52 cm (
Figure 6). The installation angle and the observation direction did not influence root counts [
46]. Based on the wide range in root distribution and seasonal dynamics in root growth of grapevine species and rootstocks, we suggest that they have adapted to the soil environmental conditions from which they originate.
5. Conclusions
We conclude that grapevine rootstocks displayed genetic variability in root development in response to changes in soil depth, developmental stage, season, year, temperature and soil moisture. We suggest that the genetic diversity of rootstocks could be drawn upon as a management tool for specific soil environments, based on these particular root distribution characteristics in different soil depths and seasonal root growth dynamics of grapevine rootstocks. In addition, climatic background of these rootstocks and their ability to adapt to different environmental conditions was one of the important aspects contributing to these variations between rootstocks. These rootstocks with differing root growth dynamics may influence whole vine performance, carbohydrate reserves and nutrient and water uptake dynamics through the season. Most importantly, our findings can be added to models that estimate whole grapevine performance and yield in changing soil environments in those grape growing regions which are the hottest.
Author Contributions
Conceptualization, B.H., S.F., Y.G., S.R. and J.S.; methodology, B.H., S.F., J.S., K.M. and S.R.; software, K.M.; validation, K.M., J.S. and S.N.; formal analysis, S.N.; investigation, K.M. and S.F.; resources, B.H., J.S., K.M.S.F.; data curation, K.M., J.S., S.F.; writing—original draft preparation, K.M.; writing—review and editing, K.M., J.S., B.H., S.N., S.F., S.R. and Y.G.; visualization, K.M. and S.N; supervision, B.H., J.S., S.R., and Y.G.; project administration, B.H.; funding acquisition, B.H.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Grape and Wine Industry Centre and Charles Sturt University, part of the PhD research. Some of root observation data utilised in this publication were from an earlier project that was supported by the Australian grape growers and winemakers through their investment body, Wine Australia, with matching funds from the Australian government.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions.
Acknowledgments
The authors would like to thank National Wine and Grape Industry Centre and Charles Sturt University and New South Wales Department of Primary Industry for their support. We also thank Rob Lamont and Helen Pan for their technical support during the study. Bernie Dominiak reviewed a pre-submission version. Bernie Dominiak reviewed an early version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mar Alsina, M.; Smart, D.R.; Bauerle, T.; de Herralde, F.; Biel, C.; Stockert, C.; Negron, C.; Save, R. Seasonal changes of whole root system conductance by a drought-tolerant grape root system. J Exp Bot 2011, 62, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Contador, M.L.; Comas, L.H.; Metcalf, S.G.; Stewart, W.L.; Porris Gomez, I.; Negron, C.; Lampinen, B.D. Root growth dynamics linked to above-ground growth in walnut (Juglans regia). Ann. Bot. 2015, 116, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Eissenstat, D.M.; Bauerle, T.L.; Comas, L.H.; Lakso, A.N.; Neilsen, D.; Neilsen, G.H.; Smart, D.R. Seasonal patterns of root growth in relation to shoot phenology in grape and apple. In Proceedings of the Vth International Symposium on Mineral Nutrition of Fruit Plants, Retamales, J.B., Ed. Acta Horticulturae; International Society Horticultural Science: Leuven 1, 2006; pp. 21–26. [Google Scholar]
- Cox, C.M.; Favero, A.C.; Dry, P.R.; McCarthy, M.G.; Collins, C. Rootstock Effects on Primary Bud Necrosis, Bud Fertility, and Carbohydrate Storage in Shiraz. American Journal of Enology and Viticulture 2012, 63, 277–283. [Google Scholar] [CrossRef]
- Atkinson, C.; Else, M. Understanding how rootstocks dwarf fruit trees. Compact Fruit Tree 2001, 34, 46–49. [Google Scholar]
- Robbins, N.S.; Pharr, D.M. Effect of restricted root growth on carbohydrate metabolism and whole plant growth of Cucumis sativus L. Plant Physiology 1988, 87, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Basile, B.; Bryla, D.R.; Salsman, M.L.; Marsal, J.; Cirillo, C.; Johnson, R.S.; DeJong, T.M. Growth patterns and morphology of fine roots of size-controlling and invigorating peach rootstocks. Tree physiology 2007, 27, 231–241. [Google Scholar] [CrossRef]
- Keller, M. Cultivars, clones, and rootstocks. In The science of grapevines: anatomy and physiology; Elsevier inc: Amsterdam, 2010; pp. 18–19. [Google Scholar]
- Di Filippo, M.; Vila, H. Influence of different rootstocks on the vegetative and reproductive performance of" Vitis vinifera" L. Malbec under irrigated conditions. Journal international des sciences de la vigne et du vin= International journal of vine and wine sciences 2011, 45, 75–84. [Google Scholar] [CrossRef]
- Comas, L.H.; Anderson, L.; Dunst, R.; Lakso, A.; Eissenstat, D. Canopy and environmental control of root dynamics in a long-term study of Concord grape. New phytologist 2005, 167, 829–840. [Google Scholar] [CrossRef]
- Keller, M.; Mills, L.J.; Harbertson, J.F. Rootstock effects on deficit-irrigated winegrapes in a dry climate: Vigor, yield formation, and fruit ripening. American Journal of Enology and Viticulture 2012, 63, 29–39. [Google Scholar] [CrossRef]
- Tandonnet, J.P.; Cookson, S.; Vivin, P.; Ollat, N. Scion genotype controls biomass allocation and root development in grafted grapevine. Australian Journal of Grape and Wine Research 2010, 16, 290–300. [Google Scholar] [CrossRef]
- Bassoi, L.H.; Grangeiro, L.C.; Silva, J.A.M.E.; Silva, E.E.G.D.A. Root distribution of irrigated grapevine rootstocks in a coarse texture soil of the São Francisco Valley, Brazil. Revista Brasileira de Fruticultura 2002, 24, 35–38. [Google Scholar] [CrossRef]
- Bhar, D.; Mason, G.; Hilton, R. In situ Observations on Plum Root Growth1. Journal of the American Society for Horticultural Science 1970, 95, 237–239. [Google Scholar] [CrossRef]
- Callejas, R.; Canales, P.; de Cortazar, V.G. Relationship between root growth of’Thompson Seedless’ grapevines and soil temperature. Chil. J. Agric. Res. 2009, 69, 496–502. [Google Scholar] [CrossRef]
- Lyr, H.; Hoffmann, G. Growth rates and growth periodicity of tree roots. In International review of forestry research; Elsevier: 1967; Volume 2, pp. 181-236.
- Teskey, R.O.; Hinckley, T.M. Influence of temperature and water potential on root growth of white oak. Physiologia plantarum 1981, 52, 363–369. [Google Scholar] [CrossRef]
- Freeman, B.; Smart, R. A root observation laboratory for studies with grapevines. American Journal of Enology and Viticulture 1976, 27, 36–39. [Google Scholar] [CrossRef]
- Hilton, R.; Khatamian, H. Diurnal variation in elongation rates of roots of woody plants. Can J Plant Sci 1973, 53, 699–700. [Google Scholar] [CrossRef]
- Southey, J.; Archer, E. The effect of rootstock cultivar on grapevine root distribution and density. The grapevine root and its environment 1988, 57–73. [Google Scholar]
- Williams, L.E.; Smith, R.J. The effect of rootstock on the partitioning of dry weight, nitrogen and potassium, and root distribution of Cabernet Sauvignon grapevines. American journal of enology and viticulture 1991, 42, 118–122. [Google Scholar] [CrossRef]
- Morlat, R.; Jacquet, A. The soil effects on the grapevine root system in several _ vineyards of the Loire valley (France),-Jo i.. by. Vitis 1993, 32, 35–42. [Google Scholar]
- Nagarajah, S. Effects of soil texture on the rooting patterns of Thompson Seedless vines on own roots and on Ramsey rootstock in irrigated vineyards. American Journal of Enology and Viticulture 1987, 38, 54–59. [Google Scholar] [CrossRef]
- Atkinson, D. The distribution and effectiveness of the roots of tree crops. Horticultural reviews 1980, 2, 424–490. [Google Scholar] [CrossRef]
- McMichael, B.; Burke, J. Soil temperature and root growth. HortScience 1998, 33, 947–951. [Google Scholar] [CrossRef]
- Bauerle, T.L.; Smart, D.R.; Bauerle, W.L.; Stockert, C.; Eissenstat, D.M. Root foraging in response to heterogeneous soil moisture in two grapevines that differ in potential growth rate. New phytologist 2008, 179, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Kuhns, M.; Garrett, H.; Teskey, R.; Hinckley, T. Root growth of black walnut trees related to soil temperature, soil water potential, and leaf water potential. Forest Science 1985, 31, 617–629. [Google Scholar]
- Bevington, K.B.; Castle, W.S. Annual root growth pattern of young citrus trees in relation to shoot growth, soil temperature, and soil water content. Journal of the American Society for Horticultural Science 1985, 110, 840–845. [Google Scholar] [CrossRef]
- Woodham, R.; Alexander, D.M.E. The effect of root temperature on development of small fruiting Sultana vines. Vitis 1966, 5, 345–350. [Google Scholar]
- Kliewer, W. Effect of root temperature on budbreak, shoot growth, and fruit-set of’Cabernet Sauvignon’grapevines. American Journal of Enology and Viticulture 1975, 26, 82–89. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Smith, J.P.; Holzapfel, B.P.; Hardie, W.J. Soil temperature moderates grapevine carbohydrate reserves after bud break and conditions fruit set responses to photoassimilatory stress. Functional Plant Biology 2011, 38, 899. [Google Scholar] [CrossRef]
- Clarke, S.J.; Lamont, K.; Pan, H.; Barry, L.; Hall, A.; Rogiers, S.Y. Spring root-zone temperature regulates root growth, nutrient uptake and shoot growth dynamics in grapevines. Australian journal of grape and wine research 2015, 21, 479–489. [Google Scholar] [CrossRef]
- Hall, A.; Jones, G.V. Spatial analysis of climate in winegrape-growing regions in Australia. Australian Journal of Grape and Wine Research 2010, 16, 389–404. [Google Scholar] [CrossRef]
- Huang, X.; Lakso, A.N.; Eissenstat, D.M. Interactive effects of soil temperature and moisture on Concord grape root respiration. J Exp Bot 2005, 56, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Dry, N. Grapevine rootstocks: selection and management for South Australian vineyards; Lythrum Press: 2007.
- Coombe, B.G. Growth stages of the grapevine: adoption of a system for identifying grapevine growth stages. Australian journal of grape and wine research 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Van Zyl, J. Response of grapevine roots to soil water regimes and irrigation systems. The grapevine root and its environment. Republic of So. Africa Dept. Agr. and Water Supply, Stellenbosch, So. Africa 1988, 30-43.
- Bassoi, L.H.; Hopmans, J.W.; de Castro Jorge, L.A.; De Alencar, C.; e Silva, J. Grapevine root distribution in drip and microsprinkler irrigation. Scientia Agricola 2003, 60, 377–387. [Google Scholar] [CrossRef]
- Comas, L.H.; Eissenstat, D.M.; Lakso, A.N. Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytologist 2000, 147, 171–178. [Google Scholar] [CrossRef]
- Ferree, D.; Scurlock, D.; Schmid, J. Root pruning reduces photosynthesis, transpiration, growth, and fruiting of container-grown French-American hybrid grapevines. HortScience 1999, 34, 1064–1067. [Google Scholar] [CrossRef]
- Bonomelli, C.; Bonilla, C.; and Nuñez, F. Soil temperature effect on root growth of cherry trees. (Prunus avium L.). VI International Cherry Symposium (ISHS-Pontificia Universidad Católica de Chile). November 15-19, 2009. Reñaca, Viña del Mar, Chile (Abstract). 2009. 15 November.
- Kramer, P.J.; Boyer, J.S. Water relations of plants and soils; Academic Press, Inc.: 1995.
- Araujo, F.; Williams, L.E.; Grimes, D.W.; Matthews, M.A. A comparative study of young ‘Thompson Seedless’ grapevines under drip and furrow irrigation. I. Root and soil water distributions. Scientia Horticulturae 1995, 60, 235–249. [Google Scholar] [CrossRef]
- Morano, L.; Kliewer, W.M. Root distribution of three grapevine rootstocks grafted to Cabernet Sauvignon grown on a very gravelly clay loam soil in Oakville, California. American Journal of Enology and Viticulture 1994, 45, 345–348. [Google Scholar] [CrossRef]
- Perry, R.; Lyda, S.; Bowen, H. Root distribution of fourVitis cultivars. Plant and Soil 1983, 71, 63–74. [Google Scholar] [CrossRef]
- Linsenmeier, A.; Lehnart, R.; Lohnertz, O.; Michel, H. Investigation of grapevine root distribution by in situ minirhizotron observation. Vitis 2010, 49, 1–6. [Google Scholar]
Figure 1.
Installation of minirhizotron tubes. The tubes were installed 20 cm from the trunk of the two centre vines in each replicate, aligned with the vine row to avoid damage from vineyard machinery, and angled under the vines at 30 degrees from vertical.
Figure 1.
Installation of minirhizotron tubes. The tubes were installed 20 cm from the trunk of the two centre vines in each replicate, aligned with the vine row to avoid damage from vineyard machinery, and angled under the vines at 30 degrees from vertical.
Figure 2.
Correlation map of air temperature, soil temperature and moisture at different depths. “av10 cm” = average soil temperature at 10 cm; “av30 cm” = average soil temperature at 30 cm; “av60 cm” = average soil temperature at 60 cm; “avt” = average air temperature; “SM10CM” = average soil moisture at 10 cm; “SM30CM” = average soil moisture at 30 cm; “SM60CM” = average soil moisture at 60 cm.
Figure 2.
Correlation map of air temperature, soil temperature and moisture at different depths. “av10 cm” = average soil temperature at 10 cm; “av30 cm” = average soil temperature at 30 cm; “av60 cm” = average soil temperature at 60 cm; “avt” = average air temperature; “SM10CM” = average soil moisture at 10 cm; “SM30CM” = average soil moisture at 30 cm; “SM60CM” = average soil moisture at 60 cm.
Figure 3.
Seasonal dynamics of soil temperature (a) and soil moisture (b) at 10 cm, 30 cm and 60 cm depths adjacent to minirhizotron tubes over two seasons (2012/2013 and 2013/2014). Values are means of four locations for each of the four genotypes. The colour of the symbol refers to winter, spring, summer and autumn.
Figure 3.
Seasonal dynamics of soil temperature (a) and soil moisture (b) at 10 cm, 30 cm and 60 cm depths adjacent to minirhizotron tubes over two seasons (2012/2013 and 2013/2014). Values are means of four locations for each of the four genotypes. The colour of the symbol refers to winter, spring, summer and autumn.
Figure 4.
Seasonal dynamics of maximum, mean and minimum air temperature over seven years. On the figure, the “avt” is average air temperature; “maxt” is maximum air temperature and “mint” is minimum temperature. Values are daily air temperature from June 2007 to June 2014. The colour of the symbol refers to winter, spring, summer and autumn.
Figure 4.
Seasonal dynamics of maximum, mean and minimum air temperature over seven years. On the figure, the “avt” is average air temperature; “maxt” is maximum air temperature and “mint” is minimum temperature. Values are daily air temperature from June 2007 to June 2014. The colour of the symbol refers to winter, spring, summer and autumn.
Figure 5.
Number of new roots and their seasonal distribution dynamics by soil depth as monitored in minirhizotron window positions over 2007/2008, 2008/2009, 2009/2010, 2012/2013 and 2013/2014. Values are means of new roots counted at each image location (depth), by observation date. SHZ = Shiraz, RUG = 140 Ruggeri, SCH = Schwarzmann, RAM = Ramsey. The italicized symbols on the left corner of the figure indicates phenological stages of grapevines and winter period (dormancy); B = Bud break, F= Flowering, V = Veraison, H = Harvest, W = Winter. No growth (zeros) not shown in this figure. Average new root numbers at each particular depth is shown on the figure legend as 1, 2 and 3 with the size of the symbol proportional to the number of new roots.
Figure 5.
Number of new roots and their seasonal distribution dynamics by soil depth as monitored in minirhizotron window positions over 2007/2008, 2008/2009, 2009/2010, 2012/2013 and 2013/2014. Values are means of new roots counted at each image location (depth), by observation date. SHZ = Shiraz, RUG = 140 Ruggeri, SCH = Schwarzmann, RAM = Ramsey. The italicized symbols on the left corner of the figure indicates phenological stages of grapevines and winter period (dormancy); B = Bud break, F= Flowering, V = Veraison, H = Harvest, W = Winter. No growth (zeros) not shown in this figure. Average new root numbers at each particular depth is shown on the figure legend as 1, 2 and 3 with the size of the symbol proportional to the number of new roots.
Figure 6.
Total number of new roots of the four genotypes and their distribution dynamics by soil depth as monitored in minirhizotron window positions. Values are the means of 2007/2008, 2008/2009, 2009/2010, 2012/2013 and 2013/2014. SHZ = Shiraz, RUG = 140 Ruggeri, SCH = Schwarzmann, RAM = Ramsey.
Figure 6.
Total number of new roots of the four genotypes and their distribution dynamics by soil depth as monitored in minirhizotron window positions. Values are the means of 2007/2008, 2008/2009, 2009/2010, 2012/2013 and 2013/2014. SHZ = Shiraz, RUG = 140 Ruggeri, SCH = Schwarzmann, RAM = Ramsey.
Table 1.
Outcome from the analysis based on Model 1 and Model 2 described in the methodology. “***; ** and *” indicate significance at the 0.1, 1 and 5 % respectively. The non-significant interaction terms are not included in the table. In Model 1, significance is shown for the factors which resulted in no root growth. In Model 2, significance is shown for the factors that affected the number of new roots produced.
Table 1.
Outcome from the analysis based on Model 1 and Model 2 described in the methodology. “***; ** and *” indicate significance at the 0.1, 1 and 5 % respectively. The non-significant interaction terms are not included in the table. In Model 1, significance is shown for the factors which resulted in no root growth. In Model 2, significance is shown for the factors that affected the number of new roots produced.
| Predictor variables |
Response |
No root growth
(Model 1) |
New roots
(Model 2) |
| Air temperature |
*** |
*** |
| Genotype |
*** |
** |
| Season |
*** |
*** |
| Depth |
* |
* |
| Phenological stages |
*** |
*** |
| Year |
*** |
*** |
| Observation date |
*** |
*** |
| Air temperature: Genotype |
*** |
*** |
| Genotype: Season |
*** |
** |
| Genotype: Phenological stages |
*** |
*** |
| Genotype : Year |
*** |
*** |
| Genotype : Observation date |
*** |
*** |
Table 2.
Outcome from the analysis based on Model 3 described in the methodology. “***; ** and *” indicate significance at the 0.1, 1 and 5 % respectively. “ns” indicates no significant interaction when a term was used. The non-significant interaction terms are not included in the table.
Table 2.
Outcome from the analysis based on Model 3 described in the methodology. “***; ** and *” indicate significance at the 0.1, 1 and 5 % respectively. “ns” indicates no significant interaction when a term was used. The non-significant interaction terms are not included in the table.
| Predictor variables |
Response |
| New roots |
|---|
| Air temperature |
** |
| Genotype |
** |
| Season |
*** |
| Depth |
* |
| Phenological stages |
** |
| Year |
ns |
| Observation date |
ns |
| Soil moisture at 10 cm |
ns |
| Soil moisture at 30 cm |
** |
| Air temperature: Genotype |
*** |
| Genotype: Soil moisture at 10 cm |
* |
| Genotype: Soil moisture at 30 cm |
** |
Table 3.
Date of onset of the major phenological stages in grapevine development over five seasons. These dates are based upon the Eichhorn and Lorenz system modified by Coombe [
36].
Table 3.
Date of onset of the major phenological stages in grapevine development over five seasons. These dates are based upon the Eichhorn and Lorenz system modified by Coombe [
36].
Phenological
stages |
Season |
| Season 1 (2007/2008) |
Season 2
(2008/2009) |
Season 3 (2009/2010) |
Season 4 (2012/2013) |
Season 5 (2013/2014) |
| Bud-break (E-L 02) |
12/09/2007 |
17/09/2008 |
8/09/2009 |
18/09/2012 |
18/09/2013 |
| Flowering (E-L 08) |
08/11/2007 |
29/10/2008 |
02/11/2009 |
30/10/2012 |
02/11/2013 |
| Veraison (E-L 31) |
02/01/2008 |
07/01/2009 |
30/12/2009 |
08/01/2013 |
30/12/2013 |
| Harvest (E-L 35) |
29/02/2008 |
10/02/2009 |
23/02/2010 |
06/03/2013 |
09/03/2014 |
Table 4.
A genotype comparison of the total number of new roots produced over a seven-year period in each of the four seasons and around the major phenological stages.
Table 4.
A genotype comparison of the total number of new roots produced over a seven-year period in each of the four seasons and around the major phenological stages.
| |
Genotypes |
| Shiraz |
140 Ruggeri |
Schwarzmann |
Ramsey |
| Seasons |
Spring |
1157 |
2621 |
1440 |
1603 |
| Summer |
254 |
470 |
414 |
333 |
| Autumn |
50 |
156 |
156 |
109 |
| Winter |
245 |
331 |
205 |
514 |
Phenological
stages
|
Bud-break |
93 |
136 |
41 |
191 |
| Flowering |
594 |
1563 |
1075 |
972 |
| Veraison |
132 |
136 |
164 |
124 |
| Harvest |
99 |
203 |
81 |
69 |
| Year total |
|
1706 |
3578 |
2215 |
2559 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).