Submitted:
20 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Genetics and epigenetics of PCOS
3. PCOS phenotypic expression
4. Total abdominal (android) fat mass
4.1. Intra-abdominal adipose
4.2. Subcutaneous abdominal adipose
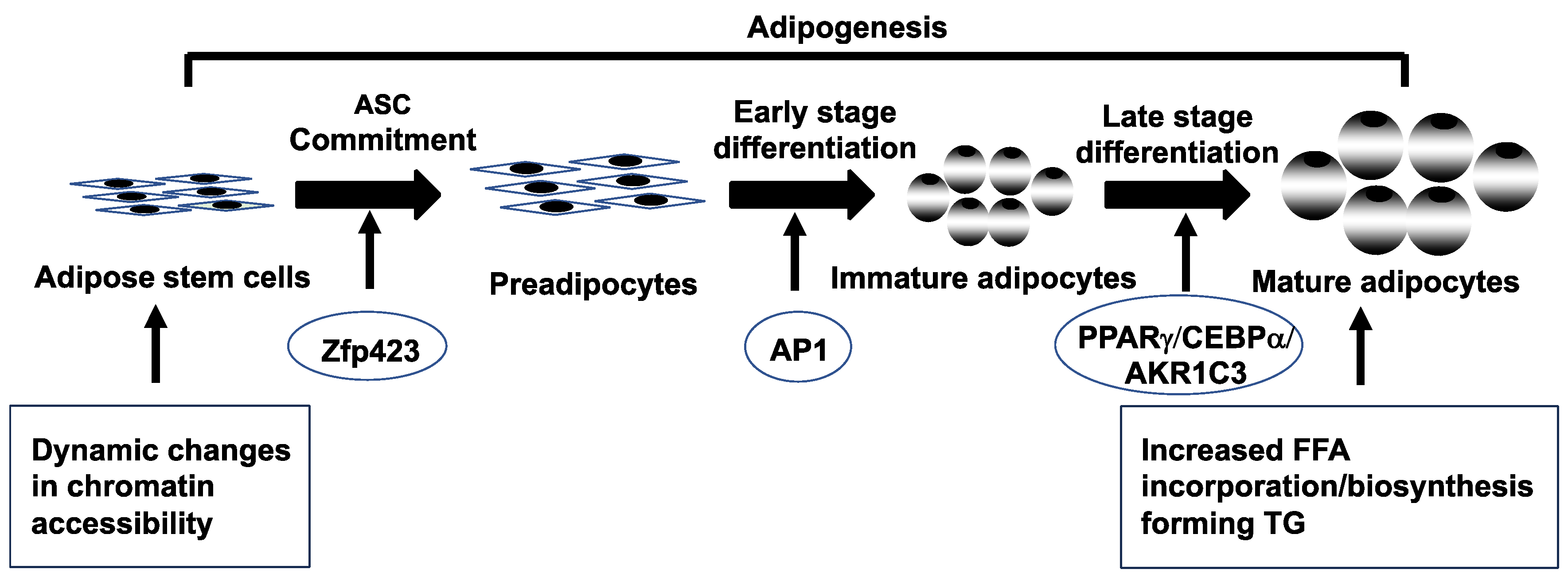
4.3. Subcutaneous abdominal stem cells and cellular reprogramming
5. Lipotoxicity
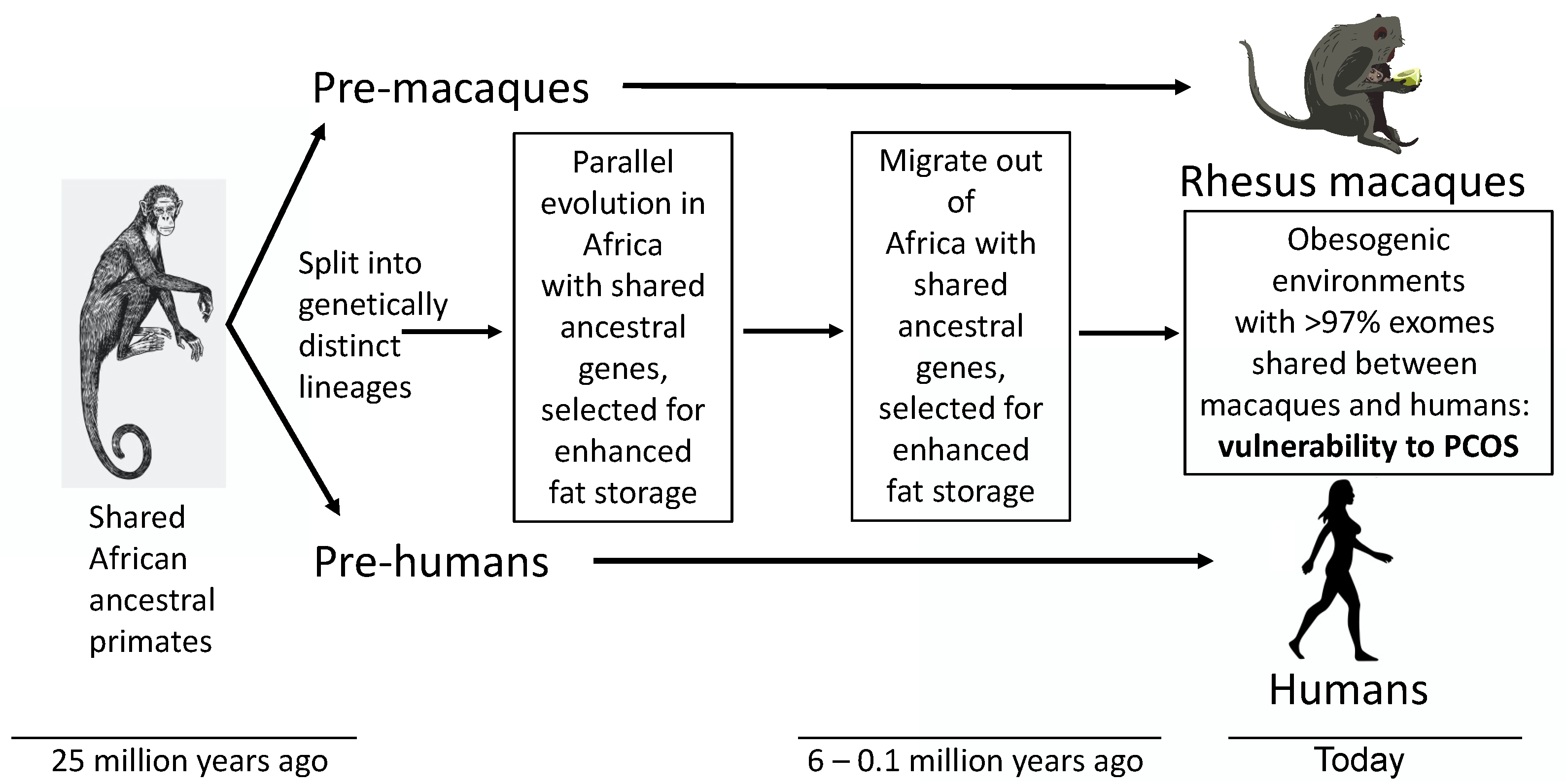
6. Parallel evolution of PCOS-like traits in naturally hyperandrogenic female rhesus macaques
7. Conclusions
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Acknowledgements
Conflicts of Interests
References
- Chang RJ, Dumesic DA. Polycystic Ovary Syndrome and Hyperandrogenic States. In Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management, 9th ed.; Strauss JF III, Barbieri RL, Dokras A, Williams CJ, Williams SZ, Eds.; Elsevier Saunders: Philadelphia, USA, 2024; pp. 517-547.
- Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr Rev. 2015,36(5),487-525. [CrossRef]
- Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Repro Update. 2010,16(4),347-363. [CrossRef]
- Carmina E, Napoli N, Longo RA, Rini GB, Lobo RA. Metabolic syndrome in polycystic ovary syndrome (PCOS): lower prevalence in southern Italy than in the USA and the influence of criteria for the diagnosis of PCOS. Eur J Endocrinol. 2006,154(1),141-145. [CrossRef]
- Dumesic DA, Hoyos LR, Chazenbalk GD, Naik R, Padmanabhan V, Abbott DH. Mechanisms of Intergenerational Transmission of Polycystic Ovary Syndrome. Reproduction, 2020,159(1),R1-R13. [CrossRef]
- Corbett S, Morin-Papunen L. Polycystic ovary syndrome and recent human evolution. Mol Cell Endocrinol. 2013,373(1-2),39-50. [CrossRef]
- Azziz R, Dumesic DA, Goodarzi M. Polycystic Ovary Syndrome: an ancient disorder? Fertil Steril 2011,95,1544-1548. [CrossRef]
- Arifin E, Shively CA, Register TC, Cline JM. Polycystic ovary syndrome with endometrial hyperplasia in a cynomolgus monkey (Macaca fascicularis). Vet Pathol. 2008,45(4),512-515. [CrossRef]
- Abbott DH, Rayome BH, Dumesic DA, Lewis KC, Edwards AK, Wallen K, Wilson ME, Appt SE, Levine JE. Clustering of PCOS-like traits in naturally hyperandrogenic female rhesus monkeys. Hum Reprod. 2017,32(4),923-936. [CrossRef]
- Abbott DH, Rogers J, Dumesic DA, Levine JE. Naturally Occurring and Experimentally Induced Rhesus Macaque Models for Polycystic Ovary Syndrome: Translational Gateways to Clinical Application. Med Sci (Basel). 2019,7(12),107. [CrossRef]
- Perelman P, Johnson WE, Roos C, Seuánez HN, Horvath JE, Moreira MA, Kessing B, Pontius J, Roelke M, Rumpler Y, Schneider MP, Silva A, O'Brien SJ, Pecon-Slattery J. A molecular phylogeny of living primates. PLoS Genet. 2011,7(3):e1001342. 1001. [CrossRef]
- Raaum RL, Sterner KN, Noviello CM, Stewart CB, Disotell TR. Catarrhine primate divergence dates estimated from complete mitochondrial genomes: concordance with fossil and nuclear DNA evidence. J Hum Evol. 2005,48(3):237-257. [CrossRef]
- Cooper EB, Brent LJN, Snyder-Mackler N, Singh M, Sengupta A, Khatiwada S, Malaivijitnond S, Qi Hai Z, Higham JP. The rhesus macaque as a success story of the Anthropocene. Elife. 2022,11:e78169. [CrossRef]
- Leakey M, Grossman A, Gutiérrez M, Fleagle JG. Faunal change in the Turkana Basin during the late Oligocene and Miocene. Evol Anthropol. 2011,20(6):238-253. [CrossRef]
- Stewart CB, Disotell TR. Primate evolution - in and out of Africa. Curr Biol. 1998,13;8(16):R582-R588. [CrossRef]
- Dumesic DA, Padmanabhan V, Levine J, Chazenbalk GD, Abbott DH. Polycystic Ovary Syndrome as a Plausible Evolutionary Metabolic Adaptation. Repro Biol Endocrinol 2022,20(1),12. [CrossRef]
- Parker J, O'Brien C, Hawrelak J, Gersh FL. Polycystic Ovary Syndrome: An Evolutionary Adaptation to Lifestyle and the Environment. Int J Environ Res Public Health. 2022,19(3),1336. [CrossRef]
- Parker J. Pathophysiological Effects of Contemporary Lifestyle on Evolutionary-Conserved Survival Mechanisms in Polycystic Ovary Syndrome. Life (Basel). 2023,13(4),1056. 4. [CrossRef]
- Tsatsoulis A, Mantzaris MD, Bellou S, Andrikoula M. Insulin resistance: an adaptive mechanism becomes maladaptive in the current environment - an evolutionary perspective. Metabolism. 2013,62(5),622-633. [CrossRef]
- Björntorp P. Metabolic implications of body fat distribution. Diabet Care. 1991,14(12):1132-1143. [CrossRef]
- Björntorp P. Visceral obesity: a "civilization syndrome". Obes Res. 1993,1(3):206-222. [CrossRef]
- López-Otín C, Kroemer G. Hallmarks of Health. Cell. 2021,184(1),33-63. [CrossRef]
- Klimentidis YC, Beasley TM, Lin HY, Murati G, Glass GE, Guyton M, Newton W, Jorgensen M, Heymsfield SB, Kemnitz J, Fairbanks L, Allison DB. Canaries in the coal mine: a cross-species analysis of the plurality of obesity epidemics. Proc Biol Sci. 2011,278(1712),1626-1632. [CrossRef]
- Terasawa E, Kurian JR, Keen KL, Shiel NA, Colman RJ, Capuano SV. Body weight impact on puberty: effects of high-calorie diet on puberty onset in female rhesus monkeys. Endocrinology. 2012,153(4),1696-1705. [CrossRef]
- Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A 95,1998,14956-14960. [CrossRef]
- Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006,91,2100-2104. [CrossRef]
- Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui HP, Zhao Z, Massart J, Ohlsson C, Lindgren E, Crisosto N, Maliqueo M, Echiburú B, Ladrón de Guevara A, Sir-Petermann T, Larsson H, Rosenqvist MA, Cesta CE, Benrick A, Deng Q, Stener-Victorin E. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019,25(12),1894-1904. [CrossRef]
- Shan D, Han J, Cai Y, Zou L, Xu L, Shen Y. Reproductive Health in First-degree Relatives of Patients With Polycystic Ovary Syndrome: A Review and Meta-analysis. J Clin Endocrinol Metab. 2022,107(1),273-295. [CrossRef]
- Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril 2001,75(1),53-58. [CrossRef]
- Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, Li Z, You L, Zhao J, Liu J, Liang X, Zhao X, Zhao J, Sun Y, Zhang B, Jiang H, Zhao D, Bian Y, Gao X, Geng L, Li Y, Zhu D, Sun X, Xu JE, Hao C, Ren CE, Zhang Y, Chen S, Zhang W, Yang A, Yan J, Li Y, Ma J, Zhao Y. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011,43(1),55-59. [CrossRef]
- Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, Zhang B, Liang X, Li T, Chen J, Shen J, Zhao J, You L, Gao X, Zhu D, Zhao X, Yan Y, Qin Y, Li W, Yan J, Wang Q, Zhao J, Geng L, Ma J, Zhao Y, He G, Zhang A, Zou S, Yang A, Liu J, Li W, Li B, Wan C, Qin Y, Shi J, Yang J, Jiang H, Xu JE, Qi X, Sun Y, Zhang Y, Hao C, Ju X, Zhao D, Ren CE, Li X, Zhang W, Zhang Y, Zhang J, Wu D, Zhang C, He L, Chen ZJ. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012,44(9),1020-1025. Nat Genet. 1025. [CrossRef]
- Goodarzi MO, Jones MR, Li X, Chua AK, Garcia OA, Chen YD, Krauss RM, Rotter JI, Ankener W, Legro RS, Azziz R, Strauss JF 3rd, Dunaif A, Urbanek M. Replication of association of DENND1A and THADA variants with polycystic ovary syndrome in European cohorts. J Med Genet. 2012,49(2),90-95. [CrossRef]
- Mutharasan P, Galdones E, Penalver Bernabe B, Garcia OA, Jafari N, Shea LD, Woodruff TK, Legro RS, Dunaif A, Urbanek M. Evidence for chromosome 2p16.3 polycystic ovary syndrome susceptibility locus in affected women of European ancestry. J Clin Endocrinol Metab. 2013,98(1),E185-E190. [CrossRef]
- Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S, Lindgren CM, Welt CK, Diamanti-Kandarakis E, Panidis D, Goodarzi MO, Azziz R, Zhang Y, James RG, Olivier M, Kissebah AH; Reproductive Medicine Network; Stener-Victorin E, Legro RS, Dunaif A. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun. 2015,6,7502. [CrossRef]
- Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, Bjonnes A, Broer L, Dunger DB, Halldorsson BV, Lawlor DA, Laval G, Mathieson I, McCardle WL, Louwers Y, Meun C, Ring S, Scott RA, Sulem P, Uitterlinden AG, Wareham NJ, Thorsteinsdottir U, Welt C, Stefansson K, Laven JSE, Ong KK, Perry JRB. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015,6,8464. [CrossRef]
- Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, Kraft P, Lin N, Huang H, Broer L, Magi R, Saxena R, Laisk T, Urbanek M, Hayes MG, Thorleifsson G, Fernandez-Tajes J, Mahajan A, Mullin BH, Stuckey BGA, Spector TD, Wilson SG, Goodarzi MO, Davis L, Obermayer-Pietsch B, Uitterlinden AG, Anttila V, Neale BM, Jarvelin MR, Fauser B, Kowalska I, Visser JA, Andersen M, Ong K, Stener-Victorin E, Ehrmann D, Legro RS, Salumets A, McCarthy MI, Morin-Papunen L, Thorsteinsdottir U, Stefansson K; 23andMe Research Team, Styrkarsdottir U, Perry JRB, Dunaif A, Laven J, Franks S, Lindgren CM, Welt CK. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018,14(12),e1007813. 1007. [CrossRef]
- Dapas M, Dunaif A. The contribution of rare genetic variants to the pathogenesis of polycystic ovary syndrome. Curr Opin Endocr Metab Res 2020,12,26-32. [CrossRef]
- Dapas M, Dunaif A. Deconstructing a Syndrome: Genomic Insights Into PCOS Causal Mechanisms and Classification. Endocr Rev. 2022,43(6),927-965. [CrossRef]
- Tian Y, Li J, Su S, Cao Y, Wang Z, Zhao S, Zhao H. PCOS-GWAS Susceptibility Variants in THADA, INSR, TOX3, and DENND1A Are Associated With Metabolic Syndrome or Insulin Resistance in Women With PCOS. Front Endocrinol (Lausanne). 2020,11,274. Front Endocrinol (Lausanne). [CrossRef]
- Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, Beaumont RN, Wittemans L, Martin S, Busch AS, Erzurumluoglu AM, Hollis B, O'Mara TA; Endometrial Cancer Association Consortium, McCarthy MI, Langenberg C, Easton DF, Wareham NJ, Burgess S, Murray A, Ong KK, Frayling TM, Perry JRB. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020,26(2),252-258. 2. [CrossRef]
- Shriner D, Tekola-Ayele F, Adeyemo A, Rotimi CN. Ancient Human Migration after Out-of-Africa. Sci Rep. 2016,6,26565. [CrossRef]
- Nielsen R, Akey JM, Jakobsson M, Pritchard JK, Tishkoff S, Willerslev E. Tracing the peopling of the world through genomics. Nature. 2017,541(7637),302-310. [CrossRef]
- Dapas M, Lin FTJ, Nadkarni GN, Sisk R, Legro RS, Urbanek M, Hayes MG, Dunaif A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020,17(6),e1003132. [CrossRef]
- Strauss JF, 3rd, McAllister JM, Urbanek M. Persistence pays off for PCOS gene prospectors. J Clin Endocrinol Metab. 2012,97(7),2286-2288. [CrossRef]
- Tee MK, Speek M, Legeza B, Modi B, Teves ME, McAllister JM, Strauss JF 3rd, Miller WL. Alternative splicing of DENND1A, a PCOS candidate gene, generates variant 2. Mol Cell Endocrinol. 2016,434,25-35. [CrossRef]
- Dapas M, Sisk R, Legro RS, Urbanek M, Dunaif A, Hayes MG: Family-based quantitative trait meta-analysis implicates rare noncoding variants in DENND1A in polycystic ovary syndrome. J Clin Endocrinol Metab 2019,104(9),3835-3850. 9. [CrossRef]
- McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, Strauss JF 3rd. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci U S A. 2014,111(15),E1519-E1527. [CrossRef]
- McAllister JM, Legro RS, Modi BP, Strauss JF 3rd. Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab. 2015,26(3),118-124. [CrossRef]
- Waterbury JS, Teves ME, Gaynor A, Han AX, Mavodza G, Newell J, Strauss JF 3rd, McAllister JM. The PCOS GWAS Candidate Gene ZNF217 Influences Theca Cell Expression of DENND1A.V2, CYP17A1, and Androgen Production. J Endocr Soc. 2022,6(7),bvac078. [CrossRef]
- Gorsic LK, Kosova G, Werstein B, Sisk R, Legro RS, Hayes MG, Teixeira JM, Dunaif A, Urbanek M. Pathogenic Anti-Mullerian Hormone Variants in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2017,102,2862-2872. [CrossRef]
- Gorsic LK, Dapas M, Legro RS, Hayes MG, Urbanek M. Functional Genetic Variation in the Anti-Mullerian Hormone Pathway in Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab 2019,104(7): 2855-2874. [CrossRef]
- Barbotin AL, Peigné M, Malone SA, Giacobini P. Emerging Roles of Anti-Müllerian Hormone in Hypothalamic-Pituitary Function. Neuroendocrinology. 2019,109(3):218-229. [CrossRef]
- Nilsson E, Benrick A, Kokosar M, Krook A, Lindgren E, Källman T, Martis MM, Højlund K, Ling C, Stener-Victorin E. Transcriptional and epigenetic changes influencing skeletal muscle metabolism in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2018,103(12),4465-4477. [CrossRef]
- Vázquez-Martínez ER, Gómez-Viais YI, García-Gómez E, Reyes-Mayoral C, Reyes-Muñoz E, Camacho-Arroyo I, Cerbón M. DNA methylation in the pathogenesis of polycystic ovary syndrome. Reproduction 2019,158(1),R27-R40. 1. [CrossRef]
- Jones MR, Brower MA, Xu N, Cui J, Mengesha E, Chen YD, Taylor KD, Azziz R, Goodarzi MO. Systems Genetics Reveals the Functional Context of PCOS Loci and Identifies Genetic and Molecular Mechanisms of Disease Heterogeneity. PLoS Genet. 2015,11(8),e1005455. 8. [CrossRef]
- Kokosar M, Benrick A, Perfilyev A, Fornes R, Nilsson E, Maliqueo M, Behre CJ, Sazonova A, Ohlsson C, Ling C, Stener-Victorin E. Epigenetic and Transcriptional Alterations in Human Adipose Tissue of Polycystic Ovary Syndrome. Sci Rep. 2016,6,22883. [CrossRef]
- McAllister JM, Han AX, Modi BP, Teves ME, Mavodza GR, Anderson ZL, Shen T, Christenson LK, Archer KJ, Strauss JF. miRNA Profiling Reveals miRNA-130b-3p Mediates DENND1A Variant 2 Expression and Androgen Biosynthesis. Endocrinology. 2019,160(8),1964-1981. [CrossRef]
- Abbott DH, Dumesic DA, Levine JE. Hyperandrogenic Origins of Polycystic Ovary Syndrome – Implications for Pathophysiology and Therapy. Expert Rev Endocrinol Metab. 2019,14(2),131-143. [CrossRef]
- Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R, Schooler TA, Okeya BL, Abbott DH, Chazenbalk GD. Hyperandrogenism Accompanies Increased Intra-Abdominal Fat Storage in Normal Weight Polycystic Ovary Syndrome Women. J Clin Endocrinol Metab. 2016,101(11),4178-4188. [CrossRef]
- Tosi F, Di Sarra D, Kaufman JM, Bonin C, Moretta R, Bonoro E, Zanolin E, Mogetti P. Total body fat and central fat mass independently predict insulin resistance but not hyperandrogenemia in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015,100(2),661-669. [CrossRef]
- Holte J, Bergh T, Berne C, Berglund L, Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab. 1994,78(5),1052-1058. [CrossRef]
- Rosenzweig JL, Ferrannini E, Grundy SM, Haffner SM, Heine RJ, Horton ES, Kawamori R. Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008,93(10),3671-3689. [CrossRef]
- Wyatt HR. Update on treatment strategies for obesity. J Clin Endocrinol Metab. 2013,98(4),1299-1306. [CrossRef]
- Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003,9(4),359-372. [CrossRef]
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endo Rev. 2012,33(6),981-1030. [CrossRef]
- Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013,14(2):95-109. [CrossRef]
- Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008,93(1),162-168. 1. [CrossRef]
- Kakoly NS, Khomami MB, Joham AE, Corray SD, Misso ML, Norman RJ, Harrison CL, Ranasinha S, Teede HJ, Moran LJ. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018,24(4),455-467. [CrossRef]
- Palaniappan LP, Carnethon MR, Fortmann SP. Heterogeneity in the relationship between ethnicity, BMI, and fasting insulin. Diabetes Care. 2002,25(8),1351-1357. [CrossRef]
- Teede HJ, Tay CT, Laven J, Dokras A, Moran LJ, Piltonen TT, Costello MF, Boivin J, M Redman L, A Boyle J, Norman RJ, Mousa A, Joham AE; International PCOS Network. Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil Steril. 2023 Aug 14:S0015-0282(23)00719-7. Epub ahead of print. [CrossRef]
- Ezeh U, Yildiz BO, Azziz R. Referral bias in defining the phenotype and prevalence of obesity in polycystic ovary syndrome. J Clin Endocrinol Metab. 2013,98(6),E1088-1096. 6. [CrossRef]
- Lizneva D, Kirubakaran R, Mykhalchenko K, Suturina L, Chernukha G, Diamond MP, Azziz R. Phenotypes and body mass in women with polycystic ovary syndrome identified in referral versus unselected populations: systematic review and meta-analysis. Fertil Steril. 2016,106(6),1510-1520.e2. [CrossRef]
- Søndergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab. 2017,102(4),1193-1199. 4. [CrossRef]
- Hershkop K, Besor O, Santoro N, Pierpont B, Caprio S, Weiss R. Adipose insulin resistance in obese adolescents across the spectrum of glucose tolerance. J Clin Endocrinol Metab. 2016,101(6):2423-2431. [CrossRef]
- Dumesic DA, Tulberg A, Leung KL, Fisch SC, Grogan TR, Abbott DH, Naik R, Chazenbalk GD. Accelerated subcutaneous abdominal stem cell adipogenesis predicts insulin sensitivity in normal-weight women with polycystic ovary syndrome. Fertil Steril. 2021,116(1),232-242. [CrossRef]
- Dumesic DA, Turcu AF, Liu H, Grogan TR, Abbott DH, Lu G, Dharanipragada D, Chazenbalk GD. Interplay of Cortisol, Testosterone, and Abdominal Fat Mass in Normal-weight Women With Polycystic Ovary Syndrome. J Endocr Soc. 2023,7(8):bvad079. [CrossRef]
- Dumesic DA, Phan JD, Leung KL, Grogan TR, Ding X, Li X, Hoyos LR, Abbott DH, Chazenbalk GD. Adipose Insulin Resistance in Normal-Weight Polycystic Ovary Syndrome Women. J Clin Endocrinol Metab. 2019,104(6),2171-2183. [CrossRef]
- McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011,96911):E1756-E1760. [CrossRef]
- Dumesic DA, Winnett C, Lu, G, Grogan TR, Abbott DH, Naik R, Chazenbalk GD. Randomized Clinical Trial: Effect of Low-Dose Flutamide on Abdominal Adipogenic Function in Normal-Weight Polycystic Ovary Syndrome Women. Fertil Steril. 2023,119(1),116-126. [CrossRef]
- Dicker A, Ryden M, Naslund E, Muehlen IE, Wiren M, Lafontan M, Arner P. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004,47(3),420-428. [CrossRef]
- Arner P. Effects of testosterone on fat cell lipolysis. Species differences and possible role in polycystic ovarian syndrome. Biochimie. 2005,87(1),39-43. 1. [CrossRef]
- Ek I, Arner P, Rydén M, Holm C, Thörne A, Hoffstedt J, Wahrenberg H. A unique defect in the regulation of visceral fat cell lipolysis in the polycystic ovary syndrome as an early link to insulin resistance. Diabetes. 2002,51(2),484-492. [CrossRef]
- Zhou MS, Wang A, Yu H. Link between insulin resistance and hypertension: What is the evidence from evolutionary biology? Diabetol Metab Syndr. 2014,6(1),12. 1. [CrossRef]
- Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unraveling the mechanism. Lancet. 2010,375(9733),2267-2277. [CrossRef]
- Chazenbalk GD, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to predipocyte formation. Steroids. 2013,78(9),920-926. 9. [CrossRef]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011,12(11),722-734. [CrossRef]
- Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annual Rev Biochem. 2012,81,715-736. 2012; 81, 715–736. [CrossRef]
- Saponaro C, Gaggini M, Carli F, Gastaldelli A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients. 2015,7(11),9453-9474. [CrossRef]
- Romacho T, Elsen M, Rohrborn D, Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol (Oxf). 2014,210(4),733-753. [CrossRef]
- Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol. 2007;192(3),585-594. [CrossRef]
- Rosenbaum D, Harber RS, Dunaif A. Insulin resistance in polycystic ovary syndrome: decreased expression of GLUT-4 glucose transporters in adipocytes. Am J Physiol. 1993,264(2 Pt 1),E197-E202. [CrossRef]
- Faulds G, Rydén M, Ek I, Wahrenberg H, Arner P. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab. 2003,88(5),2269-2273. 5. [CrossRef]
- Ek I, Arner P, Bergqvist A, Carlstrom K Wahrenberg H. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab. 1997,82(4),1147-1153. [CrossRef]
- Mannerås-Holm L, Leonhardt H, Kullberg J, Jennische E, Odén A, Holm G, Hellström M, Lönn L, Olivecrona G, Stener-Victorin E, Lönn M. Adipose tissue has aberrant morphology and function in PCOS: Enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011,96(2),E304-E311. [CrossRef]
- Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: recent advances. Mol Cell Endocrinol. 2009,301(1-2),97-103. [CrossRef]
- Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, Arlt W. Androgen generation in adipose tissue in women with simple obesity--a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5. J Endocrinol. 2004,183(2),331-342. [CrossRef]
- O’Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, Semple RK, Hebenstreit D, Dunn WB, Tomlinson JW, Arlt W. AKR1C3-Mediated Adipose Androgen Generation Drives Lipotoxicity in Women with Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2017,102(9),3327-3339. [CrossRef]
- Dumesic DA, Tulberg A, McNamara M, Grogan TR, Abbott DH, Naik R, Lu G, Chazenbalk GD. Serum Testosterone to Androstenedione Ratio Predicts Metabolic Health in Normal-Weight Polycystic Ovary Syndrome Women. J Endocr Soc. 2021,5(11),bvab158. [CrossRef]
- Fisch SC, Nikou AF, Wright EA, Phan JD, Leung KL, Grogan TR, Abbott DH, Chazenbalk GD, Dumesic DA. Precocious Subcutaneous Abdominal Stem Cell Development to Adipocytes in Normal-Weight Polycystic Ovary Syndrome Women. Fertil Steril. 2018,110(7),1367-1376. [CrossRef]
- Leung KL, Sanchita S, Pham CT, Davis BA, Okhovat M, Ding X, Dumesic P, Grogan TR, Williams KJ, Morselli M, Ma F, Carbone L, Li X, Pellegrini M, Dumesic DA, Chazenbalk GD. Dynamic changes in chromatin accessibility, altered adipogenic gene expression, and total versus de novo fatty acid synthesis in subcutaneous adipose stem cells of normal-weight polycystic ovary syndrome (PCOS) women during adipogenesis: evidence of cellular programming. Clin Epigenetics. 2020,23,12(1),181. [CrossRef]
- Spalding KL, Arner E, Westermark, PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffsted J, Naslund, E, Britton, T, Concha H, Hassan M, Rydén M, Frisén J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008,453(7196),783-787. [CrossRef]
- Tandon P, Wafer R, Minchin JE. Adipose morphology and metabolic disease. J Expt Biol. 2018,221(Pt Suppl 1),jeb164970. [CrossRef]
- Arner E, Westermark PO, Spalding, KL, Britton T, Ryden M, Frisen J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010,59(1),105-109. [CrossRef]
- Longo M, Raciti GA, Zatterale F, Parrillo L, Desiderio A, Spinelli R, Hammarstedt A, Hedjazifar S, Hoffmann JM, Nigro C, Mirra P, Fiory F, Formisano P, Miele C, Smith U, Beguinot F. Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity. Diabetologia. 2018,61(2),369-380. [CrossRef]
- Nouws J, Fitch M, Mata M, Santoro N, Galuppo B, Kursawe R, Narayan D, Vash-Margita A, Pierpont B, Shulman GI, Hellerstein M, Caprio S. Altered In Vivo Lipid Fluxes and Cell Dynamics in Subcutaneous Adipose Tissues Are Associated with the Unfavorable Pattern of Fat Distribution in Obese Adolescent Girls. Diabetes. 2019,68(6),1168-77. [CrossRef]
- Umano GR, Shabanova V, Pierpont B, Mata M, Nouws J, Tricò D, Galderisi A, Santoro N, Caprio S. A low visceral fat proportion, independent of total body fat mass, protects obese adolescent girls against fatty liver and glucose dysregulation: a longitudinal study. Int J Obes (Lond). 2019,43(4),673-682. [CrossRef]
- Brennan KM, Kroener LL, Chazenbalk GD, Dumesic DA. Polycystic Ovary Syndrome: Impact of Lipotoxicity on Metabolic and Reproductive Health. Obstet Gynecol Surv. 2019,74(4),223-231. [CrossRef]
- Virtue S, Vidal-Puig A. It's not how fat you are, it's what you do with it that counts. PLoS Biol. 2008, 6(9),e237. [CrossRef]
- Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010,1801(3),209-214. [CrossRef]
- de Zegher F, Lopez-Bermejo A, Ibáñez L. Adipose tissue expandability and the early origins of PCOS. Trends Endocrinol Metab. 2009,20(9),418-423. [CrossRef]
- Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Eng J Med. 2014;371(12),1131-1141. [CrossRef]
- Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005,90(4),1929-1935. [CrossRef]
- Dokras A, Bochner M, Hollinrake E, Markham S, Vanvoorhis B, Jagasia DH. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol. 2005,106(1),131-137. [CrossRef]
- Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, legro RS, Ghazzi MN; PCOS/Troglitazone Study Group. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006,91(1),48:-53. [CrossRef]
- Fazleen NE, Whittaker M, Mamun A. Risk of metabolic syndrome in adolescents with polycystic ovarian syndrome: A systematic review and meta-analysis. Diabetes Metab Syndr. 2018,12(6),1083-1090. 1090. [CrossRef]
- Yang R, Yang S, Li R, Liu P, Qiao J, Zhang Y. Effects of hyperandrogenism on metabolic abnormalities in patients with polycystic ovary syndrome: a meta-analysis. Reprod Biol Endocrinol. 2016,14(1),67. [CrossRef]
- Gambarin-Gelwan M, Kinkhabwala SV, Schiano TD, Bodian C, Yeh HC, Futterweit W. Prevalence of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Clin Gastroenterol Hepatol. 2007,5(4),496-501. [CrossRef]
- Macut D, Tziomalos K, Božić-Antić I, Bjekić-Macut J, Katsikis I, Papadakis E, Andrić Z, Panidis D . Non-alcoholic fatty liver disease is associated with insulin resistance and lipid accumulation product in women with polycystic ovary syndrome. Hum Reprod. 2016,31(6),1347-1353. [CrossRef]
- Vassilatou E, Vassiliadi DA, Salambasis K, Lazaridou H, Koutsomitopoulos N, Kelekis N, Kassanos D, Hadjidakis D, Dimitriadis G. Increased prevalence of polycystic ovary syndrome in premenopausal women with nonalcoholic fatty liver disease. Eur J Endocrinol. 2015,173(6),739-747. [CrossRef]
- Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004,114(2),147-152. 2. [CrossRef]
- Vassilatou E, Lafoyianni S, Vryonidou A, Ioannidis D, Kosma L, Katsoulis K, Papavassiliou E, Tzavara I . Increased androgen bioavailability is associated with non-alcoholic fatty liver disease in women with polycystic ovary syndrome. Hum Reprod. 2010,25(1),212-220. [CrossRef]
- Petta S, Ciresi A, Bianco J, Geraci V, Boemi R, Galvano L, Magliozzo F, Merlino G, Craxì A, Giordano C. Insulin resistance and hyperandrogenism drive steatosis and fibrosis risk in young females with PCOS. PLoS One. 2017,12(11),e0186136. [CrossRef]
- Jones H, Sprung VS, Pugh CJ, Daousi C, Irwin A, Aziz N, Adams VL, Thomas EL, Bell JD, Kemp GJ, Cuthbertson DJ. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012,97(10),3709-3716. 3709. [CrossRef]
- Schwartz SM, Kemnitz JW, Howard CF Jr. Obesity in free-ranging rhesus macaques. Int J Obes Relat Metab Disord. 1993,17(1),1-9. [PubMed]
- Raboin MJ, Letaw J, Mitchell AD, Toffey D, McKelvey J, Roberts CT Jr, Curran JE, Vinson A. Genetic Architecture of Human Obesity Traits in the Rhesus Macaque. Obesity (Silver Spring). 2019,27(3),479-488. [CrossRef]
- Kemnitz JW, Goy RW, Flitsch TJ, Lohmiller JJ, Robinson JA. Obesity in male and female rhesus monkeys: fat distribution, glucoregulation, and serum androgen levels. J Clin Endocrinol Metab. 1989,69(2),287-93. [CrossRef] [PubMed]
- Pound LD, Kievit P, Grove KL. The nonhuman primate as a model for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2014;21(2),89-94. [CrossRef]
- True C, Abbott DH, Roberts CT Jr, Varlamov O. Sex Differences in Androgen Regulation of Metabolism in Nonhuman Primates. Adv Exp Med Biol. 2017,1043,559-574. [CrossRef]
- Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 2003,58(3),212-219. [CrossRef]
- ishop CV, Takahashi D, Mishler E, Slayden OD, Roberts CT, Hennebold J, True C. Individual and combined effects of 5-year exposure to hyperandrogenemia and Western-style diet on metabolism and reproduction in female rhesus macaques. Hum Reprod. 2021,36(2),444-454. [CrossRef]
- Brown E, Ozawa K, Moccetti F, Vinson A, Hodovan J, Nguyen TA, Bader L, López JA, Kievit P, Shaw GD, Chung DW, Osborn W, Fu X, Chen J, Lindner JR. Arterial Platelet Adhesion in Atherosclerosis-Prone Arteries of Obese, Insulin-Resistant Nonhuman Primates. J Am Heart Assoc. 2021,10(9),e019413. [CrossRef]
- Newman LE, Testard C, DeCasien AR, Chiou KL, Watowich MM, Janiak MC, Pavez-Fox MA, Sanchez Rosado MR, Cooper EB, Costa CE, Petersen RM, Montague MJ, Platt ML, Brent LJN, Snyder-Mackler N, Higham JP. The biology of aging in a social world: insights from free-ranging rhesus macaques. bioRxiv [Preprint]. 2023 Jan 29:2023.01.28.525893. [CrossRef]
- Eisner JR, Dumesic DA, Kemnitz JW, Colman RJ, Abbott DH. Increased adiposity in female rhesus monkeys exposed to androgen excess during early gestation. Obes Res. 2003,11(2):279-286. [CrossRef]
- Barnett DK, Abbott DH. Reproductive adaptations to a large-brained fetus open a vulnerability to anovulation similar to polycystic ovary syndrome. Am J Hum Biol. 2003,15(3),296-319. [CrossRef]
- Bruns CM, Baum ST, Colman RJ, Dumesic DA, Eisner JR, Jensen MD, Whigham LD, Abbott DH. Prenatal androgen excess negatively impacts body fat distribution in a nonhuman primate model of polycystic ovary syndrome. Int J Obes (Lond). 2007,31(10),1579-1585. 10. [CrossRef]
- Zhou R, Bruns CM, Bird IM, Kemnitz JW, Goodfriend TL, Dumesic DA, Abbott DH. Pioglitazone improves insulin action and normalizes menstrual cycles in a majority of prenatally androgenized female rhesus monkeys. Reprod Toxicol. 2007,23(3),438-448. [CrossRef]
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005,11(4),357-374. [CrossRef]
- Keller E, Chazenbalk GD, Aguilera P, Madrigal V, Grogan T, Elashoff D, Dumesic DA, Abbott DH. Impaired preadipocyte differentiation into adipocytes in subcutaneous abdominal adipose of PCOS-like female rhesus monkeys. Endocrinology. 2014,155(7),2696-2703. [CrossRef]
- Barrett ES, Hoeger KM, Sathyanarayana S, Abbott DH, Redmon JB, Nguyen RHN, Swan SH. Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. J Dev Orig Health Dis. 2018,9(3),307-314. 2018; 9, 3, 307–314. [CrossRef]
- Sánchez-Ferrer ML, Mendiola J, Hernández-Peñalver AI, Corbalán-Biyang S, Carmona-Barnosi A, Prieto-Sánchez MT, Nieto A, Torres-Cantero AM. Presence of polycystic ovary syndrome is associated with longer anogenital distance in adult Mediterranean women. Hum Reprod. 2017,32(11),2315-2323. 11. [CrossRef]
- ata B, Mimouni NEH, Barbotin AL, Malone SA, Loyens A, Pigny P, Dewailly D, Catteau-Jonard S, Sundström-Poromaa I, Piltonen TT, Dal Bello F, Medana C, Prevot V, Clasadonte J, Giacobini P. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018,24(6):834-846. [CrossRef]
- Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009,71(9):776-784. [CrossRef]
- Susa JB, Neave C, Sehgal P, Singer DB, Zeller WP, Schwartz R. Chronic hyperinsulinemia in the fetal rhesus monkey. Effects of physiologic hyperinsulinemia on fetal growth and composition. Diabetes. 1984,33(7):656-660. [CrossRef]
- Warren WC, Harris RA, Haukness M, Fiddes IT, Murali SC, Fernandes J, Dishuck PC, Storer JM, Raveendran M, Hillier LW, Porubsky D, Mao Y, Gordon D, Vollger MR, Lewis AP, Munson KM, DeVogelaere E, Armstrong J, Diekhans M, Walker JA, Tomlinson C, Graves-Lindsay TA, Kremitzki M, Salama SR, Audano PA, Escalona M, Maurer NW, Antonacci F, Mercuri L, Maggiolini FAM, Catacchio CR, Underwood JG, O'Connor DH, Sanders AD, Korbel JO, Ferguson B, Kubisch HM, Picker L, Kalin NH, Rosene D, Levine J, Abbott DH, Gray SB, Sanchez MM, Kovacs-Balint ZA, Kemnitz JW, Thomasy SM, Roberts JA, Kinnally EL, Capitanio JP, Skene JHP, Platt M, Cole SA, Green RE, Ventura M, Wiseman RW, Paten B, Batzer MA, Rogers J, Eichler EE. Sequence diversity analyses of an improved rhesus macaque genome enhance its biomedical utility. Science. 2020,370(6523),eabc6617. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




