Submitted:
17 August 2023
Posted:
21 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. The analytes
2.2. Chemicals
2.3. Chromatographic equipment
2.4. Chromatographic conditions
2.5. Pharmacokinetic in silico studies
2.6. TLC-based bioatographic assay towards the AChE inhibitory activity
2.7. Molecular docking procedure
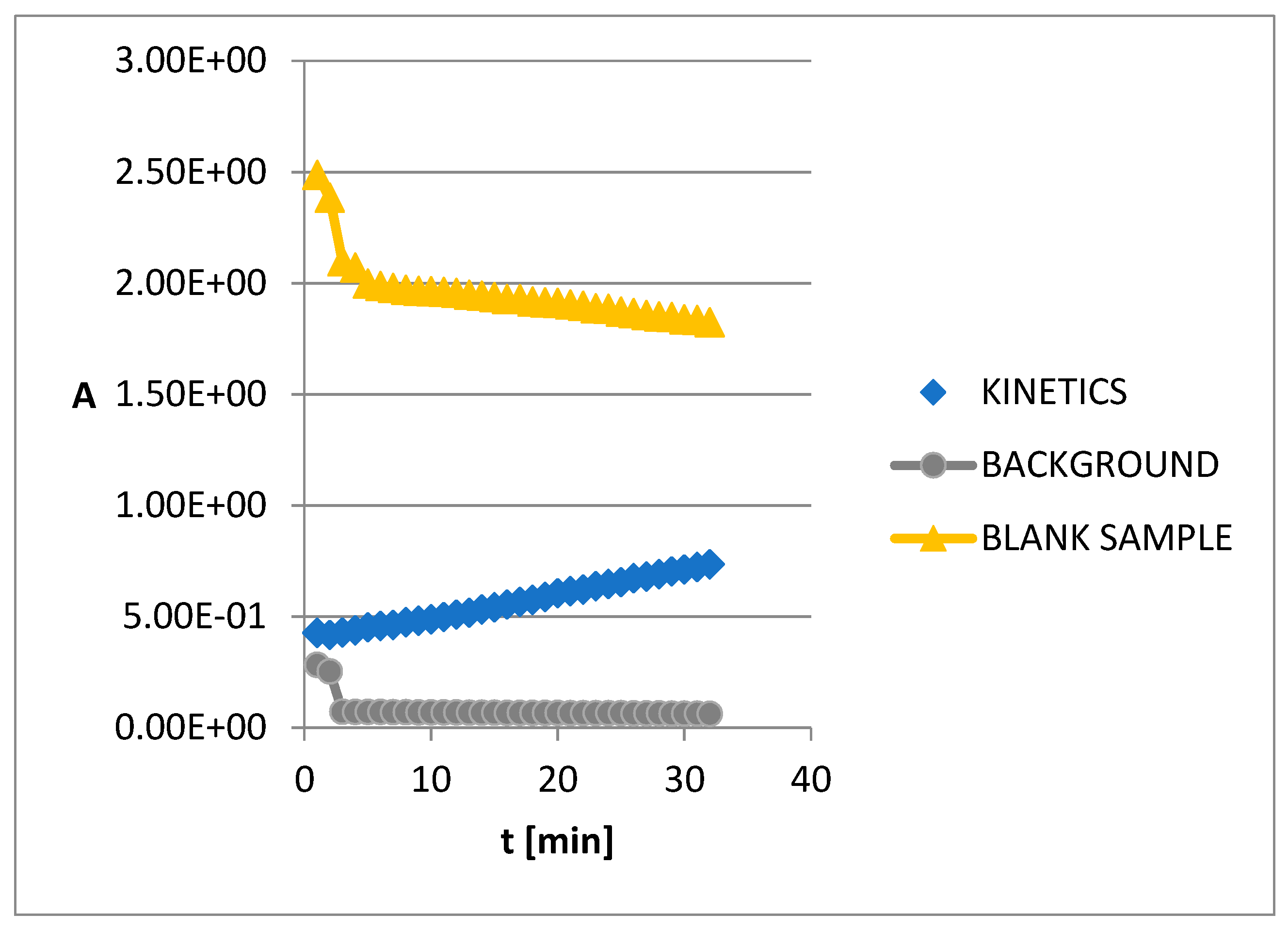
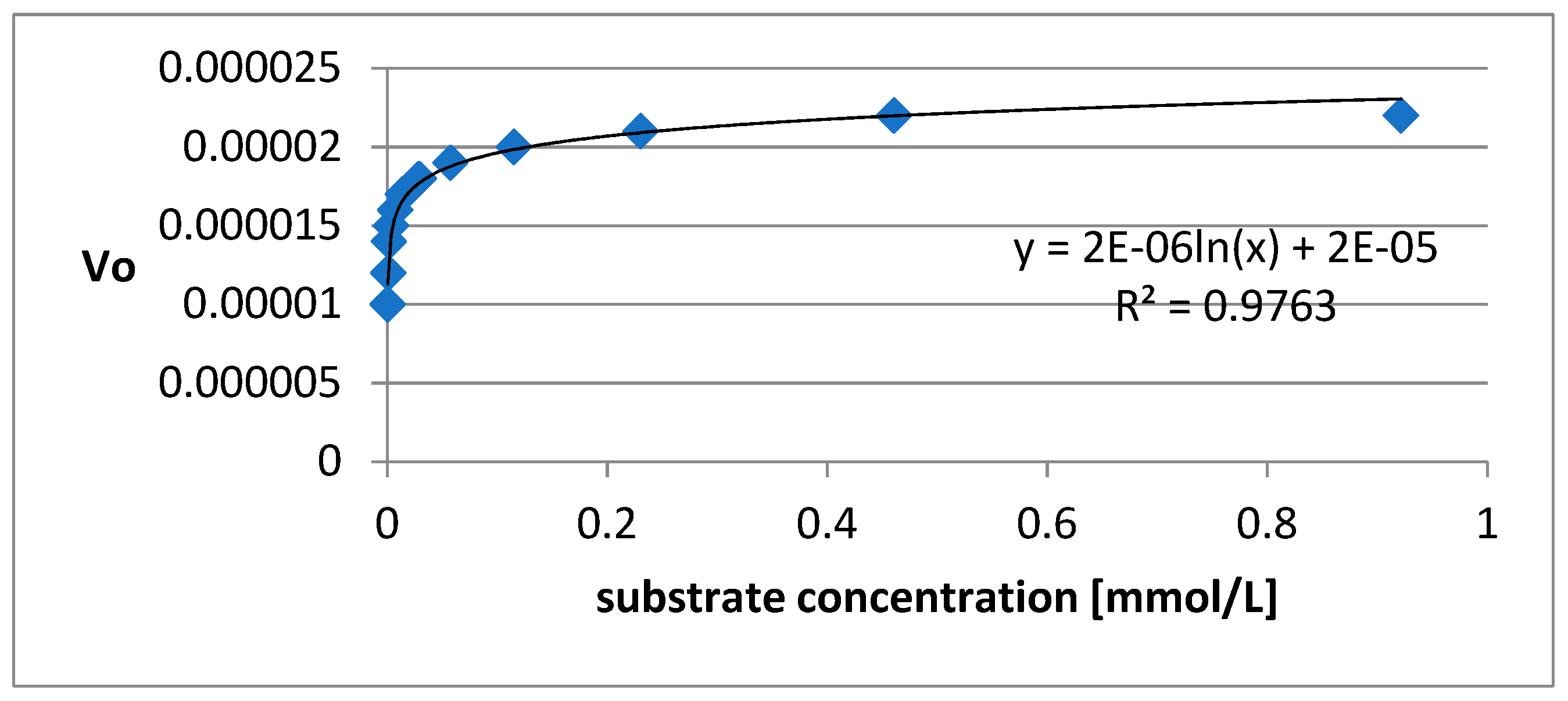
2.8. Kinetics of AChE inhibition
2.9. Toxicity assay
3. Results
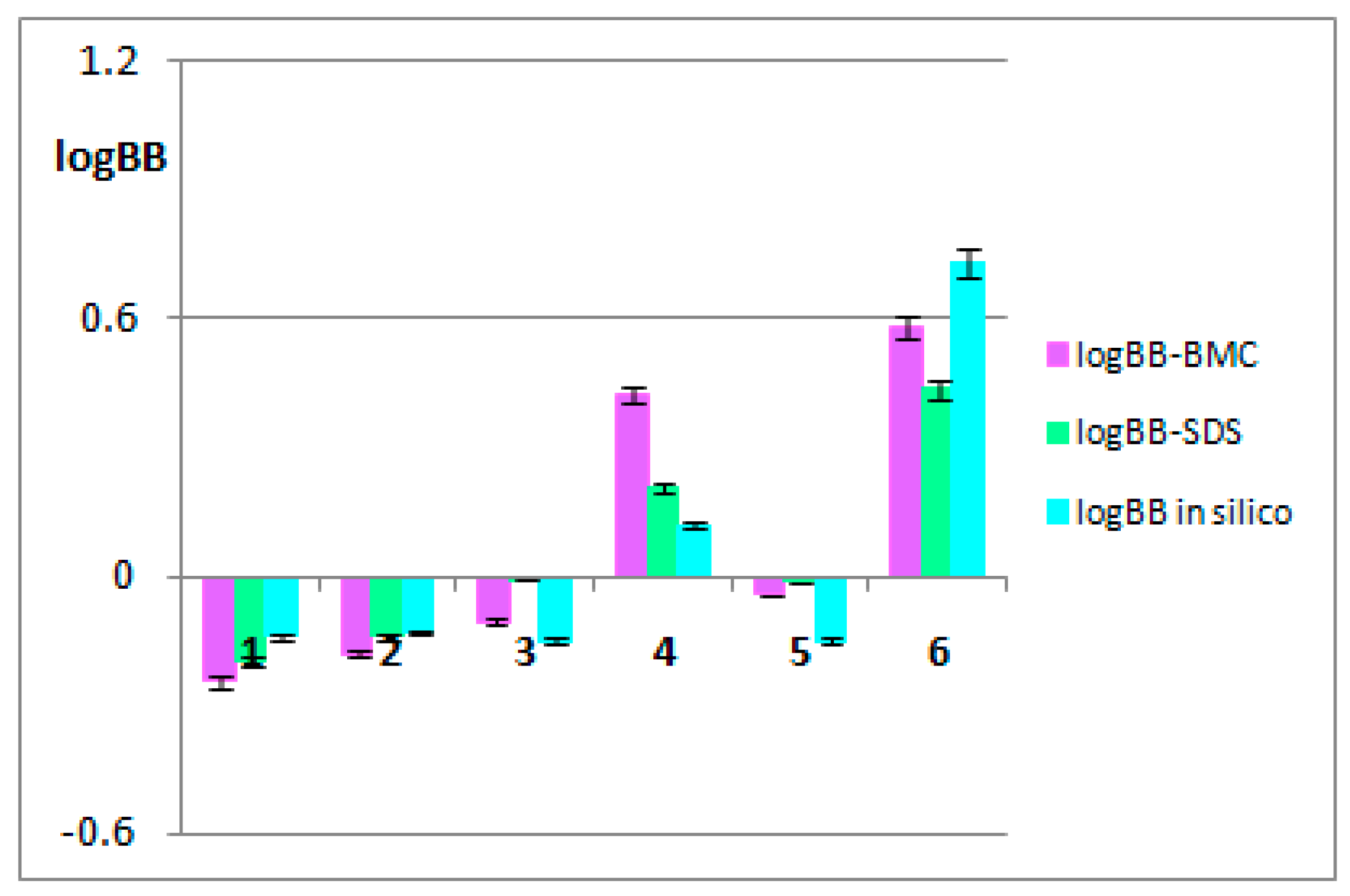
3.1. The BBB-pharmacokinetic in silico studies
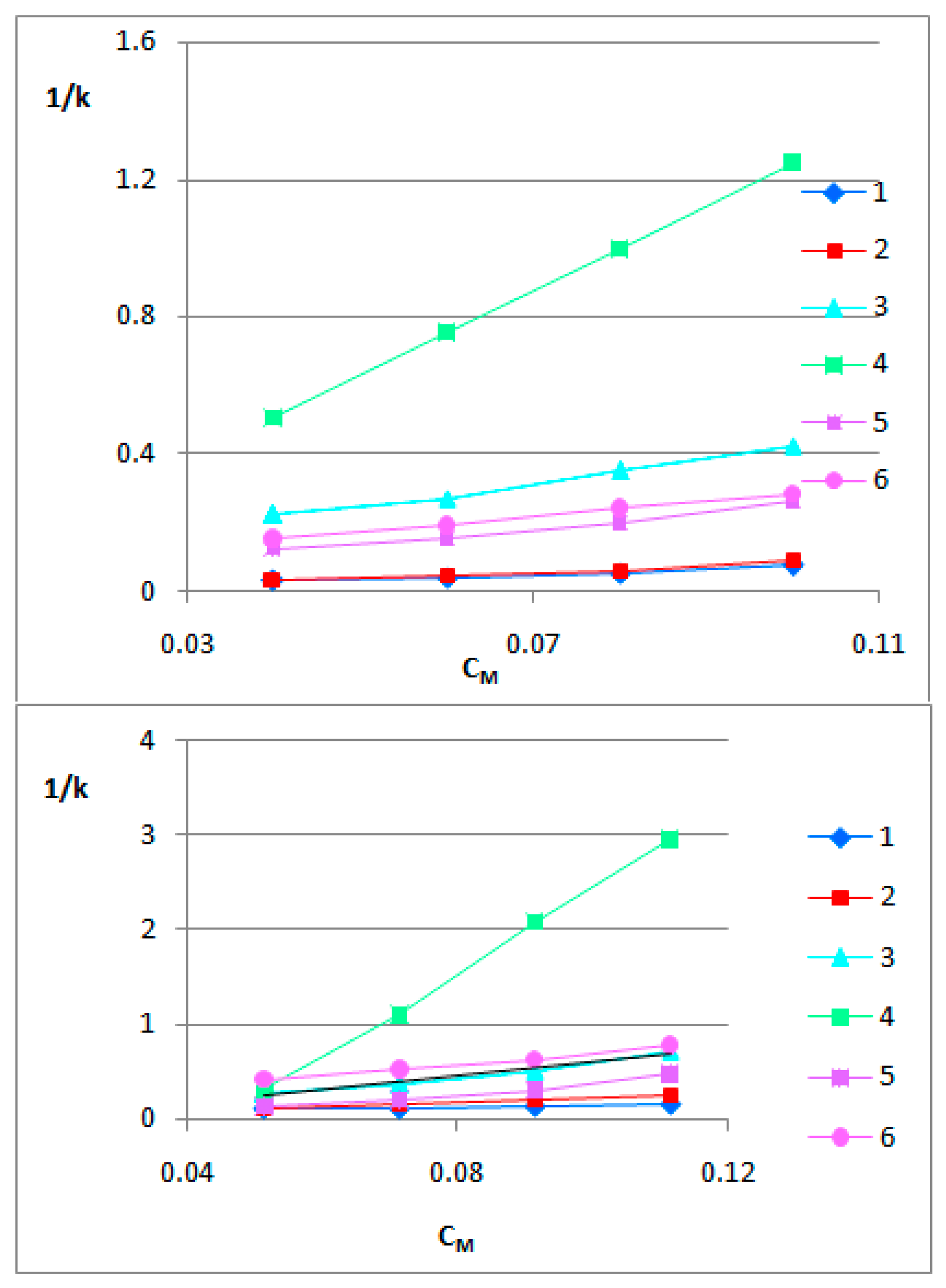
3.2. The BBB-biomimetic studies
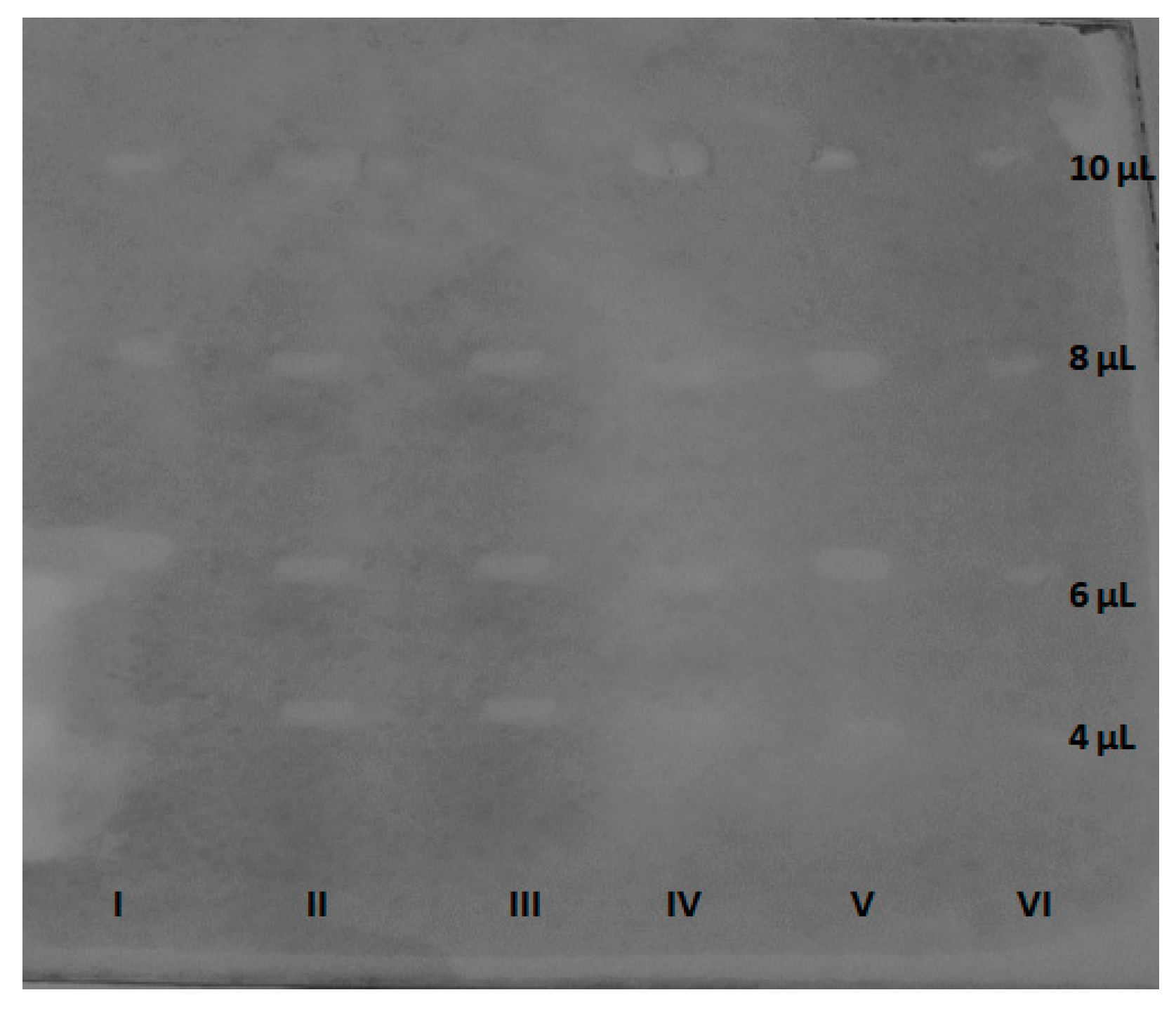
3.3. AChE inhibitory activity of the selected saponins in the TLC-bioautography assay
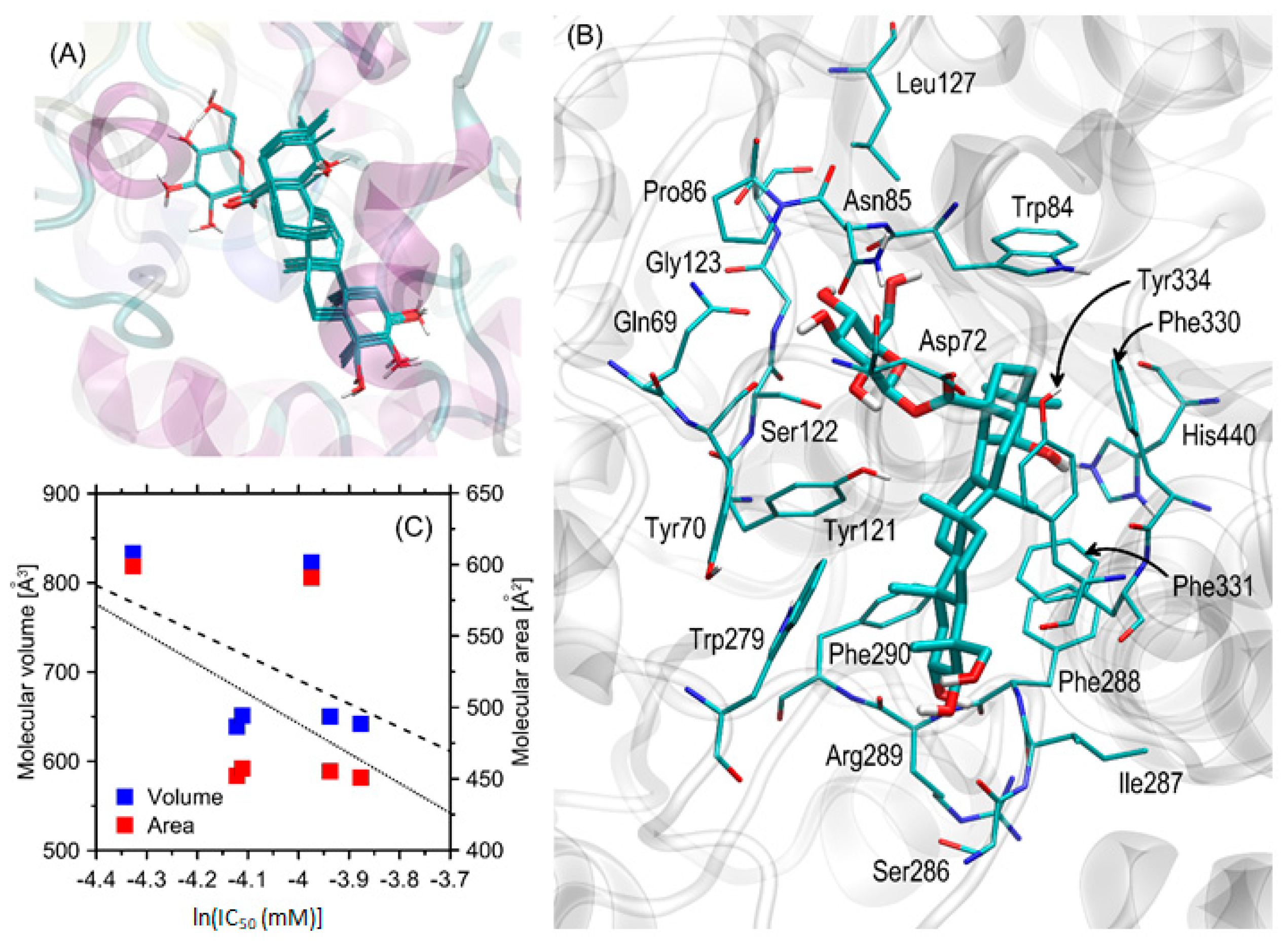
3.4. Molecular docking studies
3.5. The kinetics of AChE inhibition
3.6. In silico prediction of acute and chronic toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The WHO brochure “Global action plan on the public health response to dementia 2017 – 2025”, 2017; ISBN: 978-92-4-151348-7. Available online: https://www.who.int/publications/i/item/global-action-plan-on-the-public-health-response-to-dementia-2017---2025 (accessed on 3 October 2023).
- Wiendl, H.; Meuth, S.G. Pharmacological Approaches to Delaying Disability Progression in Patients with Multiple Sclerosis. Drugs 2015, 75, 947–977. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Cherubini, A.; Volpato, S.; et al. Acetyl-cholinesterase-inhibitors slow cognitive decline and decrease overall mortality in older patients with dementia. Sci Rep. 2022, 16, 12214. [Google Scholar] [CrossRef] [PubMed]
- Smyrska-Wieleba, N.; Mroczek, T. Natural Inhibitors of Cholinesterases: Chemistry, Structure–Activity and Methods of Their Analysis. Int. J. Mol. Sci. 2023, 24, 2722. [Google Scholar] [CrossRef]
- Coelho dos Santos, T.; Mota Gomes, T.; Araújo Serra Pinto, B.; et al. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer's Disease Therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Atanasova, M.; Georgiev, B.; et al. The Amaryllidaceae alkaloids: An untapped source of acetylcholinesterase inhibitors. Phytochem. Rev. 2022, 21, 1415–1443. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. Phytochemical and pharmacological potential of Medicago sativa: A review. Pharm. Biol. 2011, 49, 211–220. [Google Scholar] [CrossRef]
- Liu, X.-G.; Sun, Y.-Q.; Bian, J.; et al. Neuroprotective effects of triterpenoid saponins from Medicago sativa L. against H2O2-induced oxidative stress in SH-SY5Y cells. Bioorg. Chem. 2019, 83, 468–476. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Wang, L.; Liu, X.; Chang, Q.; Guo, Z.; Liao, Y.; Pan, R.; Fan, T.P. Memory-Enhancing Effects of the Crude Extract of Polygala tenuifolia on Aged Mice. Evid Based Complement Alternat Med. 2014, 2014, 392324 htpps:. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Yu, S.; et al. Triterpenoid Saponins with Neuroprotective Effects from the Roots of Polygala tenuifolia. Planta Med. 2008, 74, 133–141. [Google Scholar] [CrossRef]
- Son, I.H.; Park, Y.H.; Lee, S.I.; et al. Neuroprotective Activity of Triterpenoid Saponins from Platycodi radix Against Glutamate-induced Toxicity in Primary Cultured Rat Cortical Cells. Molecules 2007, 12, 1147–1152. [Google Scholar] [CrossRef]
- Yaidikar, L.; Thakur, S. Arjunolic acid, a pentacyclic triterpenoidal saponin of Terminalia arjuna bark protects neurons from oxidative stress associated damage in focal cerebral ischemia and reperfusion. Pharmacol. Rep. 2015, 67, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Toropov, A.A.; Toropova, A.P.; Beeg, M.; et al. QSAR model for blood-brain barrier permeation. J. Pharmacol. Toxicol. Meth. 2017, 88, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Van Bree, J.B.M.M.; De Boer, A.G.; Danhof, M.; et al. Drug transport across the blood-brain barrier. Pharm. Weekbl. 1992, 14, 305–310. [Google Scholar] [CrossRef]
- Iyer, M.; Mishra, R.; Han, Y.; et al. Predicting Blood–Brain Barrier Partitioning of Organic Molecules Using Membrane-Interaction QSAR Analysis. Pharm. Res. 2002, 19, 1611–1621. [Google Scholar] [CrossRef]
- Ekins, S.; Waller, C.L.; Swaan, P.W.; et al. Progress in predicting human ADME parameters in silico. J. Pharmacol. Toxicol. Meth 2000, 44, 251–272. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Mroczek, T. Application of hydrostatic CCC-TLC-HPLC-ESI-TOF-MS for the bioguided fractionation of anticholinesterase alkaloids from Argemone mexicana L. roots. Anal. Bioanal. Chem. 2015, 407, 2581–2589. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; et al. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J Cheminform. 2012, 13, 17 htpps. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 30, 455–461. [Google Scholar] [CrossRef]
- Tarabasz, D.; Szczeblewski, P.; Laskowski, T.; et al. The Distribution of Glucosinolates in Different Phenotypes of Lepidium peruvianum and Their Role as Acetyl- and Butyrylcholinesterase Inhibitors-In Silico and In Vitro Studies. Int. J. Mol. Sci. 2022, 27, 4858. [Google Scholar] [CrossRef]
- Ellman, G.L.; Lourtney, D.K.; Andres, V.; Gmelin, G. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Baranowska-Wójcik, E. Terpenes and Phenylpropanoids as Acetyl- and Butyrylcholinesterase Inhibitors: A Comparative Study. Curr Alzheimer Res. 2019, 16, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.P. Critical compilation of solute-micelle binding constants and related parameters from micellar liquid chromatographic measurements. Anal. Chim. Acta 1990, 231, 237–247. [Google Scholar] [CrossRef]
- Janicka, M.; Śliwińska, A.; Sztanke, M.; et al. Combined Micellar Liquid Chromatography Technique and QSARs Modeling in Predicting the Blood–Brain Barrier Permeation of Heterocyclic Drug-like Compounds. Int. J. Mol. Sci. 2022, 23, 15887. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.; Grimley, E.J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev. 2009, 21, CD003120. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.S.; Lee, D.I. Potential effects of microglial activation induced by ginsenoside Rg3 in rat primary culture: Enhancement of type a macrophage scavenger receptor expression. Arch. Pharm. Res. 2005, 28, 1164–1169. [Google Scholar] [CrossRef]
- Chen, F.; Eckman, E.A.; Eckman, C.B. Reductions in levels of the Alzheimer's amyloid β peptide after oral administration of ginsenosides. FASEB J. 2006, 20, 1269–1271. [Google Scholar] [CrossRef]
- Li, N.; Liu, B.; Dluzen, D.E.; Jin, Y. Protective effects of ginsenoside Rg2 against glutamateinduced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2007, 111, 458–463. [Google Scholar] [CrossRef]
- Joo, S.S.; Yoo, Y.M.; Ahn, B.W.; et al. Prevention of inflammation-mediated neurotoxicity by Rg3 and its role in microglial activation. Biol. Pharm. Bull. 2008, 31, 1392–1396. [Google Scholar] [CrossRef]
- Shieh, P.C.; Tsao, C.W.; Li, J.S.; et al. Role of pituitary adenylate cyclase-activating polypeptide (PACAP) in the action of ginsenoside Rh2 against betaamyloid-induced inhibition of rat brain astrocytes. Neurosci. Lett. 2008, 434, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, I.; Yasui, K. Wogonin inhibits inducible prostaglandin E2 production in macrophages. Eur. J. Pharmacol. 2000, 406, 477–481. [Google Scholar] [CrossRef]
- Park, B.K.; Heo, M.Y.; Park, H.; et al. Inhibition of TPA-induced cyclooxygenase-2 expression and skin inflammation in mice by wogonin, a plant flavone from Scutellaria radix. Eur. J. Pharmacol. 2001, 425, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Hayasaka, S.; Zhang, X.Y.; et al. Effects of baicalein, baicalin, and wogonin on interleukin-6 and interleukin-8 expression, and nuclear factor-kb binding activities induced by interleukin-1beta in human retinal pigment epithelial cell line. Exp. Eye Res. 2003, 77, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.; Lee, H.; Kang, S.S.; et al. Flavonoid baicalein attenuates activationinduced cell death of brain microglia. J. Pharmacol. Exp. Ther. 2003, 305, 638–645. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Bosetti, C. Diet and cancer risk in Mediterranean countries. Hungar. Med. J. 2007, 1, 13–23. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Lagiou, P.; Kuper, H.; et al. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 869–873. [Google Scholar]
- Visioli, F.; Grande, S.; Bogani, P.; et al. The role of antioxidant in the Mediterranean diets: Focus on cancer. Eur. J. Cancer Prev. 2004, 13, 337–343. [Google Scholar] [CrossRef]
- Ninfali, P.; Mea, G.; Giorgini, S.; et al. Antioxidant capacity of vegetables, spices, and dressings relevant to nutrition. Brit. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef]
- dos Santos-Neto, L.L.; de Vilhena Toledo, M.A.; Medeiros-Souza, P.; et al. The use of herbal medicine in Alzheimer’s disease--A systematic review. Evid.-Based Complement. Altern. Med. 2006, 3, 441–445. [Google Scholar] [CrossRef]
- Bozin, B.; Mika-Dukic, N.; Samojlik, I.; et al. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food. Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.; Tai, J. Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol. Rep. 2007, 17, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Imanshahidi, M.; Hosseinzadeh, H. The pharmacological effects of Salvia species on the central nervous system. Phytother. Res. 2006, 20, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.U.; Nizami, Q.; Asiaf, A.; et al. Anticonvulsant activity of methanolic and aqueous extracts of Melissa parviflora in experimentally induced Swiss albino mice. EXCLI J. 2012, 11, 1–6. [Google Scholar] [PubMed]
- Hosseinzadeh, H.; Ramezani, M.; Shafaei, H.; et al. Anticonvulsant effect of Berberis integerrima L. root extracts in mice. J. Acupunct. Meridian Stud. 2013, 6, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ya'u, J.; Yaro, A.H.; Malami, S.; et al. Anticonvulsant activity of aqueous fraction of Carissa edulis root bark. Pharm. Biol. 2015, 53, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Sankari, M.; Chitra, V.; Silambujanaki, P.; et al. Anticonvulsant activity of ethanolic extract of Aegle marmelos (leaves) in mice. Int. J. Pharmtech Res. 2010, 2, 640–643. [Google Scholar]
- Anaka, O.N.; Ozolua, R.I.; Ikpefan, E.O.; et al. Anticonvulsant activity of the aqueous extract of Allium cepa L.(Amaryllidaceae) in rats and mice. J. Pharm. Biores. 2014, 11, 1–7. [Google Scholar] [CrossRef]
- Showraki, A.; Emamghoreishi, M.; Oftadegan, S. Anticonvulsant Effect of the Aqueous Extract and Essential Oil of Carum Carvi L. Seeds in a Pentylenetetrazol Model of Seizure in Mice. Iran. J. Med. Sci. 2016, 41, 200–208. [Google Scholar]
- Amabeoku, G.J.; Oluchi, N.M.; Davids, T.; et al. Evaluation of the anticonvulsant activity of the leaf methanol extract of Crassula arborescens (Mill.) Willd. (Crassulaceae) in mice. J. Pharm. Pharmacol. 2014, 2, 393–403. [Google Scholar]
- Chaulya, N.C.; Haldar, P.K.; Mukherjee, A. Antidiabetic activity of methanol extract of rhizomes of Cyperus tegetum Roxb (Cyperaceae). Acta Pol. Pharm. 2011, 68, 989–992. [Google Scholar] [PubMed]
- Chinchawade, A.B.; Deshmukh, D.B.; Gaikwad, D.D.; et al. Anticonvulsant Activity of Chloroform Extract of Bark and Root of Erythrina variegata L. Int. J. Pharm. Clin. Res. 2013, 5, 23–25. [Google Scholar]
- Nishanthi, N.; Mohanambal, E.; Devdass, G.; et al. Anticonvulsant Activity of Peperomia tetraphylla (G. Forst., Hook. & Arn.). Int. J. Nov. Trends Pharm. Sci. 2012, 2, 35–38. [Google Scholar]
- Barua, C.C.; Begum, S.A.; Barua, A.G.; et al. Anxiolytic and anticonvulsant activity of methanol extract of leaves of Alternanthera brasiliana (L.) Kuntze (Amaranthaceae) in laboratory animals. Indian J. Exp. Biol. 2013, 51, 450–457. [Google Scholar]
- Khan, I.; Karim, N.; Ahmad, W.; et al. GABA-A Receptor Modulation and Anticonvulsant, Anxiolytic, and Antidepressant Activities of Constituents from Artemisia indica Linn. Evid.-based Complement. Altern. Med. 2016, 2016, 1215393. [Google Scholar] [CrossRef]
- Li, D.-Q.; Zhou, L.; Wang, D.; et al. Neuroprotective oleanane triterpenes from the roots of Bupleurum chinense. Bioorg. Med. Chem. Lett. 2016, 26, 1594–1598. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, H.; Jin, R.; et al. Antiepileptic activity of total triterpenes isolated from Poriacocos is mediated by suppression of aspartic and glutamic acids in the brain. Pharm. Biol. 2016, 54, 2528–2535. [Google Scholar] [CrossRef]
- Srivastava, G.; Sandeep, G.A.; et al. Transcriptome analysis and functional characterization of oxidosqualene cyclases of the arjuna triterpene saponin pathway. Plant Sci. 2020, 292, 110382. [Google Scholar] [CrossRef]
- Mohanty, I.R.; Borde, M.; Kumar, S.; et al. Dipeptidyl peptidase IV Inhibitory activity of Terminalia arjuna attributes to its cardioprotective effects in experimental diabetes: In silico, in vitro and in vivo analyses. Phytomedicine 2019, 57, 158–165. [Google Scholar] [CrossRef]
- Pawar, R.S.; Bhutani, K.K. Effect of oleanane triterpenoids from Terminalia arjuna – a cardioprotective drug on the process of respiratory oxyburst. Phytomedicine 2005, 12, 391–393. [Google Scholar] [CrossRef]
- Kapoor, D.; Vijayvergiya, R.; Dhawan, V. Terminalia arjuna in coronary artery disease: Ethnopharmacology, pre-clinical, clinical & safety evaluation. J. Ethnopharmacol. 2014, 155, 1029–1045. [Google Scholar] [PubMed]
- Pugazhendhi, A.; Shafreen, R.B.; Devi, K.P.; et al. Assessment of antioxidant, anticholinesterase and antiamyloidogenic effect of Terminalia chebula, Terminalia arjuna and its bioactive constituent 7-Methyl gallic acid – An in vitro and in silico studies. J. Mol. Liq. 2018, 257, 69–81. [Google Scholar] [CrossRef]
- Gupta, D.; Kumar, M. Evaluation of in vitro antimicrobial potential and GC-MS analysis of Camellia sinensis and Terminalia arjuna. Biotechnol. Rep. (Amst). 2016, 30, 19–25 hptts://. [Google Scholar] [CrossRef]
- Mandal, S.; Patra, A.; Samanta, A.; et al. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pac. J. Trop. Biomed. 2013, 12, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Dube, N.; Nimgulkar, C.; Bharatraj, D.K. Validation of therapeutic anti-inflammatory potential of Arjuna Ksheera Paka – A traditional Ayurvedic formulation of Terminalia arjuna. J. Tradit. Complement. Med. 2017, 7, 414–420. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Ahmad, S.; Gautam, B.; et al. Terminalia arjuna, a herbal remedy against environmental carcinogenicity: An in vitro and in vivo study. Egypt. J. Medical Hum. Genet. 2014, 15, 61–67. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Pal, P.K.; Ghosh, A.K.; et al. Aqueous bark extract of Terminalia arjuna protects against cadmium-induced hepatic and cardiac injuries in male Wistar rats through antioxidative mechanisms. Food Chem. Toxicol. 2019, 124, 249–264. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizojiri, K. Drug-protein binding and blood-brain barrier permeability. J Pharmacol. Exp. Ther. 1999, 288, 912–918. [Google Scholar]
- Platts, J.A.; Abraham, M.H.; Zhao, Y.H.; et al. Correlation and prediction of a large blood-brain distribution data set--an LFER study. Eur. J. Med. Chem. 2001, 36, 719–730. [Google Scholar] [CrossRef]
- Young, R.C.; Mitchell, R.C.; Brown, T.H.; et al. Development of a new physicochemical model for brain penetration and its application to the design of centrallyacting H2 receptor histamine antagonists. J. Med. Chem. 1988, 31, 656–671. [Google Scholar] [CrossRef]
- Fantini, S.; Sassaroli, A.; Tgavalekos, K.T.; et al. Cerebral blood flow and autoregulation: Current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 2016, 3, 031411. [Google Scholar] [CrossRef]
- Xingrong, L.; Smith, B.J.; Chen, C.; et al. Use of a Physiologically Based Pharmacokinetic Model to Study the Time to Reach Brain Equilibrium: An Experimental Analysis of the Role of Blood-Brain Barrier Permeability, Plasma Protein Binding, and Brain Tissue Binding. J. Pharmacol. Exp. Ther. 2005, 313, 1254–1262. [Google Scholar] [CrossRef]
- Kalvass, J.C.; Maurer, T.S.; Pollack, G.M. Use of plasma and brain unbound fractions to assess the extent of brain distribution of 34 drugs: Comparison of unbound concentration ratios to in vivo p-glycoprotein efflux ratios. Drug Metab. Dispos. 2007, 35, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund-Udenaes, M.; Fridén, M.; Syvänen, S.; et al. On the rate and extent of drug delivery to the brain. Pharm. Res. 2008, 25, 1737–1750. [Google Scholar] [CrossRef] [PubMed]
- Escuder-Gilabert, L.; Molero-Monfort, M.; Villanueva-Camañas, R.M.; et al. Potential of biopartitioning micellar chromatography as an in vitro technique for predicting drug penetration across the blood–brain barrier. J. Chromatogr. B. 2004, 807, 193–201. [Google Scholar] [CrossRef]
- Martínez-Pla, J.J.; Martín-Biosca, Y.; Sagrado, S.; et al. Evaluation of the pH effect of formulations on the skin permeability of drugs by biopartitioning micellar chromatography. J. Chromatogr. A. 2004, 1047, 255–262. [Google Scholar] [CrossRef]
- Hadjmohammadi, M.; Salary, M. Biopartitioning micellar chromatography with sodium dodecyl sulfate as a pseudo α1-acid glycoprotein to the prediction of protein–drug binding. J. Chromatogr. B. 2013, 912, 50–55. [Google Scholar] [CrossRef]
- Tsopelas, F.; Danias, P.; Pappa, A.; et al. Biopartitioning micellar chromatography under different conditions: Insight into the retention mechanism and the potential to model biological processes. J. Chromatogr. A. 2020, 1621, 461027. [Google Scholar] [CrossRef]
- Dobričić, V.; Nikolic, K.; Vladimirov, S.; et al. Biopartitioning micellar chromatography as a predictive tool for skin and corneal permeability of newly synthesized 17β-carboxamide steroids. Eur. J. Pharm. Sci. 2014, 56, 105–112. [Google Scholar] [CrossRef]
- Molero-Monfort, M.; Escuder-Gilabert, L.; Villanueva-Camanas, R.M.; et al. Biopartitioning micellar chromatography: An in vitro technique for predicting human drug absorption. J. Chromatogr. B. 2001, 753, 225–236. [Google Scholar] [CrossRef]
- Escuder-Gilabert, L.; Martinez-Pla, J.J.; Sagrado, S.; et al. Biopartitioning micellar separation methods: Modelling drug absorption. J. Chromatogr. B. 2003, 797, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Escuder-Gilabert, L.; Sanchis-Mallols, J.M.; Sagrado, S.; et al. Chromatographic quantitation of the hydrophobicity of ionic compounds by the use of micellar mobile phases. J. Chromatogr. A. 1998, 823, 549–559. [Google Scholar] [CrossRef]
- Stępnik, K. Biomimetic Chromatographic Studies Combined with the Computational Approach to Investigate the Ability of Triterpenoid Saponins of Plant Origin to Cross the Blood-Brain Barrier. Int. J. Mol. Sci. 2021, 30, 3573. [Google Scholar] [CrossRef]
- Jusril, N.; Juhari, A.M.; Abu Bakar, S.; et al. Combining In Silico and In Vitro Studies to Evaluate the Acetylcholinesterase Inhibitory Profile of Different Accessions and the Biomarker Triterpenes of Centellaasiatica. Molecules 2020, 25, 3353. [Google Scholar] [CrossRef] [PubMed]
- Jamila, N.; Khairuddean, M.; Yeong, K.K.; et al. Cholinesterase inhibitory triterpenoids from the bark of Garcinia hombroniana. J. Enzym. Inhib. Med. Chem. 2014, 30, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Stavrakov, G.; Philipova, I.; Lukarski, A.; et al. Galantamine-curcumin hybrids as dual-site binding acetylcholinesterase inhibitors. Molecules 2020, 25, 3341. [Google Scholar] [CrossRef]
| No. | Name | Chemical structure |
|---|---|---|
| 1 | Arjunic acid | 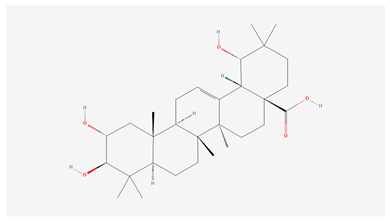 |
| 2 | Arjunolic acid | 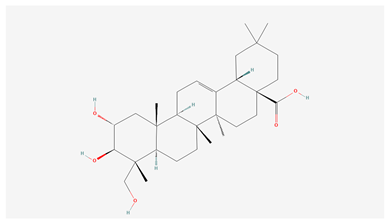 |
| 3 | Arjungenin | 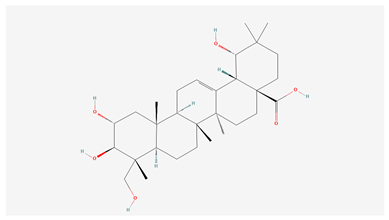 |
| 4 | Arjunglucoside I |  |
| 5 | Sericic acid |  |
| 6 | Arjunetin | 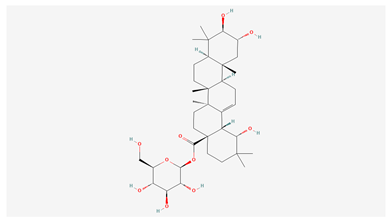 |
| Name | logBB | logPS | logPS,Fu,brain | Fu | Fb |
|---|---|---|---|---|---|
| Arjunic acid | -0.14 | -3.2 | -4.9 | 0.012 | 0.02 |
| Arjunolic acid | -0.13 | -3.2 | -5.0 | 0.012 | 0.02 |
| Arjungenin | -0.15 | -2.9 | -4.6 | 0.016 | 0.02 |
| Arjunglucoside I | 0.12 | -2.9 | -4.4 | 0.050 | 0.04 |
| Sericic acid | -0.15 | -2.9 | -4.6 | 0.016 | 0.02 |
| Arjunetin | 0.73 | -2.3 | -4.3 | 0.051 | 0.01 |
| Name | IC50 [mg/mL] | IC50 [mM] |
|---|---|---|
| 1. arjunic acid | 10.12 | 0.0207 |
| 2. arjunolic acid | 7.92 | 0.0162 |
| 3. arjungenin | 9.83 | 0.0195 |
| 4. arjunglucoside I | 8.78 | 0.0132 |
| 5. sericic acid | 8.3 | 0.0164 |
| 6. arjunetin | 12.23 | 0.0188 |
| Organism | Duration | Endpoint | Predicted value [mg/L] |
|---|---|---|---|
| Acute effects | |||
| Fish | 96 h | LC50 | 566.412 |
| Daphnia | 48 h | 1364.175 | |
| Green algae | 96 h | EC50 | 721.889 |
| Chronic effects | |||
| Fish | ChV | 56.863 | |
| Daphnia | 1385.105 | ||
| Green algae | 118.412 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





