Submitted:
20 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
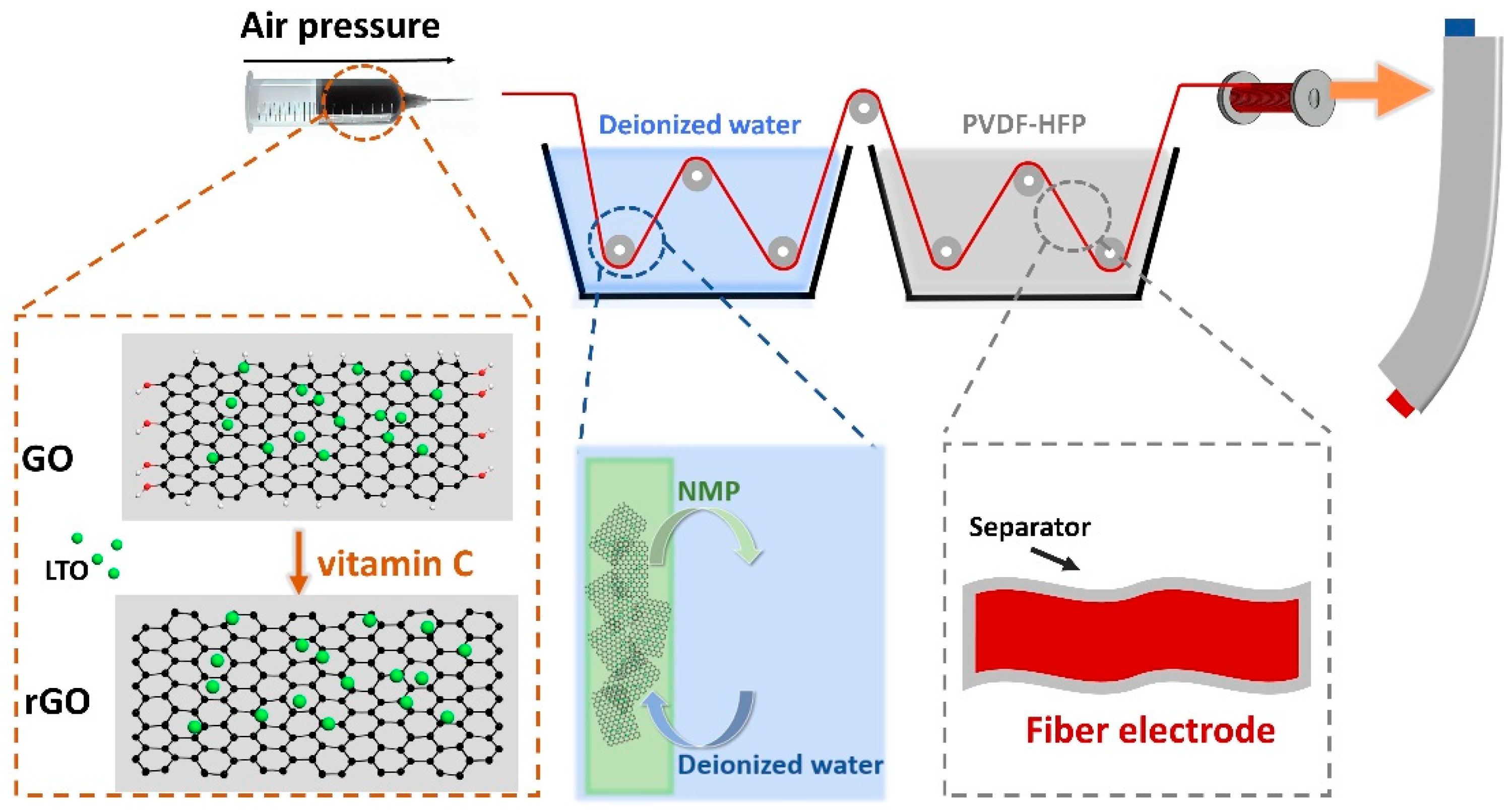
2. Materials and Methods
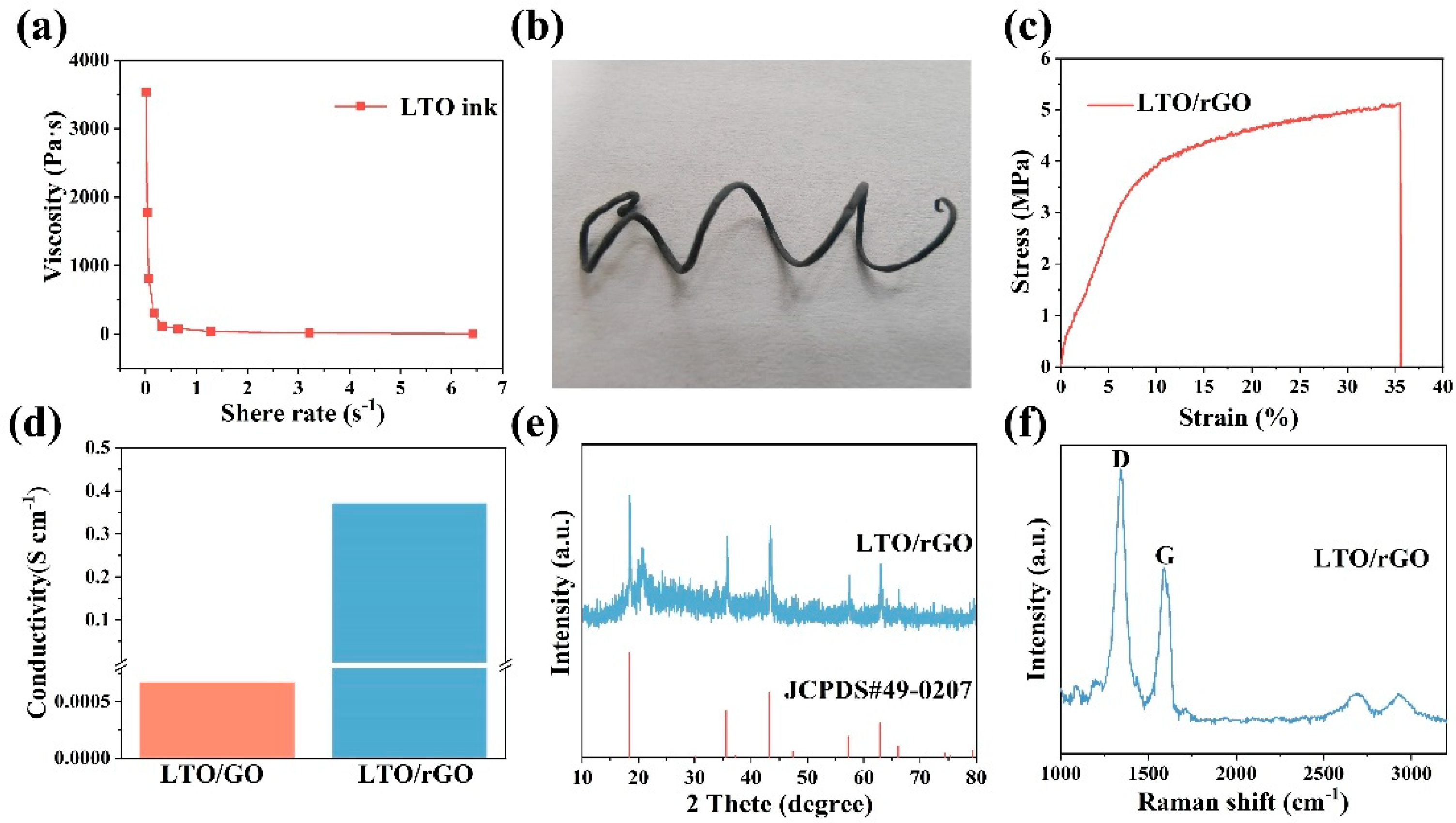
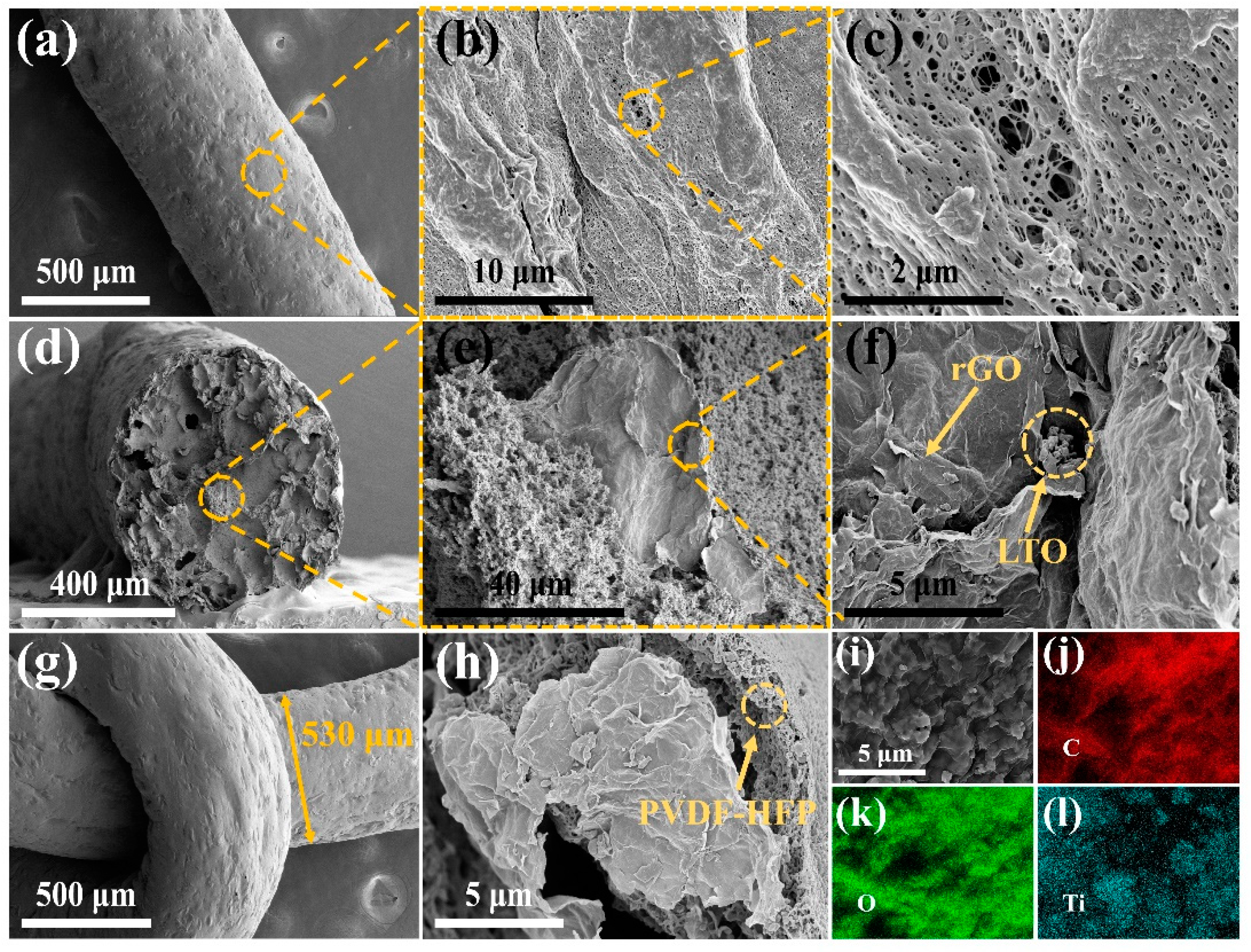
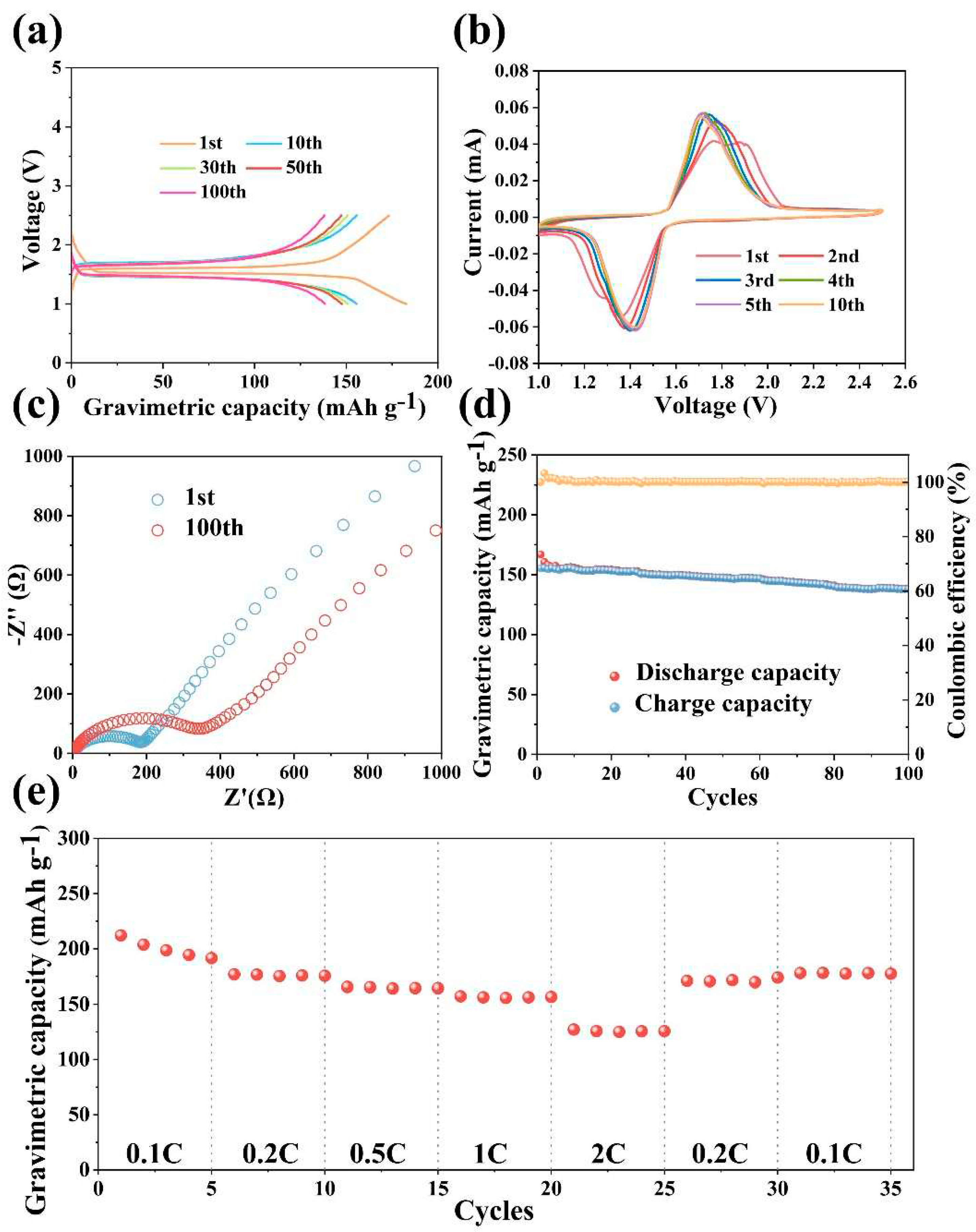
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shi, X. L.; Chen, W. Y.; Zhang, T.; Zou, J.; Chen, Z. G. Fiber-based thermoelectrics for solid, portable, and wearable electronics. Energy Environ. Sci. 2021, 14, 729–764. [Google Scholar] [CrossRef]
- Tu, J.; Torrente-Rodríguez, R. M.; Wang, M.; Gao, W. The era of digital health: a review of portable and wearable affinity biosensors. Adv. Funct. Mater. 2020, 30, 1906713. [Google Scholar] [CrossRef]
- Rahman, M. T.; Rana, S. S.; Salauddin, M.; Maharjan, P.; Bhatta, T.; Park, J. Y. Biomechanical energy-driven hybridized generator as a universal portable power source for smart/wearable electronics. Adv. Energy Mater. 2020, 10, 1903663. [Google Scholar] [CrossRef]
- Chen, X. Making electrodes stretchable. Small Methods 2017, 1, 1600029. [Google Scholar] [CrossRef]
- Lv, Z.; Li, W.; Yang, L.; Loh, X. J.; Chen, X. Custom-made electrochemical energy storage devices. ACS Energy Lett. 2019, 4, 606–614. [Google Scholar] [CrossRef]
- Song, W. J.; Lee, S.; Song, G.; Park, S. Stretchable aqueous batteries: progress and prospects. ACS Energy Lett. 2019, 4, 177–186. [Google Scholar] [CrossRef]
- Song, Z.; Ma, T.; Tang, R.; Cheng, Q.; Wang, X.; Krishnaraju, D.; Panat, R.; Chan, C. K.; Yu, H.; Jiang, H. Origami lithium-ion batteries. Nat. Commun. 2014, 5, 3140. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, X.; Zhang, X.; Yang, L.; Zhang, X.; Chen, H.; Yang, M.; Fang, D. Free-standing and flexible LiMnTiO4/carbon nanotube cathodes for high performance lithium ion batteries. J. Power Sources 2016, 321, 120–125. [Google Scholar] [CrossRef]
- Fu, K. K.; Cheng, J.; Li, T.; Hu, L. Flexible batteries: from mechanics to devices. ACS Energy Lett. 2016, 1, 1065–1079. [Google Scholar] [CrossRef]
- Bao, Y.; Hong, G.; Chen, Y.; Chen, J.; Chen, H.; Song, W. L.; Fang, D. Customized kirigami electrodes for flexible and deformable lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 780–788. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Rogers, J. A. Materials and designs for power supply systems in skin-interfaced electronics. Acc. Chem. Res. 2019, 52, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wang, C.; Hong, Y.; Zhang, Y.; Cheng, X.; Sun, H.; Huang, X.; Ye, L.; Wu, J.; Shi, X.; Kang, X.; Zhou, X.; Wang, J.; Li, P.; Sun, X.; Chen, P.; Wang, B.; Wang, Y.; Xia, Y.; Cheng, Y.; Peng, H. Industrial scale production of fibre batteries by a solution-extrusion method. Nat. Nanotechnol. 2022, 17, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lou, Z.; Jiang, K.; Shen, G. Device configurations and future prospects of flexible/stretchable lithium-ion batteries. Adv. Funct. Mater. 2018, 28, 1805596. [Google Scholar] [CrossRef]
- Mackanic, D. G.; Kao, M.; Bao, Z. Enabling deformable and stretchable batteries. Adv. Energy Mater. 2020, 10, 2001424. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Zhang, J.; Sun, X.; Peng, H. Energy harvesting and storage in 1D devices. Nat. Rev. Mater. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, F.; Yu, M.; Zhuang, X.; Feng, X. Two-dimensional materials for miniaturized energy storage devices: from individual devices to smart integrated systems. Chem. Soc. Rev. 2018, 47, 7426–7451. [Google Scholar] [CrossRef]
- Ren, J.; Bai, W.; Guan, G.; Zhang, Y.; Peng, H. Flexible and weaveable capacitor wire based on a carbon nanocomposite fiber. Adv. Mater. 2013, 25, 5965–5970. [Google Scholar] [CrossRef]
- Kwon, Y. H.; Woo, S. W.; Jung, H. R.; Yu, H. K.; Kim, K.; Oh, B. H.; Ahn, S.; Lee, S. Y.; Song, S. W.; Cho, J.; Shin, H. C.; Kim, J. Y. Cable-type flexible lithium ion battery based on hollow multi-helix electrodes. Adv. Mater. 2012, 24, 5192–5197. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, W.; Ren, J.; Weng, W.; Lin, H.; Zhang, Z.; Peng, H. Super-stretchy lithium-ion battery based on carbon nanotube fiber. J. Mater. Chem. A 2014, 2, 11054–11059. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Zhang, Y.; Fan, W.; Liu, T. Three-dimensional nanoporous graphene-carbon nanotube hybrid frameworks for confinement of SnS2 nanosheets: flexible and binder-free papers with highly reversible lithium storage. ACS Appl. Mater. Interfaces 2015, 7, 27823–27830. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.; Yan, J.; Guan, C.; Wang, J. Electrospun nanofibers for new generation flexible energy storage. Energy Environ. Mater. 2021, 4, 502–521. [Google Scholar] [CrossRef]
- Selvaraj, A. R.; Raja, I. S.; Chinnadurai, D.; Rajendiran, R.; Cho, I.; Han, D. W.; Prabakar, K. Electrospun one dimensional (1d) pseudocapacitive nanorods embedded carbon nanofiber as positrode and graphene wrapped carbon nanofiber as negatrode for enhanced electrochemical energy storage. J. Energy Storage 2022, 46, 103731. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, K.; Qi, Y.; Liu, Z. Chemical vapor deposition method for graphene fiber materials. Acta Phys.-Chim. Sin. 2020, 38, 2006046–0. [CrossRef]
- Sun, C.; Chen, S.; Li, Z. Controllable synthesis of Fe2O3-carbon fiber composites via a facile sol-gel route as anode materials for lithium ion batteries. Appl. Surf. Sci. 2018, 427, 476–484. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Xie, H.; Gao, T.; Yao, Y.; Pastel, G.; Han, X.; Li, Y.; Zhao, J.; Fu, K. K.; Hu, L. 3D-printed all-fiber li-ion battery toward wearable energy storage. Adv. Funct. Mater. 2017, 27, 1703140. [Google Scholar] [CrossRef]
- Praveen, S.; Sim, G. S.; Ho, C. W.; Lee, C. W. 3D-printed twisted yarn-type Li-ion battery towards smart fabrics. Energy Stor. Mater. 2021, 41, 748–757. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, T.; Qian, F.; Chen, W.; Chandrasekaran, S.; Yao, B.; Song, Y.; Duoss, E. B.; Kuntz, J. D.; Spadaccini, C. M.; Worsley, M. A.; Li, Y. 3D printed functional nanomaterials for electrochemical energy storage. Nano Today 2017, 15, 107–120. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, M.; Viswanathan, V. V.; Swart, B.; Shao, Y.; Wu, G.; Zhou, C. 3D printing technologies for electrochemical energy storage. Nano Energy 2017, 40, 418–431. [Google Scholar] [CrossRef]
- Sousa, R. E.; Costa, C. M.; Lanceros-Méndez, S. Advances and future challenges in printed batteries. ChemSusChem 2015, 8, 3539–3555. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Jin, J.; Yuan, S.; Chua, C. K.; Tor, S. B.; Zhou, K. Emerging 3D-printed electrochemical energy storage devices: a critical review. Adv. Energy Mater. 2017, 7, 1700127. [Google Scholar] [CrossRef]
- Zheng, X.; Smith, W.; Jackson, J.; Moran, B.; Cui, H.; Chen, D.; Ye, J.; Fang, N.; Rodriguez, N.; Weisgraber, T.; Spadaccini, C. M. Addendum: multiscale metallic metamaterials. Nat. Mater. 2017, 16, 497–497. [Google Scholar] [CrossRef] [PubMed]
- Truby, R. L.; Lewis, J. A. Printing soft matter in three dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. C.; Song, W. L.; Fang, D. Twistable Origami and Kirigami: From structure-guided smartness to mechanical energy storage. ACS Appl. Mater. Interfaces 2019, 11, 3450–3458. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V. G.; Saiz, E.; Tirichenko, I. S.; García-Tuñón, E. Direct ink writing advances in multi-material structures for a sustainable future. J. Mater. Chem. A 2020, 8, 15646–15657. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Tao, R.; Li, F.; Lu, X.; Liu, F.; Xu, J.; Kong, D.; Zhang, C.; Tan, X.; Ma, S.; Shi, W.; Mo, R.; Lu, Y. High-conductivity–dispersibility graphene made by catalytic exfoliation of graphite for lithium-ion battery. Adv. Funct. Mater. 2021, 31, 2007630. [Google Scholar] [CrossRef]
- Wang, P.; Ye, Y.; Liang, D.; Sun, H.; Liu, J.; Tian, Z.; Liang, C. Layered mesoporous Mg(OH)2/GO nanosheet composite for efficient removal of water contaminants. RSC Adv. 2016, 6, 26977–26983. [Google Scholar] [CrossRef]
- Zhang, D.; Chi, B.; Li, B.; Gao, Z.; Du, Y.; Guo, J.; Wei, J. Fabrication of highly conductive graphene flexible circuits by 3D printing. Synth Met 2016, 217, 79–86. [Google Scholar] [CrossRef]
- Gómez-Navarro, C.; Weitz, R. T.; Bittner, A. M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic transport properties of individual chemically reduced graphene oxide sheets. Nano Letters 2007, 7, 3499–3503. [Google Scholar] [CrossRef]
- De Silva, K. K. H.; Huang, H. H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D. A.; Dommett, G. H. B.; Kohlhaas, K. M.; Zimney, E. J.; Stach, E. A.; Piner, R. D.; Nguyen, S. T.; Ruoff, R. S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, G.; Lamberti, P.; Tucci, V.; Ivanova, R.; Tabakova, S.; Ivanov, E.; Kotsilkova, R.; Cimmino, S.; Di Maio, R.; Silvestre, C. Rheological and electrical behaviour of nanocarbon/poly(lactic) acid for 3D printing applications. Compos. B. Eng. 2019, 167, 467–476. [Google Scholar] [CrossRef]
- Ponnamma, D.; Sadasivuni, K. K.; Cabibihan, J. J.; Yoon, W. J.; Kumar, B. Reduced graphene oxide filled poly(dimethyl siloxane) based transparent stretchable, and touch-responsive sensors. Appl. Phys. Lett. 2016, 108, 171906. [Google Scholar] [CrossRef]
- Mo, R.; Lei, Z.; Sun, K.; Rooney, D. Facile Synthesis of anatase TiO2 quantum-dot/graphene-nanosheet composites with enhanced electrochemical performance for lithium-ion batteries. Adv. Mater. 2014, 26, 2084–2088. [Google Scholar] [CrossRef]
- Mo, R.; Li, F.; Tan, X.; Xu, P.; Tao, R.; Shen, G.; Lu, X.; Liu, F.; Shen, L.; Xu, B.; Xiao, Q.; Wang, X.; Wang, C.; Li, J.; Wang, G.; Lu, Y. High-quality mesoporous graphene particles as high-energy and fast-charging anodes for lithium-ion batteries. Nat. Commun. 2019, 10, 1474. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J. W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Terrones, M.; Martín, O.; González, M.; Pozuelo, J.; Serrano, B.; Cabanelas, J. C.; Vega-Díaz, S. M.; Baselga, J. Interphases in graphene polymer-based nanocomposites: achievements and challenges. Adv. Mater. 2011, 23, 5302–5310. [Google Scholar] [CrossRef]
- Seyssiecq, I.; Ferrasse, J. H.; Roche, N. State-of-the-art: rheological characterisation of wastewater treatment sludge. Biochem. Eng. J. 2003, 16, 41–56. [Google Scholar] [CrossRef]
- Lewis, J. A. Direct ink writing of 3D functional materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Clausen, A.; Wang, F.; Jensen, J. S.; Sigmund, O.; Lewis, J. A. Topology optimized architectures with programmable poisson’s ratio over large deformations. Adv. Mater. 2015, 27, 5523–5527. [Google Scholar] [CrossRef]
- Fathy, M.; Gomaa, A.; Taher, F. A.; El-Fass, M. M.; Kashyout, A. E. H. B. Optimizing the preparation parameters of GO and rGO for large-scale production. J. Mater. Sci. 2016, 51, 5664–5675. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, Q.; Xu, X.; Zhao, Q.; Wu, C.; Liu, J.; Wang, H. Nanochannels regulating ionic transport for boosting electrochemical energy storage and conversion: a review. Nanoscale 2020, 12, 15923–15943. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, X.; Guo, W.; Li, D.; Zhang, H.; Zheng, W. Electrochemical performances investigation of NiS/rGO composite as electrode material for supercapacitors. Nano Energy 2014, 5, 74–81. [Google Scholar] [CrossRef]
- Zhang, W.; Tu, Z.; Qian, J.; Choudhury, S.; Archer, L. A.; Lu, Y. Design Principles of functional polymer separators for high-energy, metal-based batteries. Small 2018, 14, 1703001. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z. S.; Ren, W.; Xu, L.; Li, F.; Cheng, H. M. Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Song, Z.; Ma, T.; Smith, B. B.; Tang, R.; Yu, H.; Jiang, H.; Chan, C. K. Folding paper-based lithium-ion batteries for higher areal energy densities. Nano Letters 2013, 13, 4969–4974. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Mo, R.; Xu, J.; Li, X.; Yin, Q.; Shen, L.; Lu, Y. High performance sodium ion anodes based on Sn4P3 encapsulated within amphiphilic graphene tubes. Adv. Energy Mater. 2022, 12, 2102345. [Google Scholar] [CrossRef]
- Shi, X.-L.; Chen, W.-Y.; Zhang, T.; Zou, J.; Chen, Z.-G. Fiber-based thermoelectrics for solid, portable, and wearable electronics. Energy Environ. Sci. 2021, 14, 729–764. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



