Submitted:
19 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. The Pharmacokinetic In Silico Studies on BBB Permeation
2.2. Anisotropic Membrane-Like Systems
2.3. QSAR Analysis for BBB Permeation
2.4. Acetylcholinesterase Inhibitory Activity of OA
2.5. Free energy Calculations and Molecular Docking
2.6. Inhibitory Effect of Oleanolic Acid on the SH-SY5Y Cell Viability
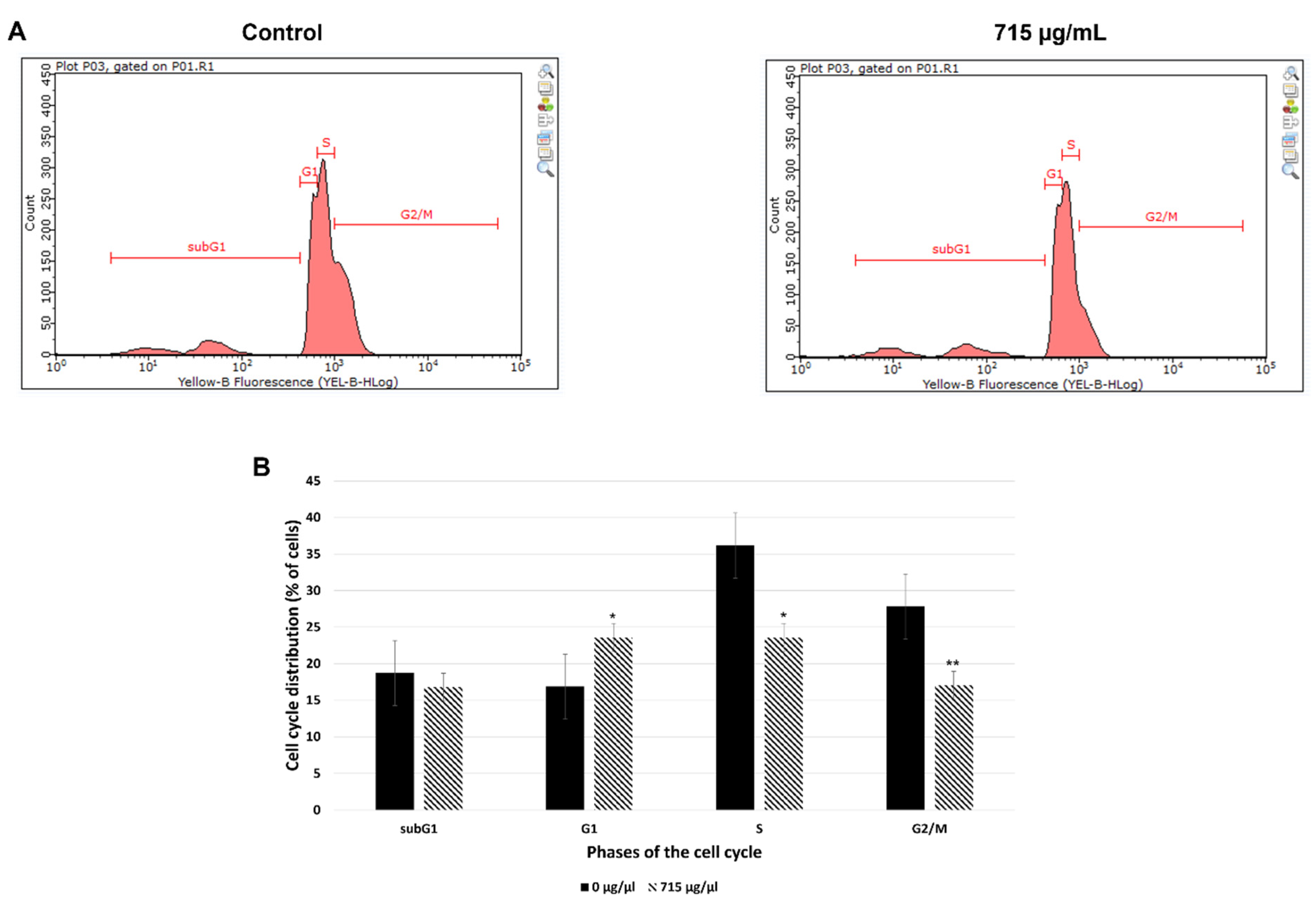
2.7. Effect of Oleanolic Acid on the Cell Cycle Analyzed with the Flow Cytometry
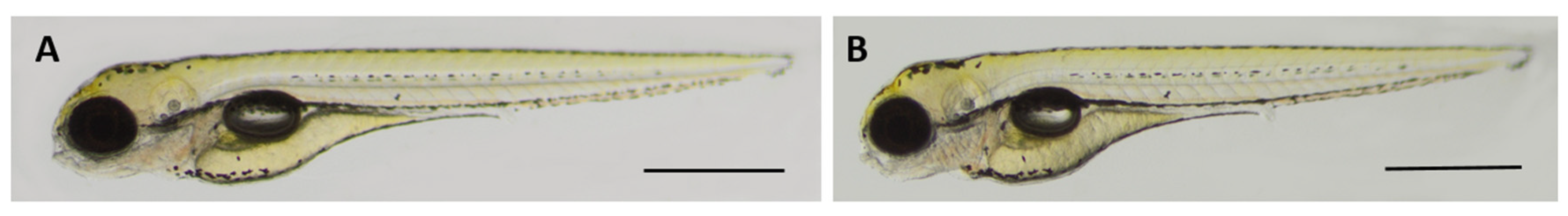
2.8. Oleanolic Acid is Non-Toxic for Developing Zebrafish
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. In Silico Determination of Blood-Brain Barrier (BBB) Pharmacokinetic Descriptors
4.3. Membrane-Like Chromatograhic Equipment and Conditions
4.4. Quantitative Structure-Activity Relationship (QSAR) studies for estimation of permeation through the BBB
4.5. TLC-Bioautography Assay towards the Inhibition of Acetylcholinesterase (AChE) Activity
4.6. Molecular Docking
4.7. Free Energy Profiles
4.8. Cytotoxicity Test in Human Neuroblastoma In Vitro
4.8.1. Cell Culture
4.8.2. MTT Assay
4.8.3. Cell Cycle Analysis
4.9. Toxicity Analysis in Zebrafish In Vivo
4.9.1. Animals
4.9.2. Zebrafish Embryo Acute Toxicity Test
4.9.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarabasz, D.; Szczeblewski, P.; Laskowski, T.; Płaziński, W.; Baranowska-Wójcik, E.; Szwajgier, D.; Kukula-Koch, W.; Meissner, H.O. The Distribution of Glucosinolates in Different Phenotypes of Lepidium peruvianum and Their Role as Acetyl- and Butyrylcholinesterase Inhibitors—In Silico and In Vitro Studies. Int. J. Mol. Sci. 2022.23 4858. [CrossRef]
- Kukula-Koch, W.; Koch, W.; Czernicka, L.; Głowniak, K.; Asakawa, Y.; Umeyama, A.; Marzec, Z.; Kuzuhara, T. MAO-A Inhibitory Potential of Terpene Constituents from Ginger Rhizomes-A Bioactivity Guided Fractionation. Molecules2018.231301. [CrossRef]
- Urbain, A.; Simões-Pires, C.A. (2023) Thin-Layer Chromatography for the Detection and Analysis of Bioactive Natural Products. In Encyclopedia of Analytical Chemistry, R.A. Meyers (Ed.). [CrossRef]
- Rajmohan, R.; Reddy, P. H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer's disease Neurons. Journal of Alzheimer's disease: JAD2017. 57975–999. [CrossRef]
- Abdykerimova, S.; Sakipova, Z.; Nakonieczna, S.; Koch, W.; Biernasiuk, A.; Grabarska, A.; Malm, A.; Kozhanova, K.; Kukula-Koch, W. Superior Antioxidant Capacity of Berberis iliensis-HPLC-Q-TOF-MS Based Phytochemical Studies and Spectrophotometric Determinations. Antioxidants 2020.9 504. [CrossRef]
- Smyrska-Wieleba, N.; Mroczek, T. Natural Inhibitors of Cholinesterases: Chemistry, Structure–Activity and Methods of Their Analysis. Int. J. Mol. Sci. 2023.24 2722. [CrossRef]
- Mroczek, T.; Dymek, A.; Widelski, J.; Wojtanowski, K.K. The Bioassay-Guided Fractionation and Identification of Potent Acetylcholinesterase Inhibitors from Narcissus c.v. 'Hawera' Using Optimized Vacuum Liquid Chromatography, High Resolution Mass Spectrometry and Bioautography. Metabolites2020. 10395. [CrossRef]
- Ouyang, C.; Ma, X.; Zhao, J.; Li, S.; Liu, C.; Tang, Y.; Zhou, J.; Chen, J.; Li, X.; Li, W. Oleanolic acid inhibits mercury chloride induced-liver ferroptosis by regulating ROS/iron overload. Ecotoxicol Environ Saf.2023. 258 114973. [CrossRef]
- Ayeleso, T. B.; Matumba, M. G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017.22 1915. 1915. [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022.14 623. [CrossRef]
- Lu, C., Zhang, C., Zhao, F., Li, D. and Lu, W. (2018), Biosynthesis of ursolic acid and oleanolic acid in Saccharomyces cerevisiae. AIChE J, 64: 3794-3802. [CrossRef]
- Soczewiński, E.; Wachtmeister, C.A. The relation between the composition of certain ternary two-phase solvent systems and RM values. J. Chromatogr. 1962.7 311–320.
- Janicka, M.; Sztanke, M.; Sztanke, K. Predicting the Blood-Brain Barrier Permeability of New Drug-like Compounds via HPLC with Various Stationary Phases. Molecules 2020.25 487.
- Stępnik, K. Biomimetic Chromatographic Studies Combined with the Computational Approach to Investigate the Ability of Triterpenoid Saponins of Plant Origin to Cross the Blood–Brain Barrier. Int. J. Mol. Sci. 2021.22 3573. [CrossRef]
- Platzer, G.; Mayer, M.; Beier, A.; Brüschweiler, S.; Fuchs, J.E.; Engelhardt, H.; Geist, L.; Bader, G.; Schörghuber, J.; Lichtenecker, R.; Wolkerstorfer, B.; Kessler, D.; McConnell, D.B.; Konrat, R.PI by NMR: Probing CH–π Interactions in Protein–Ligand Complexes by NMR Spectroscopy. Angew. Chem. Int. Ed. 2020. 59, 14861. [CrossRef]
- Ferreira de Freitas, R.; Schapira, M. A systematic analysis of atomic protein–ligand interactions in the PDB. Med. Chem.Commun.2017. 8, 1970-1981. [CrossRef]
- Nishio, M.; Umezawa, Y.; Fantini, J.; Weiss, M.S.; Chakrabarti, P. CH–π hydrogen bonds in biological macromolecules. Phys.Chem. Chem. Phys.2014, 16, 12648-12683. https://doi.org/10.1039/C4CP00099D. The WHO brochure “Global action plan on the public health response to dementia 2017 – 2025”, 2017. ISBN: 978-92-4-151348-7. [CrossRef]
- Birks, J.; Grimley, E.J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev.2009.21. [CrossRef]
- Joo, S.S.; Lee, D.I. Potential effects of microglial activation induced by ginsenoside Rg3 in rat primary culture: enhancement of type a macrophage scavenger receptor expression. Arch. Pharm. Res. 2005.281164–1169.
- Chen, F.; Eckman, E.A.; Eckman, C.B. Reductions in levels of the Alzheimer's amyloid β peptide after oral administration of ginsenosides. FASEB J2006.20 1269–1271.
- Li, N.; Liu, B.;Dluzen, D.E.;Jin, Y. Protective effects of ginsenoside Rg2 against glutamate induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2007.111458–463.
- Joo, S.S.; Yoo, Y.M.;Ahn, B,W., et al. Prevention of inflammation-mediated neurotoxicity by Rg3 and its role in microglial activation. Biol. Pharm. Bull.2008.31 1392–1396.
- Shieh, P.C.; Tsao, C.W.; Li, J.S. et al. Role of pituitary adenylate cyclase-activating polypeptide (PACAP) in the action of ginsenoside Rh2 against betaamyloid-induced inhibition of rat brain astrocytes. Neurosci. Lett. 2008.434 1–5.
- Wakabayashi, I.;Yasui, K. Wogonin inhibits inducible prostaglandin E2 production in macrophages. Eur. J. Pharmacol. 2000.406477–481.
- Park, B.K.;Heo, M.Y.; Par, H.; et al. Inhibition of TPA-induced cyclooxygenase-2 expression and skin inflammation in mice by wogonin, a plant flavone from Scutellaria radix. Eur. J. Pharmacol.2001.425 153–157.
- Nakamura, N.;Hayasaka, S.; Zhang, X.Y.; et al. Effects of baicalein, baicalin, and wogonin on interleukin-6 and interleukin-8 expression, and nuclear factor-kb binding activities induced by interleukin-1beta in human retinal pigment epithelial cell line. Exp. Eye Res.2003.77 195–202.
- Suk, K.; Lee, H.; Kang, S.S.; et al. Flavonoid baicalein attenuates activation induced cell death of brain microglia. J. Pharmacol.Exp. Ther.2003.305 638–645.
- Hosseinzadeh, H.; Ramezani, M.; Shafaei, H.; et al. Anticonvulsant effect of Berberis integerrima L. root extracts in mice. J. Acupunct. Meridian Stud. 2013.12 12–17. [CrossRef]
- Ya'u, J.; Yaro, A.H.; Malami, S.; et al. Anticonvulsant activity of aqueous fraction of Carissa edulis root bark. Pharm. Biol. 2015.531329–1338. [CrossRef]
- Bhat, J.U.; Nizami, Q.; Asiaf, A.; et al. Anticonvulsant activity of methanolic and aqueous extracts of Melissa parviflora in experimentally induced Swiss albino mice. EXCLI J2012.111–6.
- Tariq, U.; Butt, M.S.; Pasha, I.; Faisal, M.N. Neuroprotective effects of Olea europaea L. fruit extract against cigarette smoke-induced depressive-like behaviors in Sprague-Dawley rats. J Food Biochem.2021.45 e14014. [CrossRef]
- Angeloni, C.; Malaguti, M.;Barbalace, M.C.;Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017.182230. https://doi.org/10.3390/ijms18112230. [CrossRef]
- dos Santos-Neto, L.L.; de Vilhena Toledo, M.A.; Medeiros-Souza, P.; et al. The use of herbal medicine in Alzheimer’s disease - A systematic review. Evid.-Based Complement. Altern. Med. 2006.3441–445.
- Bozin, B.; Mika-Dukic, N.;Samojlik, I.; et al. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food. Chem. 2007.557879–7885.
- Imanshahidi, M.;Hosseinzadeh, H. The pharmacological effects of Salvia species on the central nervous system. Phytother.Res.2006.20 427–437.
- Visioli, F.; Grande, S.;Bogani, P.; et al. The role of antioxidant in the Mediterranean diets: focus on cancer. Eur. J. Cancer Prev.2004.13 337–343.
- Ninfali, P.; Mea, G.;Giorgini, S.; et al. Antioxidant capacity of vegetables, spices, and dressings relevant to nutrition. Brit. J.Nutr.2005.93 257–266.
- Cheung, S.; Tai, J. Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol. Rep. 2007. 17 1525–1531.
- Lin, S. X.; Curtis, M. A.; Sperry, J. Pyridine alkaloids with activity in the central nervous system. Bioorg Med Chem2020.28115820. 5820. [CrossRef]
- Arens, H.; Borbe, H.O.; Ulbrich, B.; Stöckigt, J. Detection of pericine, a new CNS-active indole alkaloid from Picralima nitida cell suspension culture by opiate receptor binding studies. Planta Med.1982.46 210-214. [CrossRef]
- Paladini, A.C.; Marder, M.; Viola, H.; Wolfman, C.;Wasowski, C.; Medina, J.H. Flavonoids and the central nervous system: from forgotten factors to potent anxiolytic compounds. J Pharm Pharmacol.1999.51 519-526. /: 519-526. htpps.
- Jäger, A. K.; Saaby, L. Flavonoids and the CNS. Molecules 16 (2011) 1471–1485. 1471–1485. [CrossRef]
- Sun, A., Xu, X., Lin, J., Cui, X. and Xu, R. Neuroprotection by Saponins, Phytother. Res. 2015.29 187– 200. [CrossRef]
- Yuan, C.S.; Wang, C.Z.; Wicks, S.M.; Qi, L. W. Chemical and pharmacological studies of saponins with a focus on American ginseng. J Gins Res 2010.34 160–167. [CrossRef]
- Hussain, G.; Huang, J.; Rasul, A.; Anwar, H.; Imran, A.; Maqbool, J.; Razzaq, A.; Aziz, N.; Makhdoom, E. U. H.; Konuk, M.; Sun, T. Putative Roles of Plant-Derived Tannins in Neurodegenerative and Neuropsychiatry Disorders: An Updated Review. Molecules2019.242213. [CrossRef]
- Takahashi, R.N.; de Lima, T.C.; Morato, G.S. Pharmacological actions of tannic acid; II. Evaluation of CNS activity in animals. Planta Med. 1986.4272-275. [CrossRef]
- Awasthi, M.; Upadhyay, A.K.; Singh, S.; Pandey, V.P.; Dwivedi, U.N. Terpenoids as promising therapeutic molecules against Alzheimer’s disease: amyloid beta- and acetylcholinesterase-directed pharmacokinetic and molecular docking analyses. Molecular Simulation2018. 44 1-11. [CrossRef]
- Lai Min, S.; Liew, S. Y.; Chear, N. J. Y.; Goh, B. H.; Tan, W. N.; Khaw, K. Y. Plant Terpenoids as the Promising Source of Cholinesterase Inhibitors for Anti-AD Therapy. Biology2022. 11 307. [CrossRef]
- Mony, T.J.; Elahi, F.; Choi, J. W.; Park, S. J. Neuropharmacological Effects of Terpenoids on Preclinical Animal Models of Psychiatric Disorders: A Review. Antioxidants 2022.11 1834. [CrossRef]
- Meckes, M.; Calzada, F.; Tortoriello, J.; González, J.L.; Martínez, M. Terpenoids Isolated from Psidium guajava Hexane Extract with Depressant Activity on Central Nervous System. Phytother. Res. 1996.10 600-603. [CrossRef]
- Mohanty, I.R.; Borde, M.; Kumar, S.; et al. Dipeptidyl peptidase IV Inhibitory activity of Terminalia arjuna attributes to its cardioprotective effects in experimental diabetes: In silico, in vitro and in vivo analyses. Phytomedicine 2019.57158-165.
- Pawar, R.S.;Bhutani, K.K. Effect of oleanane triterpenoids from Terminalia arjuna – a cardioprotective drug on the process of respiratory oxyburst. Phytomedicine 2005.12 391-393.
- Kapoor, D.;Vijayvergiya, R.; Dhawan, V. Terminalia arjuna in coronary artery disease: Ethnopharmacology, pre-clinical, clinical & safety evaluation. J. Ethnopharmacol. 2014.155 1029-1045. 1029–1045.
- Sudharsan, P.T.; Mythili, Y.; Selvakumar, E.; Varalakshmi, P. Cardioprotective effect of pentacyclic triterpene, lupeol and its ester on cyclophosphamide-induced oxidative stress. Hum Exp Toxicol. 2005.24 313-318. [CrossRef]
- Pugazhendhi, A.; Shafreen, R.B.; Devi, K.P.; et al. Assessment of antioxidant, anticholinesterase and antiamyloidogenic effect of Terminalia chebula, Terminalia arjuna and its bioactive constituent 7-Methyl gallic acid – An in vitro and in silico studies. J.Mol. Liq. 2018.257 69-81.
- Gupta, D.; Kumar, M. Evaluation of in vitro antimicrobial potential and GC-MS analysis of Camellia sinensis and Terminalia arjuna. Biotechnol. Rep. (Amst).2016.30 19-25. [CrossRef]
- Mandal, S.; Patra, A.; Samanta, A.; et al. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pac. J. Trop. Biomed. 2013.12 960-966. [CrossRef]
- Dube, N.;Nimgulkar, Ch.;Bharatraj, D.K. Validation of therapeutic anti-inflammatory potential of Arjuna KsheeraPaka – A traditional Ayurvedic formulation of Terminalia arjuna. J. Tradit. Complement. Med. 2017.7 414-420.
- Ahmad, M.S.; Ahmad, S.; Gautam, B.; et al. Terminalia arjuna, a herbal remedy against environmental carcinogenicity: An in vitro and in vivo study. Egypt. J. Medical Hum. Genet. 2014.15 61-67.
- Ruszkowski, P.;Bobkiewicz-Kozlowska, T. Natural Triterpenoids and their Derivatives with Pharmacological Activity Against Neurodegenerative Disorders. Mini-Rev Org Chem2014.11. [CrossRef]
- Bhattacharjee, B.; Pal, P.K.; Ghosh, A.K.; et al. Aqueous bark extract of Terminalia arjuna protects against cadmium-induced hepatic and cardiac injuries in male Wistar rats through antioxidative mechanisms. Food Chem. Toxicol. 2019. 124249-264.
- Tanaka, H.;Mizojiri, K. Drug-protein binding and blood-brain barrier permeability. J Pharmacol. Exp. Ther. 1999.288 912-918.
- Platts, J.A.; Abraham, M.H.; Zhao, Y.H.; et al. Correlation and prediction of a large blood-brain distribution data set--an LFER study. Eur. J. Med. Chem. 2001.36 719-730. [CrossRef]
- Sapkota, A.; Choi, J.W. Oleanolic Acid Provides Neuroprotection against Ischemic Stroke through the Inhibition of Microglial Activation and NLRP3 Inflammasome Activation. BiomolTher (Seoul). 2022.30 55-63. [CrossRef]
- Chen, C.; Ai, Q.; Shi, A.; Wang, N.; Wang, L.; Wei, Y. Oleanolic acid and ursolic acid: therapeutic potential in neurodegenerative diseases, neuropsychiatric diseases and other brain disorders. Nutr Neurosci. 2023.26 414-428. [CrossRef]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. Int J Mol Sci. 2021.22 4599. 4599. [CrossRef]
- Fantini, S.;Sassaroli, A.;Tga’valekos K.T.; et al. Cerebral blood flow and autoregulation: current measurement techniques and prospects for noninvasive optical methods. Neurophotonics2016. 3031411. [CrossRef]
- Xingrong, L.; Smith, B.J.; Chen, C.; et al. Use of a Physiologically Based Pharmacokinetic Model to Study the Time to Reach Brain Equilibrium: An Experimental Analysis of the Role of Blood-Brain Barrier Permeability, Plasma Protein Binding, and Brain Tissue Binding. J. Pharmacol. Exp. Ther. 2005.313 1254-1262. [CrossRef]
- levi, A.; Bellera, C.L. Free Drug Theory. In: The ADME Encyclopedia. Springer, Cham. 2022. [CrossRef]
- Hammarlund-Udenaes, M.; Fridén, M.; Syvänen, S.; et al. On the rate and extent of drug delivery to the brain. Pharm. Res. 2008.251737-1750. [CrossRef]
- Kalvass, J.C.; Maurer, T.S.; Pollack, G.M. Use of plasma and brain unbound fractions to assess the extent of brain distribution of 34 drugs: comparison of unbound concentration ratios to in vivo p-glycoprotein efflux ratios. Drug Metab. Dispos.2007.35660-666. [CrossRef]
- Banks, W.A.; Kastin, A.J. Peptides and the blood-brain barrier: lipophilicity as a predictor of permeability. Brain Res Bull. 1985.15287-292. /: htpps. [CrossRef]
- Chikhale, E.G.; Ng, K-Y.; Burton, P.S.; Borchardt, R.T. Hydrogen bonding potential as a determinant of the in vitro and in situ blood-brain barrier permeability of peptides. Pharm Res. 1994.11412-419. [CrossRef]
- Abbott, N.J. Prediction of blood–brain barrier permeation in drug discovery from in vivo, in vitro and in silico models. Drug Discov Today: Technologies 2004. 1407-416. [CrossRef]
- Clark, D.E. In silico prediction of blood–brain barrier permeation. Drug Discov Today 2003.8 927-933. [CrossRef]
- Stéen, E.J.L.; Vugts, D.J.; Windhorst, A.D. The Application of in silico Methods for Prediction of Blood-Brain Barrier Permeability of Small Molecule PET Tracers. Front. Nucl. Med. 2022.2 853475. [CrossRef]
- Radan, M.; Djikic, T.; Obradovic, D.; Nikolic, K. Application of in vitro PAMPA technique and in silico computational methods for blood-brain barrier permeability prediction of novel CNS drug candidates. Eur J Pharms Sci 2022.168 106056. 6056. [CrossRef]
- Yang, R.; Wei, T.; Goldberg, H.; Wang, W.; Cullion, K.; Kohane, D.S. Getting Drugs Across Biological Barriers. Adv Mater. 2017.29. /: htpps. [CrossRef]
- Oldendorf, W.H. Lipid solubility and drug penetration of the blood brain barrier. Proc Soc Exp Biol Med.1974.147 813-815. /: 813-815. htpps. [CrossRef]
- Aryal, M.; Arvanitis, C.D.; Alexander, P.M.; McDannold, N. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Deliv Rev.2014.72 94-109. [CrossRef]
- Feher, M.; Sourial, E.; Schmidt, J.M. A simple model for the prediction of blood-brain partitioning. Int J. Pharm. 2000.201239–247. [CrossRef]
- Liu, R.; Sun, H.; So, S–S. Development of quantitative structure − property relationship models for early ADME evaluation in drug discovery. 2. Blood-brain barrier penetration. J Chem. Inf. Comput. Sci. 2001.41 1623–1632. [CrossRef]
- Prasanna, S.; Doerksen, R.J. Topological polar surface area: a useful descriptor in 2D-QSAR. Curr Med Chem. 2009.16 21-41. [CrossRef]
- Van de Waterbeemd, H. In Silico Models to Predict Oral Absorption, in: Taylor, J.B., Triggle, D.J. (Eds.) Comprehensive Medicinal Chemistry II. Elsevier, Amsterdam 2007. pp. 669-697.
- Barbato, F. The Use of Immobilised Artificial Membrane (IAM) Chromatography for Determination of Lipophilicity. Curr.Comput. Drug Des.2006. 2 341–352.
- Ogden, P.B.; Coym, J.W. Retention mechanism of a cholesterol-coated C18 stationary phase: van’t Hoff and Linear Solvation Energy Relationships (LSER) approaches. J Chromatogr A2011. 12182936-2943. [CrossRef]
- Stavrakov, G.; Philipova, I.; Lukarski, A.; et al. Galantamine-curcumin hybrids as dual-site binding acetylcholinesterase inhibitors. Molecules 2020.25 3341. [CrossRef]
- Stępnik, K.; Kukula-Koch, W.; Plazinski, W.; Gawel, K.; Gaweł-Bęben, K.; Khurelbat, D.; Boguszewska-Czubara, A. Significance of Astragaloside IV from the Roots of Astragalus mongholicus as an Acetylcholinesterase Inhibitor—From the Computational and Biomimetic Analyses to the In Vitro and In Vivo Studies of Safety. Int. J. Mol. Sci. 2023. 24 9152. [CrossRef]
- Mente, S.; Lombardo, F. A recursive-partitioning model for blood–brain barrier permeation. J Comput Aided Mol Des 2005.19 465–481. [CrossRef]
- Kukula-Koch, W.;Mroczek, T. Application of hydrostatic CCC-TLC-HPLC-ESI-TOF-MS for the bioguided fractionation of anticholinesterase alkaloids from Argemonemexicana L. roots. Anal. Bioanal. Chem. 2015.407 2581-2589. [CrossRef]
- cactus.nci.nih.gov/translate.
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992.114 10024-10035.
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; et al. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform. 2012.13 17. [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010.30 455-461. [CrossRef]
- Oostenbrink, C.; Villa, A.; Mark, A.E.; Van Gunsteren, W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem.2004.25 1656-1676. [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindalhl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers.SoftwareX 2015.1-2 19-25. [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Hermans, J. Interaction Models for Water in Relation to Protein Hydration. In: Pullman, B. (eds) Intermolecular Forces. The Jerusalem Symposia on Quantum Chemistry and Biochemistry, vol 14. 1981.Springer, Dordrecht. [CrossRef]
- Kukol, A. Lipid Models for United-Atom Molecular Dynamics Simulations of Proteins.JChem Theor Comput2009. 5 615-626. [CrossRef]
- Stroet, M.; Caron, B.; Visscher, K.M.; Geerke, D.P.; Malde, A.K.; Mark, A.E. Automated Topology Builder Version 3.0: Prediction of Solvation Free Enthalpies in Water and Hexane.J Chem Theor Comput2018. 14 5834-5845. [CrossRef]
- Kumar, S.; Rosenberg, J.M.; Bouzida, D.; Swendsen, R.H.; Kollman, P.A. THE weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J. Comput. Chem. 1992.13 1011-1021. [CrossRef]
- Hub, J.S.; de Groot, B.L.; van der Spoel, D. g_wham—A Free Weighted Histogram Analysis Implementation Including Robust Error and Autocorrelation Estimates.J Chem Theor Comput2010. 12 3713-3720. [CrossRef]
- Hess, B. P-LINCS: A Parallel Linear Constraint Solver for Molecular Simulation.J Chem TheorComput2008.4 116-122. [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J Chem Phys.2007.126 014101. [CrossRef]
- Parrinello, M.;Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method.J ApplPhys1981. 52 7182–7190. [CrossRef]
- (a)Essmann, U.;Perera, L.;Berkowitz, M.L.;Darden, T.;Lee, H.;Pedersen, L.G. A smooth particle mesh Ewald method, J. Chem. Phys. 1995.103 8577–8593. 8577–8593.
- https://www.oecd-ilibrary.org/environment/test-no-236-fish-embryo-acute-toxicity-fet-test_9789264203709-en.
- Nakonieczna, S.; Grabarska, A.; Gawel, K.; Wróblewska-Łuczka, P.; Czerwonka, A.; Stepulak, A.; Kukula-Koch, W. Isoquinoline Alkaloids from Coptis chinensis Franch: Focus on Coptisine as a Potential Therapeutic Candidate against Gastric Cancer Cells. Int J Mol Sci 2022.23 10330. [CrossRef]
- Gawel, K.; Turski, W.A.; van der Ent, W.; Mathai, B.J.; Kirstein-Smardzewska, K.J.; Simonsen, A.; Esguerra, C.V. Phenotypic Characterization of Larval Zebrafish (Danio rerio) with Partial Knockdown of the cacna1a Gene. Mol Neurobiol. 2020.57 1904-1916. [CrossRef]
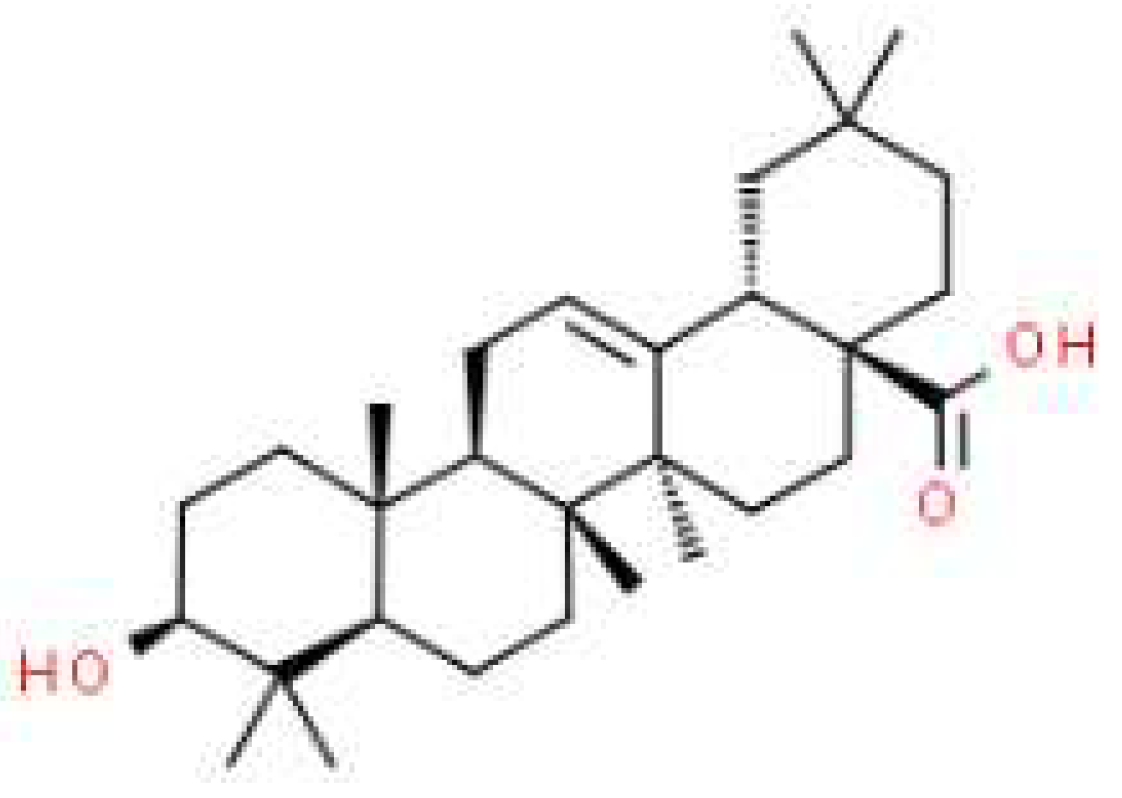
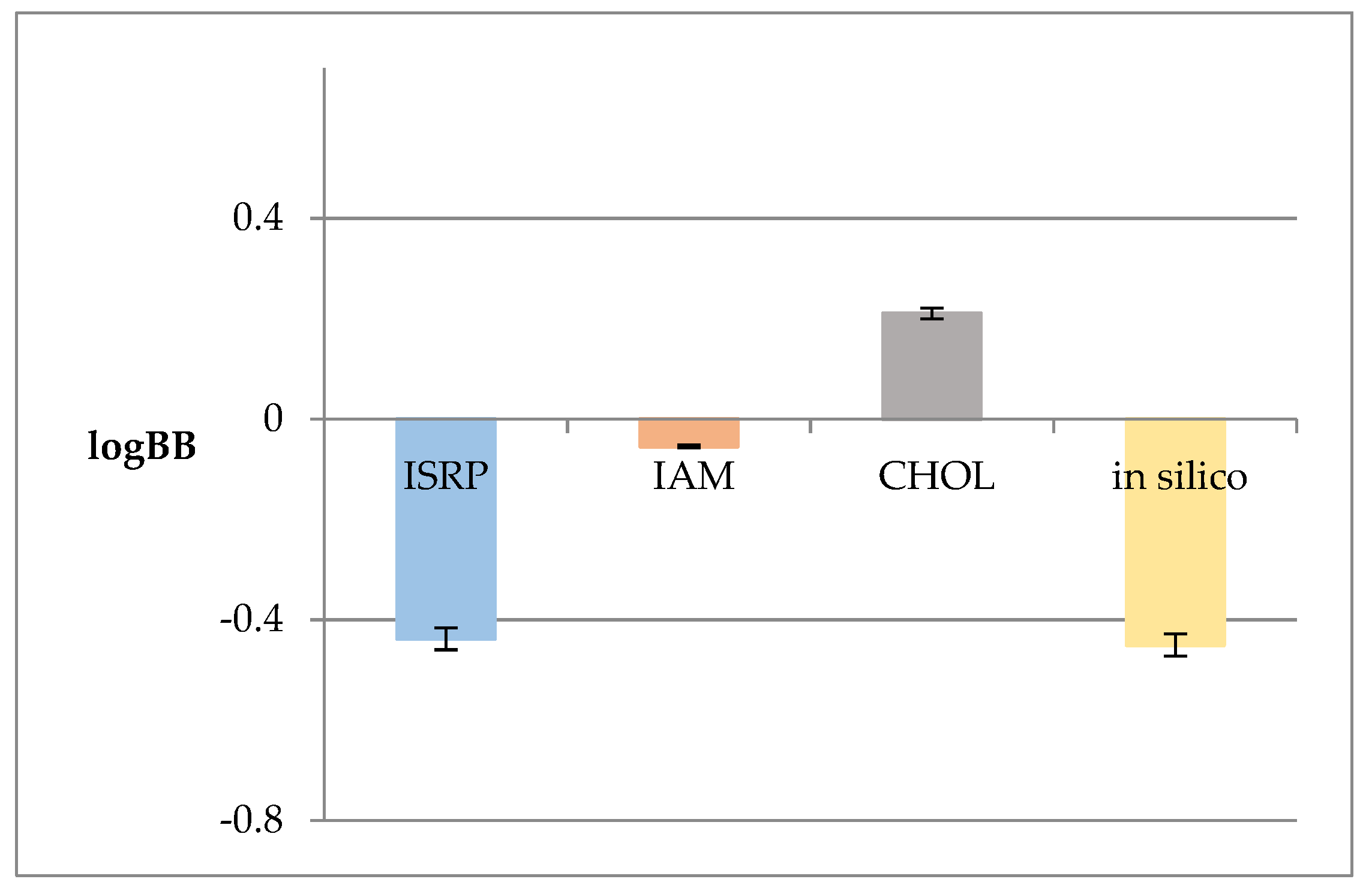


| logBB-in silico | logPS | logPSFb | Fu | Fb | logPow | TPSA[Ų] | MW[g/mol] |
|---|---|---|---|---|---|---|---|
| -0.45 | -4.3 | -6.1 | 0.0055 | 0.02 | 11.108 | 57.53 | 456.7 |
| Membrane-like system | logkw | s | R2 |
|---|---|---|---|
| IAM | 1.656 | 1.515 | 0.991 |
| CHOL | 2.361 | 2.037 | 0.993 |
| ISRP | 0.637 | 1.853 | 0.983 |
| Membrane-like system | logkw | logPcw | ΔlogP | E |
|---|---|---|---|---|
| ISRP | 0.637 | 8.995 | 8.358 | 1.46 |
| IAM | 1.656 | 7.339 | ||
| CHOL | 2.361 | 6.634 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





