Submitted:
18 August 2023
Posted:
22 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
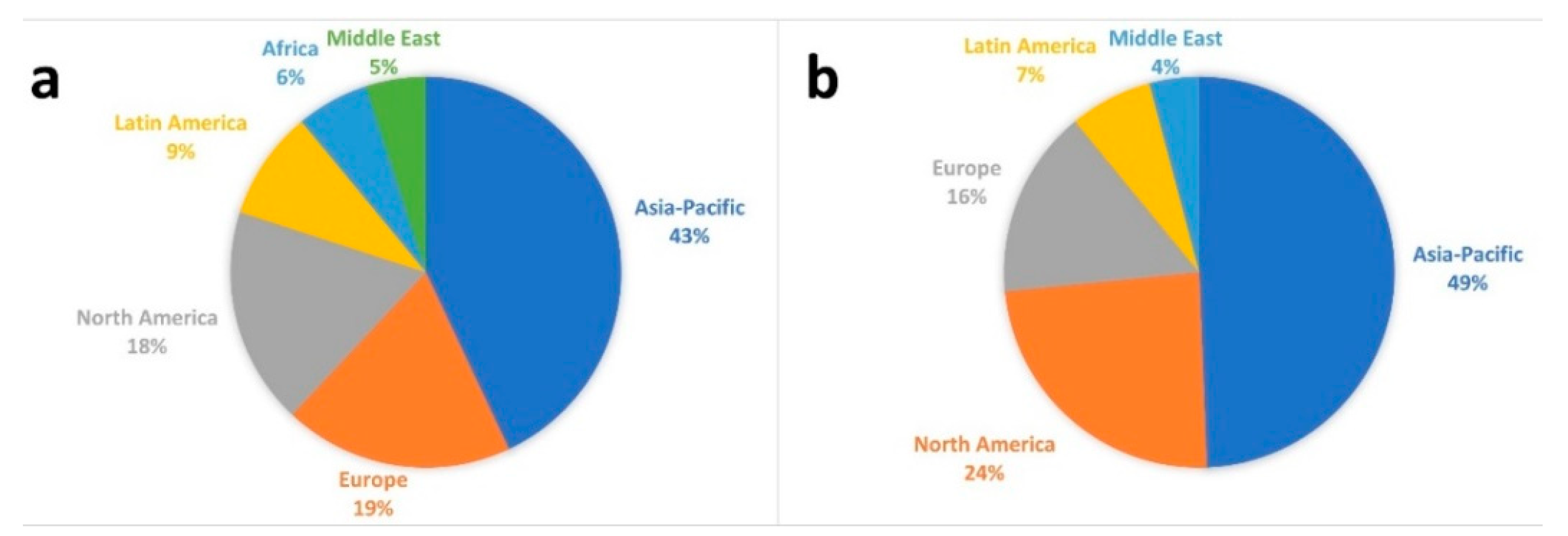
1.1. Global energy and materials scenario: the role of biolubricants
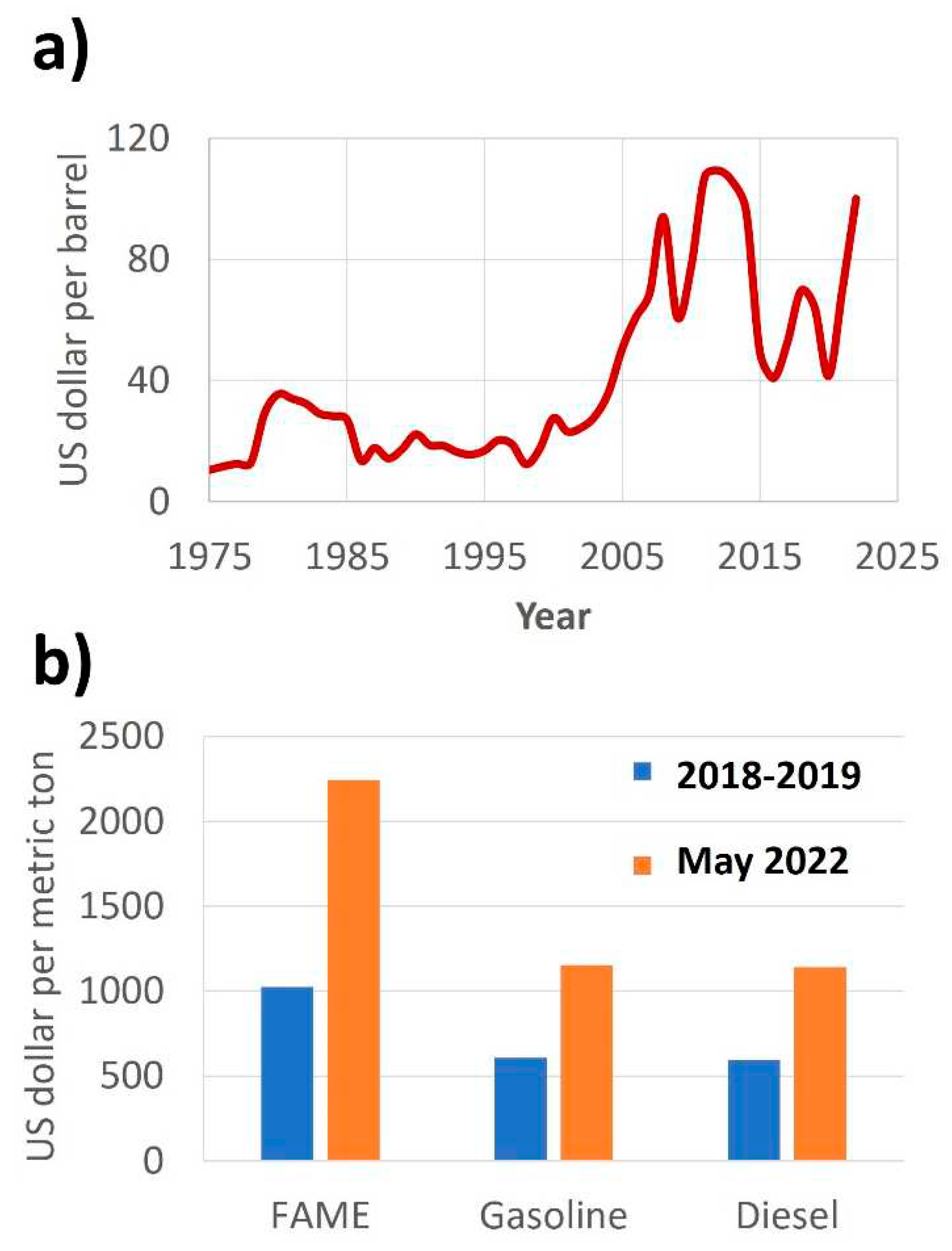
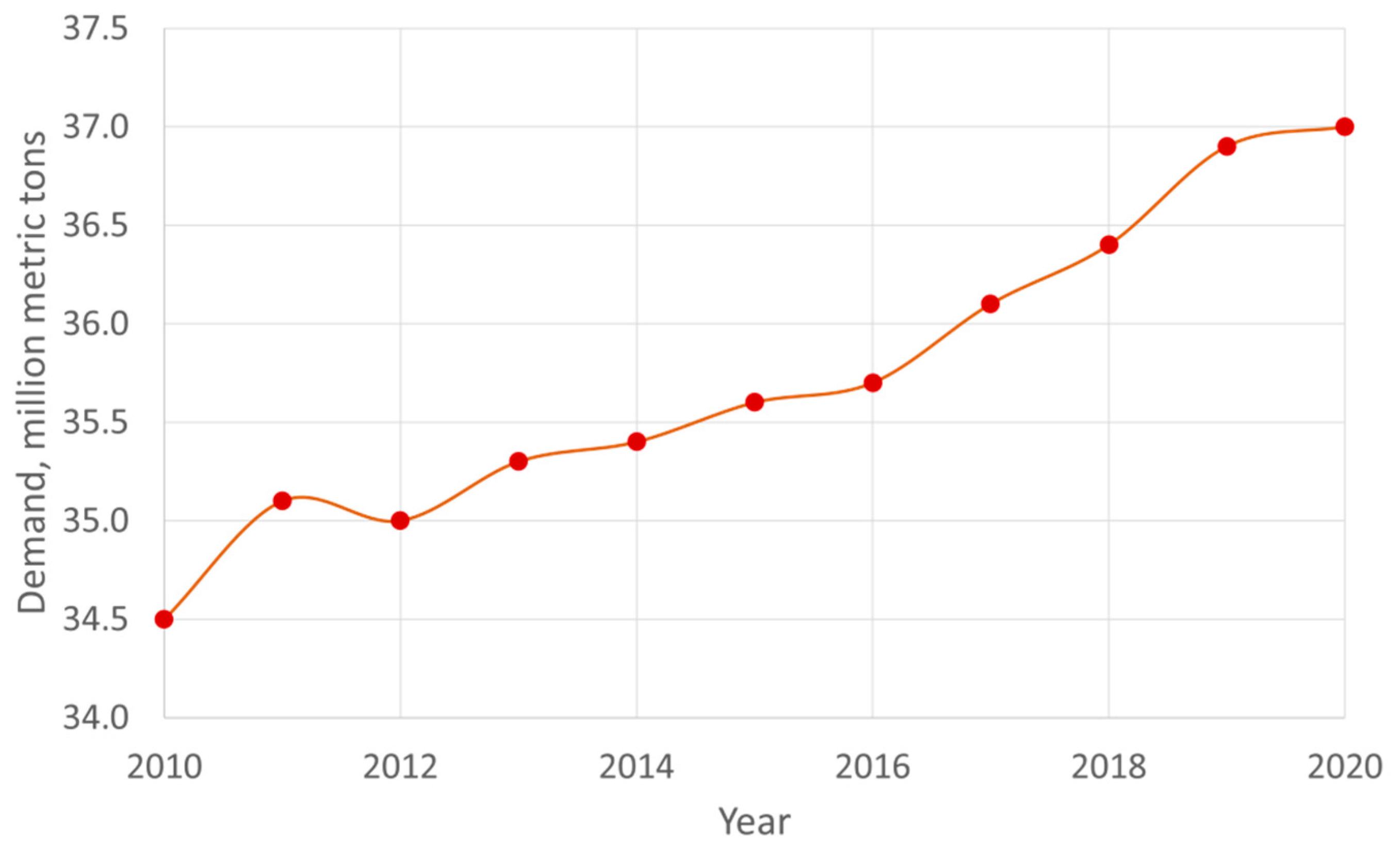
1.2. Industrial activity, lubricant demand and the subsequent opportunity for biolubricants
- Its economic growth and industrial development are becoming more and more noticeable, with the subsequent risks if environmental measures are not suitably taken.
- There is a great opportunity for a sustainable development according to the SDGs, with the subsequent decrease in economic dependence from traditional trade relations.
- For this purpose, many sustainable processes can contribute to the industrial network of developing countries.
- On the one hand and considering the abovementioned concerns from international agencies and countries, the replacement for lubricants present great potential in developed countries in Asia, Europe and North America.
- On the other hand, considering the case of Africa, the use of biolubricants can meet the demands of developing countries, where the production of vegetable oils adapted to extreme climate conditions (such as cardoon or safflower) could contribute to an increase in industrial activity in a sustainable way, making these regions less dependent on energy or imported materials (like lubricants).
1.3. Waste cooking oil: a real challenge with endless opportunities (such as biolubricant production)
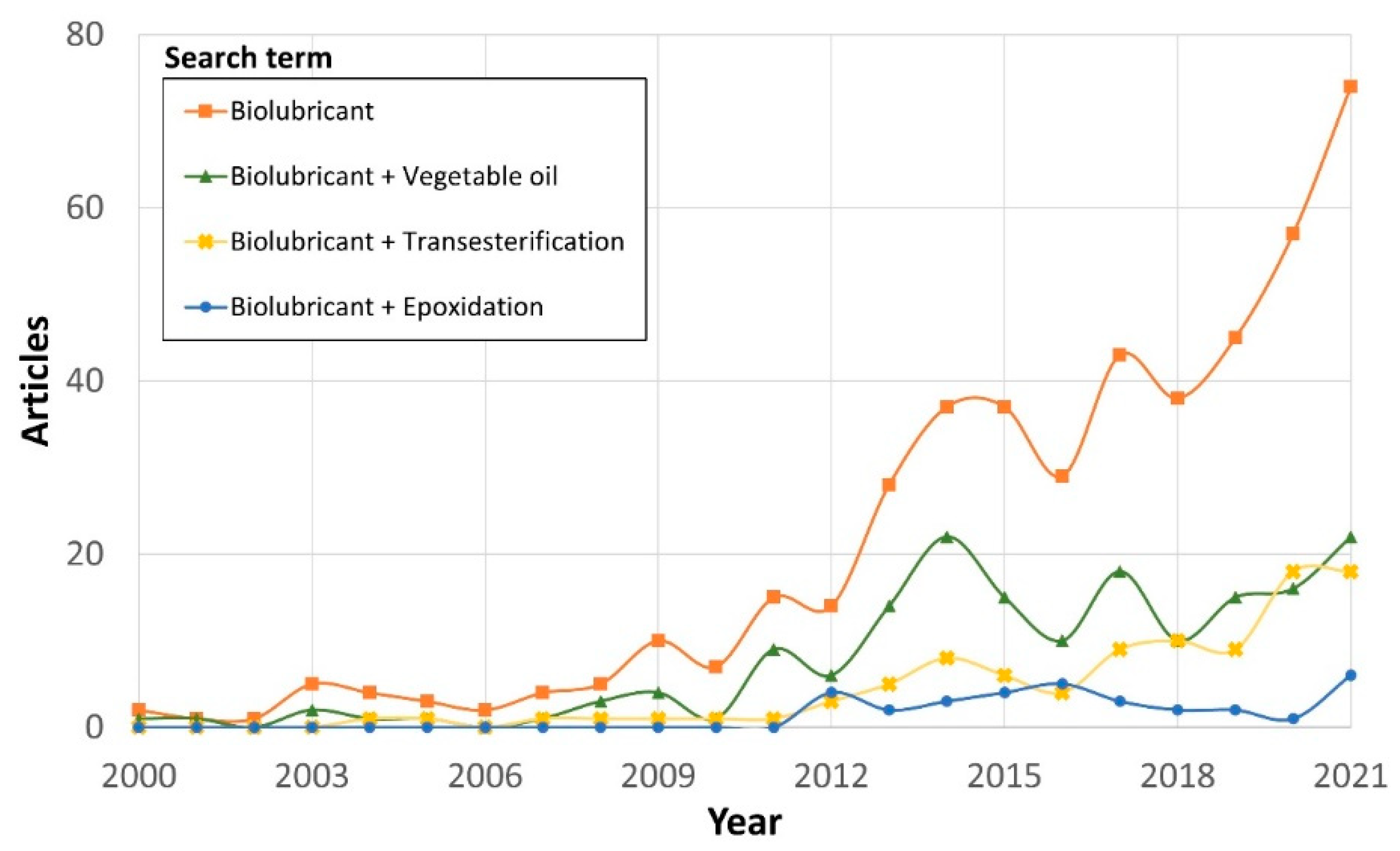
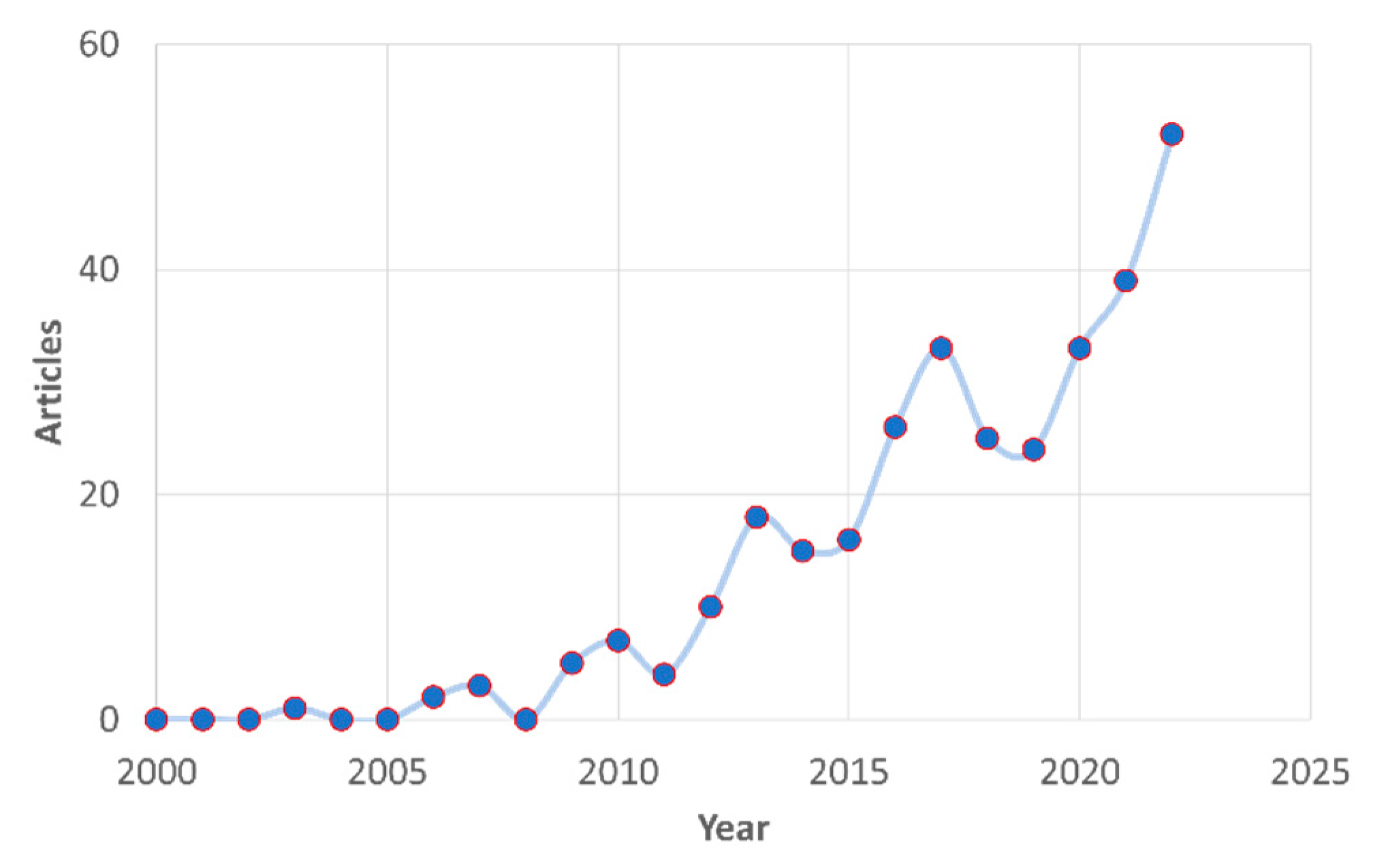
1.4. Scientific interest in biolubricants
1.5. Aim of this review
- Current use of vegetable oils in biolubricant production, especially concerning the possibilities related to the valorization of frying oil.
- Main biolubricant synthesis routes, paying special attention to transesterification processes, which offer a real potential for the implementation of biolubricant production in biorefineries based on vegetable oils.
- Use of catalysts, both homogeneous and heterogeneous, to make biolubricant production more competitive compared to the equivalent and traditional lubricant production.
- Main factors affecting quality during biolubricant production, with special focus on viscosity and oxidative stability, which are parameters that are vital to determine the use and service life of biolubricants. This way, the role of chemical conditions, as well as catalyst addition or additives such as antioxidants, will be specially enhanced.
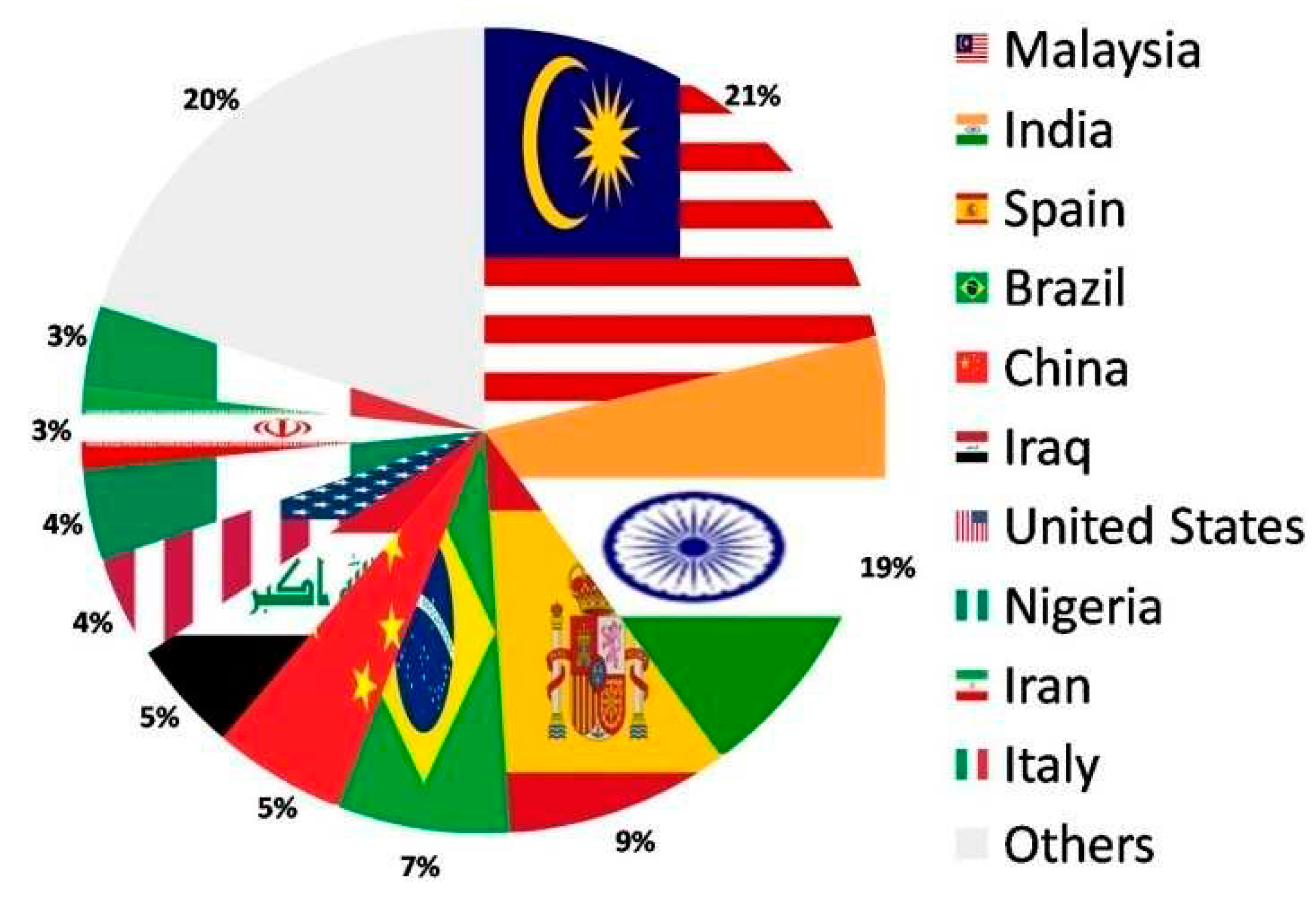
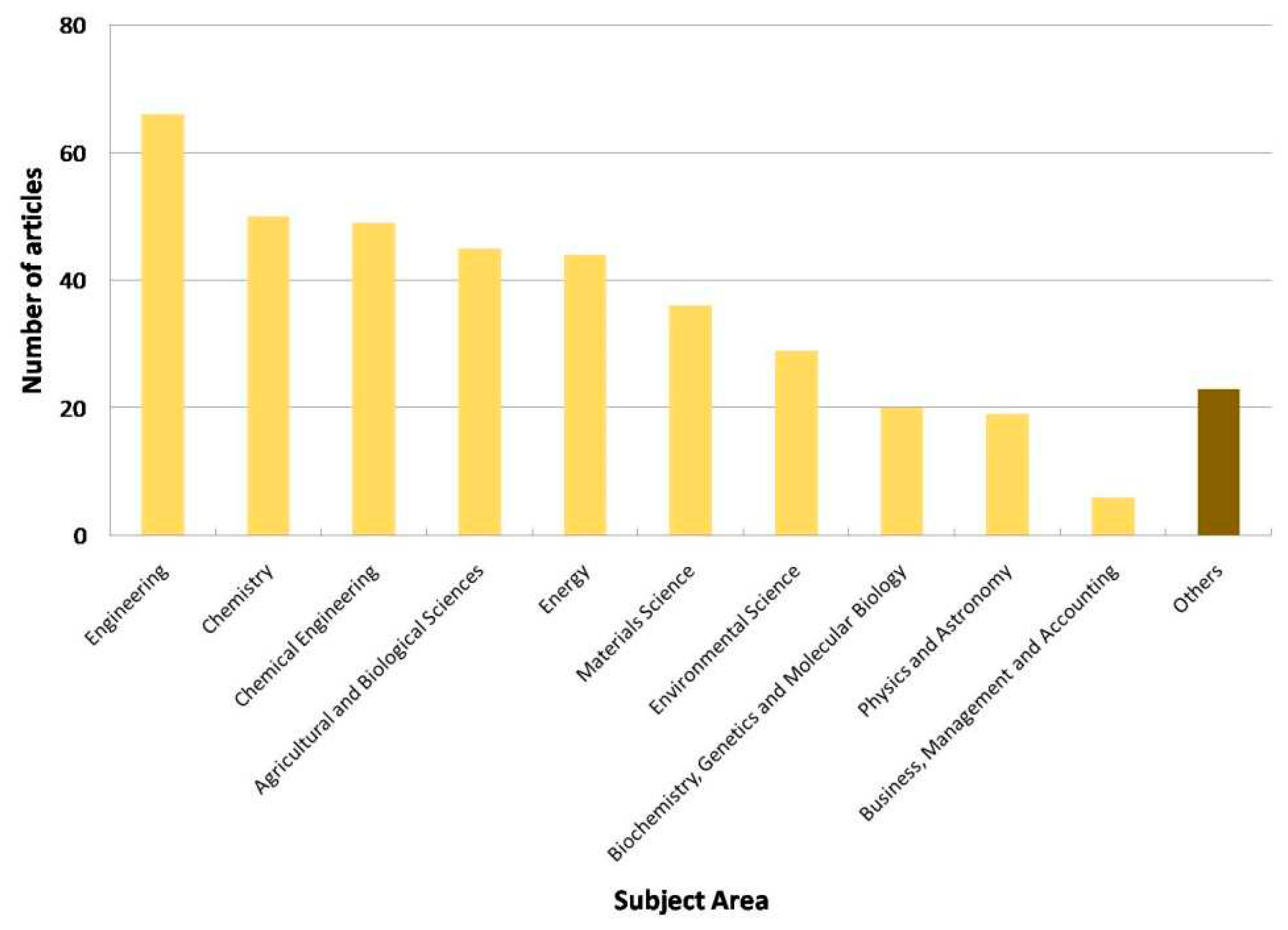
1.6. Scope and bibliometric analysis
2. Biolubricants based on vegetable oils: main sources and characteristics
2.1. Biolubricants: definition and raw materials
- They are more sustainable and biodegradable, as raw materials are natural compared to oil. Also, there are no by-products with a difficult management, as they can be used for other purposes or re-used in the same biolubricant production, as explained in further sections.
- Biolubricants usually have higher lubricity and viscosity index values (clearly exceeding 140-150, compared to the low values found for lubricants, at 90-100), which is important as it means that their viscosity is less dependent on temperature.
- They present, in general, higher flash and combustion points, which is a positive issue when it comes to safety during storage and shipping.
- However, they also present some disadvantages or challenges, like the following:
- Due to the presence of saturated fatty acids, biolubricants might present a poor performance at low temperatures, which limit their worldwide marketability.
- Hydrolysis can take place in contact with moisture, increasing the possibility of corrosion in facilities by increasing free fatty acid levels.
- They usually have a short oxidative stability, which could imply a change in their properties during storage or oxidation, which is undesirable.
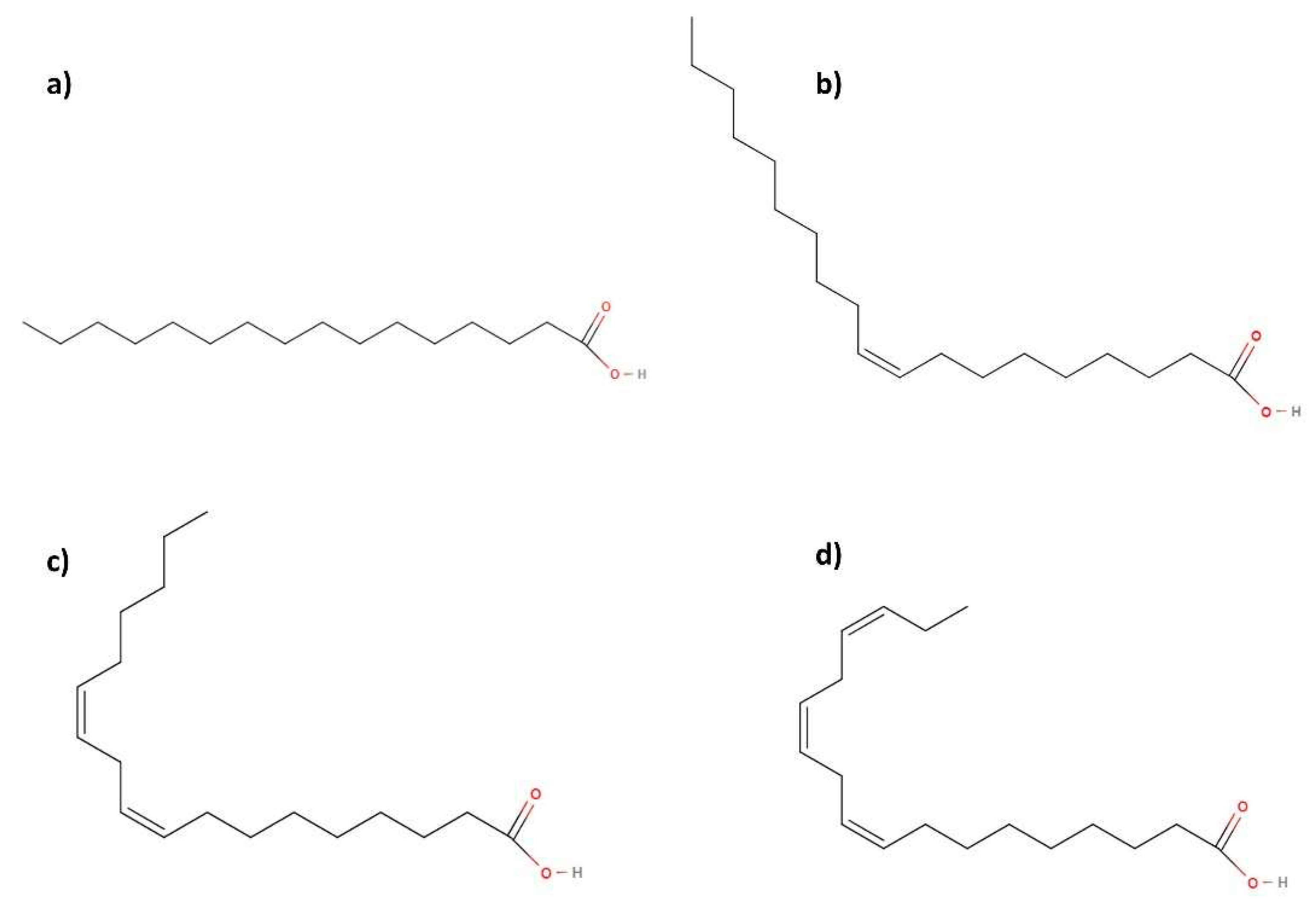
- In general, palmitic, oleic and linoleic acids are the majority fatty acids found for most vegetable oils, which points out their vital role in some properties in the final biolubricant, as explained in further sections. In that sense, the presence of double bonds in their molecular structure (see Figure 9), which can be conserved in the molecular structure of the final biolubricant (especially in the case of double transesterification of fatty acids), could determine its oxidative stability. Thus, the knowledge of the ratio of some fatty acids (such as oleic/linoleic ratio) is usually interesting to understand oxidative stability in biodiesel or biolubricants.
- Nevertheless, there are some specific oils, such as castor oil, whose main fatty acid presents some special properties, as in the case of ricinoleic acid, with an hydroxyl group that can influence the properties of the final biolubricant regarding viscosity (as it promotes intermolecular interactions like hydrogen bonds, implying an increase in viscosity). In any case, there are other oils that present high quantities of other fatty acids, such as lauric and myristic acid in the case of coconut oil or icosenoic acid for rapeseed, which could imply changes in the properties of the corresponding biolubricants.
- The use of GM crops, as in the case of safflower, might vary the properties of the corresponding oil, with a considerable increase in oleic acid (exceeding 80 %), improving some properties such as oxidative stability in the final biolubricant obtained [60]. Other studies point out the same aspect related to soybean oil, with a wide range of fatty acid contents depending on the gene technology used or the selection of soybean mutants [61]. Consequently, it is more interesting to consider FA profiles instead of kinds of vegetable oils, as it would give us a more exact idea about the raw material.
- The nature of WCO (and its subsequent fatty acid composition) might vary depending on the eating habits of the area where the research study was carried out. That is the reason why there was a wide range for oleic and linoleic acids in this table. In general, main oils used for cooking are rapeseed, sunflower, soy, and olive oil, which can vary in the diet of some regions or areas. In any case, the fatty acid composition of this waste is relatively equivalent to the rest of vegetable oils, which supports the idea that its use as biodiesel and biolubricant source is feasible if a proper pre-treatment is carried out.
2.2. Main characteristics of biolubricants
- Viscosity: This is an essential parameter for a biolubricant, as it will determine its use for specific purposes. Thus, the resistance to flow of a specific fluid (normally expressed in cSt) will influence on many factors such as the film thickness between the surfaces in contact or its permanence during lubricantion (the higher the viscosity is, the thicker the film will be). Viscosity is influenced by factors such as temperature or pressure. It can be measured by dynamic or kinematic methods (by using Cannon-Fenske or Ostwald viscosimeters) at a specific temperature (normally 40 or 100 °C, which will be useful to determine VI) [63].
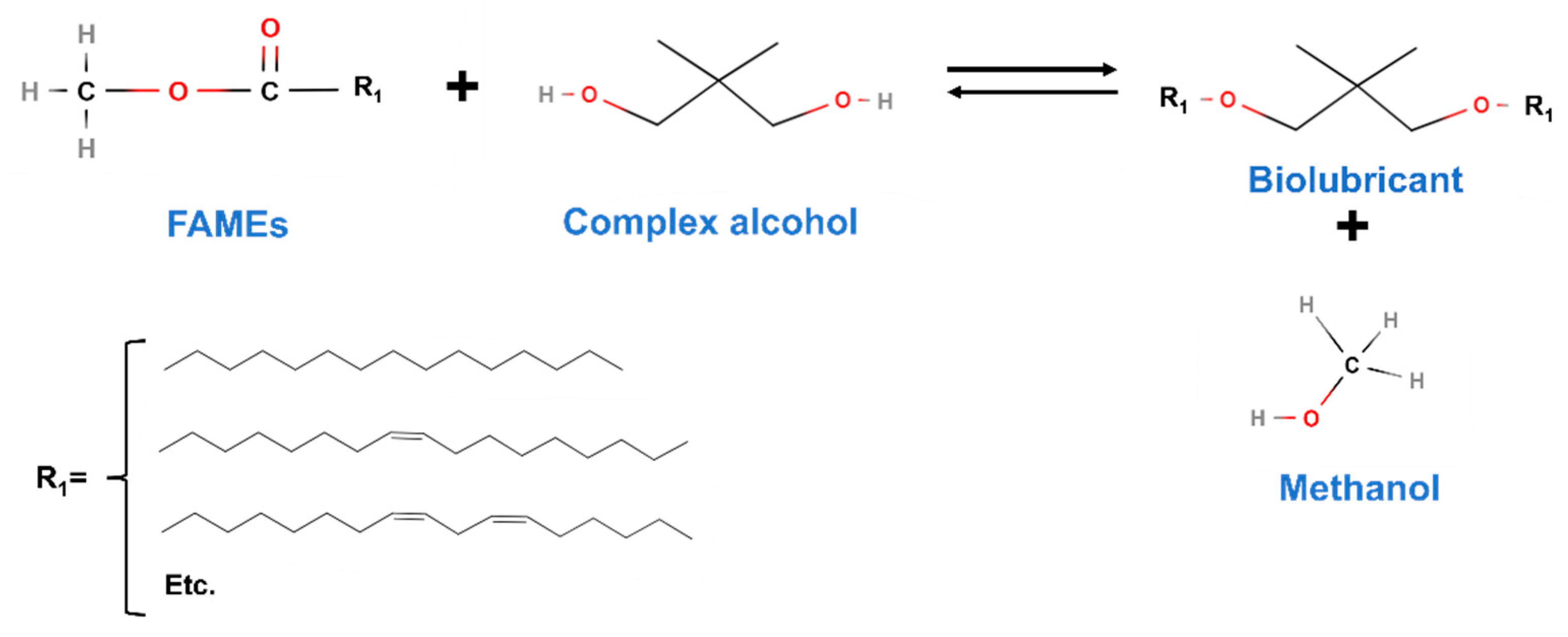
- Viscosity index (VI): This index indicates the changes in viscosity with temperature. Thus, a high viscosity index will imply a lower decrease in viscosity when temperature increases, which is a desirable effect as changes in temperature would not present a considerable influence on biolubricants [64,65]. High VI values will be obtained when the molecular structure of the final biolubricant is longer, and it will decrease with branching. That is the reason why the selection of a chemical rout or specific ragents (like complex alcohols in double transesterification) will be vital to determine this parameter. It is calculated by measuring viscosity at 40 and 100 °C.
- Oxidative stability (OS): This parameter is related to the stability of biolubricants during oxidation processes, including storage. It is expressed in hours and determined through the induction point (IP) according to the Rancimat method [66,67]. This way, free radical generation (mainly due to the presence of double bonds in biolubricants) could start a chain reaction, where propagation of free radicals could end up generating undesirable products such as free fatty acids (FFA) or polymers, among other decomposition compounds. The former could be related to the increase in acidity, which is an undesirable effect, especially when it comes to the maintenance of equipment and facilities. The latter is especially related to an increase in viscosity, as polymerization of esters generates more complex molecules, which could imply an increase in molecular interactions such as Van der Waals or hydrogen bonds and, therefore, a higher resistance to flow of biolubricants (that is, an increase in viscosity).
- Other parameters: in our opinion, the abovementioned properties are the most important ones to define the performance of a biolubricant, but there is a wide range of characteristics that should be considered like pour point (the lowest temperature at which a biolubricant pours or flows, which is desirable to be as low as possible to be useful in cold climates), lubricity (that is, the reduction of friction between two surfaces in contact when the biolubricant is used), flash and combustion points (usually higher compared to traditional lubricants, which is a great advantage when it comes to safety during storage or shipping), hydrolytic stability (resistance of esters to hydrolize, that is, to degrade in contact with water at high temperature) or biodegradability (which is considerably higher compared to petrol-based lubricants), among others.
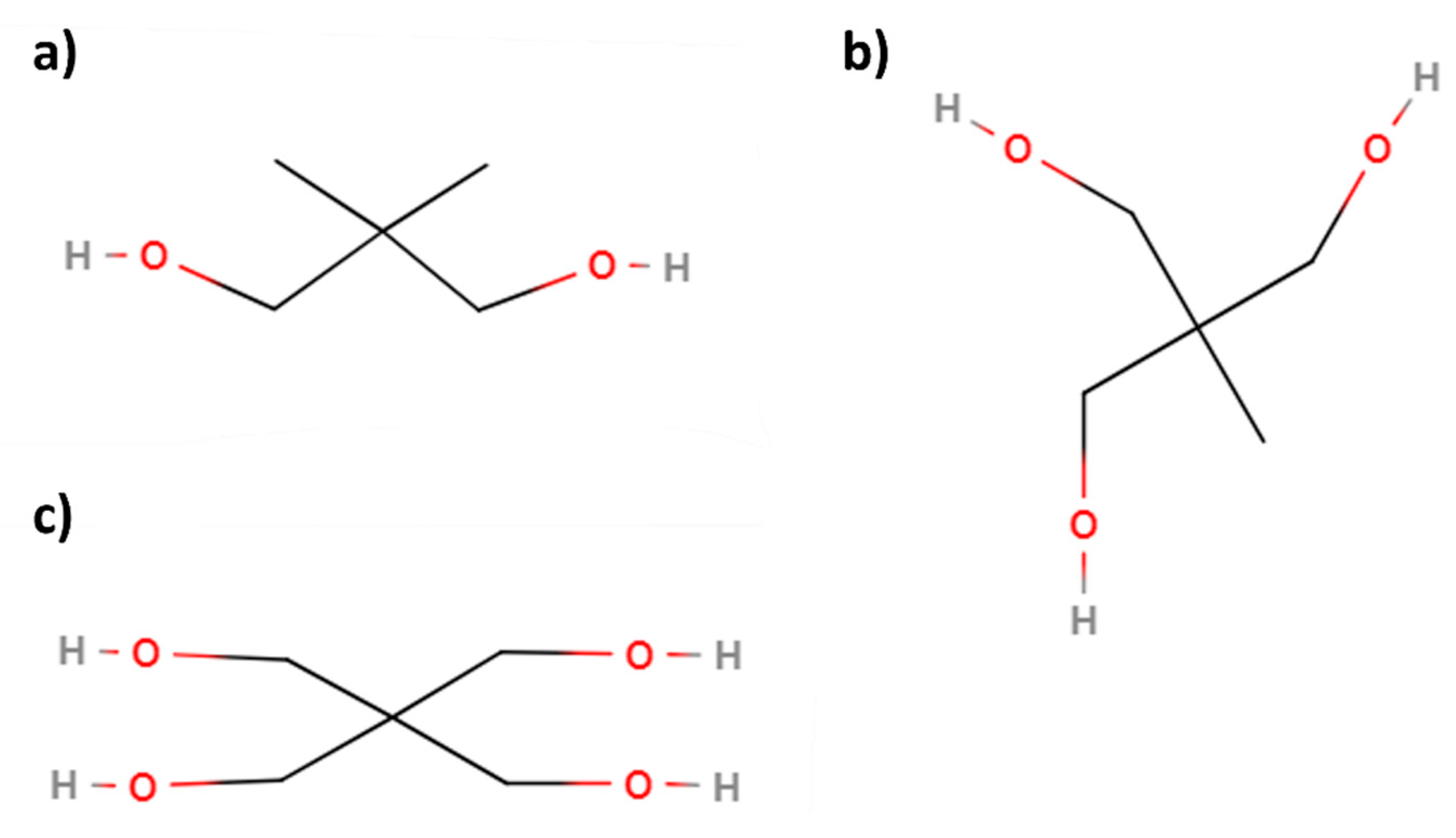
- Viscosity values are mainly influenced by the alcohol used in the second transesterification, as it is the determining factor for the final molecular structure of the biolubricant. There are some exceptions where the raw material plays an important role, as in the case of castor oil, whose majority compound (ricinoleic acid) promotes a considerable increase in viscosity by itself. Thus, biolubricants obtained with complex alcohols usually present an increasing viscosity in that order: 2-ethyl-1-hexanol < NPG < TMP < PE.
- Another important factor is the conversion of the process. Thus, low conversions will imply a mixture with biodiesel (with a viscosity range between 3 and 6, in most cases). That is the reason why the role of catalysts is so important, in order to obtain high conversion rates at mild reaction conditions.
- As previously explained, some properties of biolubricants are quite interesting, like high VI and flash points, which encourage the production of these compounds, as explained in the following section.
3. Biolubricant production
3.1. Different chemical routes for biolubricant production
- Epoxidation: This chemical route is carried out by using hydrogen peroxide, proxy acids like formic or acetic acids, and the final product is a peracid that epoxidates double bonds included in fatty acids. This way, unsaturations are removed, which can increase the oxidative stability of biolubricants obtained by this chemical route.
- Estolide formation: These compounds are usually produced by using strong acids (sulphuric acid, methanesulfonic acid or perchloric acids) as catalysts to activate alkene groups to produce estolides. Thus, they are generated by the bonding of a fatty acid’s carbonxylic acid functionality to an unsaturation of another fatty acid at relatively low temperautres (around 100 °C). This way, estolides usually have better cold flow properties and longer oxidative stability values compared to the original vegetable oil [80].
- Hydrolysis: It consists of the splitting of triglyceride molecules into fatty acids by using steam or water, implying an endothermic reaction. Some catalysts such as metal oxides (ZnO) can be used to increase conversion and yield. Also, subcritical water is another interesting way to achieve hydrolysis of vegetable oils without catalysts addition [81].
- Hydrogenation: It normally implies the total or partial reaction with molecular hydrogen, taking place an exothermic process. Partial hydrogenation can improve oxidative and thermal stability of original vegetable oils or biolubricants, whereas other properties can be unaltered (such as low temperature performance, viscosity and VI, among others). It is usually carried out at relatively low temperatures (150-210 °C) and high pressures (21-35 bar), using some catalysts such as Ni, Pa and Pt.
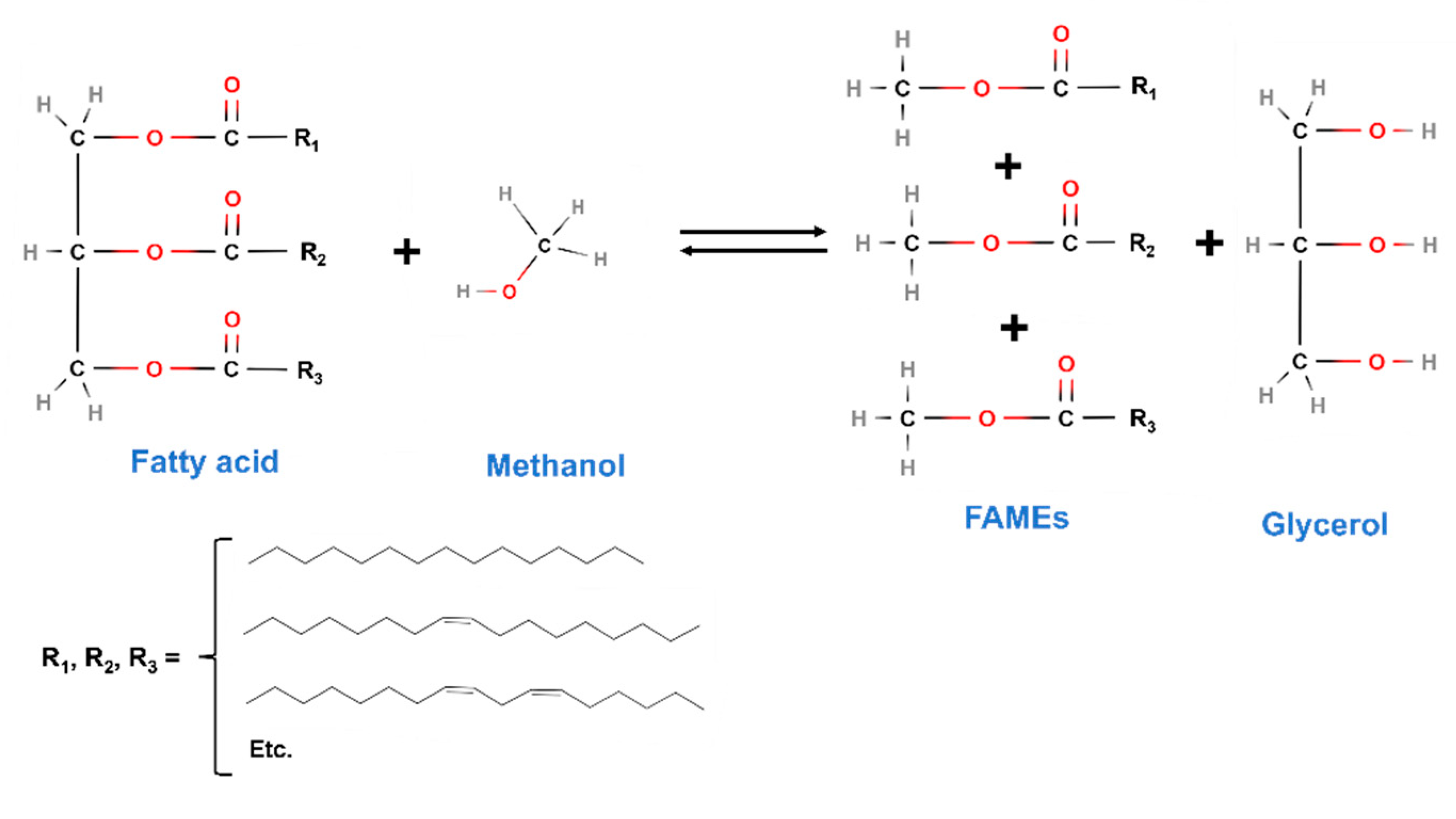
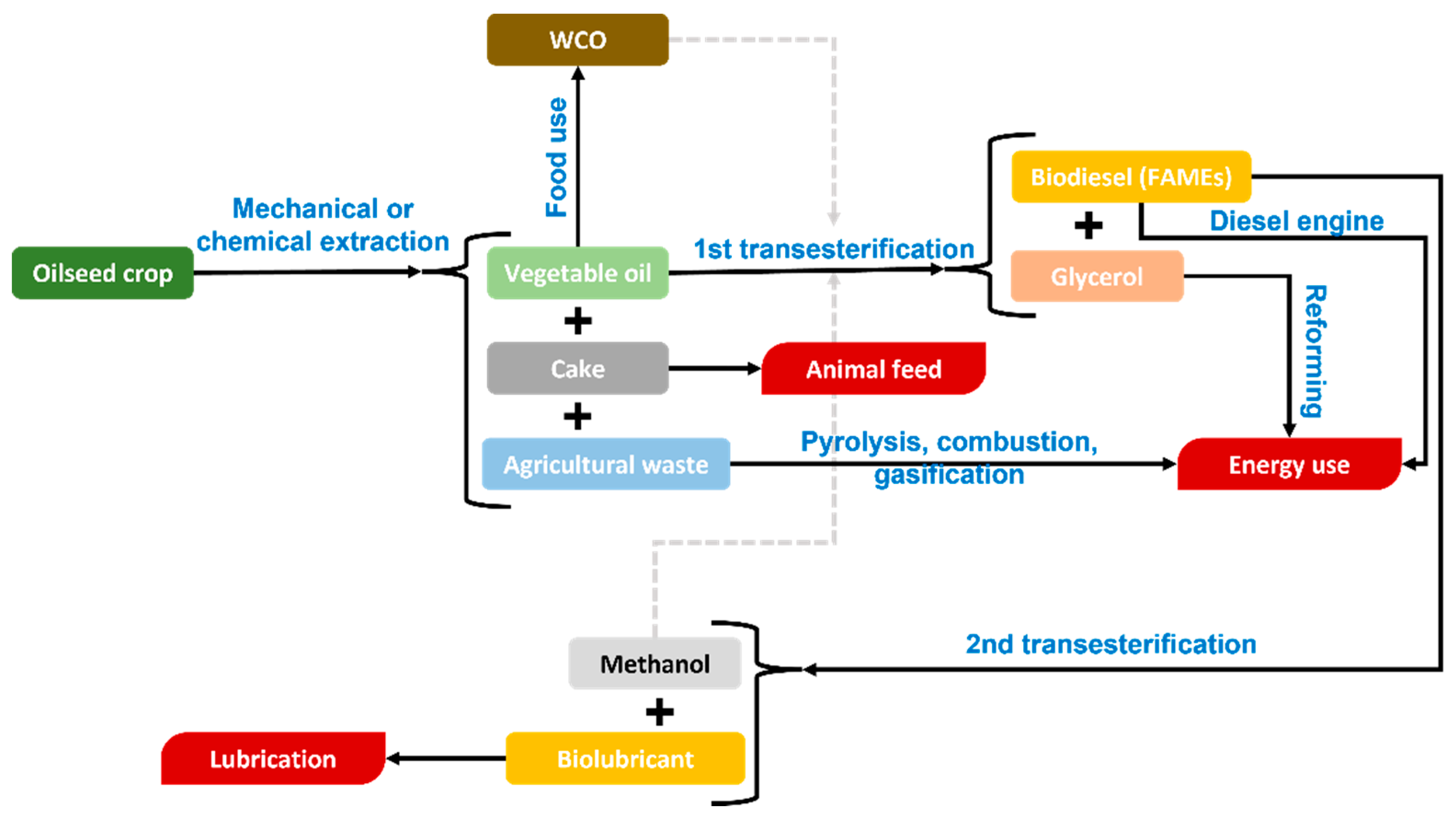
- Transesterification: This is the process generally used for biodiesel production from vegetable oils, removing glycerol from the triglyceride molecular structure. More details will be given later in this section.
3.2. Double transesterification as an interesting proposal for a biorefinery based on vegetable oils
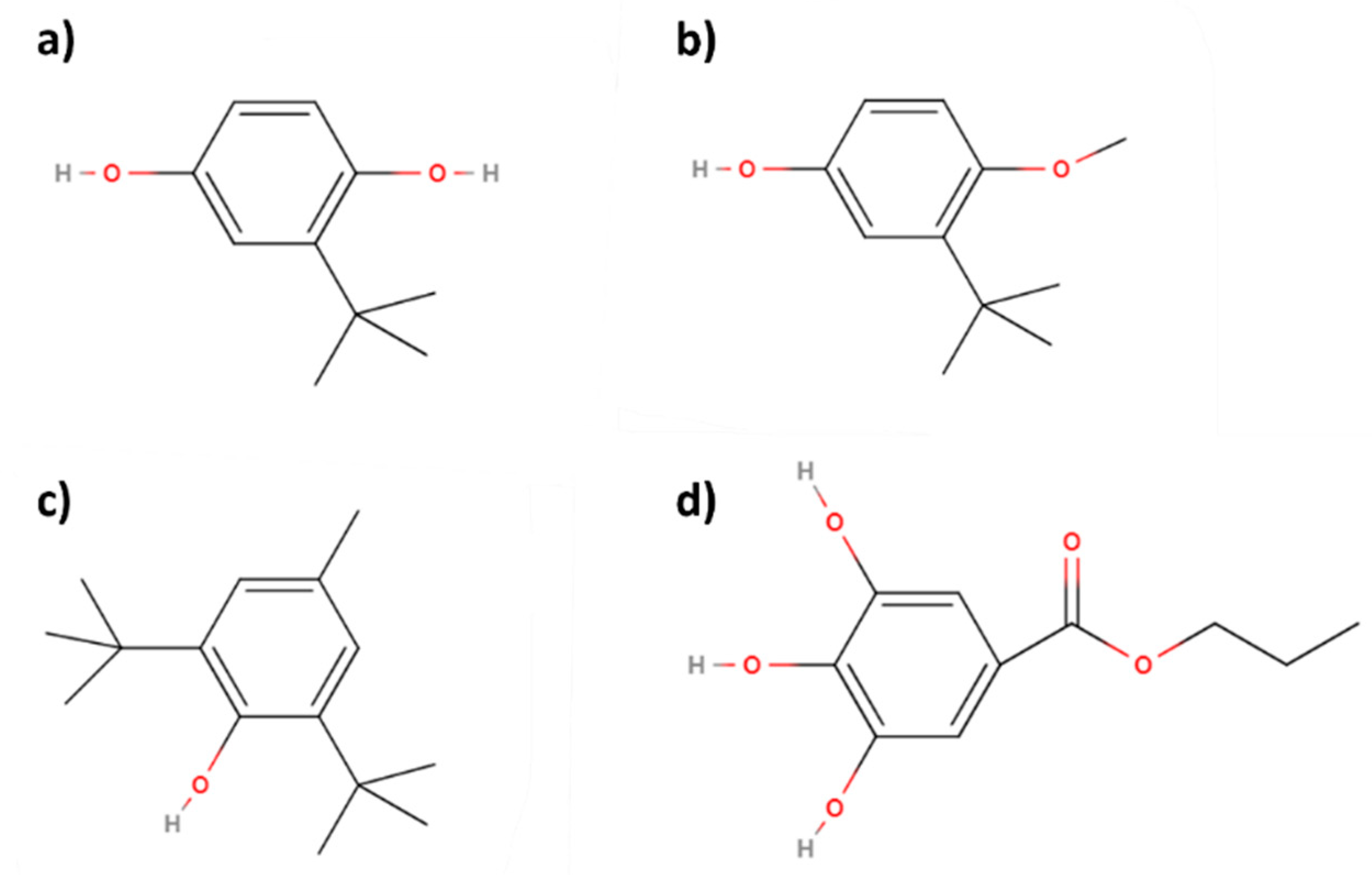
- Fatty acid methyl esters: As a result of the first transesterification, FAMEs are obtained, which can be used as fuels in Diesel engines, being the perfect replacement for traditional fuels used for that purpose. In that sense, compared to the UNE-EN 14214 standard [83], many of its requirements are clearly complied by biodiesel, especially concerning key aspects such as viscosity or cold filter plugging point (CFPP), which are essential for the correct performance in a Diesel engine. However, in many cases, and due to the same factors affecting biolubricants, the oxidative stability of FAMEs is not high enough [84,85] (not reaching 8 h, which is the lower limit of the European standard, for instance), requiring the use of alternatives such as antioxidant addition (mainly TBHQ, PG or BHA) or genetically modified (GM) crops, among others [56,86,87]. In any case, it is a very important product obtained in this process, which can increase the valorization of the whole biorefinery [88].
- Glycerol: It has been one of the most abundant and versatile by-products obtained during biodiesel production. Comparing the similarities of biodiesel and biolubricant production (indeed, FAME production is the initial stage for further biolubricant synthesis), it is no wonder that glycerol could play an important role in this case. Depending on the degree of purity of glycerol, it can be used in different ways, such as an energy source (through dry or steam reforming) or the precursor of interesting products like acrolein, propanediols or carboxylic acids, along with other products with a great interest in pharmaceutical industry) obtained from routes such as hydrogenation, oxidation, or esterification [89,90,91,92]. If the recent trend in biodiesel generation, along with the possible incorporation of biolubricant production through double transesterification, glycerol production is expected to increase, with the subsequent opportunity for its valorization through the abovementioned chemical routes. This way, a feasible technology applied to glycerol could be synthesis gas generation (a mixture of hydrogen and carbon monoxide at different ratios), which could produce green fuels as glycerol is generally obtained from raw materials [93].
- Methanol: One of the byproducts generated during the second transesterification to produce biolubricants is equally interesting, as it can be reused in the first transesterification process, where it is one of the reagents used. Thus, the concept of circular economy is really connected to this process, that could make a biorefinery based on vegetable oils more efficient. However, some factors should be taken into account during this process, like the possibility of using vacuum for a high biolubricant production yield, which could make the recovery of methanol more difficult or less economically feasible.
- Natural raw materials or wastes obtained in agricultural practices or food industries are normally used.
- Different products that are normally biodegradable are generated, as the raw materials are naturally obtained, the chemical transformations do not normally imply considerable changes in the molecular structure of the subsequent products (biodiesel and biolubricants), which make these products (in case of spillage) easily assimilable by microorganisms.
- These products can be directly used for energy production or further synthesis, or they can be upgraded to different degrees. Thus, the versatility of this technology is a strong point to compete with traditional refineries.
- Some of the byproducts generated can be directly (or indirectly through purification processes) reused in the same process, which is one of the key points of circular economy.
- With regard to these points, a biorefinery with these characteristics would perfectly fit the concept of green chemistry and circular economy, which is highly regarded by governments and society in general.
4. The use of catalysts in biolubricant production
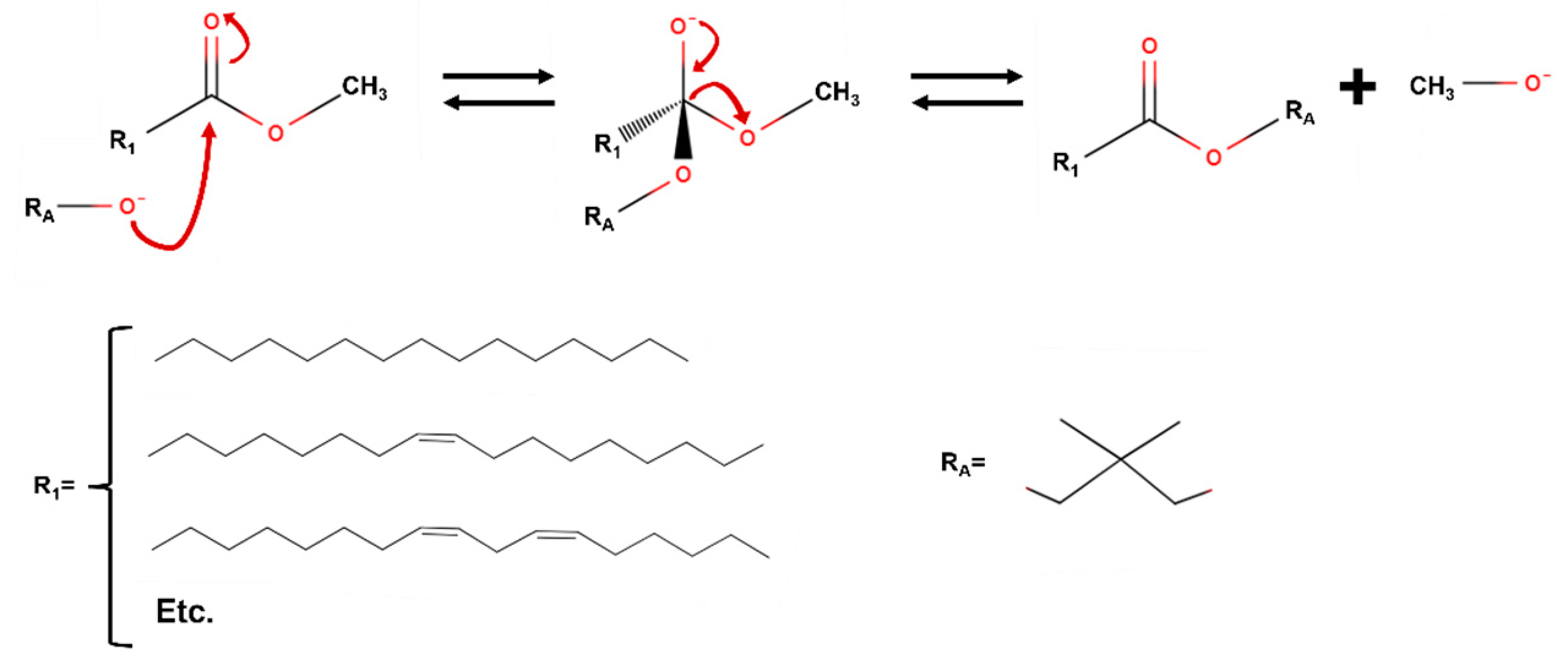
- Concerning homogeneous catalysts, they are usually classified as acidic and alkaline catalysts, which are normally more effective and provide faster reaction rates. Some examples of acidic catalysts are hydrochloric, sulfonic, sulfuric acids, etc., whereas examples of alkaline catalysts are sodium and potassium hydroxide and methoxide, among others. In the case of the latter, low moisture and FFA content in biodiesel is recommended to avoid a decrease in yield or side reactions. Figure 14 shows the main mechanism taking place when basic homogeneous catalysts are used, this way, the role of the catalyst was to generate the corresponding alkoxide (from neopentyl glycol, for instance) to carry out a nucleophilic substitution in the carboxyl group, with the final generation of the biolubricant and the release of methoxide and, subsequently, methanol.
- As far as heterogeneous catalysts are concerned, there are also acid and base catalysis, such as metal complexes, metal oxides, zeolites, membranes, or resins [102]. In this case, mass transfer phenomena, along with some important properties of the heterogeneous catalyst (such as porosimetry or reusability) should be taken into account in industrial design.
- Regarding lipases, their natural function is the hydrolysis of oils and fats to produce glycerol and free fatty acids, being one of the most resourceful enzymes in biocatalysis, as they are present in all organisms, and their variety can offer different characteristics. Lipases can be used for different purposes and chemical routes, such as esterification, transesterification, acidolysis or amidations, among others, being stable in different solvents, such as aqueous, organic or ionic [103,104,105]. In that sense, as explained in previous studies, transesterification with methanol to produce biodiesel has been successfully carried out, offering satisfactory results [57,106,107]. This way, considering the equivalence between this chemical route and double transesterification to produce biolubricants, its possible use for this purpose seems to be feasible, as explained later on.
- Regarding homogeneous catalysts, its removal from the final biolubricant might be complicated, as they are easily dissolved in the final product, increasing their level in some metal elements that could contribute to the acceleration of some degradation phenomena that can take place during storage (for instance, Na and K content due to catalyst addition could contribute to auto-oxidation acceleration). Thus, if a typical biodiesel purification (through successive washing steps) is considered as an equivalent process to be carried out in this case, it should be noted that hydrolysis could take place, which is an undesirable effect. In that sense, some alternatives could be chosen, like the avoidance of catalyst removal by adding some additives to increase the oxidative stability of biolubricants or the use of more expensive alternatives such as ion-exchange columns.
- In the case of heterogeneous catalysts, the separation process is quite simple, mainly through filtration after biolubricant production. In that sense, porous catalysts could be an interesting starting point, as its interaction with raw materials is higher [53,108,109]. However, the use of these catalysts is usually related to some inconvenients such as lower effectiveness compared to homogeneous catalysts or the low current reusability of these products, which presents a considerable room for improvement in biodiesel and biolubricant synthesis.
| Raw Material and catalyst |
Chemical conditions1 | Conversion, % | Reference |
|---|---|---|---|
| Part 1 | |||
| Rapeseed, corn and sunflower mixture, and WCO with titanium isopropoxide | Transesterification with 2-ethyl-1-hexanol at 160 °C, 1.5 % catalyst, and 1:1 molar ratio for 60 min | >96.5 | [31] |
| Palm oil with H2SO4 | Esterification of palm oil fatty acids with NPG at 138 °C, 1.12 % catalyst, and 1:2.26 molar ratio for 4.79 h | 87.6 | [110] |
| Elaeis guineensis kernel oil with H2SO4 | Transesterification with di-TMP at 150 °C, 1.7 % catalyst, and 1.6:1 molar ratio for 4.6 h | 79 | [111] |
| Methyl oleate with K2CO3 | Transesterification with TMP at 120 °C, 1.5 % catalyst, and 4:1 molar ratio for 240 min | 95.6 | [112] |
| Babassu oil with sodium methoxide | Transesterification with TMP at 65 °C, 1.0 % catalyst, and 3:1 molar ratio for 6 h at 700 mmHg | >90 | [113] |
| Palm oil and sodium methoxide | Transesterification with pentaerythritol at 158 °C, 1.19 % catalyst, and 4.5:1 molar ratio for 60 min | 40.13 | [71] |
| High-oleic safflower and sodium methoxide | Transesterification with pentaerythritol at 160 °C, 1.0 % catalyst, and 1:1/3 molar ratio for 120 min (working pressure 400 mmHg) | >94 | [60] |
| WCO and sodium methoxide | Transesterification with pentaerythritol at 160 °C, 1.0 % catalyst, and 1:1/3 molar ratio for 120 min (working pressure 260 mmHg) | 92.6 | [32] |
| WCO and zinc acetate | Transesterification with different alcohols (1-heptanol, 2-ethyl hexanol and neopentyl glycol) at 160 °C, 3.0 % catalyst, and different molar ratio for 240 min | n.d. | [59] |
| Cardoon oil and sodium methoxide | Transesterification with NG at 130 °C, 1.5 % catalyst, and 1:1 molar ratio for 120 min | >95 | [70] |
| High-oleic safflower and sodium methoxide | Transesterification with TMP at 140 °C, 1.0 % catalyst, and 1:1 molar ratio for 90 min (working pressure 400 mmHg) | >92 | [114] |
| Jatropha oil and sodium methoxide | Transesterification with TMP at 200 °C, 1.0 % catalyst, and 3.9:1 molar ratio for 3h (working pressure 10 mbar) | 47 | [115] |
| Part 2 | |||
| Rapeseed and sodium methoxide | Transesterification with TMP at 120 °C, 1.5 % catalyst, and 1:1 molar ratio for 90 min | >99 | [74] |
| High-oleic safflower and sodium methoxide | Transesterification with TMP at 100 °C, 0.3 % catalyst, and 1:1 molar ratio for 120 min and a working pressure of 210 mmHg | >94 | [73] |
| Fish oil residue with sodium ethoxide | Transesterification with TMP at 100-140 °C under vacuum | 84 | [116] |
| Litsea cubeba kernel oil with KOH | Transesterification with TMP at 130 °C, 1/4:1 molar ratio for 60 min and different working pressures | 92 | [117] |
| Cottonseed oil with sodium methoxide | Transesterification with TMP at 144 °C, 0.8 % catalyst, and 1/4:1 molar ratio for 10 h at 25 mbar | >90 | [118] |
| Jatropha oil with sodium hydroxide | Transesterification with ethylene glycol at 150 °C, 1.2 % catalyst, and 2:1 molar ratio for 120 min and vacuum | 98 | [50] |
| Mustard seed oil with KOH | Transesterification with 2-ethyl-1-hexanol at 70 °C, 2% catalyst, 2:1 molar ratio for 65 min at 0.05 bar | 93 | [69] |
| Raw material and catalyst |
Chemical conditions1 | Conversion, % | Reference |
|---|---|---|---|
| Part 1 | |||
| Castor oil and lipase | Transesterification with TMP at 40 °C, 0.4 % catalyst and atmospheric pressure, using a pervaporation membrane to remove CH4 | 59 | [119] |
| Soybean oil and lipase | Esterification with NG and TMP at 45 °C, 4 % catalyst and 6 h | 90 | [120] |
| Palm oil and solid acid catalyst | Esterification with NG at 180 °C, 2 % catalyst, 0.5:1 molar ratio and 4 h | 85 | [121] |
| Palm kernel oil with lipase | Transesterification with isoamyl alcohol at 45 °C, 4:1 molar ratio for 54 h | 99 | [78] |
| Palm oil and lipase | Esterification with TMP at 130 °C, 3% w/w catalyst, 3.45:1 molar ratio, 15.25 mbar for 48 h | 82 | [122] |
| Part 2 | |||
| WCO with CaO derived from waste cockle shell | Transesterification with TMP at 130 °C, 4% w/w catalyst, 3:1 molar ratio for 4 h | 97 | [77] |
| WCO with CaO | Transesterification with ethylene glycol at 130 °C, 1.2 % catalyst, 3.5:1 molar ratio for 1.5 h | 94 | [123] |
| Palm oil with mixed oxides of Ca and Sr on CaO | Transesterification with TMP at 180 °C, 1 %w/w mixed oxides of Ca and Sr catalyst with 5 %w/w SrO on CaO, 2 mbar and 240 min | 88 | [124] |
| Soybean oil with Zn Al hydrotalcites | Transesterification with TMP at 140 °C, 5 % catalyst, 4:1 molar ratio for 2 h | 77 | [125] |
| WCO with K2CO3-hydrotalcite | Transesterification with TMP at 160 °C, 2 % catalyst, 3:1 molar ratio and 300 Pa for 2 h | 80.6 | [126] |
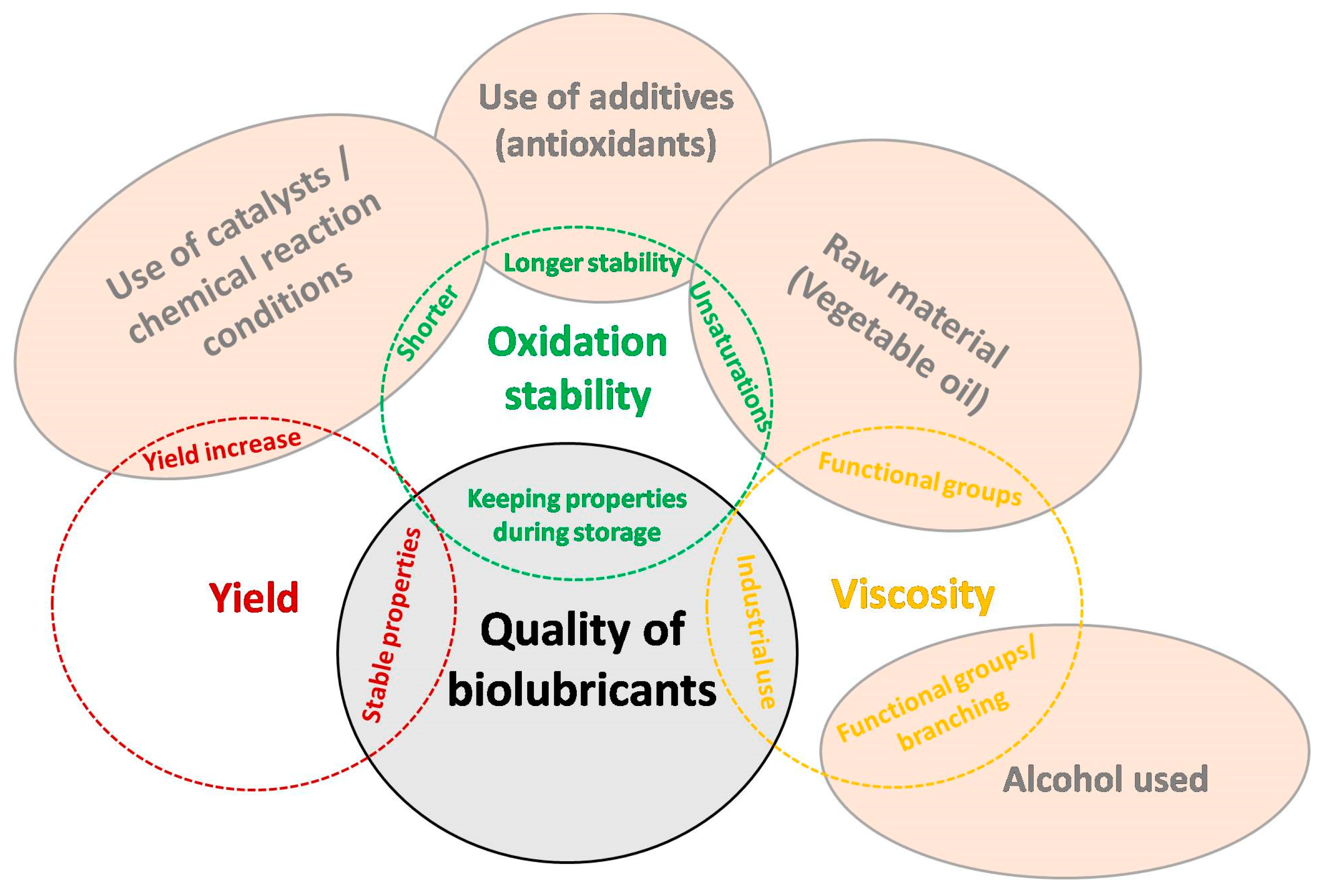
5. Influencing factors on quality parameters of biolubricants
5.1. Yield or conversion
5.2. Oxidative stability
5.3. Viscosity
5.4. Use of catalysts/chemical reaction conditions
5.5. Use of additives (antioxidants)
5.6. Raw material (vegetable oil)
5.7. Alcohols used
6. Techno-economic analyses applied to biolubricant production in a biorefinery context
- The role of raw materials is essential to determine the marketability of biolubricants, as the costs related to agricultural practices (such as harvest), along with the vegetable oil production and purification, usually imply a considerable percentage of fixed costs in a biorefinery based on vegetable oils. With this regard, again, the characteristics of WCO could be interesting, as many of the abovementioned steps could be skipped.
- One of the main strong points of biolubricant production at industrial scale is its integration in a biorefinery context, where multiple products can be obtained with the aim of adapting the performance of the biorefinery according to environmental policies or demand, among other factors.
- Another interesting aspect to consider is the reaction temperature (among other operating conditions), which usually takes a considerable percentage of energy consumption during biolubricant production. In that sense, a simple decrease of a few degrees could result in large savings. As proved in some abovementioned works, reaction temperature can easily be reduced by using some improvements such as the use of vacuum (when this technique is possible, as in many cases it can remove some reagents in the reaction medium like complex alcohols that tend to sublimate due to their molecular structure, such as 2,2-dimethyl-1,3-propanediol for double transesterification) or the use of catalysts (both heterogeneous and, homogeneous, where a considerable decrease in reaction temperature was observed).
- Indeed, the use of catalysts and their improvement related to their use in chemical routes to produce biolubricants has been widely studied in the literature. In that sense, their contribution to an increase in efficiency could be related to three aspects: First, the study of their optimum addition, to avoid extra costs; Second, the possibility of reuse (especially in the case of heterogeneous catalysts) or the purification process of biolubricants to remove homogeneous catalysts; Third, the effectiveness in reducing or improving chemical parameters that can contribute to energy or cost saving.
- Another interesting aspect to be considered is the possible real implementation of this technology by using pre-existing ones. Specifically, the use of double transesterification seems to be an interesting choice, as many of the facilities used for biodiesel production (that is, the first transesterification of fatty acids) could be perfectly adapted to the second transesterification process by adding some specific changes depending on the desired kind of biolubricant. Therefore, as similar facilities are required, the equipment acquisition would be easier and cheaper compared to other specific or customized facilities. Also, other chemical routes such as epoxidation could be easily adapted to this purpose, as explained to produce a biolubricant based on soybean oil [99].
- Finally, and even though some biolubricant productions could not be economically feasible at industrial level, there is one interesting and favorable point to tilt the balance in favor of this green technology: the high environmental value and the favorable policies carried out by national and international agencies. Thus, the market value of these products could be higher compared to traditional lubricants, as there is an increasing demand of green products by costumers in general. This fact could offset the initial and disadvantageous cost balance when these kinds of large-scale facilities.
7. Conclusions and prospects
- It has been proved the feasibility of biolubricant production from vegetable oils, obtaining high yields of this product regardless the chemical route selected and presenting some properties that are even better compared to traditional lubricants obtained from oil.
- Specifically, biolubricants through double transesterification present, in general, a higher viscosity index, which makes them more suitable to keep viscosity values under temperature changes.
- However, these products also present some challenges like the short oxidative stability, which could imply a change in very characteristic properties such as viscosity during storage or auto-oxidation processes. Nevertheless, there are accessible alternatives to avoid this problem, like the use of antioxidants such as TBHQ, BHA or PG, widely used in the scientific literature to increase the service life of this product.
- Regarding the possibility of implementation of this technology, it should be noted the potential of some wastes derived from VO such as WCO, whose management would be difficult otherwise. As a matter of fact, the properties observed for biolubricants based on WCO are equivalent to those obtained from vegetable oils, with a slight decrease in oxidative stability in some cases, which could be easily solved by antioxidant addition as explained above. This fact points out the versatility of biolubricant synthesis, being able to compete with traditional lubricant production.
- Considering the above, the possible integration of biolubricant production in biorefineries, or even the implementation of a biorefinery based on vegetable oils could be an interesting starting point for this technology, especially in the case of double transesterification from fatty acids, as many bioproducts and by products that are easily reusable can be obtained, implying a green process that should replace traditional ones.
- In that sense, the role of catalysts is becoming more and more important in biolubricant production, as its use contributes to a higher efficiency of the process, which is an essential part for the real implementation of this technology at industrial scale. This way, plenty of studies have been focused on the use of new and innovative catalysts, mainly heterogeneous and porous catalysts with the possibility of re-use in several cycles, to make the process more attractive when it comes to techno-economic analyses.
- Thus, “every detail counts”, which should be pointed out especially in the case of temperature, catalyst addition or reaction time, among other factors. Indeed, many studies addressed the use of milder reaction conditions, which will present a positive effect on techno-economic assessment but, especially, in biolubricant quality. In other words, it would be the greatest exponent of sustainability, as it would be possible to obtain better products (in this case biolubricants) under mild reaction conditions and the subsequent energy and material cost, implying a more attractive process for its implementation at industrial scale.
- Finally, and according to the techno-economic assessments carried out by the scientific literature (where the role of catalysts is essential), the industrial scale implementation of biolubricant production is feasible, pointing out the high added-value product obtained, apart from other by-products equally interesting or reusable, and showing a promising starting point for the contribution to green chemistry and circular economy.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Abbreviation | Term | Abbreviation | Term |
| BHA | Butylated hydroxyanisole | PEE | Pentaerythritol ester |
| CFPP | Cold filter plugging point | PG | Propyl gallate |
| FA | Fatty acid | PP | Pour point |
| FAE | Fatty acid esters | SDG | Sustainable development goal |
| FAME | Fatty acid methyl ester | TBHQ | Tert-Butylhydroquinone |
| FFA | Free fatty acids | TMP | Trimethylolpropane |
| FO | Frying oil | TMPE | Trimethylolpropane ester |
| GM | Genetically modified | UN | United Nations |
| IP | Induction point | VI | Viscosity index |
| NG | Neopentyl glycol | UVO | Used vegetable oil |
| NGE | Neopentyl glycol ester | VI | Viscosity index |
| OPEC | Organization of the Petroleum Exporting Countries | VO | Vegetable oil |
| OS | Oxidative stability | WCO | Waste cooking oil |
| PE | Pentaerythritol |
References
- Scarlat, N.; Dallemand, J.F.; Monforti-Ferrario, F.; Nita, V. The Role of Biomass and Bioenergy in a Future Bioeconomy: Policies and Facts. Environ Dev 2015, 15, 3–34. [Google Scholar] [CrossRef]
- UN Sustainable Development Goals. 2019.
- Statista Global Biolubricant Demand Available online:. Available online: https://www.statista.com/statistics/411616/lubricants-demand-worldwide/ (accessed on 16 March 2022).
- Johnstone, P.; McLeish, C. World Wars and Sociotechnical Change in Energy, Food, and Transport: A Deep Transitions Perspective. Technol Forecast Soc Change 2022, 174. [Google Scholar] [CrossRef]
- Johnstone, P.; McLeish, C. World Wars and the Age of Oil: Exploring Directionality in Deep Energy Transitions. Energy Res Soc Sci 2020, 69. [Google Scholar] [CrossRef] [PubMed]
- Scholten, D.; Bosman, R. The Geopolitics of Renewables; Exploring the Political Implications of Renewable Energy Systems. Technol Forecast Soc Change 2016, 103, 273–283. [Google Scholar] [CrossRef]
- Vakulchuk, R.; Overland, I.; Scholten, D. Renewable Energy and Geopolitics: A Review. Renewable and Sustainable Energy Reviews 2020, 122. [Google Scholar] [CrossRef]
- Palle, A. Bringing Geopolitics to Energy Transition Research. Energy Res Soc Sci 2021, 81. [Google Scholar] [CrossRef]
- Bricout, A.; Slade, R.; Staffell, I.; Halttunen, K. From the Geopolitics of Oil and Gas to the Geopolitics of the Energy Transition: Is There a Role for European Supermajors? Energy Res Soc Sci 2022, 88. [Google Scholar] [CrossRef]
- Umar, M.; Riaz, Y.; Yousaf, I. Impact of Russian-Ukraine War on Clean Energy, Conventional Energy, and Metal Markets: Evidence from Event Study Approach. Resources Policy 2022, 79. [Google Scholar] [CrossRef]
- Steffen, B.; Patt, A. A Historical Turning Point? Early Evidence on How the Russia-Ukraine War Changes Public Support for Clean Energy Policies. Energy Res Soc Sci 2022, 91. [Google Scholar] [CrossRef]
- Statista Comparison of Average Wholesale Motor Fuel Prices in Europe in 2018/19 and 22 as a Result of the Russia-Ukraine War, by Fuel Type (in U.S. Dollars per Metric Ton of Oil Equivalent). 20 May.
- Statista Average Annual OPEC Crude Oil Price from 1960 to 2023 (in U.S. Dollars per Barrel).
- Rowe, F.; Robinson, C.; Patias, N. Sensing Global Changes in Local Patterns of Energy Consumption in Cities during the Early Stages of the COVID-19 Pandemic. Cities 2022, 129. [Google Scholar] [CrossRef]
- Ha, L.T. Storm after the Gloomy Days: Influences of COVID-19 Pandemic on Volatility of the Energy Market. Resources Policy 2022, 79. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Su, C.W.; Zhu, M.N. Examining the Behaviour of Energy Prices to COVID-19 Uncertainty: A Quantile on Quantile Approach. Energy 2022, 239. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Su, C.W.; Khurshid, A.; Umar, M. COVID-19 Impact on Multifractality of Energy Prices: Asymmetric Multifractality Analysis. Energy 2022, 256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, R.; Li, R. Towards Smart Energy Systems—A Survey about the Impact of COVID-19 Pandemic on Renewable Energy Research. Energy Strategy Reviews 2022, 41. [Google Scholar] [CrossRef]
- Tian, J.; Yu, L.; Xue, R.; Zhuang, S.; Shan, Y. Global Low-Carbon Energy Transition in the Post-COVID-19 Era. Appl Energy 2022, 307. [Google Scholar] [CrossRef]
- Andrew, K.; Majerbi, B.; Rhodes, E. Slouching or Speeding toward Net Zero? Evidence from COVID-19 Energy-Related Stimulus Policies in the G20. Ecological Economics 2022, 201. [Google Scholar] [CrossRef]
- Vakulchuk, R.; Overland, I.; Scholten, D. Renewable Energy and Geopolitics : A Review. Renewable and Sustainable Energy Reviews 2020, 122, 109547. [Google Scholar] [CrossRef]
- Overland, I.; Bazilian, M.; Ilimbek Uulu, T.; Vakulchuk, R.; Westphal, K. The GeGaLo Index: Geopolitical Gains and Losses after Energy Transition. Energy Strategy Reviews 2019, 26, 100406. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Boachie, M.K.; Suleman, M.T.; Gupta, R. Structure Dependence between Oil and Agricultural Commodities Returns: The Role of Geopolitical Risks. Energy 2021, 219. [Google Scholar] [CrossRef]
- Statista Global Lubricant Demand Available online:. Available online: https://es.statista.com/ (accessed on 31 January 2023).
- Wan Azahar, W.N.A.; Bujang, M.; Jaya, R.P.; Hainin, M.R.; Mohamed, A.; Ngad, N.; Jayanti, D.S. The Potential of Waste Cooking Oil as Bio-Asphalt for Alternative Binder—An Overview. J Teknol 2016, 78. [Google Scholar] [CrossRef]
- Mannu, A.; Garroni, S.; Porras, J.I.; Mele, A. Available Technologies and Materials for Waste Cooking Oil Recycling. Processes 2020, 8. [Google Scholar] [CrossRef]
- Thushari, I.; Babel, S. Comparative Study of the Environmental Impacts of Used Cooking Oil Valorization Options in Thailand. J Environ Manage 2022, 310. [Google Scholar] [CrossRef] [PubMed]
- Frota de Albuquerque Landi, F.; Fabiani, C.; Castellani, B.; Cotana, F.; Pisello, A.L. Environmental Assessment of Four Waste Cooking Oil Valorization Pathways. Waste Management 2022, 138, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, J.; Martín, S.M.; Baldino, S.; Prandi, C.; Mannu, A. European Union Legislation Overview about Used Vegetable Oils Recycling: The Spanish and Italian Case Studies. Processes 2020, 8. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales, S.; González, J.F. Biorefinery Based on Different Vegetable Oils: Characterization of Biodiesel and Biolubricants. In Proceedings of the 3rd International Conference in Engineering Applications (ICEA); 2019; 2019. [Google Scholar]
- Encinar, J.M.; Nogales, S.; González, J.F. Biodiesel and Biolubricant Production from Different Vegetable Oils through Transesterification. Engineering Reports 2020, 1–10. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Cabanillas, A.G.; Romero, Á.G.; Encinar Martín, J.M. Monitoring Tert-Butylhydroquinone Content and Its Effect on a Biolubricant during Oxidation. Molecules 2022, 27, 8931. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Yan, J.; Xu, L.; Yang, M.; Yan, Y. Bioprocess Development for Biolubricant Production Using Non-Edible Oils, Agro-Industrial Byproducts and Wastes. J Clean Prod 2022, 357. [Google Scholar] [CrossRef]
- Paul, A.K.; Borugadda, V.B.; Goud, V. V. In-Situ Epoxidation of Waste Cooking Oil and Its Methyl Esters for Lubricant Applications: Characterization and Rheology. Lubricants 2021, 9, 1–14. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, H.; Song, Y.; Ma, S.; Xiong, W.; Chen, C.; Chen, B.; Zhang, X. Green Preparation of Branched Biolubricant by Chemically Modifying Waste Cooking Oil with Lipase and Ionic Liquid. J Clean Prod 2020, 274. [Google Scholar] [CrossRef]
- Dehghani Soufi, M.; Ghobadian, B.; Mousavi, S.M.; Najafi, G.; Aubin, J. Valorization of Waste Cooking Oil Based Biodiesel for Biolubricant Production in a Vertical Pulsed Column: Energy Efficient Process Approach. Energy 2019, 189. [Google Scholar] [CrossRef]
- Department for Transport (UK); UK Petroleum Industry Association Biofuel Feedstock Consumption in the United Kingdom (UK) in 2020, by Type (in Million Liters).
- Statista Number of Biodiesel Manufacturers in Japan in Fiscal Year 2021, by Type of Raw Material.
- Scopus Scopus Available online: https://www.scopus.com/home.uri.
- Mobarak, H.M.; Niza Mohamad, E.; Masjuki, H.H.; Kalam, M.A.; Al Mahmud, K.A.H.; Habibullah, M.; Ashraful, A.M. The Prospects of Biolubricants as Alternatives in Automotive Applications. Renewable and Sustainable Energy Reviews 2014, 33, 34–43. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw Materials, Chemical Modifications and Environmental Benefits. European Journal of Lipid Science and Technology 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Mobarak, H.M.; Niza Mohamad, E.; Masjuki, H.H.; Kalam, M.A.; Al Mahmud, K.A.H.; Habibullah, M.; Ashraful, A.M. The Prospects of Biolubricants as Alternatives in Automotive Applications. Renewable and Sustainable Energy Reviews 2014, 33, 34–43. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically Modifying Vegetable Oils to Prepare Green Lubricants. Lubricants 2017, 5, 1–17. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Pardal, A. Transesterification of Castor Oil under Ultrasonic Irradiation Conditions. Preliminary Results. Fuel Processing Technology 2012, 103, 9–15. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Sánchez, N.; González, J.F. Biolubricants from Rapeseed and Castor Oil Transesterification by Using Titanium Isopropoxide as a Catalyst: Production and Characterization. Catalysts 2020, 10. [Google Scholar] [CrossRef]
- Sánchez, N.; Encinar, J.M.; Nogales, S.; González, J.F. Biodiesel Production from Castor Oil by Two-Step Catalytic Transesterification: Optimization of the Process and Economic Assessment. Catalysts 2019, 9. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Plata, D.B.; Saboya, R.M.A.; de Luna, F.M.T.; Cavalcante, C.L.; Rodríguez-Castellón, E. An Overview of the Biolubricant Production Process: Challenges and Future Perspectives. Processes 2020, 8, 1–24. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Reddyhoff, T.; Gallegos, C.; Spikes, H.A. Tribological Studies of Potential Vegetable Oil-Based Lubricants Containing Environmentally Friendly Viscosity Modifiers. Tribol Int 2014. [Google Scholar] [CrossRef]
- Kania, D.; Yunus, R.; Omar, R.; Abdul Rashid, S.; Mohamad Jan, B. A Review of Biolubricants in Drilling Fluids: Recent Research, Performance, and Applications. J Pet Sci Eng 2015, 135, 177–184. [Google Scholar] [CrossRef]
- Attia, N.K.; El-Mekkawi, S.A.; Elardy, O.A.; Abdelkader, E.A. Chemical and Rheological Assessment of Produced Biolubricants from Different Vegetable Oils. Fuel 2020, 271. [Google Scholar] [CrossRef]
- Hájek, M.; Vávra, A.; De Paz Carmona, H.; Kocík, J. The Catalysed Transformation of Vegetable Oils or Animal Fats to Biofuels and Bio-Lubricants: A Review. 2021. [Google Scholar] [CrossRef]
- Arianti, N. Annisa; Widayat Widayat A Review of Bio-Lubricant Production from Vegetable Oils Using Esterification Transesterification Process. MATEC Web of Conferences 2018, 156. [Google Scholar] [CrossRef]
- Durango-Giraldo, G.; Zapata-Hernandez, C.; Santa, J.F.; Buitrago-Sierra, R. Palm Oil as a Biolubricant: Literature Review of Processing Parameters and Tribological Performance. Journal of Industrial and Engineering Chemistry 2022, 107, 31–44. [Google Scholar] [CrossRef]
- Encinar, J.M.; Pardal, A.; Martínez, G. Transesterification of Rapeseed Oil in Subcritical Methanol Conditions. Fuel Processing Technology 2012. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. Safflower Biodiesel : Improvement of Its Oxidative Stability by Using BHA and TBHQ. Energies (Basel) 2019, 19–22. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; Guiberteau, A.; Márquez, S. The Effect of Antioxidants on Corn and Sunflower Biodiesel Properties under Extreme Oxidation Conditions. JAOCS, Journal of the American Oil Chemists’ Society. [CrossRef]
- Encinar, J.M.; González, J.F.; Sánchez, N.; Nogales-Delgado, S. Sunflower Oil Transesterification with Methanol Using Immobilized Lipase Enzymes. Bioprocess Biosyst Eng 2019, 42, 157–166. [Google Scholar] [CrossRef]
- Bashiri, S.; Ghobadian, B.; Dehghani Soufi, M.; Gorjian, S. Chemical Modification of Sunflower Waste Cooking Oil for Biolubricant Production through Epoxidation Reaction. Mater Sci Energy Technol 2021, 4, 119–127. [Google Scholar] [CrossRef]
- Joshi, J.R.; Bhanderi, K.K.; Patel, J. V.; Karve, M. Chemical Modification of Waste Cooking Oil for the Biolubricant Production through Transesterification Process. Journal of the Indian Chemical Society 2023, 100. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Álvez-Medina, C.M. High Oleic Safflower Biolubricant through Double Transesterification with Methanol and Pentaerythritol: Production, Characterization, and Antioxidant Addition. Arabian Journal of Chemistry 2022, 15, 103796. [Google Scholar] [CrossRef]
- Milazzo, M.F.; Spina, F.; Cavallaro, S.; Bart, J.C.J. Sustainable Soy Biodiesel. Renewable and Sustainable Energy Reviews 2013, 27, 806–852. [Google Scholar] [CrossRef]
- Kurre, S.K.; Yadav, J. A Review on Bio-Based Feedstock, Synthesis, and Chemical Modification to Enhance Tribological Properties of Biolubricants. Ind Crops Prod 2023, 193. [Google Scholar] [CrossRef]
- UNE-EN ISO 3104/AC:1999 Petroleum Products. Transparent and Opaque Liquids. Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity (ISO 3104:1994). 1999.
- Verdier, S.; Coutinho, J.A.P.; Silva, A.M.S.; Alkilde, O.F.; Hansen, J.A. A Critical Approach to Viscosity Index. Fuel 2009, 88, 2199–2206. [Google Scholar] [CrossRef]
- ASTM-D2270-10 Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40 oC and 100 oC. 2016.
- Focke, W.W.; Westhuizen, I. Van Der; Oosthuysen, X. Biodiesel Oxidative Stability from Rancimat Data. Thermochim Acta 2016. [Google Scholar] [CrossRef]
- UNE-EN 14112 Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Oxidation Stability (Accelerated Oxidation Test) 2017.
- Chan, C.H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological Behavior of Biolubricant Base Stocks and Additives. Renewable and Sustainable Energy Reviews 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Chen, J.; Bian, X.; Rapp, G.; Lang, J.; Montoya, A.; Trethowan, R.; Bouyssiere, B.; Portha, J.F.; Jaubert, J.N.; Pratt, P.; et al. From Ethyl Biodiesel to Biolubricants: Options for an Indian Mustard Integrated Biorefinery toward a Green and Circular Economy. Ind Crops Prod 2019, 137, 597–614. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar Martín, J.M. Cardoon Biolubricant through Double Transesterification: Assessment of Its Oxidative, Thermal and Storage Stability. Mater Lett 2021, 302. [Google Scholar] [CrossRef]
- Aziz, N.A.M.; Yunus, R.; Rashid, U.; Syam, A.M. Application of Response Surface Methodology (RSM) for Optimizing the Palm-Based Pentaerythritol Ester Synthesis. Ind Crops Prod 2014, 62, 305–312. [Google Scholar] [CrossRef]
- Yunus, R.; Fakhru’l-Razi, A.; Ooi, T.L.; Omar, R.; Idris, A. Synthesis of Palm Oil Based Trimethylolpropane Esters with Improved Pour Points. Ind Eng Chem Res 2005, 44, 8178–8183. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar Martín, J.M.; Sánchez Ocaña, M. Use of Mild Reaction Conditions to Improve Quality Parameters and Sustainability during Biolubricant Production. Biomass Bioenergy 2022, 161, 106456. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Pinilla, A. Biolubricant Production through Double Transesterification: Reactor Design for the Implementation of a Biorefinery Based on Rapeseed. Processes 2021, 9, 1224. [Google Scholar] [CrossRef]
- Heikal, E.K.; Elmelawy, M.S.; Khalil, S.A.; Elbasuny, N.M. Manufacturing of Environment Friendly Biolubricants from Vegetable Oils. Egyptian Journal of Petroleum 2017. [Google Scholar] [CrossRef]
- Ocholi, O.; Menkiti, M.; Auta, M.; Ezemagu, I. Optimization of the Operating Parameters for the Extractive Synthesis of Biolubricant from Sesame Seed Oil via Response Surface Methodology. Egyptian Journal of Petroleum 2018, 27, 265–275. [Google Scholar] [CrossRef]
- Ghafar, F.; Sapawe, N.; Dzazita Jemain, E.; Safwan Alikasturi, A.; Masripan, N. Study on The Potential of Waste Cockle Shell Derived Calcium Oxide for Biolubricant Production; 2019; Vol. 19;
- Cerón, A.A.; Vilas Boas, R.N.; Biaggio, F.C.; de Castro, H.F. Synthesis of Biolubricant by Transesterification of Palm Kernel Oil with Simulated Fusel Oil: Batch and Continuous Processes. Biomass Bioenergy 2018, 119, 166–172. [Google Scholar] [CrossRef]
- Joshi, J.R.; Bhanderi, K.K.; Patel, J. V. A Review on Bio-Lubricants from Non-Edible Oils-Recent Advances, Chemical Modifications and Applications. Journal of the Indian Chemical Society 2023, 100. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q.S. Development of Biolubricants from Vegetable Oils via Chemical Modification. Journal of Industrial and Engineering Chemistry 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Ho, C.K.; McAuley, K.B.; Peppley, B.A. Biolubricants through Renewable Hydrocarbons: A Perspective for New Opportunities. Renewable and Sustainable Energy Reviews 2019, 113, 109261. [Google Scholar] [CrossRef]
- Owuna, F.J.; Dabai, M.U.; Sokoto, M.A.; Dangoggo, S.M.; Bagudo, B.U.; Birnin-Yauri, U.A.; Hassan, L.G.; Sada, I.; Abubakar, A.L.; Jibrin, M.S. Chemical Modification of Vegetable Oils for the Production of Biolubricants Using Trimethylolpropane: A Review. Egyptian Journal of Petroleum 2020, 29, 75–82. [Google Scholar] [CrossRef]
- UNE-EN 14214:2013 V2+A1:2018 Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. 2018.
- Saluja, R.K.; Kumar, V.; Sham, R. Stability of Biodiesel—A Review. Renewable and Sustainable Energy Reviews 2016. [CrossRef]
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A Comprehensive Review on Properties of Edible and Non-Edible Vegetable Oil-Based Biodiesel: Composition, Specifications and Prediction Models. Renewable and Sustainable Energy Reviews 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Tang, H.; De Guzman, R.C.; Salley, S.O.; Ng, S.K.Y. The Oxidative Stability of Biodiesel: Effects of FAME Composition and Antioxidant. Lipid Technol 2008, 20, 249–252. [Google Scholar] [CrossRef]
- Souza, A.G.; Medeiros, M.L.; Cordeiro, A.M.M.T.; Queiroz, N.; Soledade, L.E.B.; Souza, A.L. Efficient Antioxidant Formulations for Use in Biodiesel. Energy and Fuels 2014, 28, 1074–1080. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel Fuels. Prog Energy Combust Sci 2017. [CrossRef]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1–41. [Google Scholar] [CrossRef]
- Hu, Y.; He, Q.; Xu, C. Catalytic Conversion of Glycerol into Hydrogen and Value-Added Chemicals: Recent Research Advances. Catalysts 2021, 11. [Google Scholar] [CrossRef]
- Raza, M.; Inayat, A.; Abu-Jdayil, B. Crude Glycerol as a Potential Feedstock for Future Energy via Thermochemical Conversion Processes: A Review. Sustainability (Switzerland) 2021, 13. [Google Scholar] [CrossRef]
- Mota, C.; Pinto, B.P.; Lima, A.L. de Glycerol A Versatile Renewable Feedstock for the Chemical Industry; Springer International Publishing, 2017; ISBN 978-3-319-59375-3.
- Fasolini, A.; Cespi, D.; Tabanelli, T.; Cucciniello, R.; Cavani, F. Hydrogen from Renewables: A Case Study of Glycerol Reforming. Catalysts 2019, 9. [Google Scholar] [CrossRef]
- López-Bellido, L.; Wery, J.; López-Bellido, R.J. Energy Crops: Prospects in the Context of Sustainable Agriculture. European Journal of Agronomy 2014, 60, 1–12. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Alizadeh, P.; Hedjazi, S.; Hamzeh, Y. Potential of Soya as a Raw Material for a Whole Crop Biorefinery. Renewable and Sustainable Energy Reviews 2017, 75, 1269–1280. [Google Scholar] [CrossRef]
- Demichelis, F.; Fiore, S.; Pleissner, D.; Venus, J. Technical and Economic Assessment of Food Waste Valorization through a Biorefinery Chain. Renewable and Sustainable Energy Reviews 2018, 94, 38–48. [Google Scholar] [CrossRef]
- Esteban-Lustres, R.; Torres, M.D.; Piñeiro, B.; Enjamio, C.; Domínguez, H. Intensification and Biorefinery Approaches for the Valorization of Kitchen Wastes—A Review. Bioresour Technol 2022, 360. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Umesh, M.; Selvaraj, M.; Al-Shehri, B.M.; Chakraborty, P.; Duhan, L.; Sharma, S.; Pasrija, R.; Awasthi, M.K.; et al. Emerging Challenges for the Agro-Industrial Food Waste Utilization: A Review on Food Waste Biorefinery. Bioresour Technol 2022, 362. [Google Scholar] [CrossRef]
- Parente, E.J.; Marques, J.P.C.; Rios, I.C.; Cecilia, J.A.; Rodríguez-Castellón, E.; Luna, F.M.T.; Cavalcante, C.L. Production of Biolubricants from Soybean Oil: Studies for an Integrated Process with the Current Biodiesel Industry. Chemical Engineering Research and Design 2021, 165, 456–466. [Google Scholar] [CrossRef]
- Iriondo, A.; Barrio, V.L.; Cambra, J.F.; Arias, P.L.; Güemez, M.B.; Navarro, R.M.; Sánchez-Sánchez, M.C.; Fierro, J.L.G. Hydrogen Production from Glycerol Over Nickel Catalysts Supported on Al2O3 Modified by Mg, Zr, Ce or La. Top Catal 2008, 49, 46–58. [Google Scholar] [CrossRef]
- Dahdah, E.; Estephane, J.; Gennequin, C.; Aboukaïs, A.; Abi-Aad, E.; Aouad, S. Zirconia Supported Nickel Catalysts for Glycerol Steam Reforming: Effect of Zirconia Structure on the Catalytic Performance. Int J Hydrogen Energy 2020, 45, 4457–4467. [Google Scholar] [CrossRef]
- Ahmad, U.; Naqvi, S.R.; Ali, I.; Naqvi, M.; Asif, S.; Bokhari, A.; Juchelková, D.; Klemeš, J.J. A Review on Properties, Challenges and Commercial Aspects of Eco-Friendly Biolubricants Productions. Chemosphere 2022, 309. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Berenguer-Murcia, Á.; Rocha-Martin, J.; Vieira, R.S.; Fernandez-Lafuente, R. Biocatalytic Production of Biolubricants: Strategies, Problems and Future Trends. Biotechnol Adv 2023, 68, 108215. [Google Scholar] [CrossRef]
- Brêda, G.C.; Aguieiras, E.C.G.; Cipolatti, E.P.; Greco-Duarte, J.; Collaço, A.C. de A.; Costa Cavalcanti, E.D.; de Castro, A.M.; Freire, D.M.G. Current Approaches to Use Oil Crops By-Products for Biodiesel and Biolubricant Production: Focus on Biocatalysis. Bioresour Technol Rep. [CrossRef]
- De Sousa, I.G.; Mota, G.F.; Cavalcante, A.L.G.; Rocha, T.G.; Da Silva Sousa, P.; Holanda Alexandre, J.Y.N.; Da Silva Souza, J.E.; Neto, F.S.; Cavalcante, F.T.T.; Lopes, A.A.S.; et al. Renewable Processes of Synthesis of Biolubricants Catalyzed by Lipases. J Environ Chem Eng 2023, 11. [Google Scholar] [CrossRef]
- Dizge, N.; Keskinler, B. Enzymatic Production of Biodiesel from Canola Oil Using Immobilized Lipase. Biomass Bioenergy 2008. [Google Scholar] [CrossRef]
- Kumar, D.; Das, T.; Giri, B.S.; Verma, B. Preparation and Characterization of Novel Hybrid Bio-Support Material Immobilized from Pseudomonas Cepacia Lipase and Its Application to Enhance Biodiesel Production. Renew Energy 2020, 147, 11–24. [Google Scholar] [CrossRef]
- Masudi, A.; Muraza, O. Vegetable Oil to Biolubricants: Review on Advanced Porous Catalysts. Energy and Fuels 2018, 32, 10295–10310. [Google Scholar] [CrossRef]
- Kerman, C.O.; Gaber, Y.; Ghani, N.A.; Lämsä, M.; Hatti-Kaul, R. Clean Synthesis of Biolubricants for Low Temperature Applications Using Heterogeneous Catalysts. J Mol Catal B Enzym 2011, 72, 263–269. [Google Scholar] [CrossRef]
- Nor, N.M.; Salih, N.; Salimon, J. Optimization and Lubrication Properties of Malaysian Crude Palm Oil Fatty Acids Based Neopentyl Glycol Diester Green Biolubricant. Renew Energy 2022, 200, 942–956. [Google Scholar] [CrossRef]
- Bahadi, M.; Salimon, J.; Derawi, D. Synthesis of Di-Trimethylolpropane Tetraester-Based Biolubricant from Elaeis Guineensis Kernel Oil via Homogeneous Acid-Catalyzed Transesterification. Renew Energy 2021, 171, 981–993. [Google Scholar] [CrossRef]
- Xie, Q.; Zhu, H.; Xu, P.; Xing, K.; Yu, S.; Liang, X.; Ji, W.; Nie, Y.; Ji, J. Transesterification of Methyl Oleate for Sustainable Production of Biolubricant: Process Optimization and Kinetic Study. Ind Crops Prod 2022, 182. [Google Scholar] [CrossRef]
- Bezerra, R. de C. de F.; Rodrigues, F.E.A.; Arruda, T.B.M.G.; Moreira, F.B. de F.; Chaves, P.O.B.; Assunção, J.C. da C.; Ricardo, N.M.P.S. Babassu-Oil-Based Biolubricant: Chemical Characterization and Physicochemical Behavior as Additive to Naphthenic Lubricant NH-10. Ind Crops Prod 2020, 154. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González Cortés, Á. High Oleic Safflower Oil as a Feedstock for Stable Biodiesel and Biolubricant Production. Ind Crops Prod 2021, 170. [Google Scholar] [CrossRef]
- Gunam Resul, M.F.M.; Tinia, T.I.; Idris, A. Kinetic Study of Jatropha Biolubricant from Transesterification of Jatropha Curcas Oil with Trimethylolpropane: Effects of Temperature. Ind Crops Prod 2012, 38, 87–92. [Google Scholar] [CrossRef]
- Angulo, B.; Fraile, J.M.; Gil, L.; Herrerías, C.I. Bio-Lubricants Production from Fish Oil Residue by Transesterification with Trimethylolpropane. J Clean Prod 2018, 202, 81–87. [Google Scholar] [CrossRef]
- Cai, Z.; Zhuang, X.; Yang, X.; Huang, F.; Wang, Y.; Li, Y. Litsea Cubeba Kernel Oil as a Promising New Medium-Chain Saturated Fatty Acid Feedstock for Biolubricant Base Oil Synthesis. Ind Crops Prod 2021, 167. [Google Scholar] [CrossRef]
- Gul, M.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Mujtaba, M.A.; Harith, M.H.; Syahir, A.Z.; Ahmed, W.; Bari Farooq, A. Effect of TMP-Based-Cottonseed Oil-Biolubricant Blends on Tribological Behavior of Cylinder Liner-Piston Ring Combinations. Fuel 2020, 278, 118242. [Google Scholar] [CrossRef]
- Diaz, P.A.B.; Kronemberger, F. de A.; Habert, A.C. A Pervaporation-Assisted Bioreactor to Enhance Efficiency in the Synthesis of a Novel Biolubricant Based on the Enzymatic Transesterification of a Castor Oil Based Biodiesel. Fuel 2017, 204, 98–105. [Google Scholar] [CrossRef]
- Fernandes, K. V.; Cavalcanti, E.D.C.; Cipolatti, E.P.; Aguieiras, E.C.G.; Pinto, M.C.C.; Tavares, F.A.; da Silva, P.R.; Fernandez-Lafuente, R.; Arana-Peña, S.; Pinto, J.C.; et al. Enzymatic Synthesis of Biolubricants from By-Product of Soybean Oil Processing Catalyzed by Different Biocatalysts of Candida Rugosa Lipase. Catal Today 2021, 362, 122–129. [Google Scholar] [CrossRef]
- Ng, B.Y.S.; Ong, H.C.; Lau, H.L.N.; Ishak, N.S.; Elfasakhany, A.; Lee, H.V. Production of Sustainable Two-Stroke Engine Biolubricant Ester Base Oil from Palm Fatty Acid Distillate. Ind Crops Prod 2022, 175. [Google Scholar] [CrossRef]
- Abd Wafti, N.S.; Yunus, R.; Lau, H.L.N.; Choong, T.S.Y.; Abd-Aziz, S. Enzymatic Synthesis of Palm Oil-Based Trimethylolpropane Ester as Biolubricant Base Stock Catalyzed by Lipozyme 435. Energy 2022, 260. [Google Scholar] [CrossRef]
- Hussein, R.Z.K.; Attia, N.K.; Fouad, M.K.; ElSheltawy, S.T. Experimental Investigation and Process Simulation of Biolubricant Production from Waste Cooking Oil. Biomass Bioenergy 2021, 144. [Google Scholar] [CrossRef]
- Ivan-Tan, C.T.; Islam, A.; Yunus, R.; Taufiq-Yap, Y.H. Screening of Solid Base Catalysts on Palm Oil Based Biolubricant Synthesis. J Clean Prod 2017, 148, 441–451. [Google Scholar] [CrossRef]
- Shrivastava, S.; Prajapati, P.; Virendra; Srivastava, P. ; Lodhi, A.P.S.; Kumar, D.; Sharma, V.; Srivastava, S.K.; Agarwal, D.D. Chemical Transesterification of Soybean Oil as a Feedstock for Stable Biodiesel and Biolubricant Production by Using Zn Al Hydrotalcites as a Catalyst and Perform Tribological Assessment. Ind Crops Prod 2023, 192. [Google Scholar] [CrossRef]
- Sun, G.; Li, Y.; Cai, Z.; Teng, Y.; Wang, Y.; Reaney, M.J.T. K2CO3-Loaded Hydrotalcite: A Promising Heterogeneous Solid Base Catalyst for Biolubricant Base Oil Production from Waste Cooking Oils. Appl Catal B 2017, 209, 118–127. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Kotoka, F. The Potential of Castor, Palm Kernel, and Coconut Oils as Biolubricant Base Oil via Chemical Modification and Formulation. Thermal Science and Engineering Progress 2020, 16. [Google Scholar] [CrossRef]
- Sikdar, S.; Rahman, M.H.; Menezes, P.L. Synergistic Study of Solid Lubricant Nano-Additives Incorporated in Canola Oil for Enhancing Energy Efficiency and Sustainability. Sustainability (Switzerland) 2022, 14. [Google Scholar] [CrossRef]
- Ahmad, U.; Raza Naqvi, S.; Ali, I.; Saleem, F.; Taqi Mehran, M.; Sikandar, U.; Juchelková, D. Biolubricant Production from Castor Oil Using Iron Oxide Nanoparticles as an Additive: Experimental, Modelling and Tribological Assessment. Fuel 2022, 324. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Guiberteau, A.; Encinar, J.M. Effect of Tert-Butylhydroquinone on Biodiesel Properties during Extreme Oxidation Conditions. Fuel 2022, 310, 122339. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Rueda-Duran, C.A.; Ortiz-Sanchez, M.; Cardona Alzate, C.A. A Comprehensive Review on the Economic Assessment of Biorefineries: The First Step towards Sustainable Biomass Conversion. Bioresour Technol Rep 2021, 15. [Google Scholar] [CrossRef]
- Mousavi-Avval, S.H.; Sahoo, K.; Nepal, P.; Runge, T.; Bergman, R. Environmental Impacts and Techno-Economic Assessments of Biobased Products: A Review. Renewable and Sustainable Energy Reviews 2023, 180. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Laghezza, M.; Fiore, S.; Berruti, F.; Moustakas, K.; Cardona Alzate, C.A. Review of the Impact of Socio-Economic Conditions on the Development and Implementation of Biorefineries. Fuel 2022, 328. [Google Scholar] [CrossRef]
- Khan, S.; Das, P.; Quadir, M.A.; Thaher, M.; Annamalai, S.N.; Mahata, C.; Hawari, A.H.; Al Jabri, H. A Comparative Physicochemical Property Assessment and Techno-Economic Analysis of Biolubricants Produced Using Chemical Modification and Additive-Based Routes. Science of the Total Environment 2022, 847. [Google Scholar] [CrossRef] [PubMed]
- Moncada B, J.; Aristizábal M, V.; Cardona A, C.A. Design Strategies for Sustainable Biorefineries. Biochem Eng J 2016, 116, 122–134. [Google Scholar] [CrossRef]
- Budzianowski, W.M. High-Value Low-Volume Bioproducts Coupled to Bioenergies with Potential to Enhance Business Development of Sustainable Biorefineries. Renewable and Sustainable Energy Reviews 2017, 70, 793–804. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Sacia, E.R.; Sreekumar, S.; Gunbas, G.; Gokhale, A.A.; Scown, C.D.; Toste, F.D.; Bell, A.T. Novel Pathways for Fuels and Lubricants from Biomass Optimized Using Life-Cycle Greenhouse Gas Assessment. Proceedings of the National Academy of Sciences 2015, 112, 7645–7649. [Google Scholar] [CrossRef]
- Acevedo-García, B.; Santibañez-Aguilar, J.E.; Alvarez, A.J. Integrated Multiproduct Biorefinery from Ricinus Communis in Mexico: Conceptual Design, Evaluation, and Optimization, Based on Environmental and Economic Aspects. Bioresour Technol Rep 2022, 19. [Google Scholar] [CrossRef]
- Joshi, U.P.; Ham, P.G. Heterogeneous Catalyst for Transesterification and Method of Preparing Same. USRE49551 (E) 2023. [Google Scholar]
- Di Serio, M.; Gallo, F. PROCESS FOR THE PRODUCTION OF LUBRICATING BIOOILS. WO2023126789 (A1) 2023. [Google Scholar]
- Cavalcanti da Silva, J.A.; Silva, G.B.; Guimaraes Freire, D.M.; Gonçalves Aguieiras, E.C.; Cavalcanti Oliveira, E.D.; Greco Duarte, J.; Ignacio, K.L.; Soares, V.F.; Da Silva, P.R. PROCESS FOR PRODUCING ESTERS AND BIOLUBRICANTS, CATALYSED BYFERMENTED SOLID. US2021189442 (A1) 2021. [Google Scholar]
- Summers, W.A.; Williams, R.; Gulledge, D.; Tripp, R.B. System and Methods for Making Bioproducts. US2018319733 (A1) 2018. [Google Scholar]
- Salazar, J.A.; Joshi, M. METHOD FOR MAKING BIOFUELS AND BIOLUBRICANTS. CA2816018 (A1) 2012. [Google Scholar]
- Nagabhushana, K.; Mal, N.; Shinde, T.; Dapurkar, S.; Kumar, R. A PROCESS FOR PRODUCTION OF BIOLUBRICANT USING FLY ASH AS ACATALYST. WO2011007361 (A1) 2011. [Google Scholar]
- Hwan, K.I.; Won, K.J. Method for Producing Neopentyl Glycol Diester as a Biolubricant Using Enzymaticreaction. KR20220101428 (A) 2022. [Google Scholar]
| Vegetable oil | 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 18:3 | Others | References |
|---|---|---|---|---|---|---|---|---|
| Castor | 0.5-1.3 | n.d. | 0.5-1.2 | 4-5 | 2-8.4 | 0.4-1 | 83-88 | [43,44,45,46,47,48] |
| Coconut | 8-11 | n.d. | 1-3 | 5-8 | 0-1 | n.d. | 57-71 | [43,47] |
| Corn | 10.3-13 | 0.3 | 2-3 | 25-31 | 54-60 | 1 | n.d. | [43,47,49] |
| Jatropha | 13-16 | n.d. | 5-10.5 | 24-45 | 32-63 | 0.3-0.7 | 0.8-1.4 | [43,47,50,51,52] |
| Olive | 11.5-13.7 | 1.8 | 2.5 | 71-74 | 9.5-10 | 1.5 | n.d. | [43,47,51] |
| Palm | 37-47.9 | 0.4 | 3-8 | 37-45 | 1.9-10 | 0.3-0.5 | 1 | [43,47,51,53] |
| Rapeseed | 4-5 | 0.1- 0.2 | 1-2 | 56-69.8 | 20-26 | 6.2-10 | 9.1 | [43,45,47,54] |
| Safflower | 5-7 | 0.08 | 1-4 | 10-21 | 73-79 | n.d. | n.d. | [43,47,55] |
| Soybean | 9.3-12 | 0.2-1.7 | 3-4.7 | 21-27.3 | 48.5-56.3 | 5.6-8 | 2.3 | [43,47,48,50,51] |
| Sunflower | 4.9-7 | 0.1-0.3 | 1.9-5 | 18.7-68 | 21-68.6 | 0.1-1.9 | 2.2 | [43,46,47,50,56,57] |
| WCO | 6.6-36.8 | 0.21-1.9 | 3-18.4 | 17.9-37.5 | 11.8-72.1 | 0.02-2.02 | 3.3 | [31,50,58,59] |
| Biolubricant | Viscosity1, cSt | VI | Pour point, °C | Flash point, °C | IP, h | Reference |
|---|---|---|---|---|---|---|
| Rapeseed-based 2-ethyl-1-hexyl-esters | 7.97 | n.d 2 | n.d. | 196 | <6 | [31] |
| Seed-based 2-ethyl-1-hexyl-esters | 7.47 | n.d. | n.d. | 195 | >3 | [31] |
| WCO-based 2-ethyl-1-hexyl-ester | 7.40 | n.d. | n.d. | 193 | >3 | [31,59] |
| WCO-based 2-ethyl-1-hexyl-ester | 34.91 | 122 | −1 | 216 | n.d. | [59] |
| Mustard seed oil-based 2-ethyl-1-hexyl ester | 8.6 | n.d. | n.d. | n.d. | n.d. | [69] |
| Cardoon-based NPGE | 18.85 | 184 | n.d. | n.d. | <3 | [70] |
| WCO-based NPGE |
44.9 | 457 | −1 | 238 | n.d. | [59] |
| Palm-based PEE | 68.4 | 188 | −20 | 302 | n.d. | [71] |
| High-oleic safflower-based PEE | 77.7 | 155 | n.d. | 260 | 2.86 | [60] |
| WCO-based PEE | 68.5 | 144 | n.d. | 253 | 2.07 | [32] |
| Palm-based TMPE | 49.7 | 188 | −1 | n.d. | n.d. | [72] |
| High-oleic safflower-based TMPE | 73.39 | 103 | n.d. | 216 | 6.7 | [60] |
| High-oleic safflower-based TMPE | 89.11 | 131 | n.d. | 220 | >7 | [73] |
| Rapeseed-based TMPE | 75.5 | 128 | n.d. | 210 | 4.9 | [74] |
| Jatropha-based TMPE | 51.89 | 140 | −3 | n.d. | n.d. | [75] |
| Palm-based TMPE | 38.25 | 171 | 5 | 240 | n.d. | [75] |
| Sesame-based TMPE | 35.55 | 193 | −21 | 196 | n.d. | [76] |
| WCO-based TMPE |
30 | 179 | n.d. | n.d. | n.d. | [77] |
| Palm kernel-based isoamyl ester | 3-6 | 149 | n.d. | n.d. | 0.3 | [78] |
| Chemical route | Details | Advantages | Disadvantages |
|---|---|---|---|
| Epoxidation | Double bound removal and introduction of a epoxide functional group | Higher OS and lubricity | Viscosity, VO and PP usually low |
| Estolide formation | Estolide generation through different ways, reacting two acidic molecules | Low temperature reaction, higher OS and lubricity and better performance at low temperatures | Expensive |
| Esterification and Transesterification | Use of alcohols to transform fatty acids into fatty acid esters | High VI and flash point, improvement of low-temperature properties | High reaction temperatures. OS depends on fatty acid profile of the raw material. |
| Hydrogenation | Reaction with molecular hydrogen | Better OS and lower unsaturation | Possible side reactions |
| Description | Details | Reference |
|---|---|---|
| Biolubricant production from rapeseed oil through double transesterification with methanol and TMP | High conversions were obtained for first (97 %) and second (99 %) transesterification, and a reactor was designed (12 m3) for industry level (production = 5550 tm·y−1) | [74] |
| Techno-economic analysis of biolubricants through different chemical routes | Non-edible oils (karanja, jatropha, WCO) through transesterification with TMP was proved as one sustainable way to obtain biolubricants for food lubrication and as automotive oils | [134] |
| Study of different biorefineries (based on first to fourthgeneration raw materials, including edible and non-edible oils) for its design at different scale levels | The authors point out the importance of a suitable design of biorefineries depending on the purity and use of the final product obtained. Thus, high-quality products such as pharmaceutics are more adequate for small scales, whereas energy products could be useful at industrial scale | [135] |
| Biorefinery based on non-food agricultural feedstocks (vegetable pulp). | High lifecycle greenhouse gas savings can be obtained (up to 80 %) if biofuels and biolubricant production are coupled in a biorefinery context | [136,137] |
| Biorefinery based on castor oil to produce biodiesel and multiple products, including a biolubricant | This biorefinery was mainly based on biodiesel production (exceeding 40 % of total production) as well as other products, pointing out that multi-objective optimization is essential to obtain the optimal feedstock distribution and operating conditions to upgrade its performance | [138] |
| Description | Details | Reference |
|---|---|---|
| Preparation of heterogeneous catalyst for transesterification | It can be used for manufacturing commercial grade biodiesel, biolubricant and glycerol | [139] |
| Production of lubricating bio-oils | Use of soaps, WCO, animal fats with an initial hydrolysis, to react with several alcohols and produce biolubricants | [140] |
| Production of biolubricants catalyzed by fermented solid | The reaction of methyl esters or free fatty acids with a polyhydroxylated alcohol is catalyzed by a fermented solid containing lipases | [141] |
| System for making biolubricants | A process for a continuous preparation of biolubricants is described, including the use of acidic heterogeneous catalyst | [142] |
| Method for making biofuel and biolubricant | A process for producing biofuels and biolubricants from lipid material, pointing out the possibility of a biorefinery | [143] |
| Biolubricant production using fly ash as catalyst | Reaction of fatty acids with different alcohols for the production of alkyl esters with C5 to C12 alcohols in the presence of fly ash as catalyst | [144] |
| Method for producing neopentyl glycol diester as a biolubricant | Neopentyl glycol and vegetable fatty acids react using immobilized lipase | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).






