1. Introduction
Nanotechnology-based approaches are becoming widely utilized in single-molecule studies of enzymes. These approaches include atomic force microscopy (AFM) is commonly employed for estimation of activity of single enzyme molecules. For instance, in [
1], semi-contact mode of AFM was used for the monitoring of height fluctuations of lysozyme molecules adsorbed on mica. The time dependencies of the height fluctuations were obtained without scanning, upon positioning the AFM tip above lysozyme monolayer under various conditions: in buffer, in the presence of the lysozyme substrate, and in the presence of its inhibitor. The height fluctuations with an amplitude of 1 nm and a period of 50 ms were observed in the presence of the oligosaccharide substrate. In contrast, in the presence of the inhibitor, the fluctuations amplitude reduced to the level observed before the substrate addition. The interpretation of these findings suggests that the height fluctuations of lysozyme correspond to its conformational changes during hydrolysis reaction. Furthermore, these fluctuations can be influenced by differences in height and elasticity of the lysozyme-substrate transition complex. Similar approach was employed in other studies [
2,
3], in which the fluctuations of IgG antibodies, and the functioning of the chitosanase enzyme were investigated. These examples illustrate how AFM allows one to monitor enzymatic activity by tracking the oscillation of the AFM probe. Furthermore, analogous approach was employed in order to measure the activity of single molecules of cytochrome P450 BM3 enzyme [
4].
With respect to AFM, one should emphasize that the implementation of this approach requires expensive equipment, and the AFM experiments are time-consuming. Alternative nanotechnology-based approach to the registration of functioning of single enzyme molecules is based on the use of nanopores. In numerous papers, the use of natural nanopores was reported [
5,
6,
7,
8,
9,
10]. The approach employing natural nanopores has certain limitations, which are connected with the size of these nanopores. This is why the use of natural nanopores for studying a wide range of enzymes is still limited.
In contrast to the use of natural nanopores, the application of artificial solid-state nanopores overcomes the above-discussed limitation, since their diameters can be varied upon the nanopore fabrication, thus allowing one to study diverse enzymes. Thus, the technology based on solid-state nanopores with a pore diameter of about 5 nm is the most promising nanopore-based approach for investigating enzyme activity [
11]. These solid-state nanopores can be fabricated by different methods, including focused ion beam (FIB) [
11], controlled dielectric breakdown (SDB) [
12,
13], and electron beam drilling (EBD) [
14]. The material commonly used for the fabrication of solid-state nanopores is silicon nitride (SixNy, hereafter abbreviated SiN).
Enzyme systems play key roles in diverse metabolic processes in living organisms [
15]. Herein, horseradish peroxidase (HRP) enzyme, which pertains to a broad class of peroxidases, has been selected as an object owing to its well-known properties. HRP participates in catalytic oxidation reactions involving a wide range of organic and inorganic compounds in the presence of hydrogen peroxide [
16]. The molecular weight of HRP is approximately 40 kDa [
17]. The catalytic activity of HRP can be assessed using a distinctive reaction involving its substrate 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) and hydrogen peroxide, described by Sanders et al. [
18].
In our present research, the EBD technology has been employed in order to fabricate the solid-state nanopore in SiN. The HRP enzyme was inserted into the nanopore, and the ABTS oxidation reaction was started by adding hydrogen peroxide (H2O2). The change in the current flowing through the nanopore during the enzyme functioning has been monitored.
2. Results
2.1. Nanopore fabrication
According to [
19], the dimensions of horseradish peroxidase (HRP) were determined to be approximately 4.3×4.8×5.8 nm based on X-ray data [
19]. The nanopore with a diameter of 5 nm was fabricated in 40-nm-thick SiN.
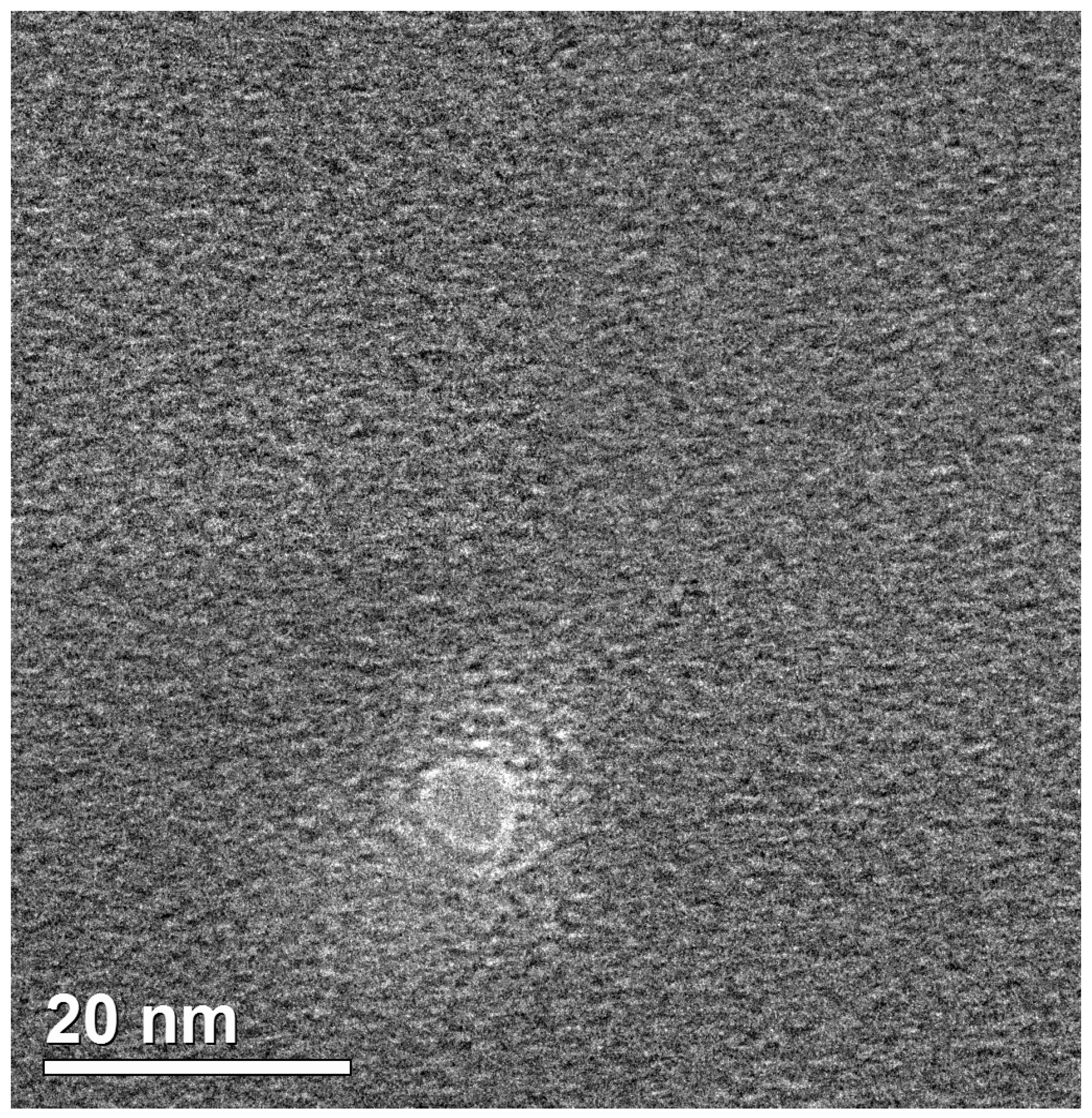
Figure 1 displays characteristic transmission electron microscopy (TEM) image of the so-fabricated nanopore.
The nanopore was observed to have a size slightly smaller than the maximum size of HRP (5.8 nm), but larger than its minimum size (4.3 nm). Accordingly, the nanopore was suitable for accommodating the HRP molecule, preventing it from passing through, while allowing for its incorporation within the nanopore.
2.2. Testing of Nanopore Performance
For the registration of the HRP enzymatic activity with the nanopore-based detector, tests were performed in order to assess the successful incorporation of HRP into the nanopore. Prior to these tests, the chip was washed with ultrapure water measurements. Subsequently, both chambers of the measuring cell were filled with 2 mM PBS-D buffer.
Control experiments were conducted in order to obtain the time dependence of the ion current through the nanopore at a voltage of -200 mV. Pure buffer was initially added to the cis chamber, followed by the addition of ABTS at a concentration of 0.3 mM. Half of the solution volume was then evacuated from the cis chamber, and 0.003% H2O2 solution was added. In this experiment, we observed no significant changes in the current signal fluctuations, which remained below 50 pA.
2.3. Nanopore Experiments
For the experiments involving HRP incorporation, a buffer solution containing HRP at a concentration of 10
-8 M was introduced into the cis part of the cuvette, while the trans part was filled with HRP-free buffer solution.
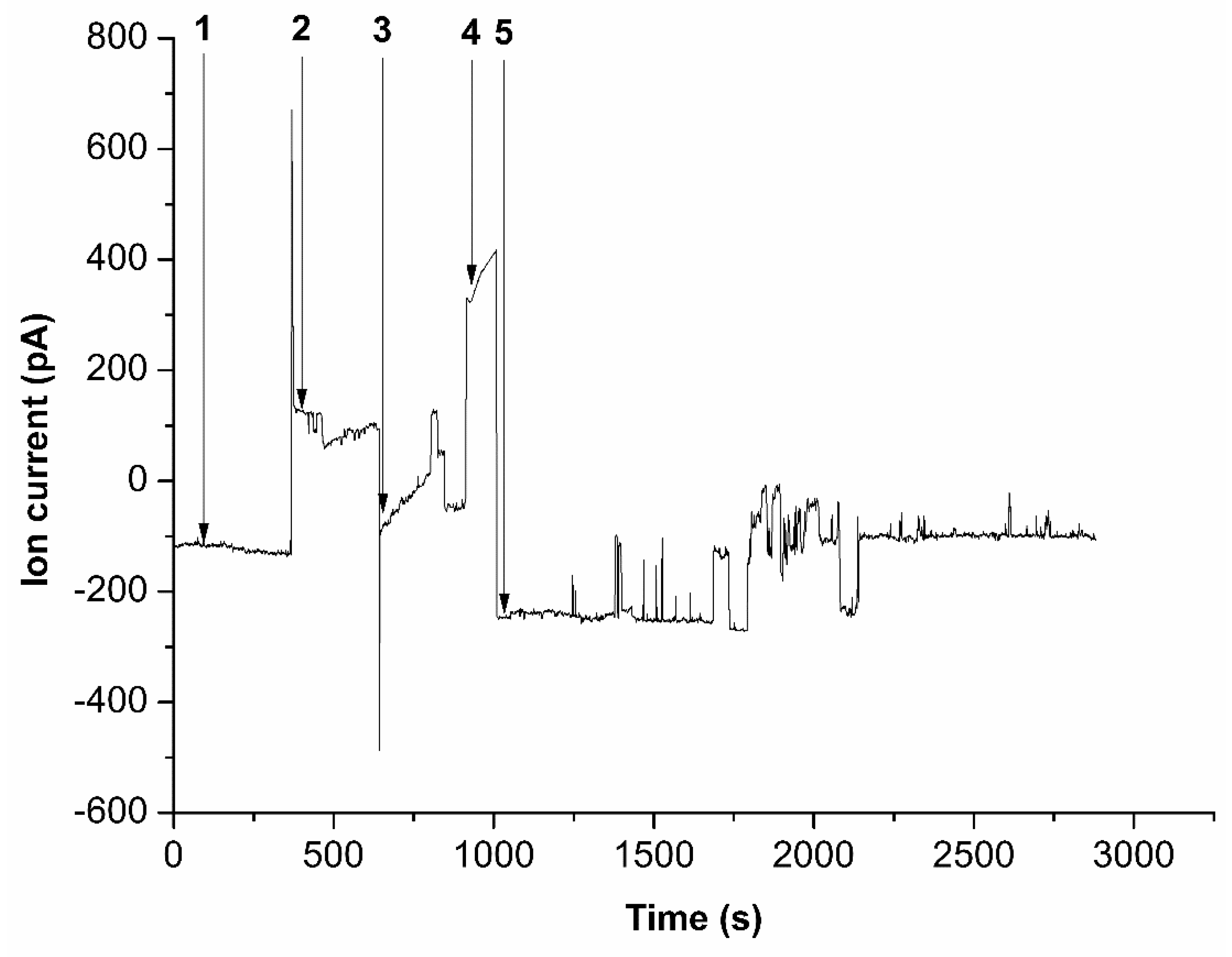
Figure 2 illustrates the ion current dependencies over time during the addition of the corresponding solution to the cis part of the measuring cell.
The curve shown in
Figure 2 indicates that at 10
-8 M HRP concentration and U=-200 mV voltage applied to the cell (cis-chamber/trans-chamber), the ion current through the nanopore was approximately -100 pA. It should be noted that HRP, at the pH of the buffer solution used, carries a negative charge, which directs the enzyme into the nanopore under the applied voltage conditions. The voltage polarity was then switched to U=200 mV (cis-chamber/trans-chamber), resulting in a change in the current flowing through the nanopore. At that, the current reached approximately 100 pA. Then the cell polarity was switched again to U=-200 mV (cis-chamber/trans-chamber), forcing the nanopore blockade, as the ion current decreased to zero. The cell was subsequently filled with ABTS solution. After subsequent evacuation of half of the ABTS solution volume, H
2O
2 was added to initiate the ABTS oxidation reaction. This led to a series of significant signal fluctuations, which persisted for about 1800 seconds, indicating the enzyme functioning.
In the spectrophotometry experiments, we observed that under the conditions described in section 4.4, the absorbance of the solution containing 1 nM HRP, reached the value of 0.4 after five minutes of ABTS oxidation reaction. This indicated that the enzyme is able to catalyze the ABTS oxidation in 2 mM PBS-D buffer, i.e. is active. No change in the solution absorbance was observed in blank spectrophotometry experiments in the absence of the enzyme.
3. Discussion
Our study demonstrates the successful use of a 5 nm SiN solid-state nanopore for the monitoring the enzymatic activity of a single HRP molecule. The latter was inserted into the nanopore. In our experiments, the time dependence of the ion current through the nanopore during the enzyme catalytic cycle was recorded. During the catalytic cycle, current pulses were observed, indicating changes in the nanopore size. The latter can be attributed to alterations in the shape of the enzyme molecule confined within the nanopore. These current fluctuations persisted for a considerably long (1800 seconds) time, and after this the enzyme was found to be still active. At that, after 1700 seconds (i.e. approximately 700 seconds after the addition of Н2О2), a decrease in the ion current was observed. This decrease can be explained by structural changes in the enzyme, leading to a substantial nanopore blockade and thus hindering the ion flow and decreasing the current. These findings demonstrate that individual HRP enzyme molecules are able to retain activity for approximately 700 seconds before undergoing structural changes, which decrease the nanopore conductivity. Control experiments were carried out without the enzyme. In the control experiments, we observed no significant current fluctuations in the presence of ABTS and Н2О2 were in the measuring cell. These results hold significant implications for comprehending enzyme functionality and enable the analysis of enzyme behaviour at the single-molecule level.
4. Materials and Methods
4.1. Chemicals and Enzyme
The enzyme (peroxidase from horseradish; Cat. #6782) and its substrate ABTS were purchased from Sigma. The experiments were performed in 2 mM Dulbecco’s modified phosphate buffered saline (hereafter PBS-D buffer) with a pH of 7.4. Ultrapure deionized water, obtained with a Simplicity UV system (Millipore, Molsheim, France), was used in all experiments. The HRP enzyme was purchased from Sigma (USA). The solution containing 0.3 mM ABTS was prepared by dissolving 8.2 mg of ABTS in 50 mL of in 2 mM PBS-D immediately prior to the experiments. Hydrogen peroxide (H2O2) solution was diluted with 2 mM PBS-D to a final concentration in the cell of 0.003% (v/v).
4.2. Nanopore-based detector
The nanopore was formed by EBD in a SiN chip. Its diameter was approximately 5 nm, and its thickness was ~40 nm. This nanopore was obtained with a JEM 2100F high-resolution transmission electron microscope. The nanopore-based detector comprised the measuring cell, which was divided into two chambers with the SiN nanopore integrated into the partition. That is, the chip with the nanopore served as the partition between the two chambers (cis- and trans-). The cell was washed with ultrapure water immediately prior to the measurements.
4.3. Electrical measurements
The both chambers (cis- and trans-) were filled with 700 μL of 2 mM PBS-D (pH 7.4). In the measurements, voltage was applied to the both chambers, and the current flowing through the nanopore was recorded. Ag/AgCl electrodes were immersed into the cis- and trans-chambers in order to apply the electric voltage for recording the ion current through the nanopore. The detector was shielded with a Faraday cell. The current flowing through the nanopore was measured with a patch-clamp amplifier with an intrinsic noise level of 0.3 fA in a frequency band of 1000 Hz. The voltage was varied within the range from -300 to 300 mV. The current signal from the nanopore was recorded at a frequency of 10 kHz using a 16-bit analog-to digital converter (ADC). This signal was then filtered through a low-pass Butterworth filter with a frequency of 1000 Hz. After the measurement cycle, the SiN chip was washed with ultrapure water in order to eliminate salt residues, and reinstalled into the measuring cell.
4.4. Spectrophotometry
Spectrophotometry measurements were performed according to the modified technique reported by Sanders et al. [
18]. In the originally reported technique, the measurements are performed in phosphate-citrate buffer [
18]. According to Drozd et al. [
20], phosphate buffers can also be successfully employed, but the pH should be acidic (5.0) in order to achieve optimal performance [
21].
Accordingly, our spectrophotometry measurements were aimed at finding out whether HRP is able to catalyze the oxidation of ABTS by H
2O
2 in 2 mM PBS-D buffer employed in the nanopore-based detector. That is, the modification of the technique consisted in the use of the PBS-D buffer instead of the phosphate-citrate one. Other experimental conditions were similar to those recommended by Sanders et al. [
18]. Namely, the concentrations of the enzyme, ABTS and H
2O
2 were 1 nM, 0.3 mM and 2.5 mM, respectively, and the absorbance of the solution was recorded at 405 nm wavelength in 1-cm-long quartz cell. An Agilent 8453 spectrophotometer was used in the measurements. The time dependencies of the absorption were recorded for five minutes in at least three technical replicates.
5. Conclusions
In the course of the research a nanopore (a diameter of approximately 5 nm) with an immobilized HRP was fabricated. The experiments conducted revealed that this nanopore can be successfully used for monitoring the enzymatic activity of a single HRP molecule (which was confined within the nanopore) against its substrate ABTS in the presence of Н2О2. Importantly, 1800 seconds exhibited observable current fluctuations during enzyme functioning, which were notably absent in the absence of ABTS and Н2О2. These findings provide valuable insights into the fundamental mechanisms governing enzyme function at the single-molecule level.
Author Contributions
Conceptualization, Yu.D.I. and A.I.A.; methodology, Yu.D.I., A.N.A., and I.S.M.; software, A.N.A; validation, I.D.S.; formal analysis, Yu.D.I.; investigation, Yu.D.I., A.N.A., I.D.S., I.A.I., N.V.V., D.V.L., and I.S.M.; resources, N.V.V., D.V.L., and I.S.M.; data curation, A.N.A., N.V.V., and I.D.S.; writing—original draft preparation, Yu.D.I., A.N.A., and I.D.S.; writing—review and editing, Yu.D.I.; visualization, I.S.M. and I.D.S.; supervision, A.I.A.; project administration, Yu.D.I.; funding acquisition, A.I.A. All authors have read and agreed to the published version of the manuscript.
Funding
The work was performed (done, carried out) within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021-2030) (No. 122030100168-2).
Data Availability Statement
Correspondence and requests for materials should be addressed to Y.D.I.
Acknowledgments
The AFM measurements were performed employing a Titanium multimode atomic force microscope, which pertains to “Avogadro” large-scale research facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Radmacher, M.; Fritz, M.; Hansma, H.G.; Hansma, P.K. Direct observation of enzyme activity with the atomic force microscope. Science 1994, 265(5178), 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Thomson, N.H.; Fritz, M.; Radmacher, M.; Cleveland, J.P.; Schmidt, Ch.F.; Hansma, P.K. Protein tracking and detection of protein motion using atomic force microscopy. Biophys J. 1996, 70, 2421–2431. [Google Scholar] [CrossRef] [PubMed]
- Arnoldi, M.; Schäffer, T.E.; Fritz, M.; Radmacher, M. Direct observation of single catalytic events of chitosanase by atomic force microscopy. J. AZoNano — Online J. Nanotechnol, 1: 1 (a108), 1-11. DOI, 2240. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Bukharina, N.S.; Pleshakova, T.O.; Frantsuzov, P.A.; Krokhin, N.V.; Ziborov, V.S.; Archakov, A.I. Atomic force microscopy visualization and measurement of the activity and physicochemical properties of single monomeric and oligomeric enzymes. BIOPHYSICS 2011, 56, 892–896. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhang, S.; Liu, L.; Wu, H.-C. Measuring enzymatic activities with nanopores. ChemBioChem 2020, 21(15), 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, Y.; Long, Y.-T.; Minteer, S.D.; Ying, Y.-L. Nanopore-based measurement of the interaction of P450cam monooxygenase and putidaredoxin at the single-molecule level. Faraday Discuss. 2022, 233, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Willems, K.; van Meervelt, V.; Wloka, C.; Maglia, G. Single-molecule nanopore enzymology. Phil. Trans. R. Soc. B. 2017, 372, 20160230. [Google Scholar] [CrossRef] [PubMed]
- Wloka, C.; van Meervelt, V.; Gelder, D.V.; Danda, N.; Jager, N.; Williams, C.P.; Maglia, G. Label-free and real-time detection of protein ubiquitination with a biological nanopore. ACS Nano 2017, 11(5), 4387–4394. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.; Eron, S.J.; Hill, M.E.; Li, X.; Fahie, M.A.; Hardy, J.A.; Chen, M. A nanopore approach for analysis of caspase-7 activity in cell lysates. Biophys. J. 2019, 117(5), 844–855. [Google Scholar] [CrossRef] [PubMed]
- Fahie, M.A.; Pham, B.; Li, F.; Chen, M. A selective activity-based approach for analysis of enzymes with an OmpG nanopore. Methods in Molecular Biology. 2021, 2186, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Stein, D.; McMullan, C.; Branton, D.; Aziz, M.J.; Golovchenko, J.A. Ion-beam sculpting at nanometre length scales. Nature 2001, 412, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Kwok, H.; Briggs, K.; Tabard-Cossa, V. Nanopore fabrication by controlled dielectric breakdown. PLoS One, 9288. [Google Scholar] [CrossRef]
- Waugh, M.; Briggs, K.; Gunn, D.; Gibeault, M.; King, S.; Ingram, Q.; Jimenez, A.M.; Berryman, S.; Lomovtsev, D.; Andrzejewski, L.; Tabard-Cossa, V. Solid-state nanopore fabrication by automated controlled breakdown. Nature Protocols 2020, 15(1), 122–143. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Rooks, M.J.; Kim, K.-B.; Rossnagel, S.M. Ionic field effect transistors with sub-10 nm multiple nanopores. Nano Lett. 2009, 9(5), 2044–2048. [Google Scholar] [CrossRef] [PubMed]
- Metzler, D.E. Biochemistry: The Chemical Reactions of Living Cells; Academic Press: Oxford, UK, 1970. [Google Scholar]
- Rogozhin, V.V.; Kutuzova, G.D.; Ugarova, N.N. Inhibition of horseradish peroxidase by N-ethylamide of o-sulfobenzoylacetic acid. Bioorganic Chem. 2000, 26(2), 156–160. [Google Scholar] [CrossRef]
- Davies, P.F.; Rennke, H.G.; Cotran, R.S. Influence of molecular charge upon the endocytosis and intracellular fate of peroxidase activity in cultured arterial endothelium. J. Cell Sci. 1981, 49(1), 69–86. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.A.; Bray, R.C.; Smith, A.T. pH-dependent properties of a mutant horseradish peroxidase isoenzyme C in which Arg38 has been replaced with lysine. Eur. J. Biochem. 1994, 224, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Berglund, G.I.; Carlsson, G.H.; Smith, A.T.; Szöke, H.; Henriksen, A.; Hajdu, J. The catalytic pathway of horseradish peroxidase at high resolution. Nature 2002, 417, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Drozd, M.; Pietrzak, M.; Parzuchowski, P.G.; Malinowska, E. Pitfalls and capabilities of various hydrogen donors in evaluation of peroxidase-like activity of gold nanoparticles. Anal. Bioanal. Chem. 2016, 408, 8505–8513. [Google Scholar] [CrossRef] [PubMed]
- Enzymatic Assay of Peroxidase (EC 1.11.1.7) 2,20-Azino-Bis(3-Ethylbenzthiazoline-6-Sulfonic Acid) as a Substrate Sigma Prod. No. P-6782. Available online: https://www.sigmaaldrich.com/RU/en/technical-documents/protocol/protein-biology/enzymeactivity-assays/enzymatic-assay-of-peroxidase-abts-as-substrate (accessed on 18 February 2022).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).