1. Introduction
Thyme (
Thymus officinalis L.), which belongs to the Lamiaceae family, is a perennial shrub that is used for food flavoring and preservation. This plant can be found in Europe, North Africa, and Asia [
1]. Thyme is rich in essential oil (EO) which is part of the secondary metabolites of the plants. It is involved in the defense mechanism of the herb (repelling the phytophagous organisms) and in attracting pollinators. Among constituents of thyme EO (carvacrol, p-cymene, α-pinene, γ-terpinene, etc.) thymol is the major one [
2]. This compound has been shown to have antioxidant and anti-inflammatory [
3], antibacterial [
4], antimicrobial [
5], antifungal [
6], antiparasitic [
7], immunological [
8], and anticancer [
9] properties. Thyme also contains polyphenolic compounds (rosmarinic, caffeic, p-hydroxybenzoic, and procatechuic acids) and carotenoids (β-carotene, lutein, and zeaxanthin) [
10,
11]. These bioactive compounds enhance the valuable properties of thymol, providing to thyme extracts a beneficial effect on the human health. Also, if added to foodstuffs, the thyme EO can improve their quality. For example, Medina et al. proved that the thyme EO decreased the oxidation in the supplemented minced beef [
2].
The extraction of EOs from plants can be performed by conventional methods, such as cold pressing [
12], hydro-distillation, and steam distillation [
13]. These methods present several drawbacks, such as long extraction times, high energy and solvent consumption, etc. [
14]. Thus, in recent years, in order to overcome these shortcomings, alternative methods have been developed. The new approaches include microwave-assisted hydro-distillation [
15,
16], ultrasound [
17] and enzymatic [
18,
19] pre-treatments before the extraction of EOs, supercritical [
20] and subcritical [
21] fluid extractions, solvent-free extraction [
22], and microwave hydro-diffusion and gravity extraction [
23,
24].
Microwave-assisted hydro-distillation (MWHD) combined with ultrasound pre-treatment of aromatic herbs to extract EOs was successfully employed [
25,
26]. This strategy combines the advantages of ultrasound and microwave extractions. The latter can provide volumetric and selective heating. During microwave irradiation, the heterogeneous extraction mixture is heated as a whole volume and the vegetal material can be heated selectively. Although the microwave heating of extraction mixture occurs rapidly, in the overall rate of the process the mass transfer is limited [
27,
28]. On the other hand, the cavitation phenomena can promote the breakage of the cellular tissue which will increase the mass transfer rate [
29].
Our work describes the extraction of EO from thyme leaves by consecutive use of ultrasound and microwave treatments. Thus, the aim of this study was to apply an ultrasound pre-treatment of the extraction mixture in order to increase the thymol content and the extraction yield of the EO obtained using MWHD. The influence of the ultrasound pre-treatment on the extraction process of EO was studied using both an ultrasound bath and an ultrasonic processor. To our knowledge, MWHD combined with ultrasound pre-treatment to extract EO was not used for thyme leaves. There are only a few studies regarding the ultrasound pre-treatment of thyme and neither of them reported the comparison between different sonication equipment. Kowalski et al. applied an ultrasonic pre-treatment of the thyme leaves using an ultrasound bath, but the EO was further extracted by conventional steam distillation [
30]. Roldan-Gutierrez et al. used an ultrasonic processor for the pre-treatment of thyme leaves, but the extraction of EO was further performed by steam distillation and superheated water methods [
31].
2. Materials and Methods
2.1. Materials
Fresh thyme (stems and leaves) was purchased from Hofigal (Bucharest, Romania). The fresh leaves and stems were dried in an air flow-heating oven at 60 °C to a constant weight. Part of the dried vegetal material was chopped into pieces of 1-2 cm, and another part was ground using an electric grinder and screened to a particle size under 0.1 cm. The ground thyme was dosed in samples of 100 g (in sealed plastic container) and stored at 4-5 °C until extraction of EO. The water content of the dried thyme leaves was 7.7 %. For the GC-MS quantification of thyme EO, the following standards were used: thymol, γ-terpinene, p-cymene, α-terpinene, and β-pinene from Merck (Darmstadt, Germany).
2.2. Methods
2.2.1. Essential Oil Extraction Procedure
The extraction of EO from thyme was performed by MWHD using a multimode microwave oven (Plazmatronika, Wroclaw, Poland). During the experiment temperature, time, and power were controlled via an operating console. Steam produced in the reactor carrying the thyme EO was directed to a modified Neo Clevenger trap with a 10 mL graduated tube. The extractions were carried out at different ratios of solvent to plant: 8/1, 10/1, and 12/1 (v/w). The particle size of vegetal material was varied, as follows: pieces of 1-2 cm or particles under 0.1 cm. The solvent used was distilled water and the mixture was subjected to extraction until no EO was obtained (80 min). The protocols for MWHD method is described in our previous work [
10]. Comparative extraction of thyme EO by conventional hydro-distillation (CHD) method was also performed. The conventional extraction was carried out for 180 min (after this time no EO was obtained) at a solvent to plant ratio of 12/1 (v/w). The separated EO was kept at 4 °C until GC-MS analysis.
2.2.2. Ultrasound pre-treatment of plant material
Before MWHD, the vegetal material mixed with the solvent (distilled water) was subjected to ultrasound pre-treatment for 30 min. The pre-treatment was performed using either an ultrasound bath (ES375H Bench Top Ultrasonic Tank, Hilsonic Birkenhead, UK) with a volume of 3 L, a power of 120 W, and a frequency of 40 kHz, either an ultrasonic processor (Vibracell VCX-750, Sonics & Materials Newtown, CT, USA) with a power of 750 W, a frequency of 20 kHz, and a titanium horn with a diameter of 13 mm. The sonication of the extraction mixture, using the ultrasonic processor, was applied with a duty cycle of 5 s on and 5 s off for an amplitude of 50% or 70%. The sonication for the ultrasonic bath was applied continuously. Control samples without any pre-treatment were directly subjected to MWHD or CHD.
2.2.3. GC-MS analysis of the thyme essential oil
The separated EO was analyzed by GC-MS method. The analyses were undertaken using a Thermo Electron Corporation Focus GC gas chromatograph with a Macrogol 20 000 R column (film thickness 0.25 μm); l = 60 m; Ø = 0.25 mm. The sample injection volume was 0.2 μL and helium with a flow rate of 1.5 mL/min was used as mobile phase. A Thermo Electron Corporation DSQII mass spectrometer was used for detection. The main constituents of thyme EO (thymol, γ-terpinene, p-cymene, α-terpinene, and β-pinene) were identified according to retention times of known standards. Identification of the other constituents of thyme EO was performed by comparing the samples spectral peaks with spectra from Wiley database. The main constituents of thyme EO were chosen based on preliminary tests that were part of a research project (PN-III-P2-PED-2019-2118, project: Technologies for obtaining of natural products with immunostimulatory properties - “IMUNOSTIM” financed by contract: PED, no. 381PED/2020). Relative percentages of the individual components were calculated based on GC peak areas.
2.2.4. Statistical analysis
All measurements were carried out in triplicate and the data were expressed as mean value ± SD (standard deviation) for triplicate of samples (
n=3). All the results obtained at different levels of process factors were subjected to univariate one-way analysis of variance (ANOVA) and multivariate principal component analysis (PCA). Statistical analysis of the data was performed using the multiple comparison Duncan’s post hoc
t-test in order to detect the significant statistical differences between the averages of the main components of two or more independent groups. To evaluate the strength of linear correlations between dependent variables, the Pearson correlation coefficient (r) was used [
32,
33]. The differences were considered statistically significant at
p < 0.05. Statistical analysis was conducted using XLSTAT Version 2019.1 (Addinsoft, New York, NY, USA).
3. Results
3.1. Microwave-assisted Hydro-distillation (MWHD) vs. Conventional Hydro-distillation (CHD)
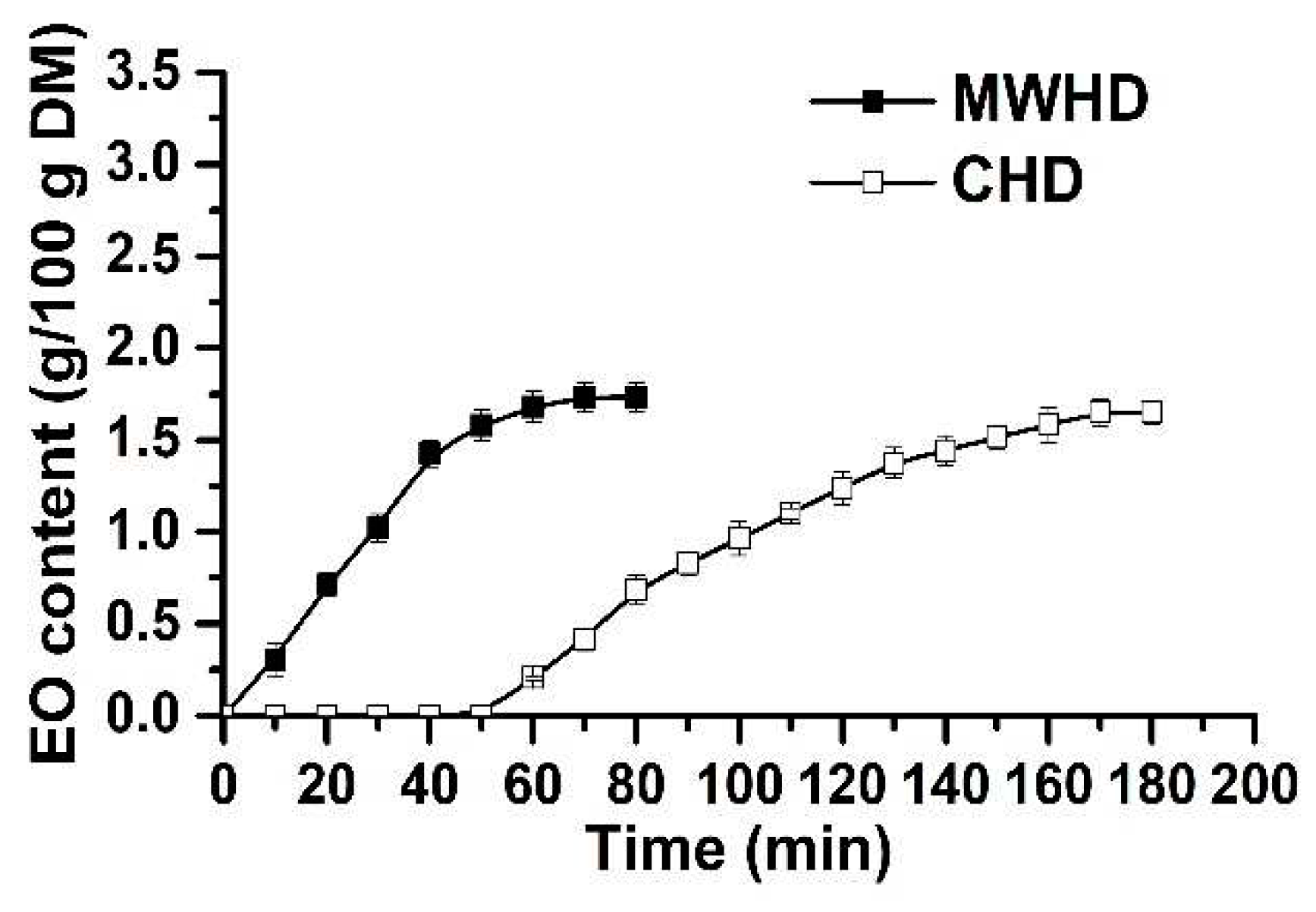
The first step to optimize the extraction process of thyme EO consists of kinetics study of the control samples obtained by MWHD and CHD (
Figure 1). As shown in
Figure 1, the heating time required for MWHD (10 min) is lower compared with CHD (60 min). This can be explained by the volumetric heating of microwaves compared with the conventional one which occurs by convection [
27]. The MWHD leads to a rapid increase in EO content in the first 40 min (which corresponds to 83% of the total yield), achieving a maximum amount after 70 min (1.73 g EO/100 g DM). For CHD, the EO content increases slowly, obtaining 1.65 g of EO/100 g DM after 170 min. Further, the experiments were performed by MWHD.
The thyme EO was analyzed and identified by GC-MS. The results, for both methods (MWHD and CHD), are shown in
Table 1.
For both methods, approximately 12 compounds were identified. As shown in
Table 1, the main constituents of thyme EO are thymol, γ-terpinene, p-cymene, α-terpinene, and β-pinene. These compounds constitute between 93% and 97% of the total amount of resulted EO. Considering the EO composition, there is only a slight difference between the two extraction methods. Thymol is extracted more efficiently by MWHD, while γ-terpinene is extracted more efficiently by CHD. This difference can be explained by the bioconversion of γ-terpinene in thymol, which is influenced by different factors, one of them being the extraction method [
34]. The same behavior can be noticed for p-cymene, α-terpinene, and β-pinene which are extracted more efficiently by CHD.
3.2. Influence of solvent to plant ratio on the extraction of thyme essential oil
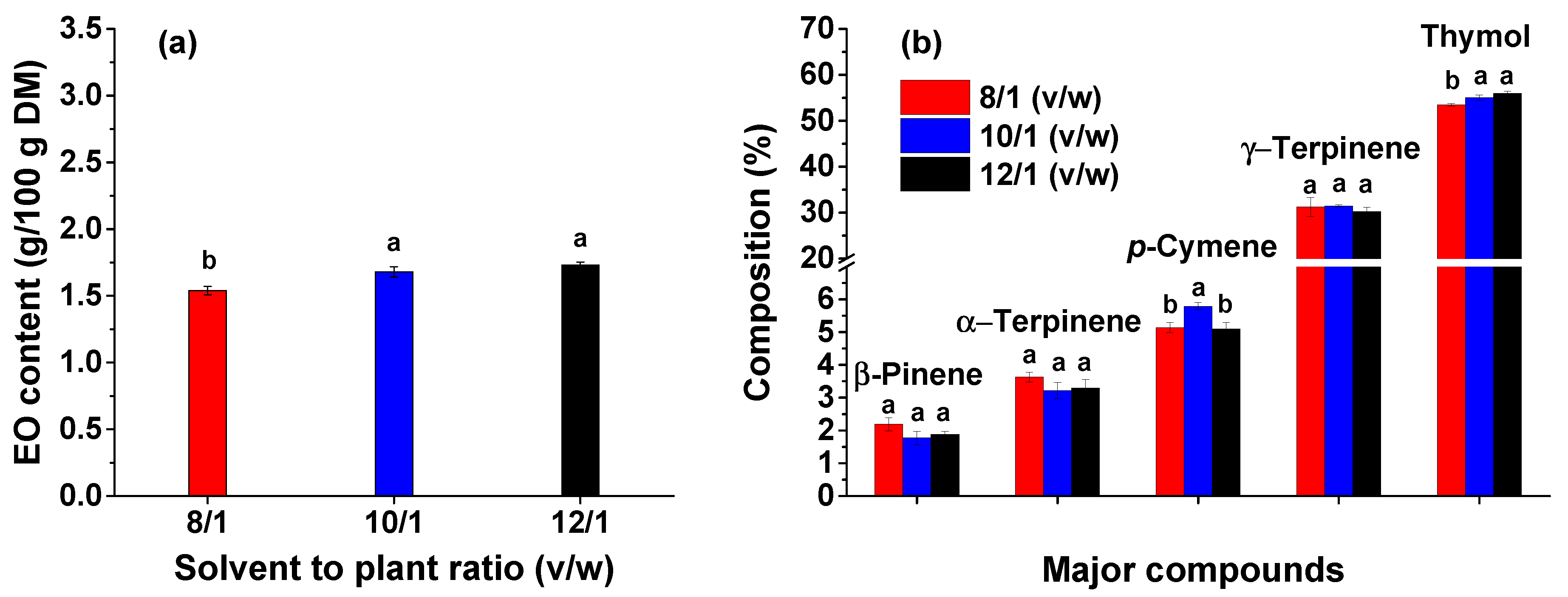
The next step to optimize the EO extraction from thyme was to study the influence of solvent to plant ratio. To establish the solvent volume required to achieve as much EO as possible, different ratios of solvent to plant material were used. Moreover, to avoid degradation of thyme leaves due to direct microwave irradiation, an adequate amount of water is required. As shown in
Figure 2a, the amount of EO increases with increasing the water volume. The most efficient solvent to plant ratio for the extraction of thyme EO by MWHD is 12/1 (v/w).
The resulted EO, for all solvent to plant ratios, was analysed by GC-MS. The main compounds of thyme EO identified by GC-MS are shown in
Figure 2(b). It can be noticed that the composition is similar for all three ratios and the amount of thymol is directly proportional with the solvent to plant ratio. Thus, a high amount of water is required to entrain the constituents with high boiling points. The one-way ANOVA analysis demonstrated that by increasing the solvent to plant ratio from 8/1 to 10/1 the EO content increases significantly (
p < 0.05). By further increase of solvent to plant ratio, the EO content increases non-significantly (
Figure 2a). Regarding the thymol extraction, the one-way ANOVA analysis showed that by increasing the solvent to plant ratio, the thymol concentration increased significantly (
p < 0.05,
Figure 2b).
Although the amount of EO is similar for both 10/1 and 12/1 ratios, the further experiments were performed for a 12/1 (v/w) ratio of solvent to plant material since the amount of the targeted compound (thymol) is higher and the specific energy (see
Table 2) is lower compared with the other ratios used.
3.3. Influence of ultrasound equipment on the extraction of thyme essential oil
Prior to the extraction of thyme EO by MWHD, the extraction mixture was subjected to ultrasound pre-treatment for 30 min using two types of equipment: an ultrasound bath (ES375H Bench Top Ultrasonic Tank) and an ultrasonic processor (Vibracell VCX -750).
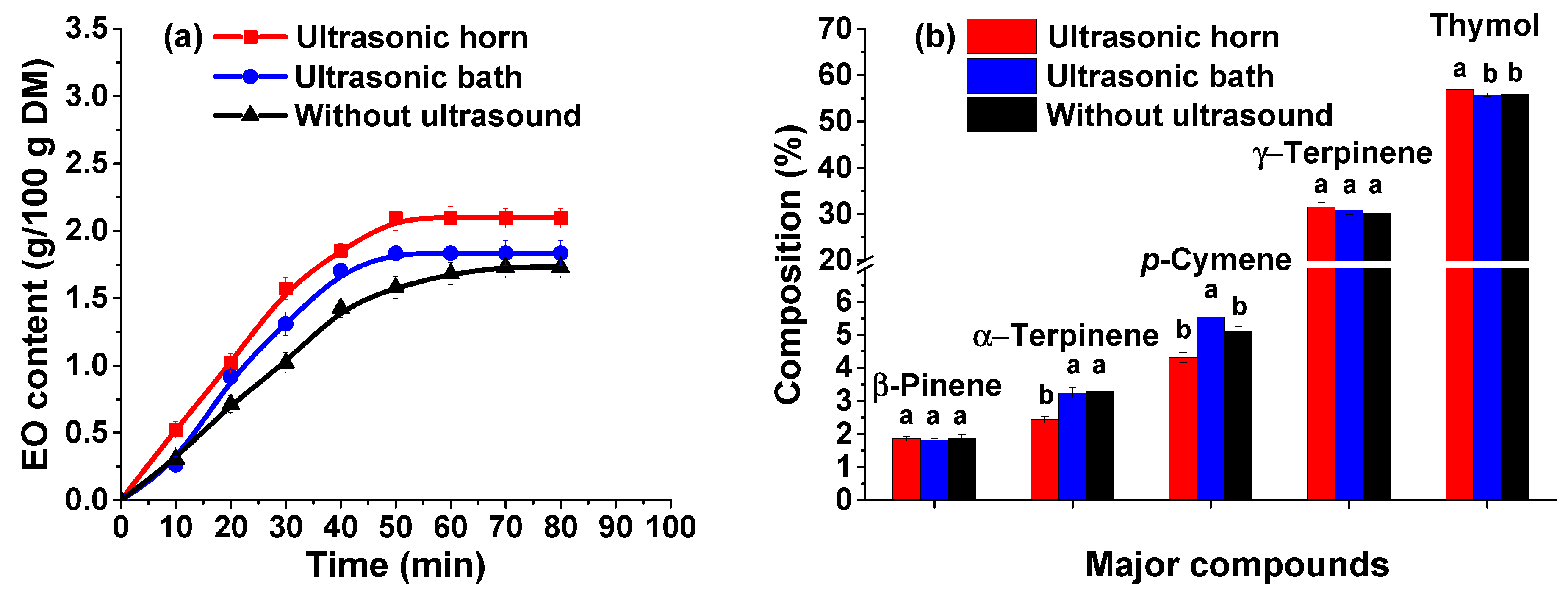
It can be noticed (
Figure 3a) that the ultrasound pre-treatment of the extraction mixture leads to higher amounts of EO compared with the experiments without pre-treatment, for both ultrasound bath (approximatively 6% higher) and horn (approximatively 21% higher). This can be due to the cavitation phenomenon which can promote the disruption of cell walls and increase the mass transfer rate. In ultrasonic bath the cavitation occurs uncontrollably. The ultrasound energy is unevenly distributed in the bath, and it is of low intensity. On the other hand, for horn, the cavitation has a high localized intensity and implicitly the sonication process is more efficient [
35]. Therefore, the higher amount of EO achieved when the ultrasound pre-treatment is carried out using the Vibracell equipment (an increase of approximately 15%), can be due to these differences between ultrasonic baths and horns. In addition, the ultrasound pre-treatment of the extraction mixture has a beneficial effect on the extraction time, achieving a maximum amount of EO in only 50 min compared with the extraction without pre-treatment when a maximum amount is achieved after 70 min. Further, the experiments were performed using the Vibracell ultrasonic processor to pre-treat the extraction mixture.
The GC-MS analyses of the EO obtained by consecutive use of ultrasound and microwave irradiation are shown in
Figure 3b. It can be noticed that the major constituents of the EO are similar for all three methods (the extraction with ultrasound pre-treatment using both ultrasonic horn and bath and the extraction without pre-treatment). As shown in
Figure 3b, the thymol is extracted more efficiently when the ultrasound pre-treatment is carried out using the ultrasonic probe. Instead, p-cymene and α-terpinene are obtained in lower amounts. This can be due to the transformation of some compounds during ultrasound irradiation, such as: isomerization, oxidation, and degradation of dimmers and polymers [
36]. Also, in water, the cavitation phenomenon can lead to the formation of free radicals (H•, OH•) which can cause the transformation of the EO constituents [
37]. The ANOVA analysis along with Duncan’s post hoc t-test showed that pre-treatment with the ultrasonic horn led to a significant increase in the thymol content, while using the ultrasonic bath the content of
p-cymene and α-terpinene increased significantly (
p < 0.05). The opposite behavior of
p-cymene and α-terpinene when the pre-treatment is performed using the ultrasonic bath, can be due to the low intensity of the cavitation in such equipment. Since the ultrasound energy for horn can be focused on specific sample area, the constituents with lower boiling points can be rapidly released and, implicitly, they can be degraded afterwards.
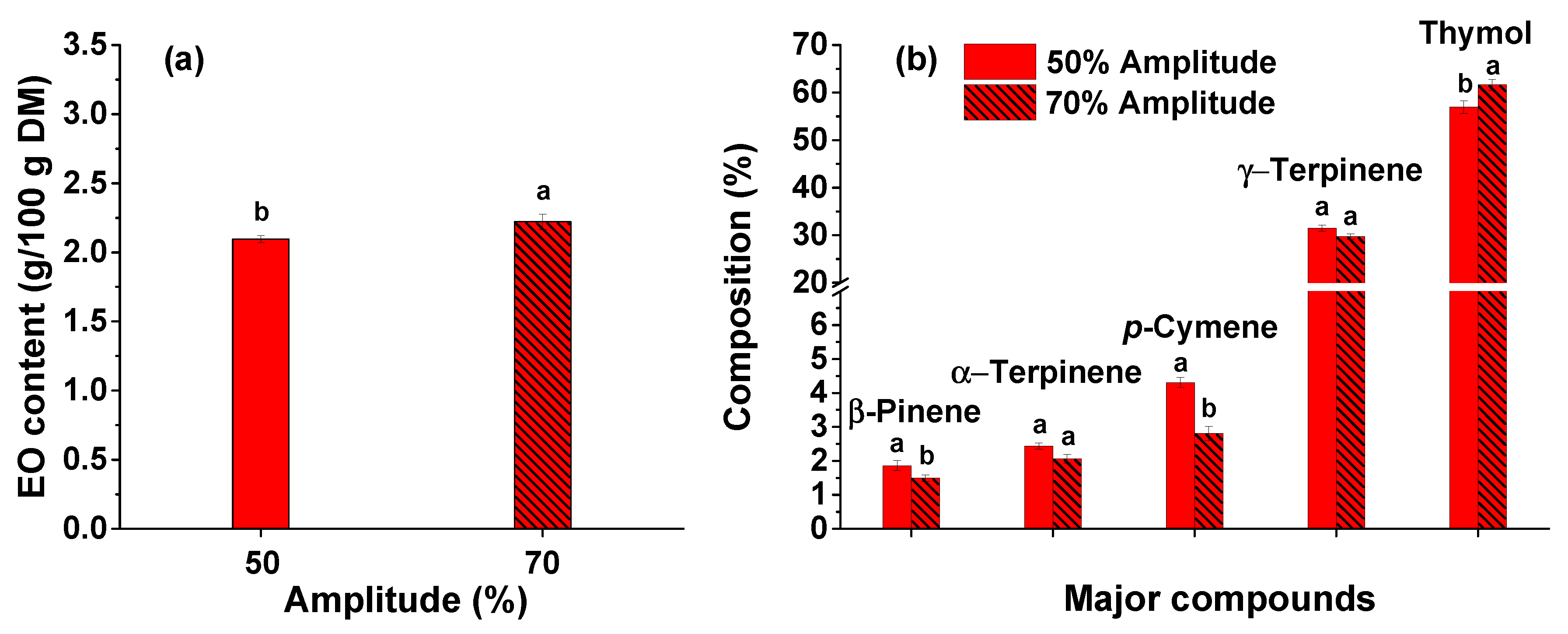
3.4. Influence of ultrasound amplitude on the extraction of thyme essential oil
The influence of ultrasound amplitude on the extraction process efficiency was also studied. Amplitudes of 50 and 70% were chosen. As shown in
Figure 4a, the amount of thyme EO is directly proportional to the ultrasound amplitude. The EO content increases by 6% compared with the pre-treatment performed at an amplitude of 50%. The extraction mechanism involves the diffusion of the solvent through the cell walls and, implicitly, their rinsing since the walls are disrupted. These phenomena can be enhanced by ultrasonic cavitation [
38]. Thus, an efficient ultrasound pre-treatment of the extraction mixture can increase the EO yield.
The thyme EO was also analyzed and identified by GC-MS. The results, for both ultrasound amplitudes, are shown in
Figure 4b. As in the previous experiments (see Figures 2b and 3b, and
Table 1) the main constituents of thyme EO (thymol, γ-terpinene,
p-cymene, α-terpinene, and β-pinene) remain unchanged. As shown in
Figure 4b, the targeted compound, thymol, is extracted more efficiently when an amplitude of 70% is used. The one-way ANOVA analysis demonstrated that by increasing the ultrasonic amplitude from 50 to 70%, the EO and thymol contents increase significantly (
p < 0.05). However, the percentages of γ-terpinene,
p-cymene, α-terpinene, and β-pinene are inversely proportional with the ultrasound power, achieving higher amounts for an amplitude of 50%. This can be due to an accentuated transformation of some constituents at high ultrasound powers.
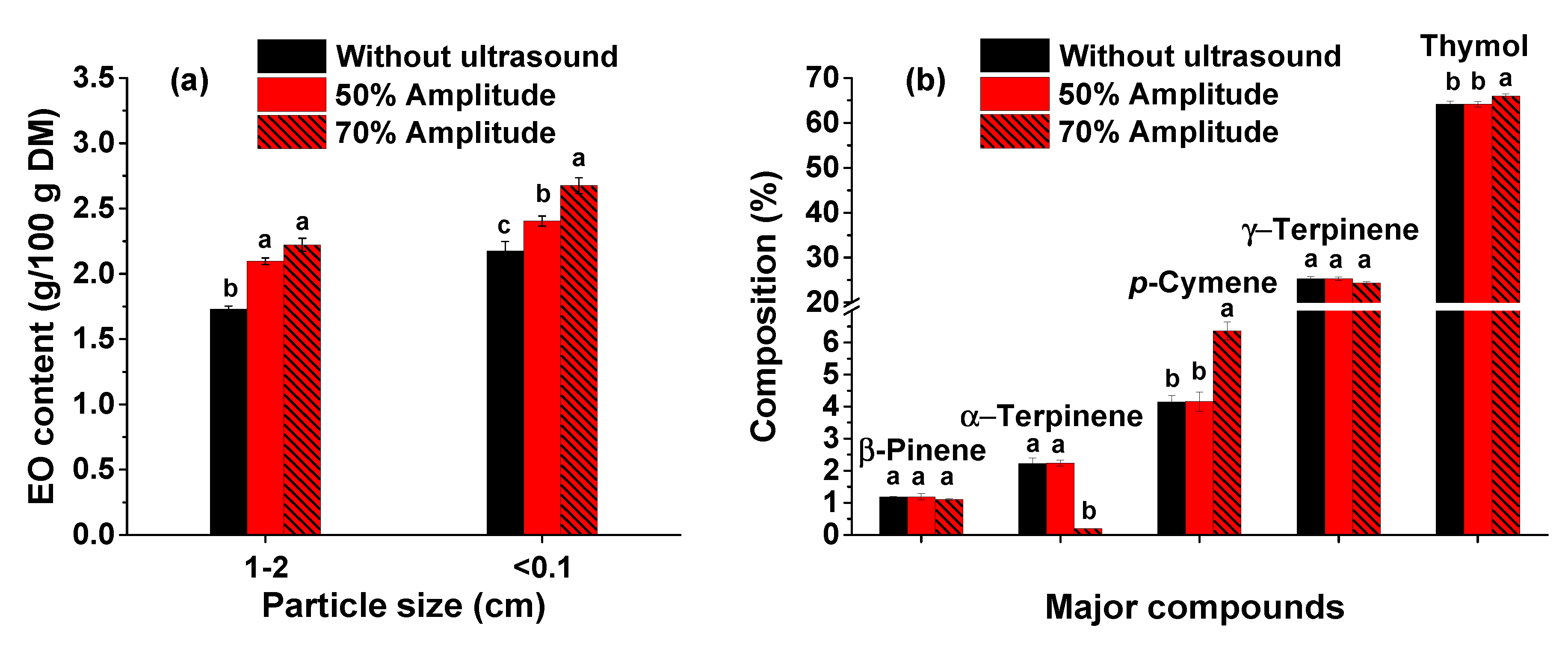
3.5. Influence of leaves particle size on the extraction of thyme essential oil
In addition to external glands which can be easily destroyed by sonication, the EO can be found in the internal secretory structures of the vegetal materials. This can lead to a mass transfer resistance. A strategy to overcome this drawback is to mill the herb in order to increase the surface area. Thus, more cells will be directly exposed to the cavitation phenomenon, thus enhancing the mass transfer of the targeted compounds from vegetal matrix to the solvent [
38]. As shown in
Figure 5a, the particle size of the thyme leaves influences to a large extent the EO content. For a particle size under 0.1 cm, higher amounts of EO are achieved for both ultrasound pre-treatment (for both amplitudes applied, i.e., 50 and 70%) and extraction without pre-treatment. Using smaller particles, the EO content is 23% higher when an ultrasound pre-treatment (with an amplitude of 70%) of the extraction mixture is applied, compared with the extraction without pre-treatment. The one-way ANOVA analysis along with Duncan’s post hoc t-test showed that EO content increased significantly (
p < 0.05) by decreasing the plant material particles size.
The composition of thyme EO for a granulation of the leaves under 0.1 cm is similar with the one for a particle size of 1-2 cm. However, the amount of the targeted compound (thymol) is higher for the herb milled to a granulation under 0.1 cm (see
Figures 3b, 4b, and 5b). This behavior can be noticed for all three methods: the extraction with ultrasound pre-treatment applying both 50 and 70% amplitudes and the extraction without pre-treatment. As shown in
Figure 5b, the thymol content is higher when an ultrasound pre-treatment with an amplitude of 70% is applied, while the α-terpinene constituent is not identified. This can be due to its degradation under severe conditions of ultrasound pre-treatment.
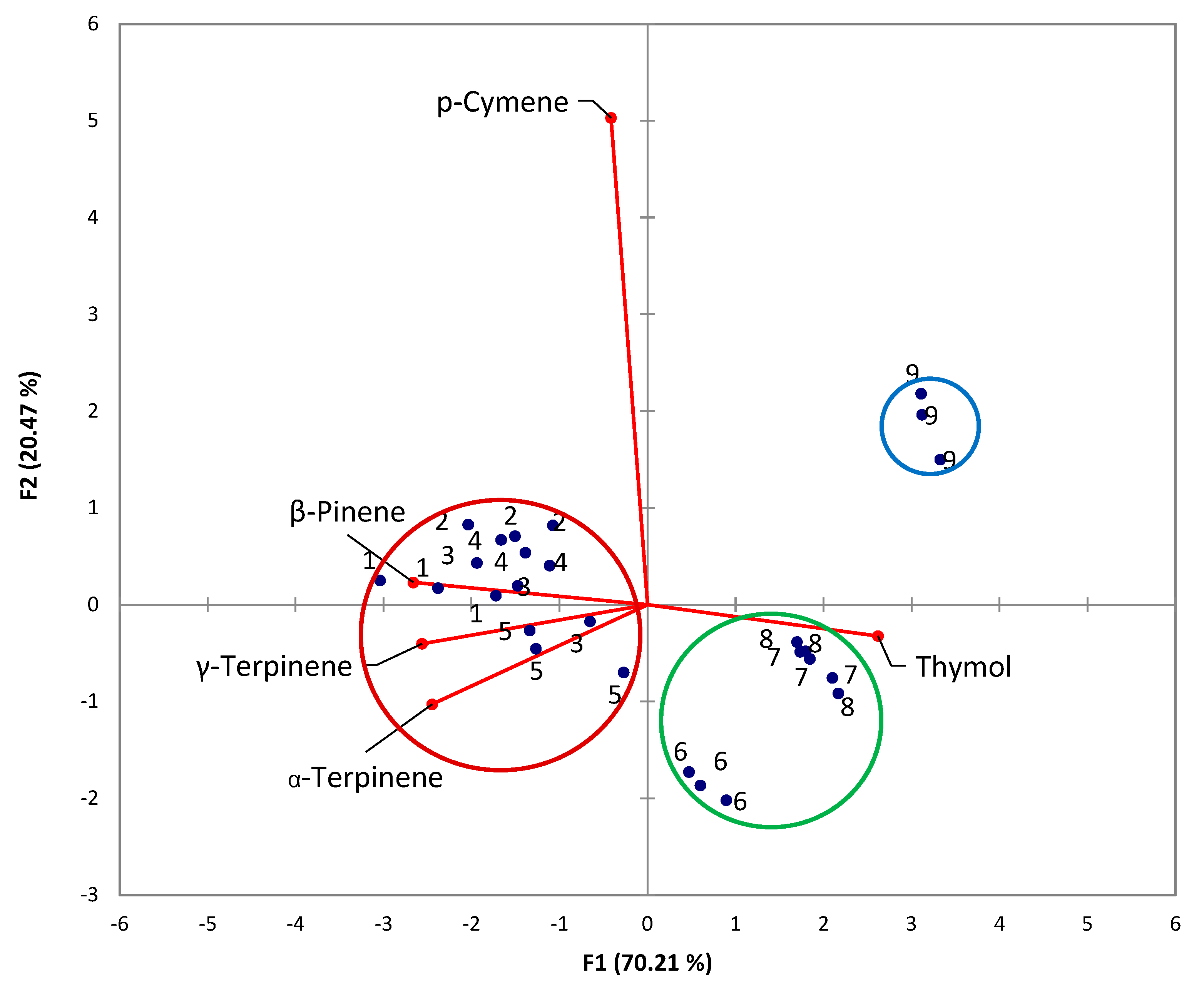
3.6. Principal Component Analysis
To evaluate the relation between the extraction method and the composition of the EO, a principal component analysis (PCA) was performed. For the multivariate analysis, the chemical compositions of the thyme EO obtained by different extraction methods were determined. The PCA results show two eigenvalues higher than 1, i.e.: those corresponding to PC1 (3.55) and PC2 (1.04). The main components of thyme EO in the plane formed by these first two PCs explain 90.68% of the variability, including 70.21% on the first axis and 20.47% on the second axis. The factor loadings (coordinates of variables on the factor-plane PC1−PC2) are shown in
Table 2, with the significant levels marked in bold. The factor scores (projections of cases on the factor-plane PC1−PC2) are summarized in
Table 3.
The PCA bi-plot and the correlation matrix are shown in
Figure 6 and
Table 4, respectively. The significant values of correlation coefficients (
r) at a significance level
α = 0.05 (two-tailed test) are highlighted in bold.
The PCA bi-plot (
Figure 6) and correlation matrix (
Table 4) indicate the following aspects:
p-Cymene is not significantly correlated with thymol, β-pinene, α-terpinene, and γ-terpinene (–0,034 ≤ r ≤ –0,179);
Thymol is significantly inversely correlated with β-pinene, α-terpinene, and γ-terpinene (–0,890 ≤ r ≤ –0,815);
β-Pinene is significantly directly correlated with α-terpinene, and γ-terpinene (–0,797 ≤ r ≤ –0, 908);
The EO obtained by methods 6-9 (highlighted using blue and green circles) had a higher content of thymol and lower contents of β-pinene, α-terpinene, and γ-terpinene compared with the EO obtained by methods 1-5 (highlighted using red circle - discrimination on PC1);
The EO obtained by method 9 (highlighted using blue circle) had a higher content of p-cymene compared with the EO obtained by methods 6-8 (highlighted using green circle - discrimination on PC2).
Moreover, the highest amount of thymol was obtained by method 9.
3.7. Energy considerations
During thyme leaves pre-treatment and extraction of EO, the microwave and ultrasound powers were recorded for all experiments. Using the above-mentioned recorded values, the total energy introduced into the system was determined. The specific energy was also calculated using the following equation:
where E
s is the specific energy [kJ/g of EO], E
total is the total energy introduced into the system [kJ], and m
EO is the amount of EO obtained by each extraction method [g].
The total energy introduced into the system and the specific energy for each method are shown in
Table 5.
As shown in
Table 5, the specific energy for MWHD (when thyme leaves with a particle size of 1-2 cm where used) decrease with increasing the solvent to plant material ratio. Also, it can be noticed that the pre-treatment performed with the ultrasonic bath is not energetically efficient. In this case, the specific energy is 62% higher as compared with the extraction without pre-treatment. However, as compared with MWHD, using the ultrasonic probe to pre-treat the thyme leaves leads to a specific energy with 14 and 16% lower for an amplitude of 50 and 70%, respectively. This difference is lower for a thyme leaves particle size under 0.1 cm (a specific energy with 6 and 13% lower for an amplitude of 50 and 70%, respectively as compared with the extraction without pre-treatment).
5. Conclusions
The purpose of this study was to investigate the influence of ultrasound pre-treatment of thyme leaves, before MWHD, on the thymol content and on the EO extraction yield. Comparative extractions, without pre-treatment, by both MWHD and CHD were also performed. The influence of several parameters (solvent to plant ratio, particle size of the vegetal material, ultrasound equipment to pre-treat the extraction mixture, and amplitude of the ultrasonic processor) on the extraction of thyme EO by MWHD was investigated. The kinetic study of MWHD and CHD showed that the heating of the extraction mixture occurred faster for microwave irradiation. For MWHD, the extraction of the EO began after 10 min, approximately 97% of the total EO content being extracted in 60 min. In comparison, for CHD, the EO extraction started after 60 min. Considering the solvent to plant ratio, a high quantity of water was required to achieve better results, i.e., 12/1 (v/w). The ultrasound pre-treatment of the extraction mixture led to a higher content of EO (2.09 and 1.83 g EO/100 g DM for ultrasonic horn and bath, respectively) achieved in a shorter time (50 min) compared with the extraction by MWHD without pre-treatment (1.73 g EO/100 g DM in 70 min). However, the extraction process was more efficient when the ultrasonic horn was used to pre-treat the thyme leaves. Milling the thyme leaves from a size of 1-2 cm to under 0.1 cm resulted in an increase of the EO content for both extractions: with (for both ultrasound amplitudes applied) or without ultrasound pre-treatment. Although the ultrasonic pre-treatment implies additional energy consumption, the specific energy was 16% lower for the EO extraction when an ultrasonic probe was used for the thyme leaves pre-treatment (for an amplitude of 70%) compared with the extraction without pretreatment. Thus, the thyme EO was extracted more efficiently for a solvent to plant ratio of 12/1 (v/w) and a particle size under 0.1 cm, applying, before MWHD, an ultrasound pre-treatment (using the ultrasonic horn at an amplitude of 70%). The composition of the EO resulted from all methods was analyzed by GS-MS, the major constituents being thymol, γ-terpinene, p-cymene, α-terpinene, and β-pinene. The targeted compound, thymol, followed the same behavior as the total EO content, achieving a higher amount by consecutive use of ultrasound (ultrasonic horn at an amplitude of 70%) and microwave treatments for a solvent to plant ratio of 12/1 (v/w) and a particle size under 0.1 cm.
Author Contributions
Conceptualization, A.I.G. and I.P.; methodology, A.I.G. and I.P.; software, I.P and O.C.P.; validation, A.I.G. and I.P.; statistical analysis, O.C.P.; formal analysis, C.G.C.-N., L.M., M.L.G.; investigation, C.G.C.-N., L.M., M.L.G.; data curation, A.I.G., O.C.P, and I.P.; writing—original draft preparation, C.G.C.-N., L.M., and M.L.G.; writing—review and editing, I.P.; supervision, A.I.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reddy V, P. Review on Thymus vulgaris Traditional Uses and Pharmacological Properties. J Medicinal Aromat Plants 2014, 03, 1000164. [CrossRef]
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants (Basel) 2020, 9, 961. [CrossRef]
- Sheorain, J.; Mehra, M.; Thakur, R.; Grewal, S.; Kumari, S. In vitro anti-inflammatory and antioxidant potential of thymol loaded bipolymeric (tragacanth gum/chitosan) nanocarrier. Int J Biol Macromol 2019, 125, 1069-1074. [CrossRef]
- Biswal, A.K.; Vashisht, I.; Khan, A.; Sharma, S.; Saha, S. Synthesis, characterization and antibacterial activity of thymol-loaded polylactic acid microparticles entrapped with essential oils of varying viscosity. J Mater Sci 2019, 54, 9745-9758. [CrossRef]
- Parkatzidis, K.; Chatzinikolaidou, M.; Koufakis, E.; Kaliva, M.; Farsari, M.; Vamvakaki, M. Multi-photon polymerization of bio-inspired, thymol-functionalized hybrid materials with biocompatible and antimicrobial activity. Polym Chem 2020, 11, 4078-4083. [CrossRef]
- Venturini, T.P.; Rossato, L.; Chassot, F.; De Azevedo, M.I.; Al-Hatmi, A.M.S.; Santurio, J.M.; Alves, S.H. Activity of cinnamaldehyde, carvacrol and thymol combined with antifungal agents against Fusarium spp. J Essent Oil Res 2021, 33, 502-508. [CrossRef]
- Felici, M.; Tugnoli, B.; Ghiselli, F.; Massi, P.; Tosi, G.; Fiorentini, L.; Piva, A.; Grilli, E. In vitro anticoccidial activity of thymol, carvacrol, and saponins. Poult Sci 2020, 99, 5350-5355. [CrossRef]
- Videla, E.A.; Giayetto, O.; Fernandez, M.E.; Chacana, P.A.; Marin, R.H.; Nazar, F.N. Immediate and transgenerational effects of thymol supplementation, inactivated Salmonella and chronic heat stress on representative immune variables of Japanese quail. Sci Rep 2020, 10, 18152. [CrossRef]
- Alam, M.M.; Malebari, A.M.; Syed, N.; Neamatallah, T.; Almalki, A.S.A.; Elhenawy, A.A.; Obaid, R.J.; Alsharif, M.A. Design, synthesis and molecular docking studies of thymol based 1,2,3-triazole hybrids as thymidylate synthase inhibitors and apoptosis inducers against breast cancer cells. Bioorg Med Chem 2021, 38, 116136. [CrossRef]
- Calinescu, I.; Asofiei, I.; Gavrila, A.I.; Trifan, A.; Ighigeanu, D.; Martin, D.; Matei, C.; Buleandra, M. Integrating Microwave Assisted Extraction of Essential Oils and Polyphenols from Rosemary and Thyme Leaves. Chem Eng Commun 2017, 204, 965-973. [CrossRef]
- Munekata, P.E.S.; Alcantara, C.; Zugcic, T.; Abdelkebir, R.; Collado, M.C.; Garcia-Perez, J.V.; Jambrak, A.R.; Gavahian, M.; Barba, F.J.; Lorenzo, J.M. Impact of ultrasound-assisted extraction and solvent composition on bioactive compounds and in vitro biological activities of thyme and rosemary. Int Food Res 2020, 134, 109242. [CrossRef]
- Ou, M.-C.; Liu, Y.-H.; Sun, Y.-W.; Chan, C.-F. The Composition, Antioxidant and Antibacterial Activities of Cold-Pressed and Distilled Essential Oils of Citrus paradisi and Citrus grandis (L.) Osbeck. Evid Based Complementary Altern Med 2015, 2015, 1-9. [CrossRef]
- Kant, R.; Kumar, A. Advancements in steam distillation system for oil extraction from peppermint leaves. Mater Today: Proc 2021. [CrossRef]
- Rombaut, N.; Tixier, A.-S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuel Bioprod Biorefin 2014, 8, 530-544. [CrossRef]
- Calinescu, I.; Gavrila, A.I.; Ivopol, G.C.; Ivopol, M.; Mircioaga, N.; Buleandra, M.; Badea, I.A.; Patrascu, M. On The Efficient Extraction Of Essential Oil From Mentha Spicata. UPB Sci Bull B: Chem Mater Sci 2014, 76, 3-14.
- Peng, X.; Feng, C.; Wang, X.; Gu, H.; Li, J.; Zhang, X.; Zhang, X.; Yang, L. Chemical composition and antioxidant activity of essential oils from barks of Pinus pumila using microwave-assisted hydrodistillation after screw extrusion treatment. Ind Crop Prod 2021, 166, 113489. [CrossRef]
- Miljanovic, A.; Bielen, A.; Grbin, D.; Marijanovic, Z.; Andlar, M.; Rezic, T.; Roca, S.; Jerkovic, I.; Vikic-Topic, D.; Dent, M. Effect of Enzymatic, Ultrasound, and Reflux Extraction Pretreatments on the Chemical Composition of Essential Oils. Molecules 2020, 25, 4818. [CrossRef]
- Calinescu, I.; Gavrila, A.I.; Ivopol, M.; Ivopol, G.C.; Popescu, M.; Mircioaga, N. Microwave assisted extraction of essential oils from enzymatically pretreated lavender (Lavandula angustifolia Miller). Cent Eur J Chem 2014, 12, 829-836. [CrossRef]
- Liu, Z.; Li, H.; Cui, G.; Wei, M.; Zou, Z.; Ni, H. Efficient extraction of essential oil from Cinnamomum burmannii leaves using enzymolysis pretreatment and followed by microwave-assisted method. Food Sci Technol 2021, 147, 111497. [CrossRef]
- Leila, M.; Ratiba, D.; Al-Marzouqi, A.-H. Experimental and mathematical modelling data of green process of essential oil extraction: Supercritical CO2 extraction. Mater Today: Proc 2021. [CrossRef]
- Guo, J.; Yang, R.; Gong, Y.; Hu, K.; Hu, Y.; Song, F. Optimization and evaluation of the ultrasound-enhanced subcritical water extraction of cinnamon bark oil. Food Sci Technol 2021, 147, 111673. [CrossRef]
- Yingngam, B.; Brantner, A.; Treichler, M.; Brugger, N.; Navabhatra, A.; Nakonrat, P. Optimization of the eco-friendly solvent-free microwave extraction of Limnophila aromatica essential oil. Ind Crop Prod 2021, 165, 113443. [CrossRef]
- Asofiei, I.; Calinescu, I.; Gavrila, A.I.; Ighigeanu, D.; Martin, D.; Matei, C. Microwave Hydrodiffusion and Gravity, a Green Method for the Essential Oil Extraction from Ginger - Energy Considerations UPB Sci Bull B: Chem Mater Sci 2017, 79, 81-92.
- Ferreira, D.F.; Lucas, B.N.; Voss, M.; Santos, D.; Mello, P.A.; Wagner, R.; Cravotto, G.; Barin, J.S. Solvent-free simultaneous extraction of volatile and non-volatile antioxidants from rosemary (Rosmarinus officinalis L.) by microwave hydrodiffusion and gravity. Ind Crop Prod 2020, 145, 112094. [CrossRef]
- Chen, F.; Liu, S.; Zhao, Z.; Gao, W.; Ma, Y.; Wang, X.; Yan, S.; Luo, D. Ultrasound pre-treatment combined with microwave-assisted hydrodistillation of essential oils from Perilla frutescens (L.) Britt. leaves and its chemical composition and biological activity. Ind Crop Prod 2020, 143, 111908. [CrossRef]
- Zhao, Y.; Wang, P.; Zheng, W.; Yu, G.; Li, Z.; She, Y.; Lee, M. Three-stage microwave extraction of cumin (Cuminum cyminum L.) Seed essential oil with natural deep eutectic solvents. Ind Crop Prod 2019, 140, 111660. [CrossRef]
- Calinescu, I.; Vinatoru, M.; Ghimpeteanu, D.; Lavric, V.; Mason, T.J. A new reactor for process intensification involving the simultaneous application of adjustable ultrasound and microwave radiation. Ultrason Sonochem 2021, 77, 105701. [CrossRef]
- Lee, C.S.; Binner, E.; Winkworth-Smith, C.; John, R.; Gomes, R.; Robinson, J. Enhancing natural product extraction and mass transfer using selective microwave heating. Chem Eng Sci 2016, 149, 97-103. [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Analyt Chem 2017, 97, 159-178. [CrossRef]
- Kowalski, R.; Wawrzykowski, J. Effect of ultrasound-assisted maceration on the quality of oil from the leaves of thyme Thymus vulgaris L. Flavour Frag J 2009, 24, 69-74. [CrossRef]
- Roldan-Gutierrez, J.M.; Ruiz-Jimenez, J.; Luque de Castro, M.D. Ultrasound-assisted dynamic extraction of valuable compounds from aromatic plants and flowers as compared with steam distillation and superheated liquid extraction. Talanta 2008, 75, 1369-1375. [CrossRef]
- Craciun, M.E.; Parvulescu, O.C.; Donise, A.C.; Dobre, T.; Stanciu, D.R. Characterization and classification of Romanian acacia honey based on its physicochemical parameters and chemometrics. Sci Rep 2020, 10, 20690. [CrossRef]
- Egri, D.; Pârvulescu, O.C.; Ion, V.A.; Raducanu, C.E.; Calcan, S.I.; Badulescu, L.; Madjar, R.; Orbeci, C.; Dobre, T.; Mot, A.; et al. Vine pruning-derived biochar for agronomic benefits. Agron 2022, 12, 1-15. [CrossRef]
- Zarshenas, M.M.; Samani, S.M.; Petramfar, P.; Moein, M. Analysis of the essential oil components from different Carum copticum L. samples from Iran. Pharmacogn Res 2014, 6, 62-66. [CrossRef]
- Panda, D.; Manickam, S. Cavitation Technology-The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Appl Sci 2019, 9, 766. [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.-S.; Chemat, F. Degradation during application of ultrasound in food processing: A review. Food Control 2013, 31, 593-606. [CrossRef]
- Ince, N.H.; Tezcanli, G.; Belen, R.K.; Apikyan, P.G. Ultrasound as a catalyzer of aqueous reaction systems: the state of the art and environmental applications. Appl Catal B 2001, 29, 167-176.
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason Sonochem 2001, 8, 303-313.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).