1. Introduction
Even in the era of novel agents that have improved outcomes of patients with multiple myeloma (MM) over the past few decades, disease relapse with subsequent therapeutic challenges remains inevitable [
1]. As the disease-free and treatment-free intervals get progressively shorter as patients with MM receive later lines of therapy, the first-line therapy is of paramount importance for their long-term survival and quality of life [
2,
3]. Therefore, autologous stem cell transplantation followed by novel agent-based induction is the preferred option for transplant-eligible patients with newly diagnosed MM (NDMM), as it provides more prolonged survival outcomes than chemotherapy alone [
4,
5,
6]. Successfully performing autologous stem cell transplantation for patients with NDMM without morbidity and mortality requires prerequisites, including acceptable tumor reduction by induction and patients’ tolerable condition [
7]. Collecting CD34+ stem cells above thresholds is also critical to ensure sustained multilineage recovery and improve long-term post-transplant outcomes. Although there is considerable heterogeneity, most clinicians agree that CD34+ stem cells of 2 × 10
6/kg or greater are required to avoid delayed or failed engraftment [
8,
9]. Meanwhile, others suggest that CD34+ stem cells of 5 × 10
6/kg or higher are optimal for more rapid platelet recovery [
10].
Until recently, CD34+ stem cell collection has been performed using steady-state mobilization of G-CSF alone and chemomobilization consisting primarily of chemotherapeutic agents such as a high intermediate dose of cyclophosphamide (Cy; 3.0–4.0 g/m
2) or an intermediate dose of etoposide (375 mg/m
2 for two days) plus G-CSF has been performed. However, these are occasionally associated with failure to collect adequate doses of CD34+ stem cells or significant toxicities, including infectious complications due to prolonged neutropenia [
11]. Therefore, there has been an unmet need for more effective and safer mobilization strategies. Recently, the pivotal trial for plerixafor in patients with MM showed that it could improve the yield of CD34+ stem cell collection without additional safety concerns [
12], but the widespread use has been limited due to its high burden. To address this issue, Costa et al. [
13] implemented the algorithm of G-CSF plus risk-adapted use of plerixafor, improving its cost-effectiveness. In addition, Park et al. [
14] showed that single-dose etoposide (375 mg/m
2 for one day) plus G-CSF could be an effective mobilization with a favorable safety profile.
Based on these studies, we conducted a prospective trial comparing the efficacy and safety of single-dose etoposide plus G-CSF (the etoposide group) versus the G-CSF alone (the G-CSF alone group), followed by risk-adapted use of plerixafor. The results of the current study are presented in this manuscript.
2. Patients and method
2.1. Study design, participants, and randomization
This study is an open-label, prospective, comparative, and randomized trial in which patients were recruited from two tertiary referral hospitals of the Catholic University of Korea, Seoul, South Korea (Seoul St. Mary’s Hospital and Eunpyeong St. Mary’s Hospital, Seoul). Eligible patients were 18 years of age or older with histologically confirmed MM who achieved a partial response (PR) or better to induction with proteasome inhibitors and/or immunomodulatory agents. Participants were required to meet the following inclusion criteria: Eastern Cooperative Oncology Group (ECOG) performance status (PS) of two or less, adequate hematologic function (neutrophil count of 1.0 × 109/kg or grater and platelet count of 75 × 109/kg or greater), and normal hepatic (total bilirubin and aspartate/alanine aminotransferase less than two half times of the upper limit of normal) and renal (estimated glomerular filtration rate of 30 mL/min/1.73m2 or greater) function. Patients were excluded if they were ineligible for transplantation, had a history of previous autologous or allogeneic stem cell transplantation for other diseases, or had relapsed after first-line therapy without the intention of transplantation. Based on a preliminary analysis of our historical cohort, we estimated that 24 participants would be needed in each group to achieve approximately 99% discriminative power at the 5% significance level on the hypothesis that the etoposide group would have a significantly higher optimal collection rate. Considering the dropout rate of 20%, we planned to enroll 30 patients in each group. Using computerized random assignment, participants were randomized 1:1 to the etoposide group and the G-CSF alone group. This trial was registered in the Clinical Research Information System of South Korea (KCT0006245).
2.2. Procedures
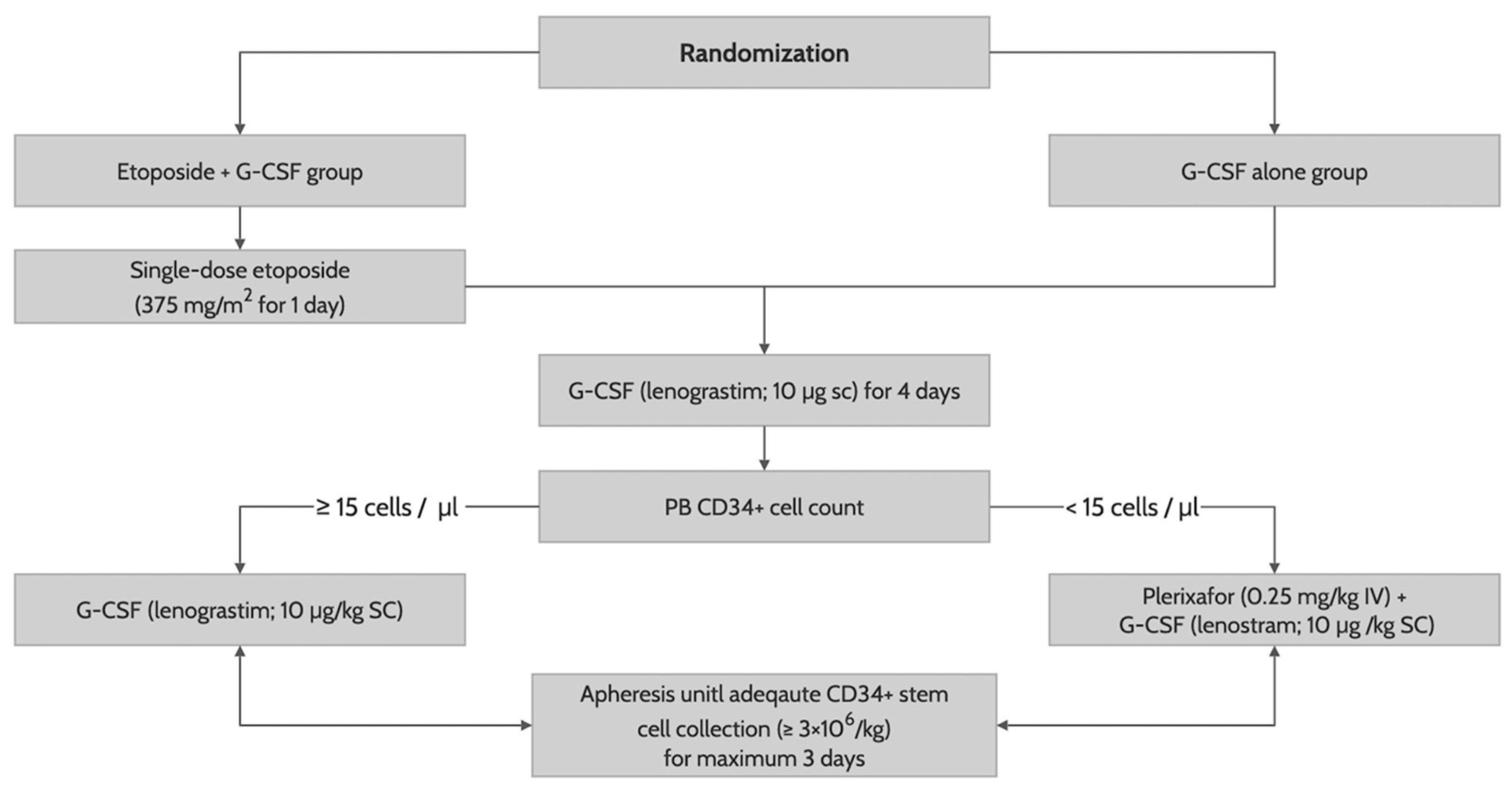
After randomization, patients received G-CSF (lenograstim; 10 µg/kg subcutaneously) until the last day of apheresis with (in the etoposide group) or without (in the G-CSF alone group) a previous single-dose etoposide (375 mg/m
2 intravenously for one day on the ninth day of G-CSF administration). On day 5 of G-CSF administration, we measured a PB CD34+ cell counts by flow cytometry. Patients with PB CD34+ cells less than 15/mm
3 received an additional risk-adapted plerixafor (0.24 mg/kg subcutaneously) until the last day of apheresis. CD34+ stem cell collection was performed in all patients using the COBE spectra (Terumo BCT, Lakewood, CO) on day 5 of G-CSF administration, with one-day delay in those determined to receive an additional risk-adapted plerixafor, for a maximum of three days, with a target CD34+ cell count of 6 × 10
6/kg (
Figure 1). All patients who successfully collected the CD34+ stem cells of 2 × 10
6/kg or greater proceeded to autologous stem cell transplantation using a melphalan-based conditioning. To facilitate neutrophil engraftment, they received G-CSF (lenograstim; 10 µg/kg intravenously) from day 5 of CD34+ stem cell infusion to achieve a sustained absolute neutrophil count of 0.5 × 10
9/l or higher. Other transplant-related procedures were performed according to institution’s protocol.
2.3. Definition
Optimal and adequate collection were defined as achieving a CD34+ stem cell count of 6 × 10
6/kg or greater and 3 × 10
6/kg or greater, respectively, by the last day apheresis. If the count of collected CD34+ stem cells was less than 2 × 10
6/kg at the completion of apheresis, it was considered a collection failure. Pre-mobilization chemotherapy response was assessed between the completion of induction and the randomization for this study according to the IMWG response criteria [
15]: using serum and 24-hour urine protein electrophoresis/immunofixation or serum-free light chain assay, with bone marrow aspiration and biopsy if patients were expected to achieve a complete response (CR) or better.
Neutrophil and platelet engraftment were defined as achieving a sustained neutrophil count of 0.5 × 109/l or greater for at least three days and a sustained platelet count of 20 × 109/l or greater for at least seven days, respectively. Adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 from the commencement of mobilization to the completion of apheresis.
2.4. Endpoints and statistical analysis
The primary endpoint of the current study was the optimal collection rate of the etoposide group and the G-CSF alone group. The secondary endpoints were adequate collection rate, collection failure rate, cumulative incidence of neutrophil and platelet engraftment, and grade I-IV (non-hematologic toxicities) or grade II-IV (hematologic toxicities) or higher adverse events in both groups. Categorical variables in baseline and disease-related characteristics were described as counts with relative frequencies, whereas continuous variables were summarized as medians with ranges. All non-time-dependent endpoints were compared using the appropriate chi-squared or Fisher’s exact test. Neutrophil and platelet engraftment were estimated as a cumulative incidence, considering disease progression and death from any cause as competing risks, which were compared using Grey’s test. Factors or covariables with P < 0.050 were considered significant using two-tailed significance testing. All statistical analyses were performed with R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria) with the ‘Survival’, ‘tidycmprsk’, ‘ggplot2’, and ‘ggsurvfit’ packages on May 15, 2023.
3. Results
3.1. Baseline and clinical characteristics
Between September 15, 2021, and October 31, 2022, 60 NDMM patients who had completed induction were enrolled. Thirty patients were randomly assigned to the etoposide group and the others to the G-CSF alone group, respectively. Four patients (three in the etoposide group and one in the G-CSF alone group) withdrew informed consent prior to mobilization. The median age of all participants at randomization was 60 years (range, 37–69). According to the Reversed International Staging System [
16], 17 (30.4%) and 27 (48.2%) patients had stages I and II–III disease, respectively. They received one course of induction, including bortezomib plus thalidomide and dexamethasone (VTD) in 29 (51.8%), bortezomib plus lenalidomide and dexamethasone in 4 (7.1%), and daratumumab plus VTD in 23 (41.1%). All baseline and disease-related characteristics were well balanced between the etoposide and the G-CSF alone groups (
P ≥ 0.199) (
Table 1).
3.2. Mobilization
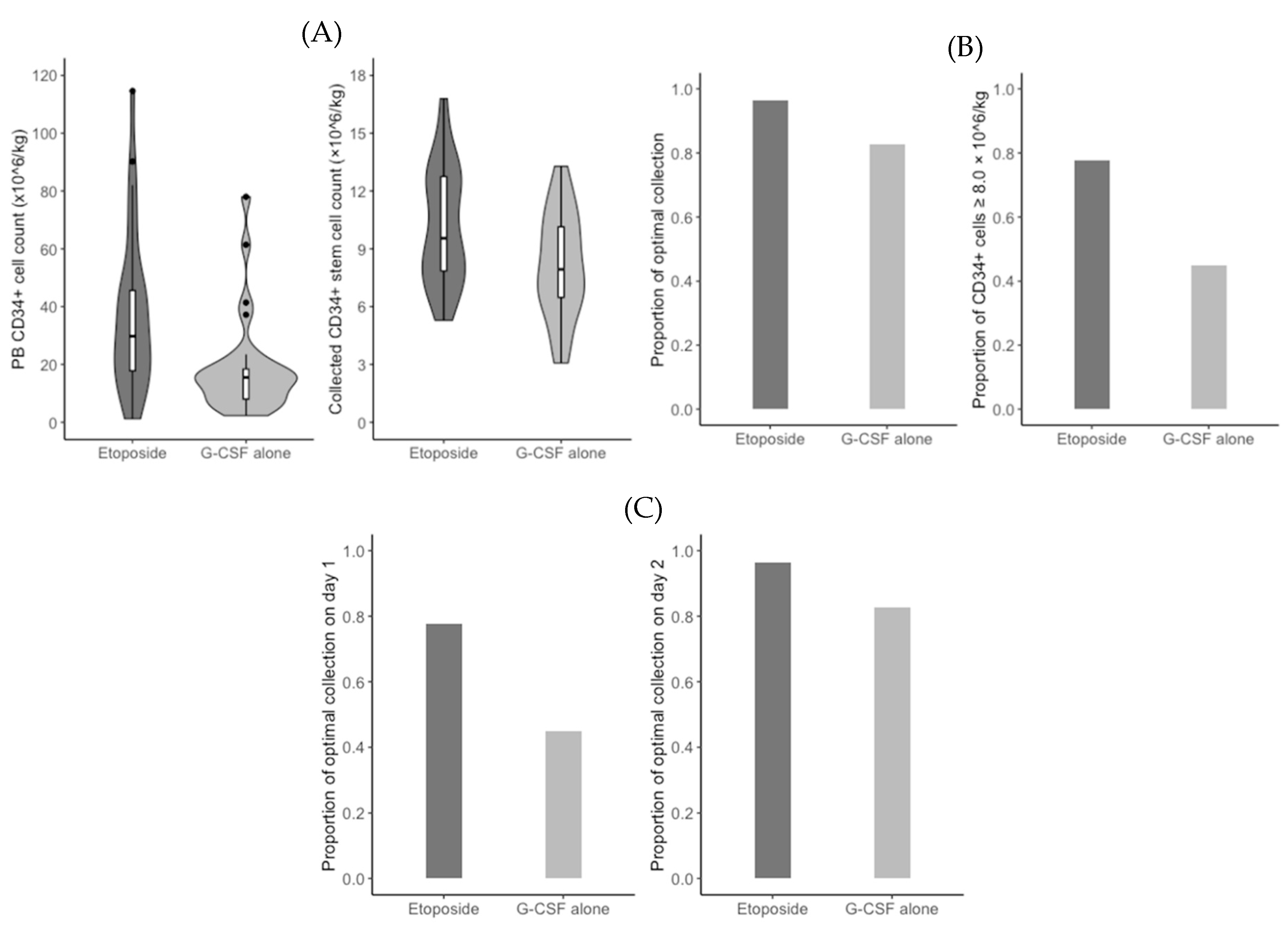
The median PB CD34+ cell count on day 5 of G-CSF administration was significantly higher in the etoposide group (29.8/mm
3 vs. 15.5/mm
3;
P = 0.001). Based on a measured count of less than 15/mm
3, 6 (22.2%) patients in the etoposide group and 15 (51.7%) patients in the G-CSF alone group received an additional risk-adapted plerixafor (
P = 0.045) (
Figure 2A). In addition, the median duration of apheresis was significantly shorter in the etoposide group than in the G-CSF alone group (1.0 days vs. 2.0 days;
P = 0.027). Consequently, the median total count of CD34+ stem cells collected was significantly higher in the etoposide group (9.5 × 10
6/kg vs. 7.9 × 10
6/kg;
P = 0.018) (
Figure 2A). However, the optimal collection rates were not significantly different between the etoposide group and the G-CSF alone group (96.3% vs. 82.8%;
P = 0.195) (
Figure 2B). When we performed the
post hoc analysis, the rate of collected CD34+ stem cells of 8.0 × 10
6/kg or grater was significantly higher in the etoposide group (77.8% vs 44.8%;
P = 0.025) (
Figure 2B). The optimal collection rate on apheresis day 1 was significantly higher in the etoposide group (77.8% vs. 44.8%;
P = 0.025), but did not differ significantly differ between the two groups on apheresis day 2 (96.3% vs. 82.8%;
P = 0.195) (
Figure 2C). CD34+ stem cell counts were 3.0 × 10
6/kg or higher in all patients, regardless of group. Baseline and disease-related characteristics did not significantly affect the rates of optimal collection and using an additional risk-adapted plerixafor (
P ≥ 0.092).
3.3. Adverse events
The rates of adverse events observed in both the etoposide and the G-CSF alone groups were relatively low, not exceeding 20.0%, except for grade II-IV thrombocytopenia of 46.4% (95% CI, 33.0–60.3). All were self-limited or manageable and resolved within three days. There were no infectious complications such as neutropenic fever or septic shock. No serious adverse events requiring hospitalization or resulting in permanent disability or death were observed.
When comparing the rates of hematologic and non-hematologic adverse events between the etoposide and the G-CSF alone groups, the rates of grade II-IV thrombocytopenia (63.0% vs. 31.0%;
P = 0.031) and grade I-IV nausea (14.8% vs. 0%;
P = 0.048) were significantly higher in the etoposide group. However, there was no significant difference in the rate of grade III-IV thrombocytopenia rate between the etoposide and G-CSF alone groups (14.8% vs. 17.2%;
P = 1.000). No patient experienced a clinical event of bleeding or hemorrhage. There were no significant differences in the rates of other adverse events between the two groups as described in
Table 2.
3.4. Neutrophil and platelet engraftment
All patients in the etoposide and the G-CSF alone groups successfully proceeded to autologous stem cell transplantation. The conditioning regimens were melphalan at 100 mg/m
2 intravenously for two days in 53 (94.6%) patients and melphalan at 70 mg/m
2 intravenously for two days plus busulfan at 3.2 mg/kg intravenously for three days in three (5.4%) patients. Of those receiving melphalan conditioning, the dose was reduced to 70 mg/m
2 in 11 patients (20.6%) due to an estimated glomerular filtration rate of less than 40 mL/min/1.73m
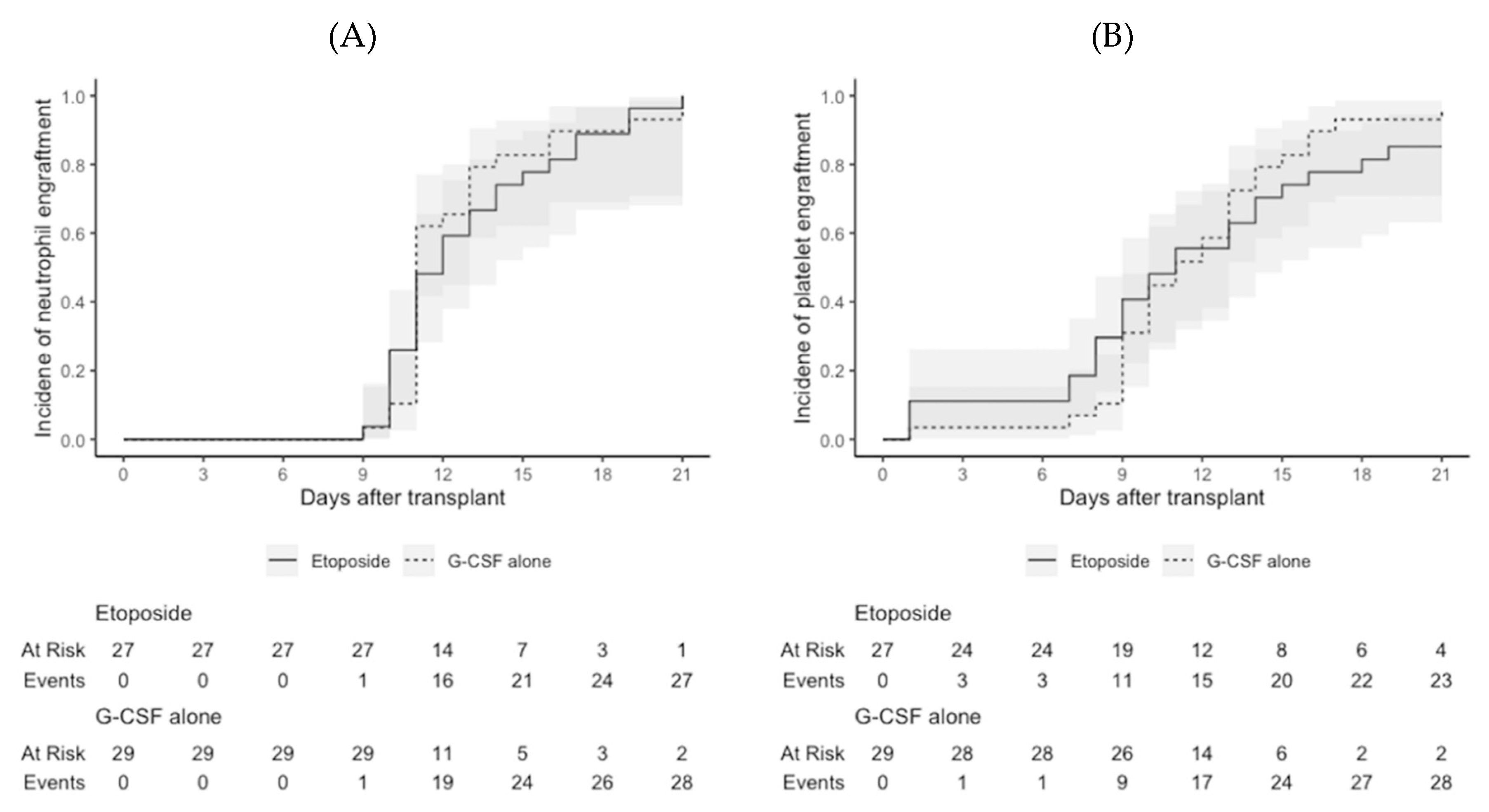
2 or other comorbidities. Median infused CD34+ stem cell counts were not significantly different between the etoposide group and the G-CSF alone group (5.8 × 10
6/kg vs. 4.9 × 10
6/kg;
P = 0.162). One patient in the etoposide group experienced delayed platelet engraftment (on day 211 after transplantation), but the median days to neutrophil engraftment (11.0 days in both;
P = 1.000) and platelet engraftment (11.0 days in both;
P = 1.000) were not significantly different between the two groups (
Figure 3). All patients who survived to the last day of follow-up maintained neutrophil and platelet engraftment.
4. Discussion
The current randomized trial comparing the mobilizations with single-dose etoposide plus G-CSF and G-CSF alone followed by risk-adapted use of plerixafor showed that optimal collection rates were not significantly different between the two groups. However, the rate of collected CD34+ stem cell of 8.0×106/kg or greater was significantly higher in the etoposide group. Patients in the etoposide group also required significantly fewer days of apheresis. The incidence of adverse events was acceptable in both the etoposide and the G-CSF alone groups. These findings suggest the considerations for the optimal mobilization strategy in NDMM patients who are expected to receive an autologous stem cell transplantation.
Steady-state mobilization with G-CSF alone and chemomobilization mainly with high-dose Cy (3.0–4.0 g/m
2) plus G-CSF have been the two mainstays for mobilizing CD34+ stem cells from bone marrow. However, when we used these traditional strategies, some challenges to collect optimal doses of CD34+ stem cells would be encountered as follows: G-CSF alone mobilization was associated with a high collection failure rate of 24–50%, despite its significantly lower rate of toxiciy [
17,
18]. While an intermediate high-dose Cy-based mobilization improved the successful collection rates up to 90%, it was associated with occasionally fatal toxicities, including neutropenic fever up to 20% and septic shock up to 20% [
19,
20,
21].
On the armamentarium to overcome these challenges, etoposide (topoisomerase II inhibitor) has been a widely used chemotherapeutic agent to facilitate the collection of CD34+ stem cells in patients with various hematologic diseases. Although the detailed mechanism to mobilize hematopoietic stem cells (HSCs) from bone marrow needs to be further elucidated, Kang et al. recently showed that the etoposide increased the interleukin-8 secretion by bone marrow stromal cells, which is responsible for the enhanced proliferation and mobilization of HSCs [
22]. In contrast, its clinical role in CD34+ stem cell mobilization has been investigated in several previous studies. Wood et al. [
23] reported that the results of CD34+ stem cell collection using intermediate-dose etoposide (375 mg/m
2 for two days) plus G-CSF mobilization in 152 patients with MM, which showed that 150 (98.7%) patients could achieve optimal collection (CD34+ cells of 5×10
6/kg or greater for one to two days), but the toxicities of neutropenia (78.9%) and neutropenic fever (9.0%) were of concern to be overcome. Subsequently, Park et al. reported the results of CD34+ cell collection in 32 patients with MM using single-dose etoposide (375 mg/m
2 for one day) plus G-CSF mobilization, which showed better safety profile of febrile neutropenia in two (6.3%) patients, without other toxicities such as mucositis, pneumonia, and bacteremia, and without decreased rates of optimal (CD34+ cells of 5×10
6/kg or higher; 71.9%) and adequate (CD34+ cells of 3 × 10
6/kg or higher; 87.5%) collection.
Plerixafor is a selective and reversible antagonist of CXCR4, which induces chemotaxis in the bone marrow through its ligand of SCF-1. In its pivotal phase III trial in 302 patients with MM, the plerixafor group showed a higher successful collection rate (CD34+ cells of 6 × 10
6/kg or greater) than the placebo group (71.6% vs. 34.4%;
P < 0.001) [
12]. The most common adverse events with plerixafor were mild gastrointestinal toxicities (diarrhea in 18.4%, nausea in 16.3%, and vomiting in 5.4%) and injection site erythema (20.4%). However, the high cost of plerixafor has limited its widespread use in real-world practice. In light of this, Costa et al. [
24] implemented a decision-making algorithm using the PB CD34+ cell count on day 5 of G-CSF administration to optimize the cost-effectiveness using risk-adapted plerixafor. When they evaluated it in a validation cohort consisting of 34 patients with MM, 33 (97.1%) achieved the pre-specified CD34+ stem cell targets (6 × 10
6/kg for patients expected to receive tandem autologous stem cell transplantation and 3 × 10
6/kg for others). Given the high applicability of this approach in real-world practice, we used it in both the etoposide and the G-CSF alone groups of the current study, using a PB CD34+ cell threshold of 15mm
3 (the highest to optimize the burden by using plerixafor, for a target CD34+ cell collection of 3 × 10
6/kg, in the study by Costa et al. [
24]).
The results of the current study did not meet the hypothesis for the primary endpoint that the optimal collection rate would be significantly higher in the etoposide group. The PB CD34+ cell count on day 5 of G-CSF administration was significantly higher in the etoposide group, suggesting that the optimal collection rates of the two groups could be significantly different if patients did not receive a risk-adapted use of plerixafor. That is, a stricter protocol-driven use of risk-adapted plerixafor in a prospective setting than in the historical real-world cohort, based on the sample size calculation in the current study, might make the optimal collection rates between the two groups not significantly different.
Nevertheless, the current study suggests that the single-dose etoposide plus G-CSF may be helpful compared to the G-CSF alone in the real-world practice with the use of risk-adapted plerixafor. First, single-dose etoposide plus G-CSF may make it possible to reduce the burden of CD34+ stem cell collection by suppressing the use of costly plerixafor, which is supported by previous studies showing that the optimal use of plerixafor contributes to reducing the cost of the CD34+ stem cell collection [
24,
25]. In addition, etoposide may not require a high burden to manage toxicities due to their low severities and incidence. Second, considering that the rate of collected CD34+ stem cells of 8.0 × 10
6/kg or greater was significantly higher in the etoposide group, single-dose etoposide plus G-CSF may be a better choice for patients with specific disease-related conditions, including patients with extra-medullary disease and high-risk cytogenetics requiring tandem autologous stem cell transplantation [
26,
27]. It should be noted that salvage autologous stem cell transplantation also showed better results compared to chemotherapy alone in relapsed/refractory MM patients with a relatively longer relapse-free interval after the first course of autologous stem cell transplantation [
28,
29,
30].
5. Conclusions
In the current study, optimal collection rates were acceptable for patients receiving single-dose etoposide plus G-CSF and G-CSF alone followed by risk-adapted plerixafor. However, the rate of collected CD34+ stem cells of 8.0×106/kg or greater was significantly higher in the etoposide group. Meanwhile, the rates of adverse events of were relatively low in both the etoposide and the G-CSF alone groups. These results suggest that single-dose etoposide plus G-CSF may be a better choice when using risk-adapted plerixafor at least for patients expected to receive a second or third course of autologous stem cell transplantation. The limitations of the current study, including the relatively small number of participants, which made it difficult to perform a detailed analysis of the factors influencing the outcome of CD34+ stem cell collection, would be overcome by well-designed studies with larger numbers of patients to provide sufficient power to address these issues.
Author contributions: Conceptualization, Chang-Ki Min and Seung-Hwan Shin; Formal analysis, Sung-Soo Park, Young-Woo Jeon, Seung-Ah Yhang and Seung-Hwan Shin; Methodology, Sung-Soo Park, Young-Woo Jeon, Seung-Ah Yhang and Seung-Hwan Shin; Project administration, Seung-Hwan Shin; Writing – original draft, Sung-Soo Park, Chang-Ki Min and Seung-Hwan Shin; Writing – review & editing, Seung-Hwan Shin.
Funding
This work was not supported by any public or commercial funding.
Institutional Review Board Statement
This study was conducted in accordance with the tenets of the Declaration of Helsinki and approved by the Institutional Review Boards of Seoul St. Mary’s Hospital (XC20MIDT0108K) and Eunpyeong St. Mary’s Hospital and (XC20MIDT0108P).
Informed Consent Statement
All enrolled patients provided written informed consent prior to randomization.
Data Availability Statement
Data will be made available upon request to the corresponding author (SHS) due to restrictions of the Personal Information Protection Act of South Korea.
Acknowledgments
We thank the data manager of CARE-MM, Jung-Min Park, for her valuable assistance. This study was supported by an investigational drug (etoposide; EPS injection®) from Boryung Pharmaceutical Co., Ltd. and by a grant from The Catholic University of Korea, Eunpyeong St. Mary's Hospital, Research Institute of Medical Science in program year 2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat Rev Dis Primers 2017, 3, 17046. [Google Scholar] [CrossRef] [PubMed]
- Acaster, S.; Gaugris, S.; Velikova, G.; Yong, K.; Lloyd, A.J. Impact of the treatment-free interval on health-related quality of life in patients with multiple myeloma: a UK cross-sectional survey. Support Care Cancer 2013, 21, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.; Delforge, M.; Driessen, C.; Fink, L.; Flinois, A.; Gonzalez-McQuire, S.; Safaei, R.; Karlin, L.; Mateos, M.V.; Raab, M.S.; et al. Multiple myeloma: patient outcomes in real-world practice. Br J Haematol 2016, 175, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med 2017, 376, 1311–1320. [Google Scholar] [CrossRef]
- Gay, F.; Musto, P.; Rota-Scalabrini, D.; Bertamini, L.; Belotti, A.; Galli, M.; Offidani, M.; Zamagni, E.; Ledda, A.; Grasso, M.; et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): a randomised, open-label, phase 2 trial. Lancet Oncol 2021, 22, 1705–1720. [Google Scholar] [CrossRef]
- Palumbo, A.; Cavallo, F.; Gay, F.; Di Raimondo, F.; Ben Yehuda, D.; Petrucci, M.T.; Pezzatti, S.; Caravita, T.; Cerrato, C.; Ribakovsky, E.; et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 2014, 371, 895–905. [Google Scholar] [CrossRef]
- Devarakonda, S.; Efebera, Y.; Sharma, N. Role of Stem Cell Transplantation in Multiple Myeloma. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Bensinger, W.; Appelbaum, F.; Rowley, S.; Storb, R.; Sanders, J.; Lilleby, K.; Gooley, T.; Demirer, T.; Schiffman, K.; Weaver, C.; et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol 1995, 13, 2547–2555. [Google Scholar] [CrossRef]
- Desikan, K.R.; Tricot, G.; Munshi, N.C.; Anaissie, E.; Spoon, D.; Fassas, A.; Toor, A.; Zangari, M.; Badros, A.; Morris, C.; et al. Preceding chemotherapy, tumour load and age influence engraftment in multiple myeloma patients mobilized with granulocyte colony-stimulating factor alone. Br J Haematol 2001, 112, 242–247. [Google Scholar] [CrossRef]
- Demirer, T.; Buckner, C.D.; Gooley, T.; Appelbaum, F.R.; Rowley, S.; Chauncey, T.; Lilleby, K.; Storb, R.; Bensinger, W.I. Factors influencing collection of peripheral blood stem cells in patients with multiple myeloma. Bone Marrow Transplant 1996, 17, 937–941. [Google Scholar]
- Arora, S.; Majhail, N.S.; Liu, H. Hematopoietic Progenitor Cell Mobilization for Autologous Stem Cell Transplantation in Multiple Myeloma in Contemporary Era. Clin Lymphoma Myeloma Leuk 2019, 19, 200–205. [Google Scholar] [CrossRef] [PubMed]
- DiPersio, J.F.; Micallef, I.N.; Stiff, P.J.; Bolwell, B.J.; Maziarz, R.T.; Jacobsen, E.; Nademanee, A.; McCarty, J.; Bridger, G.; Calandra, G.; et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol 2009, 27, 4767–4773. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Miller, A.N.; Alexander, E.T.; Hogan, K.R.; Shabbir, M.; Schaub, C.; Stuart, R.K. Growth factor and patient-adapted use of plerixafor is superior to CY and growth factor for autologous hematopoietic stem cells mobilization. Bone Marrow Transplant 2011, 46, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, D.S.; Jeon, M.J.; Lee, B.H.; Yu, E.S.; Kang, K.W.; Lee, S.R.; Sung, H.J.; Nam, M.H.; Yoon, S.Y.; et al. Single-dose etoposide is an effective and safe protocol for stem cell mobilization in patients with multiple myeloma. J Clin Apher 2019, 34, 579–588. [Google Scholar] [CrossRef]
- Durie, B.G.; Harousseau, J.L.; Miguel, J.S.; Blade, J.; Barlogie, B.; Anderson, K.; Gertz, M.; Dimopoulos, M.; Westin, J.; Sonneveld, P.; et al. International uniform response criteria for multiple myeloma. Leukemia 2006, 20, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Arora, M.; Burns, L.J.; Barker, J.N.; Miller, J.S.; Defor, T.E.; Olujohungbe, A.B.; Weisdorf, D.J. Randomized comparison of granulocyte colony-stimulating factor versus granulocyte-macrophage colony-stimulating factor plus intensive chemotherapy for peripheral blood stem cell mobilization and autologous transplantation in multiple myeloma. Biol Blood Marrow Transplant 2004, 10, 395–404. [Google Scholar] [CrossRef]
- Pusic, I.; Jiang, S.Y.; Landua, S.; Uy, G.L.; Rettig, M.P.; Cashen, A.F.; Westervelt, P.; Vij, R.; Abboud, C.N.; Stockerl-Goldstein, K.E.; et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant 2008, 14, 1045–1056. [Google Scholar] [CrossRef]
- Jang, J.E.; Cheong, J.W.; Kim, S.J.; Cho, H.; Suh, C.; Lee, H.; Eom, H.S.; Yhim, H.Y.; Lee, W.S.; Min, C.K.; et al. Selection of a mobilization regimen for multiple myeloma based on the response to induction therapy: granulocyte-colony stimulating factor (G-CSF) alone versus high-dose cyclophosphamide plus G-CSF. Leuk Lymphoma 2016, 57, 1389–1397. [Google Scholar] [CrossRef]
- Kumar, S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Gastineau, D.A.; Litzow, M.R.; Fonseca, R.; Roy, V.; Rajkumar, S.V.; et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia 2007, 21, 2035–2042. [Google Scholar] [CrossRef]
- Tuchman, S.A.; Bacon, W.A.; Huang, L.W.; Long, G.; Rizzieri, D.; Horwitz, M.; Chute, J.P.; Sullivan, K.; Morris Engemann, A.; Yopp, A.; et al. Cyclophosphamide-based hematopoietic stem cell mobilization before autologous stem cell transplantation in newly diagnosed multiple myeloma. J Clin Apher 2015, 30, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.W.; Lee, S.J.; Kim, J.H.; Lee, B.H.; Kim, S.J.; Park, Y.; Kim, B.S. Etoposide-mediated interleukin-8 secretion from bone marrow stromal cells induces hematopoietic stem cell mobilization. BMC Cancer 2020, 20, 619. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.A.; Whitley, J.; Moore, D.; Sharf, A.; Irons, R.; Rao, K.; Serody, J.; Coghill, J.; Gabriel, D.; Shea, T. Chemomobilization with Etoposide is Highly Effective in Patients with Multiple Myeloma and Overcomes the Effects of Age and Prior Therapy. Biol Blood Marrow Transplant 2011, 17, 141–146. [Google Scholar] [CrossRef]
- Costa, L.J.; Alexander, E.T.; Hogan, K.R.; Schaub, C.; Fouts, T.V.; Stuart, R.K. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone Marrow Transplant 2011, 46, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Giralt, S.; Costa, L.; Schriber, J.; Dipersio, J.; Maziarz, R.; McCarty, J.; Shaughnessy, P.; Snyder, E.; Bensinger, W.; Copelan, E.; et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant 2014, 20, 295–308. [Google Scholar] [CrossRef]
- Gagelmann, N.; Eikema, D.J.; Koster, L.; Caillot, D.; Pioltelli, P.; Lleonart, J.B.; Remenyi, P.; Blaise, D.; Schaap, N.; Trneny, M.; et al. Tandem Autologous Stem Cell Transplantation Improves Outcomes in Newly Diagnosed Multiple Myeloma with Extramedullary Disease and High-Risk Cytogenetics: A Study from the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2019, 25, 2134–2142. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Pasquini, M.C.; Blackwell, B.; Hari, P.; Bashey, A.; Devine, S.; Efebera, Y.; Ganguly, S.; Gasparetto, C.; Geller, N.; et al. Autologous Transplantation, Consolidation, and Maintenance Therapy in Multiple Myeloma: Results of the BMT CTN 0702 Trial. J Clin Oncol 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Cook, G.; Ashcroft, A.J.; Cairns, D.A.; Williams, C.D.; Brown, J.M.; Cavenagh, J.D.; Snowden, J.A.; Parrish, C.; Yong, K.; Cavet, J.; et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol 2016, 3, e340–351. [Google Scholar] [CrossRef]
- Garderet, L.; Iacobelli, S.; Koster, L.; Goldschmidt, H.; Johansson, J.E.; Bourhis, J.H.; Krejci, M.; Leleu, X.; Potter, M.; Blaise, D.; et al. Outcome of a Salvage Third Autologous Stem Cell Transplantation in Multiple Myeloma. Biol Blood Marrow Transplant 2018, 24, 1372–1378. [Google Scholar] [CrossRef]
- Michaelis, L.C.; Saad, A.; Zhong, X.; Le-Rademacher, J.; Freytes, C.O.; Marks, D.I.; Lazarus, H.M.; Bird, J.M.; Holmberg, L.; Kamble, R.T.; et al. Salvage second hematopoietic cell transplantation in myeloma. Biol Blood Marrow Transplant 2013, 19, 760–766. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).