1. Introduction
End stage renal disease (ESRD) patients have a very high cardiovascular (CV) burden, due to both traditional (hypertension, diabetes, smoking, age) and non-traditional (CKD-associated mineral bone disorder, inflammation, uremic toxins) risk factors (1). CKD associated mineral bone disease (CKD MBD) includes a vast array of imbalances including high calcium x phosphorus product, high FGF23, low vitamin D levels or high parathormone (PTH) (2). Patients on renal replacement therapies (RRT) are reported to have particular cardiovascular presentations such as arterial medial calcifications, heart valve calcifications and calcific arteriolopathy (1). Moreover, once they have occurred, calcification processes are considered largely irreversibile (3). In light of these clinical observations, the mortality of ESRD patients is largely accounted by CV factors and is 30 times higher than in the same age matched general population (4). Multiple efforts have been made in order to find therapeutic approaches.
More recently, novel molecules involved in accelerated calcifications have been identified, with vitamin K status disturbances being a key factor in the pathogenic processes.
Vitamin K deficiency is a frequent finding in CKD patients, particularly dialysis patients (5). The underlying mechanism of vitamin K deficiency is a combination between increased consumption in order to produce vitamin K dependent calcification inhibiting proteins and reduced intake, due to low potassium and phosphate diets.
Vitamin K is a fat soluble vitamine which constitutes the substrate for several vitamin K dependent proteins (VKDPs), some of them involved in the coagulation cascade, cellular adhesion and migration, bone and vascular homeostasis. The VKDPs implicated in calcification processes are Matrix Gla protein (MGP), osteocalcin (OC), bone Gla protein, growtharrest-specific protein 6, and Gla-rich protein (6). In order to assess vitamin K status, instead of measuring the plasma level of Vitamin K, that is difficult, a valid indirect and more accessible method is to measure ucOC and ucMGP through ELISA, increased levels reflecting a vitamin K deficiency
MGP is transformed in an active form through phosphorylation and vitamin K dependent carboxylations. The MGP inactive form (dephosphorylated-uncarboxylated MGP- dp-ucMGP) reflects the subject’s vitamin K status and higher levels have been associated with increased ectopic calcifications (7).
Osteocalcin (OC) is produced by osteoblasts and has a role in increasing mineral bone density. OC transformation from its uncarboxylated form to its fully functional carboxylated form is vitamin K mediated. Increased Uncarboxylated OC is, along with ucMGP, a marker of vitamin K deficiency (8).
There are several therapeutic interventions available in order to control CKD-MBD. According to the calcium-phosphate balance, PTH and (1,25) OH vitamin D levels, current guidelines (9) recommend the use of phosphate binders, calcitriol, vitamin D analogs or calcimimetics. The use of these medications has been implied extensively in order to reduce vascular calcifications and hence, cardiovascular burden. The interaction between these drugs and VKDPs has been less studied. Moreover, current vitamin K status is not routinely assessed in ESRD patients, therefore reports regarding possible interactions between concurrent medications and vitamin K deficiency are scarcer.
Vitamin K homeostasis is altered in ESRD patients requiring warfarin-based anticoagulation. Warfarin prescription for ESRD patients is implied in order to reduce stroke risk in patients with atrial fibrillation or prosthetic valves, but sometimes also in order to preserve vascular access patency. Warfarin acts through the inhibition of a vitamin K epoxide reductase enzyme and contributes to ectopic calcification processes by reducing the activity endogenous mineralization inhibitors (10).
The present study aims to assess OC and MGP levels in a cohort of hemodialysis patients and their relationship to CKD-MBD markers, nutritional status as well as concomitant medications.
2. Materials and Methods
We conducted a single center cross sectional study including 45 CKD G5D patients, all patients undergoing maintenance hemodialysis for 6 months to 10 years. All procedures were in accordance with the ethical standards of the institutional research committee and with the Declaration of Helsinki.
Clinical and biochemical evaluation
We collected pre-dialysis blood specimens in every fasting patient after prior informed consent. We obtained the following parameters: iPTH, calcium, phosphorus, serum albumin, intact OC, MGP, serum albumin, serum C reactive protein. In every patient the physical exam has been performed before the dialysis session (including assessment of weight, height, BMI, abdominal circumference).
We recorded in every included patient demographical data, (including gender, age), as well as medical history- dialysis vintage, CKD etiology, history of comorbidities such as diabetes mellitus, presence of coronary heart disease, presence of stroke, history of fractures. The use of concomitant medication for CKD associated mineral bone disease use was also recorded (including paricalcitol, phosphate binders, vitamin D supplements), as well as treatment with vitamin K antagonists.
Statistical analysis
We used MedCalc Software, version 12.5.0 (MedCalc, Mariakerke, Belgium) for the statistical analysis. We used the Kolmogrov-Smirnov test to assess the distribution of numeric variables. For variables with a normal distribution, we calculated the means and standard deviations. For group comparisons of continuous variables with a normal distribution, we used Student t tests, and we used Mann-Whitney U tests for variables with a non-normal distribution. For the correlations we used the Spearman rank correlation test.
3. Results
The study enrolled 45 patients on hemodialysis. The characteristics of the studied lot are presented in
Table 1. The mean levels of the VKDP measured in our patients were as follows: mean OC value was 169.06 +/- 128.28 ng/ml, while mean MGP was 3285.93 +/- 2092.85 pmol/l.
There was a strong statistically significant correlation of OC with CKD-MBD markers such as: iPTH (r= 0.48, p= 0.0007), serum Calcium (r=0.49, p= 0.0005) and serum Phosphorus (r= 0.29, p=0.04). We found no statistically significant correlation between MGP and iPTH, serum calcium and serum phosphorus. Only two out of 45 patients presented a history of bone fractures.
Concerning the relationship with the patients’ nutritional status, we found no statistically significant correlations of the assessed VKDP with BMI and serum albumin values. However, we did find a strong indirect statistically significant correlation of OC with the subjects’ abdominal circumference (r=-0.43, p= 0.003). Furthermore, we came across a statistically significant correlation of MGP levels with markers of inflammation (CRP) (r=0.55, p=0.004).
We found no statistically significant differences in regard to the level of VKDP of patients with and without history of different co morbidities, such as diabetes mellitus (13/ 45), coronary heart disease (18/ 45) or stroke (5/ 45).
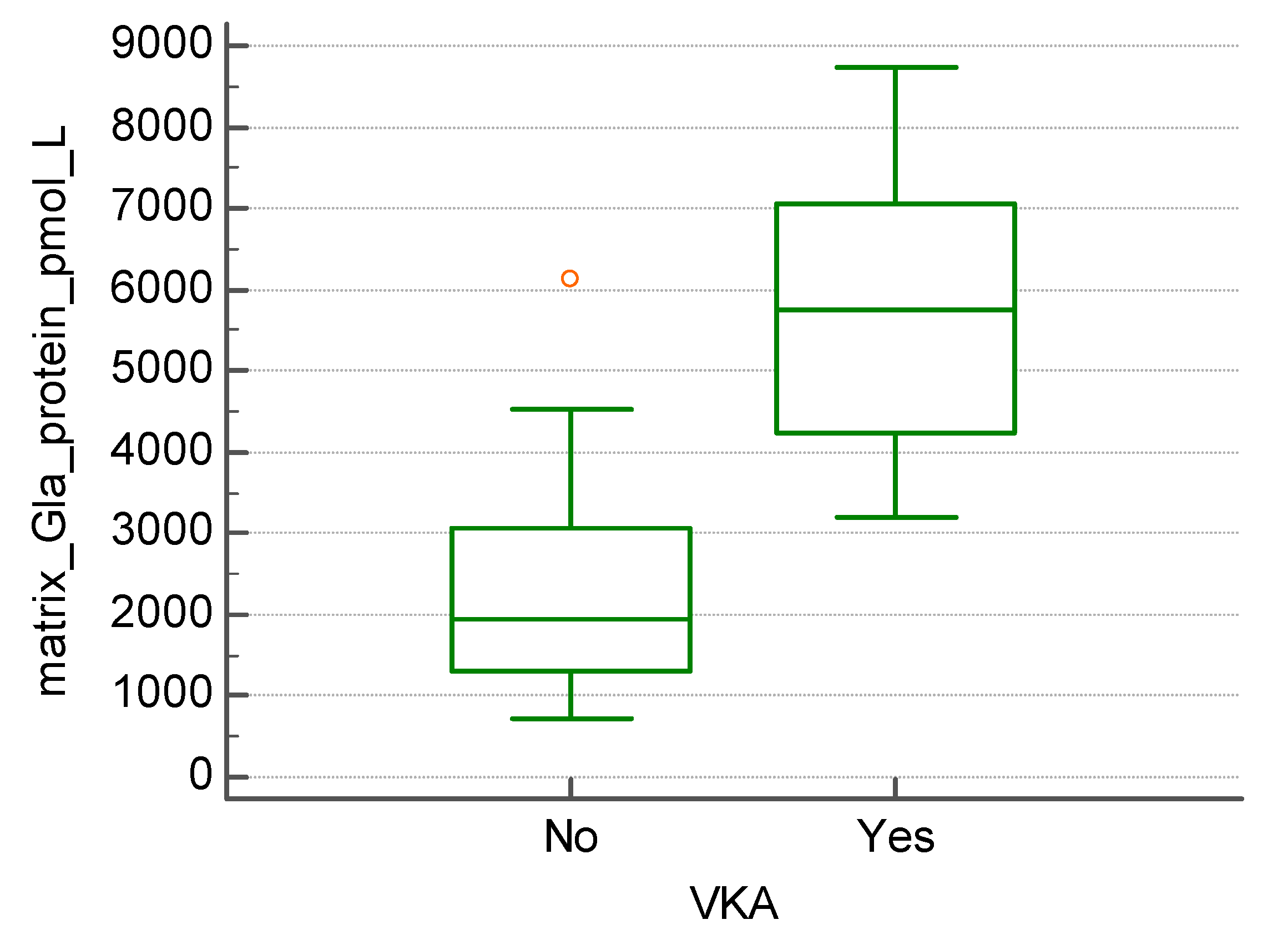
The subgroup of patients undergoing treatment with vitamin K antagonists (13 out of 45) presented with significantly higher levels of MGP (5693.0+/- 1728.64 vs. 2276.5 +/- 1232.54 pmol/l, p<0.01) (
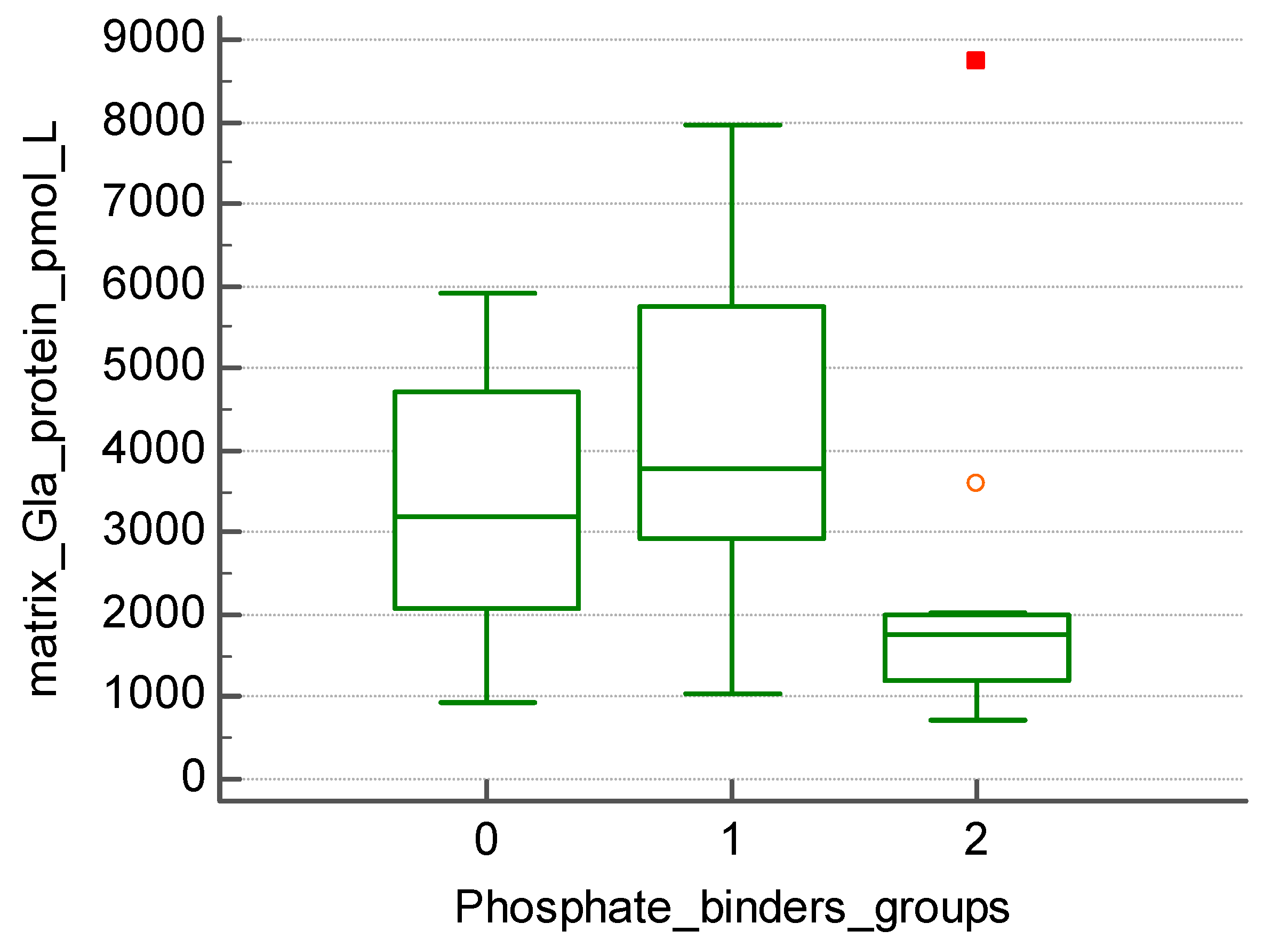
Figure 1). Those treated with non-calcium-based phosphate binders (20 patients) presented with significantly higher levels of MGP when compared to patients treated with calcium-based phosphate binders (15 patients) (4089.2 +/- 2045.79 vs. 2134.86 +/- 1944.63 pmol/l, p=0.019) (
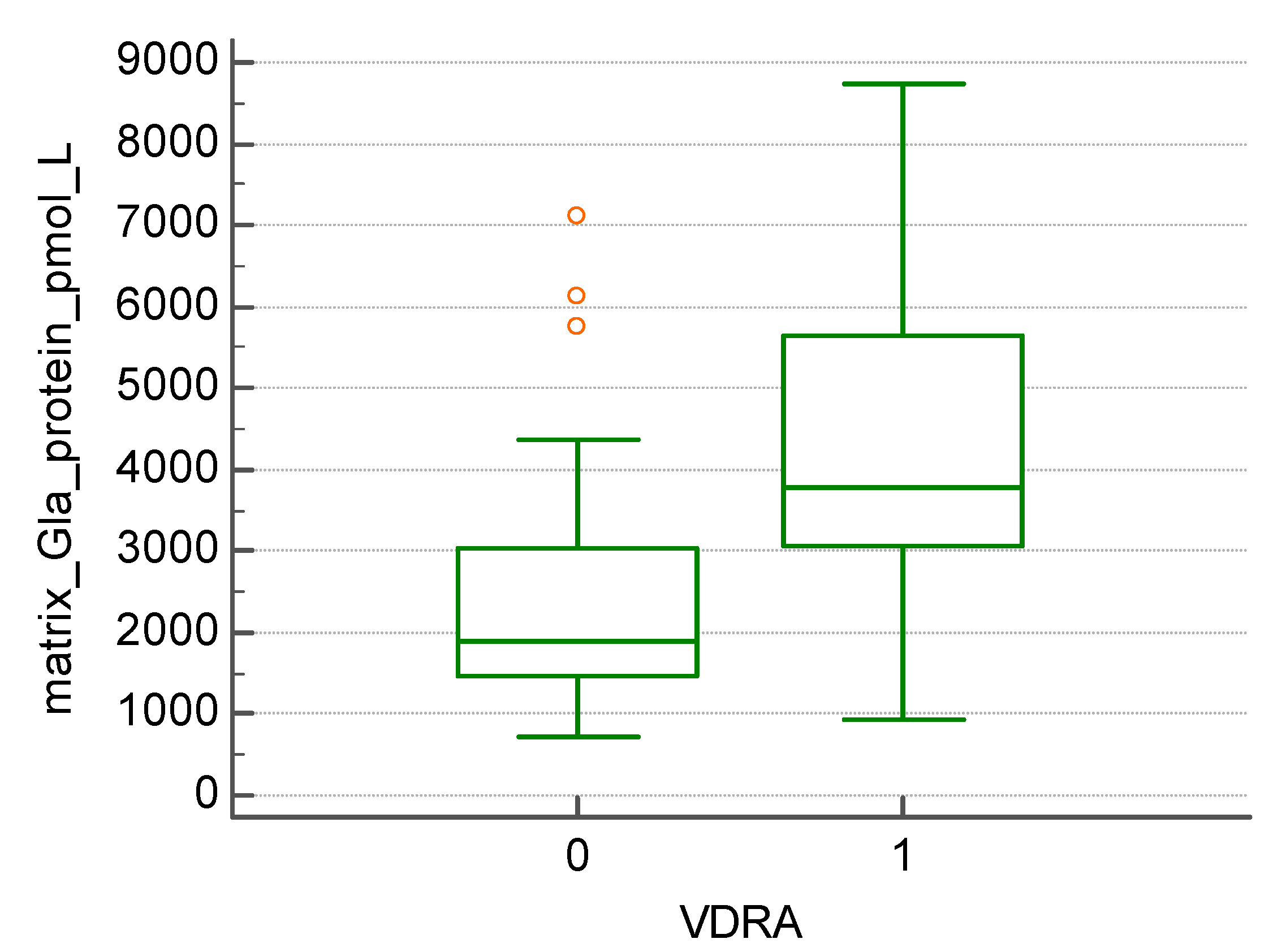
Figure 2). We found that patients treated with vitamin D receptor activator- paricalcitol (20 patients) had significantly increased levels of MGP (4224.55 +/- 2162.32 vs. 2503.75 +/- 1709.31 pmol/l, p=0.005) (
Figure 3).
4. Discussion
In the last years the central role of Vitamin K in vascular calcifications, cardiovascular and bone disease has become evident. Several studies have been performed assessing the link between vitamin K metabolism and mineral-bone disease in CKD patients. CKD patients, and especially patients treated with hemodialysis are vitamin K deficient. In a study performed by Wikstrom et al it has been shown that dialysis patients were 100% vitamin K deficient, not due to HD wash out or absorption capacity (11), but due to low vitamin K intake. Low vitamin K intake could be due to potassium sparing diets which restrict the consumption of leafy greens, rich in vitamin K. The consequences of vitamin K deficiency are mainly related to CKD- MBD.
In the present study, we obtained a significant correlation between OC, and not MGP, with markers of CKD-MBD. OC is secreted in bones by osteoblasts and plays a role in the synthesis and regulation of bone matrix (12. Similar to our results Elliott MJ et al (13)), have found that a higher calcium-phosphorus product and a higher PTH were associated to higher values of undercarboxylated OC. By contrast to our observations, Kurnatowska et al (14) found significant associations between dp-ucMGP and serum PTH and FGF23 levels, as well as higher dp-ucMGP levels in patients with higher calcium-phosphorus product.
The link between poor vitamin K status and bone health has also been proven in clinical studies assessing the incidence of fractures in CKD patients. Kohlmeier et al. showed in an observational study the relationship between low levels of Vitamin K and bone fractures (15). The VIKI study, conducted by Fusaro et al. (16), an observational study on a large group of dialysis patients, showed a direct relationship between vitamin K1 deficiency and vertebral fractures (vitamin K1 deficiency being the strongest predictor of vertebral fractures), but also with vascular calcifications. In our study we could not find any statistical correlation between vitamin K status and bone fractures because of the low number of patients with history of fractures (2 patients).
Regarding CKD-MBD treatment, we obtained significant associations between vitamin K status and non-calcium-based phosphate binders and paricalcitol. Neradova et al (17) performed an in vitro study regarding the interaction between different phosphate binders and Vitamin K2. They showed that succroferric-oxyhydroxide and sevelamer carbonate were the only medications that did not bind vitamin K2, while calcium-based phosphate binders and lanthanum carbonate ligate vitamin K2. Similar to our results, the Viki Study (16) also observed the link between concomitant treatment for CKD-MBD versus OC and MGP levels. They observed that vitamin D analogs (such as paricalcitol) increase the levels of OC and MGP. The potential link between CKD MBD and vitamin K2 metabolism may be of particular clinical interest, given the fact that the choice of medication should take into account the prevention of vascular calcifications as well. The studies regarding this topic are however scarce, and further RCTs would be useful to determine the benefit of certain medication classes over others.
Due to the fact that Vitamin K is known to be a protective factor against vascular calcifications, another consequence of vitamin K deficiency is the fact that it contributes to the high burden of vascular calcifications in CKD patients.
Multiple studies suggest that MGP accumulates in atherosclerotic calcified vessels (17,18). The great involvement of MGP in arterial calcifications has been proven in animal model studies. Thus, uremic rats displayed a larger expression of ucMGP in aortic plaques than those with normal renal function (19). Moreover, the same authors hypothesized that treatment with vitamin K may be able to reverse the calcification process.
The association between the level of VKDP and the presence of vascular calcifications, and also with the endpoint of cardiovascular or all-cause mortality has been shown across numerous cross-sectional or prospective studies performed in CKD patients.
The levels of dp-ucMGP are usually severely elevated in ESRD patients and correlate with vascular calcifications. On a cohort of predialysis patients, Schurgers et al (20) showed that dp-ucMGP increases with CKD stage and associates with abdominal aortic calcifications as well as with higher all-cause mortality. Regarding dialysis subjects, Schlieper et al. (21) showed higher all-cause mortality in patients with higher levels of dp-ucMGP. A meta-analysis conducted in 2019 by Zhang et al. including 21 articles found that elevated dp-ucMGP was associated with all-cause mortality, CV disease mortality and stroke (22).
In our study, we did not find any significant correlation between previous cardiovascular events (stroke or coronary heart disease) and levels of MGP and OC. This a surprising finding given the fact that cardiovascular events in dialysis patients rely on accelerated atherosclerosis and vascular calcifications as an underlying pathophysiologic mechanism (14).
Another key point assessed in our study was the relationship between VKDP and malnutrition-inflammation. It seems that vitamin K plays a key role in counteracting inflammation, oxidative stress and senescence. The link between malnutrition and arterial calcifications in HD patients has been studied by Zhang et al. (23). The study included 68 chronic dialysis patients, and obtained that MGP immunostaining in calcified radial arteries positively correlated to calcium x phosphorus product, albumin, and with the modified quantitative subjective global assessment. In our study, we did find a significant correlation between the inflammatory marker – CRP and VKDP. Persistent, low-grade inflammation is a novel non-traditional risk factor for accelerated atherosclerosis, either on its own, or included in the MIA syndrome (malnutrition-inflammation- atherosclerosis syndrome). Even though the molecular pathway through which vitamin K is involved in oxidative stress, DNA repairing and inflammation is well known, the relationship between inflammatory markers and Vitamin K in dialysis patients has not been extensively studied.
Regarding concomitant medication, in our study, patients treated with vitamin K antagonists (VKA) showed higher levels of MGP (p<0.01). This result is concordant to the available literature (24). Taking into account this mechanism, patients on dialysis, who are already vitamin K deplete and take warfarin are much more prone to develop vascular calcifications (25). Randhawa et al conducted a meta-analysis on patients with ESRD and atrial fibrillation and found that 22% were on warfarin treatment (26). In the context of extensive warfarin use and CKD MBD in ESRD patients, Vitamin K supplementation was mitigated to have potential benefits. Neither the RenaKvit study (27), nor the Valkyrie study (28) have showed that vitamin K2 supplementation in clinical practice can reduce vascular stiffness or calcifications in dialysis patients, and so did the VIKTORIES trial (29) in transplanted patients. Thus, there is no evidence yet to support the protective effects of vitamin K supplementation against vascular calcification in CKD patients as it has been also proven by a recently published metanalysis (30).
Regarding anticoagulation in patients on hemodialysis, considering the potential harmful effects of VKA, the question is if they could be replaced by direct oral anticoagulants (DOAC). There is no evidence that DOAC therapy would be unsafe or less effective than VKA, but most studies are retrospective studies. There are however 2 RCTs showing non-inferiority of DOAC (apixaban) vs. VKAs in hemodialysis patients regarding safety and efficacy (31,32) and one RCT (rivaroxaban vs. VKA) that shows a superior risk-benefit profile of DOACs versus VKAs and suggests that VKAs should be avoided in patients on hemodialysis (33).
The major limitations of our study are that it is a cross sectional analysis, single-centered small lot of subjects. Moreover, no quantitative or qualitative data was included regarding the presence of vascular calcifications in our study group. The associations obtained with features of malnutrition inflammation syndrome were scarce, but may be used as a hypothesis for a further more detailed analysis.
5. Conclusions
In our study, we found that vitamin K deficiency is associated to CKD- MBD and could be influenced by features of malnutrition-inflammation. Not only can vitamin K metabolism represent a hidden link between the various pathophysiological processes involved in the accelerated vascular calcifications of ESRD patients, but it can also be influenced by the use of certain medications, such as vitamin K antagonists, vitamin D receptor activators and non-calcium-based phosphate binders.
Author Contributions
Conceptualization.MP, FB ; methodology MP, RC, TMD FB, software,IG.; validation.FB; formal analysis,IGBM.; investigation.MP, SA; resources.; data curation; writing—original draft preparation,MP, IG.; writing—review and editing,FB ; visualization,IG.; supervision.FB, SA; project administration,.FB; funding acquisition LP, FB. All authors have read and agreed to the published version of the manuscript.”.
Funding “This research was funded by the University of Medicine and Pharmacy “Victor Babes” Timisoara, Centre for Molecular Research in Nephrology and Vascular Disease, Faculty of Medicine, “Victor Babeș” University of Medicine and Pharmacy, Timișoara, Romania.
Institutional Review Board Statement
“The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the University of Medicine and Pharmacy “Victor Babes” Timisoara. Approval nr 34/30.06.2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018, 33 (Suppl. 3), iii28–iii34. [Google Scholar] [CrossRef] [PubMed]
- Waziri, B.; Duarte, R.; Naicker, S.; Naicker, S. Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD): Current Perspectives. Int J Nephrol Renovasc Dis. 2019, 12, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.C.; Hsu, C.Y.; Chen, C.L. The Strategy to Prevent and Regress the Vascular Calcification in Dialysis Patients. Biomed Res Int. 2017, 2017, 9035193. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [CrossRef]
- Cranenburg, E.C.M.; Schurgers, L.J.; Uiterwijk, H.H.; Beulens, J.W.; Dalmeijer, G.W.; Westerhuis, R.; Magdeleyns, E.J.; Herfs, M.; Vermeer, C.; Laverman, G.D. Vitamin K intake and status are low in hemodialysispatients. Kidney Int. 2012, 82, 605–610. [Google Scholar] [CrossRef]
- Fusaro, M.; Tondolo, F.; Gasperoni, L.; Tripepi, G.; Plebani, M.; Zaninotto, M.; Nickolas, T.L.; Ketteler, M.; Aghi, A.; Politi, C.; La Manna, G.; Brandi, M.L.; Ferrari, S.; Gallieni, M.; Mereu, M.C.; Cianciolo, G. The Role of Vitamin K in CKD-MBD. CurrOsteoporos Rep. 2022, 20, 65–77. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Association of the Inactive Circulating Matrix Gla Protein with Vitamin K Intake, Calcification, Mortality, and Cardiovascular Disease: A Review. Int J Mol Sci. 2019, 20, 628. [Google Scholar] [CrossRef]
- Caluwé, R.; Verbeke, F.; De Vriese, A.S. Evaluation of vitamin K status and rationale for vitamin K supplementation in dialysispatients. Nephrol. Dial. Transplant. 2020, 35, 23–339. [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (2011) Erratum in: Kidney Int Suppl (2011) 2017, 7, e1. 2017, 7, 1–59. [CrossRef]
- Theuwissen, E.; Smit, E.; Vermeer, C. The role of vitamin K in soft-tissue calcification. Advances in Nutrition 2012, 3, 166–173. [Google Scholar] [CrossRef]
- Wikstrøm, S.; Aagaard Lentz, K.; Hansen, D.; Melholt Rasmussen, L.; Jakobsen, J.; Post Hansen, H.; Andersen, J.R. Causes of Vitamin K Deficiency in Patients on Haemodialysis. Nutrients. 2020, 12, 2513. [Google Scholar] [CrossRef]
- Fusaro, M.; Tondolo, F.; Gasperoni, L.; Tripepi, G.; Plebani, M.; Zaninotto, M.; Nickolas, T.L.; Ketteler, M.; Aghi, A.; Politi, C.; La Manna, G.; Brandi, M.L.; Ferrari, S.; Gallieni, M.; Mereu, M.C.; Cianciolo, G. The Role of Vitamin K in CKD-MBD. Curr Osteoporos Rep. 2022, 20, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; Booth, S.L.; Hopman, W.M.; Holden, R.M. Assessment of potential biomarkers of subclinical vitamin K deficiency in patients with end-stage kidney disease. Can J Kidney Health Dis. 2014, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Kurnatowska, I.; Grzelak, P.; Masajtis-Zagajewska, A.; Kaczmarska, M.; Stefańczyk, L.; Vermeer, C.; Maresz, K.; Nowicki, M. Plasma Desphospho-Uncarboxylated Matrix Gla Protein as a Marker of Kidney Damage and Cardiovascular Risk in Advanced Stage of Chronic Kidney Disease. Kidney Blood Press Res. 2016, 41, 231–9. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M.; Salomon, A.; Saupe, J.; Shearer, M.J. Transport of vitamin K to bone in humans. J. Nutr. 1996, 126 (Suppl. 4), 1192S–1196S. [Google Scholar] [CrossRef] [PubMed]
- Fusaro M, Noale M, Viola V, Galli F, Tripepi G, Vajente N, Plebani M, Zaninotto M, Guglielmi G, Miotto D, Dalle Carbonare L, D'Angelo A, Naso A, Grimaldi C, Miozzo D, Giannini S, Gallieni M; VItamin K Italian (VIKI) Dialysis Study Investigators. Vitamin K, vertebral fractures, vascular calcifications, and mortality: VItamin K Italian (VIKI) dialysis study. J Bone Miner Res. 2012, 27, 2271–8. https://doi.org/10.1002/jbmr.1677. Neradova A, Schumacher SP, Hubeek I, Lux P, Schurgers LJ, Vervloet MG. Phosphate binders affect vitamin K concentration by undesired binding, an in vitro study. BMC Nephrol. 2017, 18, 149. https://doi.org/10.1186/s12882-017-0560-3.
- Bjorklund, G.; Svanberg, E.; Dadar, M.; Card, D.J.; Chirumbolo, S.; Harrington, D.J.; et al. The Role of Matrix Gla Protein (MGP) in Vascular Calcification. Curr. Med. Chem. 2020, 27, 1647–1660. [Google Scholar] [CrossRef]
- Delanaye, P.; Krzesinski, J.M.; Warling, X.; et al. Dephosphorylated-uncarboxylated Matrix Gla protein concentration is predictive of vitamin K status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC Nephrol. 2014, 15, 14. [Google Scholar] [CrossRef]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Renard, C.; Magdeleyns, E.J.; Vermeer, C.; Choukroun, G.; Massy, Z.A. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010, 5, 568–75. [Google Scholar] [CrossRef]
- Schlieper, G.; Westenfeld, R.; Krüger, T.; Cranenburg, E.C.; Magdeleyns, E.J.; Brandenburg, V.M.; Djuric, Z.; Damjanovic, T.; Ketteler, M.; Vermeer, C.; Dimkovic, N.; Floege, J.; Schurgers, L.J. Circulating nonphosphorylatedcarboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol. 2011, 22, 387–95. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, L.; Bu, C. Vitamin K status and cardiovascular events or mortality: A meta-analysis. Eur J Prev Cardiol. 2019, 26, 549–553. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, G.; Cai, X.; Chen, J.; Jiang, Y.; Wang, T.; Wang, J.; Huang, H. Malnutrition, a new inducer for arterial calcification in hemodialysis patients? J Transl Med. 2013, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, C.J.; Harrington, D.J. Therapeutic warfarin use and the extrahepatic functions of vitamin K-dependent proteins. Br J Biomed Sci. 2017, 74, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Danziger, J. Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin J Am Soc Nephrol. 2008, 3, 1504–10. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.S.; Vishwanath, R.; Rai, M.P.; Wang, L.; Randhawa, A.K.; Abela, G.; Dhar, G. Association Between Use of Warfarin for Atrial Fibrillation and Outcomes Among Patients With End-Stage Renal Disease: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020, 3, e202175. [Google Scholar] [CrossRef]
- Levy-Schousboe, K.; Frimodt-Møller, M.; Hansen, D.; Peters, C.D.; Kjærgaard, K.D.; Jensen, J.D.; Strandhave, C.; Elming, H.; Larsen, C.T.; Sandstrøm, H.; Brasen, C.L.; Schmedes, A.; Madsen, J.S.; Jørgensen, N.R.; Frøkjær, J.B.; Frandsen, N.E.; Petersen, I.; Marckmann, P. Vitamin K supplementation and arterial calcification in dialysis: results of the double-blind, randomized, placebo-controlled RenaKvit trial. Clin Kidney J. 2021, 14, 2114–2123. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Caluwé, R.; Pyfferoen, L.; De Bacquer, D.; De Boeck, K.; Delanote, J.; De Surgeloose, D.; Van Hoenacker, P.; Van Vlem, B.; Verbeke, F. Multicenter Randomized Controlled Trial of Vitamin K Antagonist Replacement by Rivaroxaban with or without Vitamin K2 in Hemodialysis Patients with Atrial Fibrillation: the Valkyrie Study. J Am Soc Nephrol. 2020, 31, 186–196. [Google Scholar] [CrossRef]
- Lees, J.S.; Rankin, A.J.; Gillis, K.A.; Zhu, L.Y.; Mangion, K.; Rutherford, E.; Roditi, G.H.; Witham, M.D.; Chantler, D.; Panarelli, M.; Jardine, A.G.; Mark, P.B. The ViKTORIES trial: A randomized, double-blind, placebo-controlled trial of vitamin K supplementation to improve vascular health in kidney transplant recipients. Am J Transplant. 2021, 21, 3356–3368. [Google Scholar] [CrossRef]
- Geng, C.; Huang, L.; Pu, L.; Feng, Y. Effects of vitamin K supplementation on vascular calcification in chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Front Nutr. 2023, 9, 1001826. [Google Scholar] [CrossRef]
- Reinecke, H.; Engelbertz, C.; Bauersachs, R.; Breithardt, G.; Echterhoff, H.H.; Gerß, J.; Haeusler, K.G.; Hewing, B.; Hoyer, J.; Juergensmeyer, S.; Klingenheben, T.; Knapp, G.; Christian Rump, L.; Schmidt-Guertler, H.; Wanner, C.; Kirchhof, P.; Goerlich, D. A Randomized Controlled Trial Comparing Apixaban With the Vitamin K Antagonist Phenprocoumon in Patients on Chronic Hemodialysis: The AXADIA-AFNET 8 Study. Circulation. 2023, 147, 296–309. [Google Scholar] [CrossRef]
- Pokorney SD, Chertow GM, Al-Khalidi HR, Gallup D, Dignacco P, Mussina K, Bansal N, Gadegbeku CA, Garcia DA, Garonzik S, Lopes RD, Mahaffey KW, Matsuda K, Middleton JP, Rymer JA, Sands GH, Thadhani R, Thomas KL, Washam JB, Winkelmayer WC, Granger CB; RENAL-AF Investigators. Apixaban for Patients With Atrial Fibrillation on Hemodialysis: A Multicenter Randomized Controlled Trial. Circulation 2022, 146, 1735–1745.
- De Vriese, A.S.; Heine, G. Anticoagulation management in haemodialysis patients with atrial fibrillation: evidence and opinion. Nephrol Dial Transplant. 2022, 37, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).