1. Introduction
Scaffold-guided bone regeneration (SGBR) has been successfully translated into clinical applications in the field of regenerative medicine to advance the treatment of bone defects when the defect zone exceeds the critical size for the self-healing capacity of the organ bone [
1,
2,
3,
4,
5,
6,
7,
8]. It is important to notice that fracture healing and scaffold guided bone regeneration are two interrelated but also inherit different time course related processes during bone regeneration, each with unique characteristics and mechanisms. Several excellent reviews did summarize today’s knowledge and research regarding fracture healing, and we refer the reader to these publications [
9,
10,
11]. Hence, we focus in this publication on the histological and immunohistochemical SGBR which has been recently described in detail by our group [
12].
In the last two decades, our group has extensively been studying mPCL and mPCL-TCP
in vitro and
in vivo, with the focus on mechanical properties, degradation, geometry, surface, and fabrication optimization, as well as
in vivo biocompatibility [
11,
13,
14,
15,
16,
17,
18,
19]. Based on specimen analyses from a multitude of large animal studies [
11,
13,
16,
20,
21], we have been able to demonstrate that bone regeneration at the scaffold-tissue interface, as well as throughout the scaffold, relies on a comprehensive and complex network of processes involving hemostasis, immune response, neovascularization, callus development, and ultimately remodelling into functional, highly organized bone tissue. On closer examination, the decisive regeneration of the organ bone is influenced by the initial inflammatory state of the local microenvironment, in which macrophages facilitate the release of osteogenic cytokines and therefore affect the formation of new tissue around and throughout implanted scaffolds, also conceptualized in the more recent literature as osteoimmunomodulation [
22]. Therefore, it is imperative to investigate the entirety of the subcomponents involved to comprehend the complexity of the entire regeneration concept of SGBR, whose bone defects may take up to several years to regenerate.
The introduction of a foreign body and its inherent immune responses, particularly when implanting mPCL scaffolds, has substantial effects on the host adaptative immune system [
23]. Research indicates that there are more than 6500 genes regulating bone regeneration, which suggests a complex biological niche in which multiple cell interactions occur to promote functional bone formation [
24]. The
in vivo evaluation of the adaptative host immune responses through immunohistochemistry (IHC) analysis is an invaluable component of translational SGBR research. We have demonstrated that with ample training and experience of the interdisciplinary team in both histological and immunohistochemical analysis of segmental defect studies in sheep, a high degree of reproducibility can be achieved when validated histological and IHC protocols are developed and stringently followed [
25].
IHC analysis has been shown to be a means for identifying cellular markers that distinguish specific phenotypes for evaluating the osteoimmunological response in SGBR and in this context, is a condition
sine qua non for in-depth assessment of the spatiotemporal bone regeneration processes [
26,
27]. Mainly derived from knowledge rooted in fracture healing research, and based on extensive literature, one concludes that there are two different pathways for bone tissue regeneration. Namely, direct ossification, where new lamellar bone forms immediately and which only occurs with given proximity and stability of the fracture ends. However, more commonly, and seen in SGBR, tissue of the organ bone regenerates indirectly through endochondral (cartilaginous) ossification. This form of healing appears in five interrelating steps comprising of hematoma in combination with a stable fibrin network, granulation tissue, soft callus (cartilage), and hard callus (woven bone) formation and lastly bone remodelling [
28]. However, steps of SGBR occur both in sequential overlay and concurrent, thus exact chronological allocation to a distinct ‘state of healing’ in contrast to healing of a simple fracture is impossible. Our extensive experience from >12 studies and >350 large segmental sheep surgeries demonstrates that these aspects are critical to overall success and the ability to assess histologic and IHC findings regarding bone defect healing at different time points. These skills in preparing and evaluating histologic and immunohistological specimens reproducibly and validated are usually learned "on the job" or through the rare internship opportunities in SGBR specialized academic institutions.
The lack of conceptual replication to determine the probative value and functionality of specific biomarkers for evaluating novel SGBR approaches in a sheep model that reflects analogous conditions in humans renders IHC reproducibility challenging and is ultimately a barrier to clinical translation. To further understand the body response and action on a cellular and molecular level, our group has integrated extensive IHC analyses as a fundamental component of the histoanalytical protocol for our
in vivo studies. The aim of the present work is to signify the value of IHC for the assessment of tissue regeneration through the combined assessment of immune cellular, vascular and extracellular matrix (ECM) components whereby the context is based on work performed on our sheep large segment bone defect model [
21,
25,
29,
30,
31].
3. Results
A data set collection of SGBR relevant protein expression of investigated antibody markers, in five experimental groups with follow-up ranging from six hours to 36 months (total of 9 different time points), and their specifically assessed cells and tissues are illustrated in
Figure 1. Overall, our data set results did not identify pronounced differences in the osteoimmunological response to the different mPCL-TCP scaffolds (mPCL-TCP or mPCL-TCP-CaP), or when comparing different time points and experimental groups. COL I deposition was observed in all experimental groups and at all different time points throughout the open and fully interconnected porous architecture of the scaffolds (
Figure 1 COL I, A-M). Overlapping stages of woven bone and lamellar bone formation could be identified through a stronger and lighter intensity of COL I
+ stain, respectively (
Figure 1 COL I, G). Although the newly formed bone matrix showed some islands of remnant mineralized cartilage matrix (
Figure 1, COL II, A-M, red arrowheads) at proximity to the mPCL-TCP struts, no fibrous tissue formation was observed enclosing the mPCL-TCP struts. OC
+ expression was observed at osteoblasts (
Figure 1 OC, A, B, E, G and L, green arrow heads) and at the osteoid matrix as early as six hours, with apparent secondary osteon formation with strong OC
+ stain at cement lines at 12, 15, 21 and 36 months (
Figure 1 OC, D, F, I, K and M, black arrow heads). Neovasc ularization could be seen throughout the newly formed tissue and proximate to mPCL-TCP scaffold struts (
Figure 1, vWF A-M, green arrows). This newly stablishing vasculature, especially at the scaffold strut interfaces, as well as the osteoclast cells present in cutting cones crossing the newly bone formed at the defect site (
Figure 1 CD68 B-M, red arrows), and remnants of hypertrophic cartilage (
Figure 1 CD68 A) were continuously stained by CD68
+ (M1 and M2 macrophage marker) throughout the experimental time points. The M1 macrophage marker iNOS was observed particularly at remnants of mineralized cartilage (
Figure 1 iNOS C, F, H and L, double black arrowheads) preceding vascularization, and at blood vessels around the mPCL-TCP scaffold struts (
Figure 1 iNOS A, B, D, E, G, I and M, black arrows), however its detectability decreased overtime remaining detectable at blood vessels only. The M2 macrophage marker MR was strongly expressed throughout all time points and experimental groups (
Figure 1 MR A-M). This reactivity was located at the vessels (
Figure 1 MR F, I, J-M, double red arrowheads) and osteoclasts surrounding the outer surface of scaffold struts and cutting cones (
Figure 1 MR D, J and K, red arrowheads), at fragments of bone graft and host bone (
Figure 1 MR A, B, and M red arrows), and at remnants of mineralized cartilage (
Figure 1 MR C and H, double black arrowheads). No increase in the M1/M2 ratio indicating a chronic foreign body reaction (FBR) was observed, further corroborated with lack of α-SMA
+ marker expression. Negative controls are provided in
Supplementary Figure S2. Comprehensive and further analysis of inflammatory markers including ARG1
+, IRF5
+, CD3
+ and CD45
+, VEC markers such as VEGF
+, CD31
+, α-SMA
+ and ANG1
+, and ECM osteogenic markers comprising BMP2
+, OPG
+, ALP
+, SCL
+, OMD
+, ON
+, OPN
+, OC
+, NOG
+ and Notch 1
+ have been also studied and discussed in the following sections.
3.1. Inflammatory response (Figure 2)
One of the first macrophagic responses tested when evaluating SGBR upon mPCL-TCP implantation is staining samples for CD68+, a marker of both M1 and M2 macrophage subtypes. The immunoreactivity of CD68+ was constant and clearly identified at the endothelial tissue around the mPCL-TCP struts and within the cutting cones throughout the newly formed and remodelling bone tissue (
Figure 2 CD68, A). Local and phenotypic differences of these macrophagic immunoreaction was observed and distinguishable. Classically activated M1 macrophages increase their phagocytic activity and produce pro-inflammatory cytokines including iNOS+ and IRF5+ expression. The expression of iNOS+ and IRF5+ was mainly localized at sites of neovascularization, especially at the interface to the mPCL-TCP scaffold strut where newly formed vessels were formed, and at remnant areas of mineralized cartilage matrix (
Figure 2 iNOS and IRF5, A). Positive controls also denoted iNOS+ and IRF5+ at vascular tissue of the liver and spleen (
Figure 2 iNOS B and IRF5 B, respectively), and within vessels of the medullary canal of the contralateral tibia (Figure not shown). Conversely, M2 macrophages, including the MR marker, were strongly expressed at areas of bone resorption, and at the cells lining the surface of the newly forming bone around the mPCL-TCP scaffold struts (
Figure 2 MR, A). Although less pronounced, ARG-1+, which is also an M2 macrophage marker and known to promote fibroblast proliferation and block iNOS activity, was positively stained at the same previously described areas (
Figure 2 ARG-1, A). We further tested samples against hematopoietic cells including CD45 and CD3 markers. CD45+ expression was specifically restricted to the osteoclast mediating bone remodelling at cutting cones, throughout the newly formed bone (
Figure 2 CD45, A). Although positive control was immunoreactive for CD3+, the tested mPCL-TCP samples did not show reactivity (
Figure 2 CD3, A). It was observed that iNOS+ expression decreased, and MR expression increased overtime around the mPCL-TCP scaffold struts. However, iNOS expression was continuous throughout early and late time points at remnants of mineralized cartilage and endothelial vascular cells, respectively. All samples were tested with positive (
Figure 2 Bs) and negative (
Supplementary Figure S3) controls.
3.2. Vascularization (Figure 3)
VEGF+ and ANG1+ angiogenic immunoreactivity response were strongly observed at the endothelial tissue around the mPCL-TCP struts, and within cutting cones and haversian canals of the newly built bone tissue. Particularly, VEGF+ immunoreactivity was also detected at areas of mineralized cartilage within the area between mPCL-TCP scaffold struts (
Figure 3 VEGF, A and ANG1, A). High levels of cell adhesion molecule CD31+ were strongly observed within the sprouting walls of blood vessels of newly formed bone tissue, around mPCL-TCP struts and at mineralized cartilage islands preceding vascularization invasion (
Figure 3 CD31, A). VEGF marker was not expressed in (positive) controls of the contralateral sheep tibia, however, ANG1 was strongly positive at blood vessels, osteocytes and at the endosteal tissue lining the marrow cavity (
Figure 3 VEGF, B and ANG1, B). Vascular network maturation was indicated by strong staining for vWF at previously VEGF and ANG1 stained areas except for mineralized cartilage areas. Contralateral sheep tibiae (positive controls) were strongly immunoreactive for CD31+ at smaller and larger caliber blood vessels, whereas vWF+ was particularly strongly immunoreactive at elongated, matured blood vessels (
Figure 3 vWF, B). α-SMA+ reactivity was not observed adjacent to mPCL-TCP scaffold struts, but only seen within the wall of larger caliber blood vessels at experimental groups and contralateral sheep tibiae positive control (
Figure 3 α-SMA, A, B). NOG+ and NOTCH 1+ expressions were sparce with limited expression at endothelial tissue around the mPCL-TCP scaffold struts (
Figure 3 NOG, A and NOTCH 1). Contralateral sheep tibiae (positive controls) were faintly immunoreactive for NOG+ and NOTCH1+. All samples were tested with positive (
Figure 3 Bs) and negative (
Supplementary Figure S4) controls.
3.3. ECM (Figure 4)
COL I marker was expressed in random orientation within the scaffold macropores as well as in a parallel arranged manner on scaffold struts (
Figure 4 COL I, A). COL II+ stain was particularly observed at the interface of the newly bone formed and host bone, as well as around mPCL-TCP scaffold struts (
Figure 4 COL II, A). BMP2 marker reactivity was seen at the cells lining the outer surface of the mPCL-TCP scaffold struts, as well as at bone lining osteoblast cells within the bone tissue (
Figure 4 BMP2, A). ALP marker reactivity was mainly observed at the osteoid matrix within the cutting cones, at the peripheral cortex region near the periosteum as well as in proximity to scaffold struts (
Figure 4 ALP, A). OM marker reactivity was found at the endothelial wall of blood vessels throughout the osteoid matrix in between and surrounding scaffold struts. Further, OM was expressed on the endosteal cells lining the cortical bone and at areas of mineralized tissues (
Figure 4 OM, A). On a closer observation of our tested samples, ON+ was specifically detected at osteoblast cells within the bone matrix, as well as at the cells lining the outer surface of the mPCL-TCP scaffold struts (
Figure 4 ON, A). Further, ON marker was also present at osteocytes of the positive control samples (
Figure 4 ON, B). OPN+ and OC+ were observed at areas of randomly oriented COL I stained areas, however, where fibers were aligned in lamellar configuration appearing as osteons, OPN+ and OC+ reactivity were mainly restricted to osteocyte cells and late differentiated osteoblasts, respectively (
Figure 4 OPN, A and OC, A). Further, OC+ was clearly evident at cement lines separating osteons. Positive controls also stained OPN+ at osteocytes throughout the sample, and the endosteal tissue lining the marrow cavity (
Figure 4 OPN, B). Corroborating these results, SCL marker was positive throughout osteoid in early and especially late time points in the experimental and control samples (
Figure 4 SCL, A and B). OPG marker was strongly expressed by bone lining cells located at the outer surface of the mPCL-TCP scaffold struts, and within the osteoid matrix as well as by osteoclasts resorbing mineralized cartilage matrix areas (
Figure 4 OPG, A). Detection of OPG markers was absent within the newly formed tissue in the central area of the tubular scaffolds. Expression of OPG markers at osteocytes were observed at the host bone and positive control (contralateral tibia) (
Figure 4 OPG, A and B). All samples were tested with positive (
Figure 4 Bs) and negative (
Supplementary Figure S5) controls.
4. Discussion
Fracture healing and scaffold-guided bone regeneration are interconnected processes involved in bone regeneration, but they do possess distinct characteristics and timelines [
12]. We validated the histological and IHC protocols used in the ovine tibial segmental defect model in numerous studies as a preclinical tool to evaluate osteoimmunological responses and processes in SGBR [
25].
Disruption of local soft tissue and vascular integrity depends on the extent of surgically inflicted damage and therefore the application of protocols (e.g., for IHC) and interpretation of outcome findings are to be animal model specific. Hence, direct comparison between preclinical studies is only scientifically justified for the same type of animal model applied. Our research group established the large (3 cm) and extra-large (6 cm) segmental defect model as the “signature model” in SGBR, considering that each different type of model has a distinct tissue damage pattern which navigates the magnitude of host body inflammatory response, also and depending on the extent and type of surgical defect.
During any surgical procedure and especially in creating a large volume segmental defect a hematoma forms through disruption of surrounding blood vessels and medullary leakage, initiating several well studied and described cascadic pathway [
36]. Platelets activate the coagulation system enabling blood clot formation. Simultaneously, damage-associated molecular patterns (DAMP) are released from impaired cells, interacting with innate immune cells such as dendritic cells and macrophages, that subsequently deliberate chemokines (e.g., VEGF and ANG1) for further immune activation. Concurrently, mast cells degranulate and release further mediators (e.g., VEGF and histamine), promoting cellular attraction through increasing vascular permeability and enhancing neovascularization. Thus, the hematoma can be understood as a temporary matrix that forms and provides initial stability as well as an acidic, hypoxic and sodium- and potassium-rich environment, consisting of a fibronectin matrix, immune cells such as monocytes, macrophages, granulocytes (neutrophil and eosinophil), and lymphocytes, as well as platelets, red blood cells and mast cells [
37]. Hence, it is apparent that the host’s metabolic changes thus induced through traumatic stress during surgical procedures significantly affect the healing and regeneration process [
38]. Furthermore, in addition to the simple correlation of specific markers with a particular cell type, where the (relative) presence or absence of the cell can then be interpreted, direct correlations between markers and clinical outcomes have been studied and reported [
39,
40]. However, in contemporary literature, FBR is primarily discussed independently of the osteoimmunological processes that occur during bone healing.
Independent of SGBR treatments and time points employed, we conclude from our study results that there is a clear overlap between several processes and undoubtably, osteoimmunological responses to SGBR approaches is a combination of the immunological response firstly caused by the injury site and surgical mode of implantation, and secondly by the host response to the implant material properties. The stereotypical concepts of regeneration processes postulate that successful bone regeneration is dependent on a timely orchestrated initial osteoimmunological response, which we believe does not resolve, but evolves by continuously re-programming the widely described interrelating processes of bone regeneration in response to a changing environment. Yet, these well described processes lack adequate characterization of the osteoimmunological responses during SGBR, including macrophage polarization
in vivo, which inherently and consequently interact with biomaterials and are still part of the adaptative osteoimmunological response of bone physiological repair. Addressing and elucidating this deficiency in characterizing the re-programming of multiple cellular interactions during SGBR, emphasizes the complexity of the regulation of the osteoimmune environment, which is inherently associated with the classical (pro-inflammatory) and alternative (pro-regenerative) activations as bone restitution progresses. Therefore, the osteoimmunological responses cannot be isolated and assigned to the initial early stages of bone fracture and hematoma formation only but are to be contemplated in their entirety over the proceeding phases of bone regeneration, to adequately interpret histological and IHC data sets to dissect the mechanisms in SGBR. Based on this knowledge, we have established and optimized our protocol for histological and IHC staining, accordingly investigating the different stages of regeneration through visualization of cellular proteins that are of immunological, vascular, and matrix-derived origin (
Figure 1).
Host immune and inflammatory responses are traditionally associated with the defense process against harmful microorganisms; however, when discussing "inflammation" in the context of SGBR, it is important to destigmatize this term [
41]. This means that inflammation does not necessarily lead to chronic inflammation and ultimately implant failure [
42]. On the contrary it contributes to eubiosis [
43], and to a scaffold-host equilibrium, in which successful tissue and osteointegration is characterized by a quasi-physiological immune/inflammatory response, which is critical for peri-implant wound healing and thus allows chronic immune surveillance to maintain tissue homeostasis [
41]. However, we do not deny that it is also known that the osteoimmunological response to implants depends on the biomaterial it is made of [
44]. Rather than directly comparing study groups and time points, we subscriptively examined the osteoimmunological response to the biodegradable implants in-depth. Consistent with successful osteointegration, we observed CD68
+ staining, a marker for both M1 and M2 macrophages, on the 3D-printed mPCL-TCP scaffold strut surface at early and late time points in all experimental groups (
Figure 1 and
Figure 2). Thus, considering the importance of protein adsorption for subsequent macrophage attachment [
37,
45], we can state that the physicochemical properties of these mPCL-TCP scaffolds promote adsorption of the critical molecule(s) from wound fluids generated as a result of implantation surgery and, thereby, initiate the required osteoimmunological processes for subsequent successful tissue integration.
Moreover, IHC staining of sections with specific markers for M1 and M2 activation, namely iNOS and MR, respectively, showed labelling of the surface-adherent cells and at new blood vessels formed at the outer surface of the tested scaffold struts, at both earlier and later time points (
Figure 1 and
Figure 2). In early time point study groups, we observed an initial predominance of M1 macrophages at remnants of mineralized cartilage and blood vessels, and early prevalence of M2 macrophages at fragments of bone graft, host tissue and vascular endothelial tissue surrounding the scaffold struts. At later time points, this response evolves to an M1-prevalence at blood vessels and considerably predominance of M2 at the endothelial tissue, as well as cells populating the surface of the scaffold struts (
Figure 1 and
Figure 2). The conceptualization of an opposing phenotypic switch from M1 to M2 macrophages, implying an adequate wound healing environment, has been reported to be foster of tissue repair and regeneration, by regulating ECM formation and organization [
46,
47,
48]. Rather than categorizing M1 and M2 macrophage phenotypes into two functional sub-classes, which provides a framework for macrophage polarization, we observed that both M1 and M2 phenotypes co-exist and are continuously adapting their function in response to the repairing environment of bone regeneration. In line with previous research [
49] we can also conclude that the scaffold architecture used, and the biophysical properties of the mPCL-TCP biomaterial, result in a balanced immune response associated with a favorable pro-regenerative environment. Furthermore, iNOS, expressed by M1 macrophages, has been reported to modulate osteoclast resorption [
50,
51] and to be present in the early and late stages of vascularization, playing an important role in wound healing by up-regulating endothelial cell sprouting through VEGF synthesis, and late vascular remodelling through reinstation of the vascular network [
39,
52,
53]. These findings strongly corroborate the early expression of iNOS at mineralized remnants of cartilage preceding vascular invasion.
A reduction in iNOS has been found to correspond with delayed and non-union bone healing in mice, which can be ascribed to the decrease in nitric oxide (NO) production through iNOS [
28,
54]. NO is a free radical that is produced by different cells such as macrophages and endothelial cells promoting bone remodelling through osteoblastic activation when highly concentrated, and osteoclastogenesis when present in lower doses [
38,
39]. Thus, in the studied experimental groups, higher early expression of iNOS throughout the entire scaffolds can be interpreted as successful early neovascularization facilitating bone graft survival and new bone formation (
Figure 1 and
Figure 2). Furthermore, reduced iNOS marker concentrations at later time points throughout the experimental groups are indicative for bone remodelling through osteoclast activation and genesis (
Figure 1 and
Figure 2). Additionally, specific transcription factors have been discovered to regulate this polarization process by up- or down regulation of specific interleukins, for instance upregulation of M1 macrophages by IRF-5 [
55]. In contrary, M2 macrophages are characterized by their ability to produce anti-inflammatory cytokines and increased phagocytic activity, thus supporting the high expression of MR observed at the cells lining the outer surface of our mPCL scaffold struts at later stages of bone regeneration. Mainly expressed by macrophages, scavenger receptors among other activities exert phagocytic functions, thus playing a pivotal role in scaffold clearing and degradation. Like M1, M2 macrophages have also been reported to take part in angiogenic processes, e.g., by secreting VEGF, and by increasing collagen and fibroblast synthesis through ARG-1. However, in the assessed samples ARG-1 marker reactivity was neglected. Considering that ARG-1 is only expressed by one subtype of the M2 family (M2a), it is conceivable that other pro-regenerative subtypes (M2b-d) were more predominantly involved in the osteoimmunological processes but not captured with the antibody panel used [
37,
56,
57,
58]. In the present work, we observed significant neovascularization (
Figure 1 and
Figure 3), scaffold integration, and mineralization of the (bone) tissue matrix (
Figure 1 and
Figure 4), and therefore, based on the corroborating results compared with previous studies [
59,
60,
61], we are confident that this continuous co-existing and evolving expression of M1 and M2 macrophages, rather than the postulated stereotypical switch from M1 to M2, ensues bone regeneration, neovascularization and vascular remodelling.
A key component of bone formation is the re-establishment of the vascular network. In the initial stages of neovascularization early signaling molecules such as CD31, ANG1 and VEGF are released by monocytes adhering to the endothelium of damaged vessels [
62]. Expression of these VEC markers was observed throughout all experimental groups particularly at early time points as well as at later time points in reduced expression intensity (
Figure 3). VEC marker expression, especially VEGF (
Figure 3, VEGF A) was strong in the proximity to scaffold struts indicating that the 3D scaffold architecture provides a pro-angiogenic micro-and macroporous environment for beneficial early angiogenesis. In line with clinical studies [
1,
2,
3], our IHC findings of the presented work undoubtedly support that mPCL-TCP scaffolds in combination with different bone graft materials promote neovascularization throughout the highly porous morphology, thereby promoting bone regeneration. Furthermore, due to the more pronounced positive vWF staining at later time points throughout all experimental groups it can be presumed that the present blood vessels are of mature quality (Figures 1 and 3).
Notably, during endochondral bone formation (as in SGBR), cartilage provides a suitable material less demanding of oxygen, which temporally bridges the gap until the blood supply has sufficiently developed [
63]. As the vascular bed grows into the cartilaginous tissue, the first mineralized ECM, produced during primary bone formation, is resorbed by osteoclasts and secondary bone formation continues, which subsequently is resorbed as well. Osteoblasts express BMP2, OPG and OPN and secrete collagen fibrils in two forms: woven and lamellar [
64]. In line, new bone formation was observed in two forms of COL I deposition (
Figure 4 COL I column). First, COL I
+ stain in random orientation, and second, in parallel configuration, which resembles woven and lamellar bone, respectively. Overlapping stages of woven and lamellar bone formation were observed at early and late time points throughout the mPCL-TCP scaffold and on bone grafts (
Figure 1 COL I column). Early bone formation was further indicated by positive BMP2 marker expression suggesting recruitment of osteoblasts. At later time points, mineralized areas of cartilage depicted by COL II
+ staining were observed at the interface of the newly formed bone and host bone, as well as around mPCL-TCP scaffold struts indicating mineralization and early bone remodelling through endochondral ossification. Thus, based on favorable inflammatory response elicited by the implanted scaffolds as well as fostered early neovascularization in all study groups, the scaffold material and design facilitated
in vivo tissue integration and new bone tissue formation resulting in a sufficiently functional organ. Furthermore, osteoid matrix mineralization is up-regulated by OMD, ON, and ALP which play important roles in osteoblast cell growth and hydroxyapatite binding capacity, thus modulating collagen fiber shape and alignment. Bone mechanical properties are inherently associated with the collagen fiber architecture, strengthened by nestled hydroxyapatite mineral particles. Appropriate mineralization (strong OMD and ON marker expression) was observed at early and more pronounced at late time points, particularly at the bone graft – scaffold strut- as well as at the bone graft – host bone interface (
Figure 4). This marker expression pattern is congruous with strong osteointegration of the scaffold. Moreover, based on strong expression of OC and SCL markers at late time points, the newly formed bone throughout the scaffolds and the scaffold – host tissue interfaces point toward high quality due to osteon arrangement with uniform cement line patterns (
Figure 1 and
Figure 4).
In all study groups presented in this work, very limited degradation of the scaffolds was observed even at late time points, as indicated by scaffold struts that appeared as voids in our IHC findings due to dissolution of mPCL-TCP by xylene during sample processing (
Figure 1,
Figure 2,
Figure 3 and
Figure 4 red dashed lines). Similar findings have been described in a clinical study with mean follow-up of 248.1 ± 435.3 days using mPCL implants in 174 consecutive patients over a period of 10 years including a variety of burr hole craniotomies and reconstruction using mPCL implants (12 mm diameter, 5 mm thickness) with porosity maintained at 70% (Osteoplug and Osteoplug-C; Osteopore International Pte Ltd) [
4]. There is increasing evidence that the biocomposite TCP enhances cellular adhesion, neutralizes by-product acidity, accelerates scaffold degradation [
65], and has been found to improve osteogenesis through macrophage stimulation [
47,
65,
66]. Indeed, studies have shown that the incorporation of higher ceramic contents into scaffolds can not only improve osteogenic differentiation of osteoblasts (
Figure 4 ON, A) and bone healing effects
in vivo [
67,
68,
69], but also the mechanical strength, hydrophilicity, and degradability of polymer-based composites [
70,
71,
72].
However, it is critical to recognize that scaffold degradation during bone regeneration is a "double-edged sword" because mPCL-TCP scaffold degradation creates space for cell infiltration and ingrowth of new bone. Excessive degradation may lead to loss of integrity, which could limit osteoblast adhesion and proliferation, as well as triggering inflammation and chronic FBR by the massive release of degradational by-products [
73,
74]. Of note, ARG-1 and α-SMA are also markers frequently used for detection of fibroblasts and myofibroblasts, which play a substantial role in tissue fibrosis and scaffold encapsulation. In consideration of our IHC findings, ARG-1 reactivity was faintly found at the outer surface of scaffolds (
Figure 2), and α-SMA expression was only detected within vessel walls and not at the scaffold-bone-interface (
Figure 3), indicating no fibrous encapsulation of the biomaterial in terms of extensive FBR. While we have already initiated further studies to test the incorporation of higher ceramic contents into mPCL matrices, we are fortunate to have a reservoir of osteoimmunological protocols and findings from previous studies including those derived from different compositions of mPCL-bioceramic composites that will allow us direct comparison.
In our sheep tibial segmental large defect model standard postmortem analyses include performing plain radiography, bone volume evaluation, mechanical testing, micro-computed tomography (µCT), and histology of explanted tibial defects [
25]. However, the presented study focused on immunohistochemical analyses whereas is it noteworthy that tissue explants can be processed for usage as resin or as paraffin samples for microscopic analysis. Resin sample processing does not require decalcification and, therefore, its focus is on providing in-depth information regarding morphology of undecalcified bone samples
in toto. However, for two reasons we decided to focus on paraffin samples in the presented study. First, we are confident that it is not reasonably possible to represent both groups of methods at the highest quality level in one research paper. Second, the evaluation of paraffin samples provides a unique opportunity for a variety of different stains, particularly focusing on the osteoimmunological response. For in-depth discussion of resin sample analyses, we therefore refer the interested reader to our previous publications [
20,
25,
33]. Indeed, to the best of our knowledge, our animal model is the first large-volume animal model of segmental tibial defects in sheep that comprehensively allows extensive and highly standardized analysis of tissue sections for short- and long-term outcomes by immunohistochemical staining.
In contrast to resin embedding, larger samples such as those from large (3 cm) to extra-large (6 cm) ovine tibial segmental defects pose technical challenges for paraffin embedding and further processing of paraffin samples. When standard cassette and block size (~25 - 40 mm) is limiting, oversized cassettes, embedding molds, and slides (~50 - 70 mm) can be used to preserve the integrity across large areas or minimize site fragmentation, but performing paraffin microtomy on
in toto extra-large samples is not possible. Moreover, tissue sectioning is a critical step, and the choice of sectioning planes imparts an experimental bias that must be carefully interpreted. Notably, multiple and compound sectioning planes from various regions of interest may be necessary to thoroughly assess tissue response. Therefore, in order to achieve meaningful
ex vivo histological assessment and in particular analyze all anatomical regions in a consistent manner, we defined standardized transverse sectioning planes for the histological and immunohistological specimens and validated them for the large animal model (
Supplementary Figure S1F and G).
This study aimed at profiling the adaptative osteoimmunological responses of our benchmark sheep research model as a pre-clinical tool for evaluating 3D printed mPCL scaffolds applied to the reconstruction of critical-sized segmental bone defects, as opposed to making direct comparisons between studied groups or time points. With regard to the time points analyzed, there are limitations as although samples were assessed at earlier and later time points, we are aware that the most important changes in the cellular immune response occur within the first few weeks after surgery [
37]. It is important to mention that testing time points were selected based on the utmost beneficial gain of data in regard to the 6Rs principle [
75,
76]. This can be corroborated by the circumstance that non-histological analysis such as mechanical testing or µCT imaging does not provide sufficient information at early implantation time points. However, differences can even so be noted at chosen time points regarding immune cell phenotypes (M1 versus M2) and intercellular tissue quality (COL I versus COL II), which are to be interpreted as an evolving ongoing process of regeneration. Thus, a more accurate testimony can be made through quantification of IHC data [
26,
27], which was not the focus of this paper for the reason that emphasis was given on providing an overview on immunohistochemical staining as a method for bone regeneration analysis rather than comparing the outcome of the different study groups. However, IHC quantification was performed in our previous studies and quantitative analytical methods were published and can be used in this context [
27,
77].
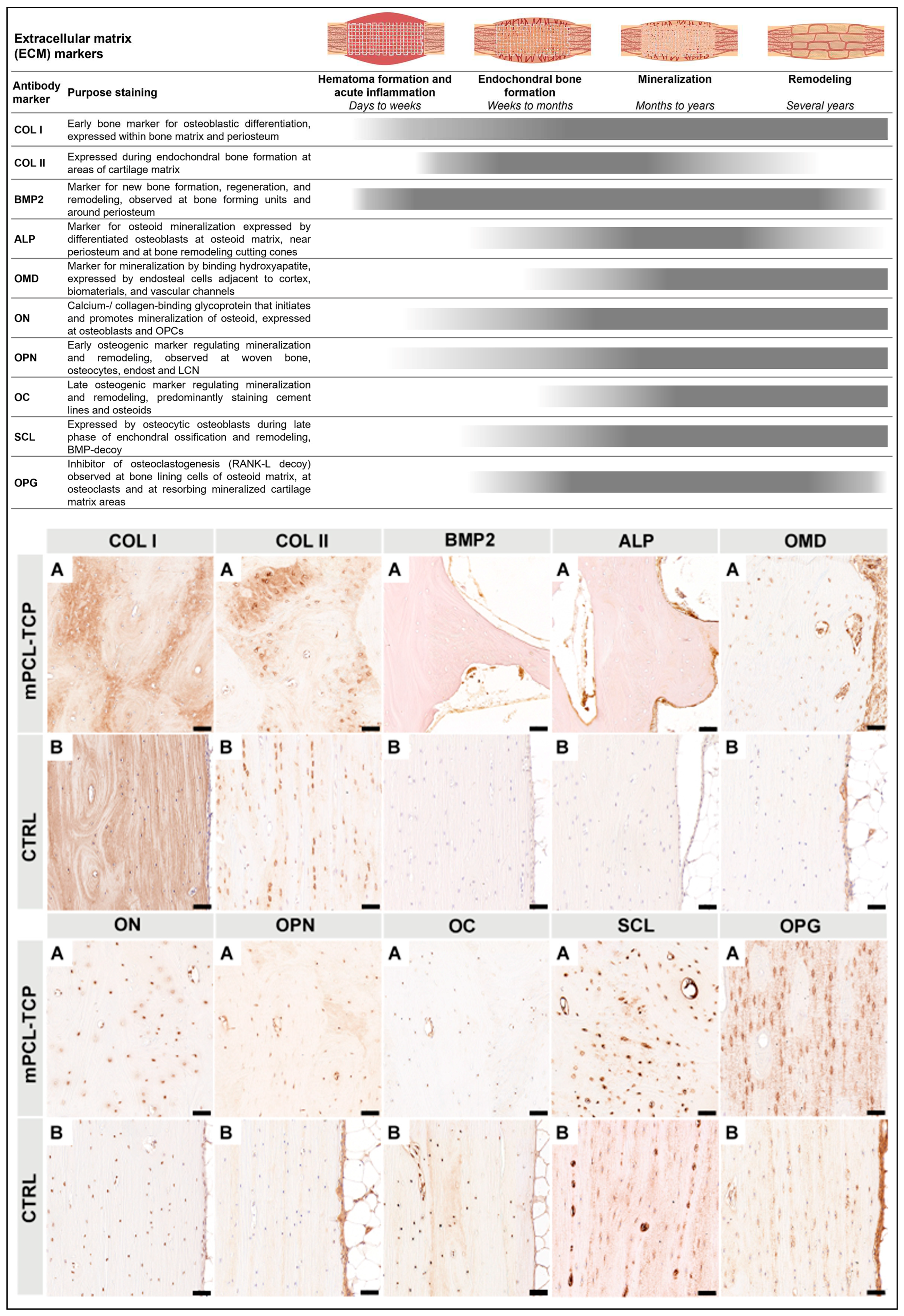
Figure 1.
Immunohistochemical analysis of protein expression in SGBR throughout the entire bone regeneration period. Foreign implanted materials, including mPCL scaffolds, activate an inflammatory response comprised of a milieu of multiple converging cytokinetic and immunological events. This includes the provision of a collagen extracellular matrix (ECM) depicted through Collagen I (COL I, A-M) deposition which is observed in all sample groups and during all time points. Clear differences in COL I stain intensity can be seen in woven bone (stronger) and lamellar bone (lighter). As bone formation in large bone defects is accomplished through osteochondral bone formation, it is not uncommon to observe the presence of a few remnant areas of mineralized cartilage going through resorption as depicted by COL II deposition near the mPCL scaffold struts as well as at the interface of host and new bone (COL II, A-M, red arrowheads). Concomitantly, up-regulation of osteocalcin (OC) is observed at osteoblast precursors within the vascular mesenchymal tissue of the cutting cones of the remodelling bone tissue (green arrowheads), throughout all time points, however with some distinct expression at later time points, which appears to be more prominent at the cement lines of the new secondary osteons formed (black arrowheads) and at osteocyte cells (OC, A-M). As regeneration progresses, neovascularization also continues to re-stablish the new vascular network, which is observed by the presence of more mature and elongated vessels through vWF+ expression throughout all time points and all experimental samples, however this expression decreases around the outer surface of the mPCL-TCP scaffold towards late time points (vWF, A-M, green arrows). As part of the innate immune system, early physiological wound healing is triggered by cytokines and cellular mediators, including macrophages. Cluster of differentiation 68 (CD68) as a M1 and M2 macrophage subset was constantly positively stained around the interconnected porous architecture of the mPCL-TCP scaffold (CD68, A-M, red arrows). The pro-inflammatory inducible nitric oxide (iNOS – M1) synthesis observed in our studies appears to be evolving overtime, yet its expression is found to be located at the remnant areas of mineralized cartilage preceding blood vessel invasion at early time points, and exclusively prevalent at blood vessels at later time points (iNOS, A-M, black arrowheads and black arrows, respectively). Ultimately, M2 macrophages depicted by mannose receptor (MR) marker, appear to self-enhance their recruitment and play a crucial role within the host-material consolidation and degradation, as observed throughout the expression and positive stain particularly at the remnants of mineralized cartilage (MR, A-M, double black arrowheads), fragments of bone graft (MR, A-M, red arrows), osteoblast precursors within the vascular mesenchymal tissue of the cutting cones (MR, A-M, red arrowheads) and at the outer surface of the mPCL-TCP scaffold struts (MR, A-M, double red arrowheads). This initial endogenous regenerative cascade, with further emerging evidence suggesting that the scaffold architecture is a niche where adaptative immune cells are decoding scaffold features, takes influence on the biocompatibility of materials, and appears to further orchestrate phenotypic macrophagic polarization by the presence of the CaP coating. Collagen type I (Col I), Collagen type II (Coll II); Osteocalcin (OC); cluster of differentiation 68 (CD68); inducible nitric oxidase synthesis (iNOS); Mannose receptor (MR); Red dashed lines: scaffold struts. Scale bars: 50µm.
Figure 1.
Immunohistochemical analysis of protein expression in SGBR throughout the entire bone regeneration period. Foreign implanted materials, including mPCL scaffolds, activate an inflammatory response comprised of a milieu of multiple converging cytokinetic and immunological events. This includes the provision of a collagen extracellular matrix (ECM) depicted through Collagen I (COL I, A-M) deposition which is observed in all sample groups and during all time points. Clear differences in COL I stain intensity can be seen in woven bone (stronger) and lamellar bone (lighter). As bone formation in large bone defects is accomplished through osteochondral bone formation, it is not uncommon to observe the presence of a few remnant areas of mineralized cartilage going through resorption as depicted by COL II deposition near the mPCL scaffold struts as well as at the interface of host and new bone (COL II, A-M, red arrowheads). Concomitantly, up-regulation of osteocalcin (OC) is observed at osteoblast precursors within the vascular mesenchymal tissue of the cutting cones of the remodelling bone tissue (green arrowheads), throughout all time points, however with some distinct expression at later time points, which appears to be more prominent at the cement lines of the new secondary osteons formed (black arrowheads) and at osteocyte cells (OC, A-M). As regeneration progresses, neovascularization also continues to re-stablish the new vascular network, which is observed by the presence of more mature and elongated vessels through vWF+ expression throughout all time points and all experimental samples, however this expression decreases around the outer surface of the mPCL-TCP scaffold towards late time points (vWF, A-M, green arrows). As part of the innate immune system, early physiological wound healing is triggered by cytokines and cellular mediators, including macrophages. Cluster of differentiation 68 (CD68) as a M1 and M2 macrophage subset was constantly positively stained around the interconnected porous architecture of the mPCL-TCP scaffold (CD68, A-M, red arrows). The pro-inflammatory inducible nitric oxide (iNOS – M1) synthesis observed in our studies appears to be evolving overtime, yet its expression is found to be located at the remnant areas of mineralized cartilage preceding blood vessel invasion at early time points, and exclusively prevalent at blood vessels at later time points (iNOS, A-M, black arrowheads and black arrows, respectively). Ultimately, M2 macrophages depicted by mannose receptor (MR) marker, appear to self-enhance their recruitment and play a crucial role within the host-material consolidation and degradation, as observed throughout the expression and positive stain particularly at the remnants of mineralized cartilage (MR, A-M, double black arrowheads), fragments of bone graft (MR, A-M, red arrows), osteoblast precursors within the vascular mesenchymal tissue of the cutting cones (MR, A-M, red arrowheads) and at the outer surface of the mPCL-TCP scaffold struts (MR, A-M, double red arrowheads). This initial endogenous regenerative cascade, with further emerging evidence suggesting that the scaffold architecture is a niche where adaptative immune cells are decoding scaffold features, takes influence on the biocompatibility of materials, and appears to further orchestrate phenotypic macrophagic polarization by the presence of the CaP coating. Collagen type I (Col I), Collagen type II (Coll II); Osteocalcin (OC); cluster of differentiation 68 (CD68); inducible nitric oxidase synthesis (iNOS); Mannose receptor (MR); Red dashed lines: scaffold struts. Scale bars: 50µm.
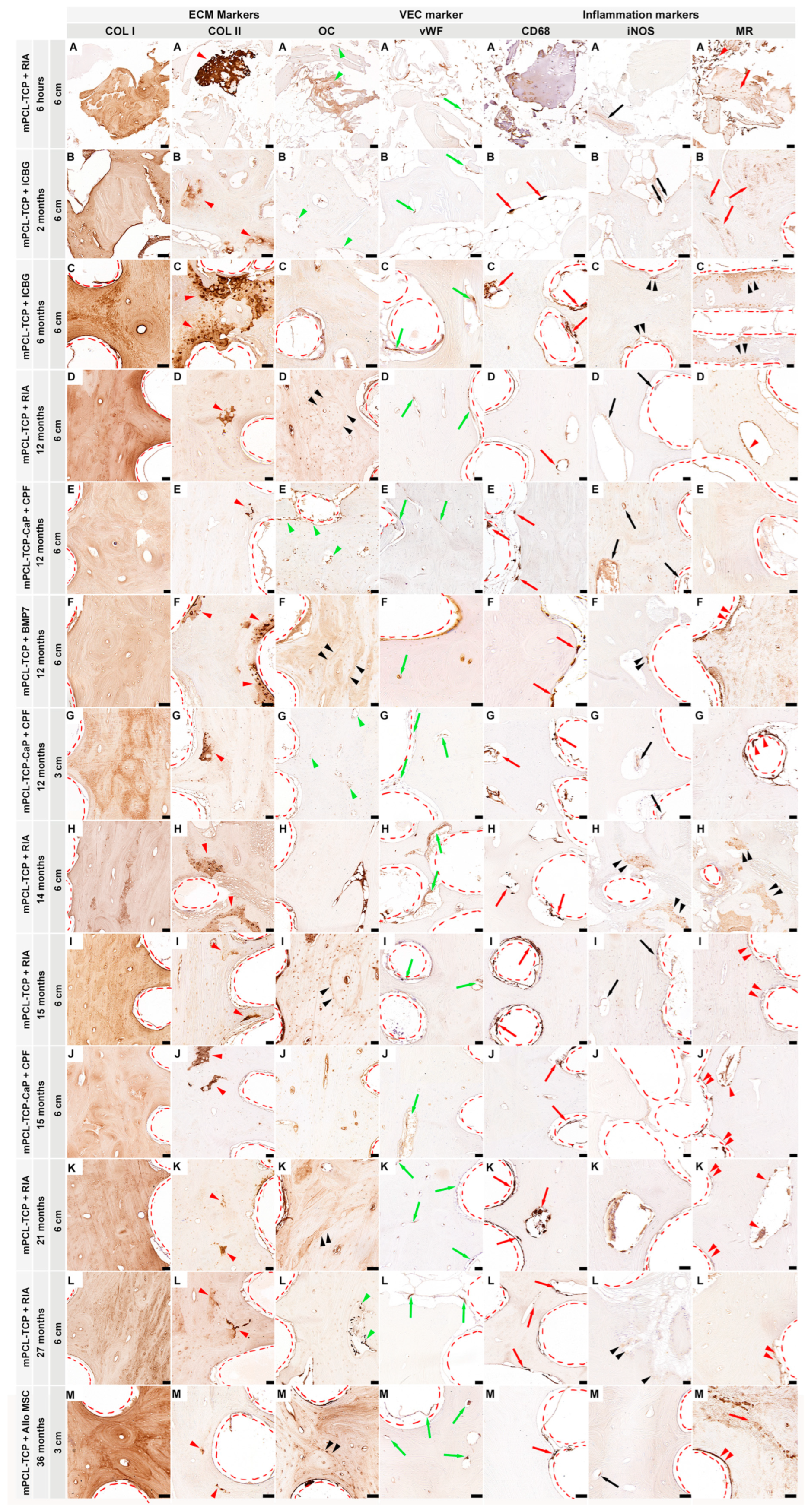
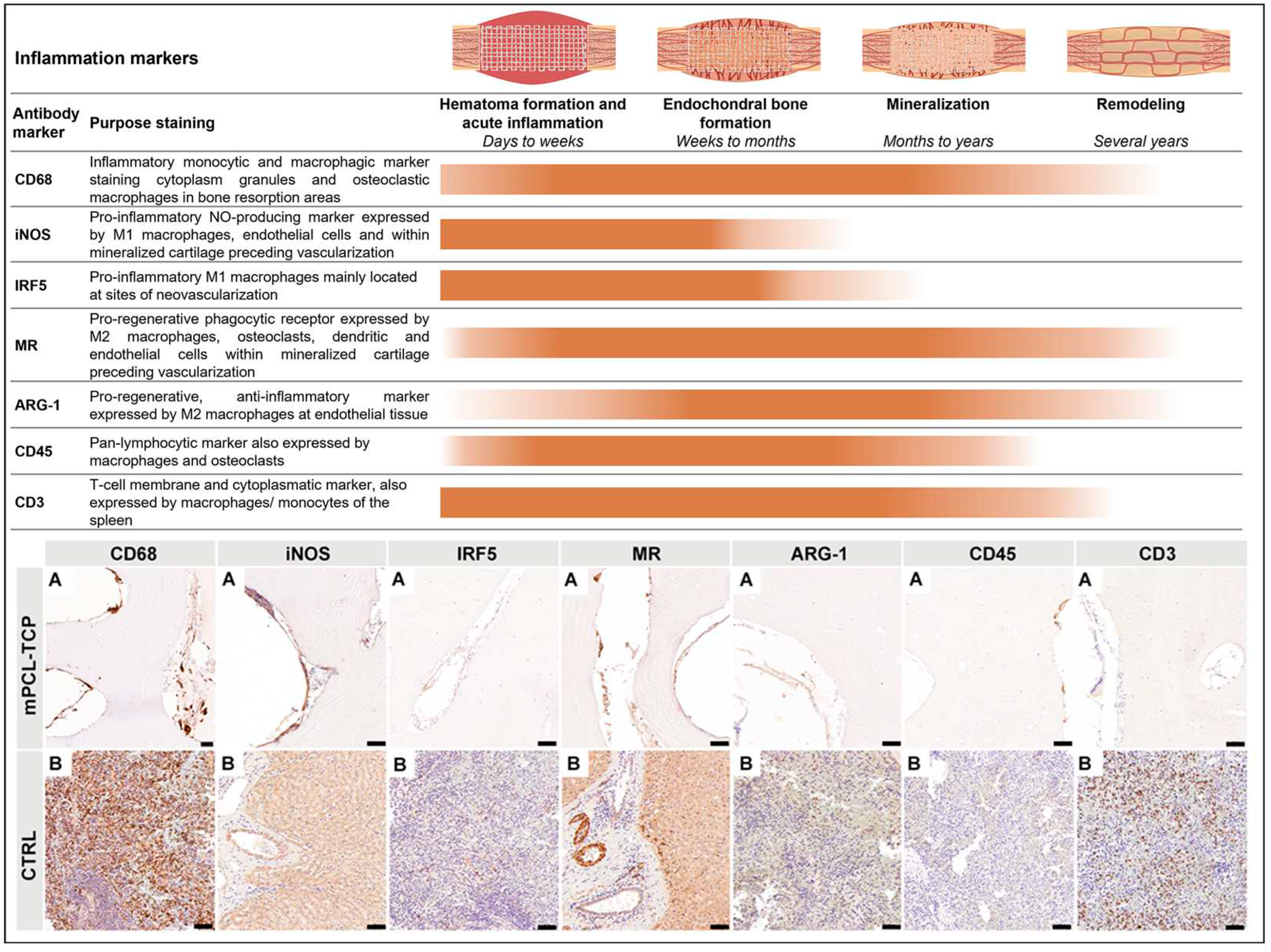
Figure 2.
The first elicited cellular reaction upon biomaterial implantation is the initial inflammatory response, caused by the release of M1 subtype macrophages (iNOS, IRF5), exerting angiogenic functions among ECM clearing. These macrophages mainly populate the outer surface of mPCL-TCP scaffolds and are present at sites of neovascularization, especially at the interface of scaffold strut and newly formed bone. As regeneration progresses, the release of anti-inflammatory cytokines, which includes the expression of multiple M2 macrophage subtypes (MR and ARG-1), is more evident and present at the outer surface of the mPCL-TCP scaffolds. NO = nitric oxide, Nitric oxide synthase (iNOS); mannose receptor (MR); Arginase-1 (ARG-1); cluster of differentiation 68 (CD68); interferon regulatory factor 5 (IRF5); cluster of differentiation 3 (CD3) and cluster of differentiation 45 (CD45). . Scale bars: 50µm.
Figure 2.
The first elicited cellular reaction upon biomaterial implantation is the initial inflammatory response, caused by the release of M1 subtype macrophages (iNOS, IRF5), exerting angiogenic functions among ECM clearing. These macrophages mainly populate the outer surface of mPCL-TCP scaffolds and are present at sites of neovascularization, especially at the interface of scaffold strut and newly formed bone. As regeneration progresses, the release of anti-inflammatory cytokines, which includes the expression of multiple M2 macrophage subtypes (MR and ARG-1), is more evident and present at the outer surface of the mPCL-TCP scaffolds. NO = nitric oxide, Nitric oxide synthase (iNOS); mannose receptor (MR); Arginase-1 (ARG-1); cluster of differentiation 68 (CD68); interferon regulatory factor 5 (IRF5); cluster of differentiation 3 (CD3) and cluster of differentiation 45 (CD45). . Scale bars: 50µm.
Figure 3.
Vessel recruitment is largely coordinated through the release of vascular endothelial growth factor (VEGF) as well as angiopoietin 1 (ANG1) through monocytes present at disrupted vessels of the injured site as well as through the pro-inflammatory environment initially created during hematoma formation. These newly formed vessels express high levels of platelet endothelial cell adhesion molecule (PECAM 1, also known as cluster of differentiation 31 (CD31)). This timely organized pro-inflammatory environment is precisely orchestrated to shift to a pro-regenerative environment, which in turn will lead to the re-establishment and remodelling of the vascular network of the defect area. Mature vessels can be detected by the expression of von Willebrand factor (vWF) within the newly formed bone tissue and around the interconnected porous architecture of the mPCL-TCP scaffold. Anti-smooth muscle actin (α-SMA) is expressed specially in myofibroblasts, which play a substantial role in scaffold encapsulation. However, it is also expressed at the endothelial wall of blood vessels and can therefore be used as a marker for vascularization. α-SMA expression in our studies was detected only at vessels formed around the mPCL-TCP scaffold struts and at larger vessels at the periosteal region of the newly formed bone tissue. In line with literature [
34,
35] we observed that NOG and Notch 1 play a regulatory role in angiogenesis and stain endothelial wall of blood vessels. BMP = bone morphogenic protein, MSC = mesenchymal stem cell; Vascular Endothelial Factor (VEGF); Angiopoietin (ANG1); Cluster of differentiation 31 (CD31); von Willebrand Factor (vWF); Anti smooth Muscle Actin (α-SMA); Noggin (NOG); Scale bars: 50µm.
Figure 3.
Vessel recruitment is largely coordinated through the release of vascular endothelial growth factor (VEGF) as well as angiopoietin 1 (ANG1) through monocytes present at disrupted vessels of the injured site as well as through the pro-inflammatory environment initially created during hematoma formation. These newly formed vessels express high levels of platelet endothelial cell adhesion molecule (PECAM 1, also known as cluster of differentiation 31 (CD31)). This timely organized pro-inflammatory environment is precisely orchestrated to shift to a pro-regenerative environment, which in turn will lead to the re-establishment and remodelling of the vascular network of the defect area. Mature vessels can be detected by the expression of von Willebrand factor (vWF) within the newly formed bone tissue and around the interconnected porous architecture of the mPCL-TCP scaffold. Anti-smooth muscle actin (α-SMA) is expressed specially in myofibroblasts, which play a substantial role in scaffold encapsulation. However, it is also expressed at the endothelial wall of blood vessels and can therefore be used as a marker for vascularization. α-SMA expression in our studies was detected only at vessels formed around the mPCL-TCP scaffold struts and at larger vessels at the periosteal region of the newly formed bone tissue. In line with literature [
34,
35] we observed that NOG and Notch 1 play a regulatory role in angiogenesis and stain endothelial wall of blood vessels. BMP = bone morphogenic protein, MSC = mesenchymal stem cell; Vascular Endothelial Factor (VEGF); Angiopoietin (ANG1); Cluster of differentiation 31 (CD31); von Willebrand Factor (vWF); Anti smooth Muscle Actin (α-SMA); Noggin (NOG); Scale bars: 50µm.
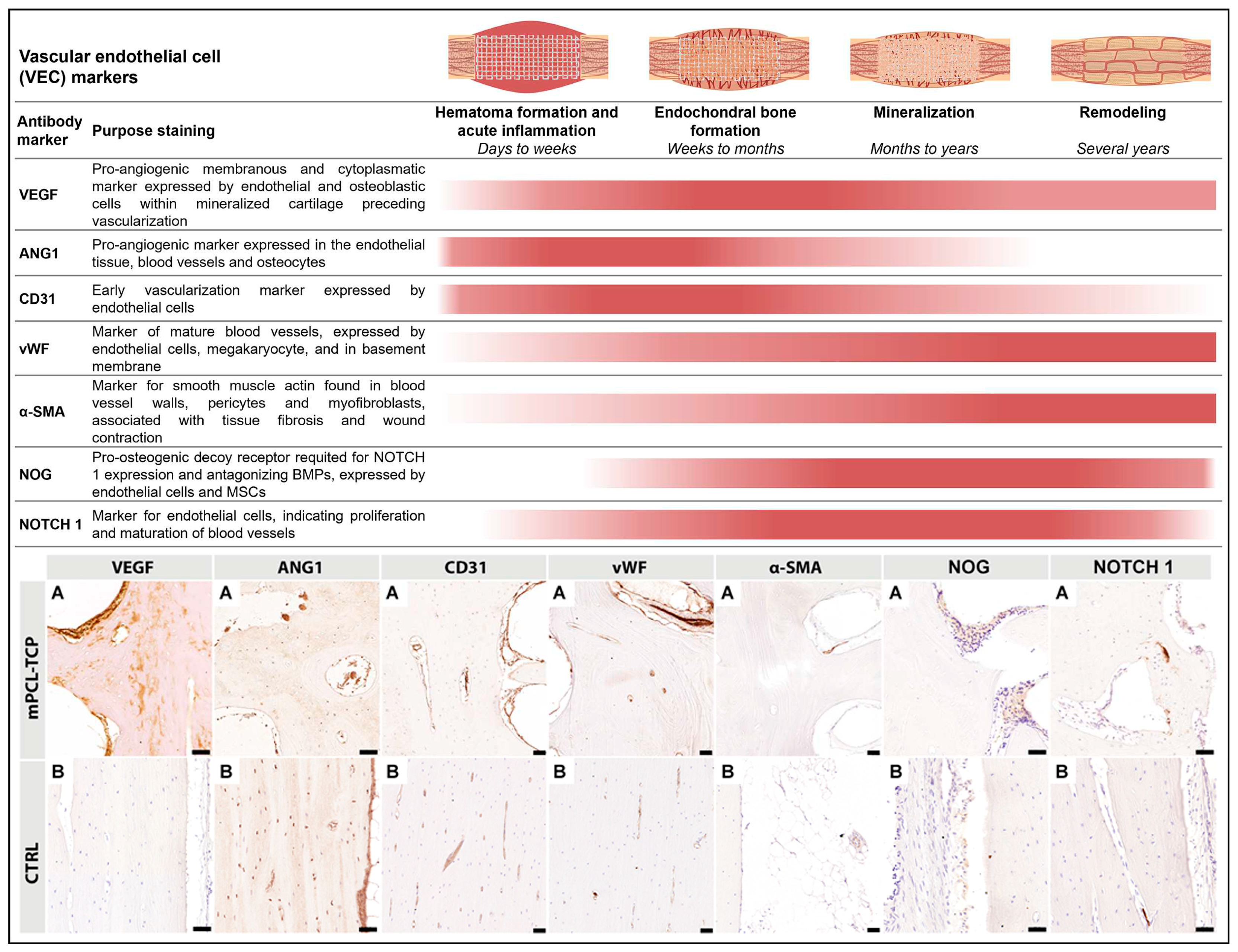
Figure 4.
The first mineralized matrix produced during primary bone formation is resorbed by osteoclasts. As cartilage is resorbed, secondary bone formation takes place. These two overlapping processes can clearly be observed through collagen type I deposition depicted by COL I+ staining throughout the newly formed bone, as well as by COL II+ staining at isolated areas of mineralized cartilage, especially around scaffold struts and at the interface of the new bone and host bone tissue. As bone regeneration continues, bone morphogenic proteins (BMP) expression, observed at osteoclasts as well as at the endothelial tissue lining the outer surface of scaffolds, stimulates osteoblast recruitment through ALP and OC signaling. ALP, an intermediate marker of osteoblast differentiation indicating bone formation and turnover (highly produced by osteoblasts and mainly seen in areas of osteoid deposition), plays an important part in local bone mineralization. Up-regulation of early osteogenic activity through OC and ON signaling follows and secondary osteon organization and mineralization takes place. ON, a glycoprotein synthetized by osteoblasts that binds calcium, also considered an intermediate marker, then initiates mineralization and promotes mineral crystal formation. ON, is positively depicted throughout the newly formed bone bordering active osteoblasts, osteocytes, osteoid and marrow progenitor cells. Correspondingly, OC expression is also evident in osteoblasts, osteoclast, osteocytes, at cement lines, as well as hypertrophic chondrocytes. Osteocytes synthesize SCL, a protein which mediates bone forming osteoblast-, bone resorbing osteoclast-, and osteocyte-cross-signaling, as well as antagonizes the activity of BMPs. Only osteocytes express SCL, which in turn acts in a paracrine fashion to inhibit bone formation. High levels of immunodetectable SCL are seen in osteoblasts that have reached the osteocytic stage, especially within the samples with late time points and at the bone cortex of the contralateral control samples. OPG is an early osteogenic marker, which has a pivotal role in inhibiting the osteoclast activation and proliferation, and therefore is essential for bone resorption. Its expression was intensely found throughout the newly formed bone, especially in the samples with late time points and at the cortex of control samples. OPCs = osteoprogenitor cells, LCN: lacuna canalicular network; Collagen type I (Col I); Collagen type II (Coll II); Bone Morphogenic Protein (BMP); Alkaline Phosphatase (ALP); Osteomodulin (OMD); Osteonectin (ON); Osteopontin (OPN); Osteocalcin (OC); Sclerostin (SCL) and Osteoprotegerin (OPG). Scale bars: 50µm.
Figure 4.
The first mineralized matrix produced during primary bone formation is resorbed by osteoclasts. As cartilage is resorbed, secondary bone formation takes place. These two overlapping processes can clearly be observed through collagen type I deposition depicted by COL I+ staining throughout the newly formed bone, as well as by COL II+ staining at isolated areas of mineralized cartilage, especially around scaffold struts and at the interface of the new bone and host bone tissue. As bone regeneration continues, bone morphogenic proteins (BMP) expression, observed at osteoclasts as well as at the endothelial tissue lining the outer surface of scaffolds, stimulates osteoblast recruitment through ALP and OC signaling. ALP, an intermediate marker of osteoblast differentiation indicating bone formation and turnover (highly produced by osteoblasts and mainly seen in areas of osteoid deposition), plays an important part in local bone mineralization. Up-regulation of early osteogenic activity through OC and ON signaling follows and secondary osteon organization and mineralization takes place. ON, a glycoprotein synthetized by osteoblasts that binds calcium, also considered an intermediate marker, then initiates mineralization and promotes mineral crystal formation. ON, is positively depicted throughout the newly formed bone bordering active osteoblasts, osteocytes, osteoid and marrow progenitor cells. Correspondingly, OC expression is also evident in osteoblasts, osteoclast, osteocytes, at cement lines, as well as hypertrophic chondrocytes. Osteocytes synthesize SCL, a protein which mediates bone forming osteoblast-, bone resorbing osteoclast-, and osteocyte-cross-signaling, as well as antagonizes the activity of BMPs. Only osteocytes express SCL, which in turn acts in a paracrine fashion to inhibit bone formation. High levels of immunodetectable SCL are seen in osteoblasts that have reached the osteocytic stage, especially within the samples with late time points and at the bone cortex of the contralateral control samples. OPG is an early osteogenic marker, which has a pivotal role in inhibiting the osteoclast activation and proliferation, and therefore is essential for bone resorption. Its expression was intensely found throughout the newly formed bone, especially in the samples with late time points and at the cortex of control samples. OPCs = osteoprogenitor cells, LCN: lacuna canalicular network; Collagen type I (Col I); Collagen type II (Coll II); Bone Morphogenic Protein (BMP); Alkaline Phosphatase (ALP); Osteomodulin (OMD); Osteonectin (ON); Osteopontin (OPN); Osteocalcin (OC); Sclerostin (SCL) and Osteoprotegerin (OPG). Scale bars: 50µm.