1. Introduction
Kiwifruit (Actinidia chinensis Planch.) is one of the most highly-appreciated fruit by consumers worldwide own to its delicious taste and nutritional value, including high content of vitamin C. As a kind of typical climacteric fruit, kiwifruit have a short storage period [
1]. There is an apparent physiological after-ripening process, and the fruit is easily infected by fungal pathogens during postharvest storage, resulting in deterioration of quality, loss of flavor and even severe rot [
2,
3]. It is extremely perishable because of its susceptibility to senescence and postharvest disease, making it challenging to store, transport and sell commercially [
4,
5].
Temperature is one of the most significant factors in maintaining the quality and extending the shelf life of fruit and vegetables [
6]. Cold storage is an effective way to decay ripening and extend the shelf life after harvest by reducing respiration and oxidase activity [
7,
8]. Cold chain transport effectively maintains the color and texture of kiwifruit [
9]. The storage of fruits and vegetables requires an optimal temperature with minimal fluctuation [
10]. However, there are inevitable temperature fluctuation (TF) during long-distance transportation, including improper operation, imprecise temperature control devices, and other problems. Current research on TF has focused on the dynamics of quality loss of frozen foods during storage [
11,
12,
13], with less research related to the impact on the quality of fresh fruit and vegetables. The seriously temperature fluctuating accelerated quality degradation of table grapes in cold chain quickly [
14]. Besides, the quality of the mushrooms, broccoli and mature-green tomatoes, including flesh browning, the loss of firmness and weight were significantly impacted by temperature changes, which were packaged in modified atmosphere [
15]. The decline of food quality caused by temperature changes is cumulative and irreversible [
16,
17].
Translucency is consodered as a symbolic signal of chilling injury symptom due to fruit flesh texture deterioration during cold storage [
18]. Liquid fills the intercellular gap in translucent flesh area, resulting in a water-soaked appearance [
19]. Transparency leads to a loss of food quality in terms of taste and flavour. Moreover, translucent fruits are more susceptible to physical damage, fungal diseases and detrimental to storage and transportation [
19]. Integrated metabolome and transcriptome analysis shows that translucency is a complex process involving genes belonging to multiple metabolic pathways and various metabolic processes [
20]. Besides, transparency may be related to metabolic dysfunction from membrane damage [
21] and the collapse of tissue adjacent [
22]. Transparency is a frequent phenomenon in the flesh of kiwifruit during storage. However, to date, little was reported previously on the transparency of kiwifruit after harvest.
The current temperature fluctuation range of cold chain land transportation is mostly 3–5 ºC, and the transportation time is up to 3 d. This is one of the major constraints faced by wholesalers and retailers. However, the study focused on the effect of TF on postharvest physiological of kiwifruit was limited. Accordingly, this study simulated temperature fluctuation patternand monitored the physiological parameters affecting postharvest life and quality of kiwifruit, including respiratory metabolism, flesh texture, ripening, the edible quality and related gene expression. The overall purpose of this research was to study the effects of different temperature fluctuation treatments on postharvest kiwifruit quality during cold storage.
2. Materials and methods
2.1. Sample collection and treatments
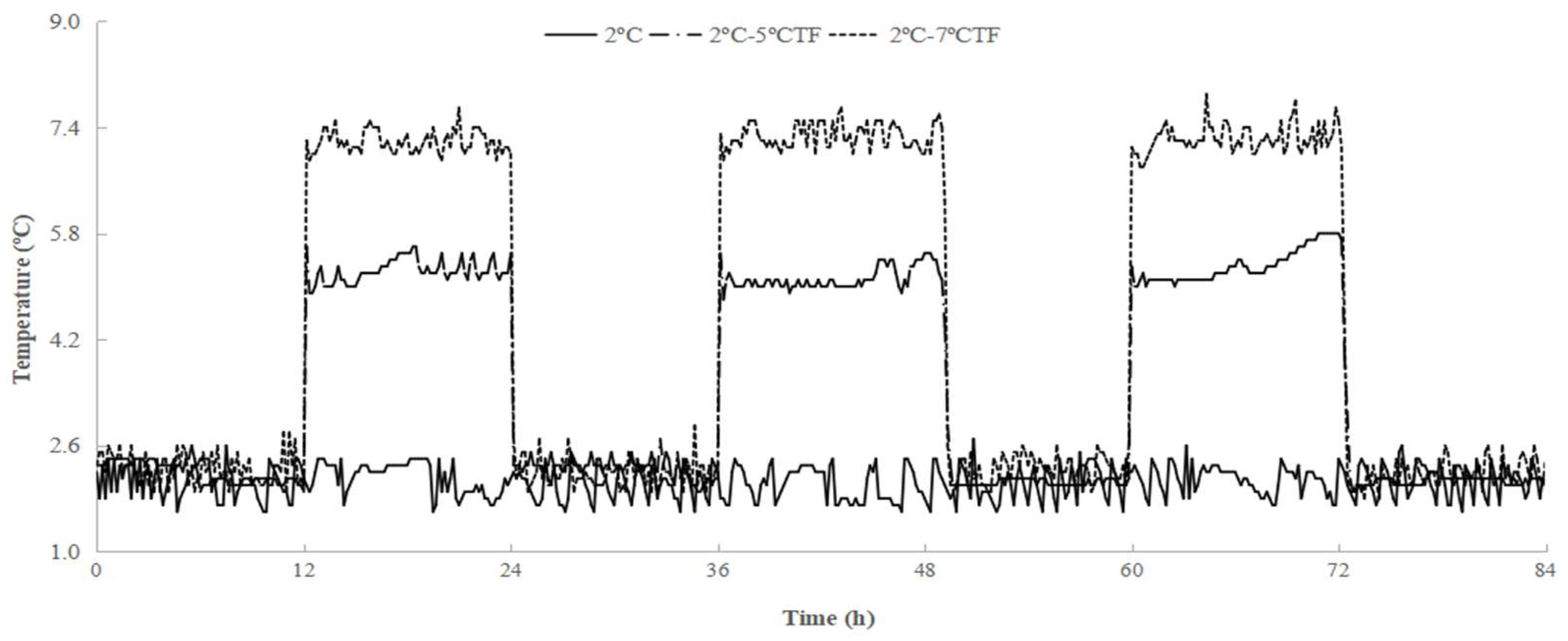
“Xu Xiang” kiwifruits were harvested at commercial maturity from a commercial orchard located in Guiyang district, Shaanxi, China. The kiwifruits were transported to the laboratory within 2 h and selected according to their uniformity of size and color. Those with visible mechanical injury or defects were discarded. The fruits were divided into three groups randomly with 540 fruits in each group. One group was stored at 2 ± 0.5 °C, served as control. One group was stored at 2 ºC or 5 ºC, alternating every 12 h, served as TF2ºC-5ºC. Another group was stored at 2 ºC or 7 ºC, alternating every 12 h, served as TF2ºC-7ºC. All treatments were performed at 85–95 % relative humidity (RH) (
Figure 1). After 72 h, all fruits were transferred to storage at 2 °C and 85–95% RH.The pulp of fruits was combined, frozen in liquid nitrogen, and stored at −80°C until assaying for gene expression.
2.2. Microscope observations
The samples for optical microscope observations were only obtained 21 d after storage. The pulp were cut into small pieces (2–3 mm2), fixed in formaldehyde–acetic acid fixative solution (50% ethanol–5%formaldehyde–5%acetic acid=18:1:1) for 24 h. Then, the pulp was dehydrated through step-wise increases in ethanol concentration (50, 60, 70, 80,90, 95 and 100%). The pulp sections were then treated with carbon dioxide to the critical drying point using a critical point drier (HCP-2; Hitachi, Tokyo, Japan). The dried pulp sections were glued to a metal table with double-sided adhesive to expose the observed parts. The samples were then coated with gold using an ion sputtering meter (E1010; Hitachi) and observed using a optical microscope (MODEL ECLIPSE Ci-S; Nikon, Tokyo, Japan).
2.3. Translucency assessment and decay incidence
The translucency incidence of the fruit pulp was visually evaluated and scored based on the percentage of total cross-sectional area containing the number of translucency, using the following grading scale: 0, no symptoms; 1, less than 25%; 2, 25%-50%; 3, more than 50%, as shown in
Figure 2. The translucency index was expressed as:
Kiwifruits from different treatment groups were collected separately every week. The total number of kiwifruit and rotten kiwifruit (rotten spot diameter ≥ 2 mm) in each treatment were counted, and the decay incidence was calculated according to the following formula:
2.4. Respiration rate and ethylene production
The respiration intensity and ethylene production were measured with 30 fruits (containing 3 replicates) every week using a portable gas analyzer (F-950; FELIX, Washington, USA). The ethylene production and respiration rate was presented as μL kg−1 h−1 and mL kg−1 h−1, respectively.
2.5. Fruit quality assessments
Firmness was measured using a handle penetrometer (FT327; FACCHINI SRL, Alfonsine, Italy) equipped with an 8 mm diameter tip. Total soluble solids (TSS) were measured using a pocket refractometer (PAL-1; ATAGO, Tokyo, Japan). Titratable acidity (TA) was determined from freshly homogenized juice samples from ten fruits per replicate using an auto titrator (809 Titrando; Metrohm, Herisau, Switzerland). The content of vitamin C was determined by high-performance liquid chromatography (HPLC) according to the methods of Gai and Wang [
23]. Weight loss was measured by weighing each fruit at harvest and each subsequent assessment day. Cumulative weight loss was expressed as a percentage value determined by deducting the initial weights from the final weights divided by the initial weights and multiplied by 100 percent (%).
2.6. Relative conductivity determination
Discs of 2 mm from kiwifruit flesh were punched out using a punch with an inner diameter of 1 cm for relative conductivity determination. The discs was put in a large beaker and then 20 mL of ultrapure water was added. The water was pour off after shaking for 10 min. Then samples were rinsed three times with ultrapure water to remove excess pectin. The discs was put in ultrapure water and the electrical conductivity was measured before and after 20 minutes of boiling. The percentage of the ratio of two values was the relative conductivity.
2.7. Reverse transcription and quantitative real-time PCR (RT-qPCR)
The RNA was extracted from fruit flesh tissue using an RNA prep Pure Plant Plus kit (Tiangen, China) according to the manufacturer’s protocol. Complementary DNA (cDNA) was synthesized by reverse transcription using the Invitrogen Super-Script™ III First-Strand Synthesis System (Carlsbad, CA, USA). RT-qPCR were performed using a 96-well plate in a Light Cycler1480II real-time PCR system (Roche, Basel, Switzerland) with Ultra SYBR mixture (CW Bio Co., Beijing, China).The amplifcation of
Pbactin sequence was used as an endogenous reference to normalize all the data. The 2
−ΔΔCt method [
24] was employed to determine gene expression. The primers were listed in Table S1. All analyses contained three technical replicates.
2.8. Statistical analysis
All tests were repeated three times, and each repeated test served as a block in the statistical design. SPSS19.0 statistical software (SPSS Inc., Chicago, IL, USA) was used to perform the analysis of variance, followed by the least significant difference (LSD) mean comparison test.
3. Results and discussion
3.1. Effect of temperature fluctuation on micromorphology of pulp cell of kiwifruit during storage
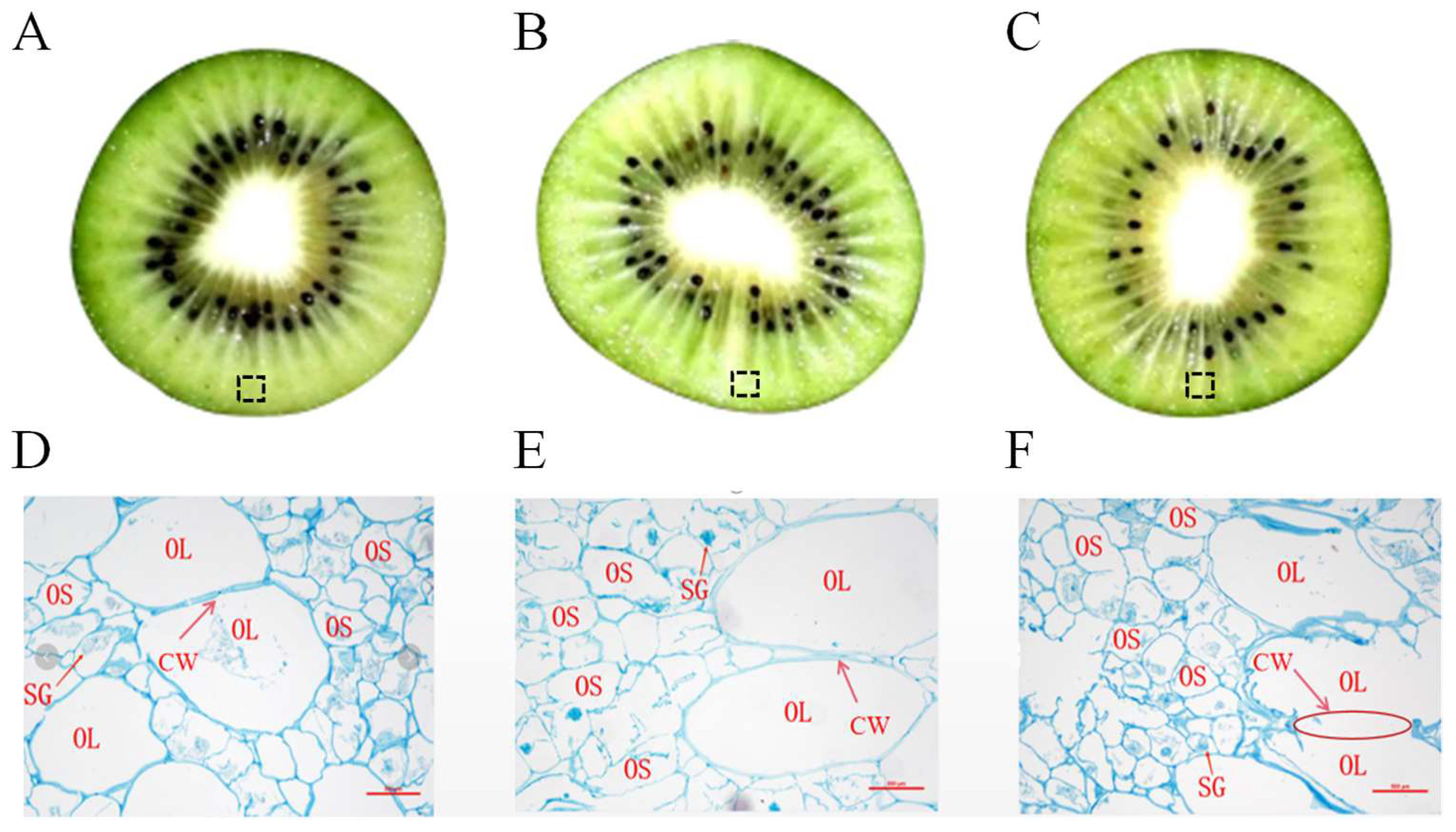
Firstly, the effect of temperature fluctuation on the flesh tissue of kiwifruit was observed. Compared to the control (
Figure 3A), the flesh of fruits in TF2ºC-5ºC group (
Figure 3B) and TF2ºC-7ºC group (
Figure 3C) turned transparent and water-soaked significantly. As the temperature fluctuation deepened, the degree of transparency became more acute. Changes in the microstructure of the cell wall of kiwifruit treated with different temperature fluctuation were observed by optical microscopy. As shown in Figures 3D, the exocarp cells of “Xu Xiang” kiwifruit were composed of thin-walled cells of both sizes, which were regularly round or oval in shape. The exocarp cells of kiwifruits stored at 2ºC for 7 d were regularly shaped and tightly arranged with the cell wall and starch granules intact. However, the starch granules of kiwifruits in TF2ºC-5ºC started to gel. The cell walls gradually loosened and the boundaries between cells became blurred (
Figure 3E). The cell damage of kiwifruits in TF2ºC-7ºC was intensified (
Figure 3F). The cell walls of kiwifruit began to degrade and disappear. In addition, the whole tissue was filled with starch matrix. From the microscopic observation results, the transparency may be related to the degradation of the cell wall, which further leads to the penetration of the cell solution. The disorders of water content and solutes such as sugar in the intercellular spaces may lead to transparency [
19]. Degradation of the cell wall could be responsible for kiwifruit softening, which contains several polysaccharide elements, including pectin, cellulose, and hemicellulose [
25]. Studies have shown that the dissolution of middle lamella and the disruption of the cell wall structure was accelerated under 5 ± 5°C TF at 30 d of the storage, while constant temperature under 5 ± 0°C maintained the integrity of the cell wall structure and firmness of apple [
26]. Acoordingly, temperature fluctuation promoted the degradation of cell wall and the rupture of starch granules, resulting in the transparency and softening of kiwifruits.
3.2. Effect of temperature fluctuation on fruit texture of kiwifruit during storage
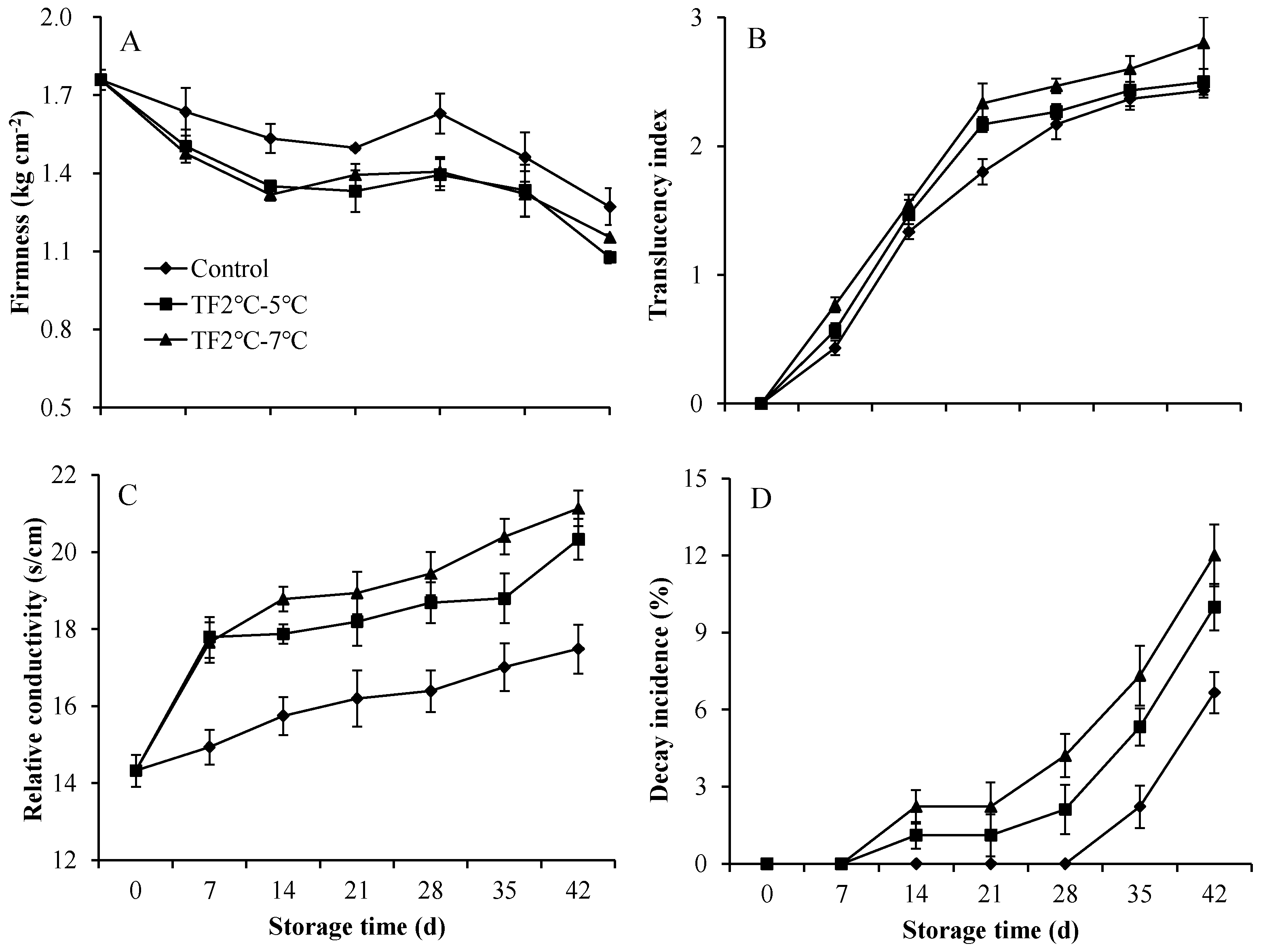
The firmness of kiwifruit in control group decreased from 1.76 kg cm
-2 to 1.27 kg cm
-2 after storage of 42 d (
Figure 4A). The treatment of TF2ºC-5ºC and TF2ºC-7ºC decreased the firmness of the kiwifruits at the end of storage by 15% and 9%, respectively. As the degree of temperature fluctuation increases, the firmness of the peaches decreases after 15 d storage [
27], which is consistent with our results. Cell wall structural compounds such as pectin and hemicellulose are grdually degraded during long-term storage, causing the decrease of fruit firmness [
28].
The translucency index of fruits treated with TF2ºC-5ºC and TF2ºC-7ºC significantly increased compared with the control group (
Figure 4B). In particularly, the translucency index was increased by 20 % and 29 % in the TF2ºC-5ºC and TF2ºC-7ºC treated fruit on 21 d, respectively. Fruit relative conductivity is often regarded as an important indicator of fruit cell membrane structure and integrity. The relative conductivity of control and treated fruits continued to increase during storage, although the control fruits always had the lowest relative conductivity (
Figure 4C). As the temperature fluctuation aggravated, the degree of relative conductivity increased. Compared to control, the relative conductivity was increased by 16 % and 21 % in the TF2ºC-5ºC and TF2ºC-7ºC treated fruit on 42 d, respectively. The increase of relative conductivity implied the damage to the cell membrane. Multiple metabolic pathways are impacted once the membranes break down, such as ion leakage, finally leading to membrane rupture and cell death [
29]. Therefore, it is essential to avoid temperature fluctuations to reduce membrane damage.
The decay incidence is one of the important indexes representing fruit storable capacity [
30]. Kiwifruits in the treatment groups began to decay within 14 d, but did not occur in the control group until 35 days (
Figure 4D). In addition, the decay incidence of the treatment groups was always significantly higher than that of the control during the entile storage period. The decay incidence of the TF2ºC-5ºC and TF2ºC-7ºC treatment group was 50% and 80% higher than that of the control group on 42 d, respectively.
TF accumulated the softening of various fruit and vegetable during storage, furthermore, the loss of firmness facilitated physical damage [
31,
26]. Besides, the increase of membrane permeability of the cells is related to the translucency of the fruit flesh [
32]. The temperature fluctuation increased the translucency index and relative conductivity of kiwifruit during cold storage, which was consistent with the effect of TF on sweet cherry [
33]. As a result, the decay indience was significantly aggravated by TF. Therefore, TF treatments should be minimized during storage.
3.3. Effect of temperature fluctuation on respiratory rate, ethylene production and quality of kiwifruit during storage
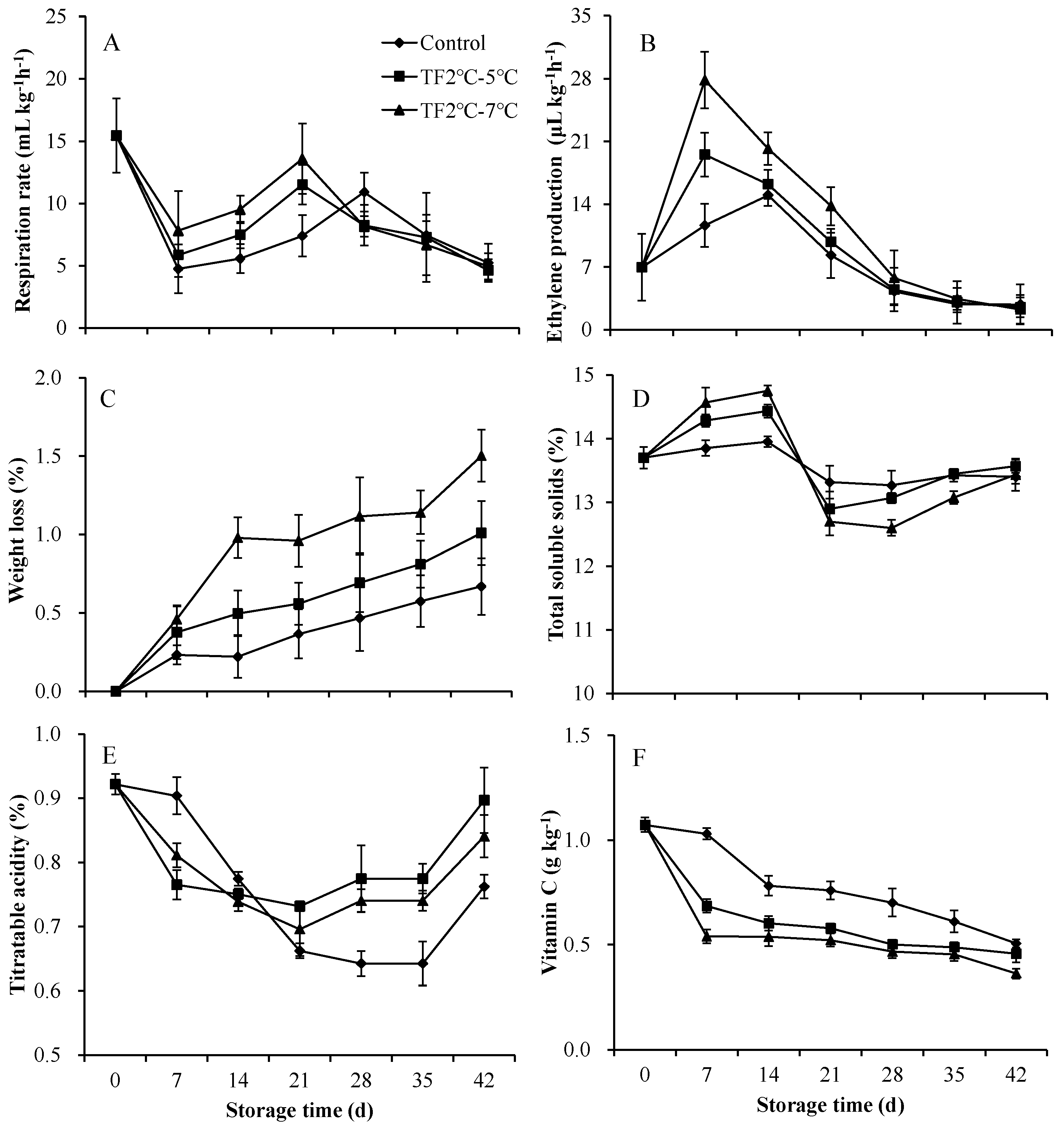
As a kind of typical climacteric fruit, kiwifruit have both obvious respiratory peak and ethylene peak during storage. The respiratory rate of control kiwifruits rapidly decreased during the first 7 d of storage and then gradually increased over the next 21 d (
Figure 5A). Subsequently, the respiratory rate decreased, eventually falling below the harvest. The respiratory rate of the fruit in TF2ºC-5ºC and TF2ºC-7ºC group was consistent with that of the control group. However, the respiratory peaks of the fruit in TF2ºC-5ºC and TF2ºC-7ºC group appeared at 21 d of storage, 7 d earlier than that of the control group. The highest peak respiratory values were found in TF2ºC-7ºC group, followed by TF2ºC-5ºC group and the lowest in the control group. The temperature fluctuation not only promoted earlier peak respiration, but also increased peak respiration values, which is not conducive to maintaining fruit quality. Similarly, the temperature fluctuation promoted an earlier peak in ethylene by 7 d and increased peak ethylene values (
Figure 5B). The ethylene peaks of the fruit in TF2ºC-5ºC and TF2ºC-7ºC group appeared at 7 d of storage, which were 30% and 86% higher than that of control, respectively. For postharvest products, respiration rate and ethylene release rate reflect the process of fruit ripening and senescence [
34]. TF accelerated the ethylene and respiration peaks in kiwifruit, at the same time, the occur of ethylene and respiration peaks in tomato [
35], “Fuji” and “Golden Delicious” apple fruit [
26] under 5 °C was accelerated by TF. Therefore, avoiding temperature fluctuation is crucial to maintain suitable respiration and ethylene production.
The water content of fruits has been regarded as predominant indicator in fruits because it directly relates to taste and physiological metabolism capacity [
36] (Jia et al., 2020). The temperature fluctuation aggravated the weight loss of the kiwifruits during storage (
Figure 5C). The weight loss rate was dramatically higher in the TF2ºC-5ºC and TF2ºC-7ºC treated fruit than that of the control by 51% and 125% on 42 d. The water loss was mainly due to the transpiration and respiration of the fruit by itself. The temperature fluctuation led to a higher peak value and lower peak value of TSS content (
Figure 5D). The TA content of control decreased after storage (
Figure 5E). The treatment with TF2ºC-5ºC and TF2ºC-7ºC increased the TA content of the kiwifruits by 18% and 10% at 42 d, respectively. Kiwifruits are an excellent dietary source of vitamin C, which is a major contributor to the total antioxidant capacity [
37]. The vitamin C content gradually reduced in control fruit during storage, which was promoted by the temperature fluctuation (
Figure 5F). In particularly, The vitamin C content of fruits treated with TF2ºC-5ºC and TF2ºC-7ºC decreased by 33% and 48% at 7 d, respectively.
Active respiration and high ethylene release cause the weight loss of fruit, furthermore, water loss is the predominant factor of mass loss. TF not only promoted respiration and ethylene release, but also significantly facilitated the mass loss of kiwifruit. The increase of total soluble solids by the treatment of TF during the first 14 d of storage may be due to the loss of water. The trend of TSS content in kiwifruits was the same as that in tomato [
35]. While, the reduce of vitamin C content was promoted by the TF, which was opposite to that of tomato [
35]. The rapid reduction of vitamin C may be as a substrate of the rapid metabolism stimulated by TF. Overall, the stable temperature is conducive to the maintenance of good fruit quality.
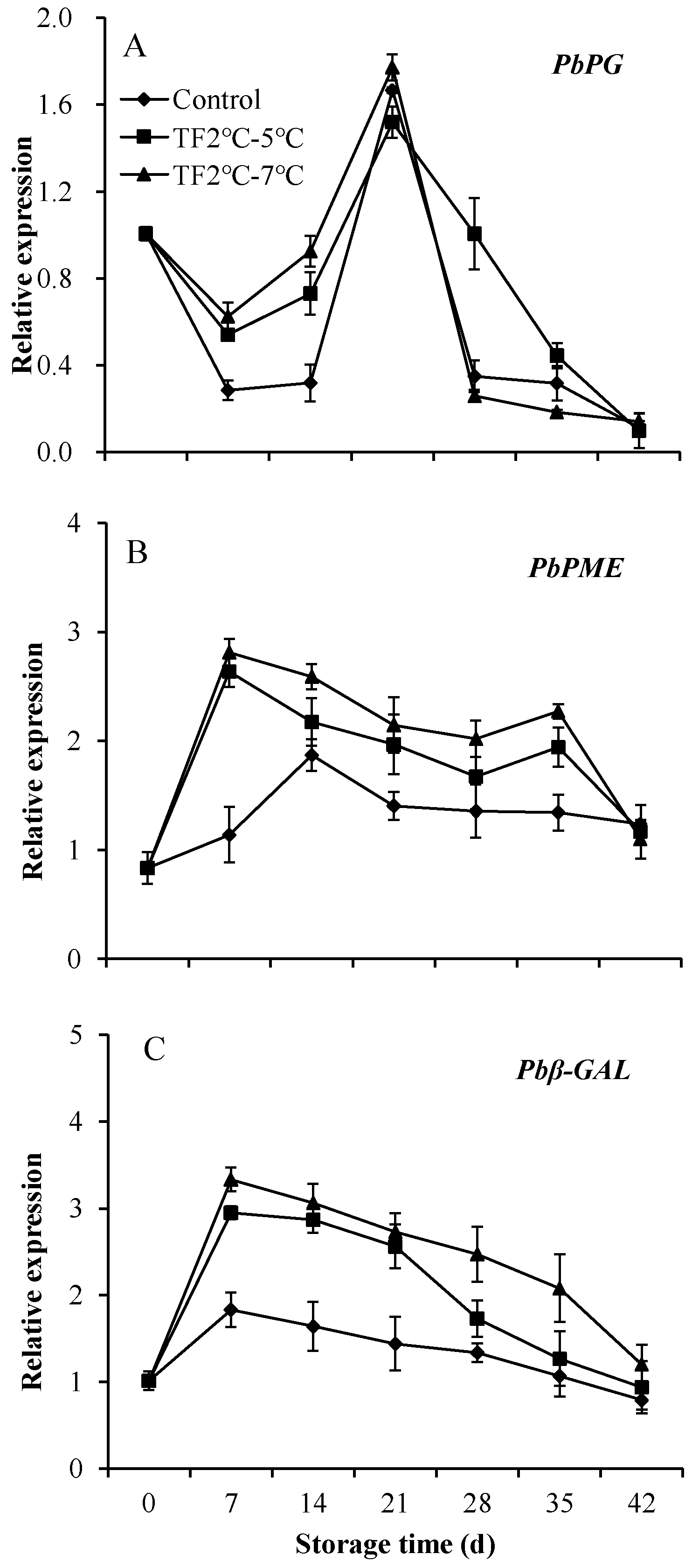
3.4. Effect of temperature fluctuation on gene expreesion of kiwifruit during storage
A few of the enzymes that work to change the structure of cell walls, especially the hydrolysis of pectin, including polygalacturonase (PG), pectin methyl esterase (PME) and β-galactosidase (β-Gal) [
38], which was significantly associated with softening. To investigate the factors influencing softening, changes in the expression levels of genes encoding these enzymes were measured by RT-qPCR. In the control group, relative expression of
PbPG decreased during the first 7 days, then rose to a peak on 21 d, and then declined again until the end of storage (
Figure 6A). The treatment of TF2ºC-7ºC promoted the expression of
PbPG in the early stages of storage. The relative expression of
PbPE in control fruits peaked at 14 d and then gradually decreased (
Figure 6B), while TF led to earlier peaks and increased peak values. The relative expression of
PbPE in TF2ºC-5ºC and TF2ºC-7ºC fruits mainly higher than that of control fruits during storage. The relative expression of
Pbβ-Gal in control kiwifruits slowly increased during the first 7 d of storage and then decreased (
Figure 6C). The relative expression of
Pbβ-Gal in the TF2ºC-5ºC and TF2ºC-7ºC groups was 1.6-fold higher and 1.8-fold higher than that in the control group at 7 d, respectively. One of the fundamental causes of softening is the structural development of pectin in the primary wall and intercellular layer of fruit under the influence of enzymes associated with cell wall metabolism, including PG, PME, and β-Gal [
39,
40]. TF accelerated the expression of
PG,
PME, and
β-Gal, which further promoted the softening of kiwifruits.
4. Conclusions
The effect of TF on the quality of kiwifruit during storage was studied. TF treatments significantly promoted the production of ethylene and CO2, which accelerated the loss of weight and vitamin C. Furthermore, the expression of PbPG, PbPME and Pbβ-GAL was up-regulated by the treatment of TF compared to the control. Hence, the softening, translucency and relative conductivity of kiwifruits were aggravated. Therefore, in order to maintain better post-harvest quality and prolong storage of kiwifruits, TF should be avoided as much as possible. The results of this study provide a theoretical basis to elucidate the mechanism underlying the effect of low-temperature fluctuation on kiwifruit quality.
Author Contributions
Ranran Xu: Data curation, formal analysis, investigation, writing-original draft preparation. Qian Chen: Writing-review and editing. Yizhao Zhang: Investigation. Jiali Li: Validation. Jiahua Zhou: Writing - review & editing. Yunxiang Wang: Validation. Hong Chang: Formal analysis. Fanxiang Meng: Validation. Baogang Wang: Conceptualization, writing-review & editing, supervision, project administration, funding acquisition.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31971808); The modern agricultural industry technology system (CARS-30); The Collaborative Innovation Center of Beijing Academy of Agricultural and Forestry Sciences (KJCX201915).
Conflicts of Interest
The authors declare no financial or personal conflicts of interest.
References
- Han, X.; Wang, X.; Shen, C.; Mo, Y.; Tian, R.; Mao, L.; Luo, Z.; Yang, H. Exogenous ABA promotes aroma biosynthesis of postharvest kiwifruit after low-temperature storage. Planta 2022, 255, 82. [Google Scholar] [CrossRef]
- Mai, Y.; Zhuang, Q.; Li, Q.; Du, K.; Wu, D.; Li, H.; Xia, Y.; Zhu, F.; Gan, R. Ultrasound-Assisted Extraction, Identification, and Quantification of Antioxidants from ‘Jinfeng’ Kiwifruit. Foods 2022, 11(6). [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Guo, W.; Du, R.; Zhou, Y. Optical properties of different kiwifruit cultivars (Actinidia deliciosa and Actinidia chinensis) and their correlation with internal quality. Infrared Phys. Technol. 2022, 123, 104113. [Google Scholar] [CrossRef]
- Hyun, J.; Lee, J.G.; Yang, K.; Lim, So.; Lee, E.J. Postharvest Fumigation of (E)-2-Hexenal on Kiwifruit (Actinidia chinensis cv. ‘Haegeum’) Enhances Resistance to Botrytis cinerea. Postharvest Biol. Technol. 2022, 187, 111854. [Google Scholar] [CrossRef]
- Eltorki, M.; Leong, R.; Ratcliffe, E. A241 kiwifruit and kiwifruit extracts for treatment of constipation: A systematic review and meta-analysis. J. Can. Asso.c Gastroenterol. 2022, 5 (Supplement1), 133–134. [Google Scholar] [CrossRef]
- Jiao, J.; Jin, M.; Liu, H.; Suo, J.; Yin, X.; Zhu, Q.; Rao, J. Application of melatonin in kiwifruit (Actinidia chinensis) alleviated chilling injury during cold storage. Sci. Hortic. 2022, 296, 110876. [Google Scholar] [CrossRef]
- Choi, H.R.; Baek, M.W.; Cheol, L.H.; Jeong, C.S.; Tilahun, S. Changes in metabolites and antioxidant activities of green ‘Hayward’ and gold ‘Haegeum’ kiwifruits during ripening with ethylene treatment. Food Chem. 2022, 384, 132490. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.G.; Lim, S.; Lee, E.J. A comparison of physicochemical and ripening characteristics of golden-fleshed ‘Haegeum’ and green-fleshed ‘Hayward’ kiwifruit during storage at 0 °C and ripening at 25 °C. Postharvest Biol. Technol. 2023, 196, 112166. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Sun, J.; Liu, Y.; Sujata, S.; Wu, Y.; Zhao, L. Effects of Actinidia yellowing ringspot virus on the yield and quality of kiwifruit. Plant Dis. 2021, 106, 800–804. [Google Scholar] [CrossRef]
- Liu, H.; Lv, Z.; Yang, W.; Li, A.; Liu, J.; Zhang, Q.; Jiao, Z. Virtual cold chain method to evaluate the effect of rising temperature on the quality evolution of peach fruit. Foods 2023, 12, 2403. [Google Scholar] [CrossRef]
- Dermesonluoglu, E.; Katsaros, G.; Tsevdou, M.; Giannakourou, M.; Taoukis, P. Kinetic study of quality indices and shelf life modelling of frozen spinach under dynamic conditions of the cold chain. J. Food Eng. 2015, 148, 13–23. [Google Scholar] [CrossRef]
- Vicent, V.; Ndoye, F.T.; Verboven, P.; Nicolaï, B.M.; Alvarez, G. Quality changes kinetics of apple tissue during frozen storage with temperature fluctuations. Int. J. Refrig. 2018, 92, 165–175. [Google Scholar] [CrossRef]
- Tao, Y.; Guo, Y.; Li, J.; Ye, K.; Zhang, Y.; Zeng, X.; Dou, H. Effect of temperature fluctuation during superchilling storage on the microstructure and quality of raw pork. Meat Sci. 2023, 198, 109096. [Google Scholar] [CrossRef] [PubMed]
- Ndraha, N.; Hsiao, H.; Vlajic, J.; Yang, M.F.; Lin, H.T.V. Time-temperature abuse in the food cold chain: Review of issues, challenges, and recommendations. Food Control 2018, 89, 12–21. [Google Scholar] [CrossRef]
- Tano, K.; Oulé, M.K.; Doyon, G.; Lencki, R.W.; Arul, J. Comparative evaluation of the effect of storage temperature fluctuation on modified atmosphere packages of selected fruit and vegetables. Postharvest Biol. Technol. 2007, 46, 212–221. [Google Scholar] [CrossRef]
- Chen, L.; Fan, K. Pulsed vacuum impregnated trehalose to improve the physicochemical quality of frozen-thawed kiwifruit. Int. J. Food Sci. Technol. 2021, 57, 268–275. [Google Scholar] [CrossRef]
- Chai, J.; Wang, Y.; Liu, Y.; Yong, K.; Liu, Z. 1-MCP extends the shelf life of ready-to-eat ‘Hayward’ and ‘Qihong’ kiwifruit stored at room temperature. Sci. Hortic. 2021, 289, 110437. [Google Scholar] [CrossRef]
- Xu, R.; Wang, L.; Li, K.; Cao, J.; Zhao, Z. Integrative transcriptomic and metabolomic alterations unravel the effect of melatonin on mitigating postharvest chilling injury upon plum (cv. Friar) Fruit. Postharvest Biol. Technol. 2022, 186, 111819. [Google Scholar] [CrossRef]
- Shu, H.; Wang, Y.; Li, K.; He, L.; Ding, L.; Zhan, R.; Chang, S. Accumulation of sugars and liquid in apoplast of fruit flesh result in pineapple translucency. Am. J. Plant Sci. 2022, 3, 576–587. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Y.; Zeng, H.; Zhang, X. Integrated Metabolome and transcriptome analysis reveals a potential mechanism for water accumulation mediated translucency in pineapple (Ananas comosus (L.) Merr.) fruit. Int. J. Mol. Sci. 2023, 24, 7199. [Google Scholar] [CrossRef]
- Luengwilai, K.; Beckles, D.M.; Siriphanich, J. Postharvest internal browning of pineapple fruit originates at the phloem. J. Plant Physiol. 2016, 202, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Techavuthiporn, C.; Boonyaritthongchai, P.; Supabvanich, S. Physicochemical changes of ‘Phulae’ pineapple fruit treated with short-term anoxia during ambient storage. Food Chem. 2017, 228, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Gai, Q.; Wang, C. Optimization and comparison of detection methods of vitamin C in dark fruits and vegetables. Food and Nutrition in China 2017, 23, 38–42. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, W.; Gong, H.; Yang, Y.; Zhang, A.; Liu, H.; Cao, J.; Guo, F.; Cui, K. Cell wall polysaccharides degradation and ultrastructure modification of apricot during storage at a near freezing temperature. Food Chem. 2019, 300, 125194. [Google Scholar] [CrossRef]
- Chen, L.; Pan, Y.; Jia, X.; Wang, X.; Yuan, J.; Li, X. Constant storage temperature delays firmness decreasing and pectin solubilization of apple during post-harvest storage. J. Food Process Pres. 2021, 45, e15655. [Google Scholar] [CrossRef]
- Pan, Y.; Li, X.; Jia, X.; Zhao, Y.; Li, H.; Zhang, L. Storage temperature without fluctuation enhances shelf-life and improves postharvest quality of peach. J. Food Process Pres. 2019, 43, e13881. [Google Scholar] [CrossRef]
- Tao, J.; Jia, H.; Wu, M.; Zhong, W.; Jia, D.; Wang, Z.; Huang, C. Genome-wide identification and characterization of the TIFY gene family in kiwifruit. BMC Genom. 2022, 23, 179. [Google Scholar] [CrossRef]
- Liang, S.; Kuang, J.; Ji, S.; Chen, Q.; Deng, W.; Min, T.; Shan, W.; Chen, J.; Lu, W. The membrane lipid metabolism in horticultural products suffering chilling injury. Food Quality and Safety 2020, 4, 9–14. [Google Scholar] [CrossRef]
- Ming, J.; Li, Y.; Jia, Y.; Ru, J.; Ling, H.; Cui, H. Effect of heat shock and potassium sorbate treatments on gray mold and postharvest quality of ‘XuXiang’ kiwifruit. Food Chem. 2020, 324, 126891. [Google Scholar]
- Mohebbi, S.; Babalar, M.; Zamani, Z.; Askari, M.A. Influence of early season boron spraying and postharvest calcium dip treatment on cell-wall degrading enzymes and fruit firmness in ‘Starking Delicious’ apple during storage. Sci. Hortic. 2020, 259. [Google Scholar] [CrossRef]
- Guillén, F.; Medina-Santamarina, J.; García-Pastor, M.E.; Chen, N.J.; Uruu, G.; Paull, R.E. Postharvest melatonin treatment delays senescence and increases chilling tolerance in pineapple. LWT 2022, 169, 113989. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, Z.; Zhang, Y.; Shi, X.; Chen,F. ; Liu, K. Effect of temperature fluctuation on colour change and softening of postharvest sweet cherry. RSC Adv. 2021, 11, 22969–22982. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Bao, Y.; Zhao, Y.; Liu, Y.; Zheng, Y.; Feng, Z.; Jin, P. Cold shock treatment enhances cold tolerance in peach fruit through modulating PpbZIP9 and PpVIP1-mediated respiratory metabolism. Postharvest Biol. Technol. 2023, 204, 112421. [Google Scholar] [CrossRef]
- Chen, L.; Pan, Y.; Li, H.; Liu, Z.; Jia, X.; Li, W.; Jia, H.; Li, X. Constant temperature during postharvest storage delays fruit ripening and enhances the antioxidant capacity of mature green tomato. J. Food Process. Pres. 2020, 44, e14831. [Google Scholar] [CrossRef]
- Jia, L.; John, F. Kennedy.; Xiao, F.; Yin, H.; Wei, C.; Zhuo, C.; Xian, W.; Xue, H. Preparation of alginate oligosaccharide and its effects on decay control and quality maintenance of harvested kiwifruit. Carbohydr. Polym. 2020, 242, 116462. [Google Scholar]
- Liao, G.; Liu, Q.; Li, Y.; Zhong, M.; Huang, C.; Jia, D.; Xu, X. Identification and expression profiling analysis of ascorbate peroxidase gene family in Actinidia chinensis (Hongyang). J. Plant Res. 2020, 133, 715–726. [Google Scholar] [CrossRef]
- Chea, S.; Yu, D.J.; Park, J.; Oh, H.D.; Chung, S.W.; Lee, H.J. Fruit softening correlates with enzymatic and compositional changes in fruit cell wall during ripening in ‘Bluecrop’ highbush blueberries. Sci. Hortic. 2019, 245, 163–170. [Google Scholar] [CrossRef]
- Liang, J.; Ren, Y.; Wang, Y.; Han, M.; Yue, T.; Wang, Z.; Gao, Z. Physicochemical, nutritional, and bioactive properties of pulp and peel from 15 kiwifruit cultivars. Food Biosci. 2021, 42, 101157. [Google Scholar] [CrossRef]
- Gwanpua, S.G.; Verlinden, B.E.; Hertog, M.L.A.T.M.; Nicolai, B.M.; Hendrickx, M.; Geeraerd, A. A transcriptomics-based kinetic model for enzyme-induced pectin degradation in apple (Malus×domestica) fruit. Postharvest Biol. Technol. 2017, 130, 64–74. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).