1. Introduction
Exposure to air pollution has, in a wide range of studies, been shown to have detrimental long-term health effects. According to the World Health Organization, ambient (outdoor) air pollution is estimated to have caused around 4.2 million premature deaths worldwide in 2019 [
1]. The long-term mortality effects associated with exposure to PM
2.5 and PM
10 have been analyzed in several previous studies. In a meta-analysis, reporting pooled estimates of the results from different studies around the world, natural-cause mortality was associated with long-term exposure to both PM
2.5 and PM
10 [
2]. Increases in all-cause mortality associated with exposure to PM
2.5 were found in other meta-analyzes including studies from a wide range of geographic areas [
3,
4]. In Europe, associations between mortality and long-term exposure to PM
2.5 were found in two multi-cohort studies [
5,
6]. Furthermore, in a systematic review based on cohort studies conducted in Australia, Mainland China, Hong Kong, Taiwan, and South Korea, an association between long-term exposure to PM
2.5 and mortality was found [
7]. Considering Sweden specifically, a multi-cohort study demonstrated a statistically significant increase in natural mortality associated with long-term exposure to PM
10, and for PM
2.5, the effect was positive, but not statistically significant [
8].
The long-term effects on mortality associated with exposure to black carbon (BC), colloquially called soot particles, have been analyzed in a few studies. BC refers to the carbonaceous fraction of particles originating from incomplete combustion, and they are usually measured by light absorption. In a large population-based French cohort, with data collected from 1989‒2017, long-term exposure to BC was associated with all-cause mortality [
9]. Long-term exposure to black smoke (BS), a previously common technique to measure soot particles, has been used in older cohort studies. One such cohort study, spanning seven cities in France, found an association between non-accidental mortality and exposure to BS (assessed in the 1970s) over a 25 year study period [
10]. Similarly, a Dutch cohort study on long-term exposure to traffic-related air pollutants, analyzed from 1987‒1996, demonstrated an association between natural mortality and exposure to BS [
11].
In addition to particulate air pollution, the mortality effects associated with long-term exposure to gaseous pollutants, including nitrogen oxides (NO
x), the sum of nitrogen monoxide (NO) and nitrogen dioxide (NO
2), and NO
2 itself, have been investigated in previous studies. For instance, associations between mortality and long-term exposure to NO
2 have been shown in several meta-analyzes based on pooled estimates from many original studies [
3,
12,
13,
14,
15]. The mortality effects associated with long-term exposure to NO
x were analyzed in a cohort in Gothenburg, Sweden, during the period from 1973‒2007, and associations were found for three different time lag windows [
16].
The long-term health effects associated with exposure to air pollutants have been explored in previous studies in a cohort in Malmö, Southern Sweden, called “The Malmö Cancer and Diet Cohort (MDC)”. In this cohort, air pollution concentrations were assessed at the participants' home addresses. The associations between cardiovascular diseases and exposure to NO
x and particles were analyzed in the MDC cohort during the period from 1991‒2016. Several statistically significant hazard ratios between exposure to NO
x or particles and cardiovascular outcomes were found [
17]. The long-term associations between a number of biomarkers and exposure to NO
x and particles were also analyzed in the MDC cohort, where several statistically significant associations were found [
18]. Statistically significant associations between long-term exposure to NO
x and particles and chronic kidney disease have also been shown in the MDC cohort [
19].
In the present study, the aim was to analyze the associations between long-term exposure to PM10, PM2.5, BC, and NOx and mortality in the MDC cohort.
2. Materials and Methods
2.1. Description of the Study Population and the Study period
This study is based on “The Malmö Diet and Cancer Cohort”, abbreviated MDC, which included those residing in Malmö, Sweden, born during the period from 1923‒1950. Initially, the main purpose of this cohort was to clarify whether a Western diet is associated with specific types of cancer while controlling for other relevant lifestyle factors [
20]. The MDC cohort has been described in more detail in a number of previous studies [
20,
21,
22,
23].
At enrollment, during the period from 1991‒1996, the participants underwent health screening in terms of questionnaires about lifestyle and diet, clinical examination, and blood sampling where a total number of 30,438 participants were selected. The cohort participants were followed from enrollment until death, or until the end of year 2016 for those who survived the entire study period. Among the total number of 30,438 participants, 17,551 (57.5%) survived the entire study period, 12,663 (41.6%) were deceased during the study period, and 264 (0.9%) moved out of the study area. The cohort participants who survived the entire study period and those who moved out of the study area were censored.
2.2. Air Pollution Exposure Assessment
The modeled concentrations of PM
10, PM
2.5, BC, and NO
x at the cohort participants' home addresses were used to calculate exposure. The air pollution concentrations in Malmö (18 km x 18 km) were modeled by the Environmental Department of the City using EnviMan software package (Opsis AB, Sweden) (
Figure 1). Each pollutant was modeled as the sum of local emissions from traffic exhaust, non-exhaust traffic emissions (mechanically generated particles from road wear, tire wear, and brake wear, including resuspension), heating, shipping, industry, households, and long-distance transported emissions. The modeled concentrations were presented as grids with a spatial resolution of 50 x 50 m. The participants` addresses during the period from 1991‒2016 were retrieved and geocoded by Statistics Sweden. The mean concentrations of PM
10, PM
2.5, BC, and NO
x for each year during the period from 1991‒2016 were assigned to each participant´s home address. In order to capture the lag effects associated with exposure and mortality, the modeled concentrations were based on the exposure lag windows of the same year (lag0), 1‒5 years (lag1‒5), and 6‒10 years (lag6‒10) (
Table 1).
2.3. Outcome Assessment
Natural cause mortality (mortality due to causes other than injuries and trauma) was used as an outcome variable, and data were retrieved from the National Cause of Death Register. Natural cause mortality was defined on the basis of the underlying cause of death according to the International Classification of Diseases, Tenth Revision (ICD-10: A00‒R99).
2.4. Covariates
All covariates were collected at enrollment. Adjustments for potential confounders were made with age and gender as two basic covariates. Education level (low < 9 years, medium = 9‒12 years, and high > 12 years) and cohabitation (yes or no) were included as adjustments for socio-economic factors. Smoking habits were included as never, former, or current smoker (occasional and regular). Systolic and diastolic blood pressure as well as the use of antihypertensive medications (yes or no) were included as covariates. Alcohol consumption was included as the self-reported quantity in terms of grams per day. Physical activity was categorized into low, medium, or high. Waist/hip ratio was also included as a covariate.
Table 2 and
Table 3 present the different covariates included in the models.
Table 2 presents the continuous variables included in the models, and
Table 3 presents the categorical variables included in the models.
2.5. Statistical Analysis
Cox proportional hazard models with time-varying air pollution exposure adjusting for possible confounders (covariates) were used to calculate the associations between long-term exposure to PM10, PM2.5, BC, and NOx and time until death among the MDC cohort participants. The time since the start of the study for each study participant was used on the time axis.
Three statistical models with varying levels of adjustment were used. Model 1 represented a crude model with air pollutants adjusted only for age at enrollment and gender. Model 2 additionally adjusted for smoking habits, educational level, and cohabitation. In addition to the covariates included in Model 1 and Model 2, Model 3 also adjusted for systolic and diastolic blood pressure, self-reported alcohol consumption, physical activity, waist/hip ratio, and the use of antihypertensive medications.
Two-pollutant models were included in cases where the pairwise air pollutants were not so highly correlated (i.e., with a correlation coefficient smaller than 0.8) that multicollinearity could occur. Multicollinearity was tested by creating a multiple regression based on all variables in each model, and the variance inflation factor (VIF) was then calculated. A VIF value below three was accepted for the inclusion in two-pollutant models. The VIF values of the air pollutants were in some cases above the value of three. Regarding the other covariates, the VIF values were below three in all cases and were, therefore, not considered to pose a multicollinearity problem.
Table A8 in
Appendix A presents correlation coefficients (Pearson) between the different air pollutants based on the exposure lag windows of the same year (lag0), 1‒5 years (lag1‒5), and 6‒10 years (lag6‒10).
All analyzes were performed using STATA 17.0 (StataCorp, Texas, TX, USA).
3. Results
3.1. Hazard Ratios Associated with Exposure
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
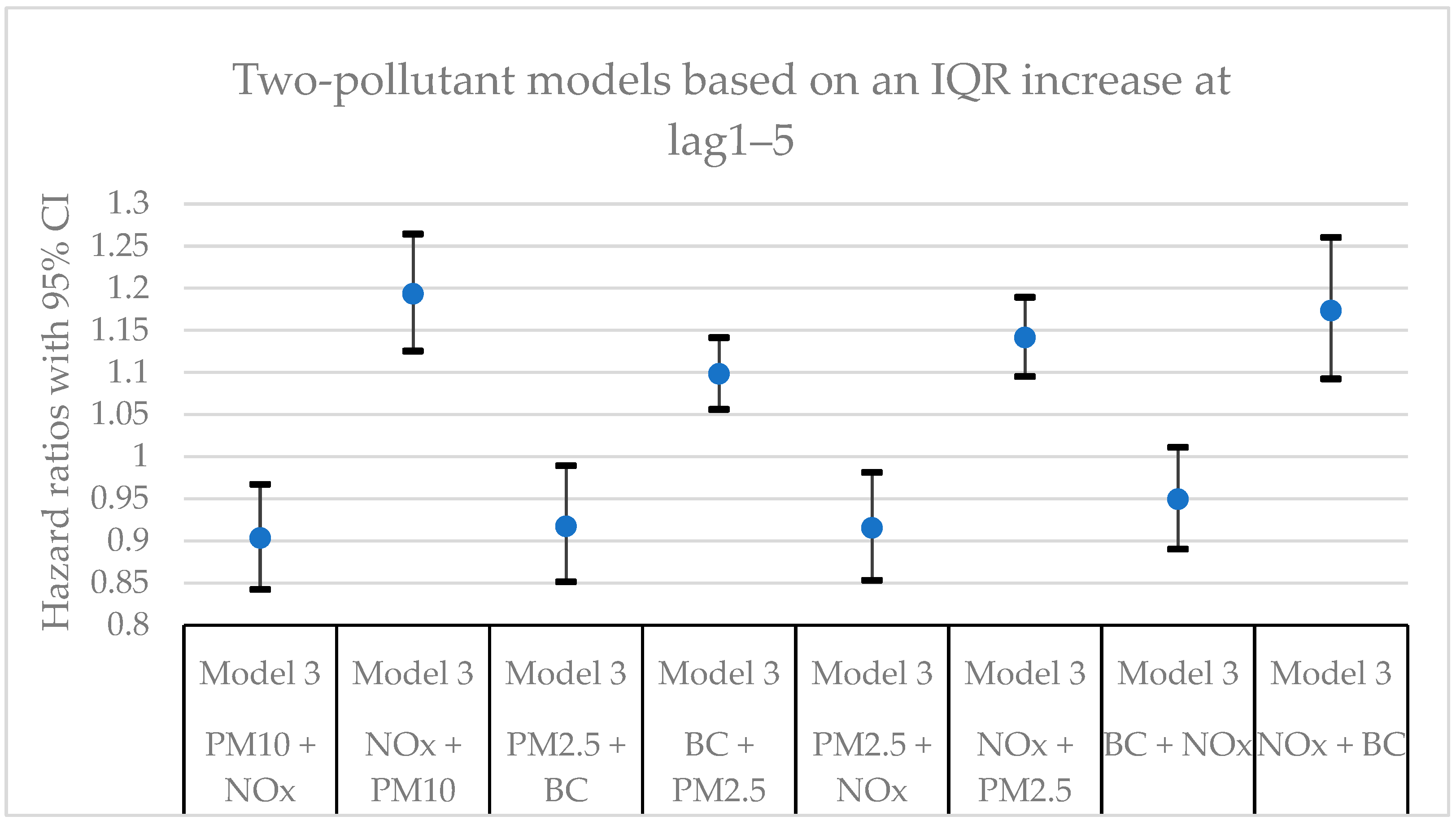
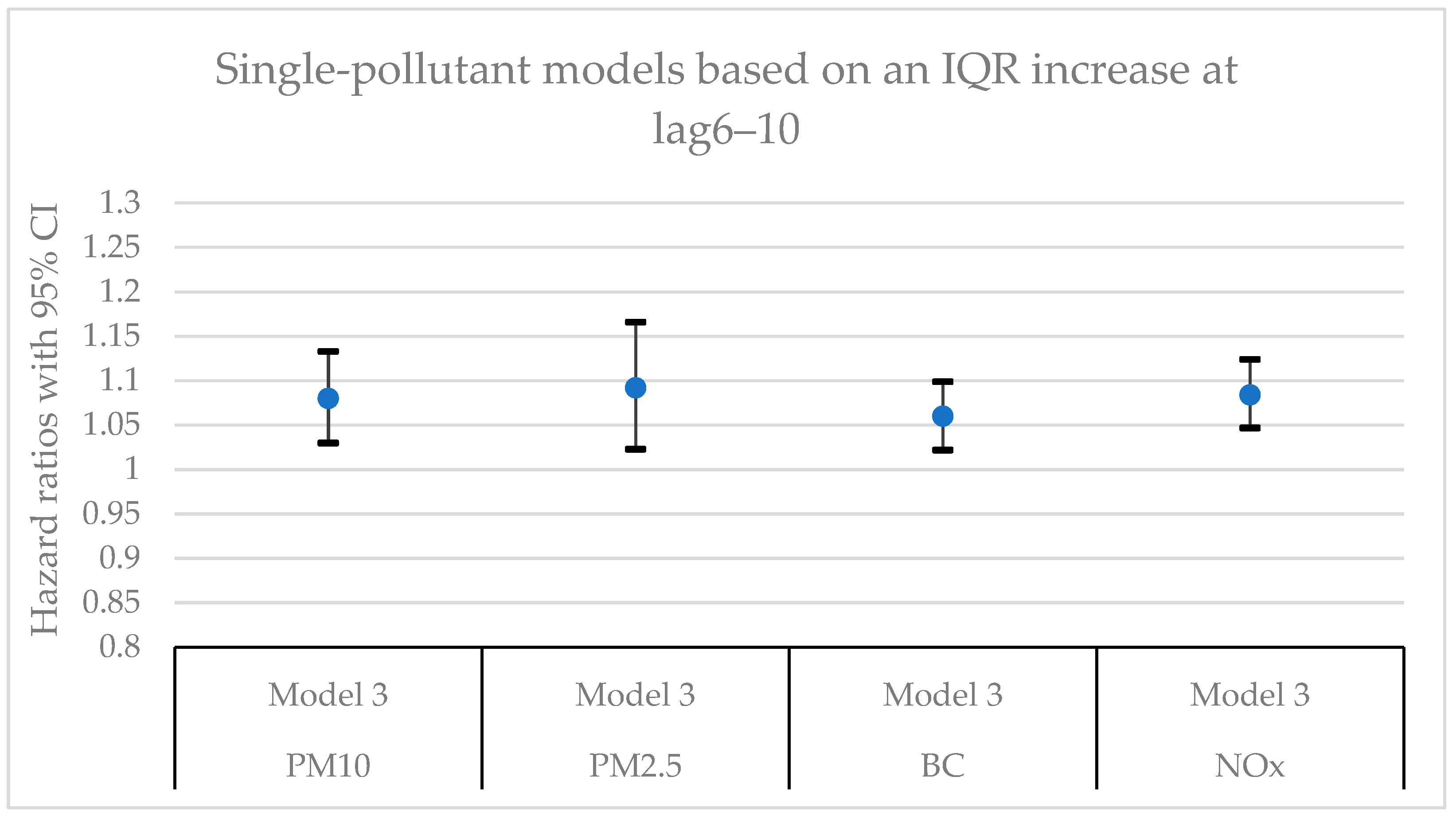
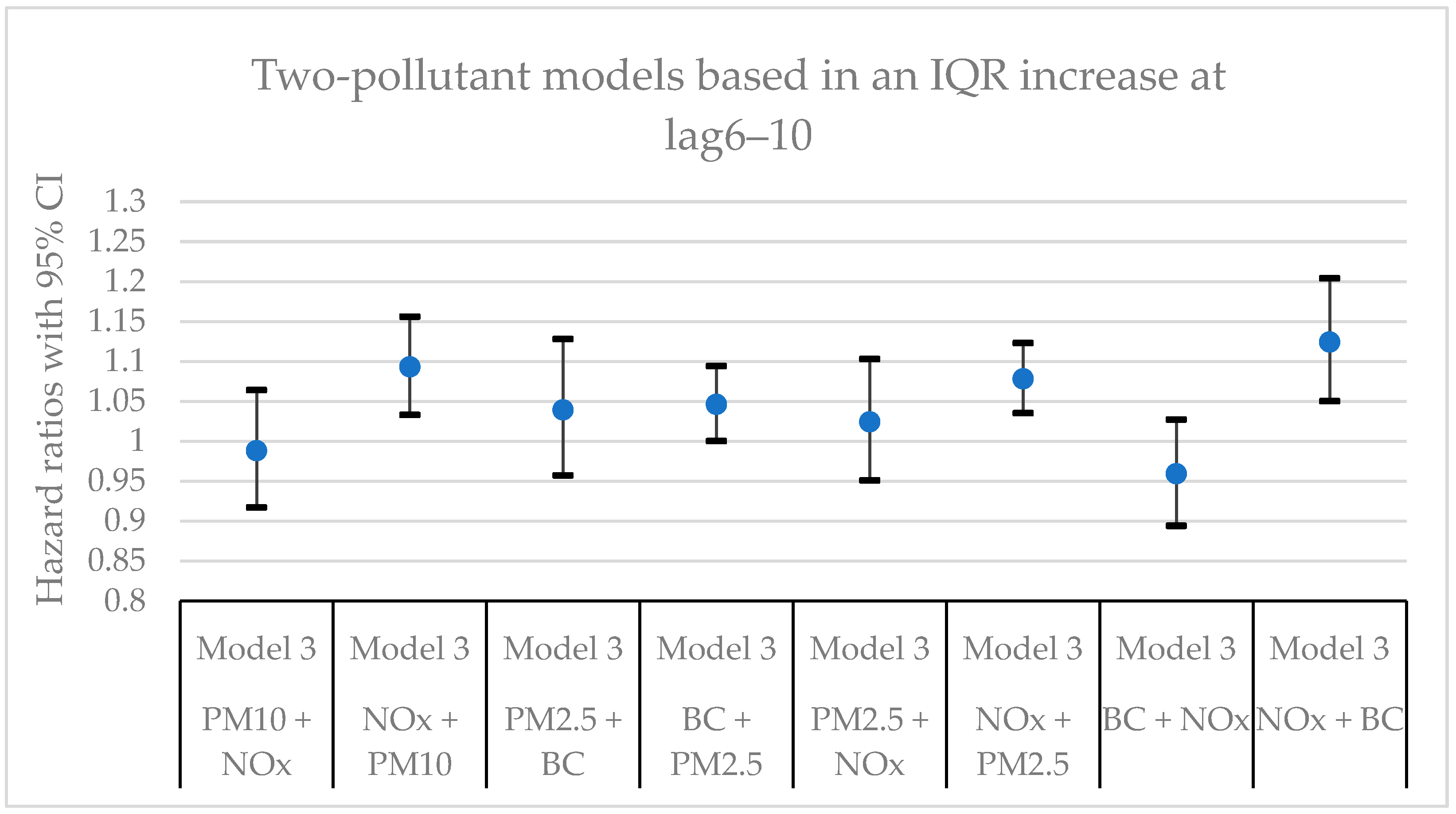
Figure 7 present hazard ratios (HRs) with 95% confidence intervals (CIs) for natural-cause mortality associated with exposure to PM
10, PM
2.5, BC, and NO
x for lag0, lag1‒5, and lag6‒10 in single- and two-pollutant models with adjustments for all covariates (Model 3). The HRs correspond to an interquartile range (IQR) increase in each air pollutant. The complete results from the Cox regressions based on all models (Models 1‒3) and all lag windows in both single- and multi-pollutant models are presented in
Table A1,
Table A2 and
Table A3.
3.2. Sensitivity analysis
In order to determine if there were any differences in the calculated HRs with respect to age, an age stratified analysis was performed. Six different age groups at enrollment were generated: Group 1 ≤ 50 years; 50 years < Group 2 ≤ 55 years; 55 years < Group 3 ≤ 60 years; 60 years < Group 4 ≤ 65 years; 65 years < Group 5 ≤ 70 years; and Group 6 > 70 years. The Cox regression model with adjustments for all covariates (Model 3) was applied for each age group based on lag1‒5 in both single- and two-pollutant models (see
Table A4 and
Table A5 in
Appendix A). The HRs were in general largest and most robust in age groups 4 and 5.
The regression models were also divided into two different periods in terms of survival time; one period for those who passed away within ten years from when the study started, and one period for those who survived more than ten years. The Cox regression model with adjustments for all covariates (Model 3) was applied for each survival time period based on lag1‒5 in both single- and two-pollutant models (see
Table A6 and
Table A7 in
Appendix A). Similar to the main models (
Table A1,
Table A2 and
Table A3), the HRs for PM
10, PM
2.5, and BC did not remain statistically significant in the two-pollutant models together with NO
x. The HRs for NO
x were more stable and robust for those who survived more than ten years.
4. Discussion
4.1. Key Results
In this cohort study from southern Sweden with > 30,000 participants from the general population and up to 25 years of follow-up, clear associations were observed between mortality and long-term residential exposure to NOx. NOx was the only air pollutant that exhibited statistically significant associations in all models and for all lags in the main models. The associations for the other air pollutants (PM10, PM2.5, and BC) did not remain statistically significant in the two-pollutant models with NOx. Adjustments for lifestyle factors and other possible confounding factors had some impact on the size of the effect estimates, but, in general, the associations remained.
4.2. The Calculated Hazard Ratios and Possible Explanations
Among the analyzed air pollutants, the HRs associated with NO
x were the most clear and robust. NO
x is an indicator of traffic emissions, and traffic is typically the major source of NO
x in urban areas [
24]. The HRs associated with PM
10, PM
2.5, and BC were in most cases statistically significant in the single-pollutant models. However, in the two-pollutant models with NO
x, no statistically significant positive were shown neither for PM
10, PM
2.5, or BC. Both NO
x and BC originate from combustion processes. However, the correlation coefficients between NO
x and BC, presented in
Table A8, were in the range of 0.4 and 0.5, indicating that they largely originate from different sources. Besides traffic, there are several sources that generate combustion-related emissions. BC can also originate from heating, shipping, industry, households, and long-distance transported emissions, and may therefore be modestly correlated with NO
x which mainly originates from traffic. The HRs associated with BC were also statistically significant in all single-pollutant models and all two-pollutant models except for those including NO
x.
In the age stratified analyzes presented in
Table A4 and
Table A5, no consistent patterns were shown. However, a certain pattern in terms of clearer and more robust HRs for group 4 and 5 were shown in several analyzes. Group 4 and 5 represent those in the age groups 60‒65 years and 65‒70 years at enrollment, respectively. The HRs in the younger age groups and those in the oldest group (> 70 years) were somewhat less clear and robust. Smaller effects in the younger age groups can be expected. Smaller effects in the oldest age group are somewhat unexpected. However, the oldest age group (> 70 years at enrollment) were in some cases older than 90 years old at the end of the cohort time period, and it is possible that air pollution exposure could have a relatively smaller impact on survival time when other age-related causes of death become more apparent.
Considering the different lags, the HRs for particles (PM
10, PM
2.5, and BC) in the single-pollutant models were in general more robust and stable at lag1‒5 and lag6‒10 compared to lag0. However, the HRs for NO
x in the single-pollutant models were in the same order of magnitude for all lag windows. The larger effects at lag1‒5 and lag6‒10 compared to lag0 for particles indicate that there could be a noticeable delay effect between exposure and mortality. Another possible explanation is that the particles were more toxic further back in time. When comparing the HRs between two survival time periods (0‒10 years and >10 years) shown in
Table A6 and
Table A7, the HRs for particles were in general larger at the survival time period of 0‒10 years, while the HRs for NO
x were larger for the survival time period of >10 years. This could possible mean that the PM
10 and PM
2.5 were more toxic during the first ten years of the cohort period. However, when considering all analyzes in this study, the HRs for PM
10 and PM
2.5 were in most cases statistically significant in the single-pollutant models, but they were non-significant, or in some cases negatively statistically significant, in the two-pollutant models. Unspecified particulate matter (PM
10 and PM
2.5) does not constitute a uniform measure of particles with respect to their chemical composition and physical properties. They originate from a variety of sources that may have large spatial and temporal variations within a city. Given that this study has analyzed the exposure effect over a period of up to 25 years, the chemical composition and physical properties of the particles, and likewise their toxic potential, to which the cohort participants have been exposed, most likely varied greatly during this time. Seasonal variations in the above-mentioned factors can also be assumed to have occurred. Road dust, which is most common during springtime, has been found to be particularly harmful to the human health [
25]. Indeed, exposure to PM
2.5-10 and PM
10 were associated with increased mortality during springtime, but not during the rest of the year, as has been shown in time-series studies performed in Stockholm [
26,
27]. The low correlations between NO
x and PM (both PM
10 and PM
2.5) in this study (
Table A8) indicate that PM
10 and PM
2.5 did not to any great extent originate from traffic. The toxic fractions of PM including road dust could be more highly correlated with NO
x, and may therefore not contribute much to the HRs when unspecified PM are used as exposure metrics.
4.3. NOx as an Indicator for Other Harmful Exposures
The largest and most robust hazard ratios in this study were shown for NO
x; however, some uncertainty remains regarding the toxicity of NO
x itself. Experimental studies with humans have demonstrated noticeable health effects after short-term exposure to NO
2 at concentrations at or above 400 µg m
-3, and health effects among patients with mild asthma could not be detected at concentrations below 200 µg m
-3 [
28]. Based on a review of several long-term studies on NO
2 exposure, increased mortality was suggested above a threshold value of 20 µg m
-3 [
29]. The modeled mean concentrations of NO
x (NO + NO
2) during the study period in this study were in the range of 25‒30 µg m
-3, and a large number of study participants may therefore have been exposed to concentrations of NO
2 that have exceeded 20 µg m
-3 during a long period of time. However, the toxicity of NO
2 itself, and its impact on mortality, has been addressed in two literature reviews. One study indicates that there is an independent effect on long-term mortality associated with exposure to NO
2 [
13]. Contrary, the other study indicates that the greater the demands placed on the studies, the less support there is for an independent effect on long-term mortality associated with exposure to NO
2 [
15]. It is thus uncertain to what extent NO
x itself would have caused the robust and statistically significant associations with mortality observed in the present study. Indeed, some other component(s) of vehicle exhaust, with similar dispersion pattern, may have been driving the negative health effects.
As previously discussed, NO
x is an established proxy for road traffic exhaust emissions. Vehicle exhaust is not a homogeneous substance, however, it is comprised of harmful components other than NO
x. For example, high correlations between NO
x and particle number count (PNC) have been shown in Gothenburg, Sweden [
30], and between NO
2 and PNC in Stockholm, Sweden [
31]. NO
x can, therefore, be considered a marker for PNC. PNC, in turn, is a marker for ultrafine particles (particles with an aerodynamic diameter smaller than or equal to 100 nm in all dimensions). Due to their small size and large surface area in relation to volume, ultrafine particles are believed to be more toxic than larger particles [
32]. From a health perspective, particles smaller than 300 nm are especially important since they are capable of diffusing rapidly in the airway mucus through the mucus pores [
33]. Ultrafine particles may, thus, be a contributing factor to the negative health effects of exposure to traffic exhaust emissions. However, fine and ultrafine particles from traffic are not only emitted from combustion processes, but can also be derived from brake and tire wear [
34].
Traffic noise is another health risk that can be correlated with exposure to NO
x. The correlation between NO
x and noise in urban areas is determined by several factors. The short-term correlations between NO
x or NO
2 and noise were analyzed in a study based on 103 urban sites with varying traffic, environment, and infrastructure characteristics. Factors that largely determined the degree of correlation were the number of lanes on the closest road, number of cars and trucks during noise sampling, and the presence of major intersections [
35]. Based on a systematic review and meta-analysis [
36], the associations between long-term exposure to traffic noise and mortality were weak, except for mortality related to ischemic heart disease. With this, authors suggested a possible threshold of 53 decibel for cardiovascular mortality from road traffic noise [
35]. In a cohort study from Gothenburg, Sweden, positive but non-significant associations were found for cardiovascular mortality and morbidity and long-term residential exposure to noise above 60 decibel, compared to 50 decibel, after adjusting for air pollution exposure [
37]. As traffic noise has not been included as a covariate in this study, it is not possible to draw conclusions regarding its impact on mortality among the MDC cohort participants.
In summary, the association between NO
x and mortality in this study is clear and robust. However, NO
x itself is not likely to be the main driver of this association. Traffic noise is also not expected to be responsible for the observed association, and there is a great need for studies that disentangle the effects of traffic-related noise and traffic-related air pollution on ill health. The ultrafine particles originating from both exhaust and from abrasion of tires, road surfaces, and brakes are probably an important factor, but more research is needed to confirm this. As road traffic is the main source of anthropogenic NO
x emissions in Europe [
38], NO
x as well as its components and correlates will continue to be important.
4.4. Strengths and Limitations of This Study
A strength of this study is that it includes more than 30,000 participants and 25 years of follow-up, which provides a robust statistical basis both in terms of measurement data and number of participants. Exposure misclassification, a possible limitation, must be considered in all epidemiological studies on long-term exposure to air pollution. In this study, exposure was modeled at the participants’ home addresses, and other sources of air pollution, such as occupational and/or indoor exposure, were not considered. This is regarded as standard practice in epidemiological studies of air pollution’s health effects. Consequently, residential mobility among the study participants and its impact on exposure to air pollutants could not be taken into account. However, a previous study in another part of Sweden has shown that residential mobility does not seem to cause major exposure misclassification [
39]. Also, exposure misclassification would have to depend on mortality to cause bias in the present study. Exposure misclassification (assumed to be non-differential) may have reduced the precision of the estimates, but low precision is not considered to be a plausible explanation for the results in the present study.
4.5. Future Research Needs and Policy Implications
The results of this study support traffic-related air pollution as an important environmental exposure with respect to premature mortality. While the number of epidemiological studies linking traffic emissions to adverse health effects continues to grow, the relative impact of specific components of pollutant mixtures generated by combustion engines have largely been overlooked. For instance, the health effects associated with exposure to specific exhaust components, e.g., particle bound or free volatile organic compounds (VOCs) and polycyclic aromatic hydrocarbons (PAHs), remain to be clarified. The role of metals that originate from engine abrasion, lubrication oils, and from the fuels themselves [
40] and bind to exhaust particles also needs to be further explored.
Additionally, the characteristics and effects of non-exhaust emissions are becoming increasingly important. Mechanically generated particles from road abrasion and brakes are present in the coarse fraction (2.5‒10 µm), the fine fraction (≤ 2.5 µm), and the ultrafine fraction (≤ 100 nm) [
34]. Epidemiological studies analyzing the health effects associated with different chemical components of particles are not abundant. However, a time-series study conducted in the U.S., focusing on the relative risks for cardiovascular and respiratory hospital admissions associated with different chemical compositions of PM
2.5, showed that elemental carbon, vanadium, and nickel contents were associated with increased risk of hospital admissions [
41]. Moreover, a literature review on exposure to road dust particles demonstrated serious health effects, especially for the respiratory system [
25]. The components of road dust that were most frequently referenced in the reviewed studies were platinum, rhodium, bohrium, aluminum, zinc, vanadium, and polycyclic aromatic hydrocarbons [
25]. Regarding brake wear particles, a toxicological study based on cell models has shown that brake abrasion particles and diesel exhaust particles are equally capable of damaging pulmonary cells [
42].
With this, future epidemiological studies on the health effects of road traffic-related emissions should aim to include ultrafine particles and carefully adjust for different components using high-quality air pollution data.
From a policy point of view, reducing emissions from traffic has not always given rise to improved air quality in Europe and the U.S. despite long-established and progressively stringent tailpipe emission limitations. Thus, the health effects associated with different components of traffic-related emissions from combustion engines, including fuel qualities like aromatic content and metal content, still need to be addressed. These aspects influence the harmfulness of PM emissions not only from diesel, but also from gasoline- and ethanol-powered vehicles, and non-road machinery. Air pollution’s chemical components and physical composition are further modified by atmospheric processes, making their regulation more difficult. Future studies need to address the above mentioned uncertainties.
5. Conclusions
In this cohort study, with roughly 30,000 participants from the general population and almost 25 years of follow-up, clear associations were observed between natural-cause mortality and long-term exposure to modeled concentrations of NOx at the residential addresses. The robust hazard ratios for NOx indicate that traffic-related air pollution had a significant association with mortality in the MDC cohort. However, it is uncertain to what extent NOx exposure in itself is the main driver of these clear and robust hazard ratios, or if it rather is an indicator of combustion-related air pollutants including ultrafine particles and their toxic components, or road traffic noise. Hence, further research is needed to clarify the importance of specific exposures related to road traffic, air pollutants and noise, especially ultrafine particles, their chemical components, and their toxic potential.
Author Contributions
Conceptualization, H.O., L.S and A.O. formal analysis, H.O. and J-O.P.; data curation, L.S. and H.K.C.; writing—original draft preparation, H.O and A.O..; writing—review and editing ALL, supervision, A.O.; funding acquisition, A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Formas under grant agreement number 2017-00898 (How is our health affected by particles from wood burning? A. Oudin), by European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 814978 (TUBE), and by the Swedish Research Council for Sustainable Development (FORMAS, number 2016–00993; Stockfelt). Open access funding was provided by Lund University.
Institutional Review Board Statement
The study was approved by the Regional Ethics Committee at the University of Lund (dnr 2016/4).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the Lund University Medical Faculty – Malmo Diet and Cancer Cohort, but restrictions apply to the availability of these data, which were used under license for the current study, and are not publicly available. Data are however available from the authors upon reasonable request and with permission of Lund University Medical Faculty – Malmo Diet and Cancer Cohort. Ethical approval from The Swedish Ethical Review Authority is needed to access the data.
Acknowledgments
We acknowledge all the participants in the Malmö Diet and Cancer Cohort (MDC), and all the researchers and research assistants who have worked with collecting data in the project.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Table A1.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in single- and two-pollutant models with an exposure lag window of the same year (lag0). In the two-pollutant models, the HRs refer to the pollutant listed first.
Table A1.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in single- and two-pollutant models with an exposure lag window of the same year (lag0). In the two-pollutant models, the HRs refer to the pollutant listed first.
| Air pollutant* |
Model†
|
IQR (µg m-3) |
HR |
95% CI |
p-Value |
| PM10
|
M1 |
2.7 |
1.05 |
1.02‒1.07 |
<0.001 |
| |
M2 |
‒ |
1.02 |
0.99‒1.05 |
0.14 |
| |
M3 |
‒ |
1.01 |
0.98‒1.04 |
0.43 |
| PM2.5
|
M1 |
1.6 |
1.01 |
0.99‒1.03 |
0.43 |
| |
M2 |
‒ |
1.00 |
0.98‒1.02 |
0.99 |
| |
M3 |
‒ |
1.00 |
0.98‒1.02 |
0.77 |
| BC |
M1 |
0.15 |
1.13 |
1.09‒1.16 |
<0.001 |
| |
M2 |
‒ |
1.06 |
1.03‒1.09 |
<0.001 |
| |
M3 |
‒ |
1.05 |
1.02‒1.08 |
0.004 |
| NOx
|
M1 |
9.7 |
1.15 |
1.12‒1.19 |
<0.001 |
| |
M2 |
‒ |
1.08 |
1.05‒1.12 |
<0.001 |
| |
M3 |
‒ |
1.08 |
1.05‒1.12 |
<0.001 |
| PM10 + BC |
M1 |
‒ |
1.00 |
0.97‒1.03 |
0.76 |
| |
M2 |
‒ |
1.00 |
0.97‒1.03 |
0.82 |
| |
M3 |
‒ |
0.99 |
0.96‒1.02 |
0.49 |
| BC + PM10
|
M1 |
‒ |
1.13 |
1.09‒1.17 |
<0.001 |
| |
M2 |
‒ |
1.06 |
1.02‒1.10 |
0.001 |
| |
M3 |
‒ |
1.06 |
1.02‒1.10 |
0.004 |
| PM10 + NOx
|
M1 |
‒ |
0.99 |
0.96‒1.02 |
0.49 |
| |
M2 |
‒ |
0.99 |
0.96‒1.02 |
0.42 |
| |
M3 |
‒ |
0.98 |
0.95‒1.01 |
0.16 |
| NOx + PM10
|
M1 |
‒ |
1.16 |
1.12‒1.20 |
<0.001 |
| |
M2 |
‒ |
1.09 |
1.05‒1.13 |
<0.001 |
| |
M3 |
‒ |
1.10 |
1.06‒1.14 |
<0.001 |
| PM2.5 + BC |
M1 |
‒ |
0.99 |
0.98‒1.01 |
0.52 |
| |
M2 |
‒ |
0.99 |
0.97‒1.01 |
0.53 |
| |
M3 |
‒ |
0.99 |
0.97‒1.01 |
0.41 |
| BC + PM2.5
|
M1 |
‒ |
1.13 |
1.09‒1.16 |
<0.001 |
| |
M2 |
‒ |
1.06 |
1.03‒1.09 |
<0.001 |
| |
M3 |
‒ |
1.05 |
1.02‒1.09 |
0.003 |
| PM2.5 + NOx
|
M1 |
‒ |
0.99 |
0.97‒1.01 |
0.32 |
| |
M2 |
‒ |
0.99 |
0.97‒1.01 |
0.34 |
| |
M3 |
‒ |
0.99 |
0.97‒1.01 |
0.24 |
| NOx + PM2.5
|
M1 |
‒ |
1.16 |
1.12‒1.19 |
<0.001 |
| |
M2 |
‒ |
1.09 |
1.05‒1.13 |
<0.001 |
| |
M3 |
‒ |
1.09 |
1.05‒1.12 |
<0.001 |
| BC + NOx
|
M1 |
‒ |
0.99 |
0.93‒1.05 |
0.67 |
| |
M2 |
‒ |
0.94 |
0.88‒1.01 |
0.08 |
| |
M3 |
‒ |
0.92 |
0.86‒0.98 |
0.02 |
| NOx + BC |
M1 |
‒ |
1.17 |
1.09‒1.25 |
<0.001 |
| |
M2 |
‒ |
1.15 |
1.07‒1.23 |
<0.001 |
| |
M3 |
‒ |
1.17 |
1.09‒1.26 |
<0.001 |
Table A2.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in single- and two-pollutant models with an exposure lag window of 1‒5 years (lag1‒5). In the two-pollutant models, the HRs refer to the pollutant listed first.
Table A2.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in single- and two-pollutant models with an exposure lag window of 1‒5 years (lag1‒5). In the two-pollutant models, the HRs refer to the pollutant listed first.
| Air pollutant* |
Model†
|
IQR (µg m-3) |
HR |
95% CI |
p-Value |
| PM10
|
M1 |
2.1 |
1.16 |
1.12‒1.21 |
<0.001 |
| |
M2 |
‒ |
1.08 |
1.03‒1.12 |
<0.001 |
| |
M3 |
‒ |
1.06 |
1.02‒1.11 |
0.007 |
| PM2.5
|
M1 |
1.7 |
1.11 |
1.05‒1.17 |
<0.001 |
| |
M2 |
‒ |
1.04 |
0.98‒1.10 |
0.24 |
| |
M3 |
‒ |
1.01 |
0.95‒1.08 |
0.69 |
| BC |
M1 |
0.14 |
1.15 |
1.11‒1.18 |
<0.001 |
| |
M2 |
‒ |
1.08 |
1.04‒1.11 |
<0.001 |
| |
M3 |
‒ |
1.07 |
1.04‒1.11 |
<0.001 |
| NOx
|
M1 |
11.4 |
1.19 |
1.15‒1.22 |
<0.001 |
| |
M2 |
‒ |
1.11 |
1.07‒1.15 |
<0.001 |
| |
M3 |
‒ |
1.11 |
1.07‒1.16 |
<0.001 |
| PM10 + NOx
|
M1 |
‒ |
0.98 |
0.92‒1.04 |
0.49 |
| |
M2 |
‒ |
0.95 |
0.89‒1.01 |
0.10 |
| |
M3 |
‒ |
0.90 |
0.84‒0.97 |
0.004 |
| NOx + PM10
|
M1 |
‒ |
1.20 |
1.14‒1.27 |
<0.001 |
| |
M2 |
‒ |
1.15 |
1.09‒1.22 |
<0.001 |
| |
M3 |
‒ |
1.19 |
1.13‒1.26 |
<0.001 |
| PM2.5 + BC |
M1 |
‒ |
0.94 |
0.88‒1.00 |
0.07 |
| |
M2 |
‒ |
0.94 |
0.87‒1.01 |
0.09 |
| |
M3 |
‒ |
0.92 |
0.85‒0.99 |
0.02 |
| BC + PM2.5
|
M1 |
‒ |
1.17 |
1.13‒1.21 |
<0.001 |
| |
M2 |
‒ |
1.10 |
1.06‒1.14 |
<0.001 |
| |
M3 |
‒ |
1.10 |
1.06‒1.14 |
<0.001 |
| PM2.5 + NOx
|
M1 |
‒ |
0.96 |
0.90‒1.02 |
0.22 |
| |
M2 |
‒ |
0.95 |
0.88‒1.01 |
0.11 |
| |
M3 |
‒ |
0.92 |
0.85‒0.98 |
0.01 |
| NOx + PM2.5
|
M1 |
‒ |
1.20 |
1.15‒1.24 |
<0.001 |
| |
M2 |
‒ |
1.13 |
1.08‒1.17 |
<0.001 |
| |
M3 |
‒ |
1.14 |
1.10‒1.19 |
<0.001 |
| BC + NOx
|
M1 |
‒ |
1.02 |
0.97‒1.09 |
0.41 |
| |
M2 |
‒ |
0.98 |
0.93‒1.05 |
0.61 |
| |
M3 |
‒ |
0.95 |
0.89‒1.01 |
0.10 |
| NOx + BC |
M1 |
‒ |
1.16 |
1.09‒1.23 |
<0.001 |
| |
M2 |
‒ |
1.13 |
1.05‒1.21 |
<0.001 |
| |
M3 |
‒ |
1.17 |
1.09‒1.26 |
<0.001 |
Table A3.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in single- and two-pollutant models with an exposure lag window of 6‒10 years (lag6‒10). In the two-pollutant models, the HRs refer to the pollutant listed first.
Table A3.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in single- and two-pollutant models with an exposure lag window of 6‒10 years (lag6‒10). In the two-pollutant models, the HRs refer to the pollutant listed first.
| Air pollutant* |
Model†
|
IQR (µg m-3) |
HR |
95% CI |
p-Value |
| PM10
|
M1 |
2.3 |
1.17 |
1.12‒1.23 |
<0.001 |
| |
M2 |
‒ |
1.09 |
1.04‒1.14 |
<0.001 |
| |
M3 |
‒ |
1.08 |
1.03‒1.13 |
0.002 |
| PM2.5
|
M1 |
1.5 |
1.18 |
1.11‒1.26 |
<0.001 |
| |
M2 |
‒ |
1.10 |
1.03‒1.17 |
0.002 |
| |
M3 |
‒ |
1.09 |
1.02‒1.17 |
0.009 |
| BC |
M1 |
0.15 |
1.14 |
1.11‒1.18 |
<0.001 |
| |
M2 |
‒ |
1.07 |
1.03‒1.10 |
<0.001 |
| |
M3 |
‒ |
1.06 |
1.02‒1.10 |
0.002 |
| NOx
|
M1 |
12.7 |
1.16 |
1.12‒1.19 |
<0.001 |
| |
M2 |
‒ |
1.08 |
1.05‒1.12 |
<0.001 |
| |
M3 |
‒ |
1.08 |
1.05‒1.12 |
<0.001 |
| PM10 + NOx
|
M1 |
‒ |
1.03 |
0.96‒1.10 |
0.42 |
| |
M2 |
‒ |
1.00 |
0.93‒1.08 |
0.94 |
| |
M3 |
‒ |
0.99 |
0.92‒1.06 |
0.74 |
| NOx + PM10
|
M1 |
‒ |
1.14 |
1.08‒1.20 |
<0.001 |
| |
M2 |
‒ |
1.08 |
1.03‒1.14 |
0.004 |
| |
M3 |
‒ |
1.09 |
1.03‒1.16 |
0.002 |
| PM2.5 + BC |
M1 |
‒ |
1.03 |
0.96‒1.11 |
0.41 |
| |
M2 |
‒ |
1.04 |
0.96‒1.13 |
0.30 |
| |
M3 |
‒ |
1.04 |
0.96‒1.13 |
0.36 |
| BC + PM2.5
|
M1 |
‒ |
1.13 |
1.08‒1.18 |
<0.001 |
| |
M2 |
‒ |
1.05 |
1.01‒1.10 |
0.02 |
| |
M3 |
‒ |
1.05 |
1.00‒1.09 |
0.05 |
| PM2.5 + NOx
|
M1 |
‒ |
1.05 |
0.98‒1.13 |
0.13 |
| |
M2 |
‒ |
1.04 |
0.97‒1.11 |
0.31 |
| |
M3 |
‒ |
1.02 |
0.95‒1.10 |
0.53 |
| NOx + PM2.5
|
M1 |
‒ |
1.14 |
1.10‒1.18 |
<0.001 |
| |
M2 |
‒ |
1.07 |
1.03‒1.12 |
<0.001 |
| |
M3 |
‒ |
1.08 |
1.04‒1.12 |
<0.001 |
| BC + NOx
|
M1 |
‒ |
1.03 |
0.97‒1.10 |
0.38 |
| |
M2 |
‒ |
0.99 |
0.92‒1.05 |
0.67 |
| |
M3 |
‒ |
0.96 |
0.89‒1.03 |
0.23 |
| NOx + BC |
M1 |
‒ |
1.13 |
1.06‒1.20 |
<0.001 |
| |
M2 |
‒ |
1.10 |
1.03‒1.17 |
0.006 |
| |
M3 |
‒ |
1.12 |
1.05‒1.20 |
0.001 |
Table A4.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in single-pollutant models divided into different age groups. All HRs are based on lag1‒5, and with adjustments for all covariates according to Model 3. .
Table A4.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in single-pollutant models divided into different age groups. All HRs are based on lag1‒5, and with adjustments for all covariates according to Model 3. .
| Air Pollutant |
Age Group |
HR |
95% CI |
p-Value |
| PM10
|
Group 1 ≤ 50 years |
1.00 |
0.84‒1.19 |
0.99 |
| |
50 years < Group 2 ≤ 55 years |
1.08 |
0.95‒1.23 |
0.23 |
| |
55 years < Group 3 ≤ 60 years |
1.02 |
0.91‒1.14 |
0.72 |
| |
60 years < Group 4 ≤ 65 years |
1.10 |
1.02‒1.20 |
0.02 |
| |
65 years < Group 5 ≤ 70 years |
1.12 |
1.01‒1.23 |
0.03 |
| |
Group 6 > 70 years |
1.02 |
0.91‒1.15 |
0.73 |
| PM2.5
|
Group 1 ≤ 50 years |
1.06 |
0.82‒1.37 |
0.65 |
| |
50 years < Group 2 ≤ 55 years |
1.05 |
0.86‒1.28 |
0.61 |
| |
55 years < Group 3 ≤ 60 years |
0.92 |
0.78‒1.08 |
0.31 |
| |
60 years < Group 4 ≤ 65 years |
1.06 |
0.94‒1.20 |
0.37 |
| |
65 years < Group 5 ≤ 70 years |
1.16 |
1.00‒1.34 |
0.05 |
| |
Group 6 > 70 years |
0.94 |
0.77‒1.15 |
0.56 |
| BC |
Group 1 ≤ 50 years |
1.04 |
0.92‒1.18 |
0.54 |
| |
50 years < Group 2 ≤ 55 years |
1.07 |
0.98‒1.18 |
0.14 |
| |
55 years < Group 3 ≤ 60 years |
1.05 |
0.96‒1.14 |
0.28 |
| |
60 years < Group 4 ≤ 65 years |
1.10 |
1.03‒1.17 |
0.004 |
| |
65 years < Group 5 ≤ 70 years |
1.09 |
1.01‒1.17 |
0.02 |
| |
Group 6 > 70 years |
1.01 |
0.93‒1.10 |
0.80 |
| NOx
|
Group 1 ≤ 50 years |
1.05 |
0.90‒1.23 |
0.52 |
| |
50 years < Group 2 ≤ 55 years |
1.11 |
1.01‒1.23 |
0.04 |
| |
55 years < Group 3 ≤ 60 years |
1.07 |
0.97‒1.17 |
0.16 |
| |
60 years < Group 4 ≤ 65 years |
1.12 |
1.05‒1.20 |
0.001 |
| |
65 years < Group 5 ≤ 70 years |
1.11 |
1.03‒1.20 |
0.006 |
| |
Group 6 > 70 years |
1.05 |
0.95‒1.15 |
0.35 |
Table A5.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in two-pollutant models divided into different age groups. All HRs are based on lag1‒5, and with adjustments for all covariates according to Model 3. In these two-pollutant models, the HRs refer to the pollutant listed first.
Table A5.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in two-pollutant models divided into different age groups. All HRs are based on lag1‒5, and with adjustments for all covariates according to Model 3. In these two-pollutant models, the HRs refer to the pollutant listed first.
| Air Pollutant |
Age Group |
HR |
95% CI |
p-Value |
| PM10 + NOx
|
Group 1 ≤ 50 years |
0.89 |
0.67‒1.17 |
0.41 |
| |
50 years < Group 2 ≤ 55 years |
0.91 |
0.73‒1.14 |
0.43 |
| |
55 years < Group 3 ≤ 60 years |
0.88 |
0.73‒1.07 |
0.20 |
| |
60 years < Group 4 ≤ 65 years |
0.97 |
0.84‒1.12 |
0.69 |
| |
65 years < Group 5 ≤ 70 years |
1.00 |
0.84‒1.18 |
0.99 |
| |
Group 6 > 70 years |
0.88 |
0.69‒1.13 |
0.32 |
| NOx + PM10
|
Group 1 ≤ 50 years |
1.14 |
0.89‒1.45 |
0.29 |
| |
50 years < Group 2 ≤ 55 years |
1.18 |
0.99‒1.40 |
0.06 |
| |
55 years < Group 3 ≤ 60 years |
1.16 |
0.99‒1.36 |
0.06 |
| |
60 years < Group 4 ≤ 65 years |
1.14 |
1.02‒1.29 |
0.03 |
| |
65 years < Group 5 ≤ 70 years |
1.12 |
0.98‒1.28 |
0.11 |
| |
Group 6 > 70 years |
1.14 |
0.94‒1.39 |
0.18 |
| PM2.5 + BC |
Group 1 ≤ 50 years |
1.02 |
0.74‒1.40 |
0.91 |
| |
50 years < Group 2 ≤ 55 years |
0.94 |
0.74‒1.20 |
0.63 |
| |
55 years < Group 3 ≤ 60 years |
0.81 |
0.67‒1.00 |
0.05 |
| |
60 years < Group 4 ≤ 65 years |
0.94 |
0.81‒1.09 |
0.40 |
| |
65 years < Group 5 ≤ 70 years |
1.08 |
0.90‒1.29 |
0.40 |
| |
Group 6 > 70 years |
0.86 |
0.65‒1.14 |
0.29 |
| BC + PM2.5
|
Group 1 ≤ 50 years |
1.04 |
0.88‒1.21 |
0.67 |
| |
50 years < Group 2 ≤ 55 years |
1.09 |
0.97‒1.23 |
0.15 |
| |
55 years < Group 3 ≤ 60 years |
1.11 |
1.00‒1.23 |
0.04 |
| |
60 years < Group 4 ≤ 65 years |
1.12 |
1.03‒1.20 |
0.004 |
| |
65 years < Group 5 ≤ 70 years |
1.06 |
0.98‒1.16 |
0.16 |
| |
Group 6 > 70 years |
1.05 |
0.94‒1.18 |
0.36 |
| PM2.5 + NOx
|
Group 1 ≤ 50 years |
1.02 |
0.76‒1.37 |
0.87 |
| |
50 years < Group 2 ≤ 55 years |
0.93 |
0.74‒1.17 |
0.54 |
| |
55 years < Group 3 ≤ 60 years |
0.83 |
0.69‒1.00 |
0.06 |
| |
60 years < Group 4 ≤ 65 years |
0.95 |
0.83‒1.10 |
0.51 |
| |
65 years < Group 5 ≤ 70 years |
1.07 |
0.90‒1.26 |
0.44 |
| |
Group 6 > 70 years |
0.82 |
0.63‒1.06 |
0.13 |
| NOx + PM2.5
|
Group 1 ≤ 50 years |
1.04 |
0.88‒1.24 |
0.63 |
| |
50 years < Group 2 ≤ 55 years |
1.13 |
1.01‒1.27 |
0.04 |
| |
55 years < Group 3 ≤ 60 years |
1.12 |
1.01‒1.25 |
0.03 |
| |
60 years < Group 4 ≤ 65 years |
1.14 |
1.05‒1.23 |
0.002 |
| |
65 years < Group 5 ≤ 70 years |
1.10 |
1.00‒1.20 |
0.04 |
| |
Group 6 > 70 years |
1.11 |
0.98‒1.25 |
0.09 |
| BC + NOx
|
Group 1 ≤ 50 years |
1.01 |
0.78‒1.32 |
0.92 |
| |
50 years < Group 2 ≤ 55 years |
0.95 |
0.79‒1.14 |
0.56 |
| |
55 years < Group 3 ≤ 60 years |
0.98 |
0.84‒1.15 |
0.84 |
| |
60 years < Group 4 ≤ 65 years |
1.02 |
0.90‒1.15 |
0.80 |
| |
65 years < Group 5 ≤ 70 years |
0.99 |
0.86‒1.14 |
0.88 |
| |
Group 6 > 70 years |
0.87 |
0.72‒1.05 |
0.15 |
| NOx + BC |
Group 1 ≤ 50 years |
1.04 |
0.76‒1.42 |
0.82 |
| |
50 years < Group 2 ≤ 55 years |
1.17 |
0.96‒1.43 |
0.12 |
| |
55 years < Group 3 ≤ 60 years |
1.09 |
0.91‒1.30 |
0.37 |
| |
60 years < Group 4 ≤ 65 years |
1.10 |
0.96‒1.27 |
0.16 |
| |
65 years < Group 5 ≤ 70 years |
1.13 |
0.96‒1.32 |
0.13 |
| |
Group 6 > 70 years |
1.21 |
0.97‒1.50 |
0.09 |
Table A6.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in single-pollutant models divided into two survival time periods. All HRs are based on lag1‒5, and with adjustments for all covariates according to Model 3.
Table A6.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in single-pollutant models divided into two survival time periods. All HRs are based on lag1‒5, and with adjustments for all covariates according to Model 3.
| Air Pollutant |
Survival Time |
HR |
95% CI |
p-Value |
| PM10
|
0‒10 years |
1.11 |
1.02‒1.20 |
0.01 |
| |
> 10 years |
1.06 |
1.01‒1.12 |
0.02 |
| PM2.5
|
0‒10 years |
1.16 |
1.00‒1.35 |
0.05 |
| |
> 10 years |
1.01 |
0.94‒1.08 |
0.76 |
| BC |
0‒10 years |
1.10 |
1.03‒1.18 |
0.003 |
| |
> 10 years |
1.06 |
1.02‒1.10 |
0.001 |
| NOx
|
0‒10 years |
1.09 |
1.03‒1.14 |
0.001 |
| |
> 10 years |
1.12 |
1.06‒1.18 |
<0.001 |
Table A7.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in two-pollutant models divided into two survival time periods. All HRs are based on lag1‒5, and with adjustments for all covariates according to Model 3. In these two-pollutant models, the HRs refer to the pollutant listed first.
Table A7.
Hazard ratios (HR) with 95% confidence intervals (CI) for the associations between natural-cause mortality and an IQR increase of the investigated air pollutants in two-pollutant models divided into two survival time periods. All HRs are based on lag1‒5, and with adjustments for all covariates according to Model 3. In these two-pollutant models, the HRs refer to the pollutant listed first.
| Air Pollutant |
Model* |
HR |
95% CI |
p-Value |
| PM10 + NOx
|
0‒10 years |
1.00 |
0.88‒1.14 |
0.95 |
| |
> 10 years |
0.92 |
0.83‒1.01 |
0.07 |
| NOx + PM10
|
0‒10 years |
1.08 |
1.00‒1.17 |
0.05 |
| |
> 10 years |
1.20 |
1.09‒1.33 |
<0.001 |
| PM2.5 + BC |
0‒10 years |
1.02 |
0.85‒1.23 |
0.82 |
| |
> 10 years |
0.93 |
0.86‒1.01 |
0.10 |
| BC + PM2.5
|
0‒10 years |
1.10 |
1.01‒1.19 |
0.03 |
| |
> 10 years |
1.08 |
1.04‒1.13 |
<0.001 |
| PM2.5 + NOx
|
0‒10 years |
1.05 |
0.89‒1.24 |
0.54 |
| |
> 10 years |
0.92 |
0.85‒1.00 |
0.04 |
| NOx + PM2.5
|
0‒10 years |
1.08 |
1.02‒1.14 |
0.01 |
| |
> 10 years |
1.15 |
1.08‒1.23 |
<0.001 |
| BC + NOx
|
0‒10 years |
1.03 |
0.91‒1.16 |
0.62 |
| |
> 10 years |
0.91 |
0.81‒1.01 |
0.09 |
| NOx + BC |
0‒10 years |
1.07 |
0.97‒1.17 |
0.18 |
| |
> 10 years |
1.27 |
1.08‒1.49 |
0.003 |
Table A8.
Correlation matrix with correlation coefficients (Pearson) between the modeled air pollutants based on lag0 (upper part), lag1‒5 (middle part), and lag6‒10 (lower part).
Table A8.
Correlation matrix with correlation coefficients (Pearson) between the modeled air pollutants based on lag0 (upper part), lag1‒5 (middle part), and lag6‒10 (lower part).
| |
PM10 lag0 |
PM2.5 lag0 |
BC lag0 |
NOx lag0 |
| PM10 lag0 |
1 |
|
|
|
| PM2.5 lag0 |
0.81 |
1 |
|
|
| BC lag0 |
0.55 |
0.25 |
1 |
|
| NOx lag0 |
0.24 |
0.03 |
0.50 |
1 |
| |
PM10 lag1‒5 |
PM2.5 lag1‒5 |
BC lag1‒5 |
NOx lag1‒5 |
| PM10 lag1‒5 |
1 |
|
|
|
| PM2.5 lag1‒5 |
0.81 |
1 |
|
|
| BC lag1‒5 |
0.85 |
0.64 |
1 |
|
| NOx lag1‒5 |
0.19 |
-0.18 |
0.41 |
1 |
| |
PM10 lag6‒10 |
PM2.5 lag6‒10 |
BC lag6‒10 |
NOx lag6‒10 |
| PM10 lag6‒10 |
1 |
|
|
|
| PM2.5 lag6‒10 |
0.90 |
1 |
|
|
| BC lag6‒10 |
0.84 |
0.66 |
1 |
|
| NOx lag6‒10 |
0.22 |
-0.09 |
0.47 |
1 |
References
- WHO, 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 22 August 2023).
- Chen, J.; Hoek, G. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Int. 2020, 143, 105974. [Google Scholar] [CrossRef] [PubMed]
- Faustini, A.; Rapp, R.; Forastiere, F. Nitrogen dioxide and mortality: review and meta-analysis of long-term studies. Eur. Respir. J. 2014, 44, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Hoek, G.; Krishnan, R.M.; Beelen, R.; Peters, A.; Ostro, B.; Brunekreef, B.; Kaufman, J.D. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ. Health. 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Beelen, R.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.; Nieuwenhuijsen, M.; et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 2014, 383, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Oftedal, B.; Chen, J.; Rodopoulou, S.; Renzi, M.; Atkinson, R.W.; Bauwelinck, M.; Klompmaker, J.O.; Mehta, A.; Vienneau, D.; et al. Long-term exposure to low ambient air pollution concentrations and mortality among 28 million people: results from seven large European cohorts within the ELAPSE project. Lancet Planet Health 2022, 6, e9–e18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mahendran, R.; Yu, P.; Xu, R.; Yu, W.; Godellawattage, S.; Li, S.; Guo, Y. Health Effects of Long-Term Exposure to Ambient PM2.5 in Asia-Pacific: a Systematic Review of Cohort Studies. Curr. Environ. Health. Rep. 2022, 9, 130–151. [Google Scholar] [CrossRef]
- Nilsson Sommar, J.; Andersson, E.M.; Andersson, N.; Sallsten, G.; Stockfelt, L.; Ljungman, P.L.; Segersson, D.; Eneroth, K.; Gidhagen, L.; Molnar, P.; et al. Long-term exposure to particulate air pollution and black carbon in relation to natural and cause-specific mortality: a multicohort study in Sweden. BMJ Open 2021, 11, e046040. [Google Scholar] [CrossRef]
- Yang, J.; Sakhvidi, M.J.Z.; de Hoogh, K.; Vienneau, D.; Siemiatyck, J.; Zins, M.; Goldberg, M.; Chen, J.; Lequy, E.; Jacquemin, B. Long-term exposure to black carbon and mortality: A 28-year follow-up of the GAZEL cohort. Environ Int. 2021, 157, 106805. [Google Scholar] [CrossRef]
- Filleul, L.; Rondeau, V.; Vandentorren, S.; Le Moual, N.; Cantagrel, A.; Annesi-Maesano, I.; Charpin, D.; Declercq, C.; Neukirch, F.; Paris, C.; et al. Twenty five year mortality and air pollution: results from the French PAARC survey. Occup. Environ. Med. 2005, 62, 453–460. [Google Scholar] [CrossRef]
- Beelen, R.; Hoek, G.; van den Brandt, P.A.; Goldbohm, R.A.; Fischer, P.; Schouten, L.J.; Jerrett, M.; Hughes, E.; Armstrong, B.; Brunekreef, B. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR study). Environ. Health Perspect. 2008, 116, 196–202. [Google Scholar] [CrossRef]
- Huangfu, P.; Atkinson, R. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ. Int. 2020, 144, 105998. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, H.; Wang, M.; Qian, Y.; Steenland, K.; Caudle, W.M.; Liu, Y.; Sarnat, J.; Papatheodorou, S.; Shi, L. Long-term exposure to nitrogen dioxide and mortality: A systematic review and meta-analysis. Sci. Total Environ. 2021, 776, 145968. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.W.; Butland, B.K.; Anderson, H.R.; Maynard, R.L. Long-term Concentrations of Nitrogen Dioxide and Mortality: A Meta-analysis of Cohort Studies. Epidemiology 2018, 29, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Stieb, D.M.; Berjawi, R.; Emode, M.; Zheng, C.; Salama, D.; Hocking, R.; Lyrette, N.; Matz, C.; Lavigne, E.; Shin, H.H. Systematic review and meta-analysis of cohort studies of long term outdoor nitrogen dioxide exposure and mortality. PLoS One 2021, 16, e0246451. [Google Scholar] [CrossRef] [PubMed]
- Stockfelt, L.; Andersson, E.M.; Molnár, P.; Rosengren, A.; Wilhelmsen, L.; Sallsten, G.; Barregard, L. Long term effects of residential NO(x) exposure on total and cause-specific mortality and incidence of myocardial infarction in a Swedish cohort. Environ. Res. 2015, 142, 197–206. [Google Scholar] [CrossRef]
- Carlsen, H.K.; Andersson, E.M.; Molnár, P.; Oudin, A.; Xu, Y.; Wichmann, J.; Spanne, M.; Stroh, E.; Engström, G.; Stockfelt, L. Incident cardiovascular disease and long-term exposure to source-specific air pollutants in a Swedish cohort. Environ. Res. 2022, 209, 112698. [Google Scholar] [CrossRef]
- Azzouz, M.; Xu, Y.; Barregard, L.; Fagerberg, B.; Zöller, B.; Molnár, P.; Oudin, A.; Spanne, M.; Engström, G.; Stockfelt, L. Air pollution and biomarkers of cardiovascular disease and inflammation in the Malmö Diet and Cancer cohort. Environ. Health. 2022, 21, 39. [Google Scholar] [CrossRef]
- Xu, Y.; Andersson, E.M.; Krage Carlsen, H.; Molnár, P.; Gustafsson, S.; Johannesson, S.; Oudin, A.; Engström, G.; Christensson, A.; Stockfelt, L. Associations between long-term exposure to low-level air pollution and risk of chronic kidney disease-findings from the Malmö Diet and Cancer cohort. Environ. Int. 2022, 160, 107085. [Google Scholar] [CrossRef]
- Berglund, G.; Elmståhl, S.; Janzon, L.; Larsson, S.A. The Malmo Diet and Cancer Study. Design and feasibility. J. Intern Med. 1993, 233, 45–51. [Google Scholar] [CrossRef]
- Manjer, J.; Carlsson, S.; Elmståhl, S.; Gullberg, B.; Janzon, L.; Lindström, M.; Mattisson, I.; Berglund, G. The Malmö Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur. J. Cancer Prev. 2001, 10, 489–499. [Google Scholar] [CrossRef]
- Rosvall, M.; Ostergren, P.O.; Hedblad, B.; Isacsson, S.O.; Janzon, L.; Berglund, G. Occupational status, educational level, and the prevalence of carotid atherosclerosis in a general population sample of middle-aged Swedish men and women: results from the Malmö Diet and Cancer Study. Am. J. Epidemiol. 2000, 152, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Drake, I.; Dias, J.A.; Teleka, S.; Stocks, T.; Orho-Melander, M. Lifestyle and cancer incidence and mortality risk depending on family history of cancer in two prospective cohorts. Int. J. Cancer 2020, 146, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Kuik, F.; Kerschbaumer, A.; Lauer, A.; Lupascu, A.; von Schneidemesser, E.; Butler, T.M. Top–down quantification of NOx emissions from traffic in an urban area using a high-resolution regional atmospheric chemistry model. Atmos. Chem. Phys. 2018, 18, 8203–8225. [Google Scholar] [CrossRef]
- Khan, R.K.; Strand, M.A. Road dust and its effect on human health: A literature review. Epidemiol. Health 2018, 40, e2018013. [Google Scholar] [CrossRef] [PubMed]
- Olstrup, H.; Johansson, C.; Forsberg, B.; Åström, C.; Orru, H. Seasonal Variations in the Daily Mortality Associated with Exposure to Particles, Nitrogen Dioxide, and Ozone in Stockholm, Sweden, from 2000 to 2016. Atmosphere 2021, 12, 1481. [Google Scholar] [CrossRef]
- Meister, K.; Johansson, C.; Forsberg, J. Estimated short-term effects of coarse particles on daily mortality in Stockholm, Sweden. Environ. Health Perspect. 2012, 120, 431–436. [Google Scholar] [CrossRef]
- Kraft, M.; Eikmann, T.; Kappos, A.; Künzli, N.; Rapp, R.; Schneider, K.; Seitz, H.; Voss, J.U.; Wichmann, H.E. The German view: Effects of nitrogen dioxide on human health–derivation of health-related short-term and long-term values. Int. J. Hyg. Environ. Health 2005, 208, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.; Hoffmann, B.; Peters, A. The Effects of Fine Dust, Ozone, and Nitrogen Dioxide on Health. Dtsch. Arztebl. Int. 2019, 116, 881–886. [Google Scholar] [CrossRef]
- Grundström, M.; Hak, C.; Chen, D.; Hallquist, M.; Pleijel, H. Variation and Co-variation of PM10, Particle Number Concentration, NOx and NO2 in the Urban Air ‒ Relationships With Wind Speed, Vertical Temperature Gradient and Weather Type. Atmospheric Environment 2015, 120, 317–327. [Google Scholar] [CrossRef]
- Olstrup, H.; Johansson, C.; Forsberg, B.; Åström, C. Association between Mortality and Short-Term Exposure to Particles, Ozone and Nitrogen Dioxide in Stockholm, Sweden. Int. J. Environ. Res. Public Health 2019, 16, 1028. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, Z.; Zeng, W.; Yang, T.; Cao, Y.; Mei, C.; Kuang, Y. Toxicity assessment of nanoparticles in various systems and organs. Nanotechnol. Rev. 2017, 6, 279–289. [Google Scholar] [CrossRef]
- Schuster, B.S.; Suk, J.S.; Woodworth, G.F.; Hanes, J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials 2013, 34, 3439–3446. [Google Scholar] [CrossRef] [PubMed]
- Fussell, J.C.; Franklin, M.; Green, D.C.; Gustafsson, M.; Harrison, R.M.; Hicks, W.; Kelly, F.J.; Kishta, F.; Miller, M.R.; Mudway, I.S.; et al. A Review of Road Traffic-Derived Non-Exhaust Particles: Emissions, Physicochemical Characteristics, Health Risks, and Mitigation Measures. Environ. Sci. Technol. 2022, 56, 6813–6835. [Google Scholar] [CrossRef]
- Davies, H.W.; Vlaanderen, J.J.; Henderson, S.B.; Brauer, M. Correlation between co-exposures to noise and air pollution from traffic sources. Occup. Environ. Med. 2009, 66, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ramakrishnan, R.; Rahimi, K. Long-term exposure to traffic noise and mortality: A systematic review and meta-analysis of epidemiological evidence between 2000 and 2020. Environ. Pollut. 2021, 269, 116222. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.M.; Ögren, M.; Molnár, P.; Segersson, D.; Rosengren, A.; Stockfelt, L. Road traffic noise, air pollution and cardiovascular events in a Swedish cohort. Environ. Res. 2020, 185, 109446. [Google Scholar] [CrossRef] [PubMed]
- Krecl, P.; Harrison, R.M.; Johansson, C.; Targino, A.C.; Beddows, D.C.; Ellermann, T.; Lara, C.; Ketzel, M. Long-term trends in nitrogen oxides concentrations and on-road vehicle emission factors in Copenhagen, London and Stockholm. Environ. Pollut. 2021, 290, 118105. [Google Scholar] [CrossRef] [PubMed]
- Oudin, A.; Forsberg, B.; Strömgren, M.; Beelen, R.; Modig, L. Impact of residential mobility on exposure assessment in longitudinal air pollution studies: a sensitivity analysis within the ESCAPE project. Sci. World J. 2012, 2012, 125818. [Google Scholar] [CrossRef]
- Ulrich, A.; Wichser, A.; Hess, A.; Heeb, N.; Emmenegger, L.; Czerwinski, J.; Kasper, M.; Mooney, J.; Mayer, A. Particle and Metal Emissions of Diesel and Gasoline Engines—Are Particle Filters Appropriate Measures? Swiss Federal Laboratories for Material Testing and Research: Dübendorf, Switzerland, 2012.
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Samet, J.M.; Dominici, F. Hospital admissions and chemical composition of fine particle air pollution. Am. J. Respir. Crit. Care. Med. 2009, 179, 1115–1120. [Google Scholar] [CrossRef]
- Selley, L.; Schuster, L.; Marbach, H.; Forsthuber, T.; Forbes, B.; Gant, T.W.; Sandström, T.; Camiña, N.; Athersuch, T.J.; Mudway, I.; et al. Brake dust exposure exacerbates inflammation and transiently compromises phagocytosis in macrophages. Metallomics 2020, 12, 371–386. [Google Scholar] [CrossRef]
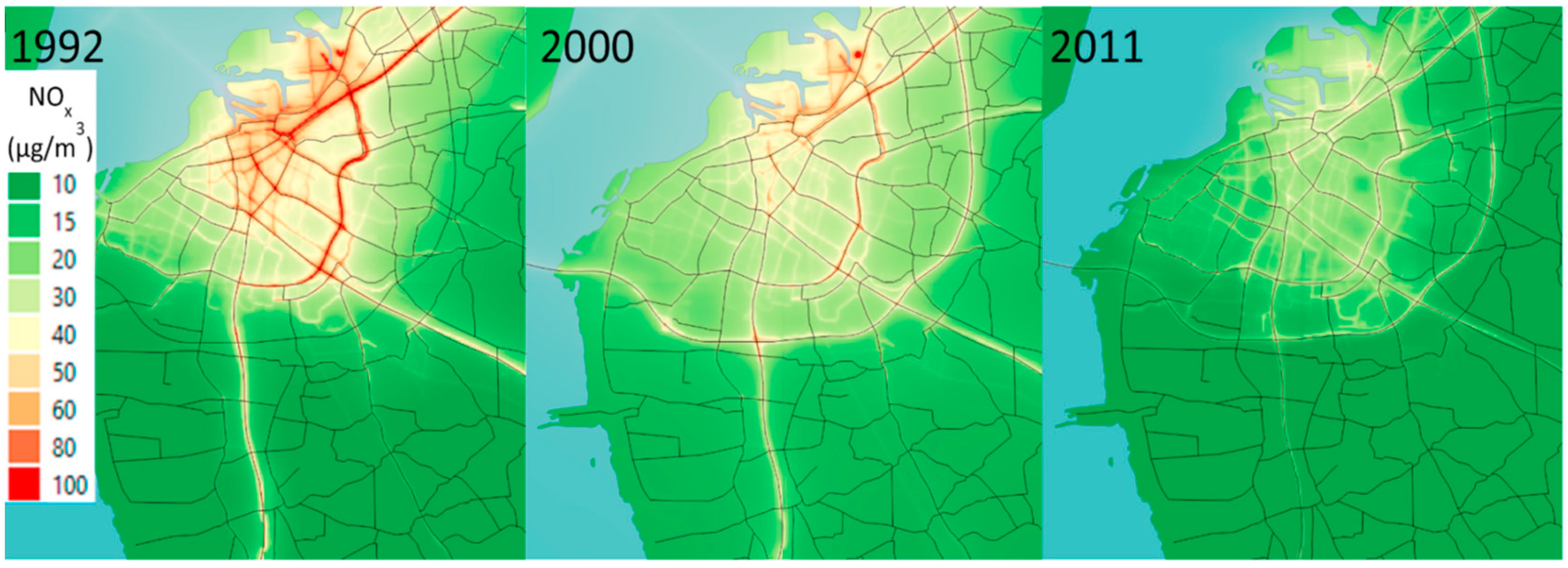
Figure 1.
The modeled concentrations (µg m
-3) of NO
x in the study area in Malmö during 1992, 2000, and 2011. Source: Carlsen et al. (2022) [
17]. .
Figure 1.
The modeled concentrations (µg m
-3) of NO
x in the study area in Malmö during 1992, 2000, and 2011. Source: Carlsen et al. (2022) [
17]. .
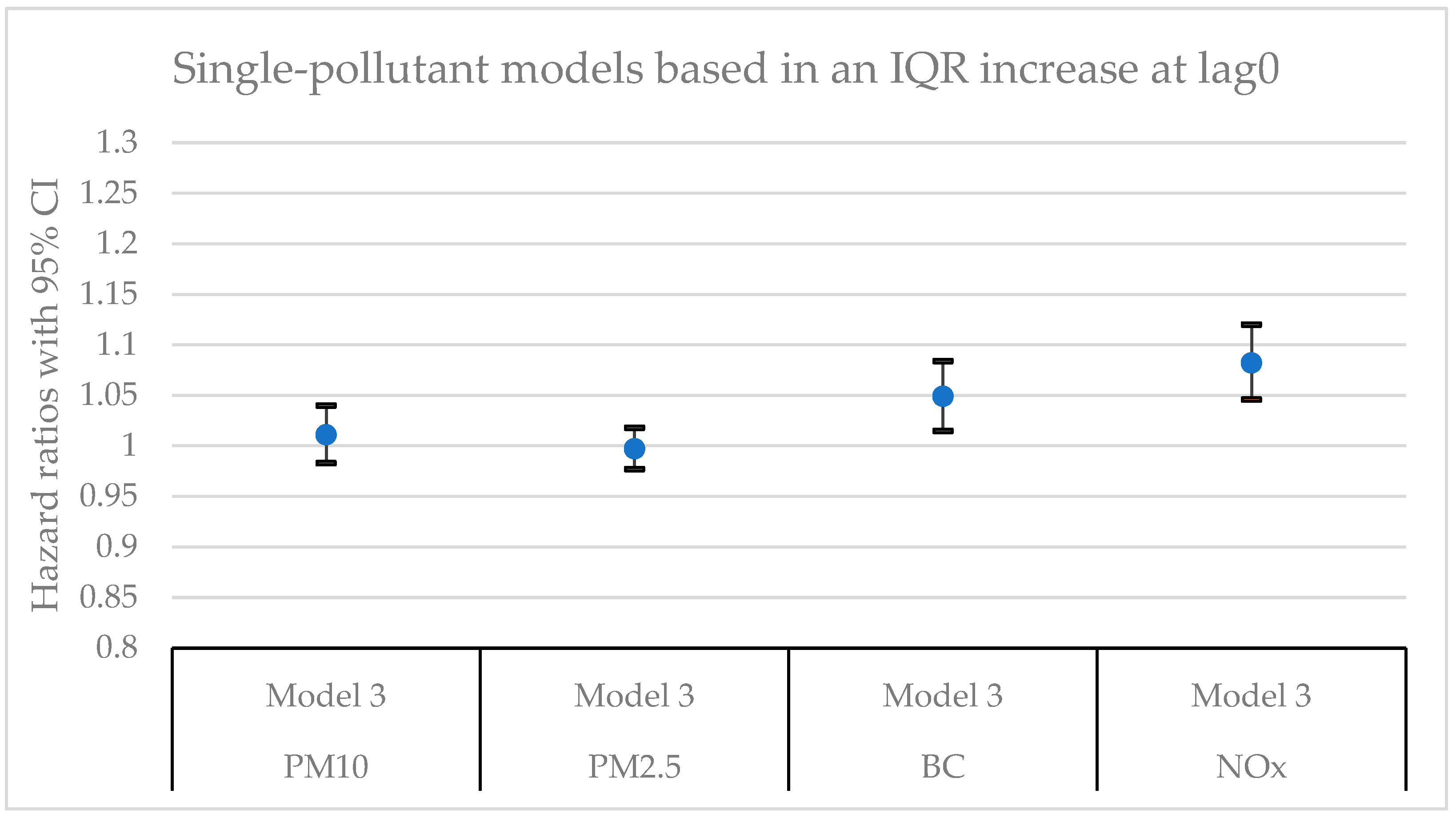
Figure 2.
Hazard ratios with 95% confidence intervals (CI) for the associations between natural-cause mortality and exposure to PM10, PM2.5, BC, and NOx based on lag0 in single-pollutant models with adjustments for all covariates (Model 3).
Figure 2.
Hazard ratios with 95% confidence intervals (CI) for the associations between natural-cause mortality and exposure to PM10, PM2.5, BC, and NOx based on lag0 in single-pollutant models with adjustments for all covariates (Model 3).
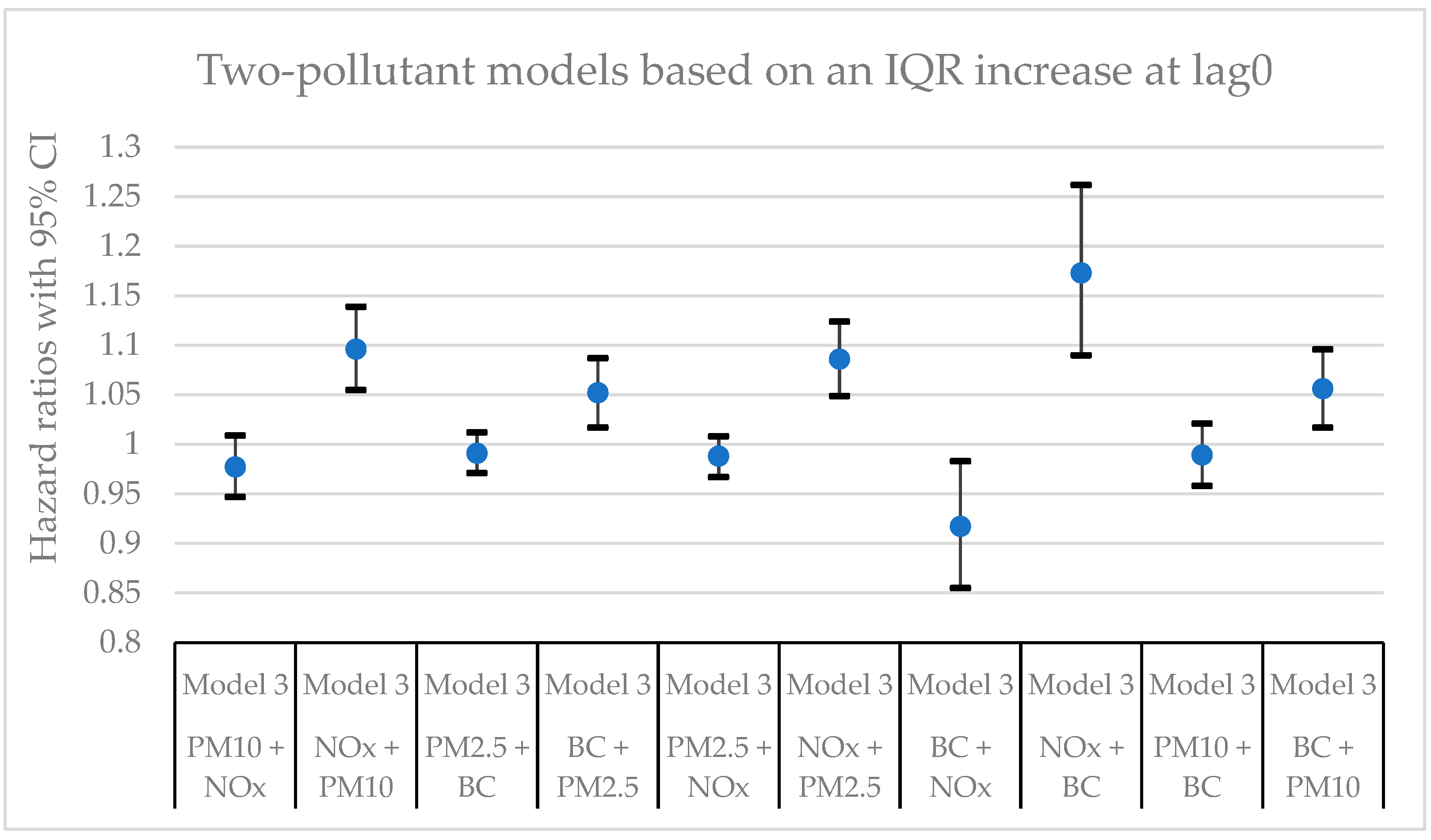
Figure 3.
Hazard ratios with 95% confidence intervals (CI) for the associations between natural-cause mortality and exposure to PM10, PM2.5, BC, and NOx based on lag0 in two-pollutant models with adjustments for all covariates (Model 3). The hazard ratios refer to the pollutant listed first.
Figure 3.
Hazard ratios with 95% confidence intervals (CI) for the associations between natural-cause mortality and exposure to PM10, PM2.5, BC, and NOx based on lag0 in two-pollutant models with adjustments for all covariates (Model 3). The hazard ratios refer to the pollutant listed first.
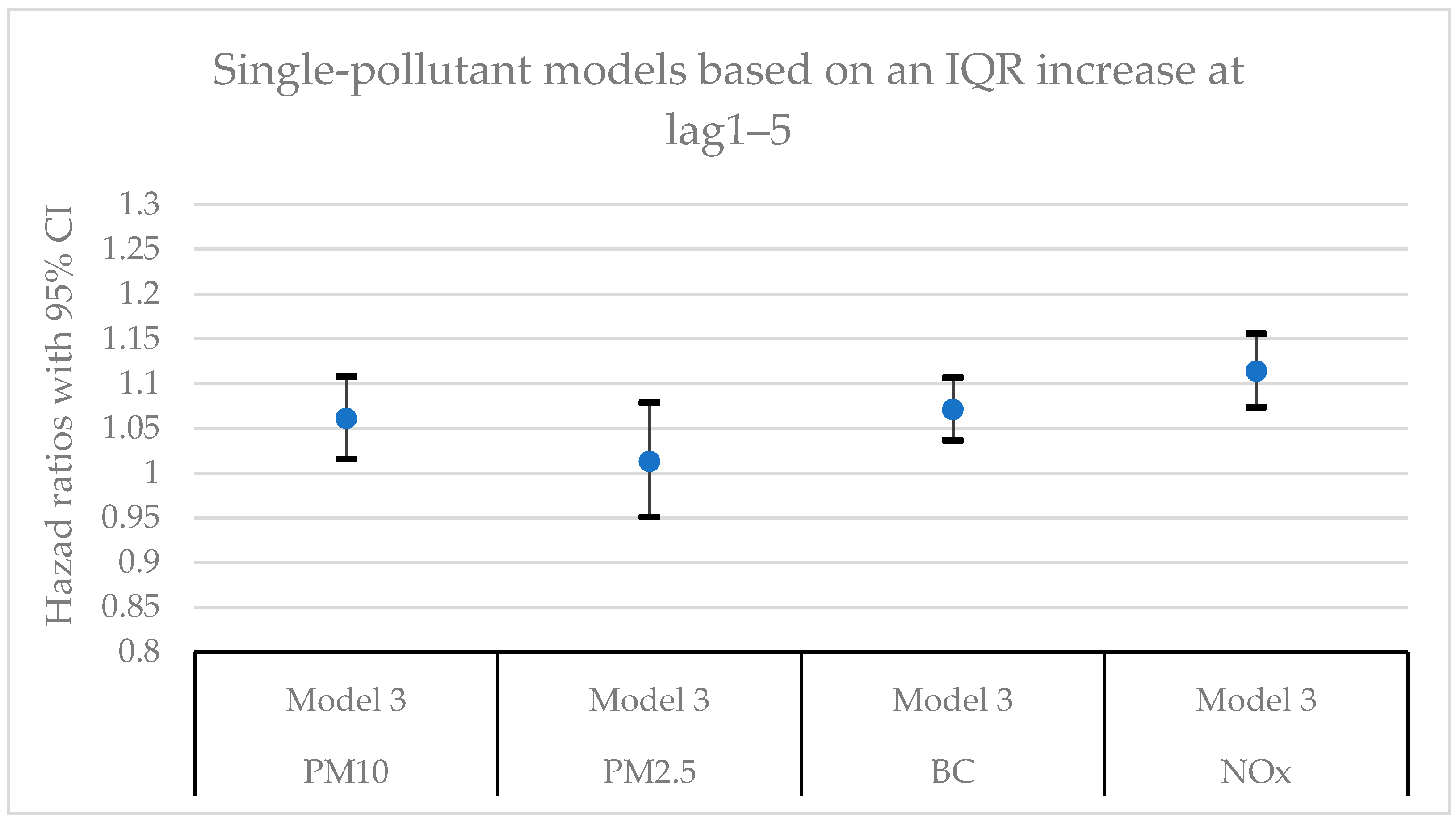
Figure 4.
Hazard ratios with 95% confidence intervals (CI) for the associations between natural-cause mortality and exposure to PM10, PM2.5, BC, and NOx based on lag1‒5 in single-pollutant models with adjustments for all covariates (Model 3).
Figure 4.
Hazard ratios with 95% confidence intervals (CI) for the associations between natural-cause mortality and exposure to PM10, PM2.5, BC, and NOx based on lag1‒5 in single-pollutant models with adjustments for all covariates (Model 3).
Figure 5.
Hazard ratios with 95% confidence intervals (CI) for the associations between natural-cause mortality and exposure to PM10, PM2.5, BC, and NOx based on lag1‒5 in two-pollutant models with adjustments for all covariates (Model 3). The hazard ratios refer to the pollutant listed first.
Figure 5.
Hazard ratios with 95% confidence intervals (CI) for the associations between natural-cause mortality and exposure to PM10, PM2.5, BC, and NOx based on lag1‒5 in two-pollutant models with adjustments for all covariates (Model 3). The hazard ratios refer to the pollutant listed first.
Figure 6.
Hazard ratios with 95% confidence intervals (CI) for the associations between natural-cause mortality and exposure to PM10, PM2.5, BC, and NOx based on lag6‒10 in single-pollutant models with adjustments for all covariates (Model 3).
Figure 6.
Hazard ratios with 95% confidence intervals (CI) for the associations between natural-cause mortality and exposure to PM10, PM2.5, BC, and NOx based on lag6‒10 in single-pollutant models with adjustments for all covariates (Model 3).
Figure 7.
Hazard ratios with 95% confidence intervals (CI) for the associations between natural-cause mortality and exposure to PM10, PM2.5, BC, and NOx based on lag6‒10 in two-pollutant models with adjustments for all covariates (Model 3). The hazard ratios refer to the pollutant listed first.
Figure 7.
Hazard ratios with 95% confidence intervals (CI) for the associations between natural-cause mortality and exposure to PM10, PM2.5, BC, and NOx based on lag6‒10 in two-pollutant models with adjustments for all covariates (Model 3). The hazard ratios refer to the pollutant listed first.
Table 1.
The modeled concentrations (µg m-3) of air pollutants in the study area during the period from 1991‒2018.
Table 1.
The modeled concentrations (µg m-3) of air pollutants in the study area during the period from 1991‒2018.
| Air pollutant |
Number of obs. |
Mean |
SD |
Median |
25
percentile |
75
percentile |
Min. |
Max. |
| PM10 lag0 |
552,608 |
15.9 |
2.2 |
15.7 |
14.5 |
17.2 |
9.7 |
27.6 |
| PM2.5 lag0 |
552,608 |
10.9 |
1.8 |
10.8 |
9.8 |
11.4 |
6.6 |
18.4 |
| BC lag0 |
552,608 |
1.0 |
0.1 |
1.0 |
0.9 |
1.1 |
0.7 |
1.9 |
| NOx lag0 |
552,608 |
26.5 |
8.8 |
24.9 |
20.8 |
30.5 |
6.8 |
130.0 |
| Air pollutant |
Number of obs. |
Mean |
SD |
Median |
25
percentile
|
75
percentile
|
Min. |
Max. |
| PM10 lag1‒5 |
534,059 |
15.8 |
1.5 |
15.9 |
14.8 |
16.9 |
11.2 |
25.8 |
| PM2.5 lag1‒5 |
534,059 |
10.9 |
0.9 |
10.8 |
10.1 |
11.8 |
8.8 |
14.9 |
| BC lag1‒5 |
534,059 |
0.9 |
0.1 |
1.0 |
0.9 |
1.0 |
0.7 |
1.7 |
| NOx lag1‒5 |
534,059 |
28.3 |
10.0 |
26.3 |
21.7 |
33.0 |
7.8 |
134.0 |
| Air pollutant |
Number of obs. |
Mean |
SD |
Median |
25
percentile
|
75
percentile
|
Min. |
Max. |
| PM10 lag6‒10 |
417,278 |
15.6 |
1.6 |
15.6 |
14.5 |
16.8 |
11.2 |
24.6 |
| PM2.5 lag6‒10 |
417,278 |
10.7 |
0.9 |
10.6 |
10.0 |
11.5 |
8.8 |
13.8 |
| BC lag6‒10 |
417,278 |
0.9 |
0.1 |
0.9 |
0.9 |
1.0 |
0.7 |
1.7 |
| NOx lag6‒10 |
417,278 |
30.1 |
10.6 |
28.1 |
22.7 |
35.4 |
8.6 |
134.0 |
Table 2.
The continuous covariates included in the calculations.
Table 2.
The continuous covariates included in the calculations.
| Covariate |
Number of obs. |
Mean |
SD |
Min. |
Max. |
| Age at enrollment |
30,438 |
58.0 |
7.6 |
44.5 |
73.6 |
| Systolic blood pressure |
30,389 |
141.1 |
20.1 |
61 |
240 |
| Diastolic blood pressure |
30,386 |
85.6 |
10.0 |
40 |
150 |
| Waist/Hip ratio |
30,362 |
0.85 |
0.1 |
0.4 |
1.9 |
| Alcohol consumption (g day-1) |
28,228 |
10.7 |
12.7 |
0 |
194 |
Table 3.
The categorical covariates included in the calculations.
Table 3.
The categorical covariates included in the calculations.
| Covariate |
Number of obs. |
Category 1 |
Category 2 |
Category 3 |
| Gender |
30,438 |
Male (39.8%) |
Female (60.2%) |
‒ |
| Smoking status |
28,557 |
Never smoker (37.9%) |
Former smoker (33.8%) |
Current smoker (28.3%) |
| Educational level |
28,492 |
Elementary school (42.0%) |
High school (35.0%) |
College (23.0%) |
| Cohabitation |
28,554 |
Yes (75.4%) |
No (24.6%) |
‒ |
| Physical activity |
30,164 |
Low (33.1%) |
Medium (33.3%) |
High (33.5%) |
| Antihypertensive drugs |
28,446 |
Yes (17.8%) |
No (82.2%) |
‒ |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).