Submitted:
19 August 2023
Posted:
23 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Diabetes Mellitus (DM)
2.1. Introduction
2.2. Type of Diabetes
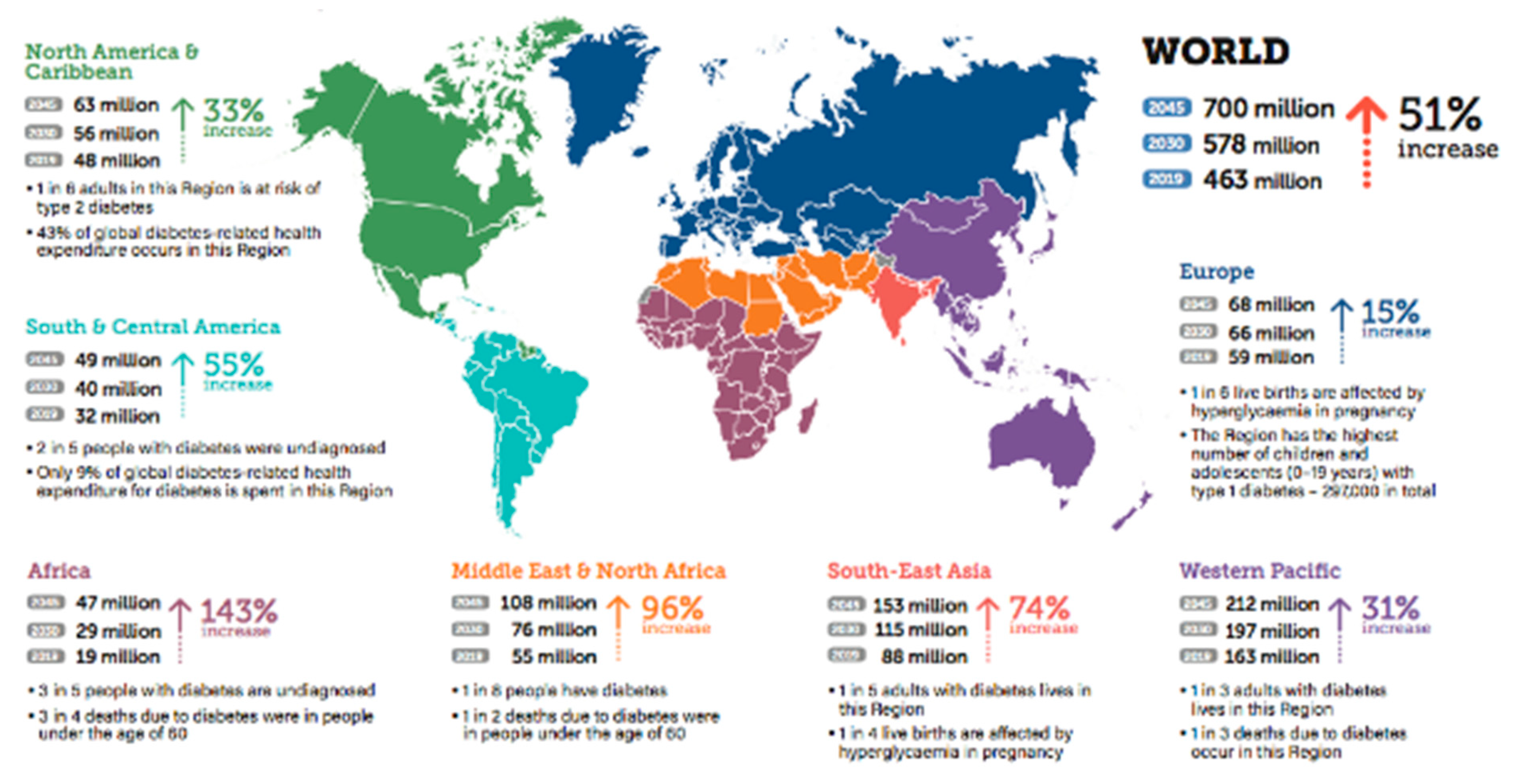
2.2.1. Type 1 diabetes
2.2.2. Type 2 diabetes
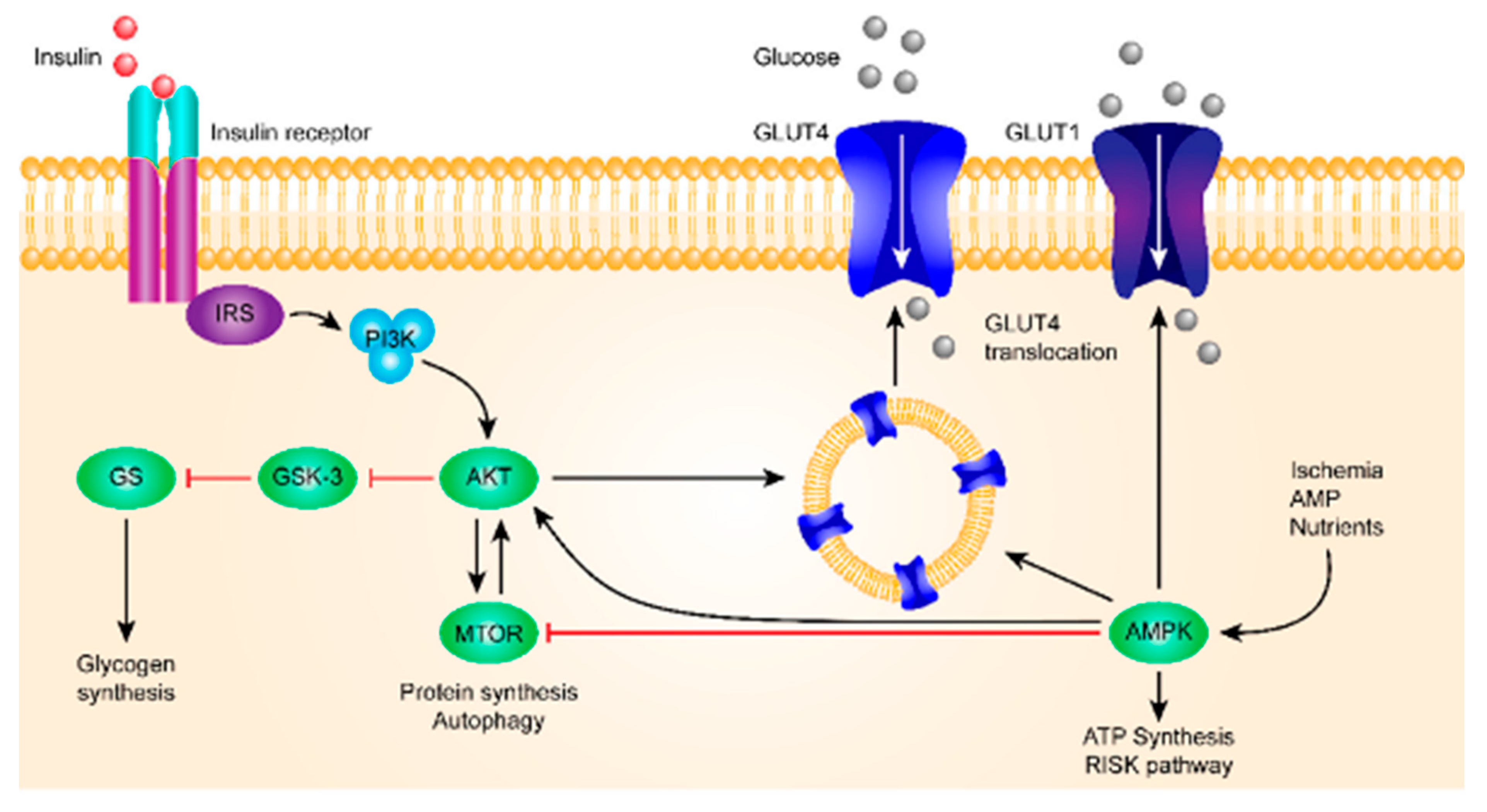
2.3. Pathology of diabetes
| Organ | HK computer | Glucose transporter | Classification | ||
|---|---|---|---|---|---|
| Erythrocyte | HK I | GLUT1 | Glucose-dependent | ||
| Brain | HK-I | GLUT1 | Glucose-dependent | ||
| GK beta-cell | HK IV B (glucokinase) | GLUT2 | Glucose-sensor | ||
| Liver | HK IV L | GLUT2 | Glucose-sensor | ||
| Kidney | ----- | GLUT3 symporter | Sodium-dependent | ||
| Gut | ----- | GLUT3 symporter | Sodium-dependent | ||
| Muscle | HK II | GLUT4 | Insulin-dependent | ||
| Adipocyte | HK II | GLUT4 | Insulin-dependent | ||
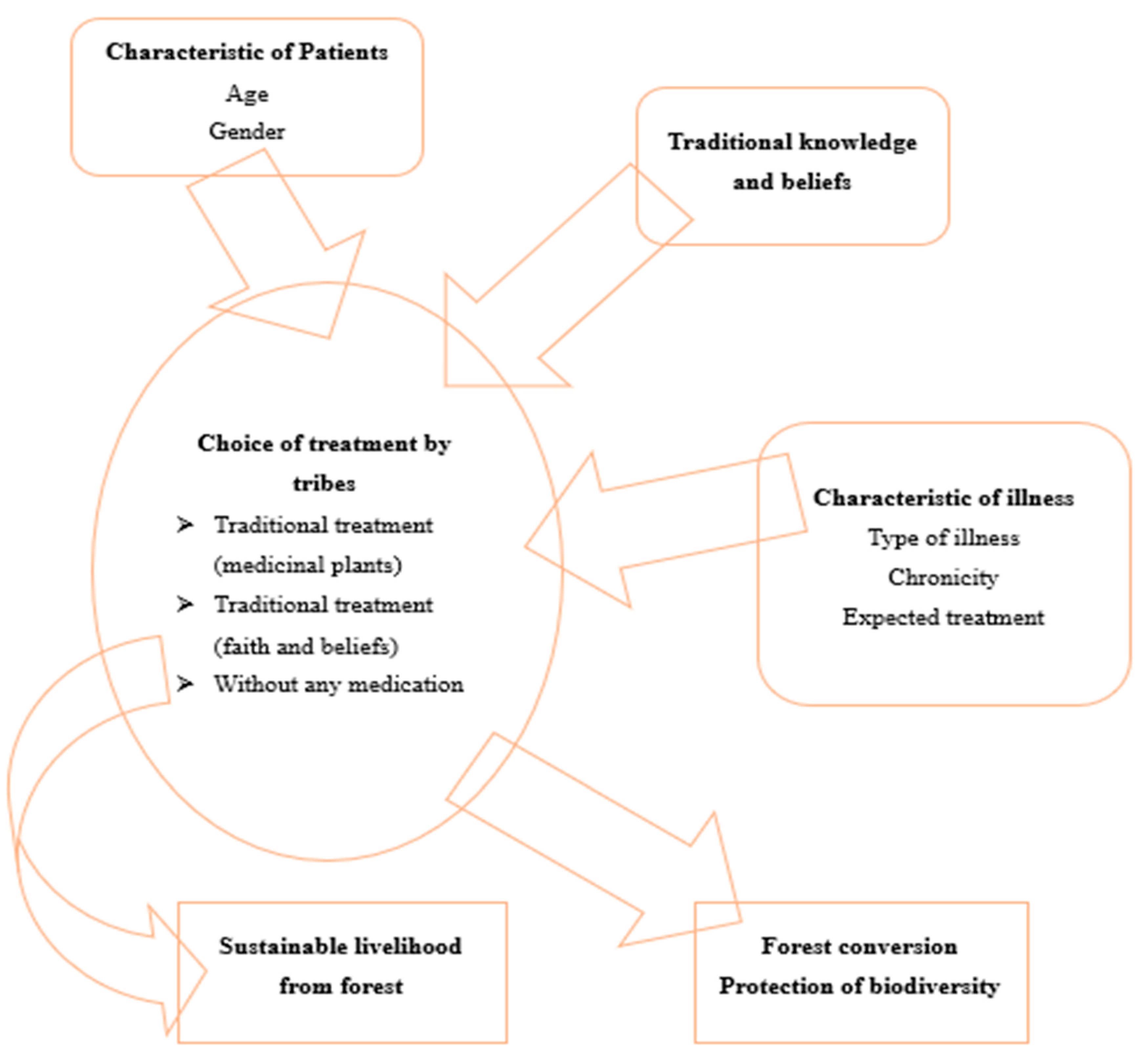
3. Traditional Medicinal Practices for Diabetes
3.1. Ethnomedicinal plants (Herbalism)
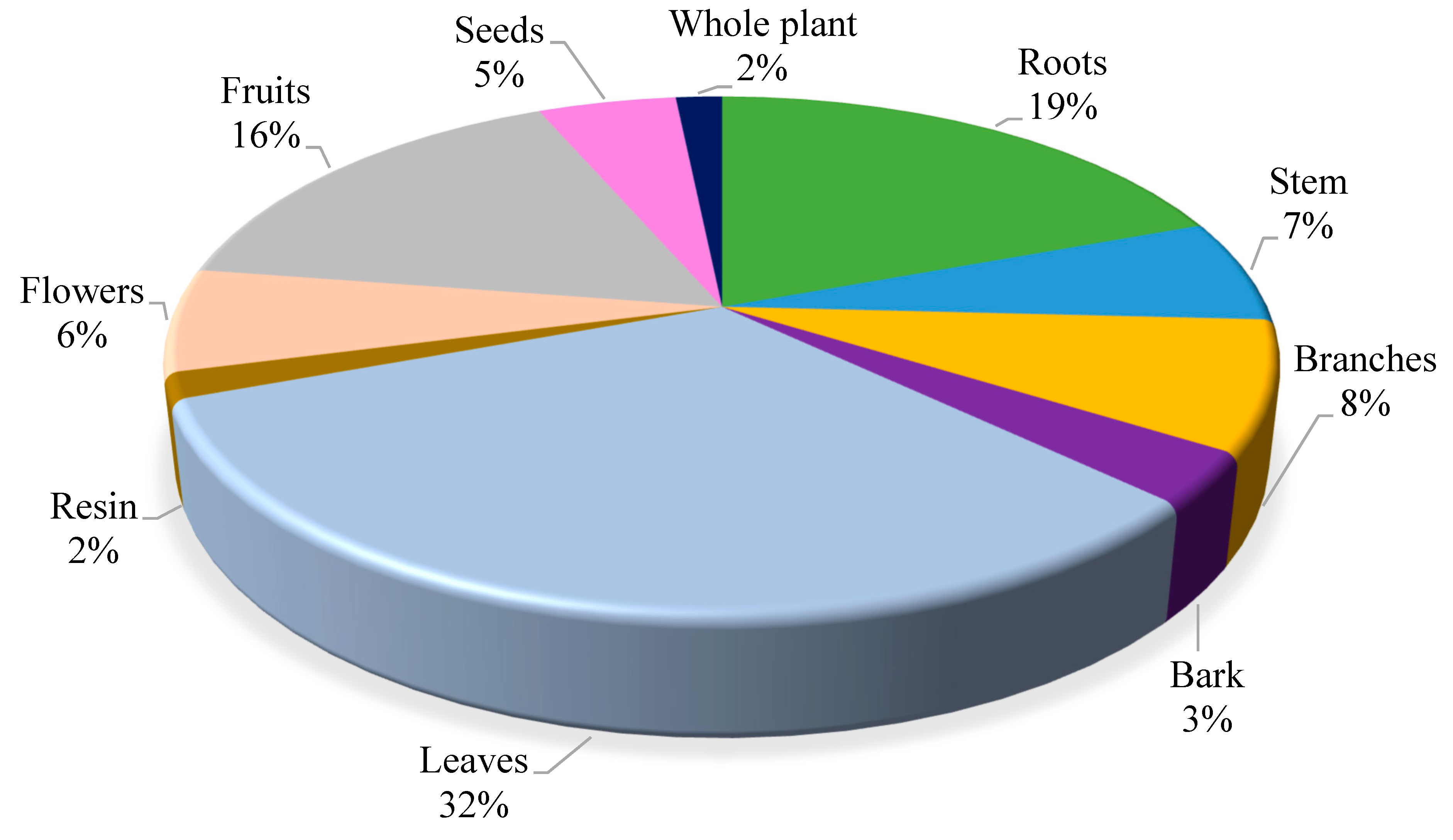
| S. No | Family | No. of species |
|---|---|---|
| 1 | Apiaceae | 2 |
| 2 | Asteraceae | 9 |
| 3 | Boraginaceae | 2 |
| 4 | Brassicaceae | 2 |
| 5 | Brassicaceae | 2 |
| 6 | Caprifoliaceae | 2 |
| 7 | Curpessaceae | 2 |
| 8 | Euphorbiaceae | 2 |
| 9 | Fumariaceae | 2 |
| 10 | Lamiaceae | 4 |
| 11 | Pinaceae | 4 |
| 12 | Ranunculaceae | 5 |
| 13 | Rosaceae | 3 |
| 14 | Rubiaceae | 2 |
| 15 | Solanaceae | 3 |
3.2. Acupuncture
| Acupuncture types and points | Effect on diabetes |
|---|---|
| Wrist-ankle acupuncture | Reduce pain caused by diabetic neuropathy |
| Electroacupuncture | Lower blood glucose levels, increase insulin sensitivity and improve pancreatic islet function |
| Herbal acupuncture | Enhance the effects of anti-diabetic medication, such as metformin |
| ST36 (Zusanli) | Improves blood glucose levels, insulin levels and glucose tolerance |
| SP6 (Sanyinjiao) | Improves blood glucose levels, insulin levels and glucose tolerance |
| LI11 (Quchi) | Improves blood glucose management, weight loss and insulin resistance |
| LI4 (Hegu) | Improves blood glucose management, weight loss and insulin resistance |
| ST25 (Tianshu) | Improves blood glucose management and weight loss |
| ST40 (Fenglong) | Improves blood glucose management and weight loss |
3.3. Massage Therapy
| Type of massage | Effects on diabetes treatments |
|---|---|
| Swedish massage | Lower blood glucose levels [53] |
| Connective tissue massage | Improve circulation in the lower limbs and slow the progression of the peripheral arterial disease [51] |
| Thai foot massage | Improve range of motion, ability to stand up, and foot sensation [53] |
| Other types of foot massage | Increase balance and mobility [53] |
| Traditional Chinese massage | Improve neuropathy symptoms [52] |
| Abdominal massage | Regulate muscle, pancreatic, and inflammatory factors, and islet function to improve disorders of lipid and glucose metabolism [54] |
| Massage at the site of insulin injection | Lower levels of blood glucose and enhance action of serum insulin in type 1 diabetes patients [52] |
3.4. Dietary interventions for diabetes prevention
4. Evidence-based Ethnomedicine for Diabetes
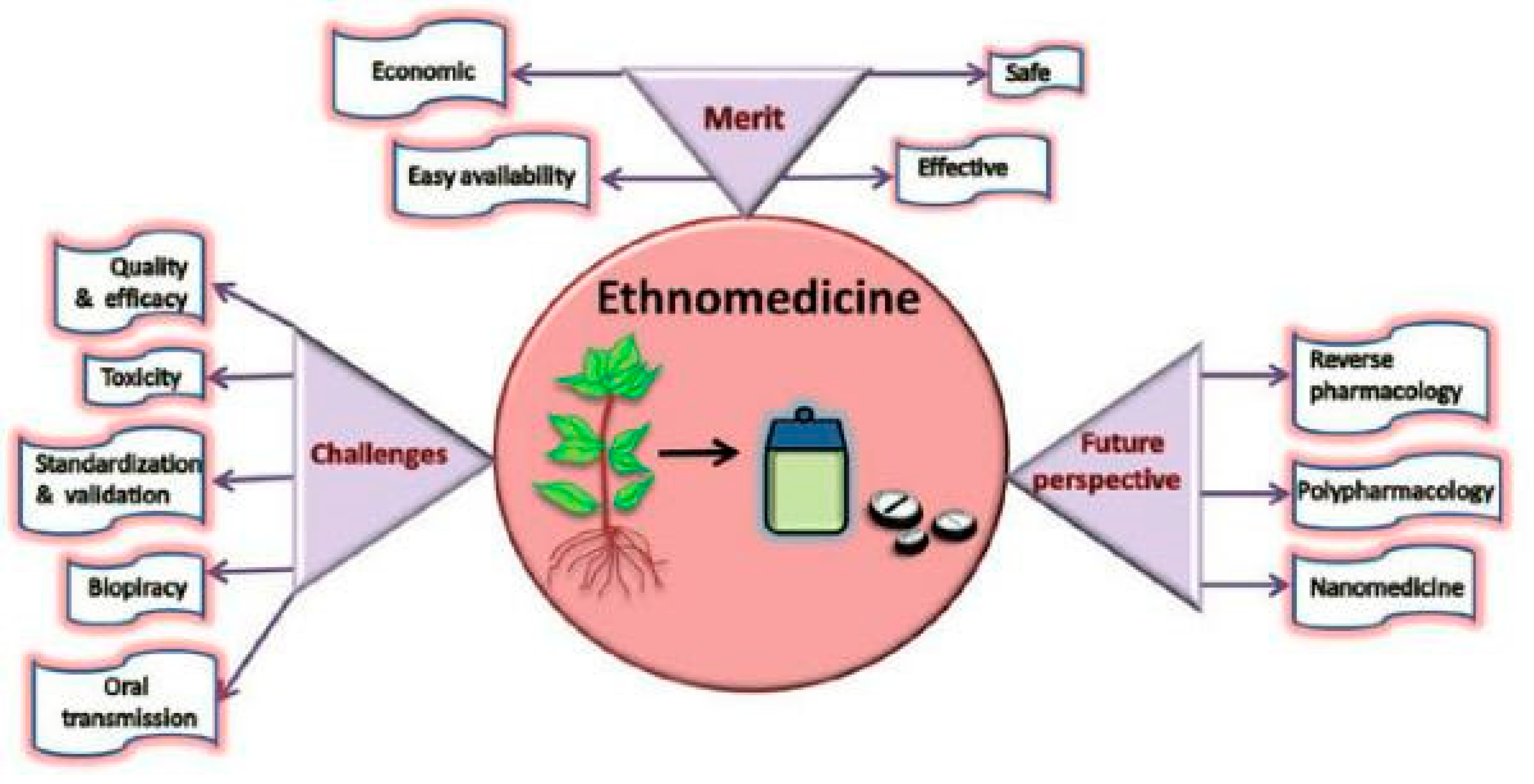
5. Herbal Remedies for Diabetes1
5.1. Momordica charantia L. (bitter melon)
5.2. Syzygium cumini (L.) Skeels (black plum)
5.3. Allium sativum L. (garlic)
5.4. Gymnema Sylvestre

5.5. Cymbopogon Citrullus
5.6. Hagenia abyssinica
5.7. Aloe vera
5.8. Clausena anisata
5.9. Cajanus cajan
5.10. Artemisia afra
5.11. Persea americana
5.12. Azadirachta indica
5.13. Catharanthus roseus
5.14. Olea europaea
5.15. Trigonella foenum-graecum
6. Dietary Interventions for Diabetes
6.1. Traditional diets for diabetes
6.1.1. Examples of key traditional types
6.1.1.1. The Mediterranean diet:
6.1.1.2. The Asian diet
6.1.1.3. The Nordic diet:
6.1.1.4. The Native American diet:
6.1.2. Some common features of these traditional diets are -
- ⇒
- Emphasize plant-based foods, like vegetables, fruits, nuts, whole grains, seeds, etc.
- ⇒
- Incorporate moderate portions of animal-based foods, such as poultry, fish, lean meats, eggs, and dairy.
- ⇒
- Limit processed foods, refined grains, added sugars, and trans fats.
- ⇒
- Use healthy fats, like canola oil, olive oil, avocado oil, and nut oils.
- ⇒
- Incorporate spices and herbs for flavour and health benefits.
6.1.3. The benefits of the traditional diets are [101,104,105,106,107,108,109,110] -
- ⇒
- Abundant in omega-3 fatty acids from nuts and fish which can lower triglyceride levels improve blood vessel function lower blood pressure and prevent or reduce insulin resistance.
- ⇒
- Rich in fibre which can slow down the digestion and absorption of carbohydrates lower blood sugar spikes after meals improve insulin sensitivity lower cholesterol levels and promote satiety.
- ⇒
- Rich in antioxidants phytochemicals and micronutrients which can protect against oxidative stress inflammation and cellular damage caused by high blood sugar levels.
- ⇒
- Moderate in protein from plant and animal sources which can help preserve muscle mass increase metabolic rate regulate appetite hormones and prevent or delay the onset of diabetic kidney disease.
6.1.4. Traditional dietary interventions for different populations and settings
6.1.4.2. Age
6.1.4.3. Ethnicity
6.1.4.4. Pregnancy
6.1.4.1. Socioeconomic status
6.1.4.5. Environmental context
6.2. Examples of evidence-based ethno-diets
6.2.1. Macronutrient’s diet
6.2.2. Micronutrients diets
6.2.2.1. Magnesium:
6.2.2.2. Zinc:
6.2.2.3. Chromium:
6.2.2.4. Vitamin D:
6.2.2.5. Vitamin B12,
6.2.3. Low-carbohydrate diets
6.2.4. Other dietary factors that influence diabetes outcomes are
6.2.4.1. Fibre:
6.2.4.2. Alcohol:
6.2.4.3. Non-nutritive sweeteners (NNS):
7. Challenges and their reason
7.1. Lack of standardization
7.2. Contamination
7.3. Lack of regulation
7.4. Variability
7.5. Lack of methodology
7.6. Adverse effects
7.7. Interactions
7.8. Limitations
7.9. Lack of evidence
7.10. Insufficient scientific evidence
7.11. Inadequate clinical trials
7.12. Limited availability and accessibility
7.13. Ethical concerns
7.14. Cultural barriers and prejudices
7.15. Lack of funding and collaboration
8. Future direction to use of ethnomedicine for Diabetes
9. Conclusion
-

- Ethnomedicine is a valuable source of knowledge and medicine for the treatment of diabetes mellitus, a chronic metabolic disorder with complex pathogenesis and varied presentation.
-

- It comprises the traditional use of plants and natural products for treating diabetes mellitus, which has been practised for centuries by different cultures and regions around the world.
-

- Glucose metabolism is influenced by ethnomedicine through various mechanisms, such as reducing absorption and production of glucose in the gut and liver respectively, increasing utilization in muscles and adipose tissues, and stimulating insulin secretion.
-

- Ethnomedicine may also have beneficial effects on other aspects of diabetes mellitus, such as oxidative stress, inflammation, lipid profile, and complications.
-

- Several ethnomedicinal plants and natural products have been evaluated in clinical trials for their efficacy and safety in treating diabetes mellitus, such as bitter melon, fenugreek, ginseng, cinnamon, garlic, ginger, turmeric, aloe vera, nopal, berberine, and ethnomedicine.
-

- Various ethnomedicinal plants and natural products contain antidiabetic compounds that work through different mechanisms, such as momordicoside, karaviloside, cucurbitacin, charantin, charantoside, cuminoside, S-allyl cysteine sulfoxide, limonoids, vindoline, vindolidine, vindolicine, vindolinine, oleuropein, oleanolic acid, vicenin-1, isoschaftoside, schaftoside, gymnemosides, gymnemagenin, pregnane glycosides and chysalodin.
-

- Low-carbohydrate diets restrict carbohydrate intake and emphasize protein and fat sources and thus show potential benefits for diabetes management and prevention by lowering blood sugar levels and reducing the need for insulin or medication. However, it is not suitable for everyone and may have some drawbacks or risks.
-

- However, despite the promising results of ethnomedicine in treating diabetes mellitus, there are still many challenges and limitations that need to be addressed before they can be widely accepted and used in clinical practice.
-

- Some of these challenges include the lack of standardization, quality control, pharmacokinetic and pharmacodynamic data, dose-response relationship, long-term safety and efficacy data, biomarker evaluation, patient compliance, regulatory approval, and intellectual property rights.
-

- Therefore, more rigorous and comprehensive research is needed to validate the ethnomedicinal plants and natural products for diabetes mellitus and to explore their mechanisms of action, optimal dosage, potential interactions, adverse effects and cost-effectiveness.
Author Contributions
Funding
Ethical Approval and Consent to Participate
Consent for publication
Availability of data and materials
Acknowledgements
Competing interests
Guarantor
Annexure 1.
| S. No. | Local Name | Taxon Name | Family | Part(s) Used | Ethnomedicinal Uses | Altitude Range; Flowering Phenology |
| 1. | Aal | Viscum album | Loranthaceae | Whole plant | Laxative And Fractures | 1000-700m. Flowering: Sept- Dec. |
| 2. | Alua | Solanumtuberosum | Solanaceae | tuber | Burns On The External Body Parts And Tightly Fastened With A Woollen Cloth. | 1600-2500, March-April |
| 3. | Anjeer | Ficus carica | Moraceae | Stem, milky | Insect Bite and Warts. Birth Rate Control, Latex, Fruit Pulp | 5,420m Flowering: May-August. |
| 4. | Bann Hulla | Tussilago farfara | Asteraceae | Leaves | Astringent, Emollient, Expectorant, Stimulant and Tonic | 2800-3800 m. Flowering: January-April. |
| 5. | Banwangun | Podophyllum hexandrum | Berberidaceae | leaves and roots | Skin Diseases, Gastric Problem | 2400-4500 m. Flowering: May-August. |
| 6. | Bazarbang | Hyoscyamus niger | Solanaceae | Seed | Tooth Ache | 2100-3300 m. Flowering: May-September |
| 7. | Bhang | Cannabis sativa | Cannabaceae | Leaves, seeds | Ear-Ache, Blood Purifier, Scabies | 2000-2500m Flowering: May-July |
| 8. | Bhuz | Betula utilis | Betulaceae | bark | Antiseptic | 4000-4,500m Flowering: April-May. |
| 9. | Bithur | Juniperus communis | Curpessaceae | Leaves | Rheumatism | 1800-3600 m. Flowering: April-May. |
| 10. | Brag Kund | Ziziphus mauritiana | Rhamnaceae | Leaves | Skin rashes | 1300-1800m; Flowering: April-May |
| 11. | Brand | Phytolacca acinosa | Phytolaccaceae | Root | Narcotic Effect, Sedative | 1500-3000m. Flowering: June-Sept. |
| 12. | Bunufsha | Viola odorata | Violaceae | Leaves, | Seeds And Flowers Respiratory Problems | 1800-2600m; Flowering: May-July |
| 13. | Chad | Pinus roxburghii | Pinaceae | Seeds and gums | General Weakness After Child Birth | 600-2300 m Flowering: March-June |
| 14. | Chella lubbar | Atropa acuminata | Solanaceae | Roots and leaves | Cough and Antispasmodic | 1800-3040 m. Flowering: June-July. |
| 15. | Choora | Angelica glauca | Apiaceae | Root | Vomiting | 1800-3700 m. Flowering: June-August |
| 16. | Daan kul | Punica granatum | Punicaceae | Seed | Jaundice and Anaemia | 2000 m-2500m Flowering: Jan–Feb |
| 17. | Daduejaid | Aquilegia fragrans | Ranunculaceae | Flowers | Indigestion | 2400-3600 m. Flowering: June-August. |
| 18. | Daech | Vitis vinifera | Vitaceae | Leaves | Skin Rashes, Sores, Eruptions | 1700-2100m; Flowering: April-May |
| 19. | Dand jari | Rhodiola himalensis | Crassulaceae | bark | Infection Of Teeth | 3300-4800 m. Flowering: June-August. |
| 20. | Danival | Coriandrum sativum | Apiaceae | Seeds | Hair Fall | 500-800m Flowering: April-May. |
| 21. | Danthiveer | Salix wallichiana | Salicaceae | Leaves | Fever, Head Ache, General Body Pain | 1900-2400, Flowering: April-June |
| 22. | Daraunm | Cynodon dactylon | Poaceae | Whole plant | Common Cold | 2600m Flowering: Aug -Oct. |
| 23. | Datur | Datura stramonium | Solanaceae | Seeds | Rheumatism, Frost Bite, Toothache, Tonic | 50-2200m Flowering: July-Sept. |
| 24. | Desibangara | Gentiana kurroo | Gentianaceae | Root | Stomach-ache and Urinary Infections | 1800-2700 m. Flowering: August-October |
| 25. | Divdar | Cedrus deodara | Pinaceae | Stem, Bark | Skin Rashes and External Ulcers | 1,500–3,200 m Flowering: May-July. |
| 26. | Doan kul | Juglans regia | Juglandaceae | Leaf, Bark | Tooth Infection, Scrofula, Rickets And Leucorrhoea | 3,000-4,000 m Flowering: March -April |
| 27. | Gautheer | Dryopteris sp | Pteridaceae | Aerial portion | Cure Kidney and Gall Stones. | 1600-2000 |
| 28. | Gulab | Rosa webbiana | Rosaceae | Flowers | Cough and Colds. | 1500 m - 4000 m. Flowering: May-July |
| 29. | Guri-dud/ Harbi | Euphorbia wallichii | Euphorbiaceae | Stem, leaves, latex | Skin Diseases | 2200-4100 m. Flowering: May-August. |
| 30. | Gurisochol, Gandi booti | Euphorbia helioscopia | Euphorbiaceae | Seeds, roots and latex | Abdominal Cramps, Cholera And Eruptions | 300-1800 m. Flowering: April-June. |
| 31. | Hand | Taraxacum officinale | Asteraceae | Roots | Back Pain, Common Cold, Chest Infection | 1600-2400, Flowering: May-July |
| 32. | Hapat makei | Arisaema jacquemontiana | Araceae | Rhizome | Muscular Strength and Skin Infections | 582 m. - 3819 m Flowering: November -February |
| 33. | Hapatfal | Sambaucus wightiana | Caprifoliaceae | Root | Leaves Chest Congestion, Boils | 1500-3600m. Flowering: June-July. |
| 34. | Jandi | Indigofera heterantha | Leguminosae | Leaves | Internal Body Disorders | 1500-3000 m. Flowering: May-June. |
| 35. | Jangli dodal | Gnaphalium affine | Asteraceae | Leaves | Antiperiodic, Antitussive, Expectorant and Febrifuge | 1200-3000 m. Flowering: Feb-Oct. |
| 36. | Jawand | Thymus serpyllum | Lamiaceae | Leaves, Seeds | Skin Eruptions; (Alopecia). Seed Powder Is Given To Children Against Worm Infection. | 1800-2300, Flowering: May-July |
| 37. | Kaenak | Triticum aestivum L. | Poaceae | Seeds | For The Treatment Of Worms | 1600-1900, Flowering: March-April |
| 38. | Kah Zaban | Arnebia benthamii | Boraginaceae | Rhizome | Common Cold, Cough, Fever, Blood Purifier | 1300-4500 m Flowering: May-July. |
| 39. | Kashkhas | Papaver somniferum | Papaveraceae | Fruit Dry | Cough, Diarrhoea | 585- 2056m Flowering: April-June |
| 40. | Kauri booti | Ajuga bracteosa | Lamiaceae | Stem, leaves | Ulcer, Colic and Jaundice | 1000-1500m. Flowering: March-December |
| 41. | Kawdach | Berberis lyceum | Berberidaceae | Roots | Indigestion, Constipation | 900-2900 m Flowering: March-June |
| 42. | Kazal-Handh | Cichorium intybus | Asteraceae | Root | Rheumatism Sore Throat, Jaundice, | 4000-5000 metres. Flowering: June- Sept. |
| 43. | Kim | Morina longifolia | Dipsacaceae | Roots | Insecticide | 3000-4000 m. Flowering: June-September |
| 44. | Kour | Picrorhiza kurrooa | Scrophulariaceae | Roots, Rhizome | Fever, Appetizer | 3300-4300 m. Flowering: June-August |
| 45. | Kown | Sambucus wightiana | Sambucaceae | roots, leaves and | Diuretic, Purgative | 1300-4500 m : Flowering: May –Nov. |
| 46. | Kraeth | Dioscorea deltoidei | Discoreaceae | Leaf | ophthalmic Infections, Urinary Infections | 450-3100 m. Flowering: May-July. |
| 47. | Kukliporte | Cuscuta | Cuscutaceae | Whole Plant | Joint Pains, Wound Healing and Falling Of Hairs | 1400 m Flowering: Dec - Feb |
| 48. | Kulhak | Nasturtium officinale | Brassicaceae | Leaf | Stomachic | 1500-4000m. Flowering: April-June. |
| 49. | kulmanch | Viburnum grandiflorum | Caprifoliaceae | Seed | Typhoid, Whooping Cough | 2700-3600 m. Flowering: April-May. |
| 50. | kulwauth | Prunella vulgaris | Lamiaceae | flower | Headache, Fever, Muscular Pain | 1600-1900m Flowering:June-July |
| 51. | kuth | Saussurea costus | Asteraceae | Rhizome | Joint Pain, Back Pain, Sole Ulcers, Dysentery, Fever, Urinary Problems | 2000-3300 m. Flowering: July-August. |
| 52. | Loothar | Galium aparine | Rubiaceae | Leaves | Jaundice, Antiseptic | 3500 m. Flowering: March-July. |
| 53. | Losdhi | Stellaria media | Caryophyllaceae | Seed | Skin Infection, Allergy | 1500-2500, Flowering: April-Sept. |
| 54. | Mazarmund | Iris kashmiriana | Iridaceae | Whole plant | Joint Pains | 1500-1800 m. Flowering: April-June |
| 55. | Meth | Trigonella foenum- graecum | Fabaceae | Seeds | Back Pain | 1300-1400m. Flowering: Jan- Apr. |
| 56. | Mongol | Senecio grandiflorus | Asteraceae | Leaves, flowers | Dermatitis, Stomach-ache | 1200 -4100 m Flowering: March-Sept. |
| 57. | Neelaan | Hackelia uncinatum | Boraginaceae | Flowers | Expectorant, Healing Wounds, Treating Tumours | 2700-4200 m. Flowering: June-August. |
| 58. | Nuner | Portulaca oleracea | Portulacaceae | leaves | For Liver Inflammation, Cough, Extract Of Whole Plant Is Taken. For Burns Crushed Plant Is Applied On Affected Area | 2000-2800 m. Flowering: March-June |
| 59. | Obej | Rumex acetosa | Fabaceae | For Stomach Problems, Whole Plant Is Eaten As Vegetable. For sting of nettles, leaves are rubbed on affected part to get relief. | 2100-4100 m Flowering: April-June | |
| 60. | Paewakh | Aconitum heterophyllum | Ranunculacea, | Root | Antidote For Snake Bites, To Treat Headache and Cough. | 2,400–4,500 m Flowering: April-May |
| 61. | Pahal gassesh | Achillea millefolium Berguer | Asteraceae | Rhizome, | Leaves Headache, Cough, Tooth Ache | 1050-3600 m. Flowering: Sept-Oct |
| 62. | Pahal-laish | Cardamine impatiens | Brassicaceae | Whole plant | Asthma, Hay Fever | 1500-4000 m. Flowering: May-July. |
| 63. | Pambechalan | Rheum emodi | Polygonaceae | Leaves | Rheumatic Pain, Wounds, Dislocated Joints, Boils | 2500-3500, June-August |
| 64. | Parglas | Asparagus officinalis | Liliaceae | whole plant, root | Toothache, Rheumatism, Female Infertility | 1,500–3,200 m Flowering: April –July |
| 65. | Phughood | Arctium lappa | Asteraceae | Leaves, root | Skin Disease, Boils, Body Pain | 2100-3700 m, Flowering: July-September. |
| 66. | Poshkar | Lamium album | Lamiaceae | Whole plant, leaves, flowers | Cough, Metrorrhagia | 1500-3700 m Flowering: April-July. |
| 67. | Pugsley, Shahtaur | Fumaria indica | Fumariaceae | Whole plan | Dyspepsia, Rheumatism | 2400 m. Flowering: April-May. |
| 68. | Rubes | Rubia cordifolia | Rubiaceae | Roots | Stomach-ache, Jaundice | 300-2800 m. Flowering: June-August. |
| 69. | Sal | Abies pindrow | Pinaceae | Bark | Rheumatism | 2100-3600 m. Flowering: April-May. |
| 70. | Sangi-harb | Corydalis govaniana | Fumariaceae | leaves | Respiratory Disorders, Chest Infections, Asthma | 2400-4800m. Flowering: May-August. |
| 71. | Shoonkar | Geum elatum | Rosaceae | Root | Astringent, Dysentery and Diarrhoea | 3500--5400 m. Flowering: June-August. |
| 72. | Soi | Urtica dioica | Urticaceae | Leaves and Roots | Rheumatism | 1000-2500 m. Flowering: Aug-Sept. |
| 73. | Sotal | Malva sylvestris | Malvaceae | seeds | Cough, Fever, Eye Sight | 2500-3500 m Flowering: April-June |
| 74. | Sozposh | Lavatera kashmiriana | Malvaceae | Flower | Mumps, Skin Irritation In Pregnant Women | 1500-3200m Flowering: July – Sept. |
| 75. | Srub | Anemone obtusiloba | Ranunculaceae | Seeds | Rheumatism | 2100-4300 m. Flowering: May-July. |
| 76. | Tethwan | Artemisia absinthium | Asteraceae | Leaves | Obesity, Diabetes, Liver Infection | 1,500-2,100 m. Flowering: June onwards |
| 77. | Tilla | Aconitum violaceum | Ranunculaceae | Root | Antidote for Snake Bites | 3600-4800 m. Flowering: July-September. |
| 78. | Trul | Impatiens glandulifera | Balsaminaceae | Leaves | Skin Burn, Joint Pain | July to August 1800–3200 meters |
| 79. | Tsok-tsen | Oxalis corniculata | Oxalidaceae ion, Diarrhoea | Whole plant, leaves. | Toothache, Convulsions, Blood Purification | 500-800m Flowering: April-June. |
| 80. | Uzmposh | Androsace rotundifolia | Primulaceae | Rhizome | Cataract | 1500-3600 m. Flowering: June-July. |
| 81. | Vangogil | Nepeta raphanorhiza | Lamiaceae | Whole plant, | Dysentery, Toothache | 1300-1500m Flowering: Jun-Sept. |
| 82. | Via-gander | Acorus calamus | Acoraceae | Rhizome | Stomachic, Diarrhoea, Cough, Swellings, Joint Pain and Piles | 1600-2800.Flowering: July-September |
| 83. | Wantamook | Verbascum Thapsus | Scrophulariaceae | Flowers and stem | Cough, Pneumonia | 2500-4500 m. Flowering: June-Aug. |
| 84. | Zakhmi hayat | Berginia ligulate | Saxifragaceae | leaves and roots | Intestine Complaints and Stomach Ulcers | 1800-4300m. Flowering: March-July. |
| 85. | Caltha alba | Ranunculaceae | Leaves | Pain And Cramps, For Menstrual Disorders | 2400-4000 m. Flowering: May-August. | |
| 86. | Juniperus recurve | Curpessaceae | Leaves | Rheumatism Insecticide | 3,000-4,000m Flowering: May-June. |
References
- Lans, C.A. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J Ethnobiol Ethnomed 2006, 2. [Google Scholar] [CrossRef]
- Kumar, M.; Rawat, S.; Nagar, B.; Kumar, A.; Pala, N.A.; Bhat, J.A.; et al. Implementation of the Use of Ethnomedicinal Plants for Curing Diseases in the Indian Himalayas and Its Role in Sustainability of Livelihoods and Socioeconomic Development. Int J Environ Res Public Health 2021, 18, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Diliarosta, S.; Sari, M.P.; Ramadhani, R.; Efendi, A.; Diliarosta, S.; Sari, M.P.; et al. Ethnomedicine Study on Medicinal Plants Used by Communities in West Sumatera, Indonesia. Natural Medicinal Plants 2021. [CrossRef]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J Diabetes 2016, 7, 354. [Google Scholar] [CrossRef]
- Tiwari, P.; Ahmad, K.; Hassan Baig, M. Gymnema sylvestre for Diabetes: From Traditional Herb to Future’s Therapeutic. Curr Pharm Des 2017, 23, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gong, J.; Huang, W.; Lu, F.; Dong, H. The Effect of Momordica charantia in the Treatment of Diabetes Mellitus: A Review. Evid Based Complement Alternat Med 2021, 2021. [Google Scholar] [CrossRef]
- Salis, S.; Virmani, A.; Priyambada, L.; Mohan, M.; Hansda, K.; de Beaufort, C. ‘Old Is Gold’: How Traditional Indian Dietary Practices Can Support Pediatric Diabetes Management. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Gray, A.; Threlkeld, R.J. Nutritional Recommendations for Individuals with Diabetes. Diabetologia 2019, 54. [Google Scholar]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and Management of Type 2 Diabetes: Dietary Components and Nutritional Strategies. Lancet 2014, 383, 1999. [Google Scholar] [CrossRef]
- Lee, I.S.; Kang, K.S.; Kim, S.Y. Panax ginseng Pharmacopuncture: Current Status of the Research and Future Challenges. Biomolecules 2020, 10. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; De, B. Indian Traditional Medicinal Systems, Herbal Medicine, and Diabetes. Diabetes Mellitus in 21st Century 2016, 125–151. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, C.; Li, X. Traditional medicine in India. Journal of Traditional Chinese Medical Sciences 2021, 8, S51–5. [Google Scholar] [CrossRef]
- Fu, Q.; Yang, H.; Zhang, L.; Liu, Y.; Li, X.; Dai, M.; et al. Traditional Chinese medicine foot bath combined with acupoint massage for the treatment of diabetic peripheral neuropathy: A systematic review and meta-analysis of 31 RCTs. Diabetes Metab Res Rev 2020, 36. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.M.; Ghoneim, M.M.; Eissa, I.H.; Elsehemy, I.A.; Mostafa, A.E.; Hegazy, M.M.; et al. Traditional ancient Egyptian medicine: A review. Saudi J Biol Sci 2021, 28, 5823. [Google Scholar] [CrossRef] [PubMed]
- Redvers, N.; Blondin, B. Traditional Indigenous medicine in North America: A scoping review. PLoS One 2020, 15, e0237531. [Google Scholar] [CrossRef] [PubMed]
- Gall, A.; Butler, T.L.; Lawler, S.; Garvey, G. Traditional, complementary and integrative medicine use among Indigenous peoples with diabetes in Australia, Canada, New Zealand and the United States. Aust N Z J Public Health 2021, 45, 664–671. [Google Scholar] [CrossRef]
- Chetty, L.; Govender, N.; Reddy, P. Traditional Medicine Use among Type 2 Diabetes Patients in KZN. Adv Public Health 2022, 2022. [Google Scholar] [CrossRef]
- Johnson, R.; Fiddler, T.; Pirozek, J.; Gordon, J.; Sodhi, S.; Poirier, J.; et al. Traditional Medicine and Type 2 Diabetes in First Nations Patients. Can J Diabetes 2022, 46, 53–59. [Google Scholar] [CrossRef]
- Kasole, R.; Martin, H.D.; Kimiywe, J. Traditional Medicine and Its Role in the Management of Diabetes Mellitus: “Patients’’ and Herbalists’ Perspectives. Evid Based Complement Alternat Med 2019, 2019. [Google Scholar] [CrossRef]
- Kirmayer, L.J. The Cultural Diversity of Healing: Meaning, Metaphor,and Mechanism. Heart Views 2013, 14, 39. [Google Scholar] [CrossRef]
- Chandra, V.; Arpita, K.; Yadav, P.; Raghuvanshi, V.; Yadav, A.; Prajapati, S. Environmental Biotechnology for Medical Waste Management: A Review of Current Practices and Future Directions. Preprint 2023. [CrossRef]
- Chandra, V.; Arpita, K.; Yadav, P.; Raghuvanshi, V.; Yadav, A.; Prajapati, S. Environmental Biotechnology for Medical Waste Management: A Review of Current Practices and Future Directions. Annals of Advanced Biomedical Sciences 2023, 6, 1–9. [Google Scholar] [CrossRef]
- Chandra V, Kumari A, Srivastava N, Yadav P, Raghuvanshi V, Rahul, et al. Comprehensive Overview of Role of Environmental Biotechnology in Reduction of Medical Waste. International Advance Journal of Engineering, Science and Management 2022, 17, 112–118. [CrossRef]
- Zhu S, Hu J, McCoy TP, li G, Zhu J, Lei M, et al. Socioeconomic Status and the Prevalence of Type 2 Diabetes Among Adults in Northwest China. Diabetes Educ 2015, 41, 599–608. [CrossRef]
- Vargas, E.; Nandhakumar, P.; Ding, S.; Saha, T.; Wang, J. Insulin detection in diabetes mellitus: challenges and new prospects. Nat Rev Endocrinol 2023. [CrossRef]
- Yadav P, Chandra V, Raghuvanshi V, Yadav A, Yadav A, Ali S, et al. Interferons for Covid-19, A Literature Review on Their Therapeutic Potential, Clinical Data and Challenges. Preprint 2023. [CrossRef]
- Raghuvanshi, V.; Yadav, P. Recent Advances in Alzheimer’s Disease Treatment. National Conference on Frontiers in Environment, Health and Biosciences, Kanpur: 2019, p. 84–84. [CrossRef]
- Yadav, P. Challenges & Solutions for Recent Advancements in Multi-Drugs Resistance Tuberculosis: A Review. Microbiol Insights 2023, 16, 1–9. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, A. Interferon Production and Potentiality as a therapeutic drug for SARS-CoV-2. Adv Pharmacoepidemiol Drug Saf 2023, 12, 1–5. [Google Scholar] [CrossRef]
- Uusitupa M, Khan TA, Viguiliouk E, Kahleova H, Rivellese AA, Hermansen K, et al. Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11. [CrossRef] [PubMed]
- Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018, 61, 2461–2498. [CrossRef] [PubMed]
- Hall, C.; Yu, H.; Choi, E. Insulin receptor endocytosis in the pathophysiology of insulin resistance. Experimental & Molecular Medicine 2020, 52, 911–920. [Google Scholar] [CrossRef]
- Arneth, B.; Arneth, R.; Shams, M. Metabolomics of Type 1 and Type 2 Diabetes. International Journal of Molecular Sciences 2019, 20, 2467. [Google Scholar] [CrossRef] [PubMed]
- Han L, Qu Q, Aydin D, Panova O, Robertson MJ, Xu Y, et al. Structure and mechanism of the SGLT family of glucose transporters. Nature 2021, 601, 274–279. [CrossRef] [PubMed]
- Solis-Herrera, C.; Triplitt, C.; Cersosimo, E.; DeFronzo, R.A. Pathogenesis of Type 2 Diabetes Mellitus 2021.
- Tsapas A, Avgerinos I, Karagiannis T, Malandris K, Manolopoulos A, Andreadis P, et al. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Ann Intern Med 2020, 173, 278–287. [CrossRef] [PubMed]
- Ojha A, Ojha U, Ojha H, Chandrashekar A, Mohammed R, Trivedi R, et al. Role of AYUSH Therapies in Modern Medicine: A Qualitative Study to Explore the Awareness and Attitudes of Doctors Towards the Utilization of Alternate System of Medicine for Diabetes Mellitus. Int J Gen Med 2020, 13, 1–8. [CrossRef] [PubMed]
- Pandey, A.; Tripathi, P.; Pandey, R.; Srivatava, R.; Goswami, S. Alternative therapies useful in the management of diabetes: A systematic review. J Pharm Bioallied Sci 2011, 3, 504. [Google Scholar] [CrossRef]
- Chen, C.; Liu, J.; Sun, M.; Liu, W.; Han, J.; Wang, H. Acupuncture for type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract 2019, 36, 100–112. [Google Scholar] [CrossRef]
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Ethnomedicinal Plants for the Management of Diabetes Worldwide: A Systematic Review. Curr Med Chem 2021, 28, 4670–4693. [Google Scholar] [CrossRef]
- Pramod Y, Vishal C, Vikas R, Amarjeet Y, Adhishree Y, Samim A, et al. Interferons as a Potential Therapeutic Drug for COVID-19, A Literature Review of Mechanisms, Current Clinical Trials, and Challenges. Journal of Community Medicine and Health Solutions 2023, 4, 048–056. [CrossRef]
- Chandra, D.r.V.; Ashraf Dr (Mohd ), T.; Yadav, P.; Raghuvanshi, M.r.V. Gene Expression Profiles of Knee Osteoarthritis Patients and Healthy Controls: A Microarray Analysis Study. Preprint -SSRN 2023. [Google Scholar] [CrossRef]
- Yadav A, Tripathi VM, Ali S, Yadav A, Raghuvanshi V, Khan S, et al. Waste-to-Hydrogen: A Sustainable Solution for Energy Generation and Waste Management in Nepal. Int J Res Appl Sci Eng Technol 2023, 11, 358–368. [CrossRef]
- Lunyera J, Wang D, Maro V, Karia F, Boyd D, Omolo J, et al. Traditional medicine practices among community members with diabetes mellitus in Northern Tanzania: An ethnomedical survey. BMC Complement Altern Med 2016, 16, 1–12. [CrossRef]
- Hospitals, P.; Athar Parwez, I.; Afsahul Kalam, I. Ethnomedicinal practices of Kashmir Valley: A Review. J Pharmacogn Phytochem 2018, 7, 278–284. [Google Scholar]
- Raghuvanshi, V.; Yadav, P.; Ali, S. Interferon production by Viral, Bacterial & Yeast system: A comparative overview in 2023. Int Immunopharmacol 2023, 120. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P. Production of Polyclonal Antibody in The Rabbit (Oryctolagus Cuniculus), Its Isolation & Quantification. master’s thesis, Chhatrapati Shahu Ji Maharaj University Kanpur, 2021. [Google Scholar] [CrossRef]
- Chandra V, Arpita K, Yadav P, Raghuvanshi V, Yadav A, Ali S, et al. Recombinant Laccase: A Promising Tool for Industrial Effluent Bioremediation. Preprint 2023. [CrossRef]
- Chandra V, Srivastava N, Kumari A, Yadav P, Raghuvanshi V, Rahul. Recent Development in Production Optimization of Recombinant Laccase Enzymes for Successful Bioremediation of Industrial Effluent: A Review. International Advance Journal of Engineering, Science and Management 2021, 15, 68–74. [CrossRef]
- Silva, N.C.M.; Chaves, É.C.L.; Carvalho, E.C.; Carvalho, L.C.; Iunes, D.H. Effect of Foot Reflexology on Capillary Blood Glucose, Tissue Temperature, and Plantar Pressure of Individuals With Diabetes Mellitus (Type 2): A Pilot Study. J Chiropr Med 2018, 17, 182–189. [Google Scholar] [CrossRef] [PubMed]
- mahluji kamran. The Effect of Massage on Diabetes and its Complications: A Systematic Review. 2019. [Google Scholar]
- Ezzo, J.; Donner, T.; Nickols, D.; Cox, M. Is massage useful in the management of diabetes: a systematic review. 2001. [Google Scholar]
- Chatchawan, U.; Eungpinichpong, W.; Plandee, P.; Yamauchi, J. Effects of thai foot massage on balance performance in diabetic patients with peripheral neuropathy: a randomized parallel-controlled trial. Med Sci Monit Basic Res 2015, 21, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Xie Y, Huan MT, Sang JJ, Luo SS, Kong XT, Xie ZY, et al. Clinical Effect of Abdominal Massage Therapy on Blood Glucose and Intestinal Microbiota in Patients with Type 2 Diabetes. Oxid Med Cell Longev 2022, 2022. [CrossRef]
- Ofuegbe, O.; Adedapo, A. Ethnomedicinal survey of some plants used for the treatment of diabetes in Ibadan, Nigeria. Asian J Med Sci 2015, 6, 36–40. [Google Scholar] [CrossRef]
- Elmi GR, Anum K, Saleem K, Fareed R, Noreen S, Wei H, et al. Evaluation of clinical trials of ethnomedicine used for the treatment of diabetes: A systematic review. Front Pharmacol 2023, 14, 822. [CrossRef] [PubMed]
- Tripathi, S. Ethnomedicine and Future Challenges. Global Journal of Archaeology & Anthropology 2019, 10. [Google Scholar] [CrossRef]
- Mignone, J.; Bartlett, J.; O’Neil, J.; Orchard, T. Best practices in intercultural health: Five case studies in Latin America. J Ethnobiol Ethnomed 2007, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, R.M.; Nepal, B.K.; Kshhetri, H.B.; Rai, S.K.; Bussmann, R.W. Ethnomedicine in Himalaya: A case study from Dolpa, Humla, Jumla and Mustang districts of Nepal. J Ethnobiol Ethnomed 2006, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Siddique Z, Shad N, Shah GM, Naeem A, Yali L, Hasnain M, et al. Exploration of ethnomedicinal plants and their practices in human and livestock healthcare in Haripur District, Khyber Pakhtunkhwa, Pakistan. J Ethnobiol Ethnomed 2021, 17, 1–22. [CrossRef]
- Bindu, J.; Narendhirakannan, R.T. Role of medicinal plants in the management of diabetes mellitus: a review. 3 Biotech 2019, 9. [Google Scholar] [CrossRef]
- Singh J, Dhupper R, Sharma S, Jindal T. TRADITIONAL KNOWLEDGE ON ETHNOMEDICINAL PLANTS AMONG LOCAL PEOPLE OF SARAIN RANGE, CHOPAL FOREST DIVISION, HIMACHAL PRADESH, INDIA. 2020, 20, 5643–5652.
- Kumar, S.; Mittal, A.; Babu, D.; Mittal, A. Herbal Medicines for Diabetes Management and its Secondary Complications. Curr Diabetes Rev 2021, 17, 437–456. [Google Scholar] [CrossRef]
- Leung, L.; Birtwhistle, R.; Kotecha, J.; Hannah, S.; Cuthbertson, S. Anti-diabetic and hypoglycaemic effects of Momordica charantia (bitter melon): a mini review. British Journal of Nutrition 2009, 102, 1703–1708. [Google Scholar] [CrossRef]
- Li Z, Xia A, Li S, Yang G, Jin W, Zhang M, et al. The Pharmacological Properties and Therapeutic Use of Bitter Melon (Momordica charantia L.). Curr Pharmacol Rep 2020, 6, 103–109. [CrossRef]
- Fang, E.F.; Froetscher, L.; Scheibye-Knudsen, M.; Bohr, V.A.; Wong, J.H.; Ng, T.B. Emerging Antitumor Activities of the Bitter Melon (Momordica charantia). Curr Protein Pept Sci 2019, 20, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Ayyanar, M.; Subash-Babu, P. Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed 2012, 2, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Santos CA, Almeida FA, Quecán BXV, Pereira PAP, Gandra KMB, Cunha LR, et al. Bioactive Properties of Syzygium cumini (L.) Skeels Pulp and Seed Phenolic Extracts. Front Microbiol 2020, 11, 990. [CrossRef] [PubMed]
- Tudu CK, Dutta T, Ghorai M, Biswas P, Samanta D, Oleksak P, et al. Traditional uses, phytochemistry, pharmacology and toxicology of garlic (Allium sativum), a storehouse of diverse phytochemicals: A review of research from the last decade focusing on health and nutritional implications. Front Nutr 2022, 9, 2258. [CrossRef] [PubMed]
- Tesfaye, A. Revealing the Therapeutic Uses of Garlic (Allium sativum) and Its Potential for Drug Discovery. The Scientific World Journal 2021, 2021. [Google Scholar] [CrossRef]
- Arumugam, G.; Manjula, P.; Paari, N. A review: Anti diabetic medicinal plants used for diabetes mellitus. Journal of Acute Disease 2013, 2, 196–200. [Google Scholar] [CrossRef]
- Jamadagni P, Pawar S, Jamadagni S, Gautam M, Gaidhani S, Prasad GP, et al. Recent Updates in Research on Gymnema sylvestre. Pharmacogn Rev 2021, 15, 128–133. [CrossRef]
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. Phytochemical and Pharmacological Properties of Gymnema sylvestre: An Important Medicinal Plant. 2014. [Google Scholar] [CrossRef]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A.S. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J Adv Pharm Technol Res 2011, 2, 3. [Google Scholar] [CrossRef]
- Tibenda, J.J.; Yi, Q.; Wang, X.; Zhao, Q. Review of phytomedicine, phytochemistry, ethnopharmacology, toxicology, and pharmacological activities of Cymbopogon genus. Front Pharmacol 2022, 13, 3388. [Google Scholar] [CrossRef]
- Assefa, B.; Glatzel, G.; Buchmann, C. Ethnomedicinal uses of Hagenia abyssinica (Bruce) J.F. Gmel. among rural communities of Ethiopia. J Ethnobiol Ethnomed 2010, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abebe, G.; Birhanu, G. A comparative study on antibacterial effects of Hagenia abyssinica oil extracted from different parts of the plant using different solvents against two selected and standardized human pathogens. Afr J Microbiol Res 2019, 13, 99–105. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, N.; Lechaba, T.; Schutte, P.J.; Hay, L.; Böhmer, L.; Megandran Govender, M. Journal of Medicinal Plants Research The effects of an aqueous leaf extract of Clausena anisata (Willd.) Hook.f.ex Benth. on blood pressure, urine output, angiotensin II levels and cardiac parameters in spontaneously hypertensive rats 2016, 10, 425–434. [Google Scholar] [CrossRef]
- Thomford, K.P.; Yorke, J.; Thomford, A.K.; Amponsah, I.K. A formulation of Clausena anisata (Willd.) Hook. f. Ex Benth and Cassia sieberiana DC. alleviates the symptoms associated with osteoarthritis: a single-blind, randomised controlled trial of a traditional Ghanaian remedy. Clinical Phytoscience 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Pal, D.; Mishra, P.; Sachan, N.; Ghosh, A. Biological activities and medicinal properties of Cajanus cajan (L) Millsp. J Adv Pharm Technol Res 2011, 2, 207–214. [Google Scholar] [CrossRef]
- Dey, J.; Saha, R.; Debnath, P.; Sinha, R.K. Comparative studies on some biochemical parameters of Cajanus scarabaeoides (L.) Thouars and Cajanus cajan (L.). Millsp 2017. [Google Scholar] [CrossRef]
- Liu, N.Q.; Van der Kooy, F.; Verpoorte, R. Artemisia afra: A potential flagship for African medicinal plants? South African Journal of Botany 2009, 75, 185–195. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. The Genus Artemisia: A Comprehensive Review. Https://DoiOrg/103109/138802092010497815 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Tcheghebe, T. O.; Nyamen, L. D.; Tatong, N. F.; Seukep, A. J. ETHNOBOTANICAL USES, PHYTOCHEMICAL AND PHARMACOLOGICAL PROFILES, AND TOXICITY OF PERSEA AMERICANA MILL. : AN OVERVIEW. Archives 2016, 3, 213–221. [Google Scholar]
- Kupnik, K.; Primožič, M.; Kokol, V.; Knez, Ž.; Leitgeb, M. Enzymatic, Antioxidant, and Antimicrobial Activities of Bioactive Compounds from Avocado (Persea americana L.) Seeds. Plants 2023, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Islas JF, Acosta E, G-Buentello Z, Delgado-Gallegos JL, Moreno-Treviño MG, Escalante B, et al. An overview of Neem (Azadirachta indica) and its potential impact on health. J Funct Foods 2020, 74, 104171. [CrossRef]
- Sanaul Moin, M.; Inam Siddiqui Assistant Professor, J.; Aftab Alam, M.; Khatoon, F.; Khan, S.; Inam Siddiqui, J.; et al. Ethnomedicinal potential of widely used plant Azadirachta indica, A. Juss: A comprehensive review. The Journal of Phytopharmacology 2021, 10, 456–467. [Google Scholar] [CrossRef]
- Pham HNT, Vuong Q Van, Bowyer MC, Scarlett CJ. Phytochemicals Derived from Catharanthus roseus and Their Health Benefits. Technologies 2020, 8, 80. [CrossRef]
- Kumar, S.; Singh, B.; Singh, R. Catharanthus roseus (L.) G. Don: A review of its ethnobotany, phytochemistry, ethnopharmacology and toxicities. J Ethnopharmacol 2022, 284. [Google Scholar] [CrossRef]
- Mehmood, A.; Murtaza, G. Phenolic contents, antimicrobial and antioxidant activity of Olea ferruginea Royle (Oleaceae). BMC Complement Altern Med 2018, 18, 1–6. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid Based Complement Alternat Med 2015, 2015. [Google Scholar] [CrossRef]
- Lulekal, E.; Asfaw, Z.; Kelbessa, E.; Van Damme, P. Ethnomedicinal study of plants used for human ailments in Ankober District, North Shewa Zone, Amhara Region, Ethiopia. J Ethnobiol Ethnomed 2013, 9, 1–13. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S.; Kumar, S.; Narashiman, B. Ethnopharmacological, phytochemical and clinical studies on Fenugreek (Trigonella foenum-graecum L. ). Food Biosci 2022, 46, 101546. [Google Scholar] [CrossRef]
- Visuvanathan, T.; Than, L.T.L.; Stanslas, J.; Chew, S.Y.; Vellasamy, S. Revisiting Trigonella foenum-graecum L.: Pharmacology and Therapeutic Potentialities. Plants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Healthy diet n.d. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 25 May 2023).
- Ćatović, A.; Ćatović, A. Dietary Patterns. Recent Updates in Eating Disorders 2022. [CrossRef]
- Glycemic Index and Diabetes - GI Diet, GI Foods & Benefits of Low GI n.d. Available online: https://www.diabetes.co.uk/diet/glycaemic-index-diet-and-diabetes.html (accessed on 25 May 2023).
- Moses, R.G.; Barker, M.; Winter, M.; Petocz, P.; Brand-Miller, J.C. Can a Low–Glycemic Index Diet Reduce the Need for Insulin in Gestational Diabetes Mellitus? A randomized trial. Diabetes Care 2009, 32, 996–1000. [Google Scholar] [CrossRef]
- Sproesser G, Ruby MB, Arbit N, Akotia CS, Alvarenga MDS, Bhangaokar R, et al. Understanding traditional and modern eating: The TEP10 framework. BMC Public Health 2019, 19, 1–14. [CrossRef]
- Sidiq, F.F.; Coles, D.; Hubbard, C.; Clark, B.; Frewer, L.J. The Role of Traditional Diets in Promoting Food Security for Indigenous Peoples in Low- and Middle-Income Countries: A Systematic Review. IOP Conf Ser Earth Environ Sci 2022, 978, 012001. [Google Scholar] [CrossRef]
- (2) (PDF) TRADITIONAL FOODS OF INDIA n.d. Available online: https://www.researchgate.net/publication/330533824_TRADITIONAL_FOODS_OF_INDIA?channel=doi&linkId=5c46b6c6299bf12be3d9fb30&showFulltext=true (accessed on 26 May 2023).
- Gokhale, J.S.; Lele, S.S.; Ananthanarayan, L. Indian Traditional Foods and Diets: Combining Traditional Wisdom with Modern Science of Nutraceuticals and Functional Foods. Nutrition, Food and Diet in Ageing and Longevity 2021, 357–392. [Google Scholar] [CrossRef]
- Huber, H.; Stoffel-Wagner, B.; Coenen, M.; Weinhold, L.; Schmid, M.; Stehle, P.; Simon, M. C. 251-OR: Impact of Vegetarian and the Nordic Diet on Glucose Metabolism: A Human Intervention Study. Diabetes 2021, 70. [Google Scholar] [CrossRef]
- Tertsunen, H.M.; Hantunen, S.; Tuomainen, T.P.; Virtanen, J.K. Adherence to a healthy Nordic diet and risk of type 2 diabetes among men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Nutr 2021, 60, 3927. [Google Scholar] [CrossRef]
- Kanerva, N.; Rissanen, H.; Knekt, P.; Havulinna, A.S.; Eriksson, J.G.; Männistö, S. The healthy Nordic diet and incidence of Type 2 Diabetes — 10-year follow-up. Diabetes Res Clin Pract 2014, 106, e34–7. [Google Scholar] [CrossRef]
- Population NRC (US) C on, Sandefur GD, Rindfuss RR, Cohen B. Diabetes Mellitus in Native Americans: The Problem and Its Implications 1996.
- Patchell, B.; Edwards, K. The Role of Traditional Foods in Diabetes Prevention and Management among Native Americans. Curr Nutr Rep 2014, 3, 340–344. [Google Scholar] [CrossRef]
- Magnusdottir, O.K.; Gunnarsdottir, I.; Birgisdóttir, B.E. Dietary guidelines in type 2 diabetes: the Nordic diet or the ketogenic diet? Curr Opin Endocrinol Diabetes Obes 2017, 24, 315–319. [Google Scholar] [CrossRef]
- Sproesser G, Ruby MB, Arbit N, Akotia CS, Alvarenga MDS, Bhangaokar R, et al. Understanding traditional and modern eating: The TEP10 framework. BMC Public Health 2019, 19, 1–14. [CrossRef] [PubMed]
- Forouhi, N.G.; Misra, A.; Mohan, V.; Taylor, R.; Yancy, W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 2018, 361. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.E.; Orr, E.; Boyer, B.B.; Thompson, B. Culturally adapting an evidence-based intervention to promote a healthy diet and lifestyle for Yup’ik Alaska native communities. Int J Circumpolar Health 2023, 82. [Google Scholar] [CrossRef]
- Subhan, F.B.; Fernando, D.N.; Thorlakson, J.; Chan, C.B. Dietary Interventions for Type 2 Diabetes in South Asian Populations—A Systematic Review. Curr Nutr Rep 2023, 12, 39–55. [Google Scholar] [CrossRef]
- Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al. Nutrition Therapy Recommendations for the Management of Adults With Diabetes. Diabetes Care 2013, 36, 3821–3842. [CrossRef] [PubMed]
- Koloverou, E.; Panagiotakos, D.B. Macronutrient Composition and Management of Non-Insulin-Dependent Diabetes Mellitus (NIDDM): A New Paradigm for Individualized Nutritional Therapy in Diabetes Patients. Rev Diabet Stud 2016, 13, 6. [Google Scholar] [CrossRef]
- Wheeler ML, Dunbar SA, Jaacks LM, Karmally W, Mayer-Davis EJ, Wylie-Rosett J, et al. Macronutrients, Food Groups, and Eating Patterns in the Management of DiabetesA systematic review of the literature, 2010. Diabetes Care 2012, 35, 434–445. [CrossRef]
- Chehade, J.M.; Sheikh-Ali, M.; Mooradian, A.D. The Role of Micronutrients in Managing Diabetes. Diabetes Spectrum 2009, 22, 214–218. [Google Scholar] [CrossRef]
- Chehade, J.M.; Sheikh-Ali, M.; Mooradian, A.D. Select Vitamins and Minerals in the Management of Diabetes. Diabetes Spectrum 2001, 14, 133–148. [Google Scholar] [CrossRef]
- Wylie-Rosett, J.; Hu, F.B. Nutritional Strategies for Prevention and Management of Diabetes: Consensus and Uncertainties. Diabetes Care 2019, 42, 727–730. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Morley, J.E. Micronutrient status in diabetes mellitus. Am J Clin Nutr 1987, 45, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.-S.; Butcher, R. Low Carbohydrate Diets for Diabetes: A Review of the Clinical Effectiveness and Guidelines. Low Carbohydrate Diets for Diabetes: A Review of the Clinical Effectiveness and Guidelines. 2019, 1–32. [Google Scholar]
- Murdoch, C.; Unwin, D.; Cavan, D.; Cucuzzella, M.; Patel, M. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. British Journal of General Practice 2019, 69, 360–361. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.M.; Strong, A.P.; Krebs, J.D. Importance of low carbohydrate diets in diabetes management. Nutr Diet Suppl 2016, 9. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Saboo B, Misra A, Kalra S, Mohan V, Aravind SR, Joshi S, et al. Role and importance of high fiber in diabetes management in India. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2022, 16, 102480. [CrossRef]
- Alcohol and Diabetes | ADA n.d. Available online: https://diabetes.org/healthy-living/medication-treatments/alcohol-diabetes (accessed on 26 May 2023).
- van de Wiel, A. Diabetes mellitus and alcohol. Diabetes Metab Res Rev 2004, 20, 263–267. [Google Scholar] [CrossRef]
- Daher, M.I.; Matta, J.M.; Abdel Nour, A.M. Non-nutritive sweeteners and type 2 diabetes: Should we ring the bell? Diabetes Res Clin Pract 2019, 155. [Google Scholar] [CrossRef]
- Manavalan, D.; Shubrook, C.; Young, C.F. Consumption of Non-nutritive Sweeteners and Risk for Type 2 Diabetes: What Do We Know, and Not? Curr Diab Rep 2021, 21. [Google Scholar] [CrossRef]
- Jana, K.; Ghosh, D.; Debidas Ghosh, A. ETHNOMEDICINE IN DIABETES MANAGEMENT: A REVIEW. World J Pharm Res 2014, 3. [Google Scholar]
- Capehorn M, Polonsky WH, Edelman S, Belton A, Down S, Gamerman V, et al. Challenges faced by physicians when discussing the Type 2 diabetes diagnosis with patients: insights from a cross-national study (IntroDia®). Diabetic Medicine 2017, 34, 1100–1107. [CrossRef]
- Kutob, R.M.; Bormanis, J.; Crago, M.; Harris, J.M.; Senf, J.; Shisslak, C.M. Cultural competence education for practicing physicians: Lessons in cultural humility, nonjudgmental behaviors, and health beliefs elicitation. Journal of Continuing Education in the Health Professions 2013, 33, 164–173. [Google Scholar] [CrossRef]
- Al Slamah, T.; Nicholl, B.I.; Alslail, F.Y.; Harris, L.; Melville, C.A.; Kinnear, D. Cultural adaptation of self-management of type 2 diabetes in Saudi Arabia (qualitative study). PLoS One 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Johnson, C.M.; D’Eramo-Melkus, G.; Vorderstrasse, A.A. Participation of Racial and Ethnic Minorities in Technology-Based Interventions to Self-Manage Type 2 Diabetes: A Scoping Review. Journal of Transcultural Nursing 2018, 29, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Finucane, M.L.; McMullen, C.K. Making diabetes self-management education culturally relevant for filipino Americans in Hawaii. Diabetes Educator 2008, 34, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Blonde, L. Current challenges in diabetes management. Clin Cornerstone 2005, 7 Suppl 3, S6. [Google Scholar] [CrossRef]
- Folashade, O.; Omoregie, H.; Ochogu, P. Standardization of herbal medicines-A review. Int J Biodivers Conserv 2012, 4, 101–112. [Google Scholar] [CrossRef]
- Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, et al. Heavy Metal Content of Ayurvedic Herbal Medicine Products. JAMA 2004, 292, 2868–2873. [CrossRef]
- Mikulski, M.A.; Wichman, M.D.; Simmons, D.L.; Pham, A.N.; Clottey, V.; Fuortes, L.J. Toxic metals in ayurvedic preparations from a public health lead poisoning cluster investigation. Int J Occup Environ Health 2017, 23, 187. [Google Scholar] [CrossRef]
- Balekundri, A.; Mannur, V. Quality control of the traditional herbs and herbal products: a review. Future Journal of Pharmaceutical Sciences 2020 6, 1 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Cox, G.F. The art and science of choosing efficacy endpoints for rare disease clinical trials. Am J Med Genet A 2018, 176, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Natural Doesn’t Necessarily Mean Safer, or Better | NCCIH n.d. Available online: https://www.nccih.nih.gov/health/know-science/natural-doesnt-mean-better (accessed on 27 May 2023).
- Toxic, Not Healthy: Surprising Liver Dangers of Herbal Products | Everyday Health n.d. Available online: https://www.everydayhealth.com/news/toxic-not-healthy-surprising-liver-dangers-herbal-products/ (accessed on 27 May 2023).
- David, S.; Cunningham, R. Echinacea for the prevention and treatment of upper respiratory tract infections: A systematic review and meta-analysis. Complement Ther Med 2019, 44, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sander, S.; White, C.M.; Rinaldi, M.; Coleman, C.I. Evaluation of echinacea for the prevention and treatment of the common cold: a meta-analysis. Lancet Infect Dis 2007, 7, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Majlesi, M.; Rafieian-Kopaei, M. Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol 2015, 4, 27. [Google Scholar] [PubMed]
- Jahromi, B.; Pirvulescu, I.; Candido, K.D.; Knezevic, N.N. Herbal Medicine for Pain Management: Efficacy and Drug Interactions. Pharmaceutics 2021, 13, 1–43. [Google Scholar] [CrossRef]
- Firenzuoli, F.; Gori, L. Herbal Medicine Today: Clinical and Research Issues. Evid Based Complement Alternat Med 2007, 4, 37. [Google Scholar] [CrossRef]
- Focus On Biomarkers Research | National Institute of Neurological Disorders and Stroke n.d. Available online: https://www.ninds.nih.gov/current-research/focus-tools-topics/focus-biomarkers-research (accessed on 27 May 2023).
- 1*WzEZ-Eb8Y0VT8iMM3pSTpA.png (1049×555) n.d. Available online: https://miro.medium.com/v2/resize:fit:1400/1*WzEZ-Eb8Y0VT8iMM3pSTpA.png (accessed on 25 May 2023).
| 1 |
Note: Apart from these described, there are other medicinal plant are also used an ethnomedicines and therefore a details list of ethnomedicinal plant is given in Annexure 1.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





