1. Introduction
Breast cancer is the most common neoplasm among women and the second leading cause of death for them. According to the American Cancer Society, approximately 1 in 8 women (13%) are diagnosed with invasive breast cancer, and 1 in 39 women (3%) will die from it [
1,
2,
3]. Breast cancer exhibits genetic heterogeneity, displaying a variety of clinical manifestations with distinct outcomes and therapeutic responses. Due to cancer control programs, the disease is detected prior to the onset of symptoms, either through screening or self-report.
Key risk factors include age – incidence increases after the age of 55, race, and ethnicity – with higher incidence in white individuals compared to African-Americans. Genetic mutations also play a role – women carrying germline mutations in BRCA1 (breast cancer gene 1) and BRCA2 (breast cancer gene 2) are more predisposed to breast cancer. Personal hereditary history of breast cancer, early menarche, and late menopause are also risk factors.
There are modifiable risk factors as well, such as excess weight and obesity, chronic alcohol consumption, smoking, number of pregnancies, breastfeeding (lower risk), oral contraceptives, postmenopausal hormone replacement therapy, and previous ionizing radiation exposure [
1,
4,
5,
6]. Obesity increases the risk of various cancers, including breast cancer. Metabolic syndrome negatively affects the breast tumor microenvironment, promoting cancer invasion.
Regarding type 2 diabetes, there is a certain association with breast cancer risk, more consistent among postmenopausal women than premenopausal ones.
According to the World Health Organization (WHO), two distinct breast cancer entities can be differentiated: in situ carcinoma – ductal or lobular, and invasive carcinoma – with over 21 subtypes. The most common histological type of invasive carcinoma is invasive ductal carcinoma, while other subtypes include lobular, tubular, mucinous, or metaplastic carcinoma, medullary carcinoma, and apocrine carcinoma.
Mucinous breast carcinoma (colloid or gelatinous carcinoma) is a rare histological type of mammary neoplasm characterized by the secretion of significant amounts of mucin into the extracellular environment. Mucinous carcinoma can be classified into two main subtypes: pure mucinous carcinoma and mixed mucinous carcinoma. It represents only 1-6% of all breast carcinomas and is more common in perimenopausal and postmenopausal women. It generally has a better prognosis compared to other malignant breast conditions, such as ductal or lobular forms. Patients typically exhibit positivity for hormonal receptors (progesterone, estrogen) and the Ki-67 proliferation index, while the HER-2/neu oncoprotein is usually negative.
2. Case Report
We report the case of a 73-year-old Caucasian woman from a rural area, retired due to age, with no history of toxic substance consumption (tobacco, alcohol). She presented to the Internal Medicine IV Clinic of the CF Cluj-Napoca University Hospital with marked dyspnea during moderate physical exercises, excessive sweating, especially at night, mild edema in the lower limbs, and bilateral lower limb paresthesia. She had no specific family history of diseases. The patient had a complex personal history of cardiometabolic disease, including insulin-requiring type 2 diabetes, essential grade II hypertension with very high additional risk, chronic ischemic heart disease, moderate mitral stenosis, severe aortic stenosis, non-rheumatic tricuspid insufficiency grade I, non-rheumatic mitral insufficiency grade I/II, aortic atheromatosis, congestive heart failure NYHA II, metabolic hepatic steatosis, under observation for nodular goiter, left kidney lithiasis, and polyarthrosis. The general physical examination revealed first-degree obesity, facial flushing, abdominal obesity, and a postoperative abdominal scar.
In May 2007, the patient was admitted to a balneology service for the treatment of associated rheumatic diseases, where routine abdominal ultrasound revealed a pancreatic cystic mass. The patient was referred for further investigations to the Medical Clinic IV - CF Cluj-Napoca Clinical Hospital, where additional abdominal ultrasound and computed tomography described a septated cystic mass of the body and tail of the pancreas with a diameter of 6.8 cm, without detecting hepatic or nodal metastases. Radical surgery was performed through pancreatectomy of the body and tail and splenectomy. Intraoperatively, a cystic tumor mass was described in the body and tail of the pancreas, measuring 10 cm in diameter, with thickened walls, a rich peritumoral vascular network, without lymph nodes, hepatic or peritoneal metastases. The morphopathological result established the diagnosis of limited mucinous cystic neoplasm of the body and tail of the pancreas. The oncology specialist recommended annual monitoring. At that time, it was already known that the patient had type 2 diabetes for about 3 months, and she was on Metformin 1000 mg/day and had first-degree obesity. The postoperative clinical course was reserved due to pancreatic function decline, with uncontrolled blood glucose levels on oral monotherapy. Hence, the decision was made to initiate insulin therapy in a basal-bolus regimen.
Ten years later, in June 2017, alongside the preexisting diagnoses, the patient presented with mixed sensory-motor polyneuropathy of the lower limbs, higher-grade obesity (obesity grade II, BMI = 35.7 kg/m2), uncontrolled diabetes (glycated hemoglobin levels above 7 g%), unstable effort angina in the setting of ischemic heart disease, and left bundle branch block.
Coronary angiography was recommended, but the patient postponed it for personal reasons. In October 2019, the patient returned to our clinic complaining of mixed dyspnea on moderate exertion, asthenia-adynamia, decreased exercise tolerance, lumbosacral pain, heel pain (talalgia), tingling sensations in the "socks" distribution, and marked excessive sweating, particularly at night. Among the main biological parameters, we noted non-specific inflammatory syndrome: elevated erythrocyte sedimentation rate (50 mm/1 h; normal range: 1-10 mm/1 h) but with physiological levels of C-reactive protein, mixed dyslipidemia with hypercholesterolemia, hyperglycemia, elevated glycated hemoglobin (8.96 g%), presence of left bundle branch block on electrocardiogram, but with unreactive cardiac markers. Reevaluation through abdominal ultrasound detected a 28 mm cystic mass in the splenorenal space, leading to an indicated abdominal computed tomography.
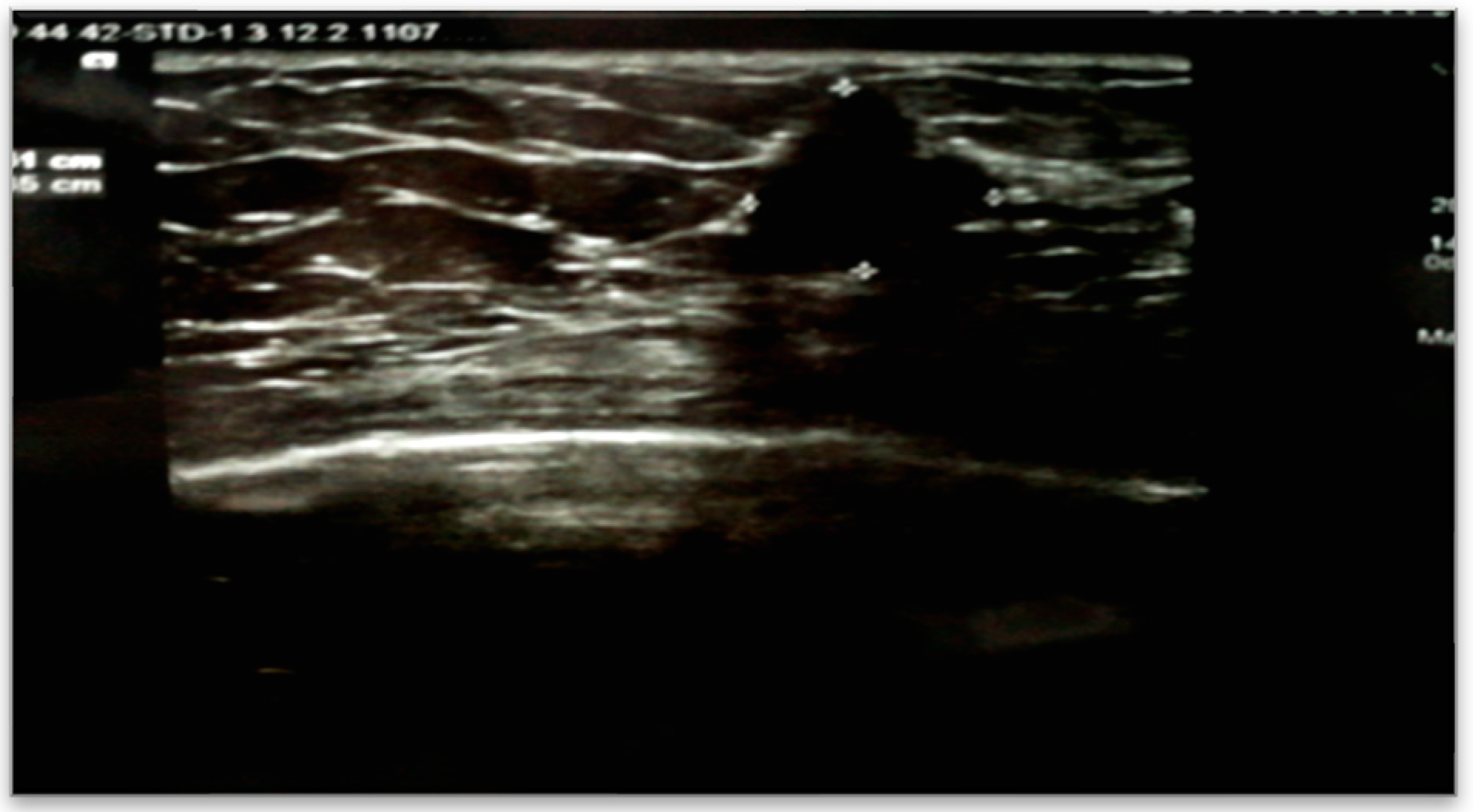
The patient underwent an abdominal CT examination, which described the presence of two accessory spleens measuring 24 mm and 19 mm, respectively, following pancreatectomy of the body and tail and splenectomy. Additionally, a left renal cystic mass measuring 28 mm was detected. Accidentally, a tumor in the right breast in the lower-outer quadrant was observed, measuring 14/15 mm. The high suspicion raised by the abdominal CT led to further imaging investigations, with breast ultrasound confirming the presence of a strongly hypoechoic spiculated mass measuring 16/13.5 mm, with irregular contours, as shown in
Figure 1.
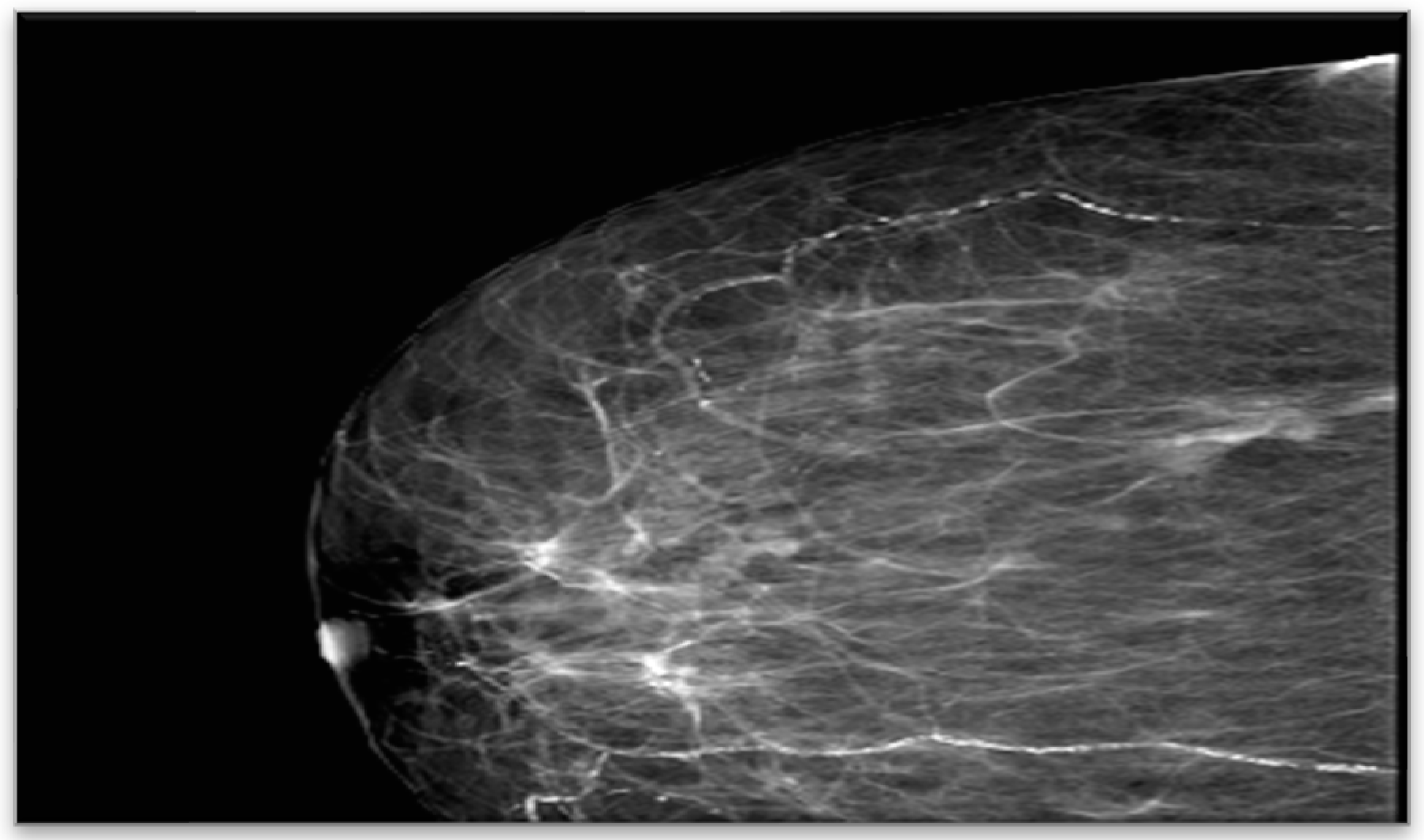
Bilateral mammography identified breasts with predominantly adipose tissue structure and symmetric appearance, without suspicious microcalcifications. In the right breast gland, a posterior nodular opacity with unclear contours was visualized (
Figure 2).
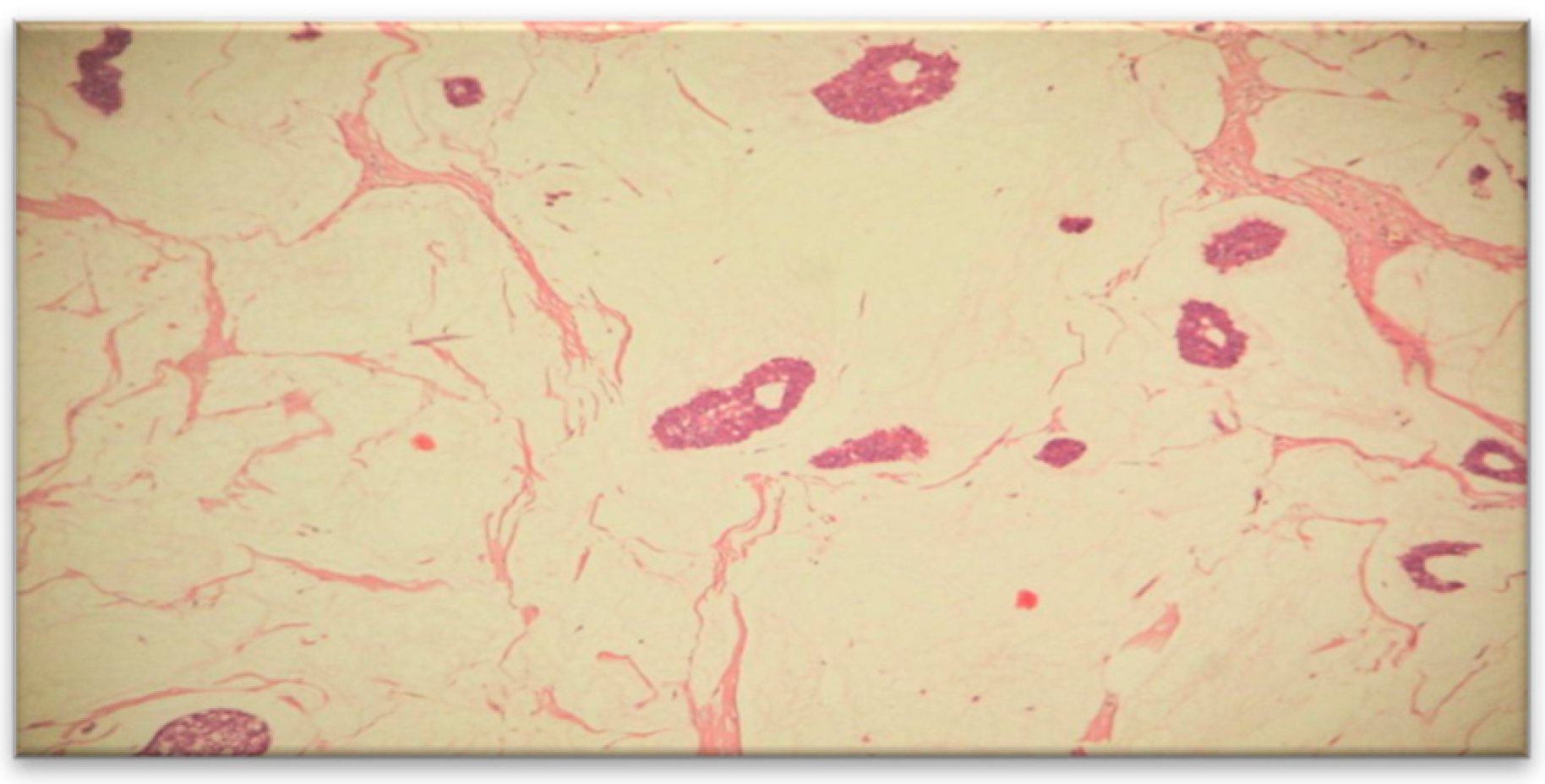
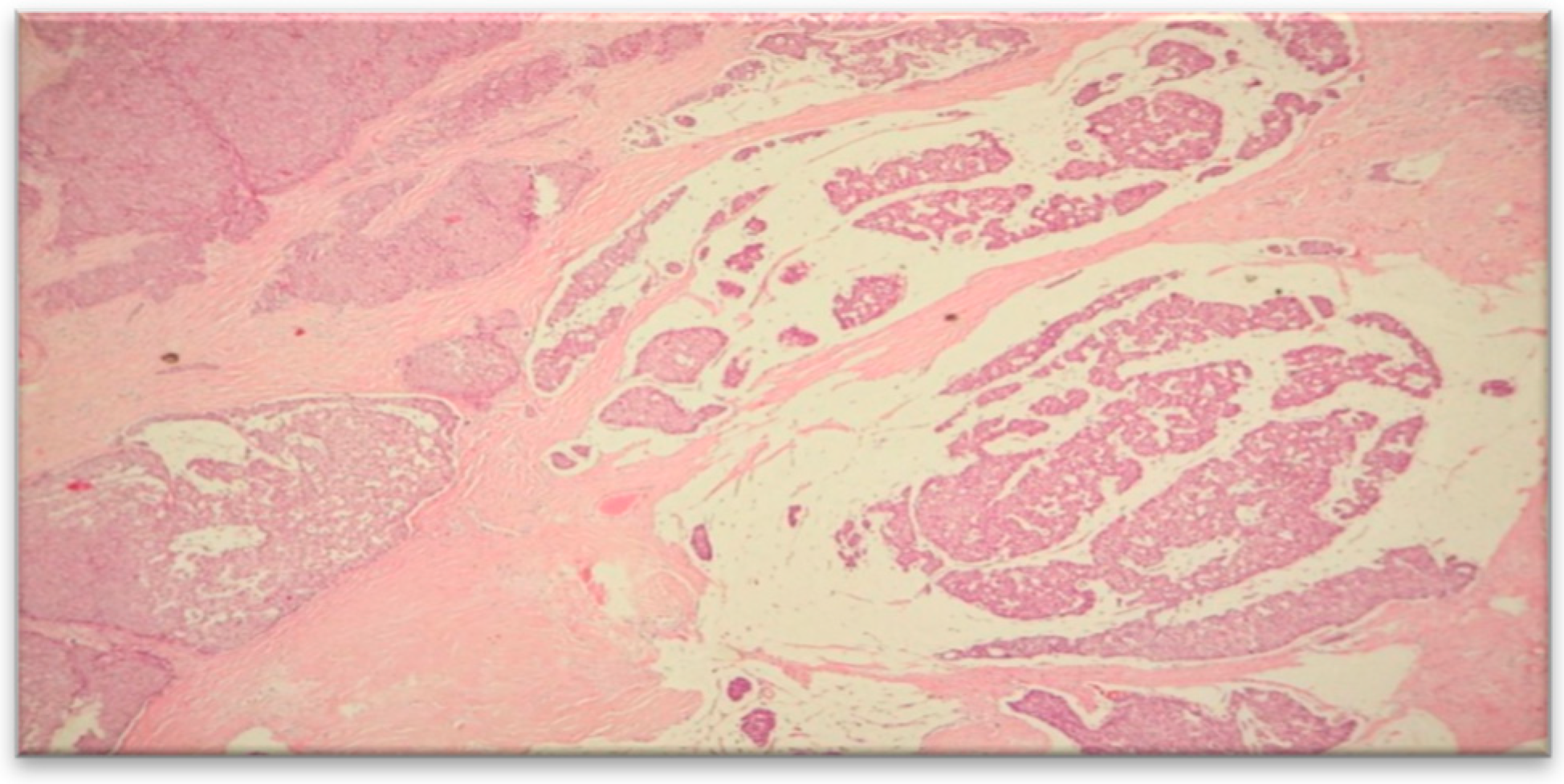
To establish a definitive diagnosis, a local anesthesia-guided core needle biopsy of the right breast was performed, obtaining 4 tissue fragments with a cumulative size of 25x1 mm. The microscopic histological structure suggested a Grade II SBR mucinous carcinoma (
Figure 3).
The tumor covered approximately 85% of the examined surface, while the cellularity of the tumor bed was around 25%.
The therapeutic decision, made through multidisciplinary collaboration between the internist, oncologist, surgeon, cardiologist, supported radical treatment by removing the tumor mass. However, considering the patient's associated cardiac symptoms, the surgical intervention was postponed until after coronary angiography. Considering the patient's history, clinical and paraclinical data, in November 2019, she was referred to an interventional cardiology clinic for coronary angiography. The procedure detected significant lesions in the left anterior descending coronary artery and the intermediate branch, which were addressed in the same stage during coronary angioplasty, with the placement of two active stents. Two days after the procedure, the patient experienced a spontaneous typical angina crisis with reactive troponins in the dynamics (troponin = 0.25 ng/ml; normal values < 0.2 ng/ml). A repeated coronary angiography revealed patent stents without additional lesions. The treatment plan was completed with a calcium channel blocker and nitrates.
In January 2020, the patient was rehospitalized at the Surgical Clinic IV of the University Hospital CF Cluj-Napoca for breast surgery. Preoperatively, she underwent reevaluation through echocardiography and a cardiac examination. The surgical intervention was performed under general anesthesia with endotracheal intubation; a lower-outer quadrantectomy of the right breast was performed with right axillary lymphadenectomy, axillary drainage, and wound drainage.
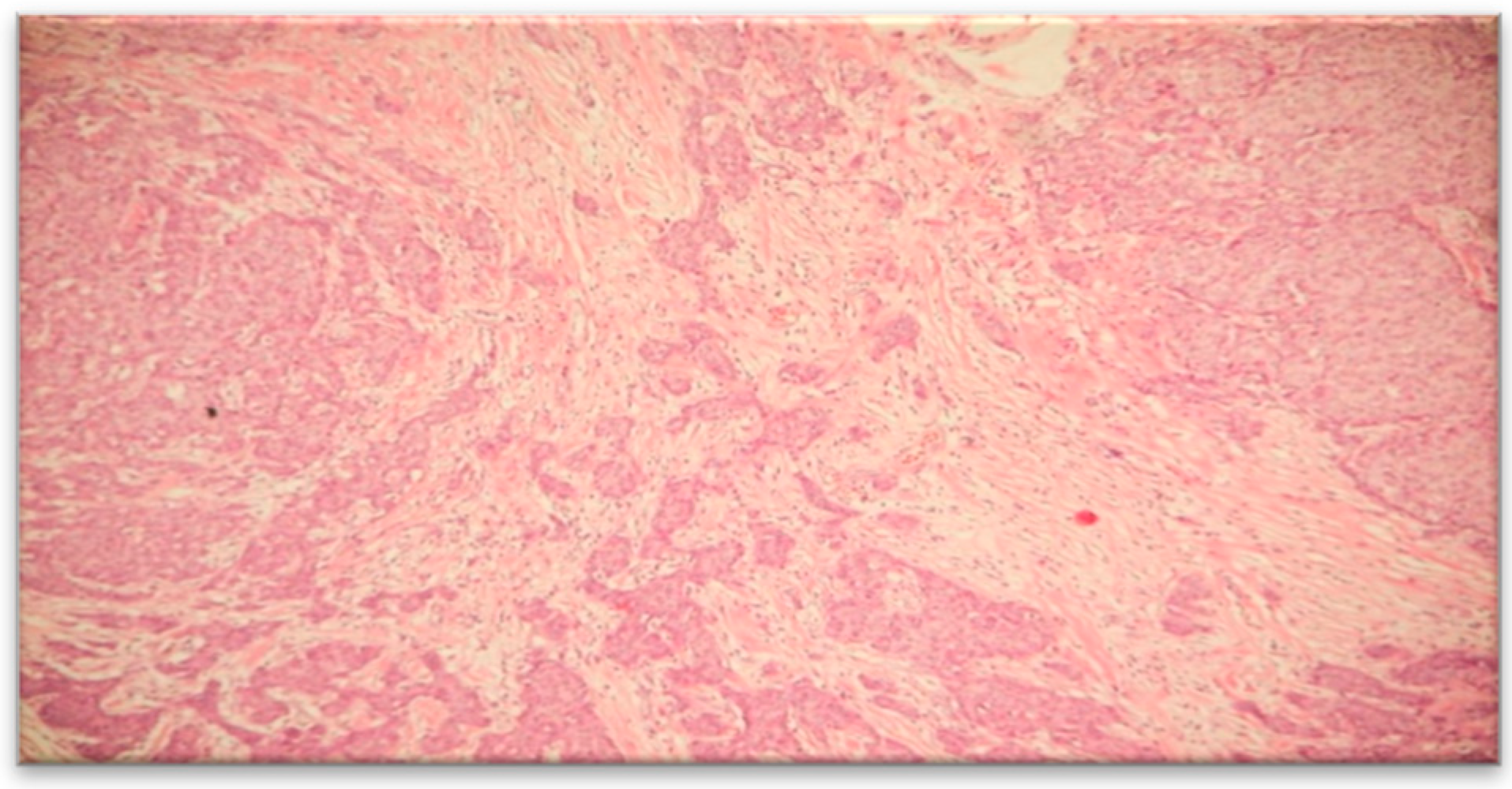
The pathological result identified macroscopically a tumor mass of 1.5x1.2 cm, situated 1 cm below the skin, 1.5 cm from the deep resection margin, and 9 cm, 7 cm, 8.5 cm, and 6 cm from the other resection margins. Multiple fragments measuring 5.5x5.2 cm in size, composed of adipose tissue containing lymph nodes, were also observed. Microscopically, the histopathological appearance corresponded to an invasive mixed mammary carcinoma – 70% mucinous carcinoma and non-specific invasive ductal carcinoma (
Figure 4 and
Figure 5), Nottingham grade II %E% (architectural grade = 3, nuclear grade = 2, mitotic grade = 1), and tumor staging pT1cN0Mx. The diagnosis of mucinous carcinoma was based on histological characteristics, namely nests and groups of tumor cells forming within a mass of extracellular mucin.
The postoperative course was favorable, with minimal drainage, allowing the removal of breast wound drains at 48 hours and axillary drains at 36 hours. The patient was discharged in good general condition, afebrile. Subsequently, she was referred to an oncology service, where adjuvant therapy was initiated after complete local healing.
3. Discussions
Age is the most important known risk factor for the development of breast cancer. Mucinous breast carcinoma usually occurs in elderly patients. The incidence of breast cancer significantly increases with age and is highest in women during perimenopause and postmenopause [
15,
16], with our 73-year-old patient having been in postmenopause for approximately 24 years.
Obesity and cancer are both major health issues in developed countries. Genetic mutations, micro- and macro-physiological environments underlie carcinogenesis. Obesity is consistently associated with an increased risk of estrogen receptor-positive breast cancer, especially in postmenopausal women, due to elevated levels of insulin and insulin-like factors in response to obesity, which can stimulate the growth of malignant cells [
15]. Current studies have found that the risk of postmenopausal breast cancer is positively associated with every 5 kg/m2 increase in body mass index [
16,
17,
18]. In some studies, the positive association between abdominal obesity and breast cancer has been shown to be independent of BMI. Studies are contradictory regarding the association of a high waist-to-hip ratio or waist circumference with an increased risk of premenopausal breast cancer [
9]. Several mechanisms underlying this association have been proposed. Among them, chronic inflammation, increased leptin secretion accompanied by systemic leptin resistance [
7], and lower levels of adiponectin in obese subjects can disrupt cell division and angiogenesis and promote cancer development [
7].
In an article published by a group of authors from Israel [
7], it is suggested that miR-10b (microRNA-10b gene), previously known to be associated with fatty liver degeneration and bladder cancer, could be a mediator between obesity and cancer in postmenopausal women, regulating several genes known to be relevant to cancer. The expression of miR-10b could have diagnostic and therapeutic implications for the incidence and prognosis of breast cancer in obese women [
7].
Diabetes mellitus, especially type 2 diabetes, tends to increase the incidence of breast cancer by 20% [
19]. Over a century ago, hyperglycemia and diabetes were categorized as risk factors for breast cancer [
9]. Diabetes induces multiple hormonal changes (insulin, estrogen) as well as cytokines and insulin-like growth factors (IGF-1, IGF-2), which raise the risk of breast cancer by their involvement in carcinogenesis [
9].
Epidemiological data suggest a strong link between metabolic syndrome and breast cancer, indicating both an increased risk for developing breast cancer and a poorer prognosis [
20,
21,
22]. Studies have already been published suggesting that metabolic syndrome paves the way for a more aggressive form of breast cancer through PAI-1. Plasminogen activator inhibitor-1 (PAI-1) is a physiological inhibitor of urokinase, a protease enzyme involved in cell promotion, migration, and invasion [
8]. Metabolic syndrome is characterized by insulin resistance, hyperinsulinism, and a state of chronic inflammation, in which, in addition to the increased risk of cardiovascular morbidity and mortality, women with this syndrome represent a group at higher risk of developing breast cancer and having a poorer prognosis [
20].
Postmenopausal women with diabetes, overweight, and obesity have an increased susceptibility to breast cancer [
23], and our patient accumulates all these risk factors.
Mucinous carcinoma commonly appears in two different subtypes, the cystic (mucinous cystadenocarcinoma) and solid (columnar cell-type mucinous carcinoma) subtypes [
24]. The most frequent locations of mucinous cystadenocarcinoma include the ovary, pancreas, and appendix [
25]. In contrast to the ovary and pancreas, mucinous cystadenoma of the breast is extremely rare and was first reported by Koenig and Tavassoli in 1998 [
24].
Mucinous cystadenomas of the pancreas are low-grade tumors and represent about 10% of all pancreatic cysts. These tumors are more common in women compared to men [
26]. This malignancy was detected in our patient 12 years before the detection of the breast carcinoma.
Regarding mucinous breast carcinoma, it represents a histological variant of infiltrating breast carcinoma that is rarely encountered in clinical practice [
25,
27]. It accounts for approximately 4% of all invasive breast cancers [
28]. Very few cases of primary mucinous cholangiocarcinoma of the breast have been reported. The first report was by Koenig, who published the first four cases in 1998 [
25]. Unfortunately, the rarity of this condition has limited the possibility of extensive studies, unlike other histological types of breast cancer. It is associated with a favorable prognosis, but its lesions often mimic benign tumors with slow growth on imaging, potentially leading to delayed diagnosis [
29]. Clinically, it presents as a relatively large cystic mass, easily palpable and firm [
25]. This type of tumor has a better prognosis than ductal or lobular carcinomas [
28,
30] and has a higher incidence in perimenopausal and postmenopausal patients [
28].
Mucinous breast carcinoma, characterized morphologically by clusters of tumor cells aggregated in an extracellular mucin-rich environment [
27], can appear in two forms: pure or mixed, depending on the proportion of the mucinous component [
28,
29,
30,
31]. The presence of mucin creates a collector environment for tumor cells and reduces the mobility of tumor cells, leading to fewer metastases and explaining the more favorable prognosis [
32]. The distinction between these subtypes is based on the quantification of cellularity. The mucinous component can vary from 30% to over 90% of the tumor [
28]. Currently, there is no established percentage for a positive diagnosis of mucinous carcinoma. However, most pathologists agree that a diagnosis of pure mucinous carcinoma should be reserved for tumors with at least 90% mucinous components [
28]. The pure type consists almost exclusively of tumor tissue with extracellular mucin production, while the mixed subtype contains other in situ or invasive components without mucin [
28]. The histopathological description in the case of our patient aligns with the mixed type, and the diagnostic classification, in line with literature data, was accurately made. A diagnostic pitfall is the mislabeling of mucinous cholangiocarcinoma as primary invasive mucinous carcinoma of the breast, a condition much more common than mucinous cholangiocarcinoma.
Histologically, unlike the invasive mucinous carcinoma cells, the cells of mucinous cholangiocarcinoma possess abundant intracellular mucin, maintaining this characteristic even in extravasated stromal mucin [
25]. Furthermore, mucinous cholangiocarcinoma is essentially negative for estrogen and progesterone receptors, while invasive mucinous carcinoma is strongly positive for these receptors [
25]. In the presented case, immunohistochemistry of the tumor cell nuclei identified a marked expression of estrogen receptor - 95% and progesterone receptor - 90%, a weakly positive Ki-67 expression (8%), and a negative HER2 expression, supporting the mixed type described in the histopathological examination of the resected specimen. Mutations in codon 12 of the KRAS gene, specific to pancreatic mucinous cholangiocarcinoma, have been observed in both non-invasive and invasive tumors, while alterations in TP53, CDKN2A (p16), and SMAD4 (DPC4) are associated with an invasive component. However, specialized literature lacks information on the genetic profile of mucinous cholangiocarcinoma, likely due to the rarity of the tumor and the absence of published data [
24].
The primary modality of the treatment protocol is surgical intervention followed by postoperative adjuvant therapy. It is important to differentiate between pure mucinous carcinoma and mixed mucinous carcinoma, as pure mucinous carcinoma tends to be a less aggressive type and has a lower rate of lymph node metastasis compared to mixed mucinous carcinoma [
31,
33,
34]. Hormone receptors are more frequently positive for mixed mucinous carcinoma, and in most cases, neoadjuvant chemotherapy is indicated [
33,
34,
35,
36,
37]. Mucinous breast carcinoma is rarely associated with lymph node involvement [
31,
38,
39]. The development of lymph node metastases negatively influences the survival rate and is considered one of the most important prognostic factors [
34]. This is why, when considering surgical strategies, mixed forms often require axillary resection.
The imaging appearance of mucinous breast carcinoma can be confused with a benign lesion as it sometimes presents as a round-shaped mass with clear margins on mammography [
30]. In some cases, mucinous breast carcinoma can be mammographically subtle or yield inconclusive mammographic results, such as calcifications, opacities, or focal asymmetries [
30]. In most cases, mucinous breast carcinoma appears on mammography as a low-density mass, round or oval in shape, with tumor margins that can range from microlobulated (high mucin content) to irregular or spiculated (low mucin content). The mucin content is correlated with peripheral features.
On ultrasound examination, mucinous carcinoma appears as a round or oval mass, isoechoic or hypoechoic compared to subcutaneous fat, often with posterior acoustic enhancement and internal echoes, along with cystic or solid components [
30].
These imaging features have contributed to shaping the diagnosis in the case of our patient.
Our patient presented with a mixed mucinous breast carcinoma without axillary lymph node invasion, with a spiculated appearance and a challenging mammographic interpretation. A definitive mammographic conclusion was only possible after repeating the computed tomography examination and performing serial mammograms with atypical features.
To date, no study has demonstrated the genetic and radiation implications in the development of mucinous carcinomas or the possible presence of mucinous carcinomas in series in the same patient. Current data are insufficient to provide recommendations for monitoring secondary malignant tumors in these patients. Further studies are needed regarding the association between mucinous pancreatic cystadenoma and mucinous breast carcinoma.
Case Specifics:
- a)
The patient was a non-smoker with no family history of neoplasms (no familial cancer pattern), but with significant cardiovascular and metabolic comorbidities.
- b)
The development of a second mucinous carcinoma – right breast carcinoma – 12 years after the first – borderline mucinous pancreatic cystadenoma.
- c)
Accidental early diagnosis of malignant tumors in a patient with multiple hospital admissions, which favored a good prognosis both in the short and long term.
- d)
Rare clinical form of breast neoplasm, as well as pancreatic neoplasm.
- e)
An extremely high risk of mortality due to the association with cardiac diseases, which initially led to a delay in specific cancer therapy.
Author Contributions
Conceptualization, T.G.A. and I.B.; methodology, T.G.A., S.F.Ț., A.C., and M.G.P.; software, O.H.O and V.N.; validation, I.B., S.F.Ț., and V.N.; formal analysis, M.G.P. and O.H.O.; investigation, T.G.A., I.O, I.D., V.N., and I.B..; resources, T.G.A. and I.O.; writing—original draft preparation, T.G.A., S.F.Ț., and I.B.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Cancer Society. Breast Cancer Facts & Figures 2019-2020; American Cancer Society, Inc.: Atlanta, 2019. [Google Scholar]
- Barbara, M.; Tsen, A.; Tenner, L.; Rosenkranz, L. Talking Genes in Breast and Pancreatic Malignancies. Mater Sociomed. 2019, 31, 146–149. [Google Scholar] [CrossRef]
- World Health Organization Regional Office for Europe. European (HFA-DB): Incidence of female breast cancer.
- Zaha, D.C.; Lazar, E.; Lazurean, C. Clinicopathologic features and five years survival analysis in molecular subtypes of breast cancer. Romanian Journal of Morphology and Embryology. 2010, 51, 85–89. [Google Scholar] [PubMed]
- National Cancer Institute: Cancer causes and prevention, Martch 7, 2019.
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press). 2019, 11, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Meerson, A.; Eliraz, Y.; Yehuda, H.; et al. Obesity impacts the regulation of miR-10b and its targets in primary breast tumors. BMC Cancer. 2019, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.M.; Whitley, B.R.; Wiesner, T.F.; et al. Breast cancer and metabolic syndrome linked through the plasminogen activator inhibitor-1 cycle. Byoessays. 2007, 29, 1029–1038. [Google Scholar] [CrossRef]
- Xue, F.; Michels, K.B. Diabetes, metabolic syndrome, and breast cancer: A review of the current evidence. The American Journal of Clinical Nutrition. 2007, 86, 823S–835S. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, O.; Davițoiu, D.; Stavarache, I.; et al. The interdisciplinary management of a patient with a rare mammary tumor - a case report. Ginecologia. 2017, 2, 44–47. [Google Scholar] [CrossRef]
- Gök, M.; Topal, U.; Öz, B.; et al. Comparison of Clinical Features and Treatment Results of Mix Mucinous Carcinomas and Other Atypical Carcinomas of the Breast. Eur J Breast Health. 2019, 15, 222–228. [Google Scholar] [CrossRef]
- Jang, Y.; Cho, E.Y.; Cho, S.Y. Human Epidermal Growth Factor Receptor 2-positive Mucinous Carcinoma with Signet Ring Cell Differentiation, Which Showed Complete Response after Neoadjuvant Chemotherapy. J Breast Cancer. 2019, 22, 336–340. [Google Scholar] [CrossRef]
- Marrazzo, E.; Frusone, F.; Milana, F.; et al. Mucinous breast cancer: A narrative review of the literature and a retrospective tertiary single-centre analysis. The Breast. 2020, 49, 87–92. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press). 2019, 11, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef] [PubMed]
- Di Saverio, S.; Gutierrez, J.; Avisar, E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008, 111, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; et al. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014, 16, 446. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Mantzoros, C.S.; Wolk, A. Diabetes mellitus and risk of breast cancer: A meta-analysis. Int J Cancer. 2007, 121, 856–862. [Google Scholar] [CrossRef]
- Tabassum, I.; Mahmood, H.; Faheem, M. Type 2 Diabetes Mellitus as a Risk Factor for Female Breast Cancer in the Population of Northern Pakistan. Asian Pacific Journal of Cancer Prevention. 2017, 17, 3255–3258. [Google Scholar]
- Hauner D, Hauner H. Metabolic Syndrome and Breast Cancer: Is There a Link? Breast Care. 2014, 9, 277–281. [CrossRef]
- Protani, M.; Coory, M.; Martin, J.M. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; et al. Body mass index and survival in women with breast cancer - systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014. [Epub ahead of print]. [Google Scholar] [CrossRef]
- Jefferson, B.; Venkatraman, I.; Kumar, R.V.; Ponnuswamy, K.; Maduraimuthu, P. Mucinous cystadenoma of pancreas with honeycombing appearance: Radiological-Pathological correlation. Indian J Radiol Imaging. 2018, 28, 327–329. [Google Scholar] [CrossRef]
- Koufopoulos, N.; Goudeli, C.; Syrios, J.; Filopoulos, E.; Khaldi, L. Mucinous cystadenocarcinoma of the breast: The challenge of diagnosing a rare entity. Rare Tumors. 2017, 9, 7016. [Google Scholar] [CrossRef] [PubMed]
- Valdespino, V.E.; Matamoros, I.L.; Valle, M.L.; et al. Mucinous cystadenocarcinoma of the breast, case report. Obstet Gynecol Rep. 2019, 3, 1–3. [Google Scholar] [CrossRef]
- Pareja, F.; Lee, J.Y.; Brown, D.N.; et al. The Genomic Landscape of Mucinous Breast Cancer. J Natl Cancer Inst. 2019, 111, 737–741. [Google Scholar] [CrossRef]
- Chaudhry, A.R.; Khoury, M.E.; Gotra, A.; et al. Imaging features of pure and mixed forms of mucinous breast carcinoma with histopathological correlation. The British Journal of Radiology. 2019, 92, 1095. [Google Scholar] [CrossRef]
- Lei, L.; Yu, X.; Chen, B.; Chen, Z.; Wang, X. Clinicopathological Characteristics of Mucinous Breast Cancer: A Retrospective Analysis of a 10-Year Study. PLoS ONE. 2016, 11, e0155132. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.B. Human mucinous breast carcinomas and their lymph node metastases. A histological review of 247 cases. Pathol Res Pract 1985, 180, 377–382. [Google Scholar] [CrossRef]
- Marrazzo, E.; Frusone, F.; Milana, F.; et al. Mucinous breast cancer: A narrative review of the literature and a retrospective tertiary single-centre analysis. Breast 2020, 49, 87–92. [Google Scholar] [CrossRef]
- Dumitru, A.; Procop, A.; Iliesiu, A.; et al. Mucinous Breast Cancer: A Review Study of 5 Year Experience from a Hospital-Based Series of Cases. Maedica (Buchar) 2015, 10, 14–18. [Google Scholar]
- Chikkannaiah, P.; Thangngeo, D.; Guruprasad, C.; Venkataramanappa, S. Clinicopathological Study of Mucinous Carcinoma of Breast with Emphasis on Cytological Features: A Study at Tertiary Care Teaching Hospital of South India. J Lab Physicians. 2020, 12, 68–75. [Google Scholar] [CrossRef]
- Ranade, A.; Batra, R.; Sandhu, G.; et al. Clinicopathological evaluation of 100 cases of mucinous carcinoma of breast with emphasis on axillary staging and special reference to a micropapillary pattern. J Clin Pathol., 2010, 63, 1043–1047. [Google Scholar] [CrossRef]
- Gök, M.; Topal, U.; Öz, B.; Akgün, H.; Akcan, A.C.; Sözüer, E.M. Comparison of Clinical Features and Treatment Results of Mix Mucinous Carcinomas and Other Atypical Carcinomas of the Breast. Eur J Breast Health 2019, 15, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Gwark, S.; Lee, H.S.; Lee, Y.; et al. Clinical Implication of HER2 Status in Hormone Receptor-Positive Mucinous Breast Cancer. Ann Surg Oncol 2019, 26, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Al Haddad, H.; Awadallah, A.; Abdel Hadi, M. Mucinous breast carcinoma: Report of four cases and review of the literature. Clin Diagn Pathol, 2017, 1, 1–4. [Google Scholar] [CrossRef]
- Dhillon, R.; Depree, P.; Metcalf, C.; Wylie, E. Screen-detected mucinous breast carcinoma: Potential for delayed diagnosis. Clin Radiol 2006, 61, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.M.; Banerjee, B. Mucinous carcinoma of breast in a 30-year-old female: A rare case report and discussion. Clin Cancer Investig J 2016, 5, 489–491. [Google Scholar] [CrossRef]
- Di Saverio, S.; Gutierrez, J.; Avisar, E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008, 111, 541–547. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).