1. Introduction
Anticoagulation therapy is the cornerstone treatment for stroke prevention in atrial fibrillation (AF) [
1,
2]. Proper use of oral anticoagulants (OACs) is crucial in the management of AF [
3]. Non-Vitamin K antagonists (NOACs) were a revolution in the anticoagulation therapy of AF, providing more predictable effects with rapid onset and offset of their action, and fewer drug and food interactions, while requiring less frequent laboratory monitoring [
4]. Even in the era of NOACs, the AF patients’ compliance to OAC therapy reported in some studies is still poor [
5], while the compliance may differ among the anticoagulation agents and different countries [
6]. Patients’ frailty status, high bleeding risk, severe renal impairment, and unwillingness to receive anticoagulation therapy are some of the main reported issues in patients who did not receive OAC from the treating physicians [
1,
2,
3,
7].
In certain individuals with “nonvalvular” AF (AF patients without mechanical prosthetic valves and/or moderate to severe mitral stenosis), left atrial appendage closure (LAAC) has shown promise as a safe treatment option in place of oral anticoagulation [
8,
9]. According to the 2020 ESC guidelines for the management of AF, LAAC may be considered for stroke prevention in patients with AF and contraindications for long-term anticoagulant treatment [
1]. However, beyond the current recommendations [
1,
2], in a post-hoc analysis of the MISOAC-AF study, almost one out of six hospitalized patients with “nonvalvular” AF may be considered eligible for LAAC [
3,
10]. In this study, we aimed to assess the anticoagulation status and LAAO indications in patients with "nonvalvular” AF from the HECMOS (Hellenic Cardiorenal Morbidity Snapshot) survey.
2. Materials and Methods
HECMOS was a multicenter cross-sectional observational snapshot survey that investigated the contemporary trends of cardiorenal morbidity among hospitalized patients in cardiology wards across Greece on an ordinary weekday, the 3rd of March 2022 [
11]. The First Cardiology Clinic of the National and Kapodistrian University of Athens (NKUA) organized it in collaboration with the 2nd and 3rd NKUA Cardiology Clinics under the auspices of INAKEN (Institute for study, research, and education of vascular, heart, brain, and kidney nosologies).
All cardiology departments of the Hellenic National Public Health System as well as high volume ones in the private sector were invited to enroll. The study included patients from 55 different departments in total, adequately covering the whole country given its geographic peculiarities. Eligibility for enrollment was offered to all adult (>18 years) inpatients able to provide informed consent by themselves or via their legal representative.
Data collection included patients’ demographics, physical characteristics, and lifestyle parameters. Details regarding the participants’ past medical history were gained, with emphasis on the presence of chronic heart failure, atrial fibrillation, chronic kidney disease, diabetes mellitus, hypertension, COVID-19 infection, stroke, severe liver dysfunction, bleeding history, chronic obstructive pulmonary disease, obstructive sleep apnea syndrome, and intracardiac devices such as a pacemaker or a defibrillator. Data collection included electrocardiographic, echocardiographic, and laboratory results synchronous to the snapshot survey. eGFR was calculated according to the 2021 CKD-EPI equation.
All data were recorded anonymously in a prespecified eCRF (electronic case report form). This form has been created through the online RedCap platform (Vanderbit University), which enables simultaneous and secure data entry. The study protocol was in accordance with the 1975 Declaration of Helsinki and was approved by the local ethical committees of each of the participating institutions. The anonymity and confidentiality of the collected data were ensured in accordance with the applicable legislation.
In this substudy, we included patients with known AF without mechanical prosthetic valves and moderate to severe mitral valve stenosis. In this report, we focus on the anticoagulation status of hospitalized AF patients. We included all the oral anticoagulation agents which were available in Greece [dabigatran, rivaroxaban, apixaban, Vitamin K antagonists (acenocumarol)]. Patients with prior stroke, history of major bleeding, poor adherence to anticoagulation therapy and end-stage renal disease (eGFR< 15 ml/min/1.73m2) were considered candidates for LAAC.
Qualitative data were presented with absolute and relative frequencies (%), while quantitative data with means, standard deviation, median and 1st – 3rd quartile. The chi-square test of independence was used to check for a correlation between qualitative characteristics of patients. The statistical significance level was set at 5% (a=0.05). The analysis was carried out with the SPSS 20.0 software package (SPSS Inc, Chicago, IL, USA).
3. Results
The total sample of the HECMOS study consisted of 918 patients from 55 hospitals per territory. Two hundred fifty-six patients (mean age 76.6±11.7, 148 males) were included in our analysis. Most of them (n=159; 62%) suffered from persistent AF (
Table 1). The mean CHA2DS2-VASc score was 4.28±1.7, while the mean HAS-BLED score was 1.47±0.9.
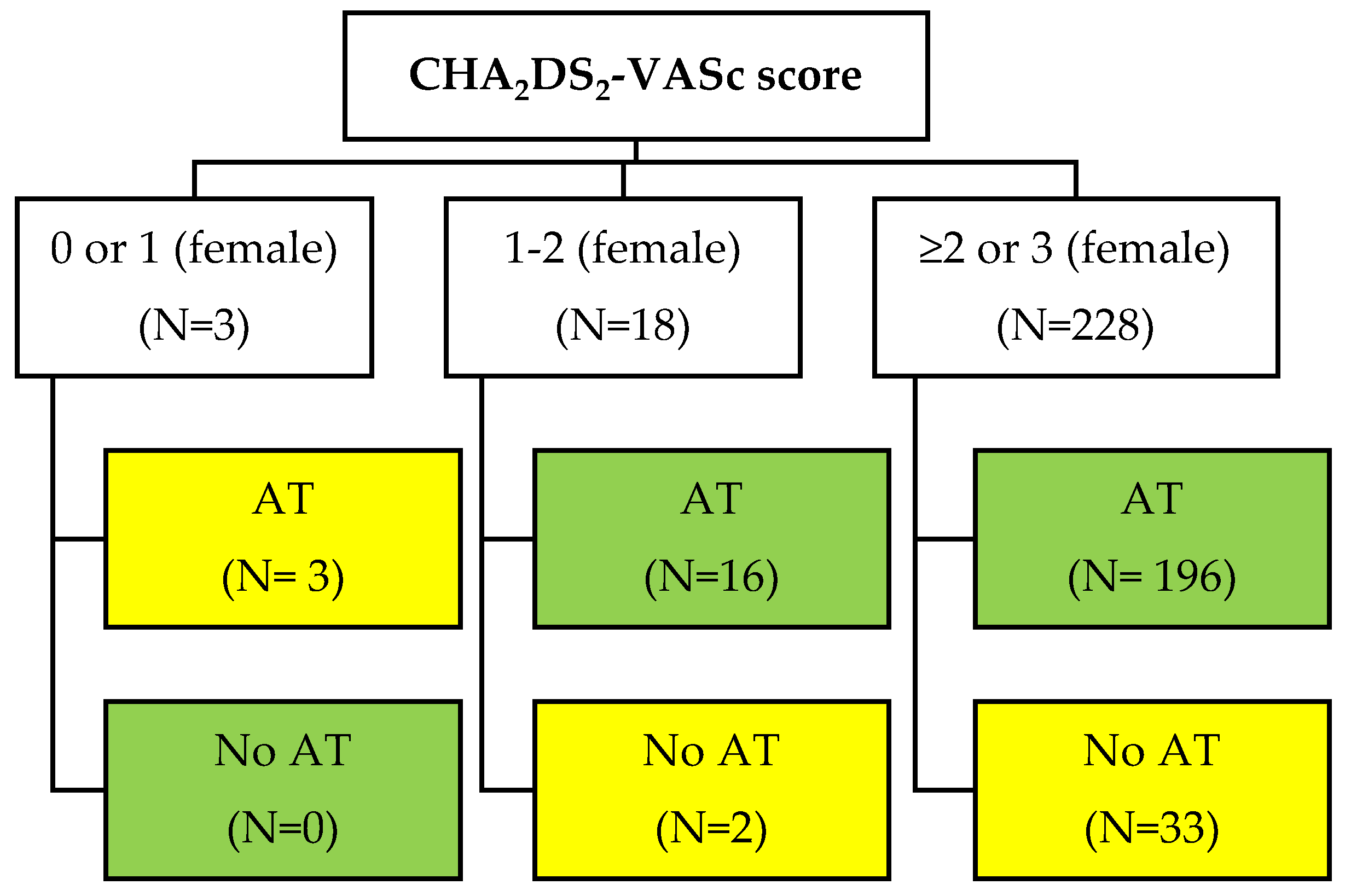
Only 3 patients had a CHA2DS2-VASc 0 or 1 (female) and all of them improperly received anticoagulants. Sixteen out of 18 patients with CHA2DS2-VASc 1 or 2 (female) were also in anticoagulants. Among 235 patients with a CHA2DS2-VASc >1 or >2 (for females) only 33 (14%) did not use anticoagulants. Regarding patients under anticoagulant therapy (n=221), 191 (86.4%) received non-vitamin K antagonist oral anticoagulants (NOACs) and 30 (13.6%) received vitamin K antagonists (acenocoumarol) (
Figure 1).
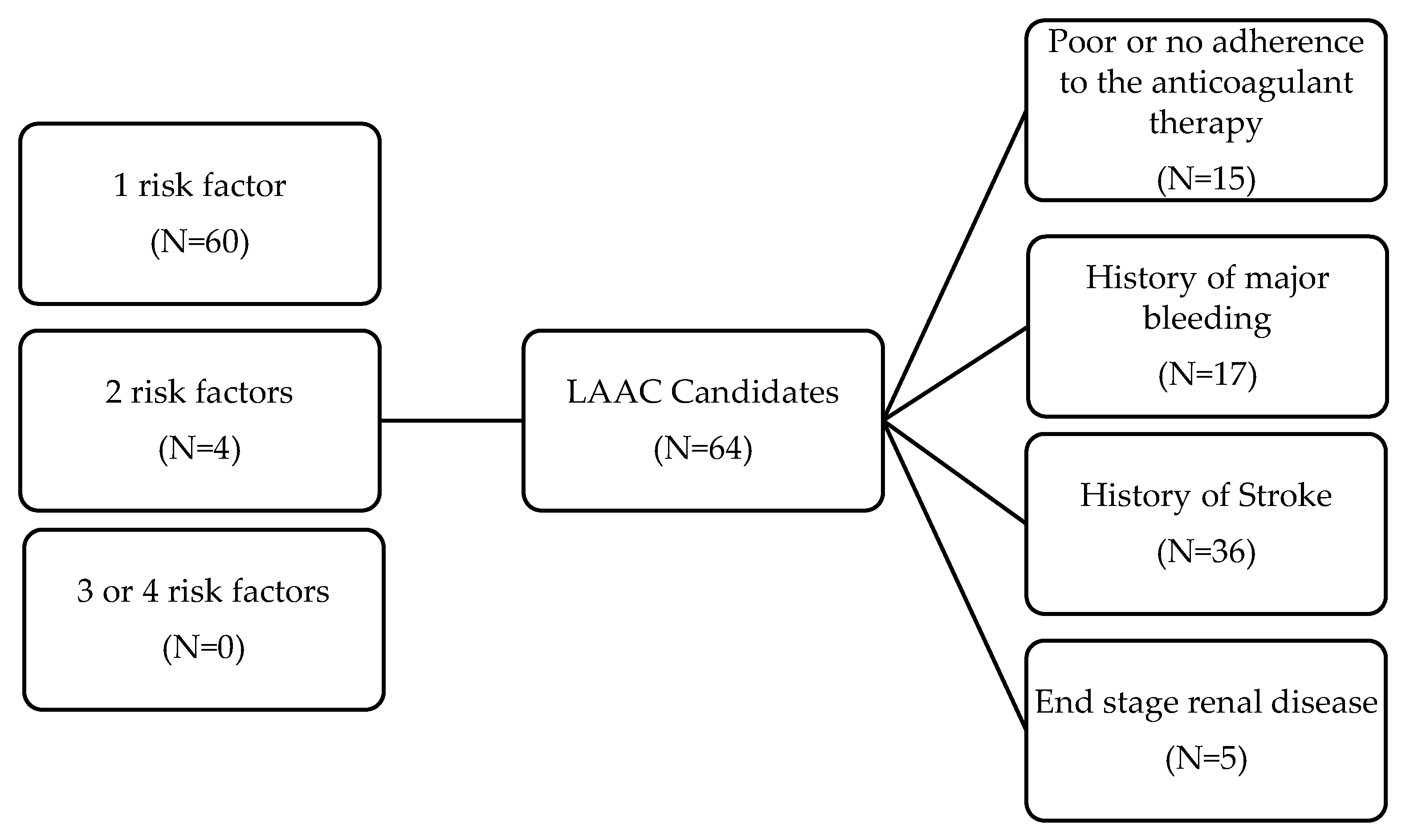
Relative indications for LAAC were present in 64 patients with NVAF (60 had only one risk factor, 4 had two and no one had three or four concurrent risk factors. In detail, 36 had a prior stroke, 17 patients had a history of major bleeding, 15 patients reported poor or no adherence to the anticoagulant therapy and 5 had eGFR< 15 ml/min/1.73m2. Moreover 33 had a HAS-BLED score ≥3. No LAAC treatment was recorded (
Figure 2).
4. Discussion
Anticoagulation status was nearly optimal in a high thromboembolic risk population of cardiology patients, mainly treated with NOACs. Our study also identified that one out of four AF patients should be screened as potentially eligible candidates for LAAC.
Adherence to anticoagulant agents had always considered a critical issue, since almost half of AF patients with high stroke risk were undertreated in the coumadin era [
12,
13]. Overall anticoagulation rates increased to 60% among eligible AF patients in the first years of NOAC use in North American registries and to 80% in European registries [
14,
15,
16]. MISOAC-AF was the first Greek trial that evaluated the real-life anticoagulation prescriptions of AF patients upon a tertiary hospital discharge [
3]. A total of 768 unselected patients with nonvalvular AF discharged between December 2015 and November 2017, had a similar mean CHA2DS2-Vasc 4.4 and HAS-BLED score 1.9. Among patients at significant stroke risk, 14.6% were not prescribed OAC in absolute accordance with our results [
3]. Towards this direction, almost all patients with a Class IIa indication for OAC [CHA2DS2-VASc 1 or 2 (female)] received anticoagulants. Suboptimal use of anticoagulation therapy in eligible patients is attributed to older age, bleeding risk, side effects and noncompliance [
5].
Our population was limited (n=3) regarding low-risk stroke patients and all of them were administered OAC. In these lines, 53% of AF patients with a CHADS score 0 at the first year of NOACs, received OAC improperly [
18]. Clinicians may be anticoagulating these patients simply because they have AF, regardless of their low rate of thrombotic events, although it is also possible that these patients may have been anticoagulated for conditions other than AF, such as valvular heart disease or venous thromboembolic events [
17].
Anticoagulant agents increase bleeding risk [
18] and although the overall risk may be lower with NOACs compared with warfarin, it is still not zero [
19]. Although LAAC scientific societies’ recommendations are weak (IIb) [
1,
2] there are an increasing number of LAAC procedures worldwide [
20]. Interestingly, there is no scientific consensus on the definitions of absolute or relative contraindications to OAC therapy for patients with AF and consequently the exact indications for LAAC. We selected potential indications for LAAC based on the last expert consensus statement on catheter based LAAC [
21]. First of all, patients with previous intracranial bleeding or stroke on adequate OAC treatment have exhibited a significant reduction in stroke/TIA and major bleeding events after LAAC, without compromising safety [
22,
23]. Patients with severe renal dysfunction (eGFR < 15 ml/min) constitute a specific AF population, since routine use of NOACs should be avoided and warfarin is harmful if the anticoagulation control is poor [
24]. LAAC even in patients GFR less than 15 ml/min, reduced significantly stroke/TIA rate and major bleeding in comparison with the expected annual risk [
25]. Nonadherence of patients and clinicians is also an important issue, whereas compliance with treatment is crucial, especially with NOACs since these drugs have a relatively short half-life [
26].
Based on the above indications, we suggested that one out of four hospitalized patients with AF might be considered a potential candidate for LAAC. If we take into account the relative stronger indications for LAAC (prior major bleeding event or prior stroke), then one out of five patients in our study were eligible, in accordance with a previous Greek study where one out of six patients with AF had these strong indications for LAAC, [
10]. Furthermore, in MISOAC-AF, 1 out of 10 patients were eligible for LAAC according to looser criteria exclusively, such as the high HAS-BLED score (≥3) or the existence of end-stage renal disease (eGFR < 15 ml/min/1.73 m
2) [
10]. The proportion of potential candidates was one out of eight in the present study considering the looser indications mentioned above.
Nonetheless, one cannot claim that 25% of hospitalized AF patients should undergo an invasive procedure with complications [
27] taking into account the next generation of anticoagulants that focus on factor XI promising reduced bleeding (factor XI has a greater role in the etiopathogenesis of thrombosis than in physiological hemostasis) [
28]. Additionally, one might suggest that in the future, discontinuation of anticoagulation therapy after a successful ablation procedure will be accompanied by elimination of stroke risk altering current recommendations regarding anticoagulation post ablation.
The generalizability of our results is confined by our study’s snapshot design. Our study included patients admitted to a cardiology ward for any reason with coexisting AF. Thus, our population cohort has a substantially higher number of comorbidities than a sample of patients with AF from the general population or another clinical site (e.g. outpatients department); yet it reflects a typical clinical practice population with AF. Snapshot design offers the power of gathering abundant information regarding the participants’ and the index admission’s characteristics in a short period of time; however, the follow-up data is missing. Missing follow-up data prevents the estimation of the importance of the observed clinical conditions and therapeutic choices in the long-term as well as the expected impact of interventions to address improve them. In the same lines, despite the fact that the geographical distribution of HECMOS study empowers it as a representative epidemiological picture of Greece for the selected population, it does not reflect on seasonal or other potential variations throughout the year.
5. Conclusions
Anticoagulation status appears to be nearly optimal in a high thromboembolic risk population of cardiology patients, mainly treated with NOACs, reflecting the increased clinicians’ and patients’ adherence this last decade. We also identified that one out of four AF patients should be screened as potentially eligible candidates for LAAC, taking into consideration absence of large randomized clinical trials comparing LAAC with NOAC therapy. Till then, a multidisciplinary team approach is required to choose the patient who may benefit more from this invasive therapy.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, D.T and K.T; D.T, I.D, P.P, M.B; data analysis, A.K; CK.A, K.D., D.T. and K.T; formal analysis, P.V, P.I, M.B, A.K, CK.A, D.D; investigation, P.V, P.I, M.B, A.K, CK.A, D.D; resources, P.V, P.I, M.B, A.K, CK.A, D.D; data curation, D.T, P.P, D.D; writing—original draft preparation, D.T, K.G, K.T; writing—review and editing, D.T.; visualization, D.T, K.G, K.T; supervision, D.T, K.G, K.T; project administration, K.T; funding acquisition. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. European heart journal 2021, 42, 373–498. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology 2019, 74, 104–132. [Google Scholar] [CrossRef]
- Kartas, A.; Samaras, A.; Vasdeki, D.; Dividis, G.; Fotos, G.; Paschou, E.; Forozidou, E.; Tsoukra, P.; Kotsi, E.; Goulas, I.; et al. Flaws in Anticoagulation Strategies in Patients With Atrial Fibrillation at Hospital Discharge. Journal of cardiovascular pharmacology and therapeutics 2019, 24, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, P.E.; Tsioufis, C.; Konstantinidis, D.; Iliakis, P.; Leontsinis, I.; Tousoulis, D. Anticoagulation in Deep Venous Thrombosis: Current Trends in the Era of Non- Vitamin K Antagonists Oral Anticoagulants. Current pharmaceutical design 2020, 26, 2692–2702. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R., Jr.; Alkhouli, M.; Reddy, V. Left Atrial Appendage Occlusion for The Unmet Clinical Needs of Stroke Prevention in Nonvalvular Atrial Fibrillation. Mayo Clinic proceedings 2019, 94, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, M.; Vidal, X.; Ballarin, E.; Rottenkolber, M.; Schmiedl, S.; Grave, B.; Huerta, C.; Martin-Merino, E.; Montero, D.; Leon-Muñoz, L.M.; et al. Adherence to Direct Oral Anticoagulants in Patients With Non-Valvular Atrial Fibrillation: A Cross-National Comparison in Six European Countries (2008-2015). Frontiers in pharmacology 2021, 12, 682890. [Google Scholar] [CrossRef]
- Papakonstantinou, P.E.; Asimakopoulou, N.I.; Papadakis, J.A.; Leventis, D.; Panousieris, M.; Mentzantonakis, G.; Hoda, E.; Panagiotakis, S.; Gikas, A. Frailty Status Affects the Decision for Long-Term Anticoagulation Therapy in Elderly Patients with Atrial Fibrillation. Drugs & aging 2018, 35, 897–905. [Google Scholar] [CrossRef]
- Alkhouli, M.; Ellis, C.R.; Daniels, M.; Coylewright, M.; Nielsen-Kudsk, J.E.; Holmes, D.R. Left Atrial Appendage Occlusion. JACC: Advances 2022, 1, 100136. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Alkhouli, M. The History of the Left Atrial Appendage Occlusion. Cardiac electrophysiology clinics 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Vrana, E.; Kartas, A.; Samaras, A.; Vasdeki, D.; Forozidou, E.; Liampas, E.; Karvounis, H.; Giannakoulas, G.; Tzikas, A. Indications for percutaneous left atrial appendage occlusion in hospitalized patients with atrial fibrillation. Journal of cardiovascular medicine (Hagerstown, Md.) 2022, 23, 176–182. [Google Scholar] [CrossRef]
- Leontsinis, I.; Farmakis, D.; Avramidis, D.; Andrikou, E.; Valatsou, A.; Gartzonikas, E.; Doundoulakis, I.; Zarifis, I.; Karpouzis, I.; Kafkala, K.; et al. Cardiorenal multimorbidity in hospitalized cardiology patients: The Hellenic Cardiorenal Morbidity Snapshot (HECMOS) study. Hellenic journal of cardiology : HJC = Hellenike kardiologike epitheorese 2023. [Google Scholar] [CrossRef]
- Hsu, J.C.; Maddox, T.M.; Kennedy, K.F.; Katz, D.F.; Marzec, L.N.; Lubitz, S.A.; Gehi, A.K.; Turakhia, M.P.; Marcus, G.M. Oral Anticoagulant Therapy Prescription in Patients With Atrial Fibrillation Across the Spectrum of Stroke Risk: Insights From the NCDR PINNACLE Registry. JAMA cardiology 2016, 1, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.Y.X.; Malo, S.; Svenson, L.W.; Wilton, S.B.; Hill, M.D. Temporal Trends in the Use and Comparative Effectiveness of Direct Oral Anticoagulant Agents Versus Warfarin for Nonvalvular Atrial Fibrillation: A Canadian Population-Based Study. Journal of the American Heart Association 2017, 6. [Google Scholar] [CrossRef]
- Marzec, L.N.; Wang, J.; Shah, N.D.; Chan, P.S.; Ting, H.H.; Gosch, K.L.; Hsu, J.C.; Maddox, T.M. Influence of Direct Oral Anticoagulants on Rates of Oral Anticoagulation for Atrial Fibrillation. Journal of the American College of Cardiology 2017, 69, 2475–2484. [Google Scholar] [CrossRef]
- Admassie, E.; Chalmers, L.; Bereznicki, L.R. Changes in Oral Anticoagulant Prescribing for Stroke Prevention in Patients With Atrial Fibrillation. The American journal of cardiology 2017, 120, 1133–1138. [Google Scholar] [CrossRef]
- Proietti, M.; Laroche, C.; Opolski, G.; Maggioni, A.P.; Boriani, G.; Lip, G.Y.H. 'Real-world' atrial fibrillation management in Europe: Observations from the 2-year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase. Europace : European pacing, arrhythmias, and cardiac electrophysiology : Journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 2017, 19, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.W.; Kim, S.; Piccini, J.P., Sr.; Ansell, J.E.; Fonarow, G.C.; Hylek, E.M.; Singer, D.E.; Mahaffey, K.W.; Kowey, P.R.; Thomas, L.; et al. Risks and benefits of anticoagulation in atrial fibrillation: Insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. Cardiovascular quality and outcomes 2013, 6, 461–469. [Google Scholar] [CrossRef]
- Kourlaba, G.; Stefanou, G.; Tsalamandris, S.; Oikonomou, E.; Papageorgiou, G.; Nikas, N.; Tousoulis, D.; Maniadakis, N. Incidence and cost of bleeding events requiring hospitalization in patients with atrial fibrillation treated with acenocoumarol in Greece. Hellenic journal of cardiology : HJC = Hellenike kardiologike epitheorese 2021, 62, 234–240. [Google Scholar] [CrossRef]
- Yang, E. A clinician's perspective: Novel oral anticoagulants to reduce the risk of stroke in nonvalvular atrial fibrillation--full speed ahead or proceed with caution? Vascular health and risk management 2014, 10, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Labori, F.; Persson, J.; Bonander, C.; Jood, K.; Svensson, M. Cost-effectiveness analysis of left atrial appendage occlusion in patients with atrial fibrillation and contraindication to oral anticoagulation. European heart journal 2022, 43, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion - an update. Europace : European pacing, arrhythmias, and cardiac electrophysiology : Journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 2020, 22, 184. [Google Scholar] [CrossRef] [PubMed]
- Tzikas, A.; Freixa, X.; Llull, L.; Gafoor, S.; Shakir, S.; Omran, H.; Giannakoulas, G.; Berti, S.; Santoro, G.; Kefer, J.; et al. Patients with intracranial bleeding and atrial fibrillation treated with left atrial appendage occlusion: Results from the Amplatzer Cardiac Plug registry. International journal of cardiology 2017, 236, 232–236. [Google Scholar] [CrossRef]
- Cruz-González, I.; González-Ferreiro, R.; Freixa, X.; Gafoor, S.; Shakir, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; et al. Left atrial appendage occlusion for stroke despite oral anticoagulation (resistant stroke). Results from the Amplatzer Cardiac Plug registry. Revista espanola de cardiologia (English ed.) 2020, 73, 28–34. [Google Scholar] [CrossRef]
- Potpara, T.S.; Ferro, C.J.; Lip, G.Y.H. Use of oral anticoagulants in patients with atrial fibrillation and renal dysfunction. Nature reviews. Nephrology 2018, 14, 337–351. [Google Scholar] [CrossRef]
- Kefer, J.; Tzikas, A.; Freixa, X.; Shakir, S.; Gafoor, S.; Nielsen-Kudsk, J.E.; Berti, S.; Santoro, G.; Aminian, A.; Landmesser, U.; et al. Impact of chronic kidney disease on left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation. International journal of cardiology 2016, 207, 335–340. [Google Scholar] [CrossRef]
- Banerjee, A.; Benedetto, V.; Gichuru, P.; Burnell, J.; Antoniou, S.; Schilling, R.J.; Strain, W.D.; Ryan, R.; Watkins, C.; Marshall, T.; et al. Adherence and persistence to direct oral anticoagulants in atrial fibrillation: A population-based study. Heart (British Cardiac Society) 2020, 106, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.; Vasdeki, D.; Tzikas, A.; Meier, B.; Saw, J. Incidence, Prevention, and Management of Periprocedural Complications of Left Atrial Appendage Occlusion. Interventional cardiology clinics 2018, 7, 243–252. [Google Scholar] [CrossRef]
- Badimon, J.J.; Escolar, G.; Zafar, M.U. Factor XI/XIa Inhibition: The Arsenal in Development for a New Therapeutic Target in Cardio- and Cerebrovascular Disease. Journal of cardiovascular development and disease 2022, 9. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).