Submitted:
22 August 2023
Posted:
23 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
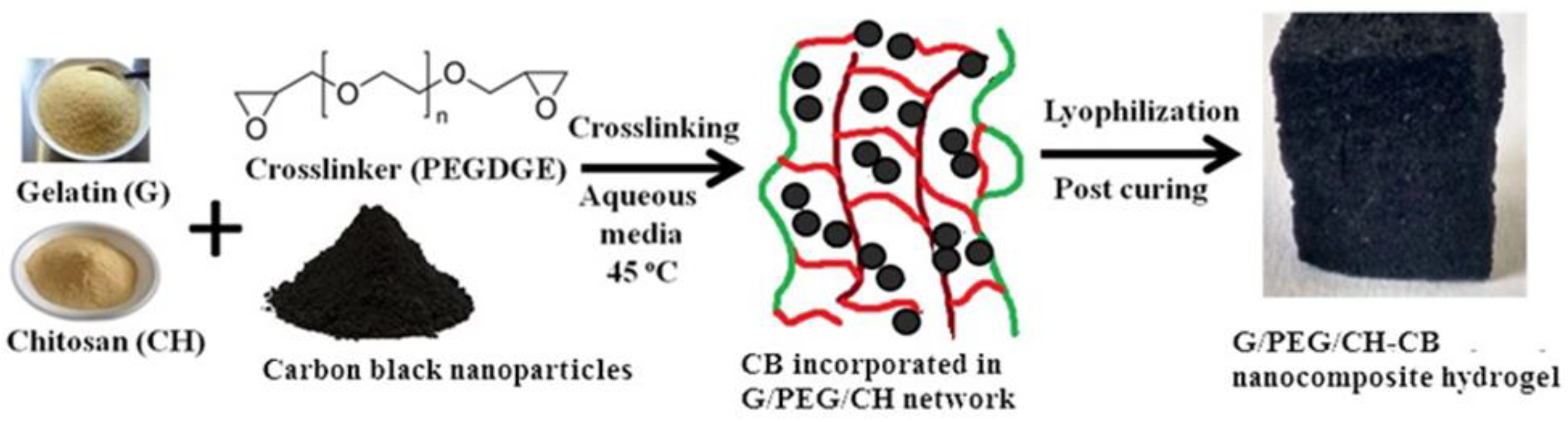
2.1. Synthesis of G/PEG/CH (CB) nanocomposite hydrogels
2.2. Density and porosity measurement
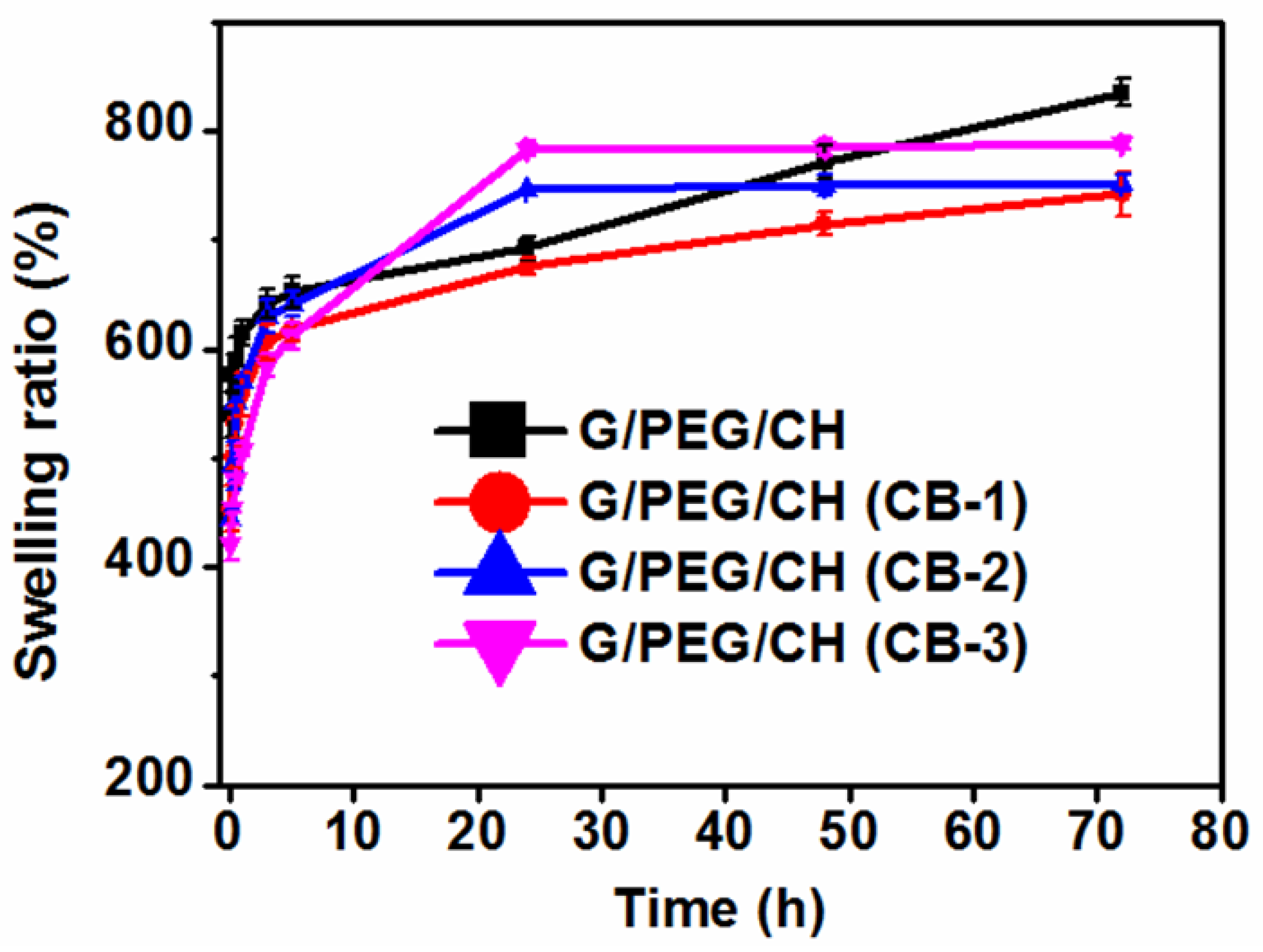
2.3. Swelling ratio (%) measurement
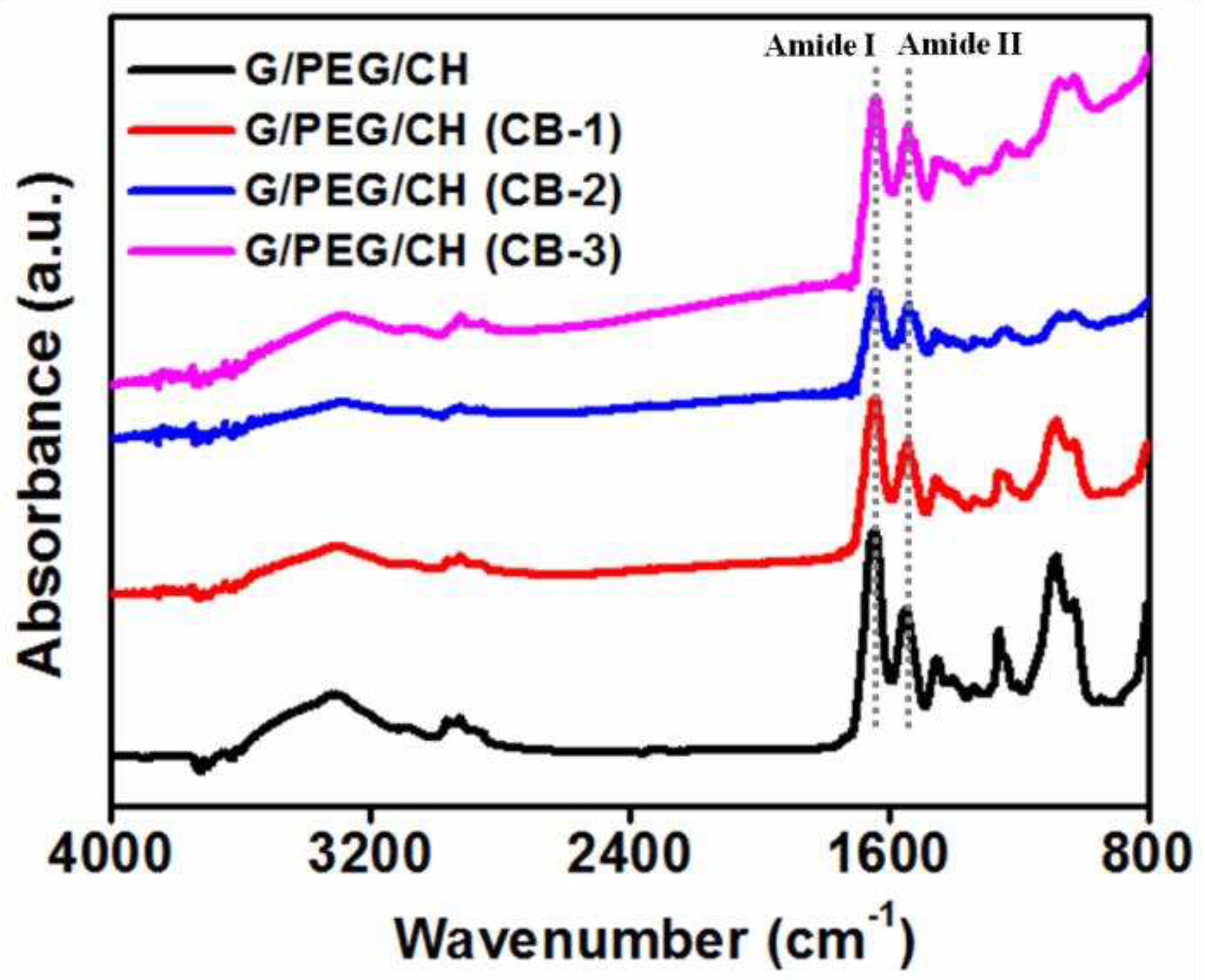
2.4. Chemical Structure Analysis
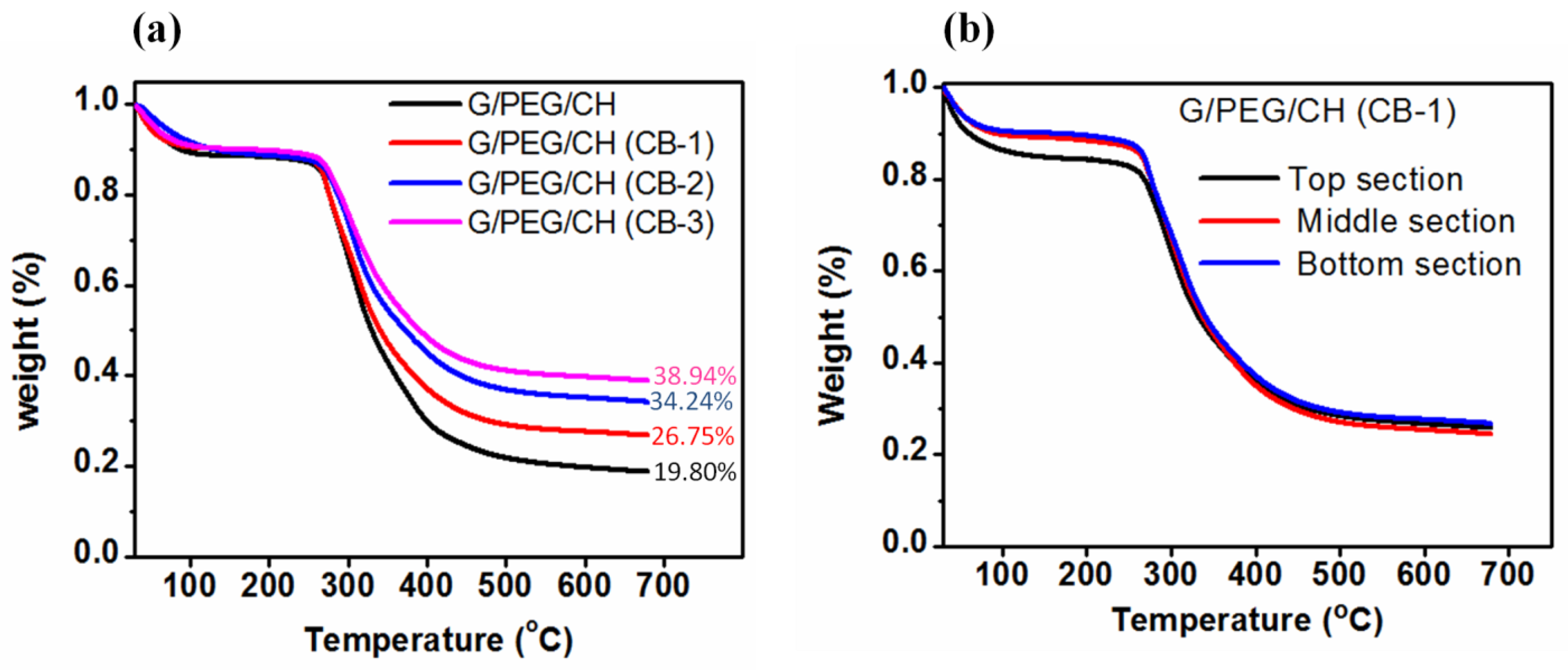
2.5. Thermogravimetric analysis
2.6. Morphology analysis
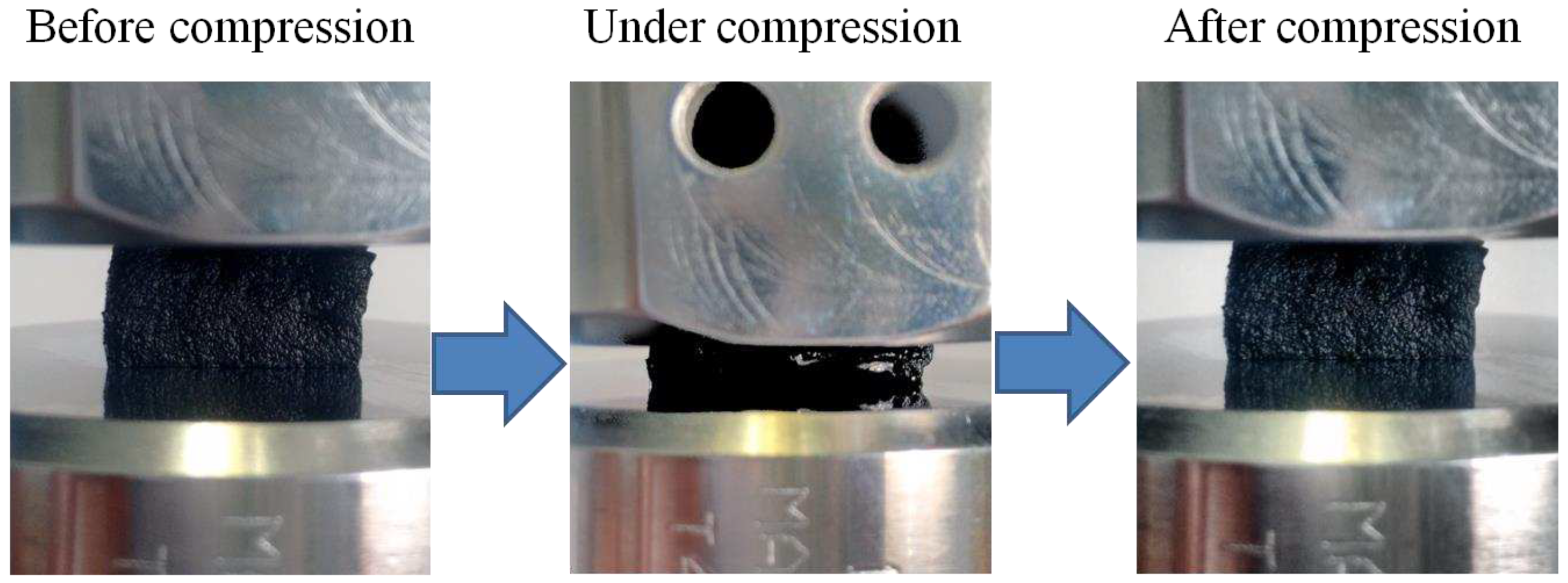
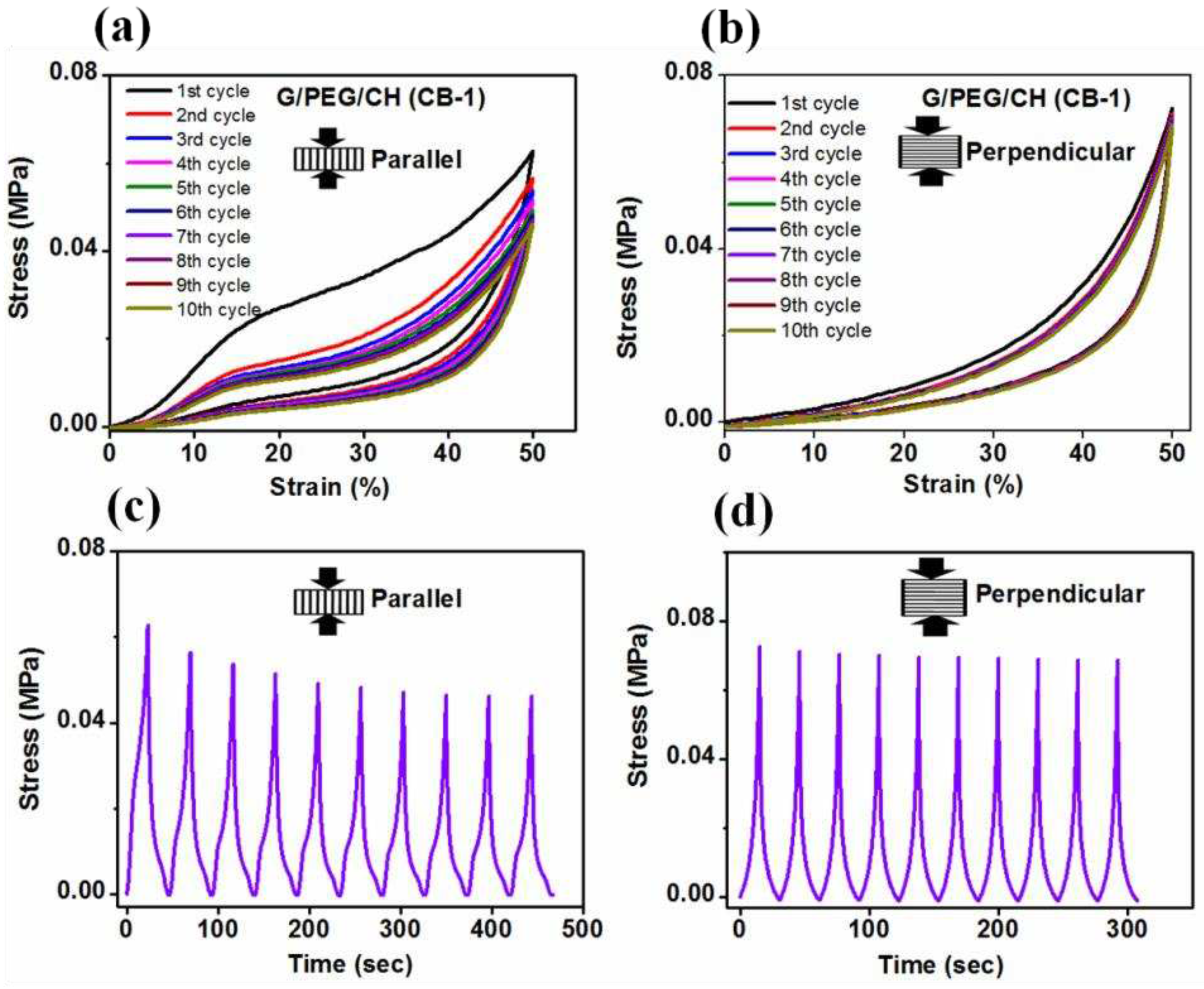
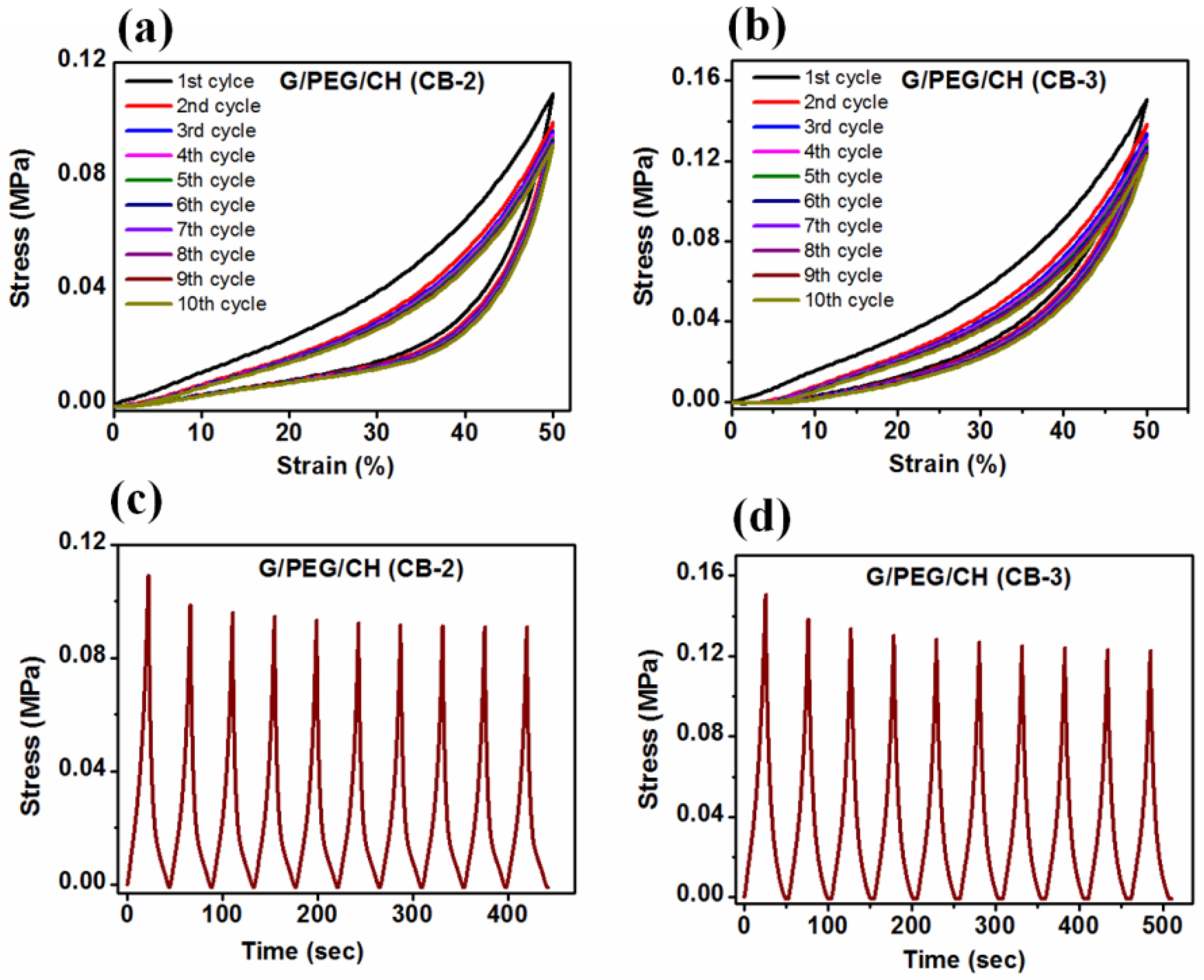
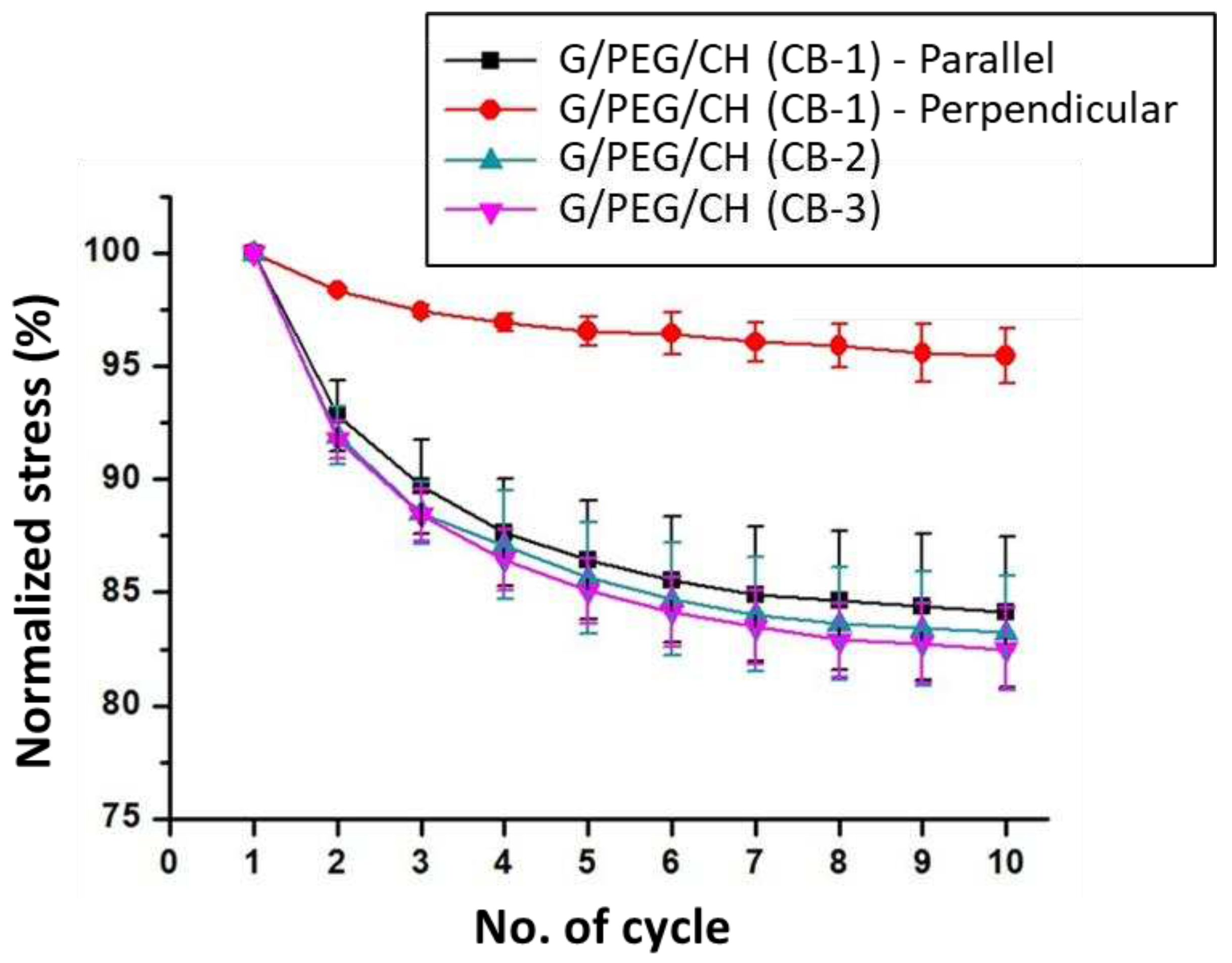
2.7. Compression and cyclic compression tests
2.8. Electrical impedance measurement
3. Results
3.1. Preparation of G/PEG/CH (CB) nanocomposite hydrogels and their physical properties
3.2. Chemical structure characterization
3.3. Apparent density, porosity and swelling ratio
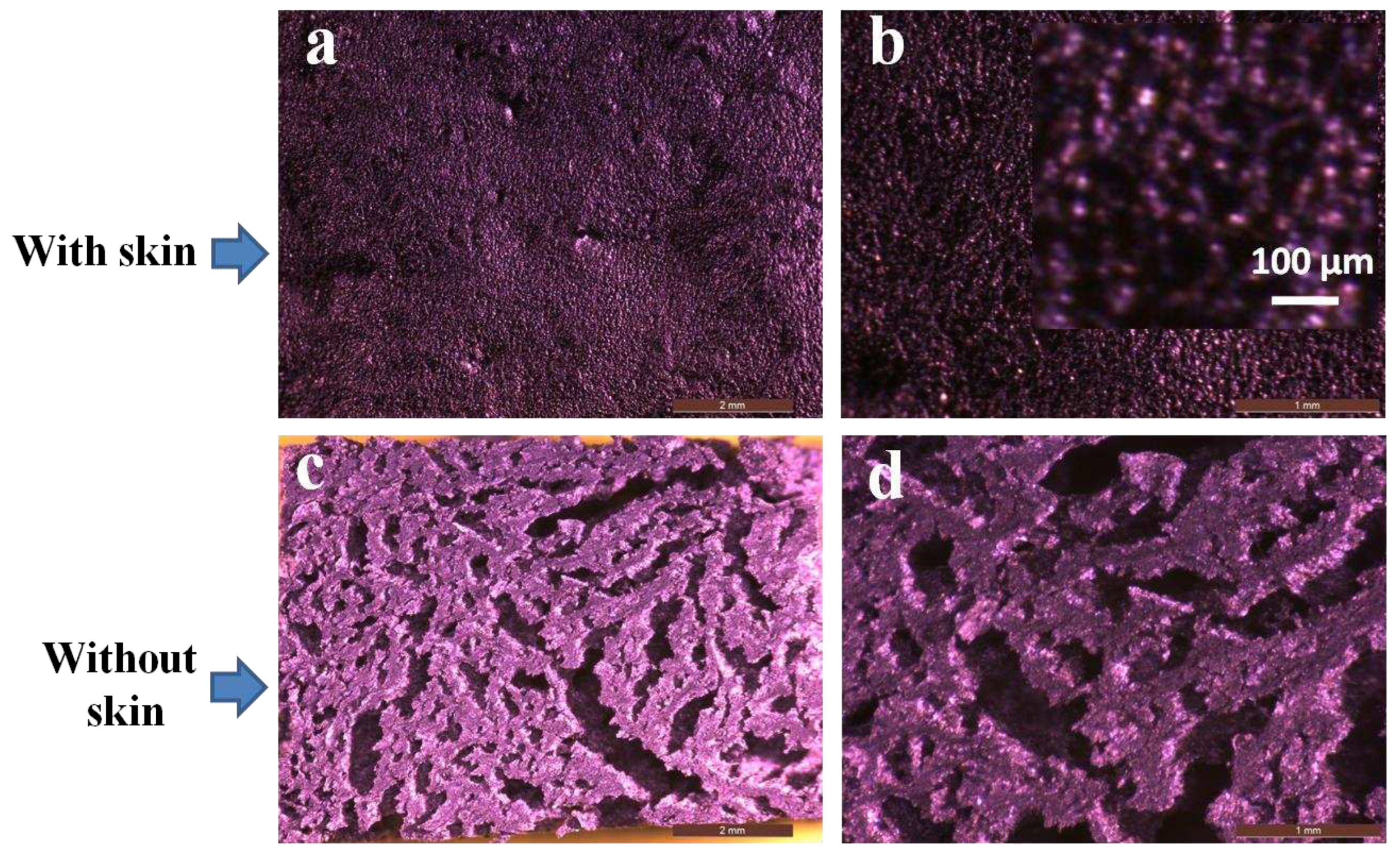
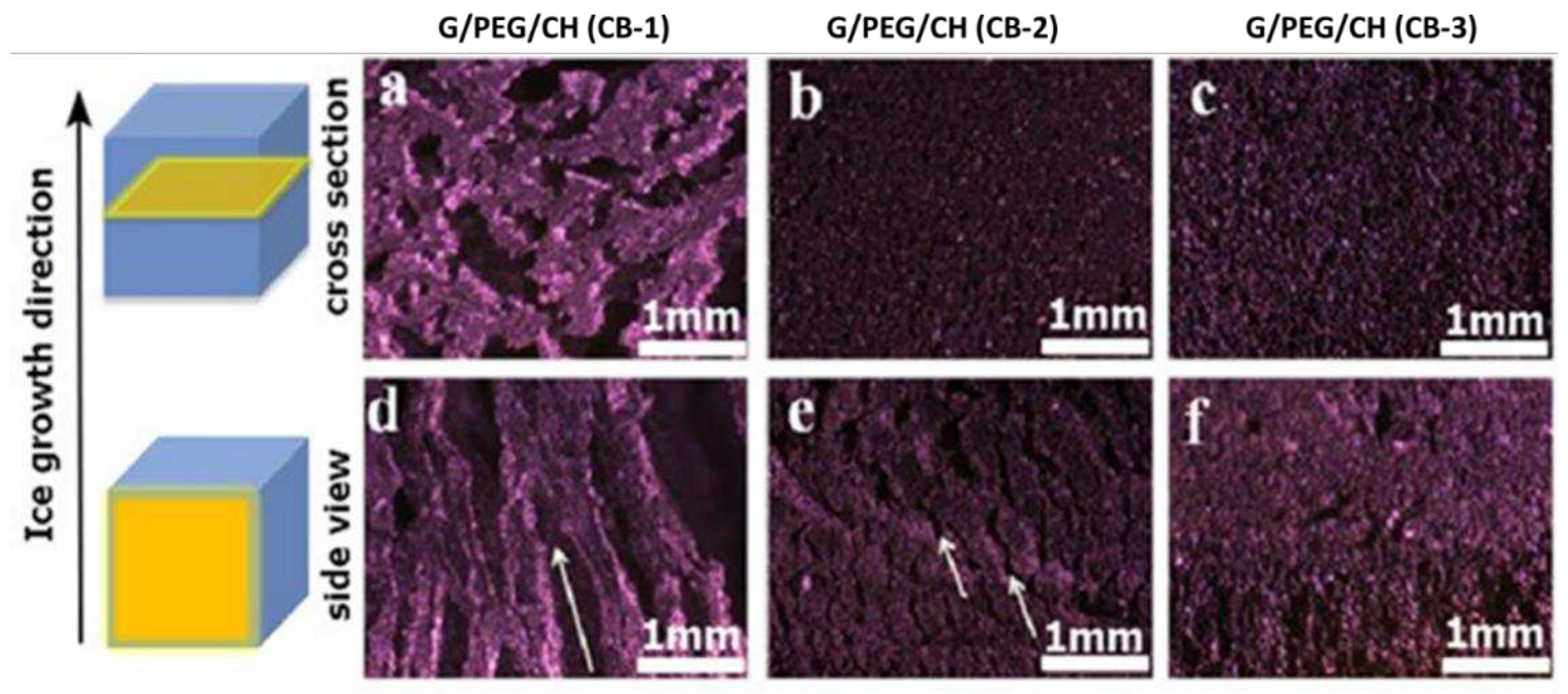
3.4. Morphological evaluation
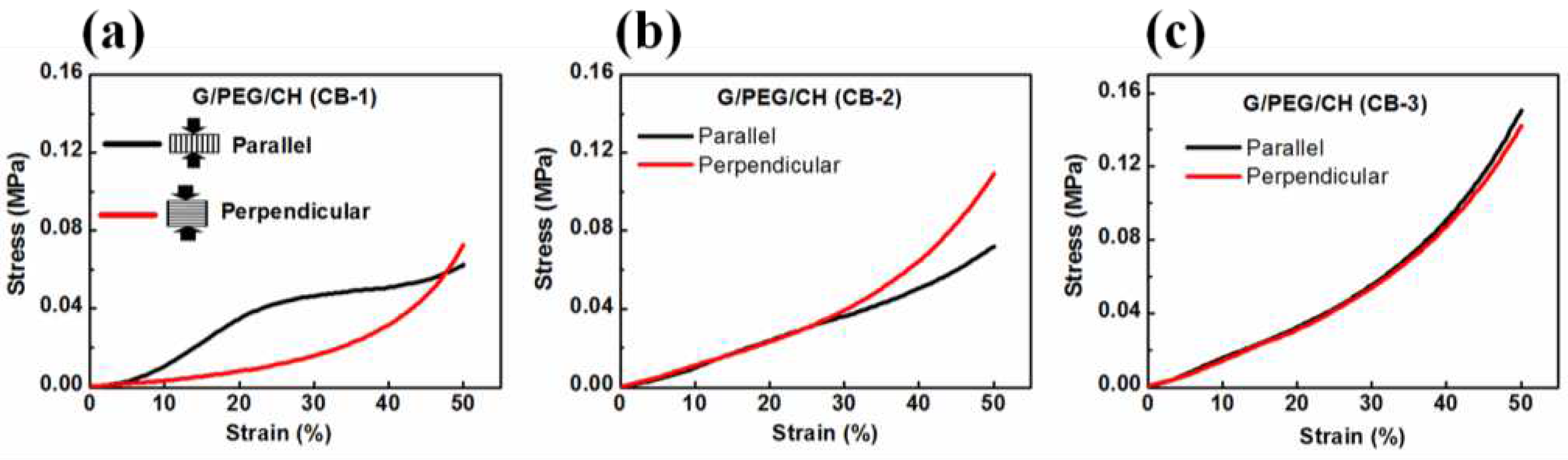
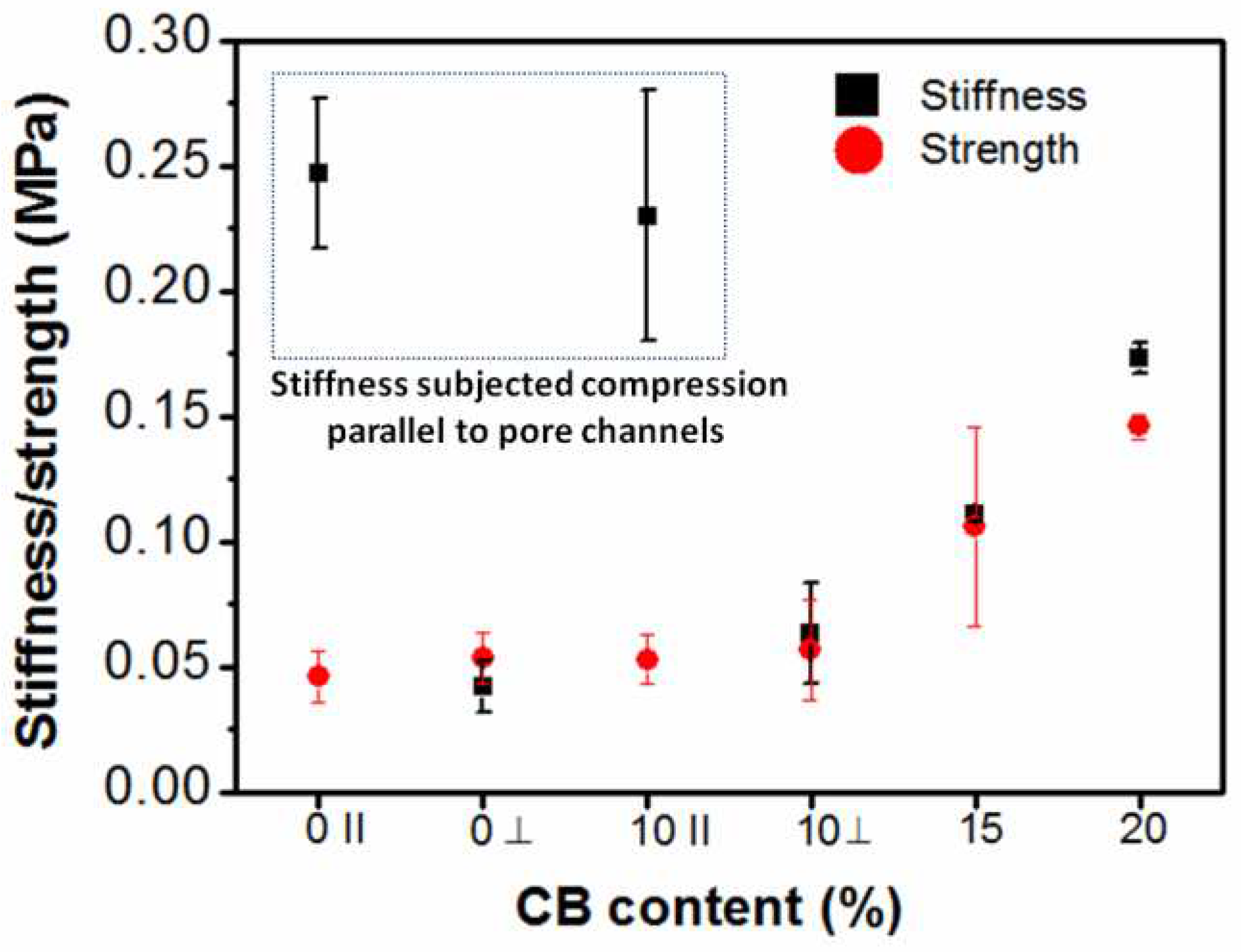
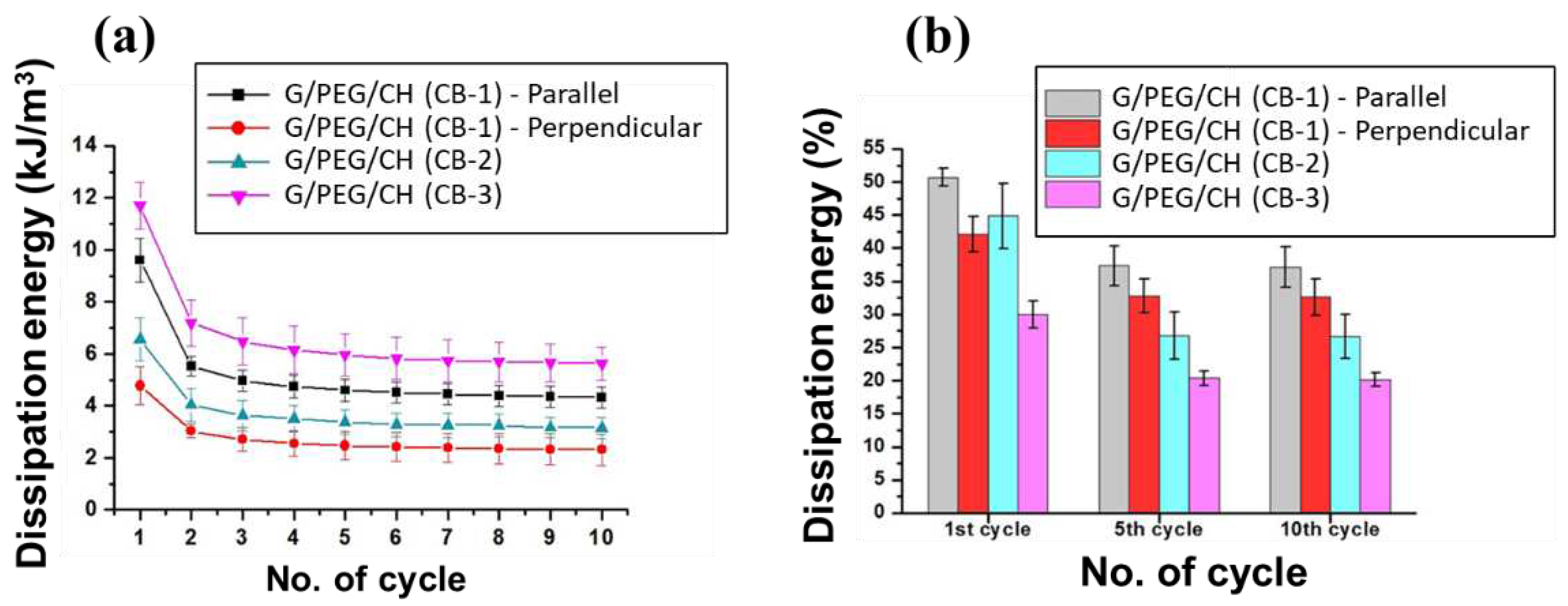
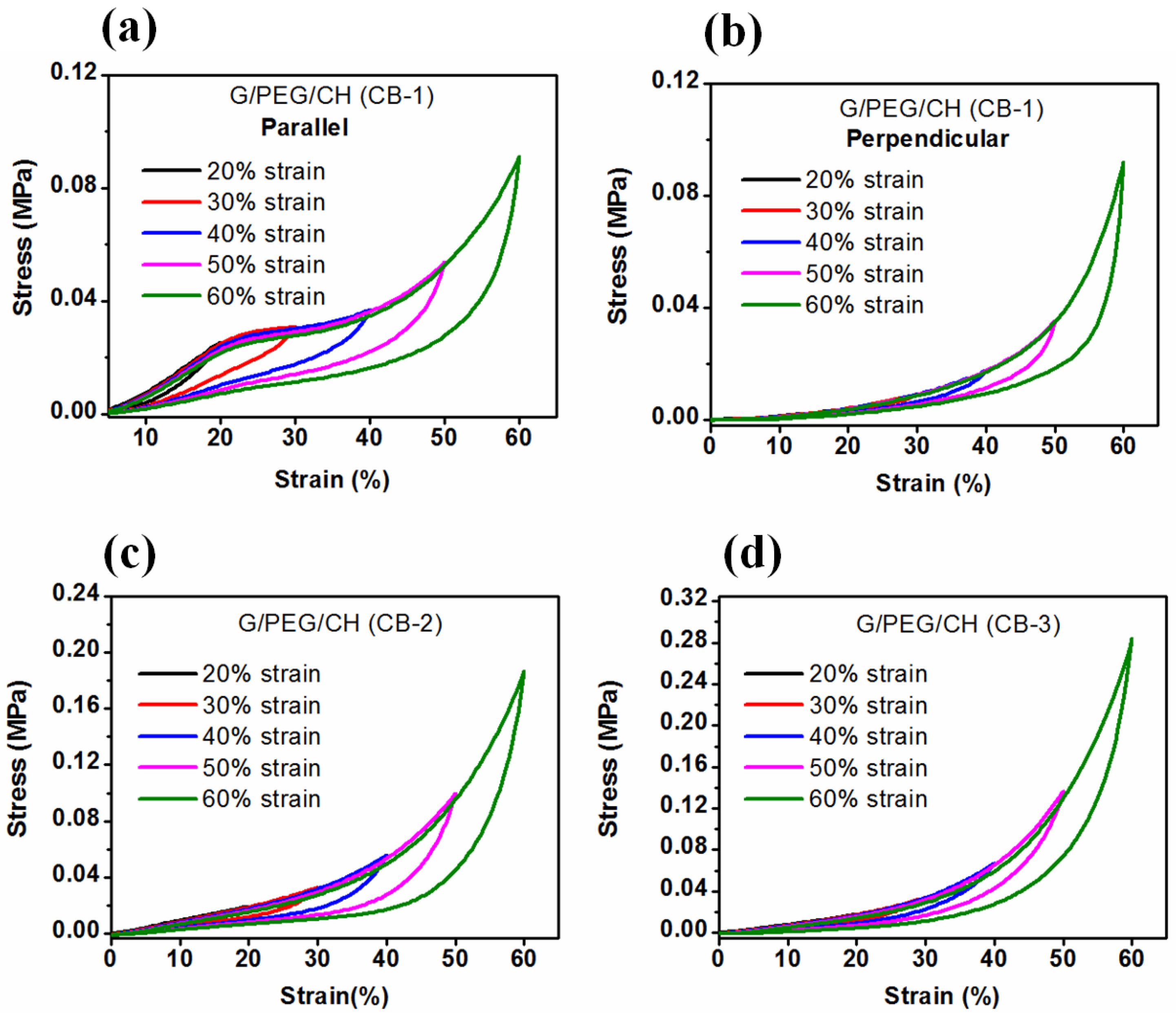
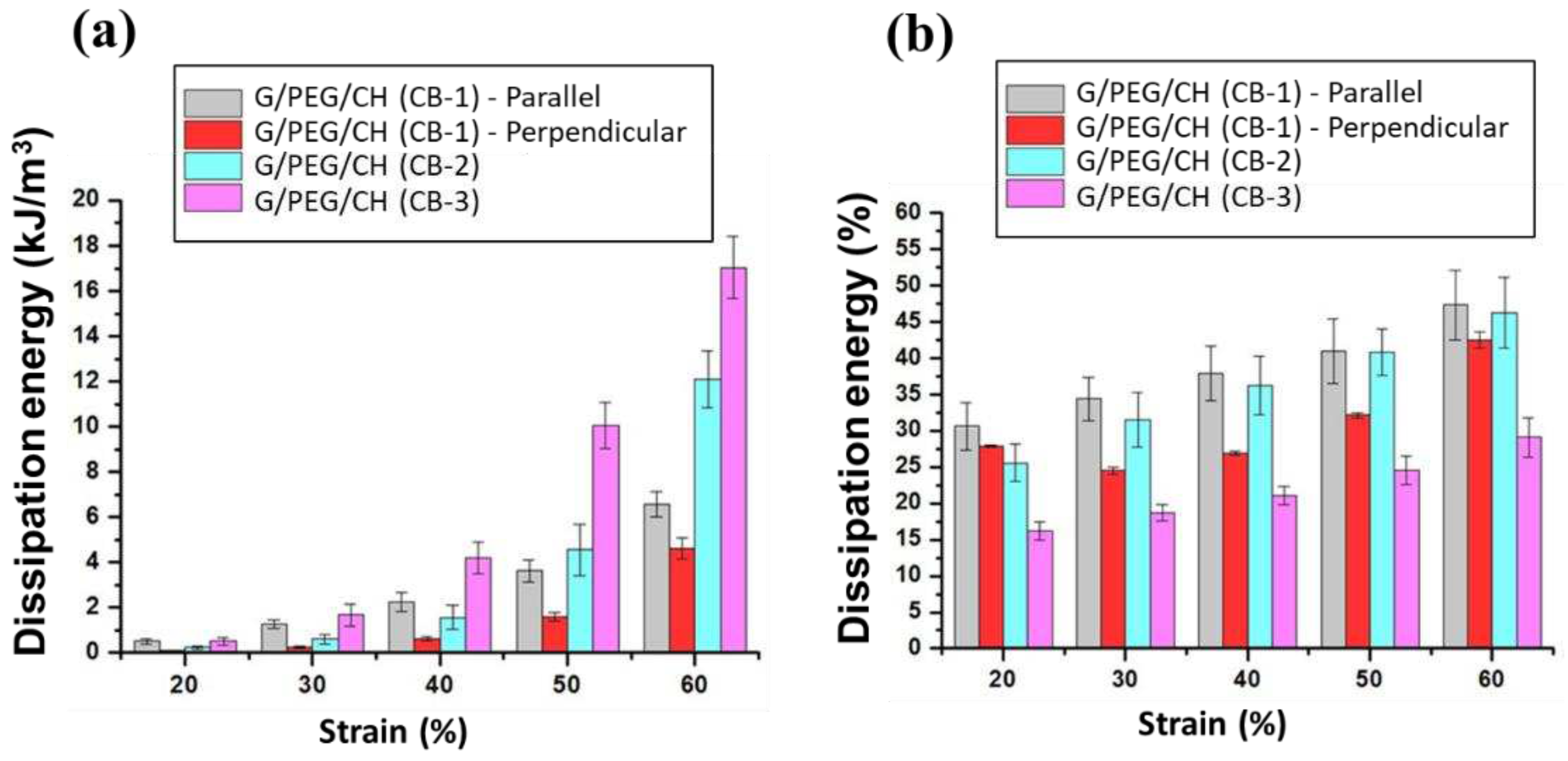
3.5. Compressive mechanical properties
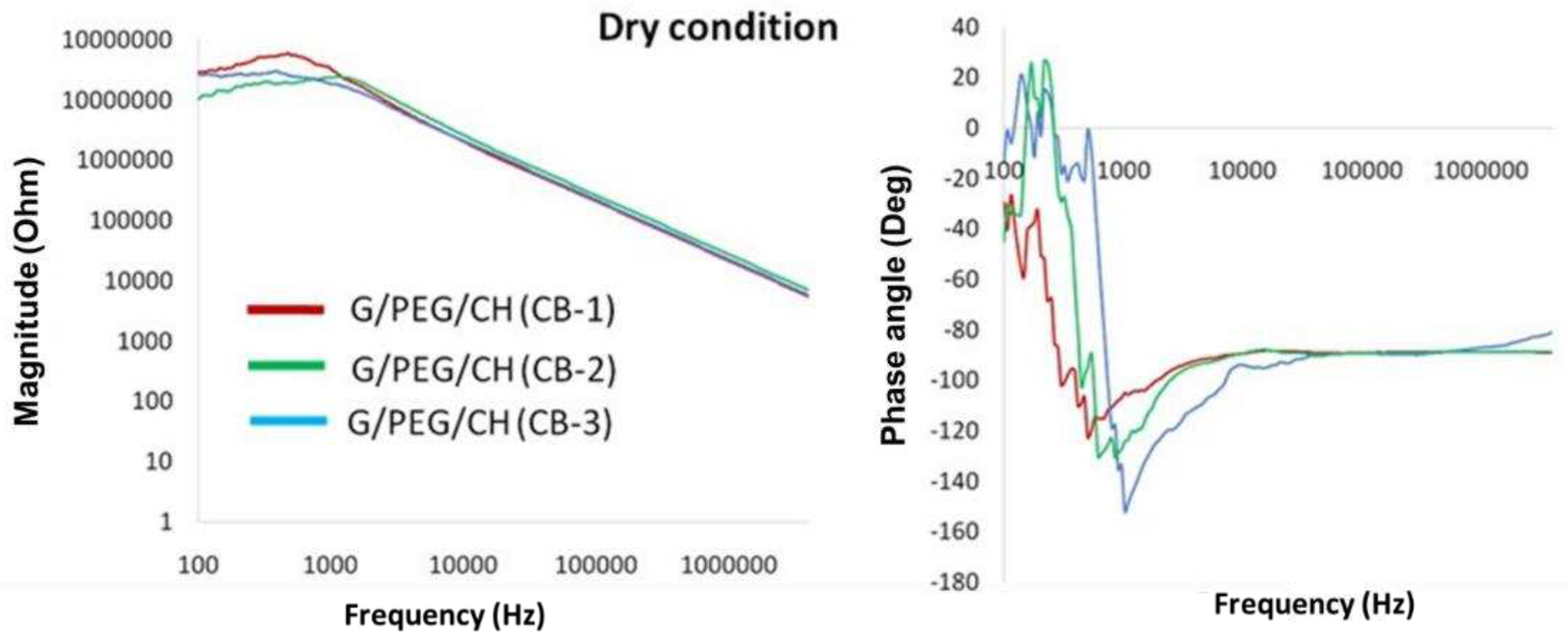
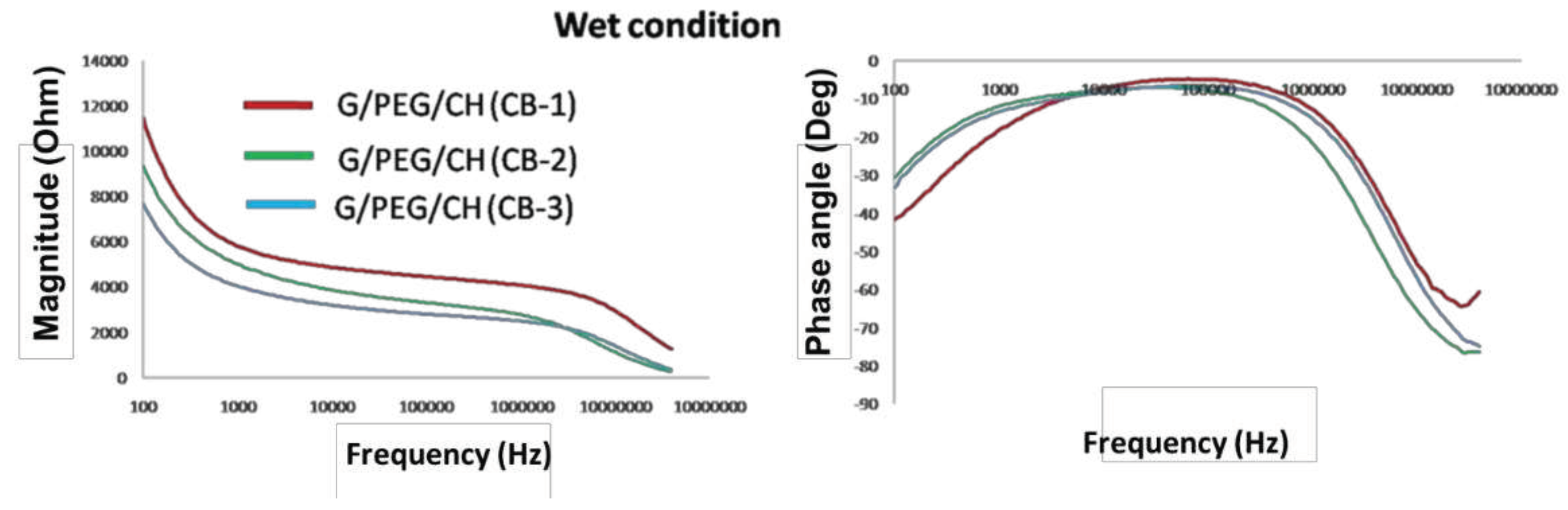
3.6. Electrical impedance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dey, K. , Roca, E., Ramorino, G., Sartore, L. Progress in the mechanical modulation of cell functions in tissue engineering. Biomater. Sci. 2020, 8, 7033–7081. [Google Scholar] [CrossRef]
- Webber, M. J. , Khan, O. F., Sydlik, S. A., Tang, B. C., Langer, R. A perspective on the clinical translation of scaffolds for tissue engineering. Ann. Biomed. Eng. 2015, 43, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M. P. , Gilbert, P. M., Blau, H. M. Designing materials to direct stem-cell fate. Nature, 2009, 462, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M. , John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Alamdari, S. G. , Alibakhshi, A., de la Guardia, M., Baradaran, B., Mohammadzadeh, R., Amini, M., Kesharwani, P., Mokhtarzadeh, A., Oroojalian, F., Sahebkar, A. (2022). Conductive and semiconductive nanocomposite-based hydrogels for cardiac tissue engineering. Adv. Healthc. Mater. 2022, 11, 2200526. [Google Scholar] [CrossRef] [PubMed]
- Guo, B. , Ma, P. X. Conducting polymers for tissue engineering. Biomacromolecules. 2018, 19, 1764–1782. [Google Scholar] [CrossRef] [PubMed]
- Afjeh-Dana, E. , Naserzadeh, P., Nazari, H., Mottaghitalab, F., Shabani, R., Aminii, N., Mehravi, B., Rostami, F.T., Joghataei, M.T., Mousavizadeh, K. Ashtari, K., 2019. Gold nanorods reinforced silk fibroin nanocomposite for peripheral nerve tissue engineering applications. Int. J. Biol. Macromol. 2019, 129, 1034–1039. [Google Scholar] [PubMed]
- Ashtari, K. , Nazari, H., Ko, H., Tebon, P., Akhshik, M., Akbari, M., Alhosseini, S.N., Mozafari, M., Mehravi, B., Soleimani, M. Ardehali, R. Electrically conductive nanomaterials for cardiac tissue engineering. Adv. Drug Deliv. Rev. 2019, 144, 162–179. [Google Scholar] [CrossRef]
- Esmaeili, H. , Patino-Guerrero, A., Hasany, M., Ansari, M.O., Memic, A., Dolatshahi-Pirouz, A. Nikkhah, M. Electroconductive biomaterials for cardiac tissue engineering. Acta Biomater. 2022, 139, 118–140. [Google Scholar] [CrossRef]
- Chopra, V. , Thomas, J., Kaushik, S., Rajput, S., Guha, R., Mondal, B., Naskar, S., Mandal, D., Chauhan, G., Chattopadhyay, N. Ghosh, D. 2023. Injectable bone cement reinforced with gold nanodots decorated rGO-hydroxyapatite nanocomposites, augment bone regeneration. Small 2023, 19, 2204637. [Google Scholar] [CrossRef]
- Arambula-Maldonado, R. Mequanint, K. 2022. Carbon-based electrically conductive materials for bone repair and regeneration. Mater. Adv. 2022, 3, 5186–5206. [Google Scholar] [CrossRef]
- Asl, M.A. , Karbasi, S., Beigi-Boroujeni, S., Benisi, S.Z. and Saeed, M. Polyhydroxybutyrate-starch/carbon nanotube electrospun nanocomposite: A highly potential scaffold for bone tissue engineering applications. Int. J. Biol. Macromol. 2022, 223, 524–542. [Google Scholar] [CrossRef] [PubMed]
- Mirmusavi, M.H. , Ahmadian, M. Karbasi, S. 2022. Polycaprolactone-chitosan/multi-walled carbon nanotube: A highly strengthened electrospun nanocomposite scaffold for cartilage tissue engineering. Int. J. Biol. Macromol. 2022, 209, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Huang, J. , Liu, F., Su, H., Xiong, J., Yang, L., Xia, J. Liang, Y. Advanced nanocomposite hydrogels for cartilage tissue engineering. Gels, 2022, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J. , Lee, G.S. Chun, H. Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci. Rep. 2016, 6, 39302. [Google Scholar] [CrossRef] [PubMed]
- Shokrani, H. , Shokrani, A., Jouyandeh, M., Seidi, F., Gholami, F., Kar, S., Munir, M.T., Kowalkowska-Zedler, D., Zarrintaj, P., Rabiee, N. Saeb, M.R. Green polymer nanocomposites for skin tissue engineering. ACS App. Bio Mater. 2022, 5, 2107–2121. [Google Scholar] [CrossRef]
- Elshishiny, F. Mamdouh, W. Fabrication of nanofibrous/xerogel layer-by-layer biocomposite scaffolds for skin tissue regeneration: in vitro study. ACS Omega, 2020, 5, 2133–2147. [Google Scholar] [CrossRef]
- Lim, C. , Park, C., Sunwoo, S.H., Kim, Y.G., Lee, S., Han, S.I., Kim, D., Kim, J.H., Kim, D.H. Hyeon, T. Facile and scalable synthesis of whiskered gold nanosheets for stretchable, conductive, and biocompatible nanocomposites. ACS Nano, 2022, 16, 10431–10442. [Google Scholar] [CrossRef]
- Zare, E.N. , Makvandi, P., Ashtari, B., Rossi, F., Motahari, A. Perale, G. Progress in conductive polyaniline-based nanocomposites for biomedical applications: a review. J. Med. Chem. 2019, 63, 1–22. [Google Scholar] [CrossRef]
- You, J.O. , Rafat, M., Ye, G.J. Auguste, D.T. Nanoengineering the heart: conductive scaffolds enhance connexin 43 expression. Nano Letters, 2011, 11, 3643–3648. [Google Scholar] [CrossRef]
- Zhao, H. , Liu, M., Zhang, Y., Yin, J. Pei, R. Nanocomposite hydrogels for tissue engineering applications. Nanoscale, 2020, 12, 14976–14995. [Google Scholar] [CrossRef]
- Mehrali, M. , Thakur, A., Pennisi, C.P., Talebian, S., Arpanaei, A., Nikkhah, M. Dolatshahi-Pirouz, A. Nanoreinforced hydrogels for tissue engineering: biomaterials that are compatible with load-bearing and electroactive tissues. Adv. Mater. 2017, 29, 1603612. [Google Scholar] [CrossRef]
- Sakr, M.A. , Sakthivel, K., Hossain, T., Shin, S.R., Siddiqua, S., Kim, J. Kim, K. 2022. Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering. J. Biomed. Mater. Res. A. 2022, 110, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. , Tang, J., Mou, Y., Zhou, J., Qu, L., Duval, K., Huang, Z., Lin, N., Dai, R., Liang, C. Chen, Z. Carbon nanotube-composite hydrogels promote intercalated disc assembly in engineered cardiac tissues through β1-integrin mediated FAK and RhoA pathway. Acta Biomater. 2017, 48, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R. , Zihlmann, C., Akbari, M., Assawes, P., Cheung, L., Zhang, K., Manoharan, V., Zhang, Y.S., Yüksekkaya, M., Wan, K.T. Nikkhah, M. Reduced graphene oxide-gelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small, 2016, 12, 3677–3689. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. , Wei, L., Lan, L., Gao, Y., Zhang, Q., Dawit, H., Mao, J., Guo, L., Shen, L. Wang, L. Conductive biomaterials for cardiac repair: A review. Acta Biomater. 2022, 139, 157–178. [Google Scholar] [CrossRef]
- Moschou, E.A. , Peteu, S.F., Bachas, L.G., Madou, M.J. Daunert, S. Artificial muscle material with fast electroactuation under neutral pH conditions. Chem. Mater. 2004, 16, 2499–2502. [Google Scholar] [CrossRef]
- Chuang, W.J. , Chiu, W.Y. Tai, H.J. Temperature-dependent conductive composites: Poly (N-isopropylacrylamide-co-N-methylol acrylamide) and carbon black composite films. J. Mater. Chem. 2012, 22, 20311–20318. [Google Scholar] [CrossRef]
- Dey, K. , Agnelli, S., Re, F., Russo, D., Lisignoli, G., Manferdini, C., Bernardi, S., Gabusi, E. Sartore, L. Rational design and development of anisotropic and mechanically strong gelatin-based stress relaxing hydrogels for osteogenic/chondrogenic differentiation. Macromol. Biosci. 2019, 19, 1900099. [Google Scholar] [CrossRef]
- Dey, K. , Agnelli, S. Sartore, L. 2023, April. Designing viscoelastic gelatin-PEG macroporous hybrid hydrogel with anisotropic morphology and mechanical properties for tissue engineering application. Micro 2023, 3, 437–457. [Google Scholar] [CrossRef]
- Dey, K. , Agnelli, S., Borsani, E. Sartore, L. Degradation-dependent stress relaxing semi-interpenetrating networks of hydroxyethyl cellulose in gelatin-PEG hydrogel with good mechanical stability and reversibility. Gels, 2021, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Manferdini, C. , Gabusi, E., Sartore, L., Dey, K., Agnelli, S., Almici, C., Bianchetti, A., Zini, N., Russo, D., Re, F. Mariani, E. Chitosan-based scaffold counteracts hypertrophic and fibrotic markers in chondrogenic differentiated mesenchymal stromal cells. J. Tissue Eng. Regen. Med. 2019, 13, 1896–1911. [Google Scholar] [CrossRef] [PubMed]
- Diani, J. , Fayolle, B. Gilormini, P. A review on the Mullins effect. Eur. Polym. J. 2009, 45, 601–612. [Google Scholar] [CrossRef]
- Re, F. , Sartore, L., Moulisova, V., Cantini, M., Almici, C., Bianchetti, A., Chinello, C., Dey, K., Agnelli, S., Manferdini, C. Bernardi, S. 3D gelatin-chitosan hybrid hydrogels combined with human platelet lysate highly support human mesenchymal stem cell proliferation and osteogenic differentiation. J. Tissue Eng. 2019, 10, 2041731419845852. [Google Scholar] [PubMed]
| Composition (w/w %) | Physical properties | ||||||
|---|---|---|---|---|---|---|---|
| Hydrogels | G | PEG | CH | CB* | CBɸ | Apparent density (g/cm3) | Porosity (%) |
| G/PEG/CH | 74 | 18 | 8 | - | - | 0.11 ± 0.03 | 77 ± 2.0 |
| G/PEG/CH (CB 1) | 67 | 16 | 7 | 10 | 6.95 | 0.12 ± 0.03 | 65 ± 10 |
| G/PEG/CH (CB 2) | 63 | 15 | 7 | 15 | 13.6 | 0.13 ± 0.01 | 64 ± 3.0 |
| G/PEG/CH (CB 3) | 60 | 14 | 6 | 20 | 18.2 | 0.13 ± 0.01 | 59 ± 10 |
| Sample name | Modulus (MPa) | Stress (MPa) at 50% strain | Compression direction | Pore morphology |
|---|---|---|---|---|
| G/PEG/CH | 0.247 ± 0.03 | 0.0462 ± 0.01 | Parallel to smaller macropore channels |
Anisotropic |
| G/PEG/CH | 0.042 ± 0.01 | 0.0534 ± 0.01 | Perpendicular to smaller macropore channels | |
| G/PEG/CH (CB-1) | 0.230 ± 0.05 | 0.053 ± 0.01 | Parallel to smaller macropore channels |
Anisotropic |
| G/PEG/CH (CB-1) | 0.063 ± 0.02 | 0.057 ± 0.02 | Perpendicular to smaller macropore channels | |
| G/PEG/CH (CB-2) G/PEG/CH (CB-2) |
0.128 ± 0.01 0.112 ± 0.01 |
0.132 ± 0.03 0.106 ± 0.04 |
Parallel to smaller macropore channels Perpendicular to smaller macropore channels |
Intermediate behaviour |
| G/PEG/CH (CB-3) | 0.173 ± 0.01 | 0.146 ± 0.01 | No observable macropore channels | Isotropic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





