Submitted:
23 August 2023
Posted:
24 August 2023
You are already at the latest version
Abstract
Keywords:
1. Background
2. What is FLASH-RT? Non-Exclusive Hypotheses to Explain FLASH Effect
3. The Beam Description in FLASH-RT to Approach Clinical Trial
3.1. High Frequency Pulsed Beams
3.2. FLASH-electron
3.3. FLASH-x-ray
3.4. FLASH-proton
4. Future of FLASH-RT
5. Conclusions
References
- Bourhis J, Montay-Gruel P, Jorge P G, Bailat C, Petit B, Ollivier J, Jeanneret-Sozzi W, Ozsahin M, Bochud F, Moeckli R, Germond J F, Vozenin M C, 2019. Clinical translation of FLASH radiotherapy: Why and how? Journal of Radiotherapy and oncology. [CrossRef]
- Lin B, Gao F, Yang Y, Wu D, Zhang Y, Feng, G, Dai T, Du, X, 2021. FLASH Radiotherapy: History and Future, Journal of Frontiers in Oncology, 11, 644400. [CrossRef]
- SubielA, MoskvinV, WelshGH, Cipiccia S, Reboredo D, DesRosiers C, Jaroszynski D A, 2017. Challenges of dosimetry of ultra-short pulsed very high energy electron beams. Journal of Physica Medica, . [CrossRef]
- Poirier Y, Mossahebi S, Becker S, Koger B, Xu J, Lamichhane N, Maxim P G, Sawant A, 2021, Radiation shielding and safety implications following linac conversion to an electron FLASH-RT unit. Journal of Medical Physics, 48, 5396-5405. [CrossRef]
- Fielding, A L, 2023. Monte-Carlo techniques for radiotherapy applications II: equipment and source modelling, dose calculations and radiobiology, Journal of Radiotherapy in Practice. 22, 1–6. [CrossRef]
- Zhang G, Zhang Zh, Gao W, Quan H, 2023. Treatment planning consideration for very high-energy electron FLASH radiotherapy, Journal of Physica Medica, 107, 102539.
- Boscolo D, Scifoni E, Durantea M, Kramer M, Fuss M C,2021. May oxygen depletion explain the FLASH effect? A chemical track structure analysis, Journal of Radiotherapy and Oncology. [CrossRef]
- Rothwell B C, Lowe M, Kirkby N F, Merchant M J, Chadwick A L, Mackay R I, Hendry J H, Kirkby K J, 2021. Oxygen Depletion in Proton Spot Scanning: A Tool for Exploring the Conditions Needed for FLASH, Journal of Radiation, 290–304. [CrossRef]
- Khabaz R, Boodaghi R, Benam M R, Zanganeh V,2018. Estimation of photoneutron dosimetric characteristics in tissues/organs using an improved simple model of linac head, Journal of Applied Radiation and Isotopes, 133, 88-94. [CrossRef]
- Boodaghi Malidarre R, Khabaz R, Benam M R, Zanganeh V, 2020. A Feasibility Study to Reduce the Contamination of Photoneutrons and Photons in Organs/Tissues during Radiotherapy, Iranian Journal of Medical Physics, 17iation therapy, Reports of Practical Oncology and Radiotherapy, 27, 344–351.
- Matuszak N, Maria Suchorska W, Milecki P, Kruszyna-Mochalska M, Misiarz A, Pracz J, Malicki J, 2022. FLASH radiotherapy: an emerging approach in radiation therapy, Reports of Practical Oncology and Radiotherapy, 27, 344–351 DOI: . [CrossRef]
- Hageman E, Che P P, Dahele M, Slotman B J, Sminia P, 2022. Radiobiological Aspects of FLASH Radiotherapy, Journal of Biomolecules, 12, 1376. [CrossRef]
- Esplen N, Egoriti L, Paley B, Planche T, Hoehr C, Gottberg A, Bazalova-Carter M, 2022. Design optimization of an electron-to-photon conversion target for ultra-high dose rate x-ray (FLASH) experiments at TRIUMF, Journal of Physics in Medicine and Biology, 105003.
- Giuliano L, Franciosini G, Palumbo L, Aggar L, Dutreix M, Faillace L, Favaudon V, Felici G, Galante F, Mostacci A, Migliorati M, Pacitti M, Patriarca A, Heinrich S, 2023. Characterization of Ultra-High-Dose Rate Electron Beams with ElectronFlash Linac, Journal of Applied Sciences, 13, 631. [CrossRef]
- Borghini A, Vecoli C, Labate L, Panetta D, Andreassi MG, Gizzi LA, 2022. FLASH ultra-high dose rates in radiotherapy: preclinical and radiobiological evidence. Journal of Radiation Biology, 98,127-135. [CrossRef]
- Patriarca A, Fouillade C, Auger M, Martin F, Pouzoulet F, Nauraye C, Heinrich S, Favaudon V, Meyroneinc S, Dendale R, Mazal A, Poortmans P, Verrelle P, De Marzi L, 2018. Experimental set-up for FLASH proton irradiation of small animals using a clinical system, International Journal Radiation Oncology Biology, Physics, 102, 619-626.
- Bohlen T T, Germond J F, Bourhis J, Vozenin M C, Ozsahin E M, Bochud F, Bailat C, Raphael Moeckli R, 2022. Normal Tissue Sparing by FLASH as a Function of Single-Fraction Dose: A Quantitative Analysis, Journal of Radiation Oncology, Biology, Physics, 114, 1032-104.
- Gao F, Yang Y, Zhu H, Wang J, Xiao D, Zhou Z, Dai T, Zhang Y, Feng G, Li J, Lin B, Xie G, Ke Q, Zhou K, Li P, Shen X, Wang H, Yan L, Lao C, Shan L, Li M, Lu Y, Chen M, Feng S, Zhao J, Wu D, Du X. 2022. First demonstration of the FLASH effect with ultrahigh dose rate high-energy X-rays. Journal of Radiotherapy and Oncology, 166, 44-50. [CrossRef]
- Subiel A, Moskvin V, Welsh G H, Cipiccia S, Reboredo D, Evans P, Partridge M, DesRosiers C, Anania M P, Cianchi A, Mostacci A, Chiadroni E, Di Giovenale D, Villa F, Pompili R, Ferrario M, Belleveglia M, Di Pirro G, Gatti G, Vaccarezza C, Seitz B, C Isaac R, Brunetti E, Wiggins S M, Ersfeld B, Islam M R, Mendonca M S, Sorensen A, Boyd M, Jaroszynsk D A, 2014. Dosimetry of very high energy electrons (VHEE) for radiotherapy applications: using radiochromic film measurements and Monte Carlo simulations, Journal of Physics in Medicine and Biology, 59, 5811.
- Schulte R, Johnstone C, Boucher S, Esarey E, Geddes C G R, Kravchenko M, Kutsaev S, Loo Jr , Méot F, Mustapha B, Nakamura K, Nanni E A, Obst-Huebl L, Sampayan S E, Schroeder C B, Sheng K, Snijders A M, Snively E, Tantawi S G, Tilborg J V, 2022. Transformative Technology for FLASH Radiation Therapy: Journal of Applied Sciences,12, x. [CrossRef]
- Hornsey S, Alper T. 1966, Unexpected dose-rate effect in the killing of mice by radiation, Nature, 210:212–3. [CrossRef]
- Field SB, Bewley DK. 1974, Effects of dose-rate on the radiation response of rat skin. International journal of radiation biology and related studies in physics, chemistry, and medicine, 26, 259–67. [CrossRef]
- Hendry JH, Moore JV, Hodgson BW, Keene JP. 1982, The constant low oxygen concentration in all the target cells for mouse tail radionecrosis. Journal of Radiation Research, 92,172–81.
- Favaudon V,Caplier L, Monceau V, Oulet F P, Sayarath M,Fouillade C, Poupon M F, Brito I, Hupe P, Bourhis J, Hall J, Fontaine J J, Vozenin M C, 2014. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice, Journal Science Translational Medicine, 6, 245.
- Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, Doenlen R, Favaudon V, Bochud F, Bailat C, Bourhis J, Vozenin MC. 2017. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Journal of Radiotherapy and Oncology 124:365–9. [CrossRef]
- Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, Petit B, Burki M, Ferrand G, Patin D, Bouchaab H, Ozsahin M, Bochud F, Bailat C, Devauchelle P, Bourhis J, 2018. The advantage of Flash radiotherapy confirmed in mini-pig and catcancer patients. Journal of Clinical Cancer Research. . CCR-17-3375. In press: clincanres.3375.2017. [CrossRef]
- Montay-Gruel P, Bouchet A, Jaccard M, Patin D, Serduc R, Aim W, Petersson K, Petit B, Bailat C, Bourhis J, Bräuer-Krisch E, Vozenin MC. 2018. X-rays can trigger the FLASH effect: ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Journal of Radiotherapy and Oncology, 129:582–8. [CrossRef]
- Bourhis J, Sozzi W J , Jorge P G , Gaide O , Bailat C, Duclos F, Patin D, Ozsahin M, Bochud F, Germond J F , Moeckli R , Vozenin M C, 2019. Treatment of a first patient with FLASH-radiotherapy, Journal of Radiotherapy and Oncology, 139:18-22. [CrossRef]
- Loo B W, Schuler E, Lartey F M, Rafat M, King G J, Trovati S, Koong A C, and Maxim P, (P003) 2017. delivery of ultra-rapid flash radiation therapy and demonstration of normal tissue sparing after abdominal irradiation of mice. International Journal of Raditherapy and Oncology, 98, E16. [CrossRef]
- Jaccard M, Durán M T, Petersson K, Germond J F, Liger P, Vozenin M C, Bourhis J, Bochud F and Bailat C, 2018 High dose-per-pulse electron beam dosimetry: Commissioning of the Oriatron eRT6 prototype linear accelerator for preclinical use, Journal of Medical Physics 45 863–7.
- Lempart M, Blad B, Adrian G, Back S, Knoos T, Ceberg C and Petersson K, 2019. Modifying a clinical linear accelerator for delivery of ultrahigh dose rate irradiation, Journal of Radiotherapy and Oncology, 139 40–.
- Montay-Gruel P, Corde S, Laissue J A, Bazalova-Carter M, 2022. FLASH radiotherapy with photon beams, Journal of Medical Physics, 49, 2055-2067. [CrossRef]
- Bazalova-Carter M, Esplen N, 2019. On the capabilities of conventional x-ray tubes to deliver ultra-high (FLASH) dose rates, Journal of Medical Physics, 46, 5690–5.
- Beyreuther E, Brand M, Hans S, Hideghety K, Karsch L, Leßmann E, Schurer M, Szabo E R, Pawelke J, 2019. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation Journal of Radiotherapy and Oncology, 139 46–50.
- Buonanno M, Grilj V and Brenner D J, 2019. Biological effects in normal cells exposed to FLASH dose rate protons, Journal of Radiotherapy and Oncology, 139 51–5.
- Darafsheh A, Hao Y, Zwart T, Wagner M, Catanzano D, Williamson J F, Knutson N, Sun B, Mutic S and Zhao T, 2020. Feasibility of proton FLASH irradiation using a synchrocyclotron for preclinical studies, Journal of Medical Physics, 47 4348–55.
- Kim MM, Verginadis II, Goia D, Haertter A, Shoniyozov K, Zou W, Maity A, Busch TM, Metz JM, Cengel KA, Dong L, Koumenis C, Diffenderfer ES. 2021. Comparison of FLASH Proton Entrance and the Spread-Out Bragg Peak Dose Regions in the Sparing of Mouse Intestinal Crypts and in a Pancreatic Tumor Model. Journal of Cancers 23, 4244. [CrossRef]
- Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, Petit B, Burki M, Ferrand G, Patin D, Bouchaab H, Ozsahin M, Bochud F, Bailat C, Devauchelle P, Bourhis J. 2019. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Journal of Clinical Cancer Research, 25,35-42. [CrossRef]
- Jorge PG, Jaccard M, Petersson K, Gondre M, Durán MT, Desorgher L, Germond JF, Liger P, Vozenin MC, Bourhis J, Bochud F, Moeckli R, Bailat C. 2019, Dosimetric and preparation procedures for irradiating biological models with pulsed electron beam at ultra-high dose-rate. Journal of Radiotherapy and Oncology, 139,34-39. [CrossRef]
- Schuler E, Trovati S, King G, Lartey F, Rafat M, Villegas M, Praxel A J, Billy W, Loo Jr, Maxim P G, 2017. Experimental platform for ultra-high dose rate flash irradiation of small animals using a clinical linear accelerator. Journal of Radiation Oncology and Biology Physics. 97, 195–203. [CrossRef]
- Levin D S, Ferretti C, Ristow N, Tecchio M, Friedman P S, Litzenberg D W, Bashkirov V, Schulte R, 2023. A Scintillator Beam Monitor for Real-Time FLASH Radiotherapy, arXiv preprint arXiv:2305.15306.
- Gao Y, Liu R, Wei C, Serdar C, Jun Z, Bradley J D, Liu T, Yang X, 2022. A potential revolution in cancer treatment: A topical review of FLASH radiotherapy, Journal Applied Clinical Medical Physics. 23,13790. [CrossRef]
- Vozenin M C, Hendry J H, Limoli C L, 2019. Biological benefits of ultra-high dose rate flash radiotherapy: sleeping beauty awoken, Journal of Clinical Oncology, 31 407–15.
- Yinghao LV, Yue LV, Wang Z, Lan T, Feng X, Chen H, Zhu J, Ma X, Du J, Hou G, Liao W,Yuan K, Wu H, 2022. FLASH radiotherapy: A promising new method for radiotherapy (Review), Journal of Oncology Letters, 24, 419. [CrossRef]
- Rezaee M, Iordachita I, Wong J W W, 2021. Ultra-high dose-rate (FLASH) x-ray irradiator for pre-clinical laboratory research, Journal of Physics in Medicine and Biology, 23, 66. [CrossRef]
- Ashraf M R, Rahman M, Zhang R, Williams B B, Gladstone D J, Pogue B W, Bruza P, 2020. Dosimetry for FLASH Radiotherapy: A Review of Tools and the Role of Radioluminescence and Cherenkov Emission, Frontiers in Physics, 8. [CrossRef]
- Zhu H, Xie D, Yang Y, Huang S, Gao X, Peng Y, Wang B, Wang J, Xiao D, Wu D, Li C, Qian C N, Deng X, 2022. Radioprotective effect of X-ray abdominal FLASH irradiation: Adaptation to oxidative damage and inflammatory response may be benefiting factors, Journal of Medical Physics, 49, 4812–4822.
- Zhang Q, Cascio E, Li C, Yang Q, Gerweck L E, Huang P, Gottschalk B, Flanz J, Schuemann J, 2020. FLASH investigations using protons: design of delivery system, preclinical setup and confirmation of FLASH effect with protons in animal systems. Journal of Radiation Research, 194, 656-664.
- Diffenderfer ES, Verginadis II, Kim MM, Shoniyozov K, Velalopoulou A, Goia D, Putt M, Hagan S, Avery S, Teo K, Zou W, Lin A, Swisher-McClure S, Koch C, Kennedy AR, Minn A, Maity A, Busch TM, Dong L, Koumenis C, Metz J, Cengel KA. 2020. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System. Journal of Radiation Oncology, Biology, Physics .106, 440-448. [CrossRef]
- Levy K, Natarajan S, Wang J, Chow S, T. Eggold J , E. Loo P, Manjappa R, Melemenidis S, M. Lartey F, Schuler E, Skinner L, Rafat M, Ko R, Kim A, H. Al-Rawi D, Eyben R V, Dorigo O, M. Casey K, E. Graves E, Bush K, S. Yu A, C. Koong A, G. Maxim P, W. Loo Jr. B, B. Rankin E, 2020. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Journal of Scientific Report, 10, 21600.
- Bley C R , Wolf F , Goncalves Jorge P, Grilj V , Petridis I , Petit B , Bohlen T T , Moeckli R , Limoli C , Bourhis J , Meier V, Vozenin M C , 2022. Dose- and Volume-Limiting Late Toxicity of FLASH Radiotherapy in Cats with Squamous Cell Carcinoma of the Nasal Planum and in Mini Pigs. Journal of Clinical Research. 28, 3814-3823. [CrossRef]
- Cooper CR, Jones D, Jones G DD, Petersson K, 2022. FLASH irradiation induces lower levels of DNA damage ex vivo, an effect modulated by oxygen tension, dose, and dose rate, British Journal of Radiology, 95, 20211150.
- 53. Gao Y, Liu R, Chang C W, Charyyev S, Zhou J, Bradley J D, Liu T, Yang X, 2022, A potential revolution in cancer treatment: A topical review of FLASH radiotherapy, Journal of Medical Physics, 23,13790. [CrossRef]
- Lin B, Huang D, Gao F, Yang Y, Wu D, Zhang Y, Feng G, Dai T and Du X, 2022. Mechanisms of FLASH effect. Journal of Frontiers in Oncology, 12, 995612. [CrossRef]
- Pratx G, SKapp D, 2019. A computational model of radiolytic oxygen depletion during FLASH irradiation and its effect on the oxygen enhancement ratio, Journal of Physics in Medicine and Biology, 64, 185005.
- Adrian G, Konradsson E, Lempart M, Back S, Ceberg C, and Petersson K. 2020, The FLASH effect depends on oxygen concentration. British Journal of Radiology, 93, 20190702.
- Khan S, Bassenne M, Wang J, Manjappa R, Melemenidis S, Breitkreutz D Y, Maxim P G, Xing L, Loo B W, Pratx G, 2021. Multicellular Spheroids as In Vitro Models of Oxygen Depletion during FLASH Irradiation. Journal of Radiation Oncology, Biology, Physics, 110, 833–844.
- Arai H, Elliott A, Xiu J, Wang J, Battaglin F, Kawanishi N, Soni S, Zhang W, Millstein J, Sohal D, Goldberg R M, Hall M J, Scott A J, Khushman M, Hwang J J, Lou E , Weinberg B A , Marshall J L , Lockhart A C, Stafford P, Zhang J, Roberto Moretto R, Chiara Cremolini C, Michael Korn W, Lenz H J, 2021. The Landscape of Alterations in DNA Damage Response Pathways in Colorectal Cancer. Journal of Clinical Cancer Research, 27, 3234–3242.
- Buonanno M, Grilj V, Brenner D J, 2019. Biological Effects in Normal Cells Exposed to FLASH Dose Rate Protons. Journal of Radiotherapy and Oncology,139, 51–55.
- Fouillade C, Curras-Alonso S, Giuranno L, Quelennec E, Heinrich S, Bonnet-Boissinot S, Beddok A, Leboucher S, Karakurt HU, Bohec M, Baulande S, Vooijs M, Verrelle P, Dutreix M, Londono-Vallejo A, Favaudon V. 2020. FLASH Irradiation Spares Lung Progenitor Cells and Limits the Incidence of Radio-Induced Senescence. Journal of Clinical Cancer Research 26, 1497–1506.
- Spitz DR, Buettner GR, Limoli CL, 2019. Response to Letter Regarding “An Integrated Physico-Chemical Approach for Explaining the Differential Impact of FLASH versus Conventional Dose Rate Irradiation on Cancer and Normal Tissue Responses”. Journal of Radiotherapy and Oncology,139, 64–65.
- Zhou G, 2020. Mechanisms Underlying FLASH Radiotherapy, a Novel Way to Enlarge the Differential Responses to Ionizing Radiation between Normal and Tumor Tissues. Radiation Medicine and Protection, 1, 35-40.
- Li T, Chen Z J, 2018. The cGAS–cGAMP–STING pathway connects DNA damage to inflammation, senescence, and cancer, Journal of Experimental Medicine, 215, 1287–1299.
- Golan T, Hammel P, Reni M, Cutsem E V, Macarulla T, Hall M J, Park J O, Hochhauser D, Arnold D, Oh D Y, Reinacher-Schick A, Tortora G, Algul H, O’Reilly E M, McGuinness D, Cui K Y, Schlienger K, Y. Locker G, L. Kindler H, 2019. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. The New England Journal of Medicine, 381, 317–327.
- Ray-Coquard I, Leary A, Pignata S, Cropet C, González-Martin A, Marth C, Nagao S, Vergote I, Colombo N, Maenpaa J, Selle F, Sehouli J, Lorusso D, Guerra Alia EM, Bogner G, Yoshida H, Lefeuvre-Plesse C, Buderath P, Mosconi AM, Lortholary A, Burges A, Medioni J, El-Balat A, Rodrigues M, Park-Simon TW, Dubot C, Denschlag D, You B, Pujade-Lauraine E, Harter P; PAOLA-1/ENGOT-ov25 investigators. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. The New England Journal of Medicine, 381, 2416–2428.
- Wu S, Zhang Q, Zhang F, Meng F, Liu S, Zhou R, Wu Q, Li X, Shen L, Huang J, Qin J, Ouyang S, Xia Z, Song H, Feng X H, Zou J, Xu P, 2019. HER2 Recruits AKT1 to Disrupt STING Signalling and Suppress Antiviral Defence and Antitumour Immunity. Journal of Nature Cell Biology 21, 1027–104.
- Catalano F, Borea R, Puglisi S, Boutros A, Gandini A, Cremante M, Martelli V, Sciallero S, Puccini A, 2022. Targeting the DNA Damage Response Pathway as a Novel Therapeutic Strategy in Colorectal, Journal of Cancer, 14, 1388. [CrossRef]
- Mauri G, Arena S, Siena S , Bardelli A, Sartore-Bianchi A, 2020. The DNA damage response pathway as a land of therapeutic opportunities for colorectal cancer, Annals of Oncology, 31, 1135-1147.
- Lozano R, Castro E, Aragon I M, Cendon Y, Cattrini C, Lopez-Casas P P, Olmos D, 2021. Genetic aberrations in DNA repair pathways: a cornerstone of precision oncology in prostate cancer, British Journal of Cancer, 124, 552–563; [CrossRef]
- Michael S. Petronek, Douglas R. Spitz, Garry R. Buettner and Bryan G. Allen, 2019. Linking Cancer Metabolic Dysfunction and Genetic Instability through the Lens of Iron Metabolism, Journal of Cancers, 11, 1077. [CrossRef]
- Hinchliffe P, Sazanov L A, 2005. Organization of Iron-Sulfur Clusters in Respiratory Complex I. Journal of Science, 309, 771–774.
- Kruszewski M, 2003. Labile iron pool: the main determinant of cellular response to oxidative stress. Journal of Mutation Research, 53, 81-92. [CrossRef]
- Zhu H, Xie D, Wang Y, Huang R, Chen X, Yang Y, Wang B, Peng Y, Wang J, Xiao D, Wu D, Qian CN, Deng X. 2022. Comparison of intratumor and local immune response between MV X-ray FLASH and conventional radiotherapies. Journal of Clinical and Translation Radiation Oncology, 38, 138-146. [CrossRef]
- Zhang Z, Liu X, Chen D, and Yu J, 2022. Radiotherapy combined with immunotherapy: the dawn of cancer treatment, Signal Transduction and Targeted Therapy, 7, 258.
- Montay-Gruel P, Acharya M M, Gonçalves Jorge P, Petit B, Petridis IG, Fuchs P, Leavitt R, Petersson K, Gondre M, Ollivier J, Moeckli R, Bochud F, Bailat C, Bourhis J, Germond JF, Limoli CL, Vozenin MC, 2021. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma That Re- 1190 duces Neurocognitive Side Effects in Mice. Clinical Cancer Research, 27, 775–784, 119. [CrossRef]
- Sarti A, De Maria P, Battistoni G, De Simoni M, Di Felice C, Dong Y, Fischetti M, Franciosini G, Marafini M, Marampon F, Mattei I, Mirabelli R, Muraro S, Pacilio M, Palumbo L, Rocca L, Rubeca D, Schiavi A, Sciubba A, Tombolini V, Toppi M, Traini G, Trigilio A and Patera V, 2021. Deep Seated Tumour Treatments With Electrons of High Energy Delivered at FLASH Rates: The Example of Prostate Cancer. Journal of Frontiers in Oncology 11, 777852. [CrossRef]
- Hornsey S, Bewley D K, 1971. Hypoxia in mouse intestine induced by electron irradiation at high dose-rates, Journal of Radiation Biology. 19, 479-483.
- 78. Di Martino F, Barca P, Barone S, Bortoli E, Borgheresi R, De Stefano S, Di Francesco M, Faillace L, Giuliano L, Grasso L, Linsalata S, Marfisi D, Migliorati M, Pacitti M, Palumbo L, Felici G, FLASH Radiotherapy With Electrons: Issues Related to the Production, Monitoring, and Dosimetric Characterization of the Beam, Journal of Frontiers in Physics, 8. [CrossRef]
- Kokurewicz K, Brunetti E, Curcio A, Gamba D, Garolfi L, Gilardi A, Senes E, Sjobak K N, Farabolini W, Corsini R, Jaroszynski D A, 2021. An experimental study of focused very high energy electron beams for radiotherapy, Communications Physics, 1-7. [CrossRef]
- 80. Jeong D H, Lee M, Lim H, Kang S K, Lee S J, Kim H C, Lee K, Kim S H, Lee D E, Jang K W, Electron beam scattering device for FLASH preclinical studies with 6- MeV LINAC, Journal of Nuclear Engineering and Technology. [CrossRef]
- Winick H, 1998, Synchrotron radiation sources—present capabilities and future directions. Journal of Synchrotron Radiation, 5, 168-175. [CrossRef]
- Zeman W, Curtis HJ, Baker CP, 1961. Histopathologic effect of high-energy-particle microbeams on the visual cortex of the mouse brain, Journal of Radiation Research, 15, 496–514.
- Eling L, Bouchet A, Nemoz C, Djonov V, Balosso J,Laissue J, Elke Brauer-Krisch E, Adam J F, Serduc R, 2019. Ultra high dose rate Synchrotron Microbeam Radiation Therapy. Preclinical Evidence in View of a Clinical Trabnsfer, Journal of Radiotherapy and Oncology, 139, 56-61.
- Hyer D E, Ding X, Rong, Y, 2021. Proton Therapy Needs Further Technological Development to Fulfill the Promise of Becoming a Superior Treatment Modality (Compared to Photon Therapy), Journal of Applied Clinical Medical Physics, 22, 4-11.
- Burnet N G, Mackay R I, Smith E D, Chadwick A L, Whitefield G A, Thomson D J, Lowe M, Kirbkby N F, Crellin A M, Kirkby K J, 2020. Proton Beam Therapy:Prospectives on the National Health Service England Clinical Service and Research Programme. British Journal of Radiology, 93, 201190873.
- Gaudin C, Lamoureux M, Rouille C, 2001. X-ray emission from a compact hot plasma: applications to radiology and mammography, Journal of Physics in Medicine and Biology, 46 835–851.
- Li D, Yang T, Wu M, Mei Z, Wang K, Lu C, Zhao Y, Ma W, Zhu K, Geng Y, Xiao C, Chen J, Lin C, Tajima T, Yan X, 2023. Introduction of Research Work on Laser Proton Acceleration and Its Application Carried out on Compact Laser–Plasma Accelerator at Peking University. Journal of Photonics 10, 132. [CrossRef]
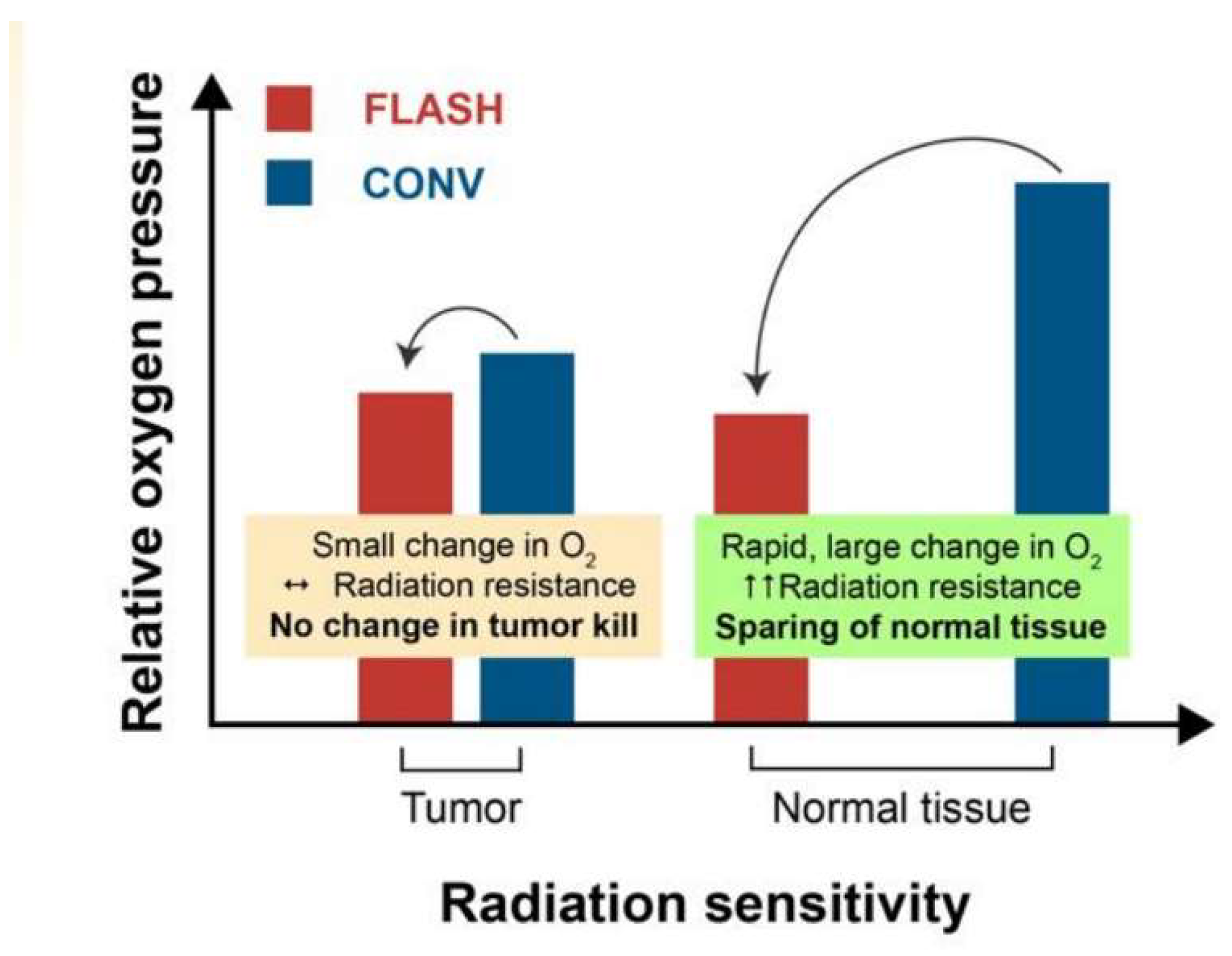
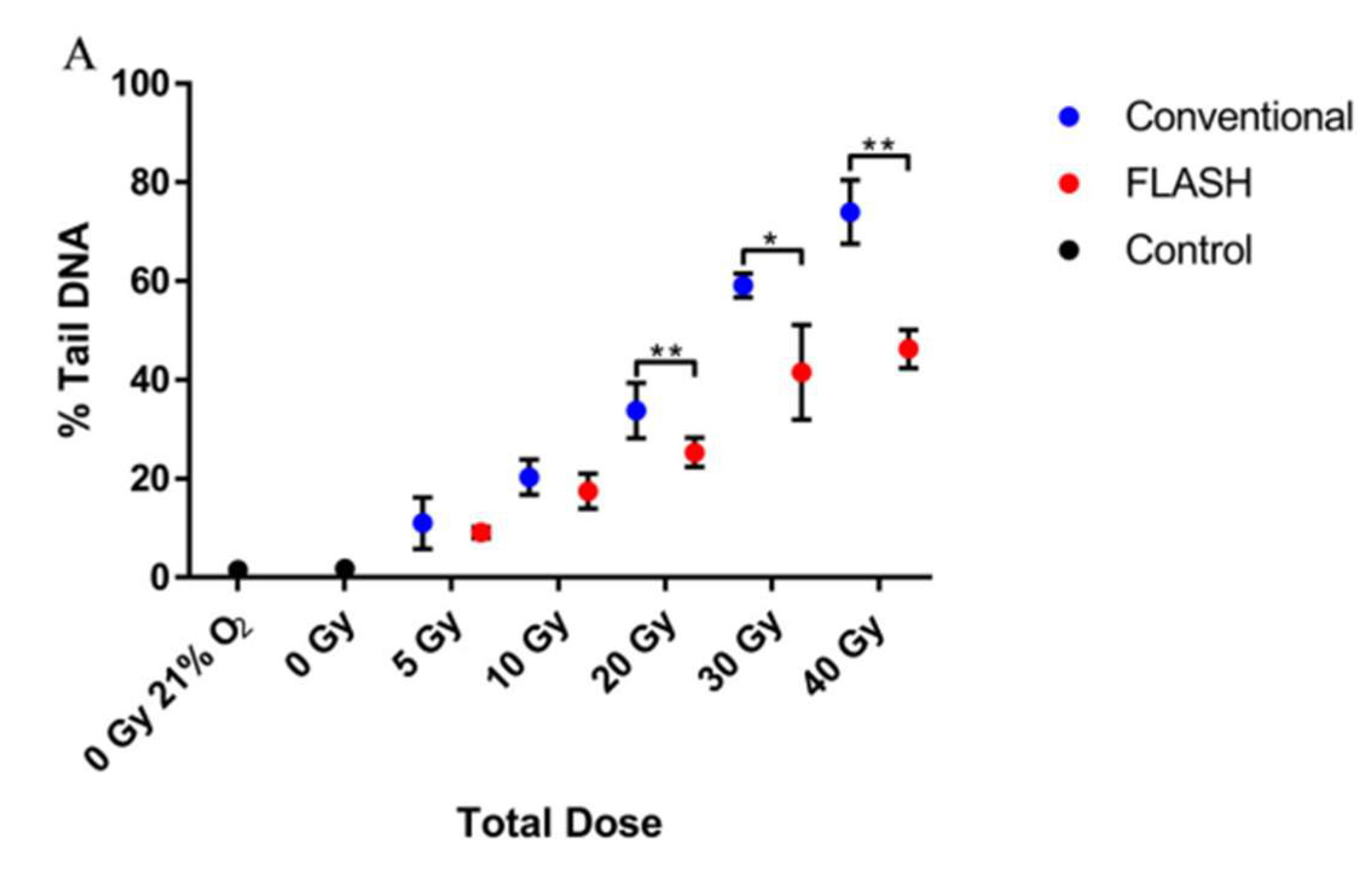
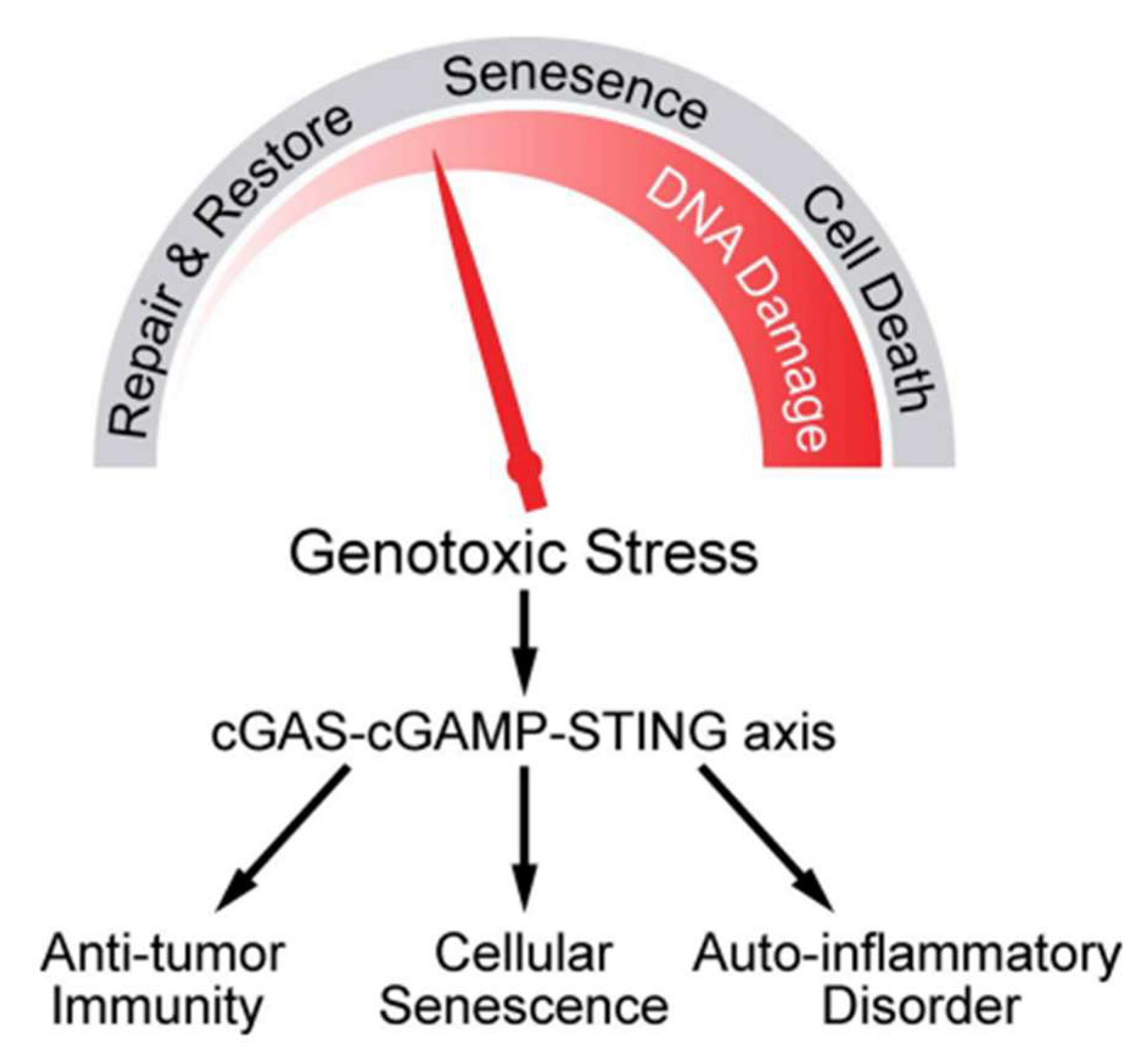

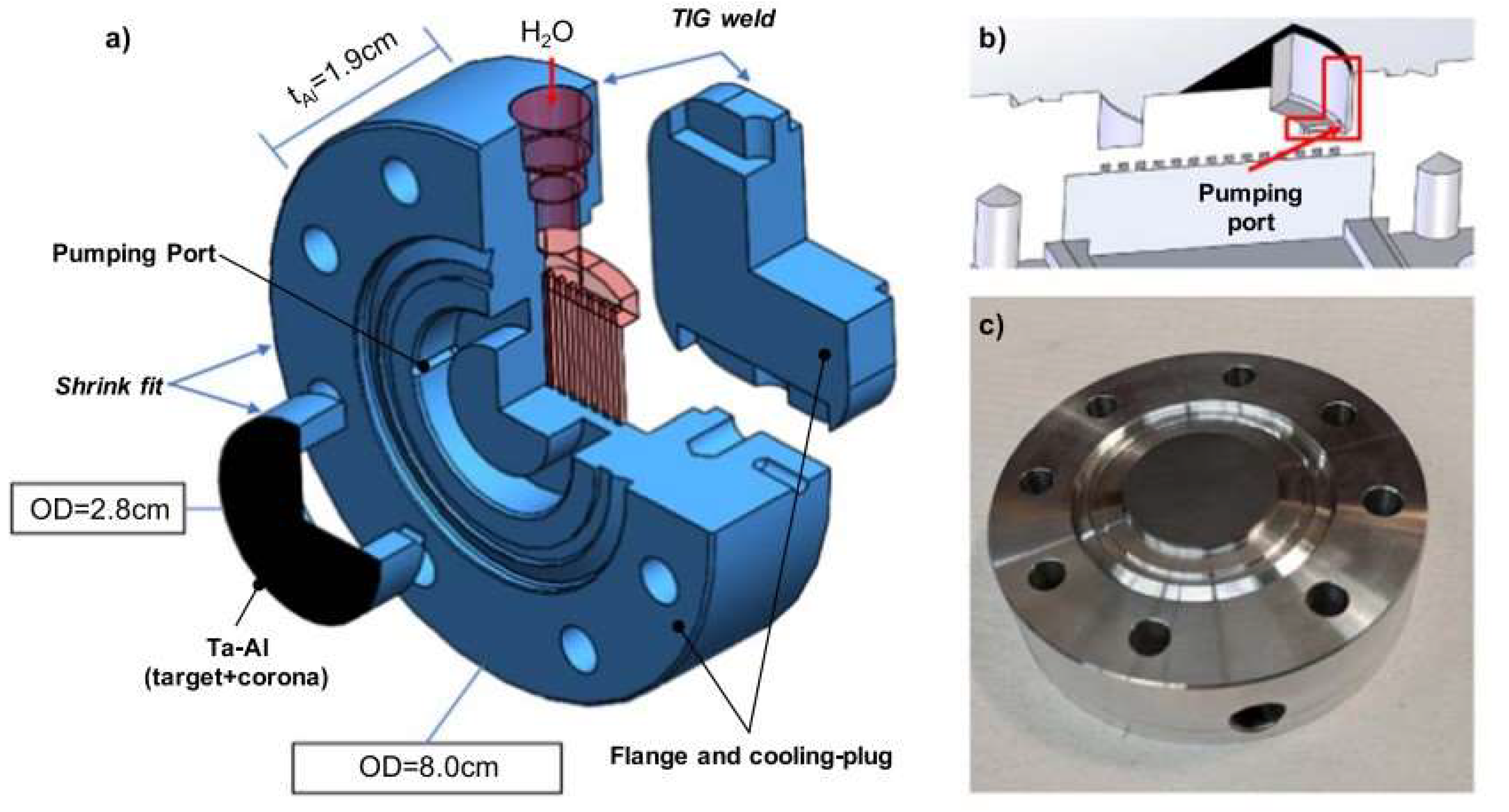
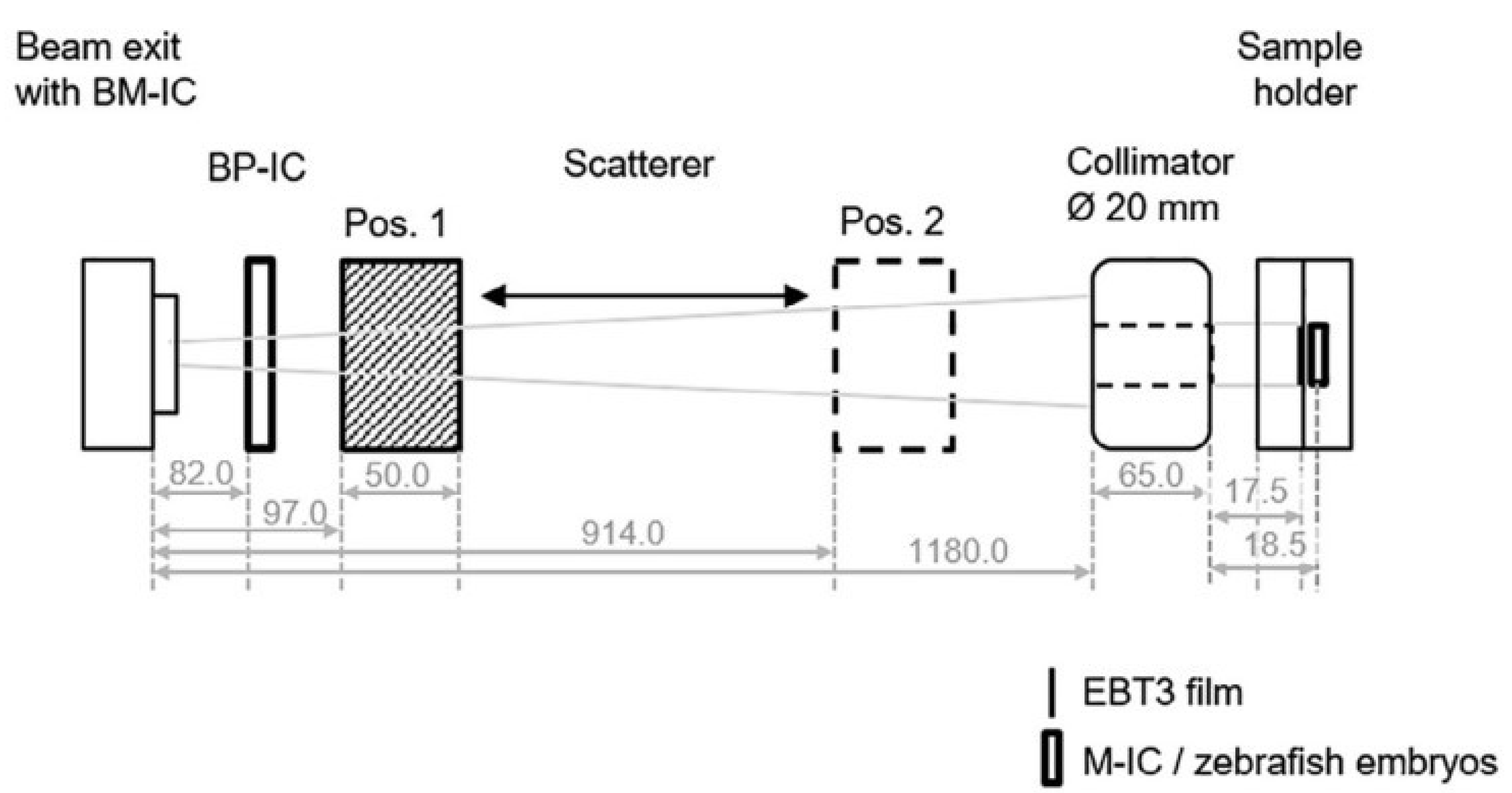
| Beam Characteristics | CONV-RT | FLASH-RT |
| Dose Per Pulse | ||
|
Dose Rate: Single Pulse |
||
|
Mean Dose Rate: Single Fraction |
||
|
Total Treatment Time T |
days. |
< 500 ms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




