1. Introduction
Preclinical studies are critical for the translation of basic scientific research into clinical practice. These studies encompass various experiments, including ex vivo or in vitro simulations and in vivo evaluations of novel therapies and devices in animal models. Ex vivo or in vitro simulations are beneficial because they allow for flexibility in preliminary testing and improvements in scientific research. However, their results may not correlate with those achieved using an in vivo animal model. Before in vivo studies are performed using an animal model, a thorough literature review should be conducted to identify the best animal model for the proposed investigation. One of the most important aspects of this step is to identify inconsistencies in previous research and where continued research is needed. Carefully designing in vivo preclinical studies is essential because an effective and well-designed protocol can prevent delays in the approval of new treatments or the potential waste of resources [
1]. An effective protocol contains detailed descriptions of the animal’s preprocedural care, surgical preparation, anesthesia, surgical procedure, and postoperative care. Here, we outline the key points that preclinical researchers should consider when optimizing the design of an in vivo preclinical study to ensure that the findings will contribute to developing safe and effective treatments.
2. Procedural Technique Development
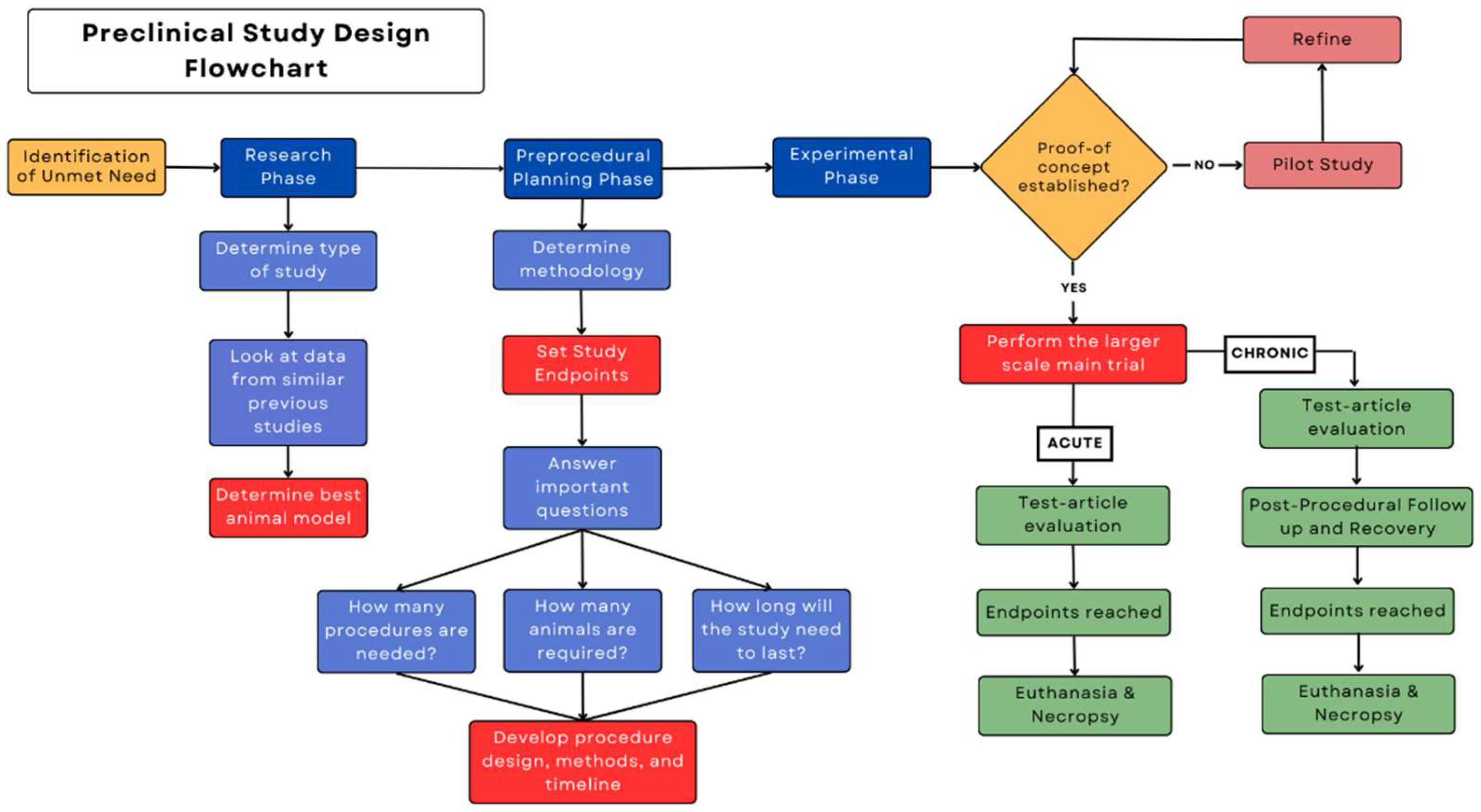
Each preclinical protocol will have a unique set of steps corresponding to the study's goals. However, all preclinical protocols should have a standard outline of the protocol's development to efficiently and effectively help the researcher reach the study’s end goal. When first considering the design of a preclinical study, it is important to focus on the critical procedural aspects of the study. The Figure outlines the general phases of a protocol that help keep the study on track towards meeting the study's goals. Completing a phase prematurely may result in the study veering from its objective, leading to an ineffective protocol and wasted time and resources.
Figure.
A phase-based diagram showing a standard outline of the steps involved in a preclinical protocol.
Figure.
A phase-based diagram showing a standard outline of the steps involved in a preclinical protocol.
3. Research Phase
3.1. Exploratory Studies
Behind every problem worth solving in medical research is the need to develop a hypothesis that describes how to produce the desired resultsand what evidence needs to be collected to support such an output. The method of developing the hypothesis and the supporting evidence is a two-step process that consists of performing two types of studies known as "exploratory" and "confirmatory" studies [
2]. Exploratory studies, also commonly referred to as pilot studies, are the first step researchers must take to establish the academic relevance of their work. These pilot studies are crucial to exploring whether the hypothesis that is being tested may be successful in the vast field of medical devices and drug therapies. The objective of an exploratory study is to establish a proof-of-concept, showing that the invention or innovation has practical potential that would aid in building a more robust grant application or investor pitch. An exploratory study is best described as a study in which the sequence of individual experiments and fine details of the design are yet to be made concrete; the hypotheses must first be solidified through a series of evolving experiments [
3].
3.2. Confirmatory Studies
Confirmatory studies are focused on collecting evidence that supports the hypothesis explored and solidified during the exploratory studies. These studies have a specific goal: providing evidence for the validity of the theory presented by using a rigid, thorough, and reproducible study design. Confirmatory studies are less common in preclinical studies because researchers are more often focused on solidifying a proof-of-concept for their hypothesis. That said, too often, researchers use exploratory studies to show proof of confirmatory results. The stark differences between exploratory and confirmatory studies are important to consider so that the researchers can make correct inferences and keep from confusing the study's goals. For a study to remain exploratory rather than confirmatory, researchers need rigor built into the study’s design to avoid the introduction of personal biases into the interpretation of the data [
4]. The relationship between data collected and results from an experiment is subject to human interpretation; therefore, for the results to be trusted, they must be peer reviewed and possibly even repeated by an outside source.
3.2.1. Good Laboratory Practice (GLP)Studies
Within the broad category of confirmatory studies, a subset known as a GLP study is heralded as one that requires a robust approach to ensuring the study’s quality, accountability, reproducibility, and accuracy. Performing a GLP study is essential for a new medical technology to be cleared by the US Food and Drug Administration (FDA) for use in human trials [
5]. The requirements of a GLP study are much more rigorous than those of nonvalidated exploratory studies because in GLP studies, the researchers are held accountable for the reliability, reproducibility, and validity of their study techniques, quality control features, and results [
6]. The foundation for a GLP study is constructed by three central figures: test facility management, the quality assurance unit, and the study director [
7]. Test facility management is usually coordinated and overseen by the principal investigator. The responsibilities of the principal investigator commonly include, but are not limited to, accepting authority and formal responsibility for the GLP compliance of the study, along with operational procedures and animal care within their facility. The quality assurance unit is vital to GLP correspondence and the general accountability of the study designers and management. In the United States, the role of the quality assurance unit is taken on by an Institutional Animal Care and Use Committee, commonly referred to as IACUC. One of this committee's primary responsibilities is reviewing and approving proposed preclinical research protocols and determining if they fit established guidelines. Lastly, the study director is the main point of contact for the study and is responsible for the entire study's compliance with GLP guidelines. Most commonly, the study director is an employee of the sponsor of the study. Each of these three figures plays a role in creating a quality study, involving other researchers to follow the study and vouch for the credibility of the data and results.
3.3. Discussion of Animal Models
Various factors contribute to planning and designing a procedural protocol for preclinical experimental studies. Because these factors are diverse, the planning and research phase is essential to creating a safe and practical study for the animals and the staff involved. One of the most considerable challenges to designing a study is selecting an animal model for the in vivo evaluation of the test article (e.g., drug therapy, surgical technique, or device). Each animal model differs from humans in several anatomic and physiologic ways. These differences may create obstacles, such as for a surgeon translating the results of a surgery in an animal study to humans in clinical trials [
7,
8]. The Table provides an overview of the best uses of standard animal models in preclinical research and lists the unique challenges they may pose to a study. Some researchers unknowingly assume that outstanding results from theoretical, computational models will translate to slightly diminished or even identical results in an animal model. However, this is rarely the case. More often, animal models provide an opportunity for in vivo surgical training and the early development of devices. When choosing an animal model, conducting a thorough literature search is necessary for multiple reasons. First, a literature search will help determine the animal model that is the best fit for the experiment, such as indicating which animal model has the most similarities to the human anatomical structures being studied. Second, a literature search can help determine that no similar study has been conducted. Additionally, a literature review helps to set up a framework on which the study’s success can determined [
9,
10].
Table.
The significance and limitations of several species of animal models used in preclinical studies.
Table.
The significance and limitations of several species of animal models used in preclinical studies.
| Animal model |
Best Uses |
Challenges |
References |
| Pig |
Human cardiovascular anatomy, device implants, Alzheimer’s disease, atherosclerosis, Type 2 diabetes mellitus, breast cancer, toxicology |
Higher purchase costs than rodents or rabbits, need for more storage space, keeping the animal settled after surgery, require specialized husbandry |
[11,12,13] |
| Cattle |
Mechanical circulatory support devices, female reproductive model, pregnancy-related issues, tuberculosis |
Largely increased purchase and maintenance costs, including feed, veterinary care, and surgery costs. Longer reproductive cycle, creating slow and expensive experiments. Calves are the most comparable to humans in size, but their quick growth limits plausible study duration. |
[12,14,15] |
| Rodents |
Drug therapy, skin wound healing, stroke model |
Large differences between mice and humans in physiology and brain composition |
[12,16,17] |
| Rabbit |
Wound healing model, drug therapy, asthma, cholesterol, cardiovascular disease, Alzheimer’s disease, stroke, cartilage repair |
Different microstructure than humans, lack of literature on required care, lack of well-equipped host facilities and expert handlers |
[11,12,16,18] |
| Guinea Pigs |
Cholesterol metabolism, asthma COPD, feto-placental development, Alzheimer’s disease, tuberculosis, vaccines |
Fewer syngenetic tumor cells lines and lack of specific immune reagents |
[12,19] |
| Hamster |
Reproductive system, micro-circulation, cancer, infection (leptospirosis), vaccines |
Fewer syngenetic tumor cells lines and lack of specific immune reagents |
[12,20] |
| Goat |
Orthopedics, mechanical circulatory support devices, stem cell and locomotor system studies |
Not prone to spontaneous arthritis like rodents, shortage in antibodies, prone to spontaneous arthritis similar to rodents, shortage in antibodies |
[12,21,22,23] |
| Sheep |
Surgical bone-to-bone healing, asthma, heart pathology, vaccines, cartilage repair, smoke inhalation, pulmonary edema, medical device testing, osteoporosis, study of main physiologic systems, abnormal fetal development, and congenital birth defects |
Relatively small chest cavity compared with humans, often have health issues not related to the study, limited availability of physiologic databases for mapping to humans |
[11,12,21,23,24,25,26] |
4. Preprocedural Planning
4.1. Methodology
After a literature review has been completed and an animal model has been selected, the next step in developing a preclinical study is to plan the preprocedural phase of the study. This phase includes determining the methodology used to administer the test article, setting study endpoints, and considering animal welfare, including preprocedural medications. Planning in this phase is essential and should be completed before beginning the experimental phase to ensure accurate and reproducible study results.
When determining how to administer and evaluate the test article in the selected animal model, it is necessary to consider the level of invasiveness, efficacy, and technical practicality of the mode of administration according to prior data. This decision, coupled with that of the location of administration, is affected by the factors mentioned above. The location and methodology used to administer the test article can pose potential stressors, add difficulty to postprocedural recovery, or even violate animal welfare guidelines if an inappropriate selection is made [
27]. Additionally, the method used to measure the effectiveness of the test article must be decided to establish study endpoints. These can vary widely among studies and must reflect the study's specific goals and purpose. For example, one study may use an objective endpoint, such as a lab test result, whereas another may use a subjective endpoint, such as the extent of pain reduction. Objective endpoints are preferable because they can be measured without bias. Ideally, endpoints are determined on the basis of clinical relevancy and application to the study goal and are easily interpreted according to preset standards to reduce individual bias. For a study to be successful, it is imperative to assess the efficacy of the test article and set clear endpoints that are easily and affordably measured [
28].
4.2. Critical Questions
Once the methodology, study endpoints, and methods to measure the endpoints have been determined, several important questions must be addressed and answered during the preprocedural planning phase. First, how many procedures are needed? Will any follow-up procedures be required, or will only one procedure be performed per animal? Alternatively, inducing a specific condition may be necessary (also called a disease model) before administering the test article, in which case multiple procedures may be required.
Determining the number of animals needed for the study is crucial in the preprocedural planning phase. A sample size that is too large can waste animals, but a sample size that is too small can lack the sensitivity to detect important biologic effects. The latter may require the study to be repeated, ultimately wasting resources. The sample size for an exploratory, hypothesis-generating study will be smaller than that of a confirmatory, hypothesis-testing study. The smaller sample size in the exploratory study is attributed to the pattern-seeking nature of an exploratory study rather than the formal testing of a hypothesis. Therefore, the sample size of an exploratory study is often determined by using data from previous studies or by using clinical judgment rather than a significance test. For a confirmatory, hypothesis-testing study, a power analysis is the most common method to determine the appropriate sample size. Power analyses can be conducted with the help of a statistician or statistical software on the basis of effect size, standard deviation, chosen significance level, chosen power, and alternative hypothesis. The accurate selection of these variables ensures the detection of only biologically meaningful effects rather than biologically irrelevant yet statistically significant results. These variables must be detailed in the protocol to justify the sample size and provide evidence to support any claims made about the hypotheses [
2,
29,
30].
Another key question that must be addressed before the experimental phase can begin is how long the study will last. The driving factors that typically determine this are endpoint measurements and institutional guidelines. If the study is an exploratory surgical study in which the endpoints are measured at the end of the procedure, this would be considered an acute nonsurvival study, usually no longer than 24 hours, and euthanasia would be performed while the animal is under anesthesia at the conclusion of the procedure. For a survival or chronic study, the animal must recover and survive the postoperative period after the test article is administered to accurately measure the study's endpoints. In either case, the animal's welfare is a primary concern; therefore, the decision between an acute or chronic study must comply with the hosting institution's animal welfare guidelines.
4.3. Animal Care
The first step of developing an experimental design and timeline is to consider preprocedural medications, the welfare of the animal, and ethical guidelines. From the time of the animal's arrival at the facility, actions must be put in place to properly prepare the animal for the study, starting with a quarantine of the animal to protect other animals in the facility from outside diseases. During this time, that animal’s baseline vitals and general condition can be assessed. Next, plans should be put in place for determining the exact dosages, routes for administration, and timing for medicating the animal before the procedure. A critical aspect of medication administration is considering potential interactions between medications and their effect on the study’s objectives. The use of each medication must be supported in the literature and have a specific goal for its administration [
27]. In addition, similar to the patient in clinical studies, the animal's welfare and the ethical implications involved are of utmost importance. Aspects of animal welfare include housing, nutrition, socializing, and environmental enrichment. When assessing the welfare of animals, baseline vitals should be regularly monitored to detect illnesses or signs of conditions that could affect the animal's qualification for the study. Although all these steps may be conducted by the organization overseeing the animal, they should still be included in the protocol to ensure that the protocol is detailed and effective [
31].
The first steps of the protocol should also describe how the animals are prepared for anesthesia, such as fasting or feeding with a particular diet, as well as surgical preparation. Researching the animal's anatomy should be done ahead of time to determine the proper surgical preparation, including cleaning and table positioning, and how to easily access the surgical site. Depending on the type of study being developed, the anesthetic selected to sedate the animal should not have any contraindications that would interfere with the study's objectives. In addition to the type of anesthetic agent, the dose of the agent must be calculated to keep the animal completely sedated without causing harm.
5. Experimental Phase
Determining the method for test article delivery requires the consideration of all variables during planning. If the method of test article delivery is surgical and an approach has already been established in the literature, that approach can be adopted for the study. If no established procedure exists, an exploratory nonsurvival pilot study may be necessary. This pilot study would require researchers to gather initial data, assess the procedure's feasibility, and identify potential areas for refinement. On the basis of the perceived results, adjustments can be made to the procedural technique; refining surgical techniques is crucial in preclinical animal studies to ensure the reproducibility and accurate evaluation of the test article [
32]. Even small variables, such as animal positioning and critical steps during the operation, should be carefully documented to ensure reproducibility. Providing detailed descriptions of all critical steps and medications used during the operation is essential to facilitate the accurate replication of the procedure in future studies.
After the procedural technique and proof-of-concept have been established and refined, chronic or survival studies can commence. These studies aim to evaluate the test article in a long-term setting so that its effectiveness can be assessed over an extended time period. The results of chronic or survival studies provide valuable insights into the test article's safety and efficacy. In addition, these data emphasize a study’s reproducibility, providing a more comprehensive understanding of its potential applications in clinical settings. This is contrasted by the acute or short-term protocols that are used in a hypothesis-generating study.
5.1. Necropsy and Euthanasia
In planning the postoperative phase, detailed protocols must be established for follow-up procedures, blood draws (for lab testing), medications, and health screenings. In addition, the administration of sedation medications or anesthesia and the unique needs of the animal model must be considered. There is no standardized set of guidelines for the recovery of animals, so it is vital to adhere to institutional regulations and rely on the experience of the testing center. When the study's endpoints are met, euthanasia must be performed humanely and ethically as set forth by the American Veterinary Medical Association (AVMA) to minimize pain and distress for the animal. After euthanasia, a necropsy can be performed to systematically examine the animal’s organs and tissue. The data obtained can then be used to understand the effects of the test article and its potential risks and benefits.
For an in vivo preclinical study, postoperative care, euthanasia, and necropsy are crucial components that must be detailed in the protocol. The proper planning and execution of these steps help ensure the ethical treatment of animals and the generation of reliable research data.
6. Pitfalls to Avoid
Before a study protocol is developed, some pitfalls must be avoided when planning and conducting the procedure. First, as described above, it is essential to clearly define the study endpoints during the planning phase before the experiments are started and to keep them in mind throughout all protocol steps. In all scientific endeavors, internal bias towards a particular outcome must be avoided to prevent compromising the reliability of the study. To eliminate these biases, guidelines for preclinical efficacy studies should be implemented [
33]. Once the endpoints have been defined and the study has begun, maintaining the endpoints prevents the results from being invalidated and preserves the reproducibility of the study. Second, when selecting a sample size in both exploratory and confirmatory studies, it is easy to underestimate the number of animals needed to obtain meaningful results. One, two, or even three animals may be enough for a pilot study to fine-tune surgical procedures or medication dosages; however, the number of animals needed to test the hypothesis of a study will increase substantially. Third, the quality of surgical techniques can vary significantly between surgeons and facilities. Cutting costs on the surgeon used should be avoided; a surgeon or any medical provider that lacks the proper technique can lead to higher rates of complications, morbidity, and mortality, affecting the scientific validity of the study [
34]. Also, the animal's welfare requires careful attention after the procedure is completed. Animals used to assess the effectiveness of a test article should be treated similar to how humans would be treated when working to obtain optimal results. The animal(s) should receive proper housing, food, water, and medical care to maintain the study's validity.
7. Conclusion
During the initial stages of developing a protocol for a preclinical in vivo study, the critical procedural aspects of the study should be the focus and basis for the decisions that follow. To best support the study’s goals, an educated decision on the choice of animal model should be made according to the available evidence. However, this decision should be made only after the protocol phases have been properly outlined. Next, focusing on the details beyond the procedure is critical. The steps taken during the preoperative, intraoperative, and postoperative phases can affect the efficacy and reliability of the study. After all phases of the study have been clearly outlined and planned according to the study's goals, time should be spent reviewing all the variables in the protocol, ensuring that every variable is considered to be significant. By beginning the planning process with a strict focus on the critical aspects of the study, the rest of the experiments should fall into place when accompanied by careful, diligent decision making.
Author Contributions
Conceptualization, A.M. and A.E.; methodology, A.M., D.D., and J.W.; investigation, D.D. and J.W.; writing—original draft preparation, A.M. D.D. and J.W.; writing—review and editing, A.M. and A.E..; visualization, A.M. and A.E..; supervision, A.M., and A.E.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Nicole Stancel, PhD, ELS(D), of the Department of Scientific Publications at The Texas Heart Institute, for her editorial contributions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ioannidis:, J.P.; Greenland, S.; Hlatky, M.A.; Khoury, M.J.; Macleod, M.R.; Moher, D.; Schulz, K.F.; Tibshirani, R. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014, 383, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Percie du Sert, N.; Vollert, J.; Rice, A.S.C. General principles of preclinical study design. Handb Exp Pharmacol 2020, 257, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Kimmelman, J.; Mogil, J.S.; Dirnagl, U. Distinguishing between exploratory and confirmatory preclinical research will improve translation. PLoS Biol. 2014, 12, e1001863. [Google Scholar] [CrossRef]
- Wagenmakers, E.J.; Wetzels, R.; Borsboom, D.; van der Maas, H.L.; Kievit, R.A. An agenda for purely confirmatory research. Perspect Psychol Sci 2012, 7, 632–638. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, J.S.; Jeon, M.S.; Lee, K.; Chung, M.K.; Song, C.W. Basic principles of the validation for good laboratory practice institutes. Toxicol. Res. 2009, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Good laboratory practice; 2001.
- Andrade, E.L.; Bento, A.F.; Cavalli, J.; Oliveira, S.K.; Schwanke, R.C.; Siqueira, J.M.; Freitas, C.S.; Marcon, R.; Calixto, J.B. Non-clinical studies in the process of new drug development - Part II: Good laboratory practice, metabolism, pharmacokinetics, safety and dose translation to clinical studies. Braz. J. Med. Biol. Res. 2016, 49, e5646. [Google Scholar] [CrossRef] [PubMed]
- van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O'Collins, V.; Macleod, M.R. Can animal models of disease reliably inform human studies? PLoS Med 2010, 7, e1000245. [Google Scholar] [CrossRef]
- Pubrica Academy. Why is it important to do a literature review in research? Available online: https://pubrica.com/academy/research/why-is-it-important-to-do-a-literature-review-in-research/ (accessed on July 12).
- Upstate University of South Carolina Library. Literature review: Purpose of a literature review. Available online: https://uscupstate.libguides.com/c.php?g=627058&p=4389968#:~:text=The%20purpose%20of%20a%20literature,questions%20left%20from%20other%20research (accessed on July 12).
- Moran, C.J.; Ramesh, A.; Brama, P.A.; O'Byrne, J.M.; O'Brien, F.J.; Levingstone, T.J. The benefits and limitations of animal models for translational research in cartilage repair. J Exp Orthop 2016, 3, 1. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: a review. Lab Anim Res 2022, 38, 18. [Google Scholar] [CrossRef]
- Walters, E.M.; Wells, K.D.; Bryda, E.C.; Schommer, S.; Prather, R.S. Swine models, genomic tools and services to enhance our understanding of human health and diseases. Lab Anim (NY) 2017, 46, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Monreal, G.; Sherwood, L.C.; Sobieski, M.A.; Giridharan, G.A.; Slaughter, M.S.; Koenig, S.C. Large animal models for left ventricular assist device research and development. ASAIO J. 2014, 60, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.; Gonzalez, L.; Blikslager, A. Large animal models: The key to translational discovery in digestive disease research. Cell Mol Gastroenterol Hepatol 2016, 2, 716–724. [Google Scholar] [CrossRef]
- Narayan, S.K.; Grace Cherian, S.; Babu Phaniti, P.; Babu Chidambaram, S.; Rachel Vasanthi, A.H.; Arumugam, M. Preclinical animal studies in ischemic stroke: Challenges and some solutions. Animal Model Exp Med 2021, 4, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Zomer, H.D.; Trentin, A.G. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef]
- Mapara, M.; Thomas, B.S.; Bhat, K.M. Rabbit as an animal model for experimental research. Dent Res J (Isfahan) 2012, 9, 111–118. [Google Scholar] [CrossRef]
- Canning, B.J.; Chou, Y. Using guinea pigs in studies relevant to asthma and COPD. Pulm. Pharmacol. Ther. 2008, 21, 702–720. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Y.; Dunmall, L.S.C.; Lemoine, N.R.; Wang, P.; Wang, Y. Syrian hamster as an ideal animal model for evaluation of cancer immunotherapy. Front. Immunol. 2023, 14, 1126969. [Google Scholar] [CrossRef]
- Burmeister, D.M.; Supp, D.M.; Clark, R.A.; Tredget, E.E.; Powell, H.M.; Enkhbaatar, P.; Bohannon, J.K.; Cancio, L.C.; Hill, D.M.; Nygaard, R.M. Advantages and disadvantages of using small and large animals in burn research: Proceedings of the 2021 research special interest group. J Burn Care Res 2022, 43, 1032–1041. [Google Scholar] [CrossRef]
- Dias, I.E.; Viegas, C.A.; Requicha, J.F.; Saavedra, M.J.; Azevedo, J.M.; Carvalho, P.P.; Dias, I.R. Mesenchymal stem cell studies in the goat model for biomedical research-a review of the scientific literature. Biology (Basel) 2022, 11, 1276. [Google Scholar] [CrossRef]
- Gregory, M.H.; Capito, N.; Kuroki, K.; Stoker, A.M.; Cook, J.L.; Sherman, S.L. A review of translational animal models for knee osteoarthritis. Arthritis 2012, 2012, 764621. [Google Scholar] [CrossRef] [PubMed]
- Banstola, A.; Reynolds, J.N.J. The sheep as a large animal model for the investigation and treatment of human disorders. Biology (Basel) 2022, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Berset, C.M.; Lanker, U.; Zeiter, S. Survey on sheep usage in biomedical research. Animals (Basel) 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Hamernik, D.L. Farm animals are important biomedical models. Anim Front 2019, 9, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.B.; Jennings, M.; Buckwell, A.; Ewbank, R.; Godfrey, C.; Holgate, B.; Inglis, I.; James, R.; Page, C.; Sharman, I.; et al. Refining procedures for the administration of substances. Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. British Veterinary Association Animal Welfare Foundation/Fund for the Replacement of Animals in Medical Experiments/Royal Society for the Prevention of Cruelty to Animals/Universities Federation for Animal Welfare. Lab. Anim. 2001, 35, 1–41. [Google Scholar] [CrossRef]
- Evans, S.R. Fundamentals of clinical trial design. J Exp Stroke Transl Med 2010, 3, 19–27. [Google Scholar] [CrossRef]
- Festing, M.F.; Altman, D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J 2002, 43, 244–258. [Google Scholar] [CrossRef]
- Ko, M.J.; Lim, C.Y. General considerations for sample size estimation in animal study. Korean J Anesthesiol 2021, 74, 23–29. [Google Scholar] [CrossRef]
- In Guidance for the Description of Animal Research in Scientific Publications; The National Academies Collection: Reports funded by National Institutes of Health; Washington (DC), 2011.
- McCulloch, P. Developing appropriate methodology for the study of surgical techniques. J. R. Soc. Med. 2009, 102, 51–55. [Google Scholar] [CrossRef]
- Henderson, V.C.; Kimmelman, J.; Fergusson, D.; Grimshaw, J.M.; Hackam, D.G. Threats to validity in the design and conduct of preclinical efficacy studies: a systematic review of guidelines for in vivo animal experiments. PLoS Med 2013, 10, e1001489. [Google Scholar] [CrossRef]
- Gelijns, A.C. In Technological Innovation: Comparing Development of Drugs, Devices, and Procedures in Medicine; Washington (DC), 1989.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).