1. Introduction
Leishmaniasis is a worldwide distributed and neglected disease. caused by protozoan parasites of the genus
Leishmania [
1]. The complex of diseases encompasses various clinical forms; from visceral to disfiguring cutaneous lesions [
2]. Genetic diversity in the
Leishmania genus plays an important role within the complex factors that lead to the wide range of clinical outcomes. In the Americas. the visceral form is caused by
Leishmania (Leishmania) infantum and the tegumentary leishmaniasis (TL) is associated with infection of 10 different species from the two subgenera (
Leishmania and
Viannia). In Brazil, the most common species causing TL are
L. (V.) braziliensis, Leishmania (V.) guyanensis and
L. (L.) amazonensis [
3]. In the Amazon region of the country TL can also be caused by other species:
L. (V.) naiffi. L. (V.) lainsoni. L. (V.) shawi and
L. (V.) lindenbergi. The species
L. (V.) utingensis, first detected in sandfly [
4] is also present in the region and has been recently detected infecting human [
5].
The diagnosis of leishmaniasis comprises the association of clinical, epidemiological and laboratory data. Clinical signs and symptoms, alone or in combination, are not always sufficient as they can be confused with other diseases. It is therefore essential to carry out the laboratory diagnosis, which can be done by parasitological diagnostic (microscopy), immunological, serological, and molecular tests. It is therefore essential to carry out the laboratory diagnosis, which can be done by direct (parasitological, culture, histopathological analysis, and PCR) or indirect methods based on the detection of anti-Leishmania antibodies - less used for TL diagnosis [
6]. As stated, the species diversity within the
Leishmania genus has a major role in the treatment, clinical outcome, and transmission cycles. Therefore, the identification of the infecting species directly in clinical samples (preferably) or by isolating the parasite, is still a relevant topic in the studies of leishmaniasis, especially in sympatric areas such as among countries and regions in the Americas.
Biological collections gather valuable material to explore microorganism diversity and its impact on the development of diagnostic and species typing tools. The Leishmania collection of Fiocruz (CLIOC; clioc.fiocruz) is a robust biobank, mainly for American
Leishmania isolates, and is also a reference center for
Leishmania species identification. The method applied for species typing is the Multilocus Enzyme Electrophosis (MLEE) - still the gold standard technique, but only applicable in successfully isolated and cultured parasites. MLEE has contributed hugely for typing [
7] and for the epidemiology of leishmaniasis [
8,
9], but its drawbacks push researchers forward to the development of a cheaper and faster substitute method. Molecular-based approaches are currently the main target, and PCR-sequencing [
10], PCR-RFLP are already applied in DNA from cultured parasites and from clinical material [
11]. Both methods, however, involve a lot of steps, including gel electrophoresis. In this regard, protocols based on quantitative real-time PCR (qPCR) are good candidates because they eliminate the need for the detection of amplified product in gel, allow many samples to be assayed simultaneously and have the potential to be automatized. Moreover, it is especially relevant for clinical samples in which the parasite load affects sensitivity and accuracy, and the technique may potentially overcome this fact by detecting and quantifying minimal amounts of nucleic acids in a wide range of samples from different sources [
12,
13]. In this context, High Resolution Melting Analysis (HRM) has been pointed as a good and practical alternative to detect and identify
Leishmania species, both as a diagnostic tool and as typing tool for laboratory work [
14,
15,
16]. HRM is an adequate technique to detect mutations, single nucleotide polymorphisms (SNPs), and epigenetic differences in DNA samples. The analysis is based on the variance between the shape of melting curves and the difference between the samples melting temperatures (Tm). The Tm value of a sample can be obtained from the dissociation curve, defined as the temperature at which 50% of each DNA molecule is denatured [
17].
A study developed by Zampieri
et al [
18] presented a HRM assay using as targets two regions of the HSP70
Leishmania gene [
19] both within a 234 bp region already used to identify species, even from clinical samples [
11]. The first pair of primers targets a conserved region of 144 bp that amplifies all
Leishmania species; a second pair targets a smaller and more variable region of 104 bp, that identifies only
Leishmania (Viannia) species [
19]. Based on HRM analysis, these assays were able to differentiate eight
Leishmania species found in the Americas:
L. (L.) infantum, L. (L.) amazonenses, L. (L.) mexicana, L. (V.) lainsoni, L. (V.) braziliensis, L. (V.) guyanensis, L. (V.) naiffi and
L. (V.) shawi; and 3 species found in Europe, Asia and Africa:
L. (L.) tropica. L. (L.) donovani and
L. (L.) major. The assays were validated in 16 experimentally infected golden hamster tissue samples, parasite strains isolated from humans, fresh tissue human samples or embedded in paraffin, experimentally infected BALB/c mice tissues and samples from naturally infected phlebotomines. The HRM analysis results were compared to previous genotyping by sequencing, and/or by MLEE, and presented a strong correlation between the methodologies. Finally, an algorithm was proposed to characterize by HRM the main
Leishmania species circulating in Brazil [
19].
Despite the potential offered by the published protocol [
19], our group raised the concern on the following specific points: first, considering the well-known intraspecific genomic variation among
L. (Viannia) strains, polymorphisms among distinct strains may affect the melting curve, melting temperature and ultimately the HRM typing efficiency. Additionally, some species were not assayed previously, and neither the intraspecific variation explored. Therefore, the effect of such diversity on the protocol must be investigated. By evaluating such aspects, we aim to appraise the potential of HRM to be applied as a substitute for MLEE. Secondly, an open question is how the accuracy, sensitivity and specificity vary when the method is applied on a panel of human clinical material displaying a broad range of parasite load. To address these subjects, we assayed a robust, distinctive and representative sampling of
Leishmania DNA available at CLIOC and, equally importantly, human clinical samples with associated previous data of parasite load obtained by a HSP70 qPCR analytical study [
20]. The samples were collected from patients with cutaneous leishmaniasis from a region known to present high intra and interspecific
Leishmania diversity [
20]. For the present study, we combine these described data and material aiming to evaluate the accuracy of the HRM protocol [
18], both as a diagnostic method and as a possible substitute to the MLEE as a species typing tool for the reference service. The approach allowed unique and comprehensive inferences to be made on the HRM potential as a species typing tool and a diagnostic method for
Leishmania.
3. Discussion
Our group has been interested in developing and validating methodologies to detect, quantify and type
Leishmania species, both as a laboratory/biobank protocol for the reference service and as a diagnostic tool to be applied in clinical material. The importance of detecting and typing
Leishmania species is widely recognized, as well as the determination of parasite load for clinical and research purposes [
20,
21]. Therefore, combined molecular approaches merging these possibilities would be valuable tools for clinicians, surveillance, and research.
CLIOC, a
Leishmania biobank, has been currently evaluating techniques with the potential to replace the MLEE, aiming to overcome the drawbacks of the technique such as its elevated cost, limited set of assayed samples per experiment, difficulty of inter-laboratory comparison, and time-consuming characteristics. High Resolution Melting analysis is simple and rapid, and its use in clinical or research samples offers many advantages, such as a lower total cost for species identification compared to MLEE. Moreover, as a real time PCR-based approach, there is no need for sequencing or gel electrophoresis to analyze the product, reducing the process and avoiding laboratory contamination with PCR products. It also reduces the need for trained personnel to analyze an electrophoretic gel or sequencing data to provide a result, and, additionally, presents the possibility of quantifying infecting parasites within samples since it can be applied as a quantitative PCR-based technique. The whole process can be automated as the analyzer software produces the result by comparing the tested sample and the reference sample profiles, which must always be included in the reactions [
22]. Based on such, we consulted the promising results from Zampieri et. al., [
18] and proposed to further evaluate the accuracy of the protocol as a typing and/or diagnostic tool.
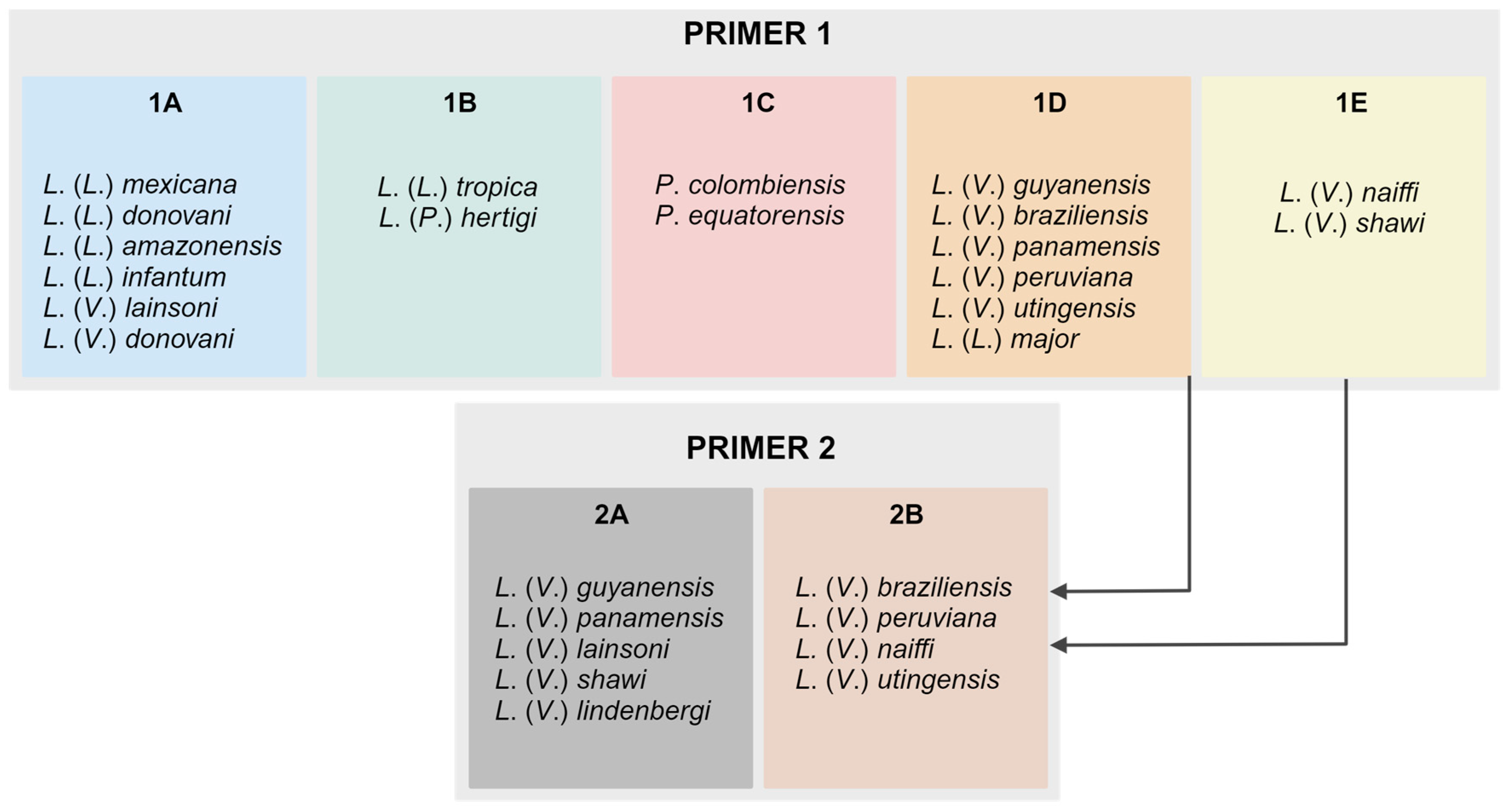
First, we tested the reproducibility of the assay proposed by Zampieri and colleagues in our lab conditions, which include different operators and equipment, among other variables. The results of a reference set of samples fully reproduced the published data, by differentiating the main species with the first set of primers (P1), except for
L. (L.) infantum vs
L. (L.) donovani, and
L. (L.) amazonensis vs
L. (L.) mexicana. We included species not yet evaluated
bonafide by the method, such as
L.
(V.
) peruviana,
L.
(V.
) panamensis,
L. (V.) lindenbergi,
L. (V.) utingensis, L. (P.) hertigi and the
Paraleishmania colombiensis and
P. equatorensis. The results precisely allowed the distinction of the main
L. (Viannia) species, additionally revealing the profiles of the above-mentioned newly assayed species (
Figure 1). By applying the second set of primers (variant 2)
L. (V.) guyanensis and
L. (V.) braziliensis were effectively differentiated, but
L. (V.) peruviana vs
L. (V.) braziliensis and
L. (V.) panamensis vs
L. (V.) guyanensis remained within the same variant and were, thus, not possible to distinguish. The separation of these species are specially relevant in sympatric regions such as Andean region in Peru [
23,
24], Colombia [
25,
26], Venezuela [
27] and Bolivia [
28]. Further steps with an additional primer design must be developed to specifically attend the purpose of typing these species.
We performed a reproducible assay with the same operator, executing experiments in distinct days and obtaining 100% reproducibility. The next necessary step was to test the efficacy of the protocol considering the intraspecific genetic variability - known to occur among
Leishmania strains. Such assay allows to evaluate the effect of unanticipated DNA polymorphism on factors that affect the HRM typing outcome, such as mismatches at the primer alignment site, the melting curve and ultimately the melt temperature [
22]. Therefore, a wider panel of samples was prepared, composed by DNA of 90 strains from widely different geographic origins representing 19
Leishmania species. Results converged with the MLEE and HSP70 sequencing data for 96.2% of samples, revealing a highly accurate outcome. Kappa index indicated
Almost perfect agreement between HRM and MLEE. Results revealed the potential for the proposed method and protocol to be applied as a typing tool on DNA from isolated and cultured
Leishmania, eventually substituting the current MLEE assay.
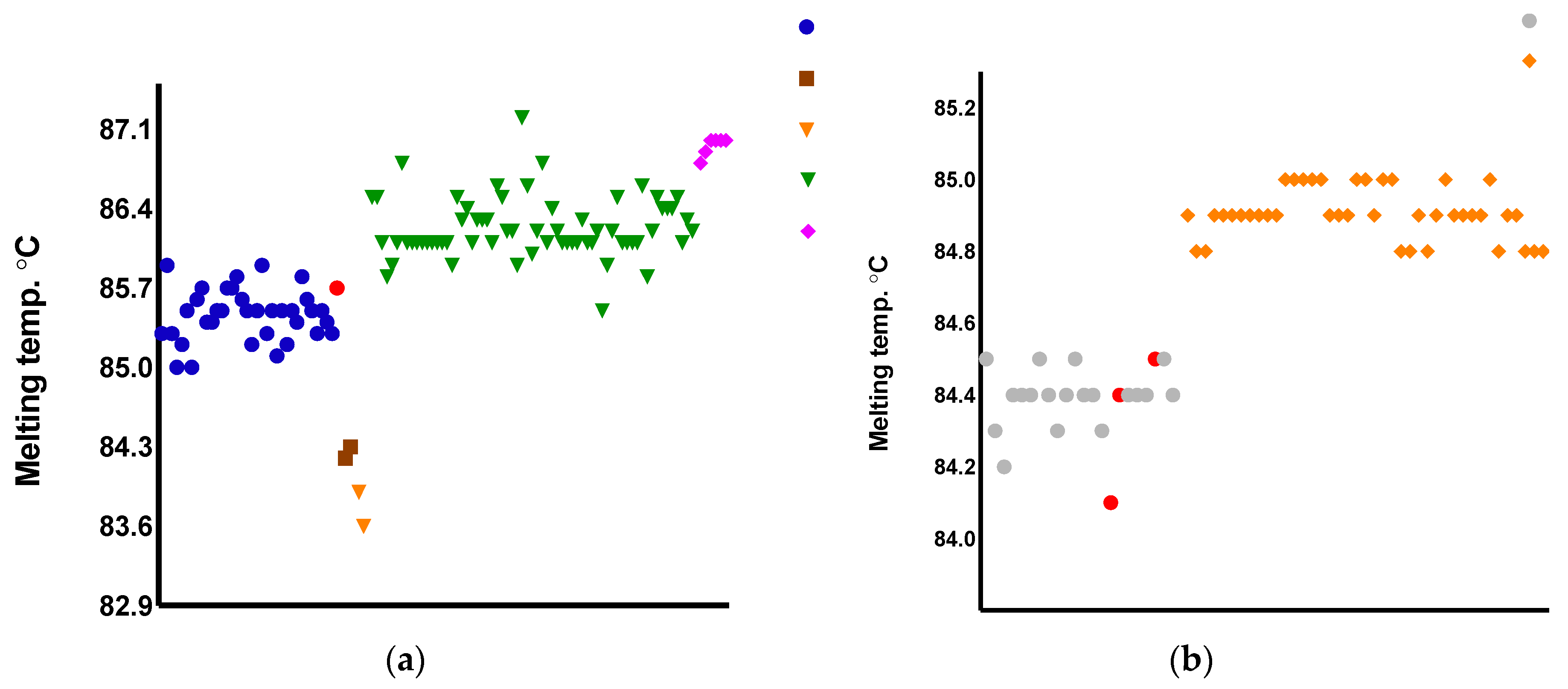
Raw data from the assays of the three divergent strains are a valuable opportunity to explore the intrinsic limiting features of HRM approach. The melting temperature is one of the parameters for the software to cluster samples within the window set defined by the reference strains profiles, though it is not the only metric. To express this subject, we plotted the Tm distribution with color-coded samples’ variants for both P1 and P2primers. The result revealed Tm as not being the only parameter to define the melting curve profile which ultimately defines the variants.
Figure 3 summarizes the limited role Tm has on the clustering of samples as divergent and convergent. It has been reported that DNA methylation, concentration of the initial template for reaction – reflected by the Ct obtained, GC content within the amplified region and stoic metrics may influence the melt curve profile. Moreover, type and quality of the DNA source material (purity and integrity), isolation method and pipetting inconsistencies, might also influence outcome [
22]. We managed to carefully avoid a few of these variables to deliberately focus on the effect of polymorphisms on melt profiles. Thus, DNAs were prepared by the same isolation method within at most six months interval; all assays were performed by the same experienced operator in the same equipment. Concentration of initial template for the reactions was not normalized, although parasites’ pellets for DNA isolation were prepared by the same laboratory protocol. Therefore, the C
t values varied slightly, mostly within the described 20-30 range presented as of optimal efficiency [
22]. Indeed, the different quantities of initial DNA template did not compromise the accuracy, whereas sequencing of the amplified region of the divergent strains revealed polymorphisms as an important, but not unique, source of divergence between typing data. These samples presented Tm close to the upper-range of the correspondent references (
Figure 3), but the G-C content and the polymorphisms position [
22] were possibly a main-factor that produced distinct melt curve profiles, ultimately leading to the divergent result (
Figure 4 and
Figure 5). Shape of melting curves may also be affected by methylation and efficiency in primer alignment, independently of polymorphisms [
22]. However, for the current data the polymorphisms and their location within the amplified region are the more parsimonious explanation for typing divergence. The intra and interspecific genetic diversity is a well-known trait of
Leishmania and indeed a challenge for species typing and for diagnostic tools development. There are geographic regions of Brazil where hosts and circulating parasites present greater chances of variability. Indeed, this was the case for the divergent samples from the validation step. These strains were isolated from dogs and from a human host, from the northern region that harbors a highly diverse parasite population [
29,
30].
Our group recently proposed an HSP70 qPCR-based approach to determine parasite load in material collected from patients with confirmed clinical diagnostic for cutaneous leishmaniasis. The patients studied were from an endemic area in the Amazon region, in which parasite population is quite diverse [
20]. Besides the HSP70 qPCR, the samples were also subjected to microscopy and conventional PCR; infecting species was identified by PCR-RFLP and/or by DNA sequencing. Herein, we applied the HSP70 HRM protocol on these samples to test the method’s accuracy as a diagnostic typing tool. Data revealed that HRM positivity percentage was the same obtained for microscopy and slightly lower than that of the HSP70 qPCR. Conventional PCR (cPCR) presented the highest positivity. To define sensitivity and specificity, microscopy and cPCR were used as gold standards. The HRM sensitivity and specificity were higher than that obtained by the HSP70 qPCR applied in these samples. By employing cPCR as the gold standard, HRM sensitivity and specificity increased significantly. These results suggest HRM represents a good diagnostic tool for
Leishmania detection, independently of the species typing effectiveness.
In contrast to the Kappa index compatible to “
Almost perfect agreement” and 96.2% concordance for the validation step, in which DNA from cultured parasites was used, for the clinical samples a “
Moderate Agreement” was achieved, and convergent typing results were attained for 76.5% of samples. The effective outcome obtained by HRM with DNA from cultured parasites is expected due to the specific nature and purity of the samples. Even though predictable, we further explored the features underlining the reduction on HRM typing success in clinical material. Coinfections, for instance, could lead to inconclusive typing result, and such possibility cannot be excluded, given the geographic region where the patients were located (sympatric area for most
Leishmania species). These conditions, however, are difficult to detect and characterize. The most likely - and testable – factor involved is the parasite load. As described in [
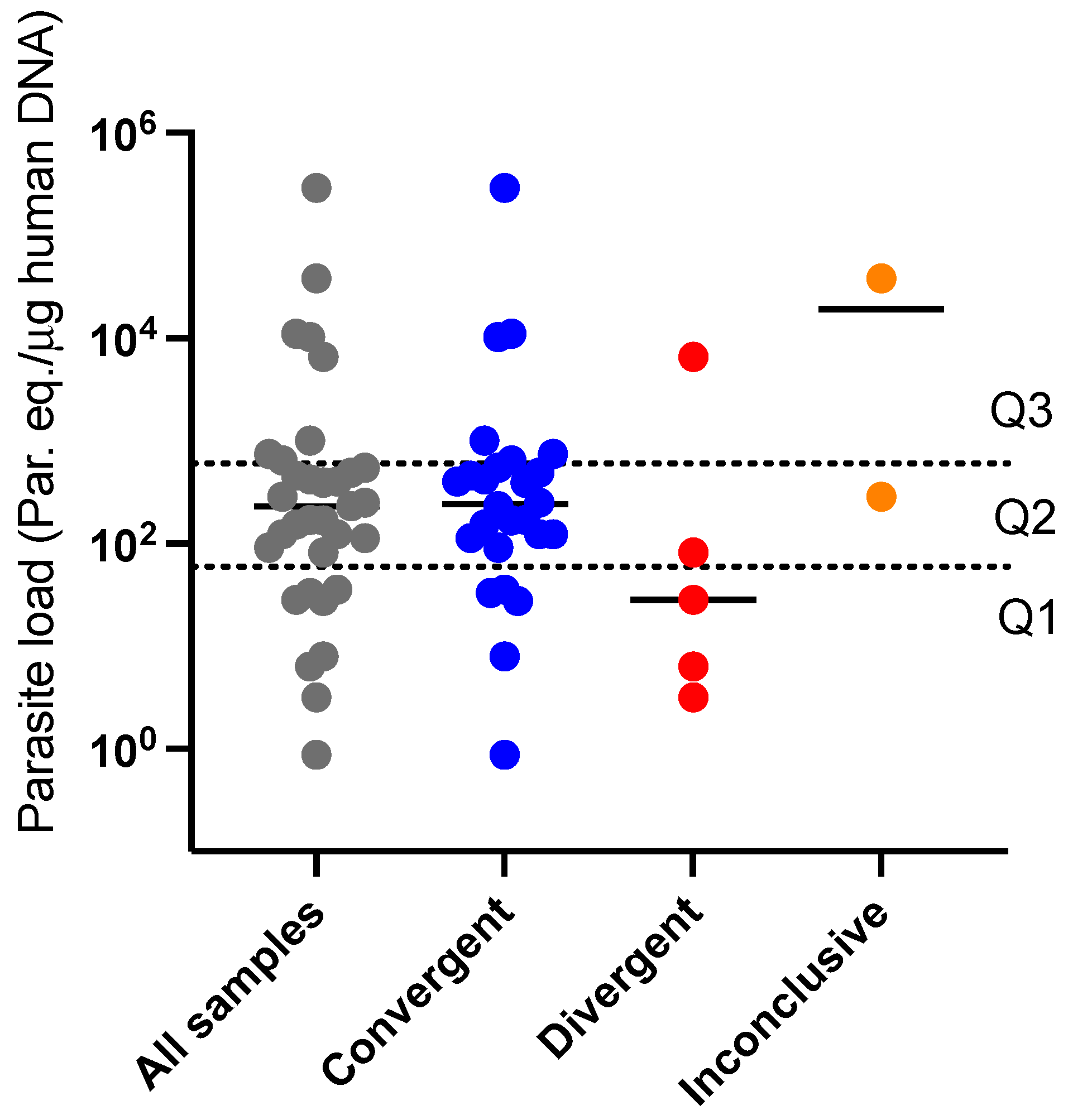
20], the clinical samples assayed presented a wide range of parasites equivalents per human DNA, thus comprising a suitable panel to be tested by HRM (
Figure 7). Therefore, to evaluate the effect of parasite load on typing accuracy, we plotted parasites equivalents per human DNA accordingly to HRM-based species typing outcome and defined the interquartile range of all samples. The result exposed the variable accuracy in each quartile, with the best outcome of 88.2% attained in a narrow range of parasite load. Therefore, HRM typing outcome was highly affected by parasite load, despite its high positivity for
Leishmania DNA. Based on this conclusion, we would suggest a clinical-sample-typing workflow that first employs the HSP70 qPCR protocol [
20] to evaluate whether the sample fits the most accurate parasite load range for HRM typing. The decision to further apply the HSP70-based HRM protocol as a typing tool must be taken considering this information.
There are recent studies in the literature addressing the use of HRM to identify
Leishmania species [
16,
31,
32]. None, however, have tested the protocol accuracy in a significantly
bonafide strains panel representing the inter and intraspecific diversity of
Leishmania. Important steps like this are vital to propose the technique as a typing tool. Moreover, the published studies avoid important validation steps by directly testing HRM-based protocols in biological material, not exploring the many factors that may influence the outcome. Herein, we reveal the great potential of the HSP70-HRM for species typing in DNA from cultured parasites. For clinical samples, however, the data raise the need for caution when using the HSP70-HRM protocol as a species typing tool. The variable accuracy dependent on parasite load imposes the need to first either determine the parasite load or, at least, establish a C
t-based cutoff value, before deciding for the typing approach.
The results presented herein open a venue of possibilities for evaluation of the HRM approach targeting other genes which might achieve better results related to
Leishmania species identification, especially those targets with higher copy number than the HSP70 gene. Among different genes employed for
Leishmania species typing, at least for neotropical regions, MPI [
30] and Cytb [
33] would be good alternatives to be tested.
Figure 1.
Schematic representation of the species groups coupled in each variant obtained by PCR reactions with primers 1 and 2; suggestion of the algorithm to differentiate the main species circulating in the Americas.
Figure 1.
Schematic representation of the species groups coupled in each variant obtained by PCR reactions with primers 1 and 2; suggestion of the algorithm to differentiate the main species circulating in the Americas.
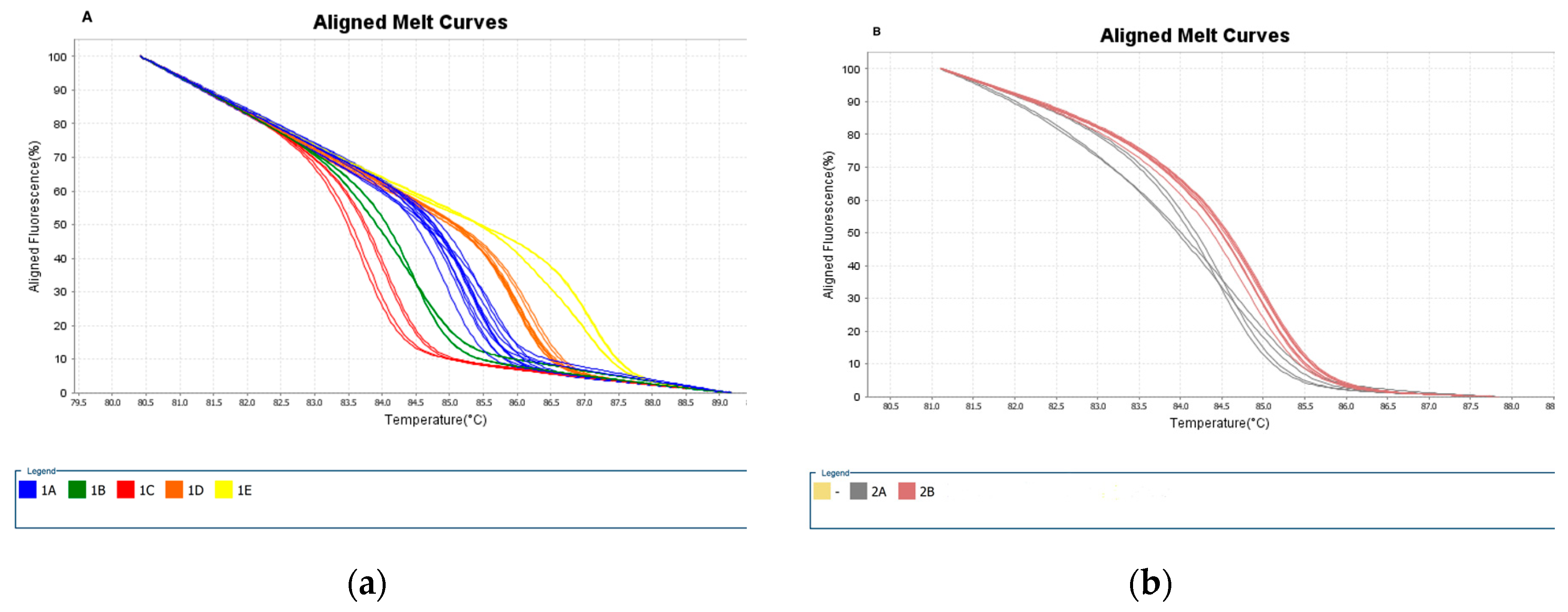
Figure 2.
Color-coded dissociation curves of reference strains selected as standards for primer 1 (a) and 2 (b). (a) The identified variants for primer 1: blue - variant 1A; green - variant 1B; red - variant 1C; orange - variant 1D; yellow - variant 1E. (b) The identified variants for Primer 2: Curves in grey - variant 2A; Curves in brown - variant 2B. Figures were exported from the High-Resolution Melting Software available at the ViiA 7 Real-Time PCR System ( Thermo Fisher Scientific).
Figure 2.
Color-coded dissociation curves of reference strains selected as standards for primer 1 (a) and 2 (b). (a) The identified variants for primer 1: blue - variant 1A; green - variant 1B; red - variant 1C; orange - variant 1D; yellow - variant 1E. (b) The identified variants for Primer 2: Curves in grey - variant 2A; Curves in brown - variant 2B. Figures were exported from the High-Resolution Melting Software available at the ViiA 7 Real-Time PCR System ( Thermo Fisher Scientific).
Figure 3.
Color-coded Melting Temperatures (Tms) accordingly to Primers 1 and 2 variants. Tms obtained during the validation assay. (a) blue circle = 1A, brown square = 1B, orange inverted triangle = 1C, green = 1D, pink diamond = 1E. (b) black circle = 2A, blue diamond = 2B. The three divergent results are represented in red: (a) red circle: IOCL 3310, L. naiffi clustered as 1A (expected to be 1E); (b) red circles correspond to the two L. braziliensis and the one L. naiffi erroneously clustered as variant 2A (expected 2B).
Figure 3.
Color-coded Melting Temperatures (Tms) accordingly to Primers 1 and 2 variants. Tms obtained during the validation assay. (a) blue circle = 1A, brown square = 1B, orange inverted triangle = 1C, green = 1D, pink diamond = 1E. (b) black circle = 2A, blue diamond = 2B. The three divergent results are represented in red: (a) red circle: IOCL 3310, L. naiffi clustered as 1A (expected to be 1E); (b) red circles correspond to the two L. braziliensis and the one L. naiffi erroneously clustered as variant 2A (expected 2B).
Figure 4.
Examples of the variants obtained by Primer 1 for different strains and species, the polymorphisms observed within the HRM analyzed region and the melting temperatures (Tm). The divergent strain IOCL 3310 is depicted in bold, marked*.
Figure 4.
Examples of the variants obtained by Primer 1 for different strains and species, the polymorphisms observed within the HRM analyzed region and the melting temperatures (Tm). The divergent strain IOCL 3310 is depicted in bold, marked*.
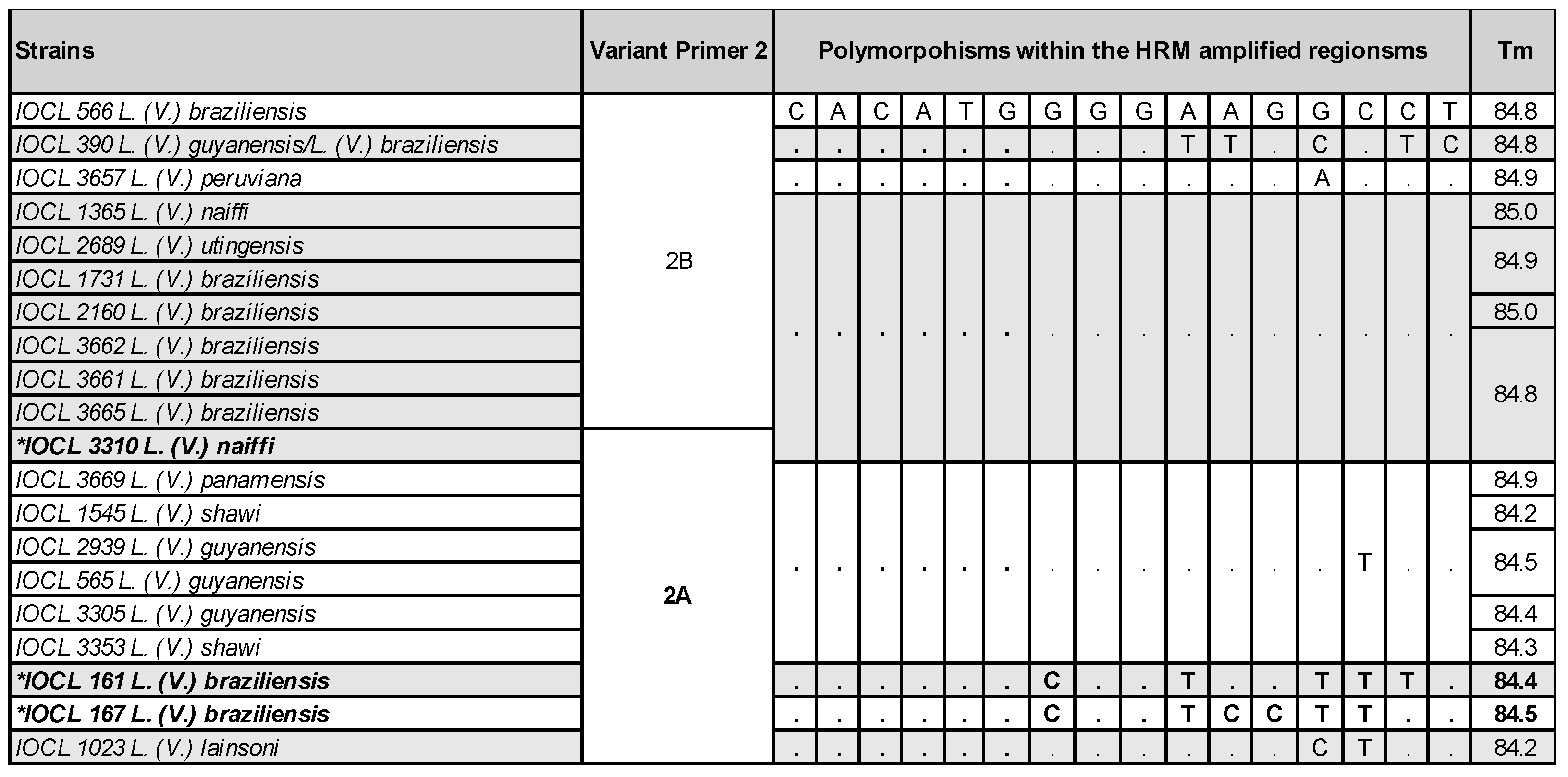
Figure 5.
Examples of the variants obtained by Primer 2 for different strains and species, the polymorphisms observed within the HRM analyzed region and the melting temperatures (Tm). The divergent strains IOCL 161, 167 and 3310 are depicted in bold, marked *.
Figure 5.
Examples of the variants obtained by Primer 2 for different strains and species, the polymorphisms observed within the HRM analyzed region and the melting temperatures (Tm). The divergent strains IOCL 161, 167 and 3310 are depicted in bold, marked *.
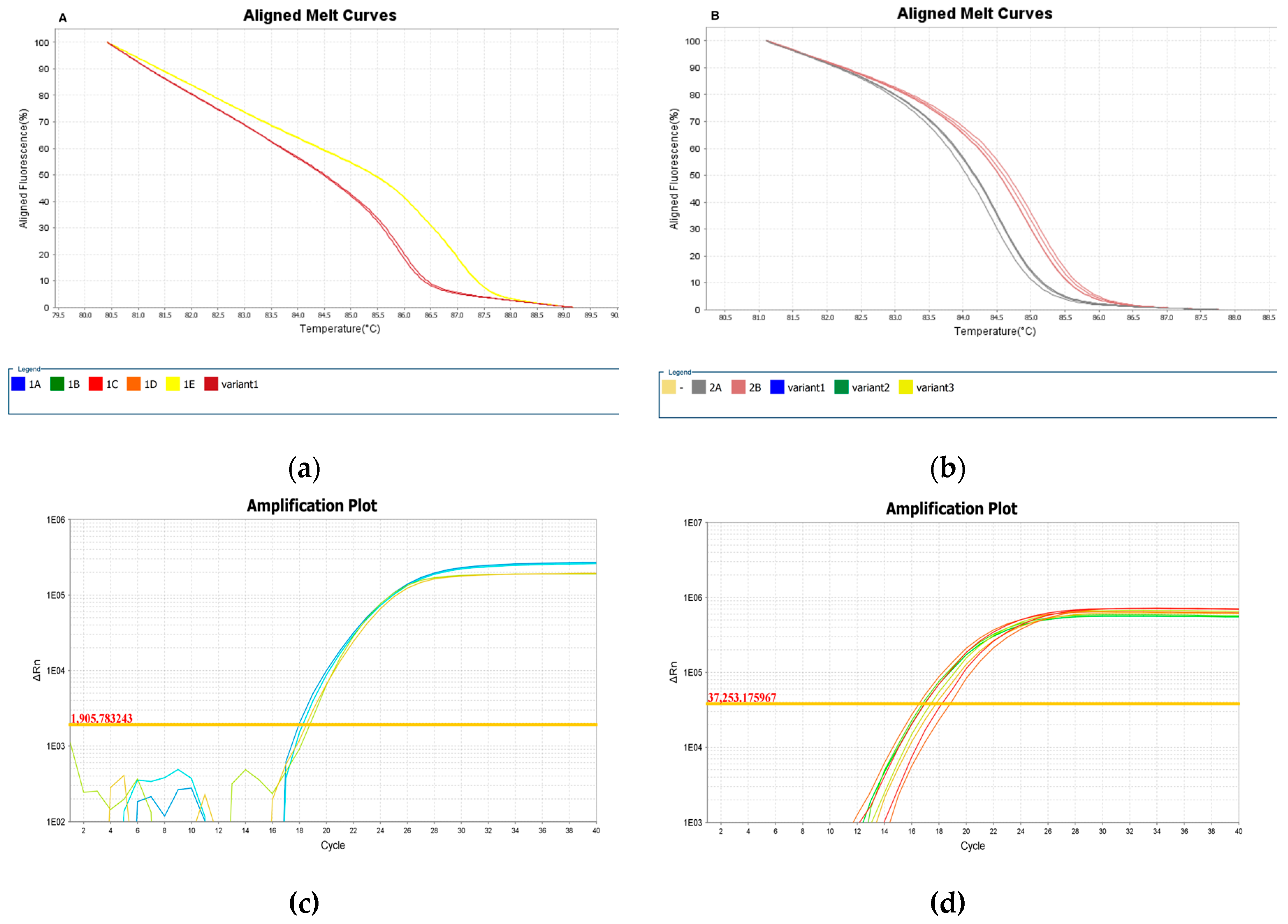
Figure 6.
(a) Aligned melt curve profiles for the divergent strain IOCL 3310 (L. naiffi) (yellow), compared to the reference strain IOCL 1365 (L. naiffi) named by the software as a new variant (brown). (b) Aligned melt curve profiles and amplification plot for the L. braziliensis strains IOCL 161 and IOCL 167 obtained from Primer 2. Melt curve for both strains are represented in grey and were assigned as a different variant from the reference strain in red. (c) and (d) Amplification plot demonstrates similar amplification efficiencies for samples and the reference, for Primer 1 and 2. Figures exported from the High-Resolution Melting Software available at the ViiA 7 Real-Time PCR System (Thermo Fisher Scientific).
Figure 6.
(a) Aligned melt curve profiles for the divergent strain IOCL 3310 (L. naiffi) (yellow), compared to the reference strain IOCL 1365 (L. naiffi) named by the software as a new variant (brown). (b) Aligned melt curve profiles and amplification plot for the L. braziliensis strains IOCL 161 and IOCL 167 obtained from Primer 2. Melt curve for both strains are represented in grey and were assigned as a different variant from the reference strain in red. (c) and (d) Amplification plot demonstrates similar amplification efficiencies for samples and the reference, for Primer 1 and 2. Figures exported from the High-Resolution Melting Software available at the ViiA 7 Real-Time PCR System (Thermo Fisher Scientific).
Figure 7.
Normalized parasite load distribution of the samples assayed plotted accordingly to species typing outcome. Convergent = HRM and RFLP convergent typing results. Divergent = HRM species typing was possible but did not match with previous RFLP characterization. Inconclusive = singular melting profile not correspondent to any of the reference strains. Doted lines mark the interquartile range of the total assayed samples separating quartiles Q1 (bottom) Q2 (in between) and Q3 (upper area).
Figure 7.
Normalized parasite load distribution of the samples assayed plotted accordingly to species typing outcome. Convergent = HRM and RFLP convergent typing results. Divergent = HRM species typing was possible but did not match with previous RFLP characterization. Inconclusive = singular melting profile not correspondent to any of the reference strains. Doted lines mark the interquartile range of the total assayed samples separating quartiles Q1 (bottom) Q2 (in between) and Q3 (upper area).
Table 1.
Reference strains, typing results during experiments 1 and 2 for Primer 1 and Primer 2; median of melting temperatures in °C (Tm) of replicates and standard variation (SD) in °C.
Table 1.
Reference strains, typing results during experiments 1 and 2 for Primer 1 and Primer 2; median of melting temperatures in °C (Tm) of replicates and standard variation (SD) in °C.
| Voucher |
Species |
Primer 1 |
Primer 2 |
Primer 1 |
Primer 2 |
| Exp 1 |
Exp.2 |
Exp 1 |
Exp.2 |
Tm |
SD |
Tm |
SD |
| IOCL 565 |
L. (V.) guyanensis |
1D |
1D |
2A |
2A |
86.1 |
0.2 |
84.1 |
0.0 |
| IOCL 566 |
L. (V.) braziliensis |
1D |
1D |
2B |
2B |
85.8 |
0.1 |
84.7 |
0.1 |
| IOCL 575 |
L. (L.) amazonensis |
1A |
1A |
- |
- |
85.2 |
0.1 |
83.9 |
0.2 |
| IOCL 579 |
L. (L.) infantum |
1A |
1A |
- |
- |
85.0 |
0.1 |
85.1 |
0.0 |
| IOCL 1023 |
L. (V.) lainsoni |
1A |
1A |
2A |
2A |
85.4 |
0.1 |
84.2 |
0.1 |
| IOCL 1365 |
L. (V.) naiffi |
1E |
1E |
2B |
2B |
85.4 |
0.1 |
84.7 |
0.2 |
| IOCL 1545 |
L. (V.) shawi |
1E |
1E |
2A |
2A |
86.7 |
0.1 |
84.0 |
0.1 |
| IOCL 2689 |
L. (V.) utingensis |
1E |
1E |
2B |
2B |
87.0 |
0.1 |
84.8 |
0.2 |
| IOCL 2690 |
L. (V.) lindenbergi |
1D |
1D |
2A |
2A |
85.9 |
0.1 |
84.1 |
0.1 |
| IOCL 3394 |
L. (V.) braziliensis |
1D |
1D |
2B |
2B |
86.1 |
0.1 |
84.6 |
0.0 |
| IOCL 3398 |
L. (V.) lainsoni |
1D |
1D |
2A |
2A |
85.7 |
0.2 |
84.1 |
0.0 |
| IOCL 3399 |
L. (L.) amazonensis |
1A |
1A |
- |
- |
85.2 |
0.3 |
84.6 |
0.0 |
| IOCL 3538 |
L. (V.) guyanensis |
1D |
1D |
2A |
2A |
86.2 |
0.1 |
84.2 |
0.0 |
Table 2.
Samples with hybrid profile by MLEE and respective variants and Tm obtained by HRM.
Table 2.
Samples with hybrid profile by MLEE and respective variants and Tm obtained by HRM.
| V 1 |
V 2 |
Voucher (IOCL) |
MLEE characterization |
DNA sequencing |
HRM |
Tm V1 |
Tm V2 |
|
| 1D |
2A |
3358 |
L. braziliensis/L. guyanensis (hybrid) |
L guyanensis |
L guyanensis |
86.5 |
84.1 |
|
| 1A |
2A |
2490 |
L. naiffi/L. lainsoni (hybrid) |
L lainsoni |
L lainsoni |
85.7 |
84.3 |
|
| 1D |
2B |
390 |
L. guyanensis/L. braziliensis (hybrid) |
L. braziliensis |
L. braziliensis |
86.5 |
84.8 |
|
Table 3.
Sensitivity and specificity values for Leishmania detection by HRM (P1) and by HSP70 qPCR, compared to cPCR and microscopy as gold standards.
Table 3.
Sensitivity and specificity values for Leishmania detection by HRM (P1) and by HSP70 qPCR, compared to cPCR and microscopy as gold standards.
| Assays |
Patient samples (n = 60) |
| HRM + |
HRM - |
Total |
| Microscopy + |
38 (82.6%) |
8 (17.4%) |
46 (100%) |
| Microscopy – |
8 (57.1%) |
6 (42.9%) |
14 (100%) |
| cPCR + |
42 (85.7%) |
7 (14.3%) |
49 (100%) |
| cPCR - |
4 (36.4%) |
7 (63.6%) |
11 (100%) |
| Assays |
HSP70 qPCR + |
HSP70 qPCR - |
Total |
| Microscopy + |
37 (80.4%) |
9 (19.6%) |
46 (100%) |
| Microscopy – |
10 (71.4%) |
4 (28.6%) |
14 (100%) |
| cPCR + |
40 (81.6%) |
9 (18.4%) |
49 (100%) |
| cPCR - |
4 (36.4%) |
7 (63.6%) |
11 (100%) |
Table 4.
Results from HRM HSP70 as a species typing tool in clinical samples.
Table 4.
Results from HRM HSP70 as a species typing tool in clinical samples.
| HRM outcome |
Samples successfully typed by RFLP (n = 45/60) |
| Inconclusive |
5/45 (11.2%) |
| Negative |
6/45 (13.3%) |
| Species typing achieved |
34/45 (75.5%) |
| |
|
| HRM outcome |
Samples with HRM species typing data (n = 34) |
| Divergent typing result |
8/34 (23.5%) |
| Convergent typing result |
26/34 (76.5%) |
| Total |
45 (100%) |
Table 5.
Primers used for HRM reactions. Amplicon sequences and references.
Table 5.
Primers used for HRM reactions. Amplicon sequences and references.
| ID |
Sequence |
Fragment |
Reference |
| P1 |
HSP70 F2 5’-GGAGAACTACGCGTACTCGATGAAG-3’HSP70C R 5’- TCCTTCGACGCCTCCTGGTTG-3’ |
144 pb |
Zampieri et al. 2016/ Graça et al. 2012 |
| P2 |
HSP70 F1 5’-AGCGCATGGTGAACGATGCGTC-3’HSP70 R1 5’-CTTCATCGAGTACGCGTAGTTCTCC-3’ |
104 pb |
Zampieri et al. 2016 |