1. Introduction
Phosphorus (P), together with nitrogen (N) and potassium (K), form part of the primary plant nutrient group. Phosphorous is a primary component of the systems responsible for enabling, storing and transferring energy and is a basic component of the macromolecular structures of interest, such as nucleic acids and phospholipids, thus it can be said that its role is generalized in all physiological processes. In other words, P cannot be substituted for any other nutrient (Fernández, 2007). The amount of P in the soil solution tends to be around 0.05 ppm, a concentration that is much lower than those absorbed by the active surfaces of the soil: from 102 a 103 . This means that plants that develop in soil only have access to a small amount of P. In contrast to other elements, the amount of available P in the soil is insufficient for plants, a deficiency that can only be regulated with the application of fertilizers that become less soluble compounds.
The root provides the plant with physical support, as well as nutrients and water from the soil. Both the development and the growth of the root depend on two factors: environmental stimuli and genetic factors (López-Bucio et al., 2003). The roots tend to develop in the direction of water and nutrients. Moreover, there are factors that can affect the growth and the development of the root system (water, temperature, soil compaction, availability of mineral substances, etc.) (Fitter, 2002).
The roots principally absorb P through the primary orthophosphate ion in the compound (H2PO4-) or through the secondary orthophosphate compound (HPO=4) (Tisdale et al., 1985). The plants´ response is specific for the deficiency of this nutrient. Some of the characteristics that roots develop for the consumption of P are: a) a rapid rate of root elongation and a high root biomass (Hill et al., 2006); b) increase in the ramification of the root and change in their angles, particularly in surface soils and regions rich in nutrients (Lynch y Brown, 2001; Rubio et al., 2003); and c) Root proteoids o root clusters in Proteaceae and in some members of the Fabaceae family such as Lupinus spp. (Dinkelaker et al., 1995; Gadner et al., 1983; Lambers et al., 2006).
The Lupinus species has the ability to improve soil fertility since it has its own source of nitrogen, present in its root clusters or proteoids. It´s been shown that Lupinus albus has the ability to mobilize and solubilize P sources that are normally unexploited for other plants (Dinkelaker et al., 1989; Hinsinger y Gilkes, 1995). The roots also have the ability to absorb metals such as Cadmium, Lead, Zinc, Mercury and Chrome, thus having phyto-remediating characteristics (De la Cruz-Landero et al., 2010).
The Lupus genus contains more than 600 described species, but only about 300 are recognized (Eastwood et al., 2008): 13 are in the old world-- originating in the Mediterranean and Northern Africa, 10 are wild and three (Lupinus albus, angustifolius and luteus) are domesticated. Mexican figures indicate that close to 110 species grow as low as sea level (state of Baja California) and as high as 4,000 meters above sea level (state of Chiapas). Some of the most abundant species are L .montanus, L. campestris, L. elegans, L. hartwegii, L. mexicanus, L. polyphyllus, L.splendens, L. silvestris y L. stipulatus (Bermúdez et al., 1999).
It is extremely important to find alternatives to help us reduce the consumption of fertilizers, as well as strategies to produce environmentally sustainable foods. The practices of intercropping is an agricultural production strategy that is principally based on the growth of two or more species on a unit of land where a total or partial combination of cycles occurs (Navas y Marín, 1995). In this vein, the maize-leguminous combinations generally exceed the performance of maize monocultures; in other words, maize monocultures require greater surface area to produce the same yield as one hectare of policulture (Mead y Willey, 1980; Vandermeer, 1989; Liebman, 1997).
Among research conducted on Lupinus-maize intercropping, little to none concentrates on the root system, which is one of the most important organs. One of the biggest problems encountered when studying plant roots is that the majority are destructive, which greatly limits the understanding of their physiology, ecology or architectural structure. Moreover, the use of plastic bags or planters for their study could potentially confound the results of any research, as they confine or limit root growth.
This research used rhizotrones, or observation chambers, to study the architecture of the roots as well as how the roots behave under different environmental conditions. Particularly in this case, rhizotrons were used to study the behavior of the root in two intercropped species (Lupinus montanus and Maize) and analyze the possibility of nutritional benefits.
2. Materials and Methods
Research was carried out at the Postgraduate College-Montecillos Campus (Colegio de Postgraduados- Campus Montecillos) in the growth chambers located in the Botany building.
2.1. Plant Material
The first stage began with the collection of Lupinus seeds (Lupinus montanus as identified in the herbarium at the Postgraduate College) the in the State of Mexico at the following coordinates: 19° 10’13.31” N, 98° 43‘ 3.73” W; 2497 msnm. The maize seeds, which were provided by the Postgraduate College´s Seed Program, are certified, trilinear hybrids, with a physiological maturity close to 150 days.
2.2. Soil
River sand was washed 15 times and sterilized during four hours to eliminate any type of nutrient in the soil. The final product was composed of 20 different samples.
Sources of Phosphorous
Dibasic and Tribasic Calcium Phosphate.
2.3. Rhizotron Design
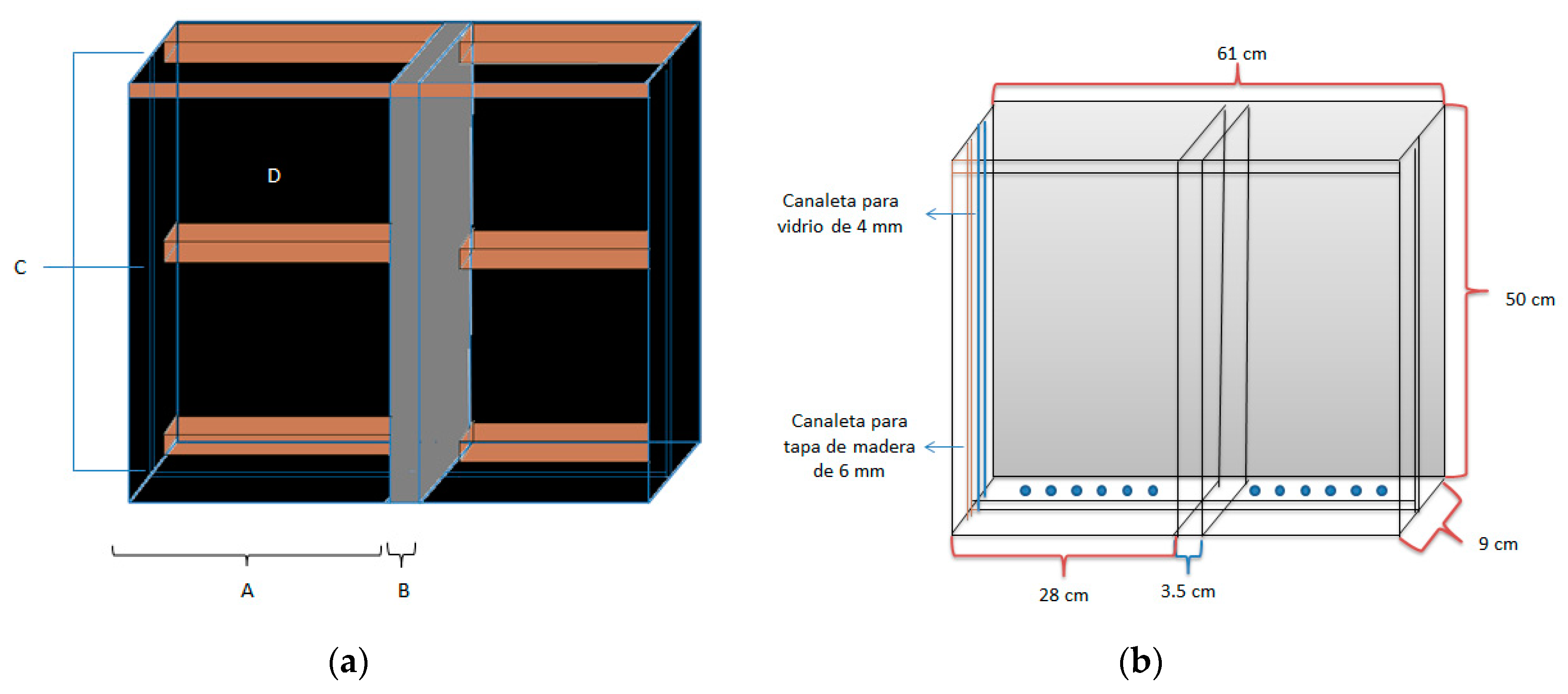
The rhizotron was constructed with wood and sliding glass that permitted the observation of the root system. As shown in
Figure 1, it consists of two compartments, each one measuring 28 x 50 cm in width (A), divided by a wooden panel 3.5cm in thickness (central divider) (B). A 3mm wooden panel (D) and three wooden strips each 2 x 5cm (C) were used as support. The lateral walls that measure 9 x 50 cm with a thickness of 7mm (D), the base and the central divider all have two tracks: a 3mm glass fits in one track and a 5mm wooden cover in the other to prevent the passage of light. The 9 x 61cm base (7mm in width) has 7mm perforations placed every 4cm for drainage (E). The system was then covered with a slab of polystyrene in order to maintain a sterile environment. To ensure that the system had no leaks, it was waterproofed with paraffin. After all of these adaptations, the rhizotron volume measured 1.2 x 10
-3 m
3.
2.4. Isolation of atmospheric nitrogen-fixing symbiotic bacteria
At the field level, Lupinus has the ability to join to bacteria from the genus Bradyrhizobium and Mesorhizobium. As such, the decision was made to isolate the bacteria from its natural environment and inoculate the Lupinus plants in the experiment. To this effect, Lupinus plants were collected from the area of San Pablo Ixayoc, Texcoco, State of Mexico that has an altitude of 2932 msnm: coordinates 19°26‘ 59.2” N, 98° 46‘ 33.4”. The plants were extracted with the root intact and then transported to the laboratory of Soil Microbiology. Five strains were selected, as they were effective at producing nodules. The inoculum was prepared and the quality was determined. The bacterial load was 1.8 x 109; the standard concentration required in the inoculants is 109 viable rhizobia cells per g o mL of support at the date of elaboration (Benintenden, 2010); therefore, the inoculant that was prepared in the laboratory satisfied the bacterial load to infect the Lupinus plants.
2.5. Identification of nodular bacteria
The extraction of DNA was carried out by the technique described by Doyle and Doyle (1990): The 16S rRNA gene was enhanced with the 8F y 1492R indicators. The sequencing reaction was carried out with the 514F y 1492R indicators.
2.6. Construction of the phylogenetic tree
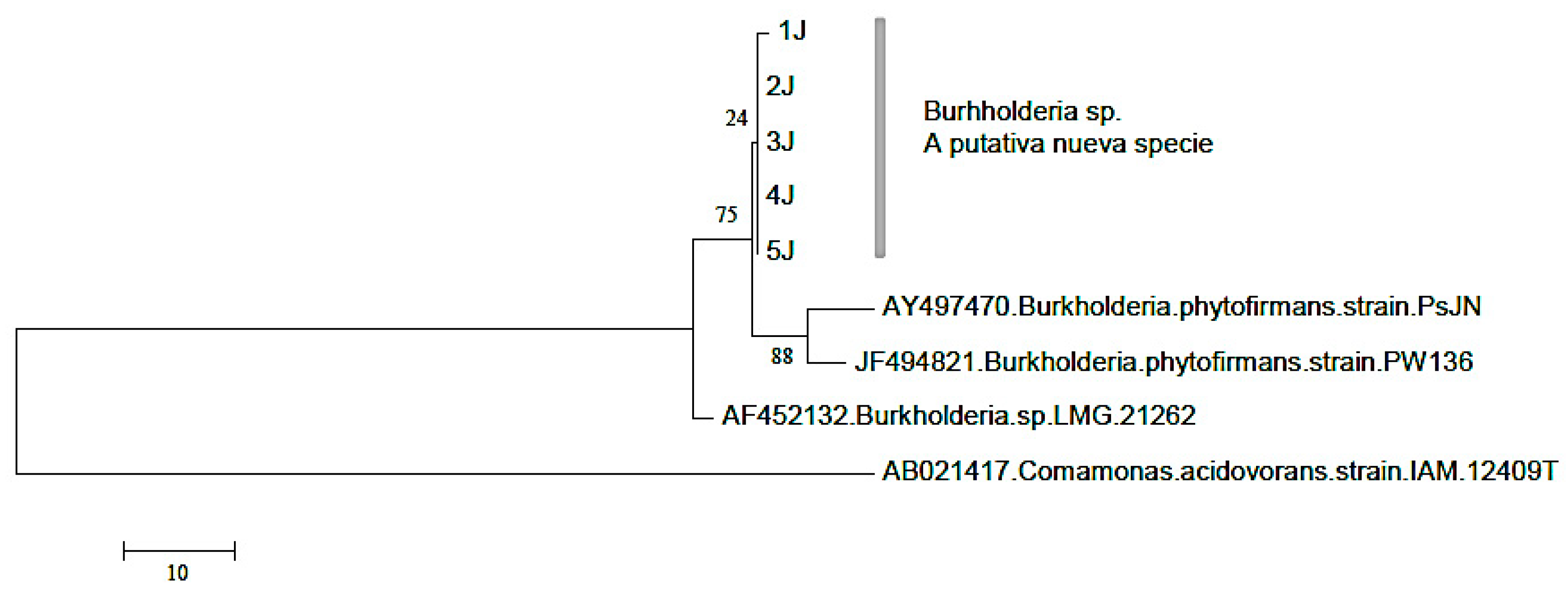
To obtain the consensus sequences, both strains were assembled with the software Bioedit, which were compared with the BLASTNucleotide option from the GenBank database from the National Center for Biotechnology Information (NCBI). The consensus sequences were aligned with the program CLUSTALW (Thompson et al., 1994), including the Mega 5 (Tamura et al., 2011). The Maxima Parsimonia method was used for the construction of the phylogenetic tree. In order to determine the reliability of the nodules, they were analyzed using the Bootstrap method with 1,000 repetitions (Felsestein, 1985).
Figure 2.
Phylogenetic tree constructed using the Maxima Parsimonia method. The obtained sequences were compared with the baseline sequences from the Genbank. .
Figure 2.
Phylogenetic tree constructed using the Maxima Parsimonia method. The obtained sequences were compared with the baseline sequences from the Genbank. .
2.7. Development of the experiment
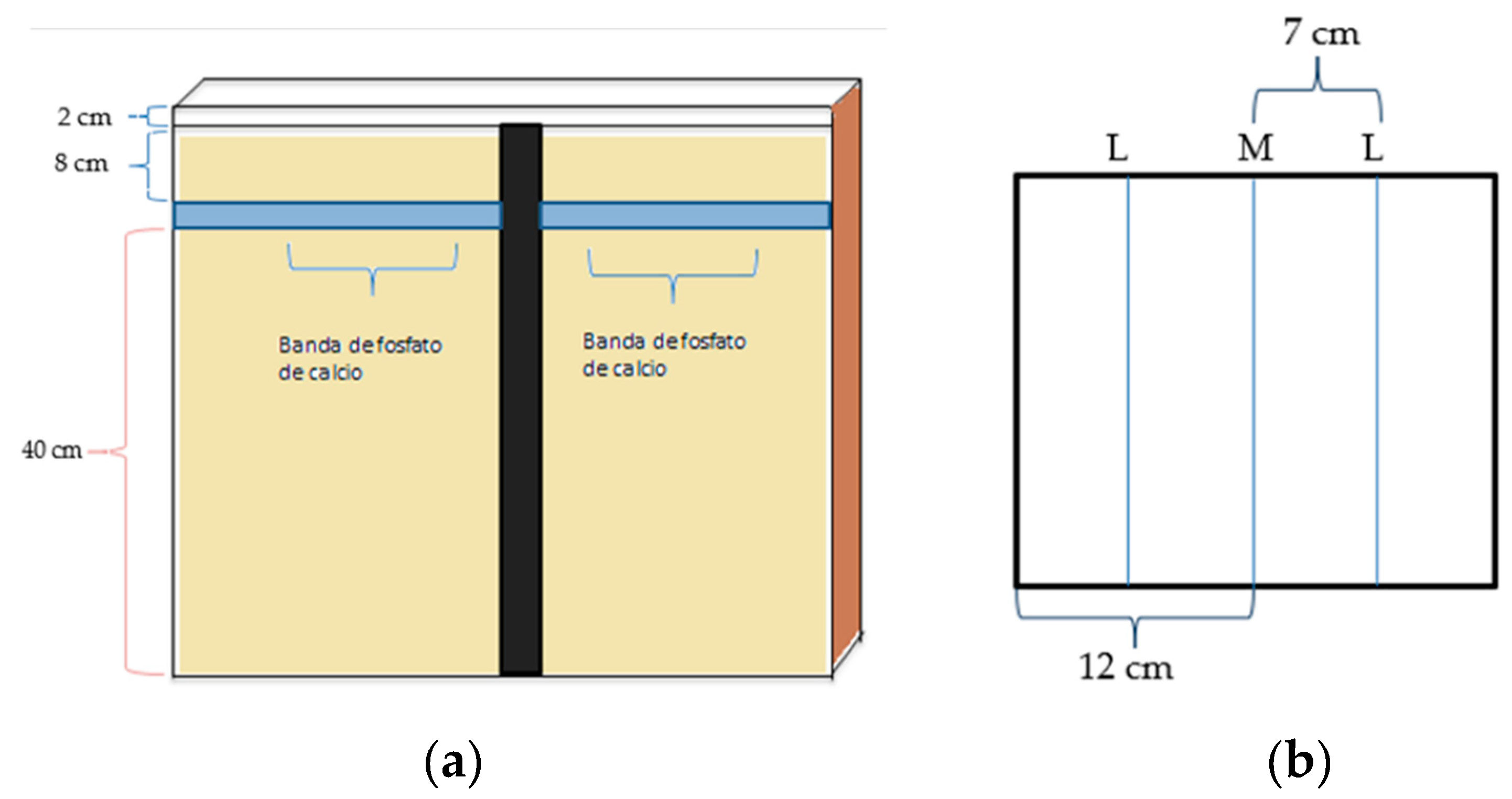
The rhizotrons were filled with river sand to a height of 40cm, at which point, calcium phosphate strips (1gr) were placed. These were then covered with sand to reach a total height of 48cm. The remaining 2cm were reserved for carrying out irrigation, as seen in
Figure 3ª.
Two
Lupinus seeds were placed for every maize; the spatial arrangement of the system is similar to that found in the field where species are interspersed. Maize was placed in the center of the compartment at 12cm from the lateral side and
Lupinus 7cm away from the maize (
Figure 3b), at a depth of 1 cm. Due to the dormancy problems faced by the legume, three seeds were placed at each point (mechanically scarified), and after germination, one was chosen. At 10 days, 1mL of symbiotic bacteria was inoculated. Because
Lupinus has a longer growing cycle, it was planted first; the maize seeds followed and were planted after 30 days. Two sources of calcium phosphate were used: Dibasic and Tribasic. Three fertilizations were carried out with Steiner modified solutions: a) (g L-1) 0.4929 Mg SO
4• 7 H
2O, 0.172 Ca SO
4• 2 H
2O, 0.609 K
2 SO4 y b) (g L-1) 0.944 Ca (NO
3)
2 •
4 H
2O, 0.404 KNO
3, 0.261 K
2 SO
4, 0.492 Mg S0
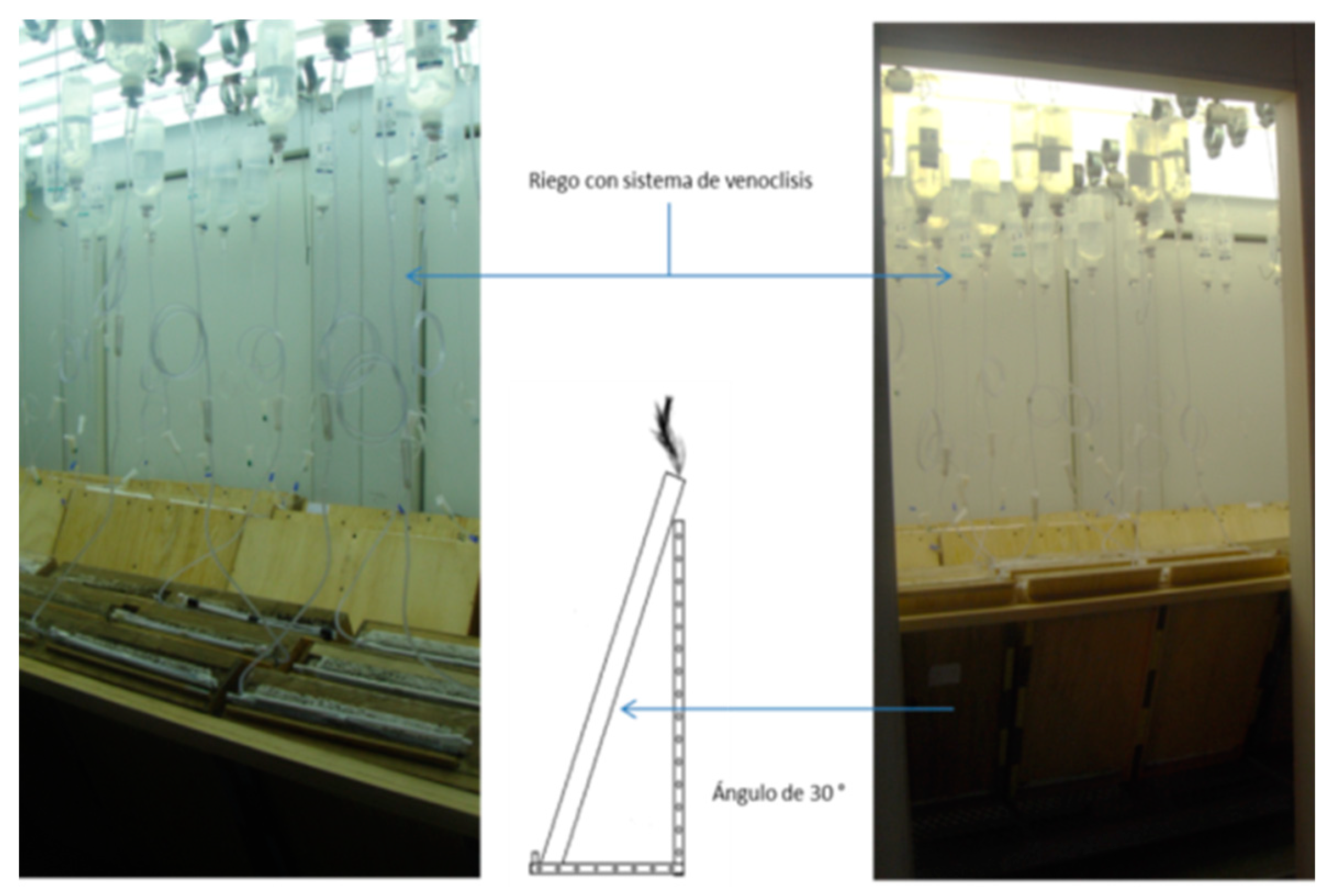
4. In the inoculated treatments, a nitrogen-free solution was applied, while the non-inoculated treatments received a nitrogen solution. During the course of the experiment, three fertilizations were carried out. The substrate used is very porous with a high ability to filtrate, and as such, a trickle irrigation system (modified IV system for medical use) was implemented. To clearly view the roots, the rhizotrons were placed at a 30 degree angle (
Figure 3), but after the first month, it was noted that this was not sufficient and the rhizotron was subsequently placed at a 45 degree angle. The research employed a completely random block design with four repetitions with two sources of P, two nutritional solutions and two cultivation systems. Growing chambers were kept at 26 °C with light for 12 hours and at 16 °C in darkness. The light emitted by the growth chamber was calculated at 500 µ E s
-1 m
-2.
In order to manage the growth of the Lupinus roots, acetates that were joined to the crystals of the rhizotron were used. Permanent markers of different colors were used for each measurement. In the case of the maize, the growth was traced over the glass, which allowed us to keep exact track of both species.
The experiment lasted two months, and the maize was only cultivated during the last month. When it was time to harvest, the plants were extracted and the aerial biomass and root biomass were weighed separately. In the inoculated treatments, the number total nodules in the Lupinus plants were calculated. The material was washed and dried in a stove at a constant temperature of 70°C and was then weighed again. In order to determine the nutritional effect on maize plant tissue, the concentration of P was analyzed through the Photochlorimetry method by reduction with molibvanadato (Bremmer, 1965) and N with the Semimicro-kjeldahl technique (Bremmer, 1965). The SAS 9.0 program was used for the statistical analysis and an ANOVA and Tukey Test were performed.
3. Results
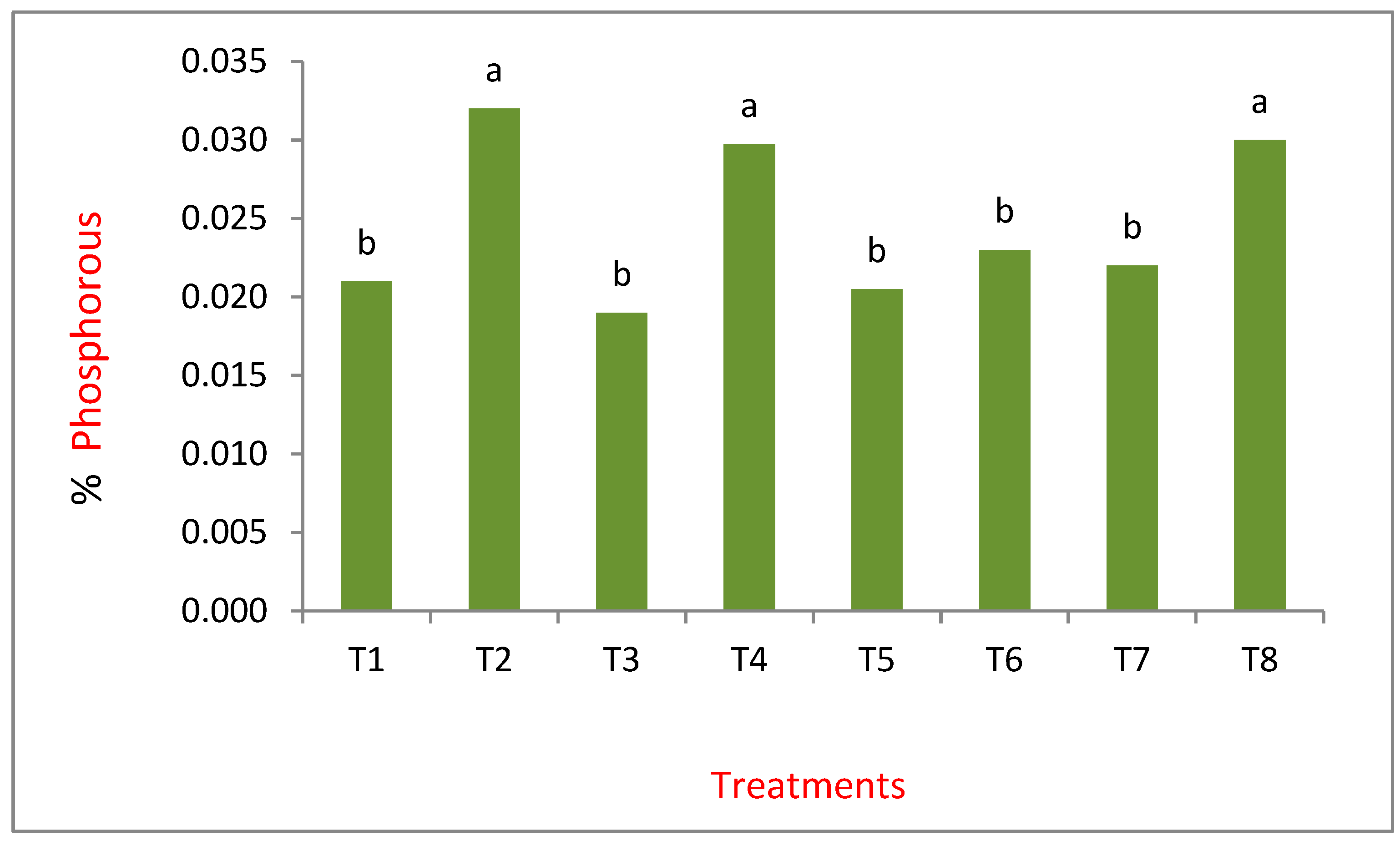
Results from the variance analysis showed that there were significant statistical differences (p<0.05), in the % P in plant tissue, which was greater in intercropping than in monoculture. Treatment 2 (T2), 4 (T4) and 8 (T8) refer to the intercropping of maize and
Lupinus, as they had the highest P values, indicating that
Lupinus releases the nutrients that it finds trapped in the calcium and makes it accessible for maize (
Figure 4). The best concentrations of P in maize were found in T2 (0.032). In this treatment, a N laced nutrient solution was added, which coincides with a study carried out by Sas (2002), which mentioned that good nutrition of N and low levels of P generate a root system that can be fundamental in the solubilization of P. The treatment T4 is relevant because this was where
Lupinus was inoculated with the bacteria; it was the second best treatment (0.030). As already mentioned above,
Burkholderia is a bacteria capable of creating a symbiosis with certain leguminous plants and in this study, we observed that the bacteria was capable of nodulating
Lupinus. It has been reported that
B. cepacia has the ability to adequately solubilize calcium phosphate, iron and aluminum (Mora y Toro, 2007).
Burkholderia bacteria have been identified in
Lupinus albus, and their presence increased senescent roots, more so in young roots (Weisskoppf
et al., 2011).
According to Jones (1998) the percentages obtained in maize tissue are less than 0.15%, a criterion that is already considered deficient. None of the treatments approach this value. It is important to remember that the plants were under P and N stress conditions, as well as being in a sandy soil (
Table 1, chemical characteristics can be observed). Tribasic calcium phosphate was the best assimilated compound by the maize plants (T2, T4). This is important because the compound has a high level of insolubility, indicating that legume has the ability to mobilize P that is trapped in the calcium, making it available for maize; this is different to what we find in monoculture circumstances.
Some studies concluded that intercropping wheat with Lupinus albus improves the concentrations of P in the plant´s biomass (Gadner y Boundy, 1983; Marschner et al., 1987; Akthar, 2004; Rodas et al., 2001).
In a study with Lupinus albus, Gardner et al. (1983) mention that the probable mechanism by which phosphorous moves in the soil/root interface is because of the excretion of citrate ions from the roots of this species. While Dinkelaker et al. (1989) were studying the excretion of citric acid and the precipitation of calcium in the Lupinus albus rhizosphere in a limestone soil, they observed that proteoid roots, which are capable of lowering pH, developed in the presence of a P deficiency. Through x-ray spectroscopy, they observed abundant white precipitates of calcium citrate. Citrate in general and acid citrate were highly effective in dissolving Tricalcium phosphate, as well as Iron and Aluminum phosphate. When Gerke et al. (1994), studied the influence of carboxylates on the movement of P, Al and Fe, they found citrate and malate in the Lupinus rhizosphere. They also found that the movement of P, Al and Fe was attributed to linked exchanges of P by citrate and the solubilization of Al and Fe as caboxylated compounds. Ozawa et al. (1995) found that L. albus plants in nutrient solutions deficient in phosphorous increased the activity of acid phosphatase.
The majority of intercropping studies use soils of natural origin, but we cannot lose sight of the fact that soils in these conditions are composed of a large amount of bacteria and that many of these bacteria have the ability to solubilize and mobilize P. Bacteria that have this capacity are: Pseudomonas, Rhizobium, Burkholderia, Achromobacter, Agrobacterium, Aereobacter, Flavobacterium, Yarowia, Streptosporangium y Erwinia (Paredes y Espinosa, 2009). The importance of the current study lies in the fact that the variable of interest was isolated in such a way that there is no possibility to attribute the solubilization of P to bacteria.
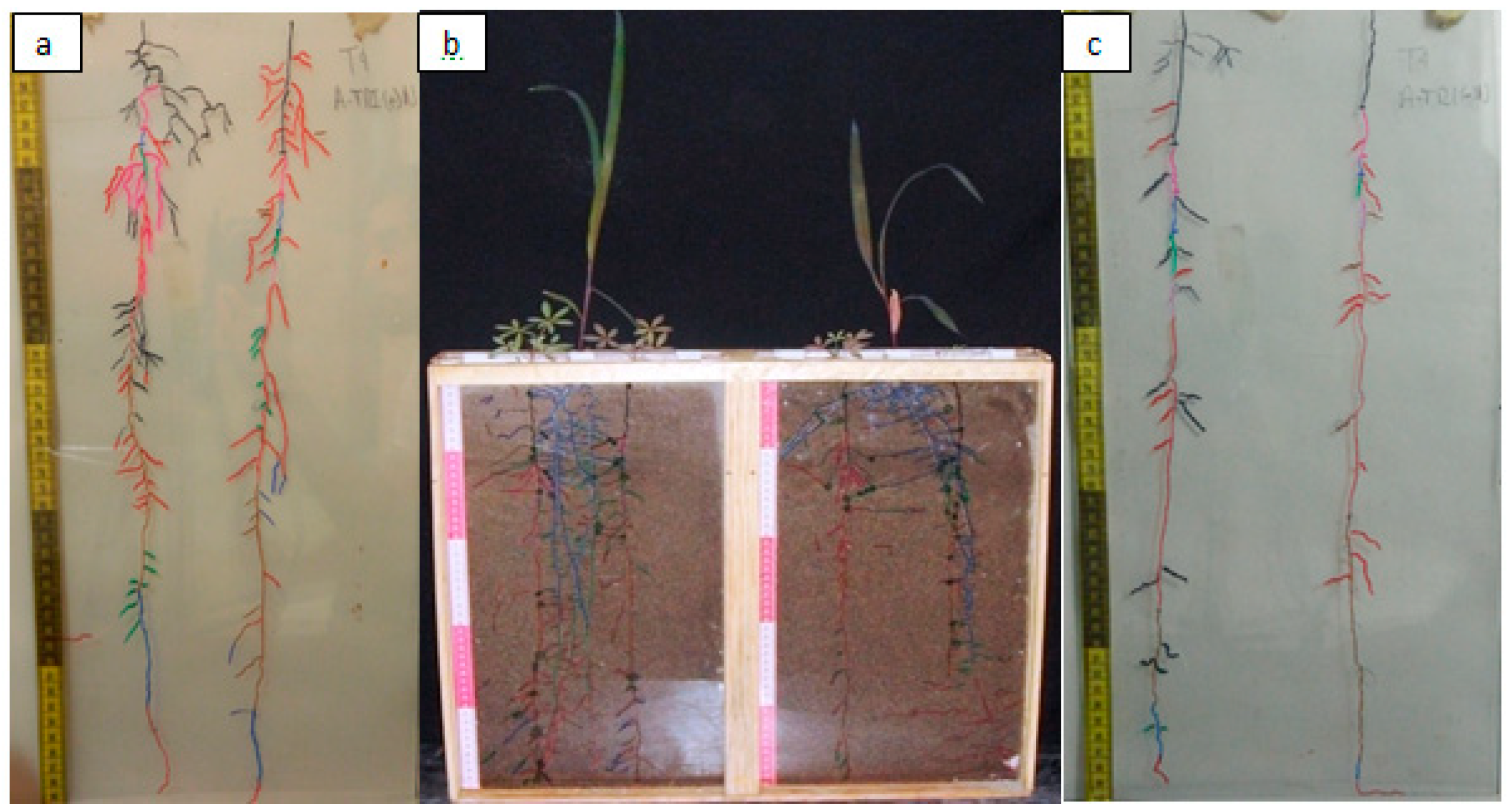
Table 5 shows the interaction of the
Lupinus roots with maize;
Figure 5b shows the
Lupinus roots that correspond to T2;
Figure 5c shows the behavior of the roots subjected to treatment T4. After analyzing the root architecture, we can observe that there are no proteoid structures as observed in
L. albus. The
Lupinus montanus root system is found in all intercropping treatments (Anexo 3). Related to this, there is clear evidence that
Lupinus angustifolius (Egle
et al., 2003) y
Lupinus mutabilis (Pearse
et al., 2006) do not produce specialized “clusters”, but they do release large quantities of carboxylates. The root structure of this species is very similar to that of a bean. .
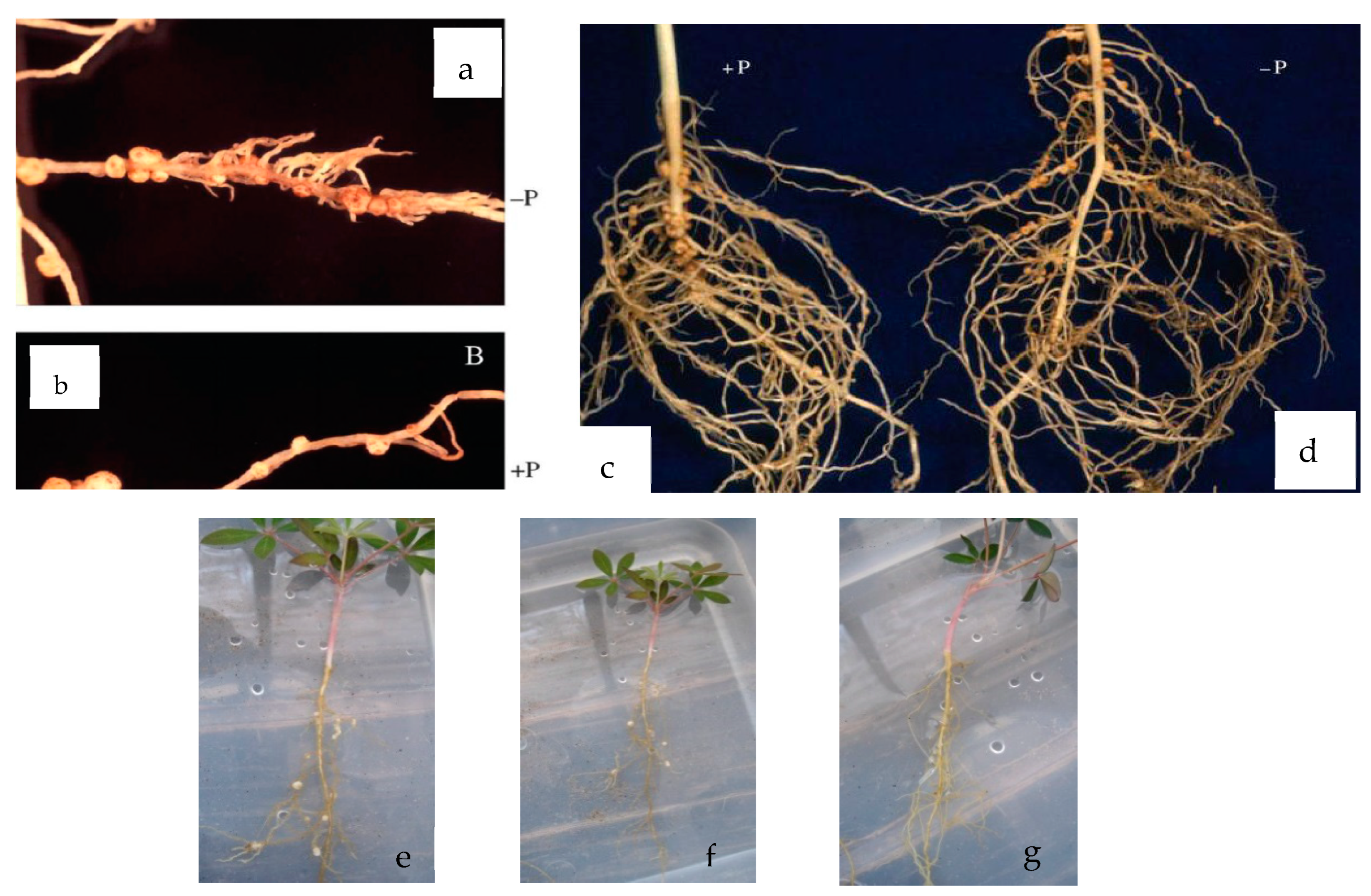
Nitrogen in plant tissue and the maize roots in sandy soil
There are no significant differences between treatments. Similar behavior is presented in the % of N in the root. The objective of inoculating and adding a nutrient solution laced with N was to observe if there were any morphological changes in the root system of the
Lupinus. While this would be reflected in the movement of P, but no such structures were present (Figura 6e, f y g). The nutrition of nitrogen can be a determining factor in the formation of proteoid roots in some wild species. Sas (2002) carried out research and examined the effect of the nutrition of N in its different forms: ammonium, nitrate and nitrate fixation under P deficiencies. The number of proteoid roots increased considerably when P was administered at 1μM, when compared to 50μM. Furthermore, under phosphate deficiency, NH
4+ is the best source of nitrogen, resulting in a high number and biomass of proteoid roots. Le-Roux
et al. (2007) mention that when confronted with P deficiencies, malate dehydrogenase is produced. Malate dehydrogenase participates in the citric acid cycle and when faced with the deficiency, the synthesis of malate can improve the formation of nodules. However, an excess of this can prevent nitrogen fixation. Schulze
et al. (2006) evaluated the formation of nodules in
Lupinus proteoid roots with low P concentrations. They observed that at day 21 (
Figure 6a, b, c and d), the number of nodules was greater in the treatment without P, but at 37 days, the nodules increased with the treatment (+) P. With this finding, a new research field has been opened, for all of the reported work already done has been inoculated with
Bradyrhizobium, whereas in this research,
Burkholderia was used. In Figure 31, the three treatments with the highest percentages of P can be observed; two of them were inoculated with the
Burkholderia.bacteria.
4. Conclusion
After analyzing the information in the current research, we can conclude the following:
1. There is an opportunity for a new field of investigation in the nodulation and nitrogen fixation of Lupinus since this research could nodulate with Burkholderia bacteria.
2. The use of Lupinus sp. is confirmed as an alternative in favor of more sustainable agricultural methods since it improves soil fertility in phosphorous deficient soils. This could potentially contribute to the wealth of knowledge used to solve Mexico´s problem of food autonomy.
3. As was observed during the experimental phase, the type and use of rhizotrons that were designed for the current study are only recommended for the evaluation of root systems in leguminous plants since crops with root systems like maize are more complicated and unreliable.
Significance Statements
Through the use of calcium phosphate , this study highlighted an enhanced phosphorus turnover in the intercrooping maize and lupins. The capability of some plant species to movilize phosphorus (P) from poorly available soil P fractions can improve P availability for P-inefficient plant species in intercrooping. These findings might help researchers and farmers to propose and apply agriculture, because the P-movilization-based facilitation by lupins to enhance P- acquisition of cooccurring plant species is determined by both available P concentration and P-sorption capacity of soil, and the root intermingling capacity among two plant partners enabling rhizosphere overlapping.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, X.X. and Y.Y.; methodology, X.X.; software, X.X.; validation, X.X., Y.Y. and Z.Z.; formal analysis, X.X.; investigation, X.X.; resources, X.X.; data curation, X.X.; writing—original draft preparation, X.X.; writing—review and editing, X.X.; visualization, X.X.; supervision, X.X.; project administration, X.X.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
Please add: “This research received no external funding” or “This research was funded by NAME OF FUNDER, grant number XXX” and “The APC was funded by XXX”. Check carefully that the details given are accurate and use the standard spelling of funding agency names at
https://search.crossref.org/funding. Any errors may affect your future funding.
Institutional Review Board Statement
In this section, you should add the Institutional Review Board Statement and approval number, if relevant to your study. You might choose to exclude this statement if the study did not require ethical approval. Please note that the Editorial Office might ask you for further information. Please add “The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of NAME OF INSTITUTE (protocol code XXX and date of approval).” for studies involving humans. OR “The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of NAME OF INSTITUTE (protocol code XXX and date of approval).” for studies involving animals. OR “Ethical review and approval were waived for this study due to REASON (please provide a detailed justification).” OR “Not applicable” for studies not involving humans or animals.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
The authors thank to Carlos Trejo, Josue Kohashi , and Miguel for their assistence at Botany Department Postgraduate College. The authors are grateful for the cooperation of Jenny Zehemer for the comments on the last version of this manuscript. This research was supported by National Council of Science and Technology (CONACYT).
Conflicts of Interest
Declare conflicts of interest or state “The authors declare no conflict of interest.” Authors must identify and declare any personal circumstances or interest that may be perceived as inappropriately influencing the representation or interpretation of reported research results. Any role of the funders in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results must be declared in this section. If there is no role, please state “The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Akhtar, M., V. Espinosa Hernández, R. Nuñez Escobar, A. Baeza. A. Mujeeb Qazi. 2004. Asociación de Lupinus silvestris- Trigo y disponibilidad de fósforo en calcisoles. Tesis de Doctorado. Colegio de Postgraduados, Montecillo, México.
- Bermúdez-Torres, K., N. Robledo-Quintos, J. Martínez-Herrera, A. Tei and M. Wink. 1999. Biodiversity of genus Lupinus in Mexico. Lupin, An Ancient Crop for the New Millenium. Proceedings of the 9th International Lupin Conference. 294-296.
- Bremer, J. M. 1965. Total nitrogen. In: C. A. Black (ed). Methods of soil analysis. Part. 2. American Society of agronomy. Madison, Wisconsin. U.S.A. Agronomy. 9: 1149-1178.
- De la Cruz-Lande, N.; Hernandez, V.; Guevara, E.; Lopez-Lope, M.; Santos, A.; Ojeda-Trej, E.; Alderete-C, A. Lupinus versicolor Response in Soils Contaminated with Heavy Metals From a Petroleum Extraction Field. J. Appl. Sci. 2010, 10, 694–698. [Google Scholar] [CrossRef]
- Dinkelaker, B.; Hengeler, C.; Marschner, H. Distribution and Function of Proteoid Roots and other Root Clusters. Bot. Acta 1995, 108, 183–200. [Google Scholar] [CrossRef]
- Dinkelaker, B.; Romheld, V.; Marschner, H. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant, Cell Environ. 1989, 12, 285–292. [Google Scholar] [CrossRef]
- Eastwood, R.J., C. S. Drummond, M.T. Schifino-Wittmann and C.E. Hughes. 2008. Diversity and evolutionary history of lupins-insights new phylogenies. “Lupins for Health and Wealth”. Proceedings of the 12th International Lupin Conference. 346-354.
- Egle, K. Römer and H. Keller. 2003. Exudation of low molecular weight organic acids by Lupinus albus L., Lupinus angustifolius L. and Lupinus luteus L. as affected by phosphorus supply. Agronomie 23: 511-518. [CrossRef]
- Fernández. M.T. 2007. Fósforo: amigo o enemigo. ICIDCA. Sobre los derivados de la caña de azúcar, vol. 41: 51-57.
- Fitter, A. 2002. Characterisitics and funtions of root systems. In: Waisel, Y., A. Eshel, U. Kafkafi. Plant Roots: The Hidden Half. 3rd Edition. New York, Marcel Dekker. pp. 2-20.
- Gardner, W. K. and A. Boundy. 1983. The acquisition of phosphorus by Lupinus albus L. IV. The effect of interplanting wheat and White lupin on the growth and mineral composition of the two especies. Plant and soil: 70: 391-402.
- Gardner, W. K. and A. Boundy. 1983. The acquisition of phosphorus by Lupinus albus L. IV. The effect of interplanting wheat and White lupin on the growth and mineral composition of the two especies. Plant and soil: 70: 391-402.
- Gerke, J. Romer, A. Jungk. 1994. The excretion of citric and malic acid by proteoid roots of Lupinus albus effect on soil solution concentration of phosphate, Fe and Al in the proteoid rhizhosphere in samples of an oxisol and luvisol. Z Pflanzen Bodenk 157: 289-294. [CrossRef]
- Hill, J.O.; Simpson, R.J.; Moore, A.D.; Chapman, D.F. Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 2006, 286, 7–19. [Google Scholar] [CrossRef]
- Hinsinger, P. and R. J. Gilkes. 1995. Root –induced disolution of phosphate rock in the rizhosphere of lupins grown in alkaline soil. Australian Journal of Soil Research 33: 477-489. [CrossRef]
- Jones, J. B. 1998. Interpretation of plan analysis for several agronomic crops. In soil tetsing and plant analysis, Part II, Plant analysis. p 49-58.
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root Structure and Functioning for Efficient Acquisition of Phosphorus: Matching Morphological and Physiological Traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Liebman, M. 1997. Sistemas de policultivos. Pp. 133-141. En: Agroecología: Bases científicas para una agricultura sustentable. M. A. Altieri (ed). CLADES-Grupo Gestor Asociación Cubana de Agricultura Orgánica, ACAO, La Habana, Cuba.
- López-Bucio, J.; Cruz-Ramı́rez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Brown, K.M. Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Marschner, H., V. Romheld, I. Kakmak. 1987. Root induced changes in nutrient availablity in the rizhosphere. J. Plant Nutr. 10: 1175-1184.
- Mead, R.; Willey, R.W. The Concept of a ‘Land Equivalent Ratio’ and Advantages in Yields from Intercropping. Exp. Agric. 1980, 16, 217–228. [Google Scholar] [CrossRef]
- Mora, E. y M. Toro. 2007. Estimulación del crecimiento vegetal por Burkholderia cepacia, una cepa nativa de suelos ácidos de sabanas venezolanas. Agronomía Trop. 57(2): 123-128.
- Navas, P. B. y D. Marín. 1995. Comportamiento ecofisiológico de la asociación canavalia-maíz con y sin aplicación de nitrógeno y con diferentes arreglos cronológicos. Agronomía Trop. 45(4):609-635.
- Paredes-Mendoza, M., D. Espinosa-Victoria. 2009. Ácidos orgánicos producidos por rizobacterias que solubilizan fosfato: Revisión Crítica. Terra Latinoamericana 28: 61-70.
- Pearse, S. J. J. Veneklaas, G. Cawthray, M. D. A. Bolland, and H. Lambers. 2007. Carboxylate composition of root exudates does not relate consistently to a crop species’ ability to use phosphorus from aluminium, iron or calcium phosphate sources. new phytologist 173:181-190.
- Rodas, C. A., R. Núñez Escobar, V. Espinosa Hernández. y G. Alcántar González. 2001. Asociación Lupinus-maiz en la nutrición fosfatada en un andosol. Terra. 19: 141-154.
- Rubio, G., H. Liao, X. L. Yan, J. Lynch. 2003. Topsoil foraging and its role in plant competitiveness for phosphorus in common bean. Crop Sci 43:598–607.
- Sas, L. Rengel and C. Tang. 2002. The effect of nitrogen nutrition on cluster root formation andproton extrusion by Lupinus albus. Annals of Botanic 89: 435- 442. [CrossRef]
- Schulze, J.; Temple, G.; Temple, S.J.; Beschow, H.; Vance, C.P. Nitrogen Fixation by White Lupin under Phosphorus Deficiency. Ann. Bot. 2006, 98, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, S.L., W. L. Nelson, and J.D. Beaton. 1985. Soil Fertility and Fertilizers. 4 ed. MacMillan Publishing Company Co. New York. 30 p.
- Simmonds, N.W.; Vandermeer, J. The Ecology of Intercropping. J. Appl. Ecol. 1989, 26, 1107. [Google Scholar] [CrossRef]
- Weisskopf, L. Heller and L. Eberl. 2011. Burkholderia species are major inhabitans of White Lupin clusters roots. Applied and enviromental microbiology. Vol. 77, 21: 7715-7720. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).