Submitted:
23 August 2023
Posted:
24 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
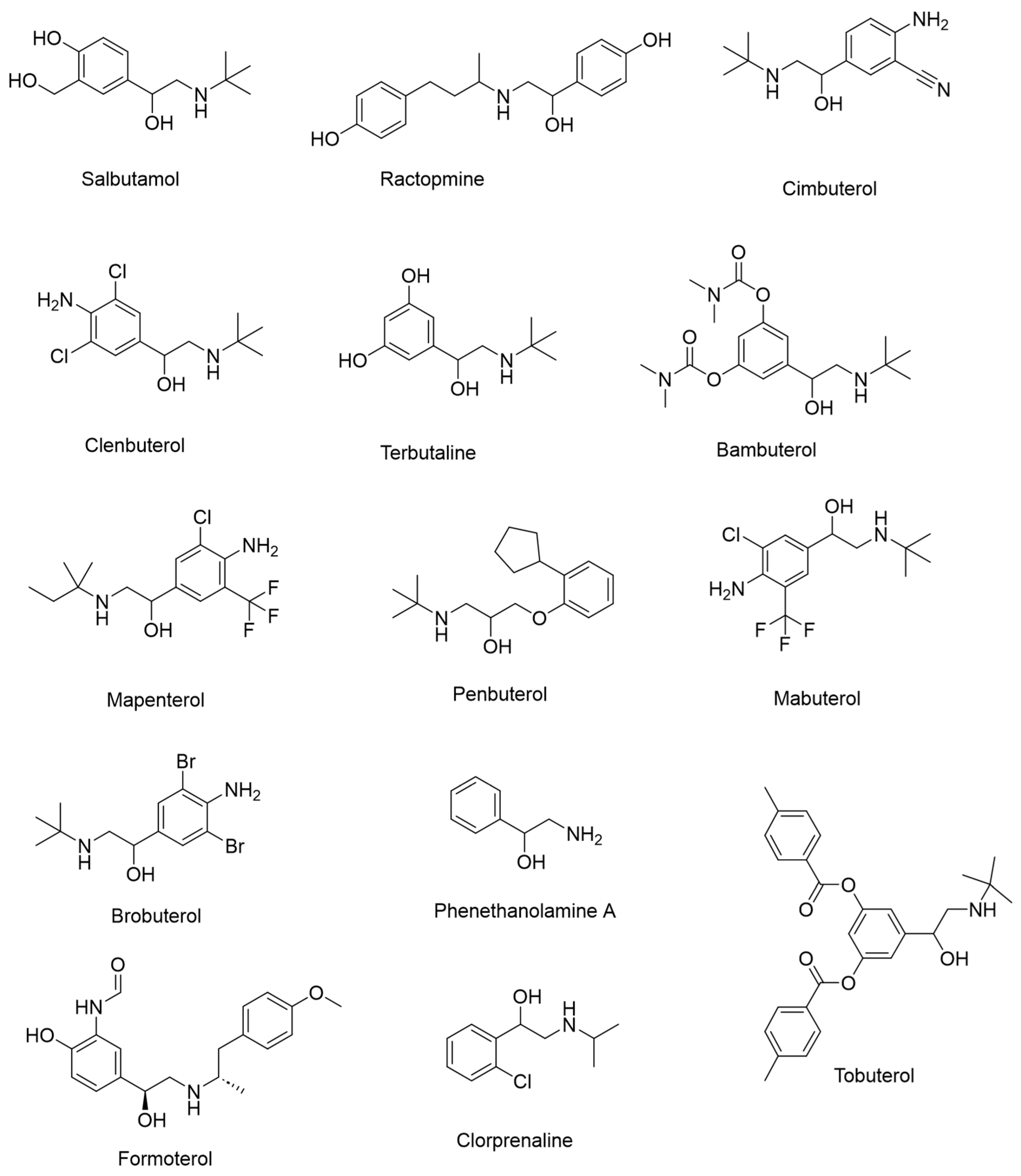
2.1. Chemicals and Reagents
2.2. Standard Solutions
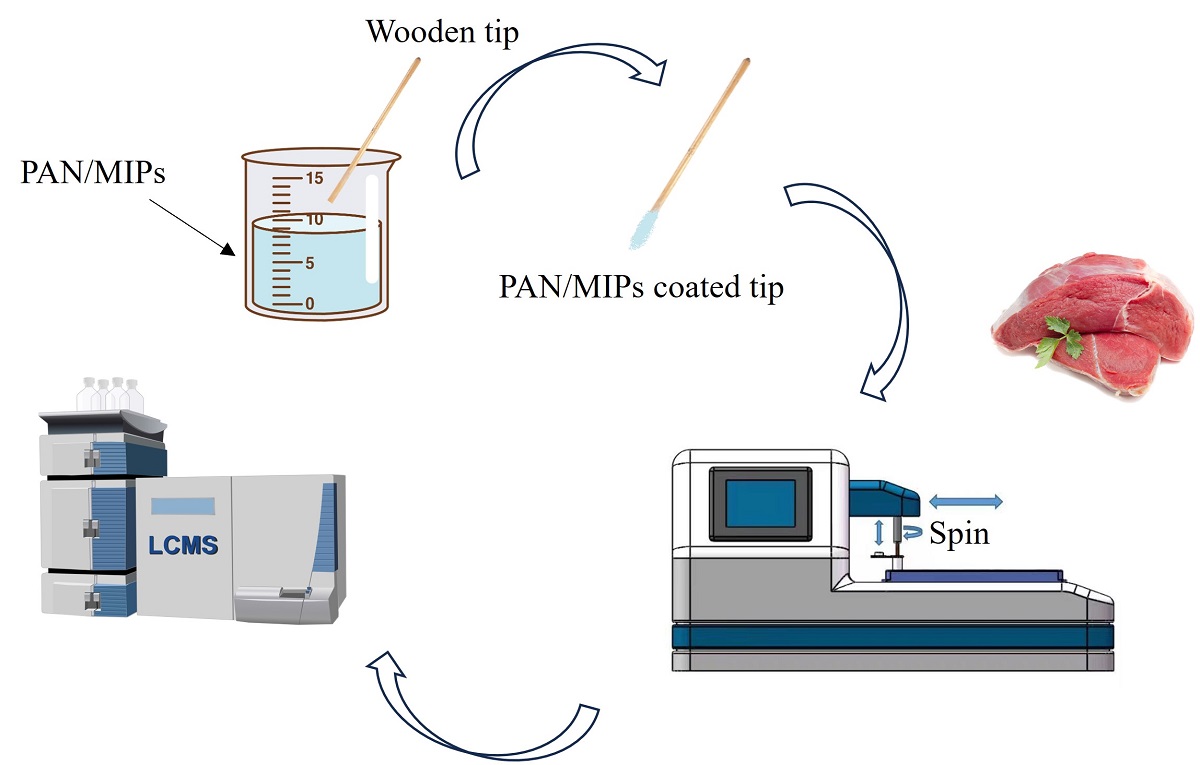
2.3 Preparation of PAN/MIP-Coated Wooden-Tip-Based SPME
2.4 Sample Preparation
2.5 Automated Wooden-tip SPME Procedure
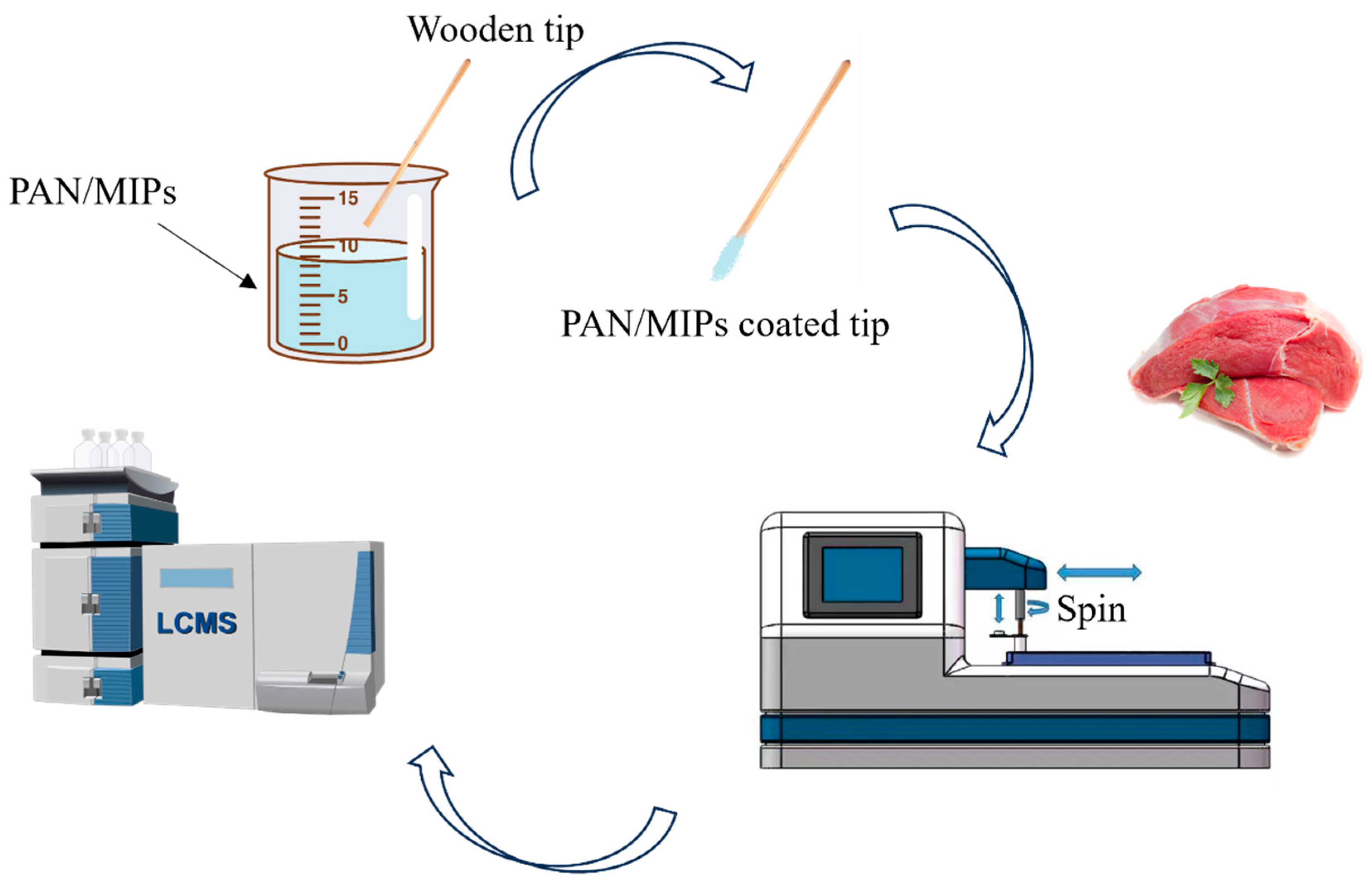
2.6 LC-MS/MS Analysis
3. Results and Disscussion
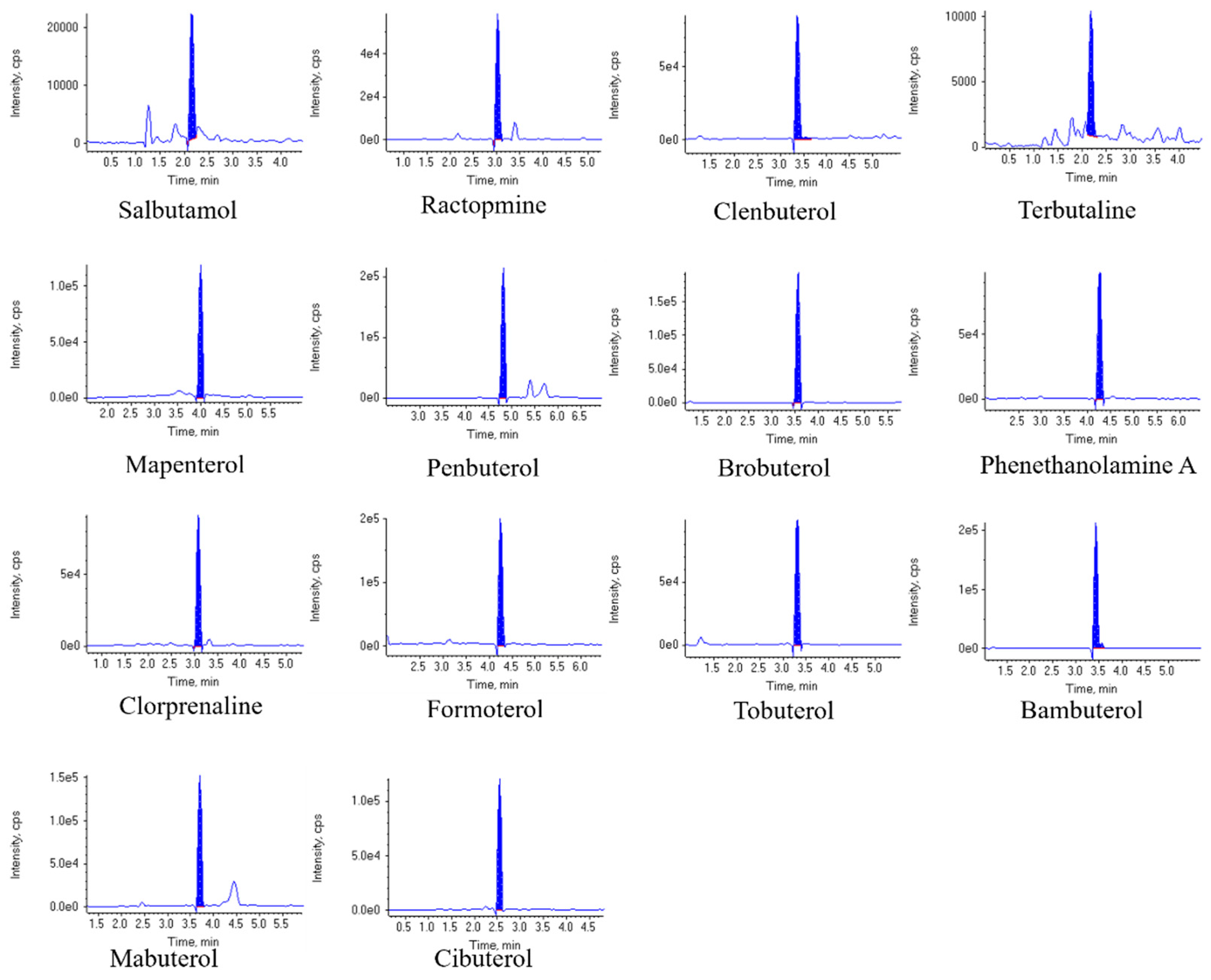
3.1 Wooden tip SPME surface coating optimization
3.2 Method validation
3.3 Analysis of positive sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, Y.; Ning, J.; Cheng, X.; Lv, Q.; Teng, S.; Wang, W. Rapid and High-Throughput Determination of Sixteen β-agonists in Livestock Meat Using One-Step Solid-Phase Extraction Coupled with UHPLC-MS/MS. Foods 2022, 12, 76. [Google Scholar] [CrossRef]
- Juan, C.; Igualada, C.; Moragues, F.; León, N.; Mañes, J. Development and validation of a liquid chromatography tandem mass spectrometry method for the analysis of β-agonists in animal feed and drinking water. J. Chromatogr. A. 2010, 1217, 6061–6068. [Google Scholar] [CrossRef]
- Centner, T.J.; Alvey, J.C.; Stelzleni, A.M. Beta agonists in livestock feed: Status, health concerns, and international trade. J. Anim. Sci. 2014, 92, 4234–4240. [Google Scholar] [CrossRef]
- Brambilla, G.; Cenci, T.; Franconi, F.; Galarini, R.; Macrı, A.; Rondoni, F.; Strozzi, M.; Loizzo, A. Clinical and pharmacological profile in a clenbuterol epidemic poisoning of contaminated beef meat in Italy. Toxicol. Lett. 2000, 114, 47–53. [Google Scholar] [CrossRef]
- Giannetti, L.; Ferretti, G.; Gallo, V.; Necci, F.; Giorgi, A.; Marini, F.; Gennuso, E.; Neri, B. Analysis of beta-agonist residues in bovine hair: Development of a UPLC–MS/MS method and stability study. J. Chromatogr. B. 2016, 1036-1037, 76–83. [Google Scholar] [CrossRef]
- Guo, P.; Wan, J.; Zhan, C.; Zhu, C.; Jiang, W.; Ke, Y.; Ding, S.; Wang, D. A simplified sample pretreatment for the rapid determination of 22 β-agonist residues in swine muscle and liver tissues by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B. 2018, 1096, 122–134. [Google Scholar] [CrossRef]
- Kuiper, H.A.; Noordam, M.Y.; van Dooren-Flipsen, M.M.H.; Schilt, R.; Roos, A.H. Illegal use of β-adrenergic agonists: European Community. J. Anim. Sci. 1998, 76, 195–207. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin (Text with EEA relevance).
- GB/T5009.192-2003 Determination of 4-Amino-3,5-dichloro-alpha-[(tert-butylamino)methyl]-benzyl alcohol (clenbuterol) residues in animal foods.
- Zhang, W.; Wang, P.; Su, X. Current advancement in analysis of β-agonists. TrAC Trends Analyt. Chem. 2016, 85, 1–16. [Google Scholar] [CrossRef]
- He, Z.; Fan, H. Research progress of Electrochemical Detection of β-Agonists: a mini-review. Int. J. Electrochem. Sci. 2019, 14, 9449–9458. [Google Scholar] [CrossRef]
- Cao, Z.; Cao, G.; Zhang, Y.; Liu, X.; Hua, Z. Determination of multi-residue β-agonists in cooked meat by isotope dilution-gas chromatography-tandem mass spectrometry. J. Food Saf. Qual. 2020, 11, 9140–9146. [Google Scholar]
- Zhu, B.; Cai, L.; Jiang, Z.; Li, Q.; Guo, X. Simultaneous stereoselective determination of seven β-agonists in pork meat samples by ultra-performance liquid chromatography-tandem mass spectrometry. Microchem. J. 2019, 150, 104082. [Google Scholar] [CrossRef]
- Kaufmann, A.; Widmer, M.; Maden, K.; Butcher, P.; Walker, S. High resolution mass spectrometry-based detection and quantification of β-agonists at relevant trace levels in a variety of animal-based food matrices. Food Addit. Contam. Part A 2021, 38, 1350–1363. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Zheng, F.; Liu, J.; Wu, D. Emerging nanosensing technologies for the detection of β-agonists. Food Chem. 2020, 332, 127431. [Google Scholar] [CrossRef]
- Castilla-Fernández, D.; Moreno-González, D.; Beneito-Cambra, M.; Molina-Díaz, A. Critical assessment of two sample treatment methods for multiresidue determination of veterinary drugs in milk by UHPLC-MS/MS. Anal. Bioanal. Chem. 2019, 411, 1433–1442. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Jung, W.-T.; Lee, H.-L. Novel vinylene-based covalent organic framework as a promising adsorbent for the rapid extraction of beta-agonists in meat samples. Anal. Chim. Acta 2023, 341492. [Google Scholar] [CrossRef]
- Yang, M.; Yi, J.; Hu, X.; Dong, Q.; Xu, Q.; Li, S.; Lu, Y.; Jiang, X.; Tu, F. Determination of 14 β-agonists in infant formulas using QuEChERS-high performance liquid chromatography-tandem mass spectrometry. China Dairy Ind. 2019, 47, 52–64. [Google Scholar]
- Wang, L.-Q.; Zeng, Z.-L.; Su, Y.-J.; Zhang, G.-K.; Zhong, X.-L.; Liang, Z.-P.; He, L.-M. Matrix effects in analysis of β-agonists with LC-MS/MS: influence of analyte concentration, sample source, and SPE type. J. Agric. Food Chem. 2012, 60, 6359–6363. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Z.; Wang, X.; Yang, J.; Chen, Z.; He, L. Multiresidue analysis of nine β-agonists in animal muscles by LC-MS/MS based on a new polymer cartridge for sample cleanup. J. Sep. Sci. 2013, 36, 1843–1852. [Google Scholar] [CrossRef]
- Kulikovskii, A.V.; Lisitsyn, A.B.; Gorlov, I.F.; Slozhenkina, M.I.; Savchuk, S.A. Determination of growth hormones (β-agonists) in muscle tissue by HPLC with mass spectrometric detection. J. Anal. Chem. 2016, 71, 1052–1056. [Google Scholar] [CrossRef]
- Mastrianni, K.R.; Metavarayuth, K.; Brewer, W.E.; Wang, Q. Analysis of 10 β-agonists in pork meat using automated dispersive pipette extraction and LC-MS/MS. J. Chromatogr. B. 2018, 1084, 64–68. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, S.; Wu, C.; Feng, Y.; Zhao, X.; Li, J.; Zhang, Q. Self-assembly preparation of Zn2+-immobilized silica hybrid monolith and application in solid-phase micro-extraction of β-agonists. J. Sep. Sci. 2022, 45, 4460–4468. [Google Scholar] [CrossRef]
- Gong, X.; Li, K.; Xu, W.; Jin, L.; Liu, M.; Hua, M.; Tian, G. Determination of Nitrofuran Metabolites in Complex Food Matrices Using a Rough, Cheap, Easy-Made Wooden-Tip-Based Solid-Phase Microextraction Probe and LC-MS/MS. J. Chem. 2022, 2022. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Bojko, B.; Pawliszyn, J. High throughput quantification of prohibited substances in plasma using thin film solid phase microextraction. J. Chromatogr. A. 2014, 1374, 40–49. [Google Scholar] [CrossRef]
- GB/T 31658.22-2022 National food safety standard-Determination of β-agonists residues in animal derived food by liquid chromatograpy-tandem mass spectrometric method.
- GB/T 27404-2008 Criterion on quality control of laboratories-Chemical testing of food.
| Analyte | Precursor ion (m/z) | Product ion (m/z) | CE(V) | DP(V) |
| salbutamol | 240.1 | 148.2*, 222.1 | 30, 15 | 80 |
| ractopmine | 302.2 | 164.0*,284.1 | 25, 15 | 80 |
| clenbuterol | 277.1 | 203.1*,258.9 | 25, 15 | 120 |
| terbutaline | 226.2 | 152.1*,107.1 | 23, 43 | 45 |
| mapenterol | 324.8 | 237.1*,217.1 | 27, 25 | 65 |
| penbuterol | 293.2 | 236.2*,201.1 | 22, 28 | 65 |
| brobuterol | 367.0 | 293.0*,348.9 | 26, 17 | 65 |
| phenethanolamine A | 345.2 | 150.2*,327.0 | 33, 19 | 65 |
| clorprenaline | 214.1 | 154.1*,195.9 | 27, 18 | 65 |
| formoterol | 345.1 | 148.9*,326.8 | 27, 19 | 65 |
| tobuterol | 228.1 | 153.8*,172.2 | 22, 16 | 65 |
| bambuterol | 368.2 | 294.1*,312.1 | 27, 21 | 65 |
| mabuterol | 311.0 | 237.1*, 217.1 | 24, 37 | 65 |
| cibuterol | 234.2 | 159.8*,143.2 | 21, 35 | 65 |
| Analytes | Linear range (ng/L) |
Calibration equation | Correlation coefficient (r2) | Matrix effect (%) |
LOD (μg/kg) |
LOQ (μg/kg) |
| salbutamol | 1-20 | Y=1.08×106X | 0.9993 | 93 | 0.22 | 0.52 |
| ractopmine | 1-20 | Y=7.63×105X | 0.9996 | 81 | 0.18 | 0.49 |
| clenbuterol | 1-20 | Y=7.4×105X | 0.9999 | 82 | 0.09 | 0.36 |
| terbutaline | 1-20 | Y=4.46×105X | 0.9997 | 85 | 0.39 | 0.98 |
| mapenterol | 1-20 | Y=9.72×105X | 0.9995 | 95 | 0.15 | 0.45 |
| penbuterol | 1-20 | Y=1.38×104X | 0.9989 | 120 | 0.12 | 0.29 |
| brobuterol | 1-20 | Y=1.49×106X | 0.9999 | 87 | 0.18 | 0.38 |
| phenethanolamine A | 1-20 | Y=1.12×106X | 0.9999 | 99 | 0.22 | 0.49 |
| clorprenaline | 1-20 | Y=1.32×106X | 0.9998 | 82 | 0.28 | 0.52 |
| formoterol | 1-20 | Y=4.55×104X | 0.9997 | 81 | 0.13 | 0.28 |
| tobuterol | 1-20 | Y=1.45×106X | 1.0000 | 81 | 0.36 | 0.99 |
| bambuterol | 1-20 | Y=2.78×106X | 0.9994 | 93 | 0.11 | 0.27 |
| mabuterol | 1-20 | Y=2.02×106X | 0.9999 | 83 | 0.18 | 0.37 |
| cibuterol | 1-20 | Y=2.17×106X | 0.9994 | 84 | 0.19 | 0.42 |
| Analyte | Intraday | Interday | ||||||||||
| 1.0 μg/kg | 5.0 μg/kg | 10.0 μg/kg | 1.0 μg/kg | 5.0 μg/kg | 10.0 μg/kg | |||||||
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | |
| salbutamol | 78.3 | 10.8 | 79.5 | 9.7 | 79.6 | 9.4 | 77.2 | 11.1 | 78.2 | 10.4 | 78.1 | 10.2 |
| ractopmine | 74.3 | 11.2 | 74.5 | 10.6 | 73.4 | 10.8 | 73.3 | 11.9 | 73.9 | 11.2 | 73.1 | 10.9 |
| clenbuterol | 75.2 | 11.3 | 77.3 | 10.7 | 74.5 | 9.6 | 74.7 | 10.9 | 76.8 | 10.2 | 74.2 | 9.9 |
| terbutaline | 80.9 | 12.5 | 77.4 | 10.3 | 78.2 | 10.1 | 79.2 | 13.4 | 76.7 | 10.9 | 77.9 | 11.6 |
| mapenterol | 81.1 | 11.1 | 81.1 | 10.3 | 80.1 | 10.1 | 80.8 | 12.1 | 81.8 | 10.9 | 81.9 | 10.7 |
| penbuterol | 76.8 | 12.1 | 73.1 | 11.8 | 72.3 | 10.4 | 76.5 | 12.5 | 73.5 | 11.9 | 72.5 | 11.1 |
| brobuterol | 82.2 | 12.9 | 79.3 | 10.1 | 78.1 | 9.2 | 82.3 | 12.1 | 79.1 | 10.6 | 78.9 | 9.8 |
| phenethanolamine A | 80.3 | 11.3 | 78.1 | 11.6 | 76.1 | 10.9 | 80.4 | 11.6 | 78.9 | 11.4 | 77.1 | 11.6 |
| clorprenaline | 79.4 | 12.2 | 76.8 | 11.4 | 73.6 | 11.1 | 79.2 | 11.3 | 76.3 | 10.2 | 74.1 | 10.4 |
| formoterol | 77.4 | 12.9 | 74.1 | 11.7 | 76.1 | 10.9 | 77.2 | 12.2 | 75.2 | 12.1 | 76.2 | 10.8 |
| tobuterol | 76.1 | 12.8 | 75.1 | 10.3 | 73.3 | 10.9 | 75.2 | 12.6 | 73.9 | 11.2 | 73.8 | 10.6 |
| bambuterol | 77.2 | 12.1 | 76.5 | 12.3 | 71.6 | 10.9 | 78.1 | 12.7 | 76.9 | 12.7 | 72.9 | 11.7 |
| mabuterol | 79.8 | 11.8 | 74.8 | 10.2 | 72.6 | 9.7 | 79.1 | 12.8 | 74.1 | 11.3 | 73.1 | 10.8 |
| cibuterol | 79.8 | 12.3 | 74.8 | 11.6 | 72.6 | 10.8 | 79.1 | 11.3 | 75.1 | 11.1 | 73.4 | 11.4 |
| Analyte | Sample NO. | Concentration (μg/kg) | True value | |
| GB method | This work | (μg/kg) | ||
| clenbuterol | 58 | 4.05 | 4.18 | 4.20 |
| ractopmine | 5.12 | 5.44 | 5.40 | |
| salbutamol | 40.71 | 42.70 | 43.37 | |
| Parameter | Proposed method (per sample) | GB/T 31658.22-2022 |
| adsorbent material cost | 3 CNY | 30 CNY |
| solvent consumption | 3.5 mL solvent + 1.0 mL water | 9 mL solvent |
| purification time | 8 min | 40-60 min |
| automation | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




