1. Introduction
Peters anomaly (PA) is a congenital anomaly that affects the anterior segment of the eye. It is characterized by corneal opacity due to defects in the corneal endothelium and Descemet’s membrane, accompanied by iridocorneal or lenticulocorneal adhesions [
1,
2]. PA is estimated to be the most prevalent disease causing anterior segment dysgenesis with corneal opacity, occurring at an incidence rate of 1.1–1.5 cases per 100,000 people [
1]. Homeotic genes controlling the differentiation of primordial cells are responsible for PA. Damage to several homeotic genes (
B3GLCT,
PAX6,
PITX3,
FOXE3, and
CYP1B1) has been reported to cause PA. Notably, most PA cases are solitary; however, autosomal recessive and dominant inheritance patterns have also been reported [
3,
4]. Homeotic genes are involved in the development of the eye and other body structures; therefore, PA is sometimes accompanied by systemic disease. Systemic involvement may present as head anomalies, facial dysmorphism, ear abnormalities, and so on [
2]. Approximately half of patients with PA have concurrent glaucoma. However, there have been very few reports on the features and treatment of glaucoma secondary to PA due to the difficulty of treatment and the rarity of the disease. This study aimed to describe the characteristics of glaucoma associated with PA as well as the treatment outcomes and prognostic factors.
2. Materials and Methods
2.1. Study design and participants
In this study, we reviewed the records of all patients diagnosed with PA at the Department of Ophthalmology, Hiroshima University Hospital, between August 2009 and September 2021 and followed up for at least 6 months. The associations between glaucoma onset and the stage of anterior segment dysgenesis, visual outcomes, and postoperative results of glaucoma surgery were retrospectively examined. This study was conducted according to the principles of the Declaration of Helsinki and was approved by the Institutional Research Ethics Committee (No. E2113). All treatment procedures also followed the principles of the Declaration of Helsinki.
2.2. Peter’s anomaly diagnosis and classification
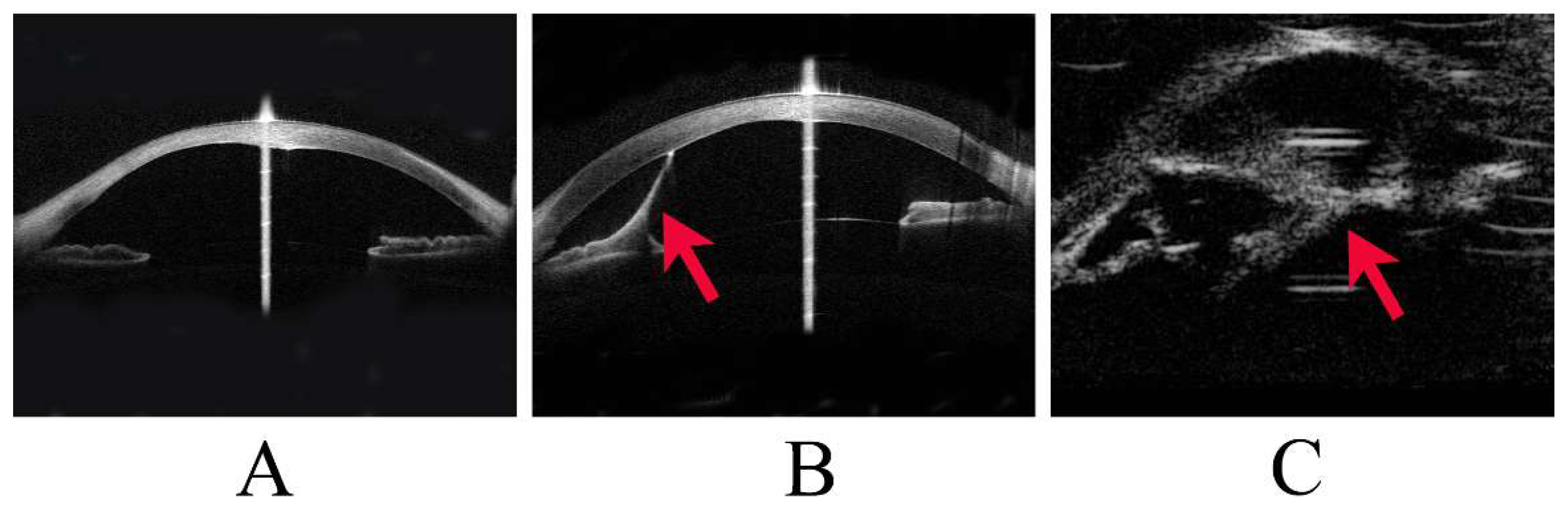
PA was diagnosed clinically and classified as Stages 1, 2, or 3 according to the severity of anterior segment dysgenesis. Stage 1 was defined as the presence of a posterior corneal defect only; Stage 2 as a posterior corneal defect with corneal iris adhesion; and Stage 3 as a posterior corneal defect with lens abnormalities, including lenticulocorneal adhesion (
Figure 1). In addition, cases of PA with accompanying systemic disease were classified as Peters-plus syndrome. Corneal staphyloma referred to thinning of the cornea with a focal bulge.
This image shows representative cases of Stage 1 to Stage 3 Peters anomaly (PA).
(A) Stage 1 PA, with anterior segment optical coherence tomography (OCT) showing a posterior corneal defect.
(B) Stage 2 PA, with anterior segment OCT showing a strand (arrowhead) from the iris to the cornea and a posterior corneal defect.
(C) Stage 3 PA, with ultrasound biomicroscopy showing a strand (arrowhead) between the lens and the cornea.
2.3. Glaucoma diagnosis
The diagnosis of glaucoma was based on intraocular pressure (IOP), changes in optic disc corneal diameter, and intraocular axis length. Cases in which the IOP was ≥21 mmHg on more than two occasions and those in which the corneal diameter or ocular axis length were increased were diagnosed with glaucoma [
5]. Most PA cases were diagnosed in infancy. We could not observe the optic disc in some participants because they also had corneal opacities. Visual acuity (VA) and visual field measurements were impossible at the initial presentation. Furthermore, most participants underwent optotype (Landolt ring) testing at the final follow-up to evaluate VA. IOP was measured mainly with the Icare (Revenue, Vantaa, Finland), Tono-pen (Reichert, Depew, NY), and Perkins applanation tonometers (Haag-Streit UK, London, UK) when a close examination was performed under general anesthesia. Palpation was also performed because it was difficult to accurately measure IOP in cases with strong corneal deformation.
2.4. Glaucoma surgical procedures
Glaucoma surgery was performed when the IOP was >21 mmHg or in cases of corneal diameter or ocular axis length increases. External trabeculotomy (TLO) was selected as first-line surgery for glaucoma secondary to PA, and trabeculectomy (TLE) with mitomycin and glaucoma drainage device (GDD) implantation was performed when TLO was ineffective. The surgical procedures were performed using previously described standard techniques [
6,
7,
8,
9]. For TLO, a 4 mm × 4 mm scleral flap with a four-fifths thickness of the sclera was created to identify the Schlemm canal. Then, a hairpin trabeculotome was used to make a 120˚ incision in the inner wall of the Schlemm canal. The selected GDDs were the Baerveldt glaucoma implant (BG 101-205, Johnson & Johnson Vision, Santa Ana, CA, USA) in one of the four pediatric patients, Ahmed FP8 (New World Medical, Rancho Cucamonga, California, USA) in the other three pediatric cases, and Ahmed FP7 (New World Medical, Rancho Cucamonga, California, USA) in one adult case. A GDD tube was inserted approximately 2 mm from the limbus and placed in the anterior chamber parallel to the iris. In all cases, the donor sclera covered the tube to decrease the erosion risk. In the Baerveldt implantation procedures, the silicone tube was occluded with an 8-0 absorbable vicryl suture to minimize the risk of early postoperative hypotony. Sherwood slits were created in the tube with a 10-0 nylon needle to reduce the frequency of early postoperative IOP elevation.
2.5. Statistical analysis
This study defined successful glaucoma surgery as a postoperative IOP <21 mmHg or Tn (normal tension by palpation) without ocular complications that could affect visual function. For statistical analysis, a log-rank test was used to determine the association between TLO outcomes and (i) the stage of anterior segment dysgenesis and (ii) corneal staphyloma. The Cochran-Armitage trend test was used to assess the association between glaucoma development and the severity of anterior segment dysgenesis. The Mann–Whitney U test was used to analyze the association between glaucoma and visual outcomes. Statistical significance was set at P <0.05. All statistical analyses were performed using the JMP version 14 statistical package program (Cary, North Carolina, USA).
3. Results
3.1. Patient characteristics and Peters anomaly diagnosis
Thirty-one eyes of 20 patients were diagnosed with PA. The median age at initial presentation was 0.88 years (interquartile range [IQR]: 0.053–2.9 years), and the median follow-up time was 8.1 years (IQR: 5.6–9.1 years). The bilateral to unilateral ratio was 11:9, and seven patients were diagnosed with Peters-plus syndrome (
Table 1), including two with agenesis of the corpus callosum, one with cardiac disease, two with developmental disorders, one with trisomy 13, and one with congenital deafness.
3.2. Incidence of secondary glaucoma and association with Peters anomaly
Sixteen eyes of 10 patients developed glaucoma secondary to PA. Additional ocular findings affecting the visual outcomes of the patients with PA included corneal staphyloma, microphthalmia, persistent fetal vasculature (PFV), and aniridia (10). In this study, three eyes had corneal staphyloma, one had PFV, and two had aniridia; all those eyes developed glaucoma.
Of the 16 eyes with glaucoma, none, thirteen, and three were classified as Stages 1, 2, and 3 PA, respectively. Of the 15 eyes without glaucoma, 2, 12, and 1 were classified as Stages 1, 2, and 3 PA, respectively. Stage 1 PA had no glaucoma, 52% of Stage 2 had glaucoma, and 75% of Stage 3 had glaucoma. Secondary glaucoma did not occur in the eyes with Stage 1 PA, and the incidence of secondary glaucoma tended to increase as the disease progressed (P=0.052;
Table 2). Thirteen eyes in the glaucoma group and 10 in the non-glaucoma group were examined at the final follow-up. All three corneal staphyloma eyes had glaucoma and were Stage 2.
3.3. Association between glaucoma and visual outcomes
Regarding the final VAs, the eyes with glaucoma had the highest percentage of no light perception, and more than half had VA below the finger counting level. In contrast, the non-glaucoma group had the highest percentage of VA better than 20/200, and no cases of blindness were observed (
Table 3). The final median VA was 1.8 logMAR (IQR: 1.4–2.0 logMAR) and 1.3 logMAR (IQR: 0.64–1.8 logMAR) in the glaucoma and non-glaucoma groups, respectively. VA was poorer for the eyes with glaucoma than for those without glaucoma (P=0.0292;
Table 3).
3.4. External trabeculotomy outcomes
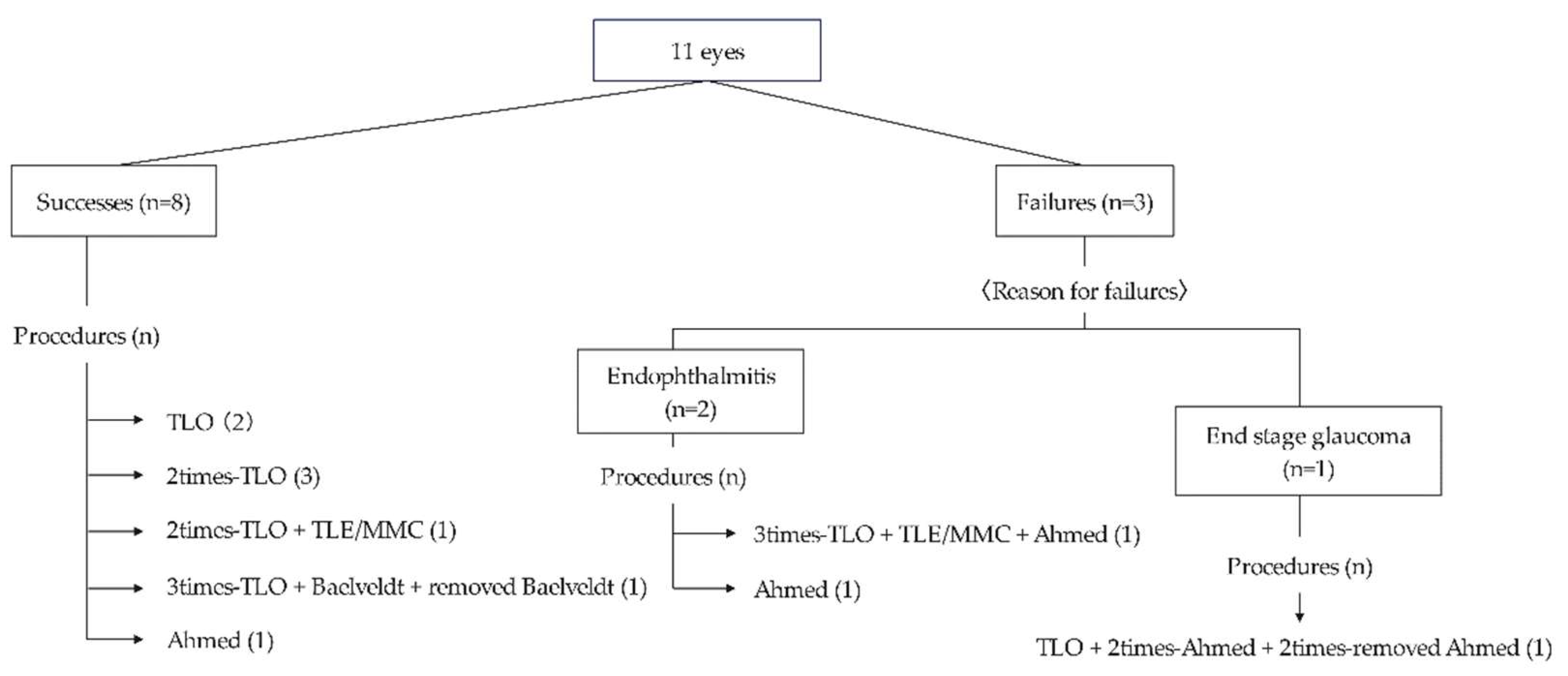
Of the 16 eyes with glaucoma, two had good IOP control with medication, 11 underwent glaucoma surgery, and three were ineligible for surgery due to poor general health or ocular conditions (
Figure 2). Postoperatively, eight eyes (73%) had well-controlled IOP until the final visit. Among these eyes, three (two underwent TLO and one underwent Ahmed implantation) achieved good IOP control with a single surgery, while five required multiple surgeries. The mean number of surgeries performed was 2.1±1.4 (range 1–5).
TLO was the initial surgery for nine eyes, and five achieved IOP control with TLO performed once or multiple times. Of the five eyes that achieved IOP control by TLO, three were classified as Stage 2 PA and two were classified as Stage 3 PA. In contrast, all four eyes that did not achieve IOP control with TLO alone were classified as Stage 2 PA (P=0.212). None of the five eyes that achieved IOP control with TLO alone had corneal staphyloma, whereas among the four eyes where TLO was ineffective, three had corneal staphyloma (P=0.0331). Among the three eyes with poor postoperative outcomes, two developed endophthalmitis after GDD implantation, and one did not achieve IOP control despite multiple surgeries.
This figure provides details of the surgical procedures for the 11 eyes that underwent glaucoma surgery. Eight of the eleven eyes had good outcomes. However, three had poor outcomes: one due to poor IOP control and two due to postoperative endophthalmitis. Among the eight eyes with good results, three (two underwent TLO and one underwent Ahmed implantation) achieved good IOP control with a single surgery, while five required multiple surgeries.
4. Discussion
Approximately half of patients with PA develop glaucoma, and glaucoma secondary to PA is an important complication that can affect visual prognosis [
11]. There are few reports on the visual prognosis and outcomes of treatment for PA with glaucoma. Furthermore, to the best of our knowledge, no studies have specifically investigated the relationship between the severity of anterior segment dysgenesis and the onset of glaucoma in patients with PA. This study investigated the relationship between glaucoma onset and PA stage and found that the incidence of glaucoma tended to increase as morphological changes progressed.
Adhesion between the lens and the cornea may occur as the severity of anterior segment dysgenesis increases. A consequent anterior shift of the lens can cause angle closure, which may be involved in the development of glaucoma. The mechanism of glaucoma onset in PA involves aqueous outflow tract abnormalities and angle closure due to anterior segment adhesions. Glaucoma secondary to PA often develops in infancy, and all patients in this study were diagnosed with glaucoma on their initial visits. However, previous reports [
12,
13] have shown that glaucoma can develop during childhood or later. Therefore, patients with severe anterior segment dysgenesis, particularly those with Stage 3 disease, should be carefully monitored for glaucoma onset.
In the existing literature, there are two reports on the treatment outcomes of glaucoma in PA. Kara et al. [
14] reported that 34 of 58 eyes with PA developed glaucoma, and 20 of the 34 eyes underwent glaucoma surgery. Fifteen of the 20 eyes (75%) achieved good IOP postoperatively. Twelve of these 15 eyes achieved IOP control with GDD implantation. In contrast, additional TLO surgery was required in eight cases due to poor IOP control in the early postoperative period. Yang et al. [
12] showed that 12 of 34 eyes (32%) with PA that underwent glaucoma surgery achieved IOP control. The success rate of the initial surgery was two of seven eyes (28.6%) for TLO and four of four eyes (100%) for GDD. In the present study, of the 16 eyes with glaucoma, glaucoma surgery was performed in 11 eyes, and eight (73%) achieved good IOP control. First-line surgery was mainly TLO, and five of nine eyes achieved good IOP control with TLO alone.
In childhood glaucoma, angle surgeries, such as goniotomy and TLO, are considered first-choice surgical procedures, with high success rates of 70–95% reported for primary pediatric glaucoma [
15,
16]. In contrast, for secondary childhood glaucoma, the success rates of goniotomy and TLO are reported to be lower [
17,
18]. A previous study reported a lower success rate for angle surgery in patients with primary childhood glaucoma with enlarged ocular dimensions (corneal diameter >14 mm) [
19]. Another report suggested that corneal staphyloma is one of the poor prognostic factors for visual outcomes in patients with PA [
20]. In the present study, patients with corneal staphyloma had poorer outcomes with TLO.
During eye enlargement due to elevated IOP, the structure of the aqueous outflow tract may change. Furthermore, even if the trabecular meshwork and Schlemm canal are adequately opened in TLO, the Schlemm canal may not function properly. In previous reports [
12,
14], corneal staphyloma was not considered when evaluating surgical outcomes. Therefore, comparing the effect of corneal staphyloma on the outcomes of TLO between this study and previous studies is difficult. However, based on the results of this study, corneal staphyloma was more closely related to the outcomes of TLO than to the stage of anterior segment dysgenesis.
5. Conclusions
In conclusion, PA cases tend to develop glaucoma as anterior segment dysgenesis progresses. Therefore, careful follow-up of patients with PA is necessary, especially those in Stage 3. TLO is effective for glaucoma secondary to PA; however, its effectiveness may be limited to cases without corneal staphyloma.
Author Contributions
Conceptualization, Y.K.; methodology, C.Y., K.H., Y.K.; formal analysis, C.Y., K.H., N.O.; investigation, C.Y., Y.K.; resources, Y.K.; data curation, C.Y.; writing—original draft preparation, C.Y.; writing—review and editing, C.Y., K.H., Y.K.; visualization, C.Y.; supervision, Y.K.; project administration, Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Research Ethics Committee of Hiroshima University Hospital (permission number: No. E2113) for studies involving humans.
Informed Consent Statement
The Institutional Research Ethics Committee of Hiroshima University Hospital exempted us from obtaining informed consent from the patients due to the retrospective and observational nature of this study. However, the patients were given the opportunity to express their choice for their data to be used using an opt-out system through the hospital website.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
We thank Editage for editing a draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kurilec, J.M.; Zaidman, G.W. Incidence of Peters anomaly and congenital corneal opacities interfering with vision in the United States. Cornea 2014, 33, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Shigeyasu, C.; Yamada, M.; Mizuno, Y.; Yokoi, T.; Nishina, S.; Azuma, N. Clinical features of anterior segment dysgenesis associated with congenital corneal opacities. Cornea 2012, 31, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Chesneau, B.; Aubert-Mucca, M.; Fremont, F.; Pechmeja, J.; Soler, V.; Isidor, B.; Nizon, M.; Dollfus, H.; Kaplan, J.; Fares-Taie, L.; Rozet, J.M. First evidence of SOX2 mutations in Peters’ anomaly: Lessons from molecular screening of 95 patients. Clin Genet 2022, 101, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Ferri, S.; Whittaker, B.; Liu, M.; Lazzaro, D.R. Peters anomaly: Review of the literature. Cornea 2011, 30, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Grajewski, A.; Papadopoulos, M.; Grigg, J.; Freedman, S. 9th Consensus meeting: Childhood glaucoma. Amsterdam: Kugler Publications 2013, 1–270.
- Scuderi, G.; Iacovello, D.; Pranno, F.; Plateroti, P.; Scuderi, L. Pediatric glaucoma: A literature’s review and analysis of surgical results. Biomed Res Int 2015, 2015, 393670. [Google Scholar] [CrossRef] [PubMed]
- McPherson Jr, S.D.; McFarland, D. External trabeculotomy for developmental glaucoma. Ophthalmology 1980, 87, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Netland, P.A.; Walton, D.S. Glaucoma drainage implants in pediatric patients. Ophthalmic Surg 1993, 24, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Molteno, A.C.; Ancker, E.; Van Biljon, G. Surgical technique for advanced juvenile glaucoma. Arch Ophthalmol 1984, 102, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Kim, J.H.; Kim, S.J.; Yu, Y.S. Long-term clinical course and visual outcome associated with Peters’ anomaly. Eye (Lond) 2012, 26, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, G.W.; Flanagan, J.K.; Furey, C.C. Long-term visual prognosis in children after corneal transplant surgery for Peters anomaly type I. Am J Ophthalmol 2007, 144, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.L.; Lambert, S.R.; Lynn, M.J.; Stulting, R.D. Surgical management of glaucoma in infants and children with Peters’ anomaly: Long-term structural and functional outcome. Ophthalmology 2004, 111, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Walton, D.S. Primary congenital open angle glaucoma: a study of the anterior segment abnormalities. Trans Am Ophthalmol Soc 1979, 77, 746–768. [Google Scholar] [PubMed]
- Dolezal, K.A.; Besirli, C.G.; Mian, S.I.; Sugar, A. , Moroi, S.E.; Bohnsack, B.L. Glaucoma and cornea surgery outcomes in Peters anomaly. Am J Ophthalmol 2019, 208, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R. Trabeculotomy compared to goniotomy for glaucoma in children. Ophthalmology 1983, 90, 805–806. [Google Scholar] [CrossRef] [PubMed]
- McPherson Jr, S.D.; Berry, D.P. . Goniotomy vs external trabeculotomy for developmental glaucoma. Am J Ophthalmol 1983, 95, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, S.A.; Brandt, J.D. Options in pediatric glaucoma after angle surgery has failed. Curr Opin Ophthalmol 2006, 17, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, B.L.; Freedman, S.F. Surgical outcomes in childhood uveitic glaucoma. Am J Ophthalmol 2013, 155, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.; Edmunds, B.; Fenerty, C.; Khaw, P.T. Childhood glaucoma surgery in the 21st century. Eye (Lond) 2014, 28, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Kim, J.H.; Kim, S.J.; Yu, Y.S. Long-term clinical course and visual outcome associated with Peters’ anomaly. Eye (Lond) 2012, 26, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).