1. Introduction
The Hawthorn berry is an economically crucial seasonal fruit with many beneficial health effects [
1]. This plant is a member of the Rosaceae family and belongs to the
Crataegus genus [
2]. Although hawthorn fruit has numerous varieties locally, the most popular species is
Crataegus tanacetifolia in Turkey, which we also used in our study. Hawthorn species (
Crataegus spp.) were commonly consumed in North America, Europe, and Asia worldwide and used as a leaf, flower, and fruit of the plant for years [
3].
Hawthorn fruit has various beneficial properties such as reducing blood pressure, regulating blood glucose and lipid metabolism [
4], and averting diabetes mellitus and cardiovascular diseases [
5]. Furthermore, it is an antioxidant superfood because of its rich flavonoids and polyphenolic compound contents. Due to those substances, it could show anti-inflammatory [
6], antibacterial [
7], and antioxidant [
8], and immune-modulator [
9], anti-cancerogenic [
10], activity.
The hawthorn fruit's total phenolic and flavonoid compounds are assumed to be directly proportional to scavenging the body's free radicals [
11]. A one Wang et al. [
12] study, in the group given hawthorn fruit extract to senescence-accelerated mice, while SOD (Superoxide dismutase), CAT (Catalase), and GPx (Glutathione peroxidase) antioxidant parameters increased in plasma, liver, and brain, MDA decreased lipid oxidation. Based on this result, it was concluded that the possible antioxidant mechanism of the hawthorn plant in vivo was realized by increasing the endogenous antioxidant enzymes. The formation of many diseases, such as cancer, Alzheimer's, and diabetes mellitus, happens with an excessive increase in free radicals. After oxidative damage, unaccompanied antioxidants are insufficient to prevent tissue damage. Therefore, it is vital with natural supplementary food sources before damage occurs [
13].
The World Health Organization (WHO) defines vinegar as a “liquid for human consumption produced by a two-stage fermentation process, first alcohol and then acid fermentation, from suitable raw materials containing starch or sugar” [
14]. Vinegar produced from fruits with high carbohydrate content, such as grapes, apples, mulberries, and hawthorn, undergo fermentation by yeast and bacteria, and the resulting alcohol becomes acetic acid. Since ancient times, vinegar has traditionally been utilized as a flavoring and preservative in various foods and in treating some diseases [
15,
16]. In this research, it was expected that thermal pasteurization and ultrasound would enhance the antioxidant properties of vinegar produced using conventional techniques. Conventional thermal pasteurization and sterilization are the most prevalent methods for inactivating and preserving microorganisms in foods for extended periods. In thermal applications, as the temperature rises, undesirable changes occur in food's nutritional value, flavor, and sensory qualities [
17]. Nevertheless, with the ultrasound technique, among the novel non-thermal technologies increasingly utilized today, the product's loss of flavor and taste is minimal. In contrast, the product's nutritional value is enhanced [
18]. Ultrasound thermal and pasteurization positively affect the antioxidant and phenolic content of fruit, fruit juices, and vinegar [
19].
Hyperlipidemia is a noteworthy risk factor associated with atherosclerosis and stroke. Current medications used to treat hyperlipidemia are incapable of repairing the oxidative stress damage caused by the disease [
20]. In this context, there has been a shift towards natural foods, and hawthorn fruit is a superior option; it can also reduce blood cholesterol levels, manage blood glucose, and provide antioxidants, and its efficacy in preventing cardiovascular disease has recently become an essential component in nutrition and nutraceuticals [
21,
22].
Our hypothesis aims to examine the effects of oral gavage administration of various hawthorn fruit vinegar methods on antioxidant parameters (SOD, GPx, and MDA) in liver and plasma concentrations, lipid profile, and immunohistochemical changes in adult rats.
2. Material & Methods
2.1. Animals and Experimental Design
Mature Wistar-Albino rats, weighing 230-270 g, aged 6-7 months, housed in Tekirdag Namık Kemal University’s (Turkey) animal housing room at a constant temperature (25 ± 1˚C) for 12 h with fixed darkness and light and humidity was maintained 50% to 60%. Animals were fed ad libitum with a standard rodent pellet chow and mains water. All animal experiments were carried out after obtaining local ethical guidelines, and the Ethical Committee approved the experiments for Animal Experiments at Tekirdag Namık Kemal University. (Approval No. T2021-609). A total of 56 mature rats were randomly divided into the following 7 groups. The animals in the research were arranged into 7 groups, 8 each, 1 control group, and 6 experimental groups. Hawthorn vinegar (HV) was given to the rats oral gavage every day at the same hour. Groups were designed as below;
1. Group: Control (No oral HV)
2. Grup: HVN0.5 (Hawthorn Vinegar Normal orally 0.5 ml/kg)
3. Grup: HVN1 (Hawthorn Vinegar Normal orally 1 ml /kg)
4. Grup: HVP0.5 (Hawthorn Vinegar Pasteurized treatment orally 0.5 ml/kg)
5. Grup: HVP1 (Hawthorn Vinegar Pasteurized treatment orally 1 ml/kg)
6. Grup: HVU0.5 (Hawthorn Vinegar Ultrasound treatment orally 0.5 ml/kg)
7. Grup: HVU1 (Hawthorn Vinegar Ultrasound treatment orally 1 ml/kg)
Before the study, the animals were fed with standard pellets for a 1-week period in order to acclimate them to the environment. Subsequently, the animals were subjected to a care regimen lasting 45 days, during which the experimental groups received hawthorn vinegar by oral gavage as a supplementary component to the pellet, in adherence to the predetermined dosage. The animals were sacrificed at the end of the experiment to collect the samples.
2.2. Obtaining Vinegar Material
For each trial, the study used 5 kg of Hawthorn fruit (Crataegus tanacetifolia) as the raw material, which was supplied from Turkey/Bursa. Vinegar was produced using conventional vinegar manufacturing methods. The process involved the maceration of Hawthorn berries in order to extract their essence. The jar contained a 50% mixture of Hawthorn and distilled water. A quantity of two teaspoons of sugar and around six to seven chickpeas were added into the stage of fermentation and afterward covered with a cloth. The substance was stored for a minimum duration of one month within a dimly lit location. Undergoing filtration, the vinegar was subsequently transferred to another container, rendering it prepared for utilization in the treatment phase.
2.3. Processing of Vinegar Group Treatments
Vinegar production was produced by modifying the previous method [
23]. The obtained vinegar was processed by 3 methods:
1. Sample without any treatment (Groups; HVN0.5 and HVN1).
2. Thermal pasteurization obtained the samples in a jar in a water bath at 65˚C for 30 minutes (Groups; HVP0.5 and HVP1).
3. The samples were treated with the Response Surface Method and Ultrasound and the application of temperature and time with the best bioactive components as a result of optimization (Groups; HVU0.5 and HVU1).
2.4. Determination of Vinegar’s Bioactive Compounds
The samples' total phenolic contents (TPCs) samples were detected using the Folin–Ciocalteu method [
24]. All analyses were performed in triplicate. The absorbance was measured on a UV–VIS spectrophotometer (SP-UV/VIS-300SRB, Spectrum Instruments, Victoria, Australia) at a wavelength of 765 nm. The total flavonoid content (TFC) was determined according to the colorimetric technique [
25]. The concentrations were calculated colorimetrically using a UV spectrophotometer (Spectrum Instrument, SP-UV/VIS-300SRB, Victoria, Australia) at 510 nm. The ascorbic acid content of the samples was calculated by AOAC 961.27 vitamin preparation and ascorbic acid 2.6 dichlorophenol indophenol-titrimetric method [
26]. The antioxidant capacity of hawthorn vinegar was determined using the CUPRAC method (Cu (II) ion-reducing antioxidant capacity), previously described by [
27]. The DPPH scavenging activity method was used to determine the antioxidant activity of the samples, as described by [
28].
2.5. Blood Samples
Blood samples were collected from puncturing the heart under isoflurane anesthesia at the end of the study and taken to K3 EDTA tubes. Blood samples were centrifuged on the same day at 3000 rpm for 10 minutes to obtain the plasma, and then the plasma was transferred to microtubes. Samples were stored at -80°C until the analysis day.
2.6. Tissue Sample Preparation
Liver tissue samples for ELISA were cut into pieces of appropriate size and weighed before homogenization. The tissues were placed in PBS (pH 7.4) and adjusted to be tissue weight (g): PBS (ml) =1:9 volume. Then, homogenates were prepared by homogenizing with a homogenizer (Interlab, Turkey). Tissue homogenates were centrifuged at 5000xg for 5 minutes and the supernatants were transferred to microtubes.
The liver tissues for immunohistochemistry were fixed in 10% formalin for 48 hours. After fixation, the samples were processed for routine histological protocols and embedded in paraffin. The sections were taken at 4 μm and stained with immunohistochemical staining.
2.7. Antioxidant Analyses
SOD, MDA, and CAT antioxidant parameters were determined with the Enzyme-linked immunosorbent assay (ELISA) method for plasma and hepatic tissues. Samples were measured by a microplate reader (Biotek Epoch, USA) Rat SOD Cat No. 201-11-0169, Rat MDA Cat No. 201-11-016, Rat CAT Cat No. 201-11-5106, using commercial ELISA kits (Sun Redbio Hotechology Company, Shanghai, China). Forty-two animals in total (6 for each group) were used for those assays. Specimens were placed in microplate wells coated with antioxidant antibodies. The biotinylated antioxidant antibody was added and bound to antioxidants in the sample. It was then bound to the biotinylated antioxidant antibody by adding Streptavidin-HRP to the wells. The microplate was incubated for 1 hour at 37°C. After incubation, unbound Streptavidin-HRP was removed during the wash step. Then, substrate solutions were added and color change was observed in proportion to the amount of antioxidants. The reaction is terminated by adding an acidic stop solution and absorbance was measured at 450 nm and determined optical density (OD value). Then the standard curve was constructed by plotting the average OD. The calculated results were composed in units of nmol/ml (MDA), ng/ml (CAT and SOD).
2.8. Biochemical Parameters
Total cholesterol, and Triglyceride (Ref No: C20T5 and Ref No: TG381, and BEN Biochemical Enterprise, Italy), HDL and LDL (Ref No: HD320 and LDL348 BEN Biochemical Enterprise, Italy) AST and ALT (Ref No: 80025 and 80027 Biolabo, France) levels were measured by using spectrophotometric and colorimetric methods. Absorbances were read using a microplate reader (Biotek, Epoch, USA). Results were computed according to manual instructions.
2.9. Immunohistochemistry Method
The streptavidin-biotin peroxidase complex (strepABC) method was applied to investigate CAT and Mn-SOD immunoreactivity in the liver tissue. The sections were collected on adhesive slides. Following deparaffinization and rehydration, sections were processed in citrate buffer solution (pH 6.0) for 10 min in a microwave oven at 700 watts and incubated in 3% hydrogen peroxide (H2O2) for 10 min. In order to inhibit nonspecific bindings sections were blocked in Ultra V Block (Thermo Scientific, Ultravision Large Volume Detection System Anti-Polyvalent, HRP, TA – 125 - UB, Deutsch, Germany) for 10 min at room temperature. Afterward, the sections were incubated with CAT primary antibody 6 (EPR1928Y, ab76110, Abcam, Cambridge, MA 02139-1517 USA, 1/200 dilution), and Mn-SOD primary antibody (Santa Cruz, B-1: sc-133254, diluted at a rate of 1/100 dilution) in a humid environment at the ambient temperature for 1 h. Seconder antibody and after streptavidin was dripped on the sections for 30 min. The 3,3′-Diaminobenzidine tetrahydrochloride (DAB) was used as chromogen for 10 min then Mayer’s hematoxylin was used for the background staining. Rabbit serum without primer antibody served as the negative control. Sections were evaluated using research microscope (Olympus BX51, Tokyo, Japan). Evaluation of immunoreactivity of CAT and MN-SOD were scored. Immunoreactive cells were categorized as having negative, mild, moderate, and intensive.
2.10. Statistical Analyses
Statistical analyses were performed with SPSS (Version 20.0; Chicago, IL). Data were analyzed for normality distribution and variance homogeneity assumptions (Shapiro-Wilk test). A One-way ANOVA test was used if the data were normally distributed, and the post hoc Tukey test was used for differences between groups. The means and standard errors of the groups were calculated and shown in the relevant tables. Differences were considered significant at P < 0.05 and median values (min-max) were calculated.
3. Results
Hawthorn fruit vinegar (HFV) is a rich source of flavonoid antioxidants. Ultrasound applied method has the highest values of the total phenolic and flavonoid compounds, DPPH and CUPRAC amounts are given in
Table S1. However, antioxidant contexts of thermal pasteurization processing method (DPHH:54.86%; CUPRAC: 60.22 %; Flavonoid: 13.18 mgCE/100 ml; Total Phenolic: 104.22 mgGAE/100ml) are lower than normal vinegar processing (DPHH:57.39%; CUPRAC:63.55 %; Flavonoid: 14.22 mgCE/100 ml; Total Phenolic: 110.58 mgGAE/100ml)
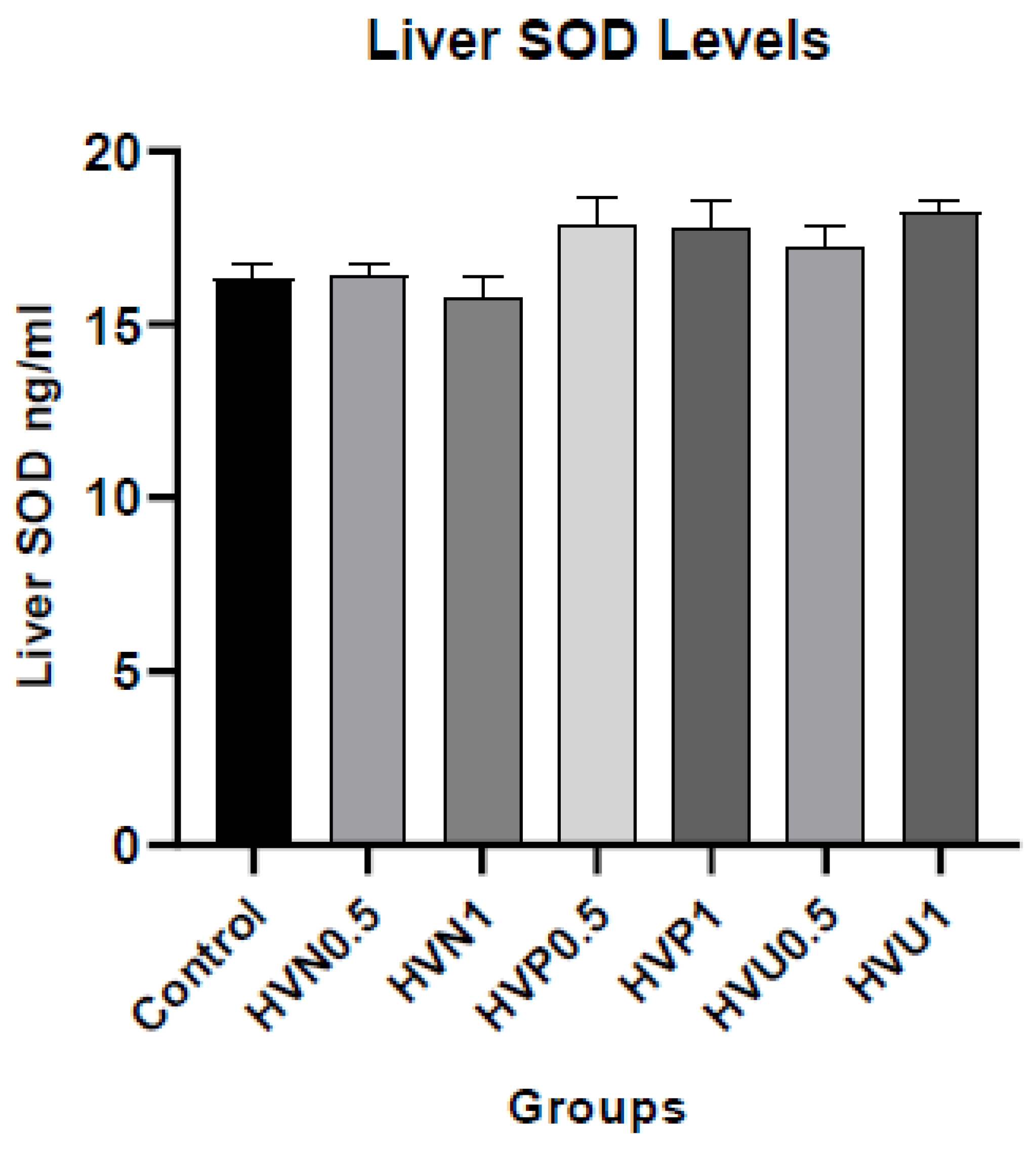
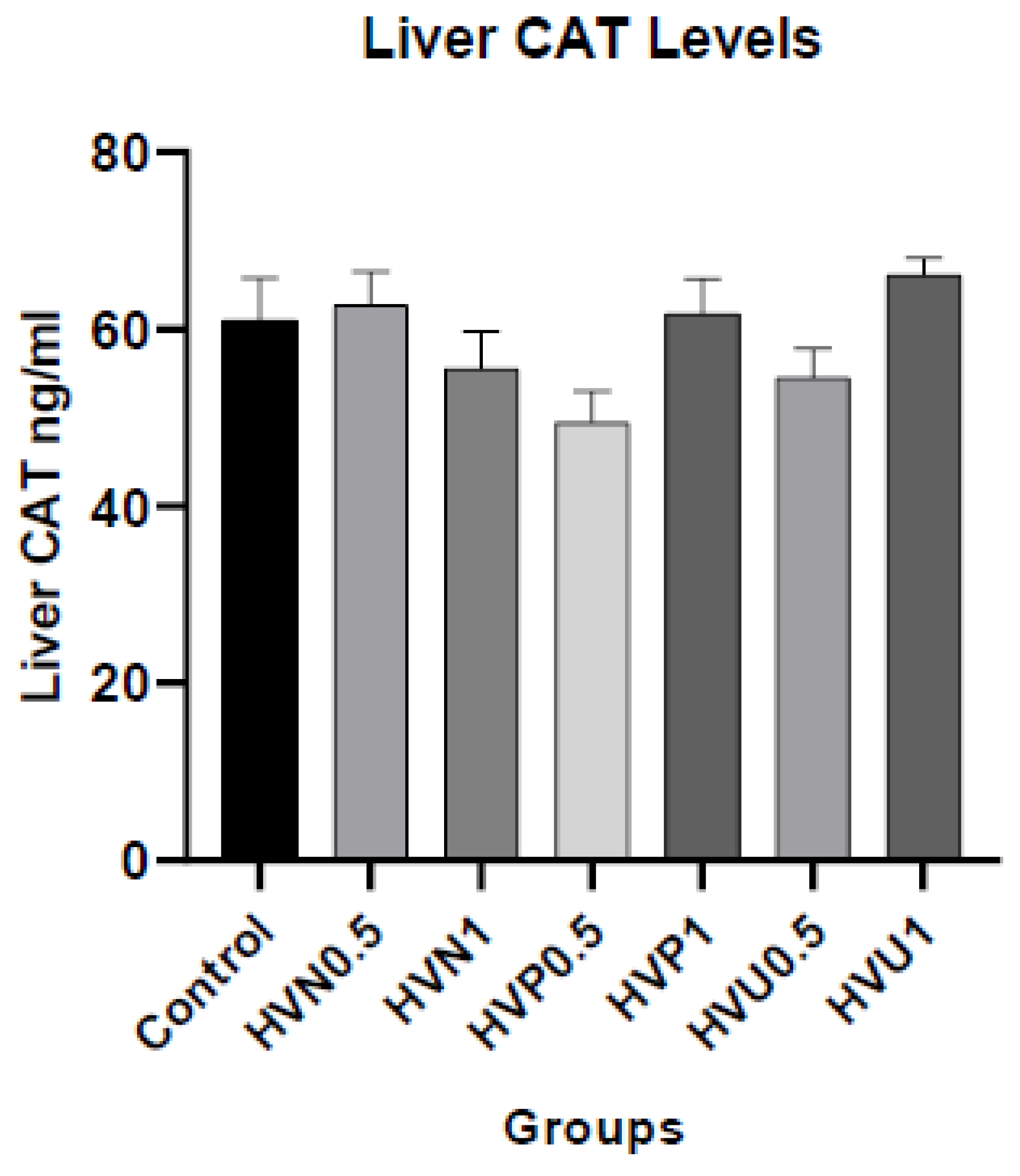
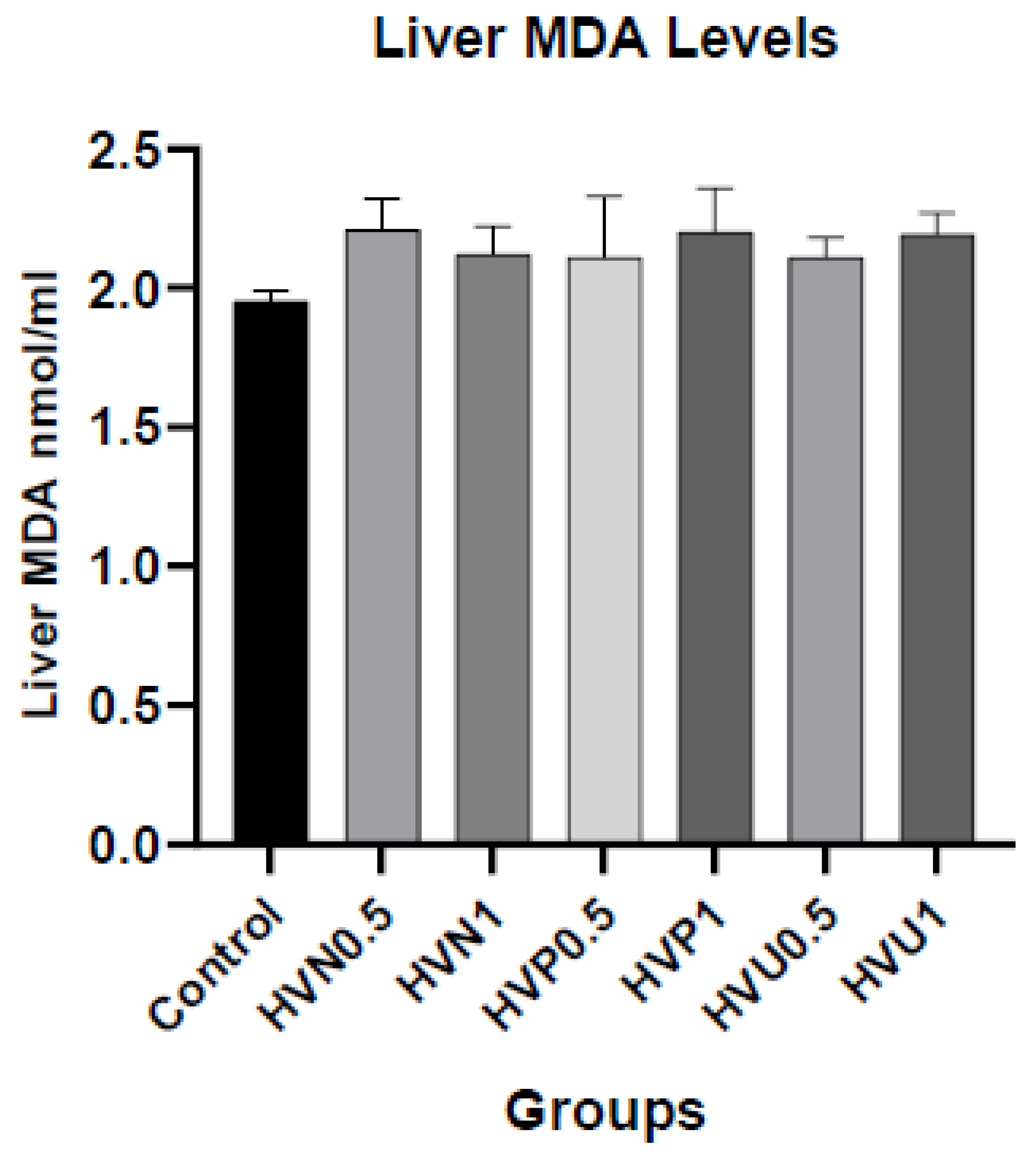
We measured the enzymatic activities of CAT, SOD, and the content of MDA in plasma and liver homogenates of rats to investigate the antioxidant capacity of Hawthorn vinegar, and they are presented in
Table S2. The results showed that plasma and hepatic tissue CAT levels in the ultrasound treatment group 1ml/kg (HVU1) were the highest according to the control and other treatment groups. However, there was no statistical difference in MDA plasma and hepatic tissue levels (p>0.05). If we look at the liver SOD levels, it was assigned that the highest values were in the HVU1 (1ml/kg) group similar to liver CAT levels but the amounts are not statistically significant (p>0.05). The plasma concentrations of CAT and SOD were superior in the thermally pasteurized groups compared to the other experimental and control groups. (
Figure 1,
Figure 2 and
Figure 3)
The lipid profile (Total cholesterol, Triglyceride, HDL, and LDL), AST, and ALT levels are displayed in
Table S3. The biochemical characteristics showed us that the HVP0.5 group had the lowest total cholesterol ratio. Moreover, it was observed that it was lower in the groups treated with ultrasound and thermal pasteurization compared to the control group. Additionally, the highest HDL value was found in the HVU1 cohort. LDL levels were similarly low in the thermal pasteurized and ultrasonic treatment groups, consistent with HDL levels and statistically significant (p≤0.05). Triglyceride levels were not statistically distinct (p>0.05). Upon examining the hepatic enzymes, there was no difference in ALT values between the groups, and the HVU1 group had the highest AST level compared to the control group, although this difference was not statistically significant.
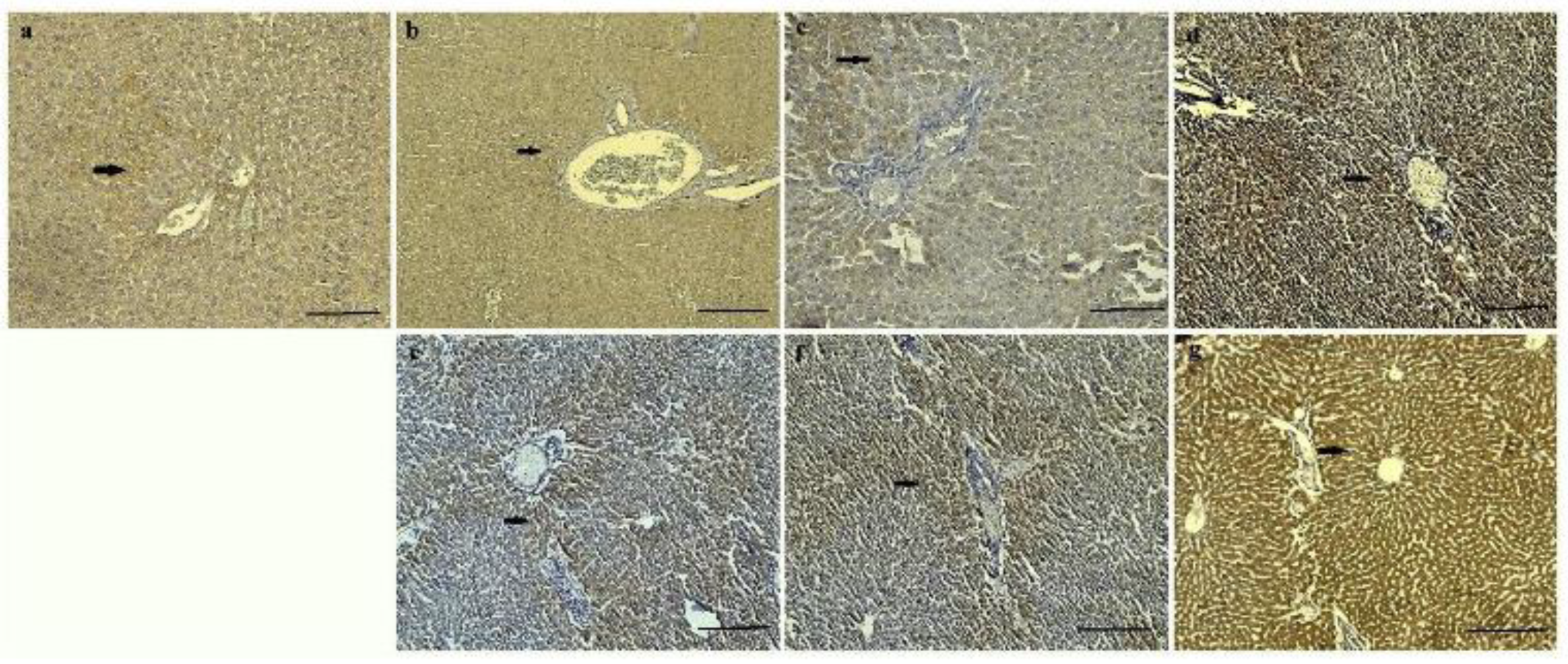
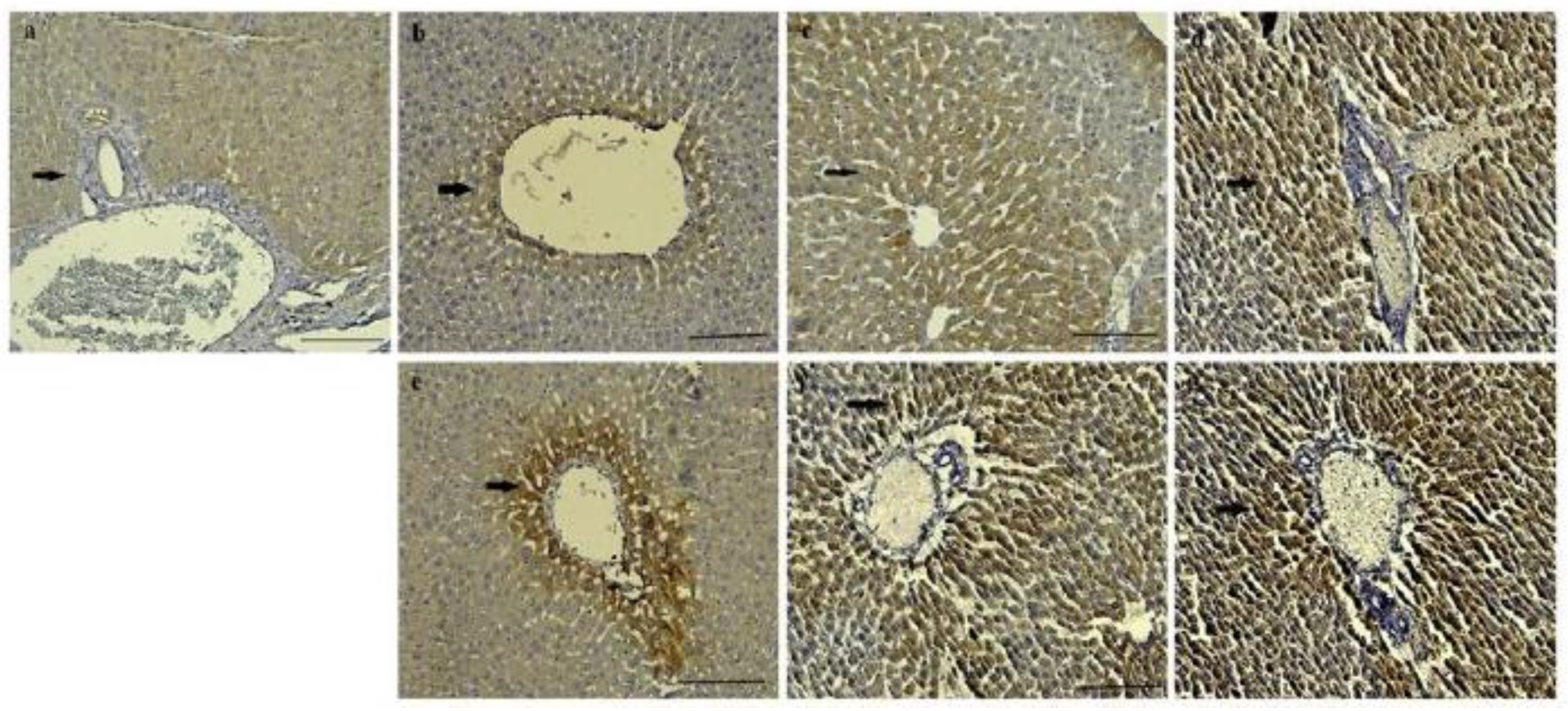
It was determined that CAT and SOD expression intensities of control, HVN and HVP, were less intense than HVU group in both 0.5 and 1 mg/kg of all groups. Mild CAT and SOD expression were observed in hepatocytes of control groups (
Figure 4a and
Figure 5a). It was pointed to CAT and SOD expressions were observed especially around the blood vessels of all groups (
Figure 4 and
Figure 5). While mild CAT expression was detected in hepatocytes in the HVN group (0.5 mg/kg), moderate expression was noted in the HVN group (1 mg/kg), (
Figure 4b,e). In the HVP group, moderate CAT intensity was detected in hepatocytes (
Figure 4c,f). Also, intensive expression was detected in HVU (0.5, and 1 mg/kg) (
Figure 4d,g) Mild SOD expression was observed in hepatocytes of HVN group rats (0.5 mg/kg) (
Figure 5b) and intensive expression was observed in HVN (1 mg/kg) group (
Figure 5e). In the HVP group, moderate expression was observed in 0.5 mg/kg and intensive expression was noted in 1 mg/kg group (
Figure 5f). It was determined intensive SOD expression in HVU group both 0.5 mg/kg and 1 mg/kg (
Figure 5d,g).
4. Discussion
Globally, diabetes mellitus, cardiovascular disease, cancer, and Alzheimer's disease are on the rise posing a grave risk to public health. Diet quality has been shown to play a crucial role in averting these metabolic disorders. As a consequence of oxidative stress, dietary nutrition leads to potential cellular damage. The presence of complex endogenous defense systems that rectify and repair, increasing free radical production and decreasing antioxidant guard complicates the measurement of oxidative stress status [
29]. Consequently, it is feasible to investigate the consumption of antioxidants as a biomarker of oxidative stress by measuring the decrease or increase in antioxidant levels [
30]. At this point, consuming foods rich in antioxidants and incorporating more of these foods into our diets is essential. In this regard, hawthorn fruit is an excellent superfood choice for components and also it is among the earliest medicinal plants and is extensively distributed throughout China and Europe [
31]. In addition, in many cultures for thousands of years, vinegar from fruit or plants has been used for medicinal therapy as well as nutrition. Researchers reported that vinegar and its processing methods are important for good nutrition. There have been several experimental and human studies about the vinegar effects on health, usage, and identify their optimal doses. The current study provides evidence of the antioxidant efficiency of hawthorn vinegar that is produced by several methods, especially ultrasound processing. In addition, present findings reported highest antioxidant activity is found in ultrasound processing of vinegar modeling.
Crataegus plants have in vitro and in vivo antioxidant properties, they can scavenge superoxide anion, hydroxyl radical, and hydrogen peroxides and also inhibit lipid peroxidation [
30]. The one of most important species of this plant named Hawthorn fruit is a traditional fruit in Europe, Asia, and China. During the last decades, food and health scientific insight has attention to this potential food. However, there have been limited studies on hawthorn fruit and extract. Feng et al. [
33] found that MDA levels were significantly reduced in rats treated orally with hawthorn fruit acid (HFA). The total antioxidant capacity in the serum and liver of rats treated with HFA was substantially elevated, as were the activities of the CAT, GSH-px, and SOD enzymes. Similar to this study, in the current study, there were increases in plasma and liver CAT and SOD values at the ultrasound method group (HVU 1 mg/kg), compared to control and other groups, although non-significantly. However, no decrease was found in MDA values in the HVU (1 mg/kg) compared to other groups. Also, the plasma levels of CAT and SOD were higher in the thermally pasteurized groups than the other experimental and control groups in our study. When hepatic tissue and blood plasma samples are compared in terms of antioxidant parameters, the liver has a higher concentration, while high rates are observed in the same cohort as a whole. The application of ultrasound has been found to have a beneficial impact on the levels of antioxidants and phenolic compounds in many commodities, including fruit, fruit juices, and vinegar. In the field of literature research, it has been shown that using the ultrasound method in food processing results in little degradation of bioactive components and nutritional characteristics of the goods. Nevertheless, while reviewing the existing research, it becomes apparent that ultrasonic investigations have mostly focused on enhancing the quality of vinegar [
19]. In this regard, it can be asserted that ultrasound processing of vinegar improves the antioxidant compounds of hawthorn, and thereby boosts liver activity and health in the recent study.
It has been demonstrated that vinegar, commonly used as a condiment, also has some medical applications. Acetic acid is the primary ingredient in vinegar. Other constituents include anthocyanin, flavanols, vitamins, mineral ions, amino acids, and nonvolatile organic acids [
34]. In animal studies, vinegar has exhibited various effects, including enhancing glycogen replenishment, preventing hypertension, and reducing serum total cholesterol and triglyceride. It is possible to use vinegar to treat the biochemical risk factors of atherosclerosis, given that vinegar is a safe, widely available, and inexpensive substance. Patients with a high cardiovascular risk respond favorably to the metabolic effects of hawthorn vinegar [
35]. Kadas et al. [
36] indicated that hawthorn vinegar consumption, decreased body weight, blood pressure, serum glucose, HbA1c, cholesterol, LDL, and triglyceride levels. Similarly, the study revealed a significant rise in HDL and a decline in total cholesterol/HDL. All of these data indicate that vinegar likely possesses a protective effect.
Hawthorn fruit and its extract have been demonstrated numerous health benefits, including cardioprotective, hypotensive, and hypocholesterolemic properties. Zhang et al. [
37] studied in rodents fed a diet containing 30% polyunsaturated canola oil, supplementation with 2% hawthorn fruit powder. They reported that increased serum-tocopherol levels by 18–20% compared to the control group animals. It has also been established that vinegar, commonly used as a condiment, also has some medical applications. Acetic acid is the primary ingredient in vinegar. Other constituents include Anthocyanin, flavones, vitamins, mineral ions, amino acids, and nonvolatile organic acids [
34]. In animal studies, vinegar has demonstrated a variety of effects, including enhancement of glycogen replenishment, prevention of hypertension, and reduction of serum total cholesterol and triglyceride. It is possible to use vinegar to treat the biochemical risk factors of atherosclerosis, given that vinegar is a safe, widely available, and inexpensive substance. A study focused on hawthorn vinegar, it was found that patients with a high cardiovascular risk respond favourably to the metabolic effects of the vinegar [
35]. Nevertheless, Kadas et al. [
36] proved that hawthorn vinegar supplementation decreased body weight, serum glucose, cholesterol, LDL, and triglyceride levels. In addition, the study revealed a significant rise in HDL value and a decline in the total cholesterol/HDL ratio. Also, they reported that hyperlipidemia is a prevalent risk factor for the development of atherosclerosis and stroke. It is commonly characterized by raised levels of total cholesterol and/or triglycerides, as well as reduced levels of HDL. They implied that a portion of the mechanism for the cardioprotective effects of hawthorn fruit may also entail direct protection of LDL from oxidation or indirect protection by maintaining the concentration of -tocopherol in LDL. In addition, a study on rats with HFD-induced hyperlipidemia revealed that the TC and TG levels in HFA-treated groups decreased progressively over the course of treatment. These results indicate that the administration of HFA can substantially enhance the lipid levels of rats [
33]. Consistent with our findings, while statistically insignificant. It was seen that plasma total cholesterol and HDL values were increased in all vinegar groups compared to the control, except group HVU0,5. This may be due to the sample size. On the other hand, the LDL values decreased in all groups compared to the control significantly. However, no significant differences were seen between the groups regarding TG levels, and liver enzyme activities. According to recent results, it was indicated that vinegar likely possesses a protective effect on health.
Due to their essential health benefits, consumers frequently select products derived from fruits abundant in phenolic compounds. Hawthorn fruit is rich in organic acids, vitamins, and minerals, as well as phenolic compounds with antioxidant activity and other bioactive compounds [
38]. As a result of the bioactive compounds in the hawthorn fruit used in our study, vinegar made from this fruit has a very high nutritional value. In the analysis of vinegar employed in our study, total phenolic and flavonoid compound antioxidant DPPH and CUPRAC ratios were determined to be greatest in the ultrasonography technique. A tangerine juice conducted study assigned that the implementation of ultrasound technology is more effective in total antioxidant and food safety than alternative methods [
39]. As preservation alternatives for fruit juices and vinegar, ultrasound technology has yielded positive results in maintaining the concentration of bioactive compounds and the physicochemical, microbiological, and sensory qualities [
40]. The consumption of fruit vinegar with an extended shelf life and more antioxidant characteristics will be preferred by the general public.
The liver, as the primary organ responsible for detoxification and protein synthesis, is involved in a multitude of enzymatic processes. The assessment of liver health can be facilitated by analyzing the concentrations of hepatic enzymes and proteins in the bloodstream. Likewise, heightened concentrations of AST, ALT, ALP, and GGT are indicative of hepatic damage. In a study conducted on rats fed a high-fat diet (HFD) and given hawthorn to an experimental group, AST and ALT levels were found to be relatively high in the HFD experimental group however, there was no change in hepatic enzymes in the hawthorn group. Similarly, in our study, there was no statistically significant difference in AST and ALT levels, while ALT levels were higher in the HVU1 group.
In our investigation, we conducted an immunohistochemistry analysis of liver tissue. We observed the presence of CAT intensity in hepatocytes. Furthermore, we saw a significant expression of both CAT and SOD in HVU groups at doses of 0.5 and 1 mg/kg. It was observed that groups that applied the ultrasound method to vinegar had more satisfactory tissue antioxidant activity. In a study similar to our work, it was revealed that hawthorn fruit acid increased the levels of antioxidant enzymes (SOD, CAT) in the liver tissue of hyperlipidemic rats [
33].