Submitted:
22 August 2023
Posted:
25 August 2023
You are already at the latest version
Abstract
Keywords:
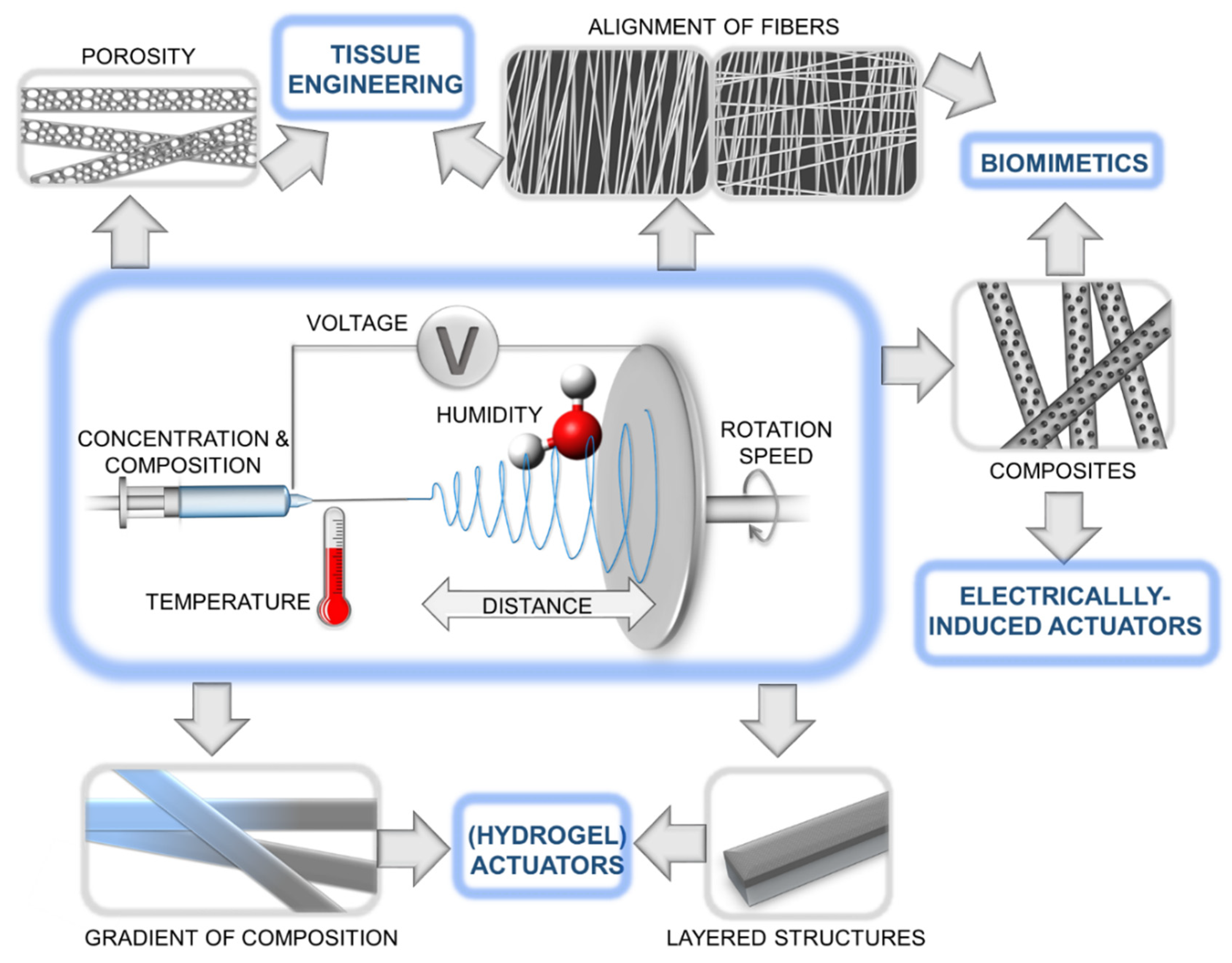
1. Introduction
2. Biomimetic inspiration for synthetic actuators
3. Electrospun hydrogel actuators
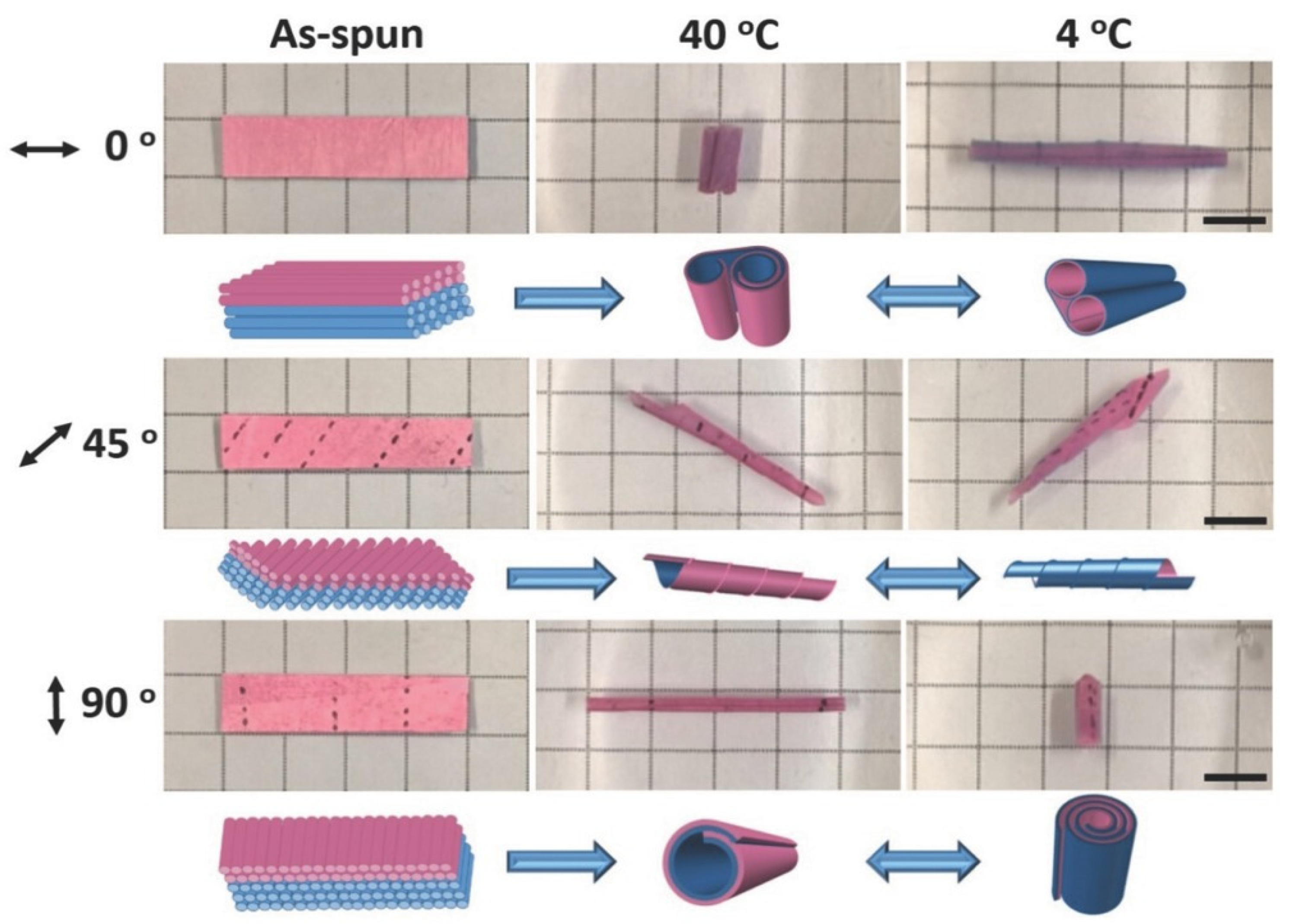
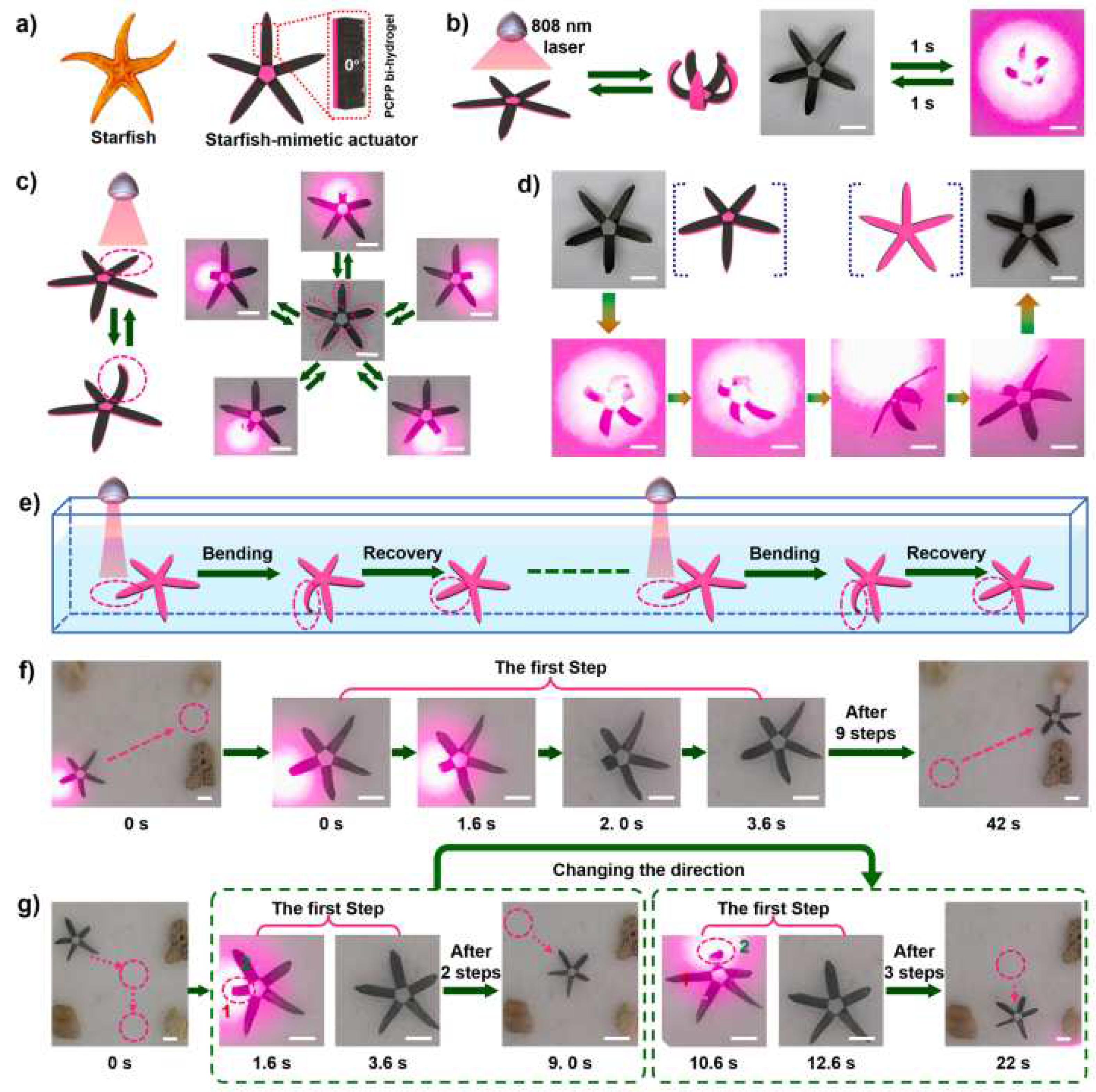
3.1. Water-, temperature-, light-, and electric field-responsive hydrogel actuators
3.2. Hydrogel actuators with multi-stimuli response
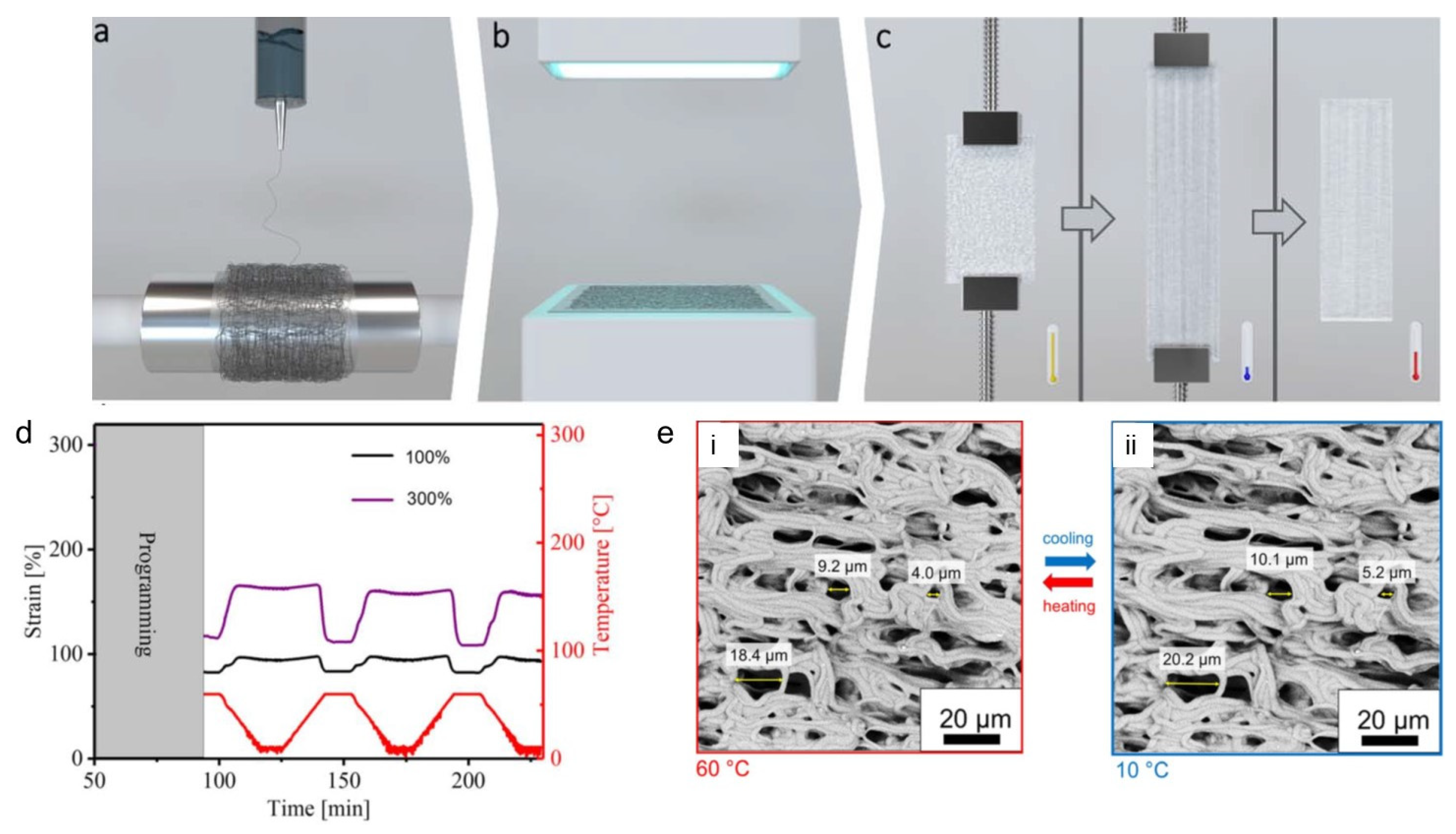
4. Electrospun shape-memory polymers (SMP) actuators
5. Electrospun electroactive actuators
6. Electrospun actuators based on liquid crystal elastomers (LCE)
7. Summary and outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Poppinga, S.; Zollfrank, C.; Prucker, O.; Rühe, J.; Menges, A.; Cheng, T.; Speck, T. Toward a New Generation of Smart Biomimetic Actuators for Architecture. Adv. Mater. 2018, 30, e1703653. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Matsuhisa, N.; Liu, Z.; Wang, M.; Luo, Y.; Cai, P.; Chen, G.; Zhang, F.; Li, C.; Liu, Z.; et al. An on-demand plant-based actuator created using conformable electrodes. Nat. Electron. 2021, 4, 134–142. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.W. Plant-inspired adaptive structures and materials for morphing and actuation: a review. Bioinspiration Biomimetics 2017, 12, 11001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; et al. Light-driven bimorph soft actuators: design, fabrication, and properties. Mater. Horizons 2021, 8, 728–757. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Li, C.; Yang, X.; Chen, W. Flexible Actuators for Soft Robotics. Adv. Intell. Syst. 2020, 2, 1900077. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, Y.; Zhang, B.; Zhang, C.; Wang, J.; Wang, Z. Recent Progress on Plant-Inspired Soft Robotics with Hydrogel Building Blocks: Fabrication, Actuation and Application. Micromachines 2021, 12, 608. [Google Scholar] [CrossRef]
- Mirfakhrai, T.; Madden, J.D.W.; Baughman, R.H. Polymer artificial muscles. Mater. Today 2007, 10, 30–38. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Cho, M.; Ahn, S.-H. Soft Tendril-Inspired Grippers: Shape Morphing of Programmable Polymer–Paper Bilayer Composites. ACS Appl. Mater. Interfaces 2018, 10, 10419–10427. [Google Scholar] [CrossRef]
- I Osotsi, M.; Zhang, W.; Zada, I.; Gu, J.; Liu, Q.; Zhang, D. Butterfly wing architectures inspire sensor and energy applications. Natl. Sci. Rev. 2021, 8, nwaa107. [Google Scholar] [CrossRef]
- Lepora, N.F.; Verschure, P.; Prescott, T.J. The state of the art in biomimetics. Bioinspiration Biomimetics 2013, 8, 13001. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, J.; Guo, X.; Yang, S.; Ozen, M.O.; Chen, P.; Liu, X.; Du, W.; Xiao, F.; Demirci, U.; et al. Multi-stimuli-responsive programmable biomimetic actuator. Nat. Commun. 2019, 10, 4087. [Google Scholar] [CrossRef]
- Liu, H.; Liu, R.; Chen, K.; Liu, Y.; Zhao, Y.; Cui, X.; Tian, Y. Bioinspired gradient structured soft actuators: From fabrication to application. Chem. Eng. J. 2023, 461, 141966. [Google Scholar] [CrossRef]
- Beregoi, M.; Beaumont, S.; Evanghelidis, A.; Otero, T.F.; Enculescu, I. Bioinspired polypyrrole based fibrillary artificial muscle with actuation and intrinsic sensing capabilities. Sci. Rep. 2022, 12, 15019. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhao, Q.; Zhang, L.; Du, X. Intelligent Polymer-Based Bioinspired Actuators: From Monofunction to Multifunction. Adv. Intell. Syst. 2020, 2, 2000138. [Google Scholar] [CrossRef]
- Carpi, F.; Kornbluh, R.; Sommer-Larsen, P.; Alici, G. Electroactive polymer actuators as artificial muscles: are they ready for bioinspired applications? Bioinspiration Biomimetics 2011, 6, 45006. [Google Scholar] [CrossRef] [PubMed]
- Marsudi, M.A.; Ariski, R.T.; Wibowo, A.; Cooper, G.; Barlian, A.; Rachmantyo, R.; Bartolo, P.J.D.S. Conductive Polymeric-Based Electroactive Scaffolds for Tissue Engineering Applications: Current Progress and Challenges from Biomaterials and Manufacturing Perspectives. Int. J. Mol. Sci. 2021, 22, 11543. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, M.Y.; Gonzalez-Gutierrez, J.; Mertz, G.; Ruch, D.; Schmidt, D.F.; Westermann, S. 4D Printing of Multicomponent Shape-Memory Polymer Formulations. Appl. Sci. 2022, 12, 7880. [Google Scholar] [CrossRef]
- Gracias, D.H. Stimuli responsive self-folding using thin polymer films. Curr. Opin. Chem. Eng. 2013, 2, 112–119. [Google Scholar] [CrossRef]
- Krause, S.; Dersch, R.; Wendorff, J.H.; Finkelmann, H. Photocrosslinkable Liquid Crystal Main-Chain Polymers: Thin Films and Electrospinning. Macromol. Rapid Commun. 2007, 28, 2062–2068. [Google Scholar] [CrossRef]
- Mu, Q.; Zhang, Q.; Yu, W.; Su, M.; Cai, Z.; Cui, K.; Ye, Y.; Liu, X.; Deng, L.; Chen, B.; et al. Robust Multiscale-Oriented Thermoresponsive Fibrous Hydrogels with Rapid Self-Recovery and Ultrafast Response Underwater. ACS Appl. Mater. Interfaces 2020, 12, 33152–33162. [Google Scholar] [CrossRef]
- Agarwal, S.; Jiang, S.; Chen, Y. Progress in the Field of Water- and/or Temperature-Triggered Polymer Actuators. Macromol. Mater. Eng. 2019, 304, 1800548. [Google Scholar] [CrossRef]
- Lendlein, A.; Kelch, S. Shape-memory polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Mather, P.T.; Luo, X.; Rousseau, I.A. Shape Memory Polymer Research. Annu. Rev. Mater. Res. 2009, 39, 445–471. [Google Scholar] [CrossRef]
- Behl, M.; Razzaq, M.Y.; Lendlein, A. Multifunctional Shape-Memory Polymers. Adv. Mater. 2010, 22, 3388–3410. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Y.; Leng, J. Progress of shape memory polymers and their composites in aerospace applications. Smart Mater. Struct. 2020. [Google Scholar] [CrossRef]
- Ebadi, S.V.; Fashandi, H.; Semnani, D.; Rezaei, B.; Fakhrali, A. Electroactive actuator based on polyurethane nanofibers coated with polypyrrole through electrochemical polymerization: a competent method for developing artificial muscles. Smart Mater. Struct. 2020, 29, 45008. [Google Scholar] [CrossRef]
- Hong, C.-H.; Ki, S.-J.; Jeon, J.-H.; Che, H.-L.; Park, I.-K.; Kee, C.-D.; Oh, I.-K. Electroactive bio-composite actuators based on cellulose acetate nanofibers with specially chopped polyaniline nanoparticles through electrospinning. Compos. Sci. Technol. 2013, 87, 135–141. [Google Scholar] [CrossRef]
- Montero De Espinosa, L.; Meesorn, W.; Moatsou, D.; Weder, C. Bioinspired Polymer Systems with Stimuli-Responsive Mechanical Properties. Chem. Rev. 2017, 117, 12851–12892. [Google Scholar] [CrossRef]
- Mazurek-Budzyńska, M.; Razzaq, M.Y.; Tomczyk, K.; Rokicki, G.; Behl, M.; Lendlein, A. Poly(carbonate-urea-urethane) networks exhibiting high-strain shape-memory effect. Polym. Adv. Technol. 2017, 28, 1285–1293. [Google Scholar] [CrossRef]
- Verpaalen, R.C.P.; Engels, T.; Schenning, A.P.H.J.; Debije, M.G. Stimuli-Responsive Shape Changing Commodity Polymer Composites and Bilayers. ACS Appl. Mater. Interfaces 2020, 12, 38829–38844. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Serpe, M.J. Stimuli-Responsive Polymers for Actuation. ChemPhysChem 2017, 18, 1451–1465. [Google Scholar] [CrossRef] [PubMed]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A Review of Shape Memory Polymers and Composites: Mechanisms, Materials, and Applications. Adv. Mater. 2021, 33, 2000713. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Severt, S.Y.; Maxwell, S.L.; Bontrager, J.S.; Leger, J.M.; Murphy, A.R. Mimicking muscle fiber structure and function through electromechanical actuation of electrospun silk fiber bundles. J. Mater. Chem. B 2017, 5, 8105–8114. [Google Scholar] [CrossRef]
- Laramée, A.W.; Lanthier, C.; Pellerin, C. Electrospinning of Highly Crystalline Polymers for Strongly Oriented Fibers. ACS Appl. Polym. Mater. 2020, 2, 5025–5032. [Google Scholar] [CrossRef]
- Huang, C.; Soenen, S.J.; Rejman, J.; Lucas, B.; Braeckmans, K.; Demeester, J.; De Smedt, S.C. Stimuli-responsive electrospun fibers and their applications. Chem. Soc. Rev. 2011, 40, 2417–2434. [Google Scholar] [CrossRef]
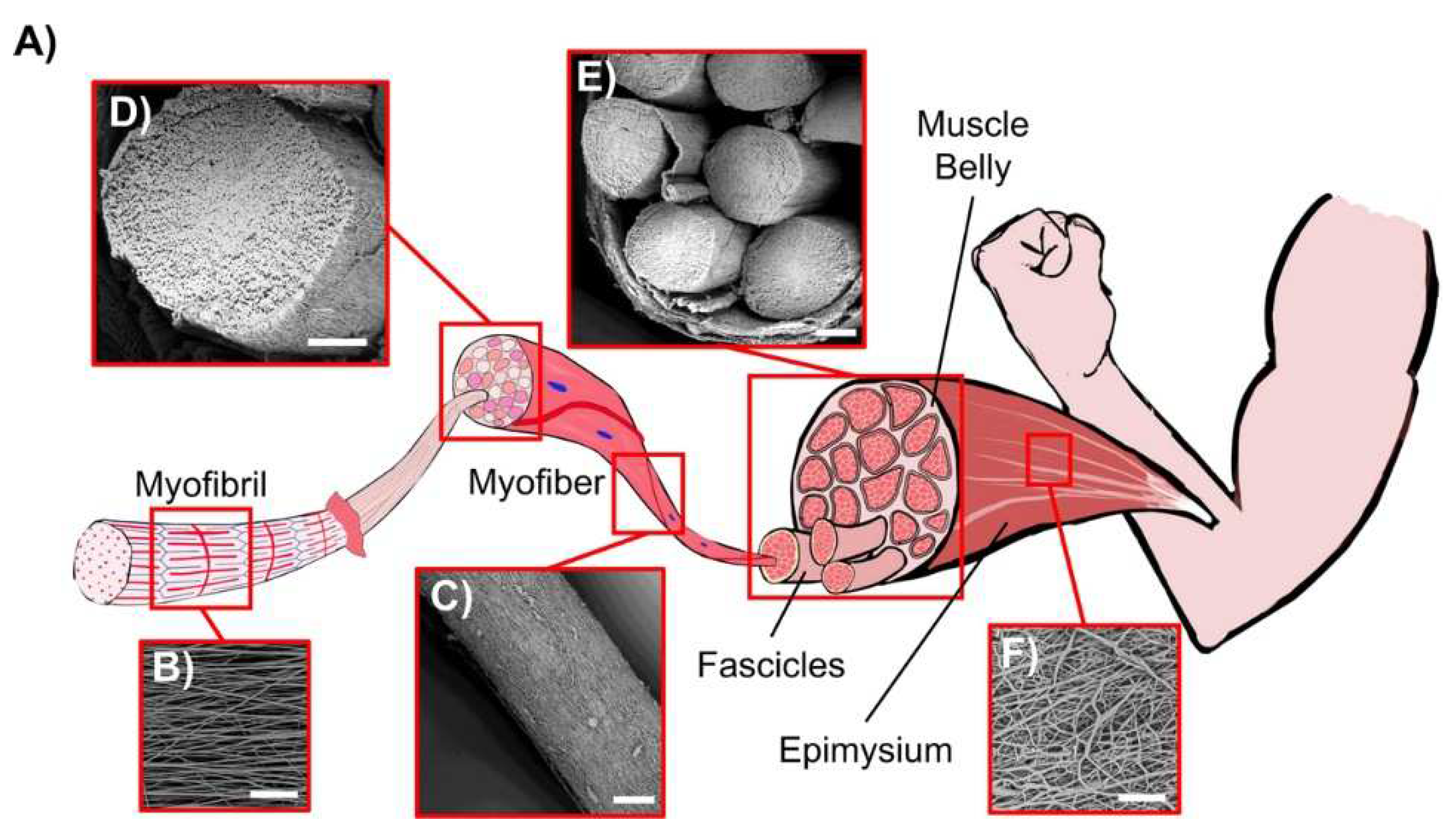
- Gotti, C.; Sensini, A.; Zucchelli, A.; Carloni, R.; Focarete, M.L. Hierarchical fibrous structures for muscle-inspired soft-actuators: A review. Appl. Mater. Today 2020, 20, 100772. [Google Scholar] [CrossRef]
- Kim, I.H.; Choi, S.; Lee, J.; Jung, J.; Yeo, J.; Kim, J.T.; Ryu, S.; Ahn, S.-K.; Kang, J.; Poulin, P.; et al. Human-muscle-inspired single fibre actuator with reversible percolation. Nat. Nanotechnol. 2022, 17, 1198–1205. [Google Scholar] [CrossRef]
- Tynan, L.; Naik, G.; Gargiulo, G.D.; Gunawardana, U. Implementation of the Biological Muscle Mechanism in HASEL Actuators to Leverage Electrohydraulic Principles and Create New Geometries. Actuators 2021, 10, 38. [Google Scholar] [CrossRef]
- Sharabi, M. Structural Mechanisms in Soft Fibrous Tissues: A Review. Front. Mater. 2022, 8. [Google Scholar] [CrossRef]
- Abrams, M.J.; Basinger, T.; Yuan, W.; Guo, C.-L.; Goentoro, L. Self-repairing symmetry in jellyfish through mechanically driven reorganization. Proc. Natl. Acad. Sci. 2015, 112, E3365–E3373. [Google Scholar] [CrossRef]
- Scorza, L.C.T.; Dornelas, M.C. Plants on the move: Towards common mechanisms governing mechanically-induced plant movements. Plant Signal. Behav. 2011, 6, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Liscum, E.; Askinosie, S.K.; Leuchtman, D.L.; Morrow, J.; Willenburg, K.T.; Coats, D.R. Phototropism: Growing towards an Understanding of Plant Movement. Plant Cell 2014, 26, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G.; Pinnock, M.-R.; Lowe, D.C.; Gay, M.S.; Markin, V.S. Complete hunting cycle of Dionaea muscipula: Consecutive steps and their electrical properties. J. Plant Physiol. 2011, 168, 109–120. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Swanson, S.J.; Gilroy, S. Touch Sensing and Thigmotropism. Plant Tropisms 2007, 91–122. [Google Scholar] [CrossRef]
- Farhan, M.; Klimm, F.; Thielen, M.; Rešetič, A.; Bastola, A.; Behl, M.; Speck, T.; Lendlein, A. Artificial Tendrils Mimicking Plant Movements by Mismatching Modulus and Strain in Core and Shell. Adv. Mater. 2023, 35, e2211902. [Google Scholar] [CrossRef]
- Li, J.; Tian, A.; Sun, Y.; Feng, B.; Wang, H.; Zhang, X. The Development of a Venus Flytrap Inspired Soft Robot Driven by IPMC. J. Bionic Eng. 2023, 20, 406–415. [Google Scholar] [CrossRef]
- Scherzer, S.; Federle, W.; Al-Rasheid, K.A.S.; Hedrich, R. Venus flytrap trigger hairs are micronewton mechano-sensors that can detect small insect prey. Nat. Plants 2019, 5, 670–675. [Google Scholar] [CrossRef]
- Kareklas, K.; Nettle, D.; Smulders, T.V. Water-induced finger wrinkles improve handling of wet objects. Biol. Lett. 2013, 9, 20120999. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Yeom, E.; Seo, S.-J.; Kim, K.; Kim, H.; Lim, J.-H.; Lee, S.J. Journey of water in pine cones. Sci. Rep. 2015, 5, 9963. [Google Scholar] [CrossRef] [PubMed]
- Reyssat, E.; Mahadevan, L. Hygromorphs: from pine cones to biomimetic bilayers. J. R. Soc. Interface 2009, 6, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Chang, Y.-C.; Tsai, M.-T.; Yang, T.-W.; Huang, M.-T.; Wu, S.-H.; Wang, C. Electrospinning of Aqueous Solutions of Atactic Poly(N-isopropylacrylamide) with Physical Gelation. Gels 2022, 8, 716. [Google Scholar] [CrossRef] [PubMed]
- Schoolaert, E.; Ryckx, P.; Geltmeyer, J.; Maji, S.; Van Steenberge, P.H.M.; D’hooge, D.R.; Hoogenboom, R.; De Clerck, K. Waterborne Electrospinning of Poly(N-isopropylacrylamide) by Control of Environmental Parameters. ACS Appl. Mater. Interfaces 2017, 9, 24100–24110. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.; Lee, J. Highly sensitive humidity-responsive actuator comprising aligned electrospun fibers containing metal–organic framework nanoparticles. Sensors Actuators B: Chem. 2021, 332, 129520. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, F.; Lerch, A.; Ionov, L.; Agarwal, S. Unusual and Superfast Temperature-Triggered Actuators. Adv. Mater. 2015, 27, 4865–4870. [Google Scholar] [CrossRef]
- Xu, Y.; Ajji, A.; Heuzey, M.-C. Tunable two-step shape and dimensional changes with temperature of a PNIPAM/CNC hydrogel. Soft Matter 2022, 18, 4437–4444. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, S.; Sun, Y.; Agarwal, S. Giving Direction to Motion and Surface with Ultra-Fast Speed Using Oriented Hydrogel Fibers. Adv. Funct. Mater. 2016, 26, 1021–1027. [Google Scholar] [CrossRef]
- Chen, T.; Bakhshi, H.; Liu, L.; Ji, J.; Agarwal, S. Combining 3D Printing with Electrospinning for Rapid Response and Enhanced Designability of Hydrogel Actuators. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Wei, X.; Xue, Y.; Sun, Y.; Chen, L.; Zhang, C.; Wu, Q.; Peng, S.; Ma, C.; Liu, Z.; Jiang, S.; et al. A robust anisotropic light-responsive hydrogel for ultrafast and complex biomimetic actuation via poly(pyrrole)-coated electrospun nanofiber. Chem. Eng. J. 2023, 452. [Google Scholar] [CrossRef]
- Miranda, D.O.; Dorneles, M.F.; Oréfice, R.L. One-step process for the preparation of fast-response soft actuators based on electrospun hybrid hydrogel nanofibers obtained by reactive electrospinning with in situ synthesis of conjugated polymers. Polymer 2020, 200, 122590. [Google Scholar] [CrossRef]
- Shang, J.; Le, X.; Zhang, J.; Chen, T.; Theato, P. Trends in polymeric shape memory hydrogels and hydrogel actuators. Polym. Chem. 2019, 10, 1036–1055. [Google Scholar] [CrossRef]
- Löwenberg, C.; Balk, M.; Wischke, C.; Behl, M.; Lendlein, A. Shape-Memory Hydrogels: Evolution of Structural Principles To Enable Shape Switching of Hydrophilic Polymer Networks. Accounts Chem. Res. 2017, 50, 723–732. [Google Scholar] [CrossRef]
- Gu, X.; Mather, P.T. Water-triggered shape memory of multiblock thermoplastic polyurethanes (TPUs). RSC Adv. 2013, 3, 15783–15791. [Google Scholar] [CrossRef]
- Ang, J.Y.; Chan, B.Q.Y.; Kai, D.; Loh, X.J. Engineering Porous Water-Responsive Poly(PEG/PCL/PDMS Urethane) Shape Memory Polymers. Macromol. Mater. Eng. 2017, 302. [Google Scholar] [CrossRef]
- Wei, X.; Chen, L.; Wang, Y.; Sun, Y.; Ma, C.; Yang, X.; Jiang, S.; Duan, G. An Electrospinning Anisotropic Hydrogel with Remotely-Controlled Photo-Responsive Deformation and Long-Range Navigation for Synergist Actuation. Chem. Eng. J. 2022, 433, 134258. [Google Scholar] [CrossRef]
- Liu, L.; Bakhshi, H.; Jiang, S.; Schmalz, H.; Agarwal, S. Composite Polymeric Membranes with Directionally Embedded Fibers for Controlled Dual Actuation. Macromol. Rapid Commun. 2018, 39, e1800082. [Google Scholar] [CrossRef]
- Cho, K.; Kang, D.; Lee, H.; Koh, W.-G. Multi-stimuli responsive and reversible soft actuator engineered by layered fibrous matrix and hydrogel micropatterns. Chem. Eng. J. 2022, 427, 130879. [Google Scholar] [CrossRef]
- Liu, W.; He, Y.; Leng, J. Humidity-Responsive Shape Memory Polyurea with a High Energy Output Based on Reversible Cross-Linked Networks. ACS Appl. Mater. Interfaces 2023, 15, 2163–2171. [Google Scholar] [CrossRef]
- Ren, L.; Li, B.; Song, Z.; Liu, Q.; Ren, L.; Zhou, X. Bioinspired fiber-regulated composite with tunable permanent shape and shape memory properties via 3d magnetic printing. Compos. Part B: Eng. 2019, 164, 458–466. [Google Scholar] [CrossRef]
- Ren, L.; Li, B.; Song, Z.; Liu, Q.; Ren, L.; Zhou, X. 3D printing of structural gradient soft actuators by variation of bioinspired architectures. J. Mater. Sci. 2019, 54, 6542–6551. [Google Scholar] [CrossRef]
- Salaris, V.; Leonés, A.; Lopez, D.; Kenny, J.M.; Peponi, L. Shape-Memory Materials via Electrospinning: A Review. Polymers 2022, 14, 995. [Google Scholar] [CrossRef]
- Zare, M.; Davoodi, P.; Ramakrishna, S. Electrospun Shape Memory Polymer Micro-/Nanofibers and Tailoring Their Roles for Biomedical Applications. Nanomaterials 2021, 11, 933. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, F.; Wang, L.; Liu, Y.; Leng, J. 18 - Electrospun shape-memory polymer fibers and their applications. In Woodhead Publishing Series in Composites Science and Engineering, Y. Dong, A. Baji, and S. B. T.-E. P. and C. Ramakrishna, Eds. Woodhead Publishing, 2021, pp. 567–596.
- Zhang, Q.; Rudolph, T.; Benitez, A.J.; Gould, O.E.C.; Behl, M.; Kratz, K.; Lendlein, A. Temperature-controlled reversible pore size change of electrospun fibrous shape-memory polymer actuator based meshes. Smart Mater. Struct. 2019, 28, 055037. [Google Scholar] [CrossRef]
- Sauter, T.; Kratz, K.; Farhan, M.; Heuchel, M.; Lendlein, A. Design and fabrication of fiber mesh actuators. Appl. Mater. Today 2022, 29, 101562. [Google Scholar] [CrossRef]
- Izraylit, V.; Heuchel, M.; Kratz, K.; Lendlein, A. Non-woven shape-memory polymer blend actuators. MRS Adv. 2021, 6, 781–785. [Google Scholar] [CrossRef]
- Banitaba, S.N.; et al. Biopolymer-based electrospun fibers in electrochemical devices: versatile platform for energy, environment, and health monitoring. Mater. Horizons 2022, 9, 2914–2948. [Google Scholar] [CrossRef]
- Liu, L.; Xu, W.; Ding, Y.; Agarwal, S.; Greiner, A.; Duan, G. A review of smart electrospun fibers toward textiles. Compos. Commun. 2020, 22, 100506–100506. [Google Scholar] [CrossRef]
- Wang, X.-X.; Yu, G.-F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar] [CrossRef]
- Kosidlo, U.; et al. Nanocarbon based ionic actuators—a review. Smart Mater. Struct. 2013, 22, 104022. [Google Scholar] [CrossRef]
- García-Gallegos, J.C.; Martín-Gullón, I.; Conesa, J.A.; Vega-Cantú, Y.I.; Rodríguez-Macías, F.J. The effect of carbon nanofillers on the performance of electromechanical polyaniline-based composite actuators. Nanotechnology 2016, 27, 15501. [Google Scholar] [CrossRef] [PubMed]
- Bar-Cohen, Y.; Anderson, I.A. Electroactive polymer (EAP) actuators—background review. Mech. Soft Mater. 2019, 1, 5. [Google Scholar] [CrossRef]
- Deng, Q.; Jia, H.; An, C.; Wu, S.; Zhao, S.; Hu, N. Progress and prospective of electrochemical actuator materials. Compos. Part A: Appl. Sci. Manuf. 2023, 165, 107336. [Google Scholar] [CrossRef]
- Martins, P.; Correia, D.M.; Correia, V.; Lanceros-Mendez, S. Polymer-based actuators: back to the future. Phys. Chem. Chem. Phys. 2020, 22, 15163–15182. [Google Scholar] [CrossRef]
- Serrano-Garcia, W.; Bonadies, I.; Thomas, S.W.; Guarino, V. New Insights to Design Electrospun Fibers with Tunable Electrical Conductive–Semiconductive Properties. Sensors 2023, 23, 1606. [Google Scholar] [CrossRef]
- Zong, H.; Xia, X.; Liang, Y.; Dai, S.; Alsaedi, A.; Hayat, T.; Kong, F.; Pan, J.H. Designing function-oriented artificial nanomaterials and membranes via electrospinning and electrospraying techniques. Mater. Sci. Eng. C 2018, 92, 1075–1091. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Conductive Electrospun Nanofiber Mats. Materials 2019, 13, 152. [Google Scholar] [CrossRef]
- Bittencourt, J.C.; Gois, B.H.d.S.; de Oliveira, V.J.R.; Agostini, D.L.d.S.; Olivati, C.d.A. Gas sensor for ammonia detection based on poly(vinyl alcohol) and polyaniline electrospun. J. Appl. Polym. Sci. 2019, 136, 47288. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, K.; Sun, B.; Fang, J.; Zhang, K.; Ei-Hamshary, H.; Al-Deyab, S.S.; Mo, X. The aligned core–sheath nanofibers with electrical conductivity for neural tissue engineering. J. Mater. Chem. B 2014, 2, 7945–7954. [Google Scholar] [CrossRef]
- Bhang, S.H.; Jeong, S.I.; Lee, T.-J.; Jun, I.; Bin Lee, Y.; Kim, B.-S.; Shin, H. Electroactive Electrospun Polyaniline/Poly[(L -lactide)-co- (ε -caprolactone)] Fibers for Control of Neural Cell Function. Macromol. Biosci. 2012, 12, 402–411. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.; Zhang, H.; Zhang, Q.; Wang, L.; Li, C.; Dai, B.; Yang, J.; Liu, J.; Sun, D. Electrically-responsive core-shell hybrid microfibers for controlled drug release and cell culture. Acta Biomater. 2017, 55, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Laforgue, A.; Robitaille, L. Production of Conductive PEDOT Nanofibers by the Combination of Electrospinning and Vapor-Phase Polymerization. Macromolecules 2010, 43, 4194–4200. [Google Scholar] [CrossRef]
- Ismail, Y.A.; Shin, M.K.; Kim, S.J. A nanofibrous hydrogel templated electrochemical actuator: From single mat to a rolled-up structure. Sensors Actuators B Chem. 2009, 136, 438–443. [Google Scholar] [CrossRef]
- Li, J.; Vadahanambi, S.; Kee, C.-D.; Oh, I.-K. Electrospun Fullerenol-Cellulose Biocompatible Actuators. Biomacromolecules 2011, 12, 2048–2054. [Google Scholar] [CrossRef]
- Qian, L.; Chen, C.; Huang, Y.; Ren, H.; Cao, X.; He, B.; Li, J. Nanocellulose-based electroactive actuators and their performance with various ions. Cellulose 2023, 30, 4455–4468. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Wei, Y.; MacDiarmid, A.G.; Lelkes, P.I. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [Google Scholar] [CrossRef]
- Ismail, Y.A.; Martínez, J.G.; Al Harrasi, A.S.; Kim, S.J.; Otero, T.F. Sensing characteristics of a conducting polymer/hydrogel hybrid microfiber artificial muscle. Sensors Actuators B: Chem. 2011, 160, 1180–1190. [Google Scholar] [CrossRef]
- Ismail, Y.A.; Shin, S.R.; Shin, K.M.; Gil Yoon, S.; Shon, K.; Kim, S.I.; Kim, S.J. Electrochemical actuation in chitosan/polyaniline microfibers for artificial muscles fabricated using an in situ polymerization. Sensors Actuators B Chem. 2008, 129, 834–840. [Google Scholar] [CrossRef]
- Florczak, S.; Lorson, T.; Zheng, T.; Mrlik, M.; Hutmacher, D.W.; Higgins, M.J.; Luxenhofer, R.; Dalton, P.D. Melt electrowriting of electroactive poly(vinylidene difluoride) fibers. Polym. Int. 2019, 68, 735–745. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.; Roca, M.I.; Martinez, J.G.; Meseguer-Olmo, L.; Cenis, J.L.; Moraleda, J.M.; Otero, T.F. Fabrication of conductive electrospun silk fibroin scaffolds by coating with polypyrrole for biomedical applications. Bioelectrochemistry 2012, 85, 36–43. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, X.; Xu, Y.; Shu, H.; Jia, W. Effect of carboxymethyl cellulose on the output force and electrochemical performance of sodium alginate ion electric actuator. Sensors Actuators A: Phys. 2022, 339, 113269. [Google Scholar] [CrossRef]
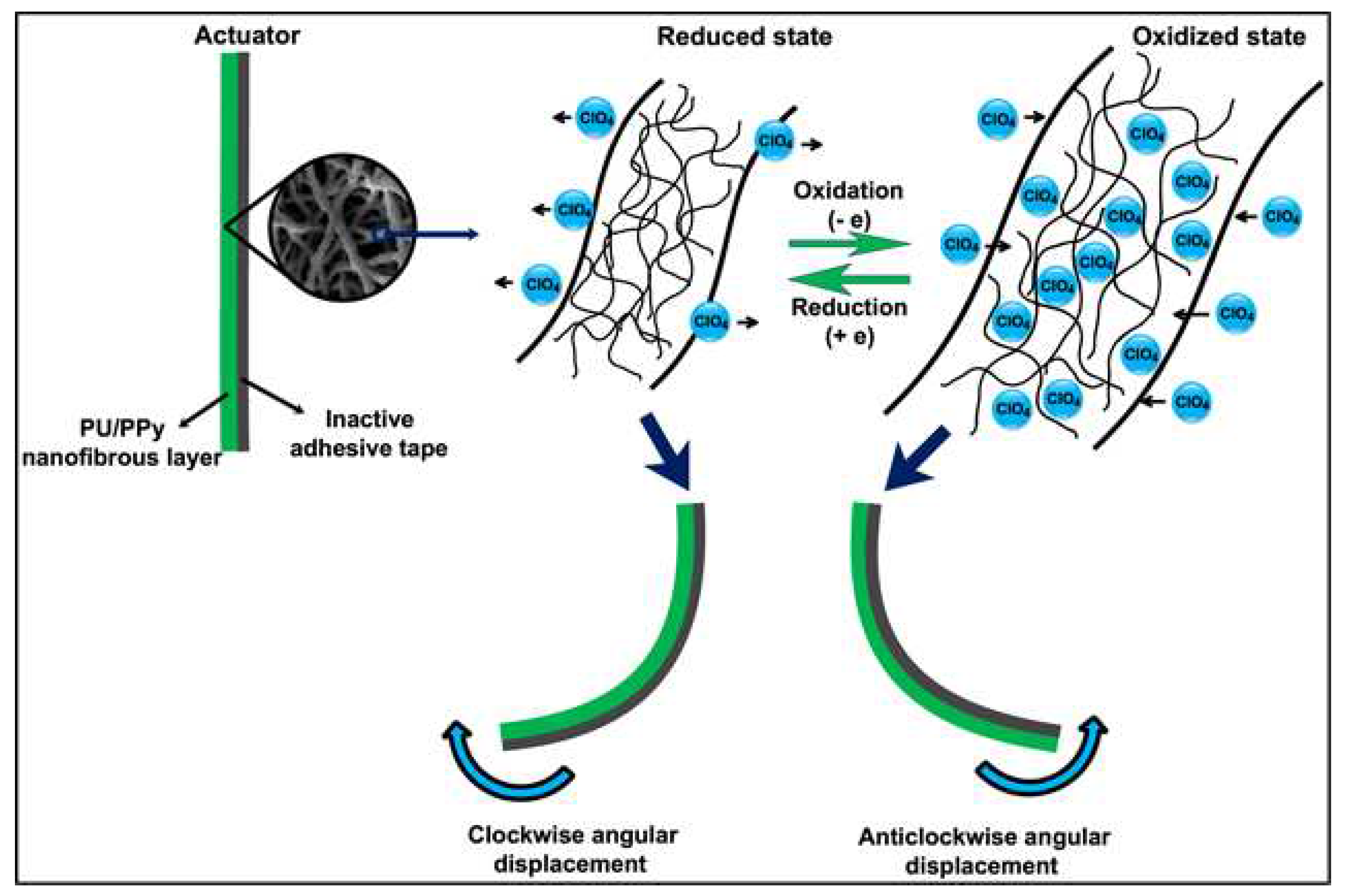
- Ebadi, S.V.; Semnani, D.; Fashandi, H.; Rezaei, B.; Fakhrali, A. Gaining insight into electrolyte solution effects on the electrochemomechanical behavior of electroactive PU/PPy nanofibers: Introducing a high-performance artificial muscle. Sensors Actuators B Chem. 2020, 305. [Google Scholar] [CrossRef]
- Ebadi, S.V.; Semnani, D.; Fashandi, H.; Rezaei, B. Synthesis and characterization of a novel polyurethane/polypyrrole-p-toluenesulfonate (PU/PPy-pTS) electroactive nanofibrous bending actuator. Polym. Adv. Technol. 2019, 30, 2261–2274. [Google Scholar] [CrossRef]
- Mottaghitalab, V.; Xi, B.; Spinks, G.M.; Wallace, G.G. Polyaniline fibres containing single walled carbon nanotubes: Enhanced performance artificial muscles. Synth. Met. 2006, 156, 796–803. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Martinez, J.G. Ionic exchanges, structural movements and driven reactions in conducting polymers from bending artificial muscles. Sensors Actuators B Chem. 2014, 199, 27–30. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G.; Arias-Pardilla, J. Biomimetic electrochemistry from conducting polymers. A review: Artificial muscles, smart membranes, smart drug delivery and computer/neuron interfaces. Electrochim. Acta 2012, 84, 112–128. [Google Scholar] [CrossRef]
- Beregoi, M.; Busuioc, C.; Evanghelidis, A.; Matei, E.; Iordache, F.; Radu, M.; Dinischiotu, A.; Enculescu, I. Electrochromic properties of polyaniline-coated fiber webs for tissue engineering applications. Int. J. Pharm. 2016, 510, 465–473. [Google Scholar] [CrossRef]
- Beregoi, M.; Evanghelidis, A.; Matei, E.; Enculescu, I. Polyaniline based microtubes as building-blocks for artificial muscle applications. Sensors Actuators B Chem. 2017, 253, 576–583. [Google Scholar] [CrossRef]
- Gu, B.K.; Ismail, Y.A.; Spinks, G.M.; Kim, S.I.; So, I.; Kim, S.J. A Linear Actuation of Polymeric Nanofibrous Bundle for Artificial Muscles. Chem. Mater. 2009, 21, 511–515. [Google Scholar] [CrossRef]
- Fengel, C.V.; Bradshaw, N.P.; Severt, S.Y.; Murphy, A.R.; Leger, J.M. Biocompatible silk-conducting polymer composite trilayer actuators. Smart Mater. Struct. 2017, 26, 055004. [Google Scholar] [CrossRef]
- Kim, S.-S.; Kee, C.-D. Electro-active polymer actuator based on PVDF with bacterial cellulose nano-whiskers (BCNW) via electrospinning method. Int. J. Precis. Eng. Manuf. 2014, 15, 315–321. [Google Scholar] [CrossRef]
- Beregoi, M.; Beaumont, S.; Jinga, S.I.; Otero, T.F.; Enculescu, I. Chemical sensing and actuation properties of polypyrrole coated fibers. Smart Mater. Struct. 2022, 31. [Google Scholar] [CrossRef]
- Beregoi, M.; Evanghelidis, A.; Diculescu, V.C.; Iovu, H.; Enculescu, I. Polypyrrole Actuator Based on Electrospun Microribbons. ACS Appl. Mater. Interfaces 2017, 9, 38068–38075. [Google Scholar] [CrossRef] [PubMed]
- Bunea, M.-C.; Beregoi, M.; Evanghelidis, A.; Galatanu, A.; Enculescu, I. Direct and remote induced actuation in artificial muscles based on electrospun fiber networks. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Kwon, J.K.; Yoo, H.J.; Cho, J.W. Conducting core-sheath polyurethane-PEDOT nanofibres for conducting polymer actuator. Int. J. Nanotechnol. 2013, 10, 661. [Google Scholar] [CrossRef]
- Harjo, M.; Zondaka, Z.; Leemets, K.; Järvekülg, M.; Tamm, T.; Kiefer, R. Polypyrrole-coated fiber-scaffolds: Concurrent linear actuation and sensing. J. Appl. Polym. Sci. 2020, 137, 48533. [Google Scholar] [CrossRef]
- Beregoi, M.; Preda, N.; Evanghelidis, A.; Costas, A.; Enculescu, I. Versatile Actuators Based on Polypyrrole-Coated Metalized Eggshell Membranes. ACS Sustain. Chem. Eng. 2018, 6, 10173–10181. [Google Scholar] [CrossRef]
- Gotti, C.; Sensini, A.; Fornaia, G.; Gualandi, C.; Zucchelli, A.; Focarete, M.L. Biomimetic Hierarchically Arranged Nanofibrous Structures Resembling the Architecture and the Passive Mechanical Properties of Skeletal Muscles: A Step Forward Toward Artificial Muscle. Front. Bioeng. Biotechnol. 2020, 8, 767. [Google Scholar] [CrossRef]
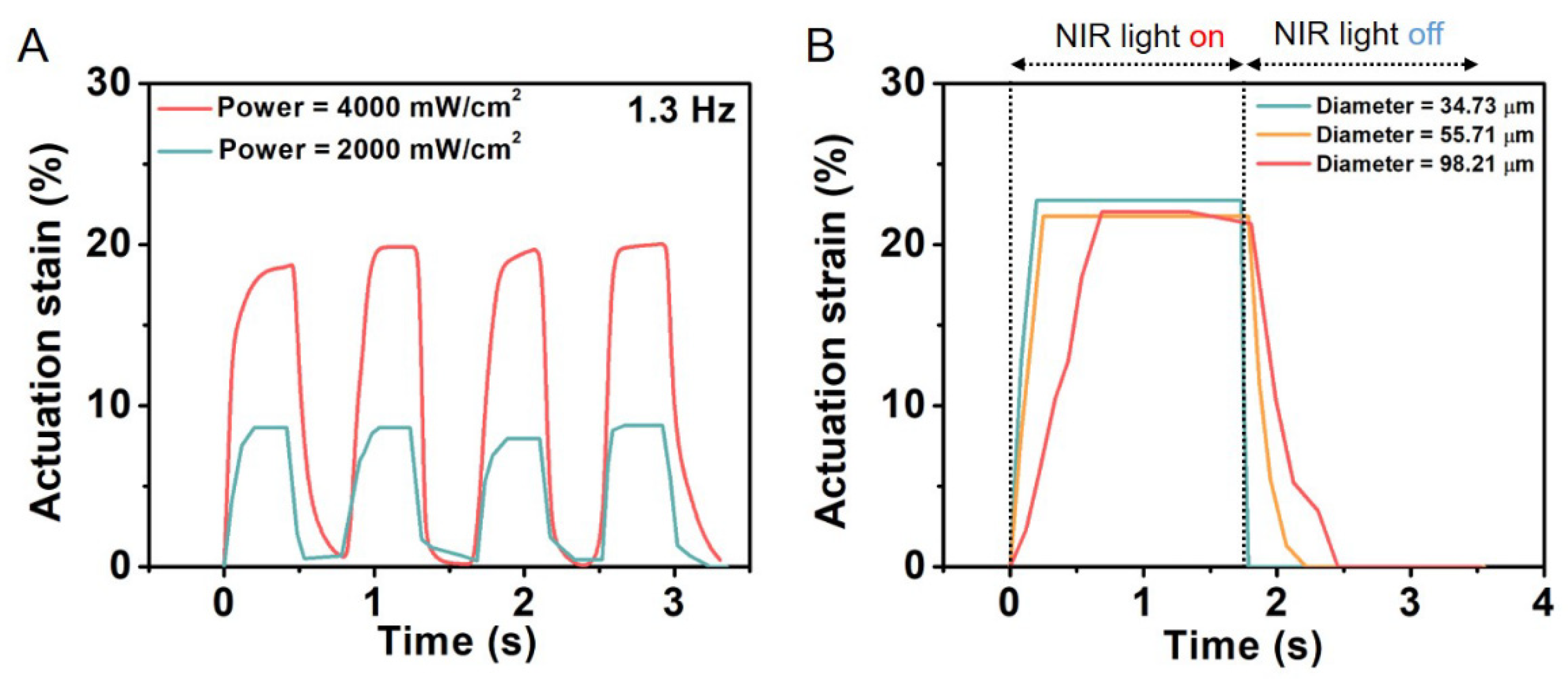
- He, Q.; Wang, Z.; Wang, Y.; Wang, Z.; Li, C.; Annapooranan, R.; Zeng, J.; Chen, R.; Cai, S. Electrospun liquid crystal elastomer microfiber actuator. Sci. Robot. 2021, 6, eabi9704. [Google Scholar] [CrossRef] [PubMed]
- Löwenberg, C.; Balk, M.; Wischke, C.; Behl, M.; Lendlein, A. Shape-Memory Hydrogels: Evolution of Structural Principles To Enable Shape Switching of Hydrophilic Polymer Networks. Accounts Chem. Res. 2017, 50, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- El-Sayed, H.; Vineis, C.; Varesano, A.; Mowafi, S.; Carletto, R.A.; Tonetti, C.; Taleb, M.A. A critique on multi-jet electrospinning: State of the art and future outlook. Nanotechnol. Rev. 2019, 8, 236–245. [Google Scholar] [CrossRef]
- Lee, J.; Moon, S.; Lahann, J.; Lee, K.J. Recent Progress in Preparing Nonwoven Nanofibers via Needleless Electrospinning. Macromol. Mater. Eng. 2023, 2300057. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




