Introduction
Inorganic soil nutrients are the fundaments of almost all life on Earth. Plants take up inorganic nutrients and then build photosynthetic reactors to fix carbon (C), the skeleton of organic compounds. In a complex food web, plants, as primary producers, are the basic food of consumers, especially that of arthropods and large mammals.
Herbivory by arthropods is a common biotic stress influencing plant growth and productivity. On one side, herbivory can have a direct effect by removing plant tissues. On the other side, herbivory can indirectly affect plant growth by changing the host plants´ primary or secondary metabolic profile. In order to survive, plants have evolved defense strategies that can prevent or mitigate damage by herbivory. Resistance and tolerance are two defense strategies of plants against pathogens and pests (Rosenthal and Kotanen, 1994; Strauss and Agrawal, 1999, illustrated in
Figure 1). The resistance strategy aims to diminish the performance or host preference of herbivores (Belsky, 1993; Strauss and Agrawal, 1999). The tolerance strategy comprises plant regrowth after damage by herbivores; this is also known as ‘compensatory growth’ (Belsky, 1993; Strauss and Agrawal, 1999). There is a common theory that trade-offs exist between resistance-and tolerance-defense mechanisms. Moreover, increasing empirical evidence suggests that plants have evolved a mixed pattern of resistance-tolerance strategies (Carmona and Fornoni, 2013; Leimu and Koricheva, 2006). In other words, plants can allocate resources to both strategies simultaneously (Leimu and Koricheva, 2006). The engagement of different defense strategies suggests allocation of nutrient elements to divergent metabolites. As reported recently, nutrients and herbivory can jointly determine grassland seed bank abundance (Eskelinen et al., 2023).
So far, there is still less known about how nutrients play a role in plant-herbivore and microbe-plant-herbivore interactions. Moreover, many studies demonstrate that microbes can induce plant resistance to herbivore damage, compared to fewer studies on plant tolerance strategy. In this review, I summarize the role of nutrients in plant defense response to herbivory and further strengthen the mechanism of beneficial microbes involving in plant defense responses on plant physiological and molecular level. This review will give more comprehensive insights into the role of nutrients and microbes, as critical players, in plant defense response. Knowledge about the relation between nutrients and defense may affect the methodology of applying differentiated nutrients to mitigate or compensate pest damage and maximize crop production.
Nutrient availability affects plant-herbivore interaction
Plants respond to herbivore feeding attack by conveying resistance and tolerance responses. Plant nutrient status and associated soil microbes can affect these responses.
Nutrient supply from the soil or artificially fertilized substrates
Soil fertilization changes plant nutrient content and consequently impacts on the preference and performance of insects (Sarfraz et al., 2009). There are two ways of nutrients impacting on herbivore performance. First, enhanced nutrient supply directly improves herbivore performance due to better nutritional quality of plant material available for the herbivores. For example, a positive correlation between foliar content of nitrogen (N) and phosphorus (P) and insect herbivores performance was found, also suggesting a potential role for magnesium (Mg), sodium (Na) and potassium (K) in plant-insect interactions (Joern et al., 2012). Similarly, P in the diet is likely to affect the larval growth rate and population dynamics (Perkins et al., 2004). Moreover, the decline of P content of lupin is associated with decreased larval growth and survival, which leads to an observed negative relationship between herbivore abundance and host density (Apple et al., 2009). Furthermore, the availability of N in soils is limited by P, so the effects of plant tissue quantity or quality mediated by P are vital to the growth of insect herbivores in the N and P co-limited conditions (Bishop et al., 2010).
Second, nutrients affect the production of defensive compounds in plants, thereby indirectly influencing herbivore performance. As shown for
Plantago spp., fertilized plants have lower total iridoid glycosides (defense compounds) and lower aucubin (one of the iridoid glycosides) levels than unfertilized plants (Prudic et al., 2005). Similarly, a high dose of P decreases the alkaloid content and thus weakens the effectiveness of the defensive system in a natural grassland (Graff et al., 2020). Utilization of K fertilizer, however, minimizes the incidence of diseases and pest invasion in most cases (Amtmann et al., 2008). Apart from the above cases, there are numerous studies showing that different elements lead to distinct effects on plant defenses (
Table S1). Admittedly, these responses also highly depend on the context of bioassays used.
Nutrient supply from the microbes interacting with plant roots
Beneficial microbes, such as phosphate solubilizing microorganisms, nitrogen-fixing microbes and symbiotic microorganisms, can promote plant nutrient uptake. Besides the direct effect of fertilization on plant defense responses, the interaction of host plant roots with beneficial microbes may affect plant-herbivore interactions via nutrients. For example, nutrient elements promote herbivory and pathogen intrusion in diversely mycorrhiza-colonized tree monocultures and mixtures (Ferlian et al., 2021). Adequate nutrient provision may drive shifts among plant defense strategies, which can lead to decreased allocation to active antiherbivore defenses but increased investment in regrowth and tolerance to herbivory (Coley et al., 1985). Under nutrient-impoverished environments, AM (arbuscular mycorrhizal) fungi can improve the accessibility of plants to nutrients, and also AM-colonized plants are armed to defend against pathogens (Chen et al., 2019). In return, nutrient-rich plants directly provide palatable food for herbivores, which is in exchange for fungal-acquired nutrients (Wilkinson et al., 2019). Moreover, in addition to improving access to N and P, AM fungi can also enhance the acquisition of other elements which are important for plant defense. For example, when silicon in the soil is limiting, AM fungi can increase the uptake of silicon and thus augment silicon-based resistance to herbivores (Frew et al., 2017).
Apart from AM fungi, other microbes are also involved in the provision of nutrient uptake or transport. The growth-promoting rhizobacteria GB03 induce the transcripts encoding for sulfur (S)-rich aliphatic and indolic glucosinolates (Aziz et al., 2016). As a result, GB03-infested plants enhance S assimilation and then strengthen the sophisticated involvement of microbial signaling in plant defense against
Spodoptera exigua (Aziz et al., 2016). Virus-infection of host plants causes an almost 30-fold rise in overall phloem amino acids (AAs) but the phloem composition rapidly changes upon the arrival of whiteflies, and finally, the effect of colonization by whiteflies outcompetes the effect of the virus on AA composition (Ángeles-López et al., 2016). Root colonization by nitrogen-fixing bacteria is more abundant on silicon-supplemented plant roots, which makes
Medicago sativa more susceptible to aphids because of the increase of essential foliar AAs due to high levels of root nodulation (Johnson et al., 2017). There are greater levels of total N, essential AAs and vitamins of the B-group (VBs) in fungal tissue and fungus-infected leaves, so caterpillars prefer to feed on black poplar foliage infected by fungus (Eberl et al., 2020). There is a prediction that endophytes with varied alkaloid-producing levels are favored under different levels of herbivory and soil nutrients in terms of N limitations to the host plants (Faeth and Fagan, 2002). Apart from the above examples, other elements or nutrients involved in microbe-plant-herbivore interactions are listed in
Tables S2 and S3.
Overall, it is highly important to interpret herbivore performance with their ecological stoichiometry (Huberty and Denno, 2006). Differentiated nutrient levels contribute to the adjustment of the herbivore population. An increase in plant or soil nutrient heterogeneity within plant and microbe communities could help to maintain the sustainable control of insect pests in ecosystems (Wetzel et al., 2016). Therefore, from an agricultural perspective, increasing crop varieties by enlarging genetic diversity within varieties or by mixing varieties, following with high nutrient variance, would mitigate the loss of crop production by pests.
Herbivory drives nutrient exchange between plant and microbial communities
From the concepts of herbivory in ecosystem dynamics, there are two points: on one side, herbivory can decelerate nutrient cycling and reduce plant abundance; on another side, herbivory can facilitate nutrient cycling, which may lead to a higher plant abundance (Belovsky and Slade, 2000). From the large number of recent studies (Bell et al., 2022; Eskelinen et al., 2023; Li et al., 2022; Zeng et al., 2022), here I discuss that herbivory contributes to the nutrient exchange in multitrophic levels (
Figure 2).
In the presence of microbes that interact with plants, N, C and P are the most frequent nutrients addressed in the selected studies of microbe-plant-herbivore interactions (
Figure 3). There are probably other nutrients to be analyzed, how they are affected by tripartite interactions. Nevertheless, it is well known that soil microbes affect plant nutrient uptake either in a positive or negative manner. To see the overall effect of herbivory on plant nutrient content in the plant-microbe interactions, I further selected the studies with approachable digital data of plant N and C content, and performed a meta-analysis (
Figure 4). It is visible that herbivory has a slightly positive overall effect on plant N and C content, but not statistically significant (
Figure 4). The results advance our current understanding of taking advantage of soil microbes in crop production.
The impact of belowground herbivory on nutrient exchange
Belowground herbivory can affect nutrient shifts between plants, herbivores and soil organisms. The effect of belowground herbivory on plant growth performance depends on the nutrient conditions of substrates and the composition of microbial communities. For instance, the belowground herbivory by nematodes enhances plant growth and nutrient uptake under C-and N-abundant substrates, owing to pivotal interactions between plants and soil microbial communities which increases nutrient availability (Gebremikael et al., 2016). The combined interaction of the root-knot nematode Medloidogyne incognita and endogenic earthworm Octolasion tyrtaeum with roots of Brassica oleracea, leads to increased N uptake, shoot biomass and microbial biomass in soil (Wurst et al., 2006). However, earthworms mobilize more soil N than litter N while nematodes are inclined to improve the microbial biomass in soil (Wurst et al., 2006). Indeed, there exists a complex nutrient flow and cycling between soil substrates (including organic and inorganic matter), soil microbial communities and plants. As shown, the belowground root-feeding beetle larvae increase plant photosynthetic products in soil; however, they lead to an 8% decrease in total soil carbon along with a 13% and 16% increase of C and N in microbial biomass, respectively (Gan et al., 2018). Root herbivores speed up C flow into soil, and thus facilitate the decomposition of existing soil organic matter (Gan et al., 2018). Additionally, nematodes affect belowground N fluxes and induce N transfer from legumes to non-N-fixing plants via the soil microbial community (Ayres et al., 2007).
In a certain number of cases, however, root herbivores can downregulate plant defense responses and have adverse effects on plant growth. For instance, root herbivory and soil fertilizer affect the defensive chemistry of Citrus aurantium, with increased root protein and peroxidase content but decreased activities of chitinase and glucanase related to the resistance to microbial pathogens (Borowicz et al., 2003). Root herbivory has an overall strong negative effect on plant growth and reproduction, indicating direct negative effects over any potential indirect benefits (Barber et al., 2015). Moreover, the effects of root herbivory on root defensive chemistry may not align with its effects on leaf chemical profile, underlining the need for research that integrates aboveground and belowground interactions (Borowicz et al., 2003).
The impact of aboveground herbivory on nutrient exchange
Aboveground herbivory does not only mediate the nutrient exchange in the direct uptake pathway via root epidermis or root hairs but also in the indirect pathway via associated microbes. Meanwhile, aboveground herbivory does not only impact on the soil nutrient availability for plants, but also on the way of plants´ nutrient acquisition (Barthelemy et al., 2017). For example, oak seedlings mitigate insect herbivore damage with a set of allocation shifts - increased foliar C, remained C rhizodeposition and N assimilation, and shifted N resources to storage in taproot and stem tissues (Frost and Hunter, 2008). In the indirect pathway, the positive effect of herbivore removal/feeding on plant growth is accomplished via the changes in soil properties and thus it can solve the high grazing pressure on plants (Barthelemy et al., 2019). Moreover, the aboveground herbivory can reduce the allocation of carbon to shoots but increase C fluxes belowground including roots, root exudates and rhizosphere respiration, thus enhancing C resources available to microbial populations (Holland, 1995; Holland et al., 1996). Strikingly, soil legacy by grazing can exert long-lasting effects on plant productivity and ecosystem functioning even after grazing has ceased (Barthelemy et al., 2019).
Aphid has been investigated as a model aboveground herbivore organism in microbe-plant-insect interactions. Aphids decrease the plant N uptake by the direct pathway as a result of microbial immobilization and by the indirect pathway probably due to the interaction of microbial immobilization and C stress (Katayama et al., 2014). Interestingly, another study shows that plants in low N conditions and exposed to aphids (without herbivore damage) promote N-fixing activity (Zekveld and Markham, 2011). This suggests that the effect of herbivores on plants needs to be separated from the presence of herbivores and their removal of plant tissues (Zekveld and Markham, 2011). Under resource shortage and herbivore attack by grasshoppers, the strategy of C partitioning between aboveground and belowground changes the relationship of the source and sink to compensate for the removal of the aboveground tissue (Potthast et al., 2021). Besides, severe short-term herbivory increases ecosystem N cycling by N immobilization and by efficiently preventing N draining (Potthast et al., 2021).
Carbon-nutrient balance theory provided by Bryant et al. (1983) conveys the concept that in nutrient-limited conditions chemical defenses are largely determined by C: In abundant nutrient or low-carbon environments carbon-based defenses decline, but nitrogen-based defenses become pronounced. Nutrient availability can dominate the impact of mammals on soil carbon and nitrogen pools in grassland, and it shows that grazed and fertilized zones retain the highest mean soil C and N pools (Sitters et al., 2020). Surplus fixed carbon, as a consequence of growth limitation imposed by insufficient nutrient or water supply, or low temperature or elevated atmospheric CO2, drives allocation and plant-soil interactions (Prescott et al., 2020).
Soil nutrients, invasive plants and insect herbivores jointly determine the aboveground and belowground responses, and the combined effects may be independent or interdependent, which vary with the scale (Wright et al., 2014). Overall, it is necessary to integrate aboveground and belowground organisms into a dynamic and sophisticated ecosystem while studying the effect of nutrients on multitrophic interactions.
Herbivory drives nutrient exchange between plant and mycorrhizal fungi
The reciprocal nutrient exchange between plants and mycorrhizal fungi is the driving force of AM associations. AM fungi contribute to the transfer of inorganic and organic N into host root cells (Hodge and Fitter, 2010; Miransari, 2011), whereas the phosphate (Pi) level of plants is an essential signal for AM symbiosis establishment and maintenance (Kobae et al., 2016). Nutrients taken up by AM fungi, such as N, P, K, and S, are transferred to the plant in exchange for C (Wang et al., 2017). AM fungi have a biotrophic lifestyle and demand C from the host plant. So far, there are two forms of C transportation reported, “sugar pathway” and “lipid pathway” (Luginbuehl et al., 2017; Manck-Götzenberger and Requena, 2016). However, the relative contribution of each pathway in an individual is still less known. The extent of mycorrhizal colonization within grass host plants is strongly affected by C assimilation and allocation (Wearn and Gange, 2007).
The plant regrowth, mycorrhizal symbionts, and aboveground herbivores act as competitors for the host´s C reserves (Piippo et al., 2011). Aphids affect the balance of nutrient exchange between plants and AM fungi because plants transfer less C to mycorrhizal while fungal P supply to plants remains (Charters et al., 2020). The presence of nematodes disrupted C for nutrient exchange between plants and AMF, with plant C and mycorrhiza-mediated P overwhelmingly obtained by the nematodes (Bell et al., 2022). Moreover, foliar herbivory alters direct and indirect Pi uptake pathways (Zeng et al., 2022). Notably, salinity, as environmental stress, alter the relationship between plants and AMF and thereafter also moderates animal grazing pressure (Ba et al., 2012). The researchers should not ignore that some abiotic factors like salinity and light might fine-tune the course of nutrient exchange.
There is a contradictory case which says that the attractiveness of beans to aphids is positively correlated with AM fungi though neither P treatment nor leaf P content affects the attractiveness of plants to aphids; instead, the mechanism is likely to manipulate by AM colonization-induced plant systemic signaling (Babikova et al., 2014). The mycelial network could act as a pipeline to transmit signals; among them, defense enzymes, volatile organic compounds, C and N content are involved in the defense process (Yu et al., 2022). In conclusion, herbivory would probably be a major driver of symbiont prevalence in native plant populations.
Mutual effects of nutrients on rhizobia-plant-herbivore interactions
Herbivory also affects nutrient fluxes between plants and another beneficial microbe – rhizobia. As reported, herbivory drives distinct plant allocation strategies across soil nitrate levels, advancing our understanding of how rhizobia influence legumes both aboveground and belowground (Thompson and Lamp, 2021). Under nutrient-limited conditions, the increased compensatory response of hosts under herbivory pressure may be attributed to enhanced nutrient provisions made by the rhizobia association (Ballhorn et al., 2017). Additionally, plant-rhizobia interactions impact on the honeydew composition of aphids, leading to 160% more total sugars than the one collected from non-nodulating plants (Whitaker et al., 2014). However, high leaf quality promoted by belowground rhizobium symbiont results in plant susceptibility to a spider mite (Katayama et al., 2010). But the authors predict that the soil N and rhizobia may independently affect the reproductive performance of the spider mite (Katayama et al., 2010). Overall, rhizobia are another key player involved in the nutrient cycling of ecosystems.
Molecular evidence of plant tolerance
The molecular underpinning of plant tolerance to herbivory is still less demonstrated. JA and its derivates are essential phytohormones of plant defense against abiotic and biotic stress, including salt, drought, osmotic, herbivore attack and pathogen infection stress. AM fungi-colonized Medicago truncatula displays a lower JAs burst upon herbivory compared to the JA level of non-colonized plants, presenting greater tolerance to herbivory by regulating JA and Pi signaling pathways (Zeng et al., 2022). Generally, after an herbivore attack, high jasmonic acid-isoleucine (JA-Ile) levels of plants lead to allocation of more resources into defense responses than into growth (He et al., 2022). However, this antagonistic relationship of growth-defense trade-offs is not simply an outcome of resource shifts but is regulated on the transcription level via hardwiring the molecular network of growth and jasmonate signaling (Campos et al., 2016). The further research of the molecular mechanism of growth-defense trade-offs can help to develop concepts of how to disconnect this balance and eventually improve plant growth performance.
The enzyme GLUCOSE-6-PHOSPHATE DEHYDROGENASE 1 (G6PD1), as a key regulator, contributes to the phenomenon of overcompensation via its role in the oxidative pentose phosphate pathway (OPPP) (Siddappaji et al., 2013). The invertase family of enzymes hydrolyze sucrose to glucose and fructose, whereby the glucose produced is shunted into the OPPP and presumably supports plant regrowth, development, and ultimately compensation. Invertases are essential not only for average plant growth and development but also for plants´ abilities to regrow and eventually compensate for fitness following apical damage (Siddappaji et al., 2015). Furthermore, herbivory downregulates the transcripts of SNF1-related kinase SnRK1, which thus increases C to roots; this silencing alters resource allocation and shows increased tolerance (Schwachtje et al., 2006). It is found that induced JA signaling, root carbohydrate responses, and defoliation tolerance are closely linked, but highly species-specific, even among closely related species (Machado et al., 2017).
Conclusion and future perspectives
The aboveground and belowground herbivores, soil nutrient availability and plant associated-microbes are highly dependent on each other in ecosystems. Changing nutrient availability, either through the type of elements or fertilization level, can potentially alter plant and herbivore performance. Meanwhile, coordinating the plant-microbe interactions can also manipulate the nutrient provision to plants and consequently influence the plant defense strategy. To date, some studies reveal plant tolerance to herbivore damage and activated plant compensatory growth. However, very little molecular evidence of tolerance strategy has been identified. In addition, many studies focus on the C-, N-and P-based resource exchange. The function of other elements mediated by microbes is still less known. Furthermore, considering the complex food web and biodiversity in the tripartite interactions, the competition for nutrients in limited conditions among the same trophic level should also deserve more attention. Soil legacy, herbivory history and plant rotation are good moderators when studying plant-herbivore interactions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: The effect of nutrients on plant-herbivore interactions; Table S2: Nutrients involved in microbe-plant-herbivore interactions; Table S3: Nutrients involved in AM fungi-or rhizobia-plant-herbivore interactions; Method S1: Literature search for
Figure 3,
Figure 4, Table S2 and S3; Method S2: Data analysis.
Author Contributions
MZ designed and performed the data analysis, and wrote the manuscript.
Data Availability Statement
Acknowledgments
This review was supported by iDiv funded by the German Research Foundation (DFG–FZT 118, 202548816). I gratefully acknowledge Prof. Dr. Bettina Hause and Prof. Dr. Nicole M. van Dam for their critical reading and revision. I acknowledge Dr. Beatriz Ramírez-Serrano for contribution of their raw data. I gratefully acknowledge Dr. Mei Li for her comments. Figures (1-3) were illustrated with BioRender (
https://biorender.com/).
Conflicts of Interest
The author has no competing interests to declare that are relevant to the content of this article.
References
- Amtmann, A., Troufflard, S., Armengaud, P., 2008. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 133, 682–691. [CrossRef]
- Ángeles-López, Y.I., Rivera-Bustamante, R.F., Heil, M., 2016. Colonization by Phloem-Feeding Herbivore Overrides Effects of Plant Virus on Amino Acid Composition in Phloem of Chili Plants. J. Chem. Ecol. 42, 985–988. [CrossRef]
- Apple, J.L., Wink, M., Wills, S.E., Bishop, J.G., 2009. Successional change in phosphorus stoichiometry explains the inverse relationship between herbivory and lupin density on Mount St. Helens. PLoS One 4, e7807. [CrossRef]
- Ayres, E., Dromph, K.M., Cook, R., Ostle, N., Bardgett, R.D., 2007. The influence of below-ground herbivory and defoliation of a legume on nitrogen transfer to neighbouring plants. Funct. Ecol. 21, 256–263. [CrossRef]
- Aziz, M., Nadipalli, R., Xie, X., Sun, Y., Surowiec, K., Zhang, J.-L., Pare, P.W., 2016. Augmenting Sulfur Metabolism and Herbivore Defense in Arabidopsis by Bacterial Volatile Signaling. Front. Plant Sci. 7, 458. [CrossRef]
- Ba, L., Ning, J., Wang, D., Facelli, E., Facelli, J.M., Yang, Y., Zhang, L., 2012. The relationship between the diversity of arbuscular mycorrhizal fungi and grazing in a meadow steppe. Plant Soil 352, 143–156. [CrossRef]
- Babikova, Z., Gilbert, L., Bruce, T., Dewhirst, S.Y., Pickett, J.A., Johnson, D., 2014. Arbuscular mycorrhizal fungi and aphids interact by changing host plant quality and volatile emission 375–385.
- Ballhorn, D.J., Elias, J.D., Balkan, M.A., Fordyce, R.F., Kennedy, P.G., 2017. Colonization by nitrogen-fixing Frankia bacteria causes short-term increases in herbivore susceptibility in red alder (Alnus rubra) seedlings. Oecologia 184, 497–506. [CrossRef]
- Barber, N.A., Milano, N.J., Kiers, E.T., Theis, N., Bartolo, V., Hazzard, R. V, Adler, L.S., 2015. Root herbivory indirectly affects above- and below-ground community members and directly reduces plant performance. J. Ecol. 103, 1509–1518. [CrossRef]
- Barthelemy, H., Dorrepaal, E., Olofsson, J., 2019. Defoliation of a grass is mediated by the positive effect of dung deposition, moss removal and enhanced soil nutrient contents: results from a reindeer grazing simulation experiment. Oikos 128, 1515–1524. [CrossRef]
- Barthelemy, H., Stark, S., Kytoviita, M.-M., Olofsson, J., 2017. Grazing decreases N partitioning among coexisting plant species. Funct. Ecol. 31, 2051–2060. [CrossRef]
- Bell, C.A., Magkourilou, E., Urwin, P.E., Field, K.J., 2022. Disruption of carbon for nutrient exchange between potato and arbuscular mycorrhizal fungi enhanced cyst nematode fitness and host pest tolerance. New Phytol. 234, 269–279. [CrossRef]
- Belovsky, G.E., Slade, J.B., 2000. Insect herbivory accelerates nutrient cycling and increases plant production. Proc. Natl. Acad. Sci. U. S. A. 97, 14412–14417. [CrossRef]
- Belsky, A.J., 1993. Overcompensation by plants : herbivore optimization or red herring ? Evol. Ecol. 7, 109–121. [CrossRef]
- Bishop, J.G., O’Hara, N.B., Titus, J.H., Apple, J.L., Gill, R.A., Wynn, L., 2010. N-P co-limitation of primary production and response of arthropods to N and P in early primary succession on Mount St. Helens Volcano. PLoS One 5, e13598. [CrossRef]
- Borowicz, V.A., Albrecht, U., Mayer, R.T., 2003. Effects of nutrient supply on citrus resistance to root herbivory by Diaprepes abbreviatus L. (Coleoptera: Curculionidae). Environ. Entomol. 32, 1242–1250.
- Bryant, J.P., Chapin, F.S., Klein, D.R., Carbon, D.R., 1983. Carbon / Nutrient Balance of Boreal Plants in Relation to Vertebrate Herbivory Author ( s ): John P . Bryant , F . Stuart Chapin , III and David R . Klein Latitudes . Proceedings of a Symposium Held 14-18 September , 1981 , at Kevo , Finland Published by 40, 357–368.
- Campos, M.L., Yoshida, Y., Major, I.T., De Oliveira Ferreira, D., Weraduwage, S.M., Froehlich, J.E., Johnson, B.F., Kramer, D.M., Jander, G., Sharkey, T.D., Howe, G.A., 2016. Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat. Commun. 7, 12570. [CrossRef]
- Carmona, D., Fornoni, J., 2013. Herbivores can select for mixed defensive strategies in plants. New Phytol. 197, 576–585. [CrossRef]
- Charters, M.D., Sait, S.M., Field, K.J., 2020. Aphid Herbivory Drives Asymmetry in Carbon for Nutrient Exchange between Plants and an Arbuscular Mycorrhizal Fungus. Curr. Biol. 30, 1–8.
- Chen, Q., Wu, W.W., Qi, S.S., Cheng, H., Li, Q., Ran, Q., Dai, Z.C., Du, D.L., Egan, S., Thomas, T., 2019. Arbuscular mycorrhizal fungi improve the growth and disease resistance of the invasive plant Wedelia trilobata. J. Appl. Microbiol. 130, 582–591. [CrossRef]
- Coley, P.D., Bryant, J.P., Chapin, F.S., 1985. Resource availability and plant antiherbivore defense. Science 230, 895–899. [CrossRef]
- Contreras-Cornejo, H.A., Macias-Rodriguez, L., Real-Santillan, R.O., Lopez-Carmona, D., Garcia-Gomez, G., Galicia-Gallardo, A.P., Alfaro-Cuevas, R., Gonzalez-Esquivel, C.E., Najera-Rincon, M.B., Adame-Garnica, S.G., Rebollar-Alviter, A., Alvarez-Navarrete, M., Larsen, J., 2021. In a belowground multitrophic interaction, Trichoderma harzianum induces maize root herbivore tolerance against Phyllophaga vetula. PEST Manag. Sci. 77, 3952–3963. [CrossRef]
- Eberl, F., de Bobadilla, M.F., Reichelt, M., Hammerbacher, A., Gershenzon, J., Unsicker, S.B., 2020. Herbivory meets fungivory: insect herbivores feed on plant pathogenic fungi for their own benefit. Ecol. Lett. 23, 1073–1084. [CrossRef]
- Eskelinen, A., Jessen, M.T., Bahamonde, H.A., Bakker, J.D., Borer, E.T., Caldeira, M.C., Harpole, W.S., Jia, M., Lannes, L.S., Nogueira, C., Olde Venterink, H., Peri, P.L., Porath-Krause, A.J., Seabloom, E.W., Schroeder, K., Tognetti, P.M., Yasui, S.L.E., Virtanen, R., Sullivan, L.L., 2023. Herbivory and nutrients shape grassland soil seed banks. Nat. Commun. 14, 3949. [CrossRef]
- Faeth, S.H., Fagan, W.F., 2002. Fungal endophytes: Common host plant symbionts but uncommon mutualists. Integr. Comp. Biol. 42, 360–368. [CrossRef]
- Ferlian, O., Lintzel, E.-M., Bruelheide, H., Guerra, C.A., Heklau, H., Jurburg, S., Kuehn, P., Martinez-Medina, A., Unsicker, S.B., Eisenhauer, N., Schaedler, M., 2021. Nutrient status not secondary metabolites drives herbivory and pathogen infestation across differently mycorrhized tree monocultures and mixtures. BASIC Appl. Ecol. 55, 110–123. [CrossRef]
- Fernandez-Conradi, P., Jactel, H., Robin, C., Tack, A.J.M., Castagneyrol, B., 2018. Fungi reduce preference and performance of insect herbivores on challenged plants. Ecology 99, 300–311. [CrossRef]
- Frew, A., Powell, J.R., Allsopp, P.G., Sallam, N., Johnson, S.N., 2017. Arbuscular mycorrhizal fungi promote silicon accumulation in plant roots, reducing the impacts of root herbivory. Plant Soil 419, 423–433. [CrossRef]
- Frost, C.J., Hunter, M.D., 2008. Herbivore-induced shifts in carbon and nitrogen allocation in red oak seedlings. New Phytol. 178, 835–845. [CrossRef]
- Gan, H., Liang, C., Wickings, K., 2018. Root herbivores accelerate carbon inputs to soil and drive changes in biogeochemical processes. Rhizosphere 6, 112–115.
- Gebremikael, M.T., Steel, H., Buchan, D., Bert, W., De Neve, S., 2016. Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci. Rep. 6, 32862.
- González, J.B., Petipas, R.H., Franken, O., Kiers, E.T., Veblen, K.E., Brody, A.K., 2018. Herbivore removal reduces influence of arbuscular mycorrhizal fungi on plant growth and tolerance in an East African savanna. Oecologia 187, 123–133.
- Graff, P., Gundel, P.E., Salvat, A., Cristos, D., Chaneton, E.J., 2020. Protection offered by leaf fungal endophytes to an invasive species against native herbivores depends on soil nutrients. J. Ecol. 108, 1592–1604.
- He, Z., Webster, S., He, S.Y., 2022. Growth–defense trade-offs in plants. Curr. Biol. 32, R634–R639.
- Hodge, A., Fitter, A.H., 2010. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. 107, 31.
- Holland, J.N., 1995. EFFECTS OF ABOVEGROUND HERBIVORY ON SOIL MICROBIAL BIOMASS IN CONVENTIONAL AND NO-TILLAGE AGROECOSYSTEMS. Appl. Soil Ecol. 2, 275–279.
- Holland, J.N., Cheng, W., Crossley, D.A., 1996. Herbivore-induced changes in plant carbon allocation: Assessment of below-ground C fluxes using carbon-14. Oecologia 107, 87–94.
- Huberty, A.F., Denno, R.F., 2006. Consequences of nitrogen and phosphorus limitation for the performance of two planthoppers with divergent life-history strategies. Oecologia 149, 444–455.
- Joern, A., Provin, T., Behmer, S.T., 2012. Not just the usual suspects: Insect herbivore populations and communities are associated with multiple plant nutrients. Ecology 93, 1002–1015.
- Johnson, S.N., Hartley, S.E., Ryalls, J.M.W., Frew, A., DeGabriel, J.L., Duncan, M., Gherlenda, A.N., 2017. Silicon-induced root nodulation and synthesis of essential amino acids in a legume is associated with higher herbivore abundance. Funct. Ecol. 31, 1903–1909.
- Katayama, N., Nishida, T., Zhang, Z.Q., Ohgushi, T., 2010. Belowground microbial symbiont enhances plant susceptibility to a spider mite through change in soybean leaf quality. Popul. Ecol. 52, 499–506.
- Katayama, N., Silva, A.O., Kishida, O., Ushio, M., Kita, S., Ohgushi, T., 2014. Herbivorous insect decreases plant nutrient uptake: The role of soil nutrient availability and association of below-ground symbionts. Ecol. Entomol. 39, 511–518.
- Kobae, Y., Ohmori, Y., Saito, C., Yano, K., Ohtomo, R., Fujiwara, T., 2016. Phosphate Treatment Strongly Inhibits New Arbuscule Development But Not the Maintenance of Arbuscule in Mycorrhizal Rice Roots. Plant Physiol. 171, 566–579.
- Leimu, R., Koricheva, J., 2006. A meta-analysis of tradeoffs between plant tolerance and resistance to herbivores : combining the evidence from ecological and agricultural studies. Oikos 112, 1–9.
- Li, Y., Gao, Y., van Kleunen, M., Liu, Y., 2022. Herbivory may mediate the effects of nutrients on the dominance of alien plants. Funct. Ecol. 36, 1292–1302.
- Luginbuehl, L.H., Menard, G.N., Kurup, S., Van Erp, H., Radhakrishnan, G. V., Breakspear, A., Oldroyd, G.E.D., Eastmond, P.J., 2017. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science (80-. ). 356, 1175–1178.
- Machado, R.A.R., Zhou, W., Ferrieri, A.P., Arce, C.C.M., Baldwin, I.T., Xu, S., Erb, M., 2017. Species-specific regulation of herbivory-induced defoliation tolerance is associated with jasmonate inducibility. Ecol. Evol. 7, 3703–3712.
- Manck-Götzenberger, J., Requena, N., 2016. Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the potato SWEET sugar transporter family. Front. Plant Sci. 7, 487.
- Miransari, M., 2011. Arbuscular mycorrhizal fungi and nitrogen uptake. Arch. Microbiol. 193, 77–81.
- Perkins, M.C., Woods, H.A., Harrison, J.F., Elser, J.J., 2004. Dietary phosphorus affects the growth of larval Manduca sexta. Arch. Insect Biochem. Physiol. 55, 153–168.
- Piippo, S., Markkola, A., Harma, E., Tuomi, J., 2011. Do compensatory shoot growth and mycorrhizal symbionts act as competing above- and below-ground sinks after simulated grazing? PLANT Ecol. 212, 33–42.
- Potthast, K., Meyer, S., Tischer, A., Gleixner, G., Sieburg, A., Frosch, T., Michalzik, B., 2021. Grasshopper herbivory immediately affects element cycling but not export rates in an N-limited grassland system. Ecosphere 12, e03449.
- Prescott, C.E., Grayston, S.J., Helmisaari, H.S., Kaštovská, E., Körner, C., Lambers, H., Meier, I.C., Millard, P., Ostonen, I., 2020. Surplus Carbon Drives Allocation and Plant–Soil Interactions. Trends Ecol. Evol. 35, 1110–1118.
- Prudic, K.L., Oliver, J.C., Bowers, M.D., 2005. Soil nutrient effects on oviposition preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia 143, 578–587.
- Rosenthal, J.P., Kotanen, P.M., 1994. Terrestrial plant tolerance to herbivory. Tree 9, 145–148.
- Sarfraz, R.M., Dosdall, L.M., Keddie, A.B., 2009. Bottom-up effects of host plant nutritional quality on Plutella xylostella (Lepidoptera: Plutellidae) and top-down effects of herbivore attack on plant compensatory ability. Eur. J. Entomol. 106, 583–594.
- Schwachtje, J., Minchin, P.E.H., Jahnke, S., Van Dongen, J.T., Schittko, U., Baldwin, I.T., 2006. SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proc. Natl. Acad. Sci. U. S. A. 103, 12935–12940.
- Siddappaji, M.H., Scholes, D.R., Bohn, M., Paige, K.N., 2013. Overcompensation in response to herbivory in Arabidopsis thaliana: The role of glucose-6-phosphate dehydrogenase and the oxidative pentose-phosphate pathway. Genetics 195, 589–598.
- Siddappaji, M.H., Scholes, D.R., Krishnankutty, S.M., Calla, B., Clough, S.J., Zielinski, R.E., Paige, K.N., 2015. The role of invertases in plant compensatory responses to simulated herbivory. BMC Plant Biol. 15, 278.
- Sitters, J., Wubs, E.R.J., Bakker, E.S., Crowther, T.W., Adler, P.B., Bagchi, S., Bakker, J.D., Biederman, L., Borer, E.T., Cleland, E.E., Eisenhauer, N., Firn, J., Gherardi, L., Hagenah, N., Hautier, Y., Hobbie, S.E., Knops, J.M.H., MacDougall, A.S., McCulley, R.L., Moore, J.L., Mortensen, B., Peri, P.L., Prober, S.M., Riggs, C., Risch, A.C., Schutz, M., Seabloom, E.W., Siebert, J., Stevens, C.J., Veen, G.F. (Ciska), 2020. Nutrient availability controls the impact of mammalian herbivores on soil carbon and nitrogen pools in grasslands. Glob. Chang. Biol. 26, 2060–2071.
- Strauss, S.Y., Agrawal, A.A., 1999. The ecology and evolution of plant tolerance to herbivory. Tree 14, 179–185.
- Tao, L., Ahmad, A., de Roode, J.C., Hunter, M.D., 2016. Arbuscular mycorrhizal fungi affect plant tolerance andchemical defences to herbivory through different mechanisms. J. Ecol. 104, 561–571.
- Thompson, M.N., Lamp, W.O., 2021. Herbivory enhances legume-rhizobia symbioses function, increasing aboveground allocation of biologically fixed nitrogen, but only in soils without additional nitrate. Plant Soil 465, 301–316.
- Wang, W., Shi, J., Xie, Q., Jiang, Y., Yu, N., Wang, E., 2017. Nutrient Exchange and Regulation in Arbuscular Mycorrhizal Symbiosis. Mol. Plant 10, 1147–1158.
- Wearn, J.A., Gange, A.C., 2007. Above-ground herbivory causes rapid and sustained changes in mycorrhizal colonization of grasses. Oecologia 153, 959–971.
- Wetzel, W.C., Kharouba, H.M., Robinson, M., Holyoak, M., Karban, R., 2016. Variability in plant nutrients reduces insect herbivore performance. Nature 539, 425–427.
- Whitaker, M.R.L., Katayama, N., Ohgushi, T., 2014. Plant-rhizobia interactions alter aphid honeydew composition. Arthropod. Plant. Interact. 8, 213–220.
- Wilkinson, T.D.J., Miranda, J.P., Ferrari, J., Hartley, S.E., Hodge, A., 2019. Aphids influence soil fungal communities in conventional agricultural systems. Front. Plant Sci. 10, 895.
- Wright, P., Cregger, M.A., Souza, L., Sanders, N.J., Classen, A.T., 2014. The effects of insects, nutrients, and plant invasion on community structure and function above- and belowground. Ecol. Evol. 4, 732–742.
- Wurst, S., Langel, R., Rodger, S., Scheu, S., 2006. Effects of belowground biota on primary and secondary metabolites in Brassica oleracea. Chemoecology 16, 69–73.
- Yu, L., Zhang, W., Geng, Y., Liu, K., Shao, X., 2022. Cooperation With Arbuscular Mycorrhizal Fungi Increases Plant Nutrient Uptake and Improves Defenses Against Insects. Front. Ecol. Evol. 10, 833389.
- Zekveld, C., Markham, J., 2011. Exposure to aphids increases alder growth and nitrogen fixation. Botany 89, 255–261.
- Zeng, M., Hause, B., van Dam, N.M., Uthe, H., Hoffmann, P., Krajinski, F., Martínez-Medina, A., 2022. The mycorrhizal symbiosis alters the plant defence strategy in a model legume plant. Plant Cell Environ. 45, 3412–3428.
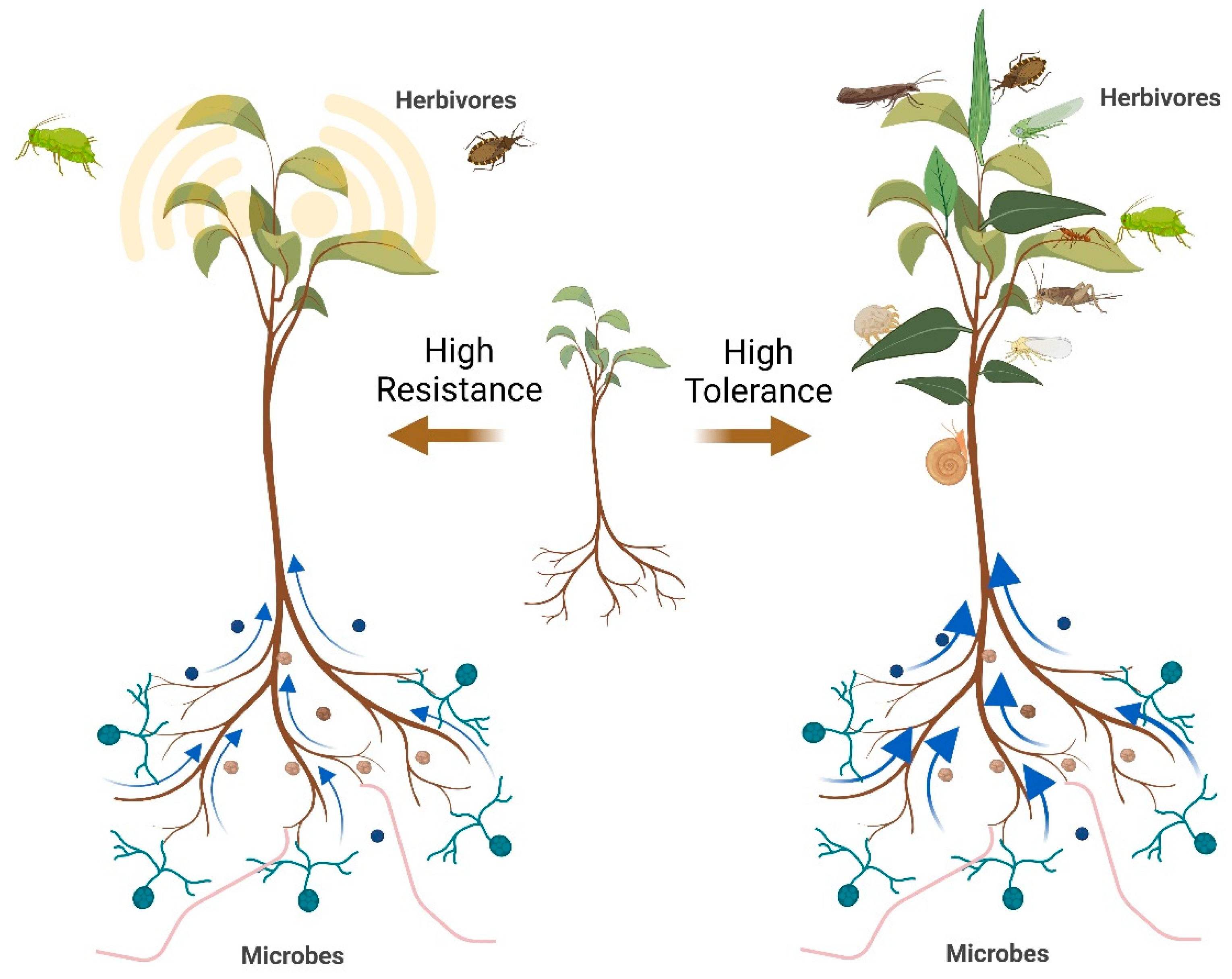
Figure 1.
Two defense strategies of plants. At the left, microbes enhance the levels of shoot defensive metabolites constitutively or induced, which significantly reduces herbivore damage. At the right, microbes provide the nutrients, allowing plants to tolerate herbivore attack by compensatory growth. As described, plants have a mixed pattern of these two strategies. The blue arrows indicate the nutrient flow from belowground to aboveground.
Figure 1.
Two defense strategies of plants. At the left, microbes enhance the levels of shoot defensive metabolites constitutively or induced, which significantly reduces herbivore damage. At the right, microbes provide the nutrients, allowing plants to tolerate herbivore attack by compensatory growth. As described, plants have a mixed pattern of these two strategies. The blue arrows indicate the nutrient flow from belowground to aboveground.
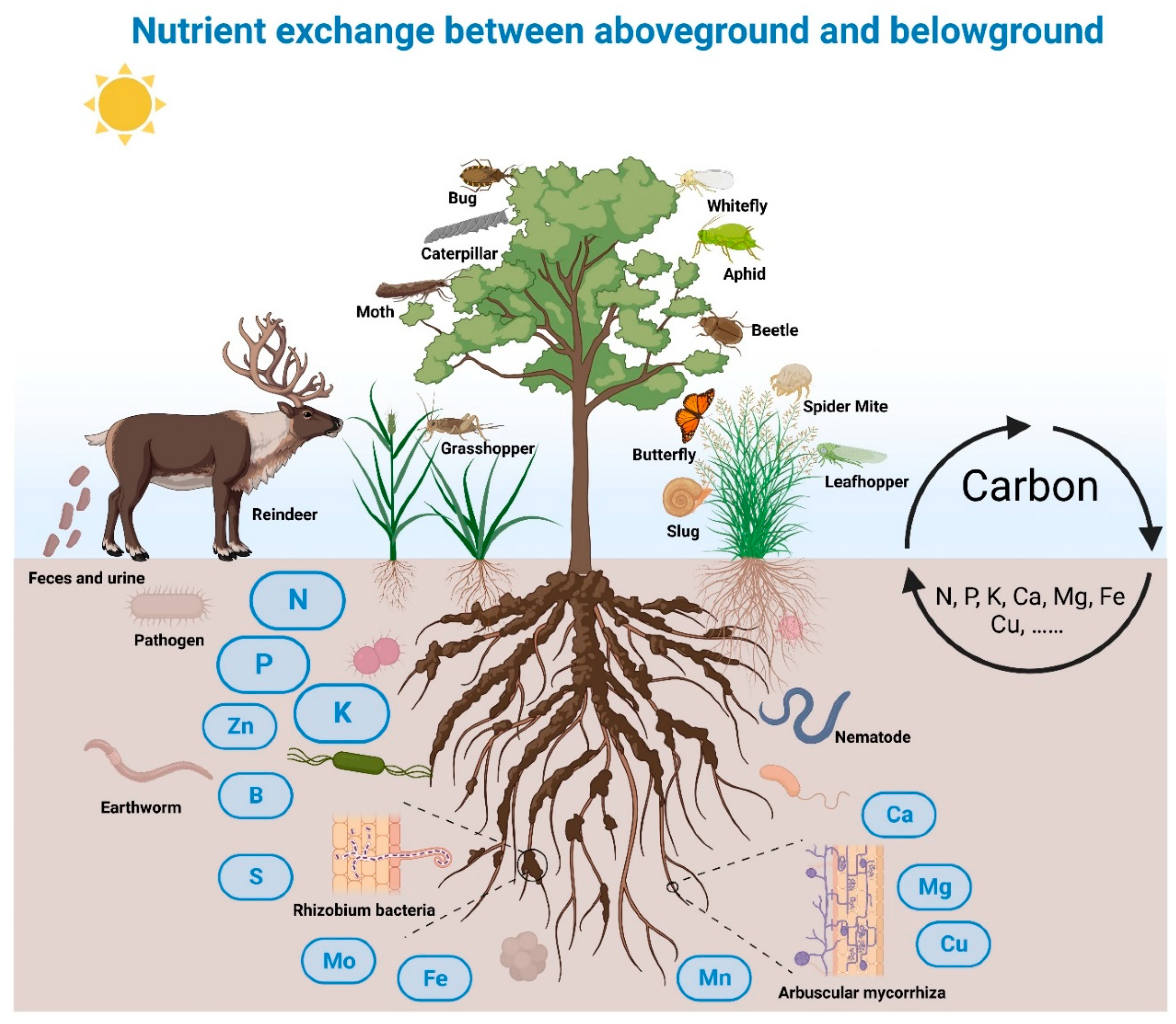
Figure 2.
Graphical illustration of herbivory driving nutrient exchange between aboveground and belowground. Plants live in a dynamic and complex ecosystem where roots can interact with many organisms including pathogens, earthworms, nematodes, plant growth promoting bacteria, and arbuscular mycorrhiza; shoots suffer from attack by herbivores including bugs, caterpillars, moths, whiteflies, aphids, beetles, slugs, and mammals. In these multitrophic interactions, herbivory affects the nutrient flow between aboveground and belowground. Herbivores obtain C resources for maintaining their life cycles. Meanwhile, plant roots acquire inorganic nutrients including N, P, K, Ca, Mg, Fe and others for their growth needs, directly from soil substrates or indirectly from the interacting microbes or degraded by organic matter, to support the formation of photosynthetic assimilates.
Figure 2.
Graphical illustration of herbivory driving nutrient exchange between aboveground and belowground. Plants live in a dynamic and complex ecosystem where roots can interact with many organisms including pathogens, earthworms, nematodes, plant growth promoting bacteria, and arbuscular mycorrhiza; shoots suffer from attack by herbivores including bugs, caterpillars, moths, whiteflies, aphids, beetles, slugs, and mammals. In these multitrophic interactions, herbivory affects the nutrient flow between aboveground and belowground. Herbivores obtain C resources for maintaining their life cycles. Meanwhile, plant roots acquire inorganic nutrients including N, P, K, Ca, Mg, Fe and others for their growth needs, directly from soil substrates or indirectly from the interacting microbes or degraded by organic matter, to support the formation of photosynthetic assimilates.
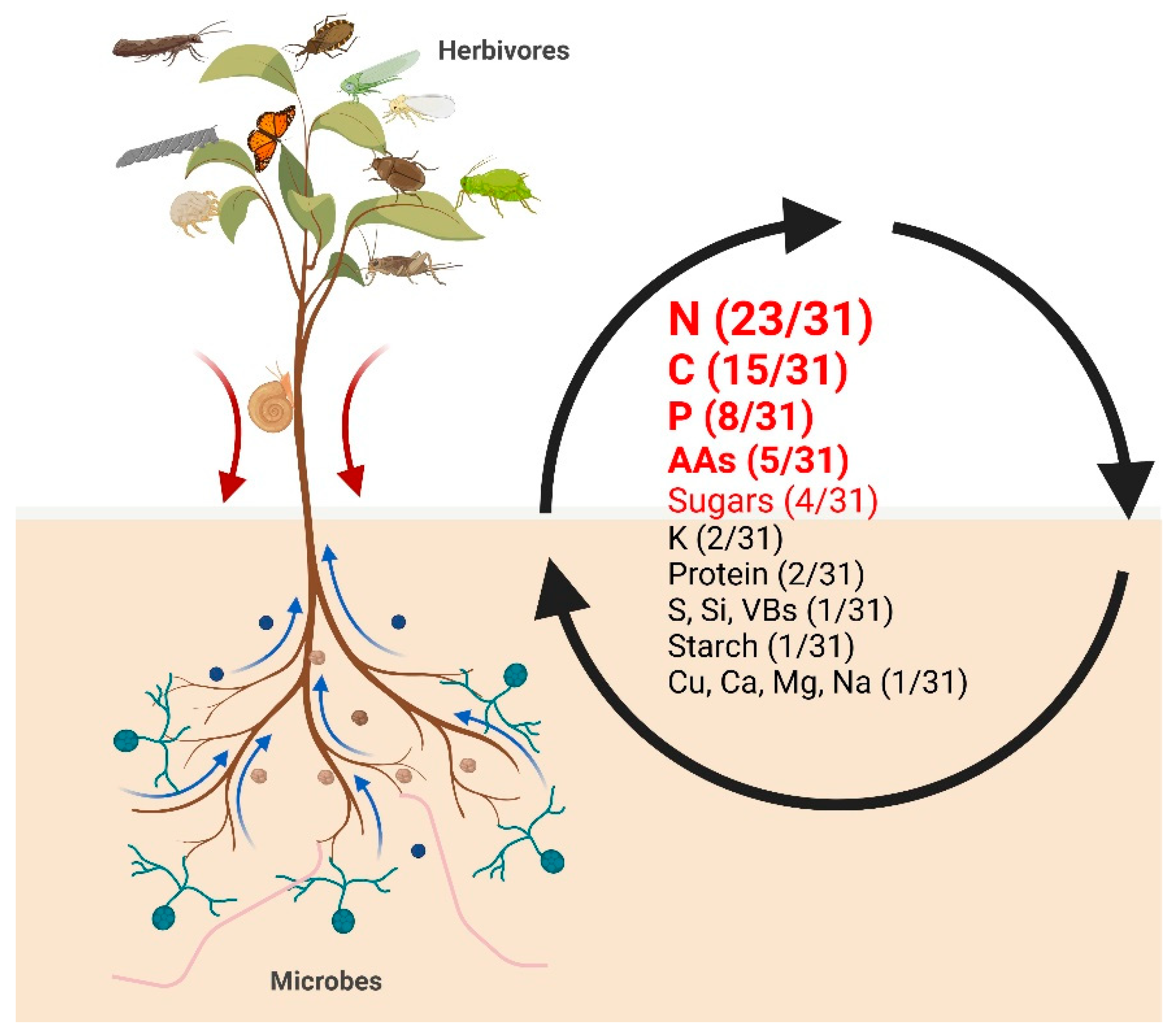
Figure 3.
The frequency of nutrients involved in the selected studies of microbe-plant-herbivore interactions. Plants interact with herbivores aboveground and microbes belowground. The brown arrows indicate C flow from aboveground to belowground; the blue arrows indicate the flow of inorganic nutrients from belowground to aboveground. In the right part of the figure, the numbers of studies, in which the respective nutrient appears, are shown. There are 31 studies in total which meet the criteria. The method of how to screen these articles from “Web of Science” database is listed in Method S1. AAs, amino acids; VBs, vitamins of the B-group.
Figure 3.
The frequency of nutrients involved in the selected studies of microbe-plant-herbivore interactions. Plants interact with herbivores aboveground and microbes belowground. The brown arrows indicate C flow from aboveground to belowground; the blue arrows indicate the flow of inorganic nutrients from belowground to aboveground. In the right part of the figure, the numbers of studies, in which the respective nutrient appears, are shown. There are 31 studies in total which meet the criteria. The method of how to screen these articles from “Web of Science” database is listed in Method S1. AAs, amino acids; VBs, vitamins of the B-group.
Figure 4.
The effect size of herbivory on plant N (A) and C (B) content in the presence of microbes that interact with plants. Error bars denote 95% confidence intervals (CIs). The size of each blue square indicates biological replicates in relation to the overall mean difference. The black diamond shows the overall effect size. Vertical solid lines show Hedges=0. The effect is significant when the 95% CI does not include zero. The method of data analysis is described in Method S2.
Figure 4.
The effect size of herbivory on plant N (A) and C (B) content in the presence of microbes that interact with plants. Error bars denote 95% confidence intervals (CIs). The size of each blue square indicates biological replicates in relation to the overall mean difference. The black diamond shows the overall effect size. Vertical solid lines show Hedges=0. The effect is significant when the 95% CI does not include zero. The method of data analysis is described in Method S2.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).