1. Introduction
Freshwaters ecosystems, such as streams, rivers, lakes, wetlands, and manmade reservoirs/dams, comprise only 2.3% of the Earth's surface, but these water bodies harbor an disproportionately high species richness relative to other ecosystems on the earth (Saunders et al. 2002; Dudgeon et al. 2006; Strayer and Dudgeon 2010; Reid et al. 2019; Stewart et al. 2022). Freshwater environments are among the most threatened ecosystems on the planet (Stewart et al. 2022). Aquatic organisms in these freshwater environments face numerous threats e.g., habitat degradation, hydrology alterations, pollution, over-exploitation, and invasive species (Dudgeon et al. 2006; Davis et al. 2013; Reid et al. 2019; Stewart et al. 2022; Saemi-Komsari et al. 2023). Reid et al. (2019) recognized several new arising threats to freshwater biological diversity that have either exacerbated or started since 2006. The threats include biological invasions, e-commerce (e.g., easily internet marketing of novel invasive species, by individual hobbyists, collectors, and breeders), infectious diseases, harmful algal blooms, emerging contaminants such as engineered nanomaterials, nanoplastics, and microplastics, light, and noise pollutions, expanding hydropower, freshwater salinization, diminishing calcium levels, and climate change. These known threats are disrupting the life cycles or phenology of aquatic organisms (Reid et al. 2019; Stewart et al. 2022). The first of these is likely a global threat affecting world biodiversity.
Introduction of an exotic organism outside its native range and the problems caused by such invasive (known as biological pollution/biopollution) is unprecedentedly changing the world's natural communities, ecological characteristics, and functions following establishment (Elliott 2003; Santamarina et al. 2019). Due to world globalization, an increase in biological invasions/bioinvasions has proliferated in the last few years, a pattern likely to continue in the future and can be considered a major threat to biodiversity (Barbet-Massin et al. 2018). Based on several pieces of evidence, invasive species have main role in declining native and endemic species diversity and their displacement worldwide (Ricciardi et al. 2013; Bellard et al. 2016; Renault et al. 2022). It has led to changes in environmental regimes (Brooks et al., 2004) and has increased risks to human well-being (Ogden et al. 2019). Moreover, invasive species also are very costly to the worldwide economy (Barbet-Massin et al. 2018; Soto et al. 2022).
In particular, non-indigenous/exotic tilapias especially the Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) are among the most successful and harmful fish invaders globally. The Nile tilapia is a cichlid fish native to Africa that has been successfully introduced to at least 100 countries, including countries of the Arabian Peninsula, for aquaculture due to its high growth rate, resistance to diseases, tolerance to various environmental conditions, high meat quality, and high production (Grammer et al. 2012; Freyhof et al. 2020; Shuai and Li 2022). At present, it is one of the most important freshwater species used in aquaculture worldwide (Shuai and Li 2022). Due to their capacity to cause a series of environmental and ecological problems, i.e. changes in water quality, habitat degradation, trophic cascades, and modifications of ecosystem function (Zengeya et al. 2013; Shuai and Li 2022), O. niloticus is currently known as one of the most hazardous invasive fish in the tropical and subtropical areas of the world (Shuai and Li 2022; Stauffer et al. 2022).
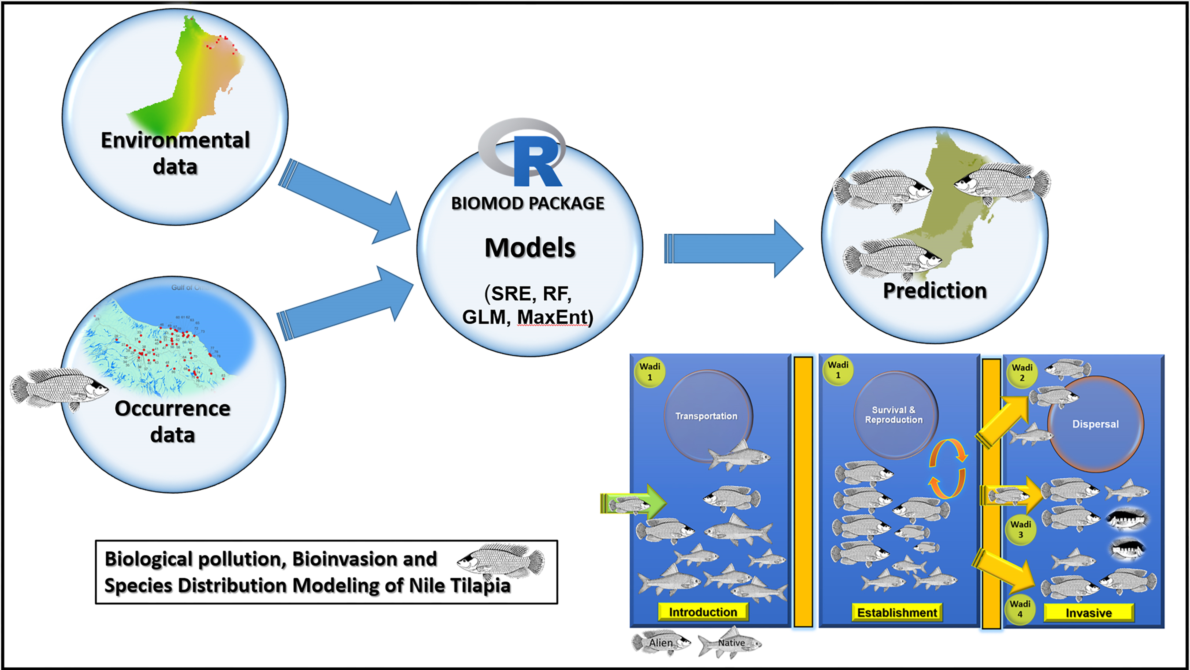
Increase in number and population size of invasive species and their impacts on biological diversity and ecosystem function raise numerous management and control concerns (Lodge et al. 2006; Hulme et al. 2009; Barbet-Massin et al. 2018). It has been documented that prevention of the establishment of an invasive species and its further spread is a more effective and less costly conservation plan strategy than eradication, containment, and control of an invasive population. Because when the invasive species established a breeding population specially in highly stressed ecosystems such as the inland and coastal water bodies of the Arabian Peninsula, a number of stressors can threaten its ecological integrity and sustainability (Hamza and Munawar 2009; Esmaeili et al. 2022; Freyhof et al. 2020). Recently species distribution models (SDMs) are progressively being applied in bioinvasion and ecological investigations to predict and understand the geographical distribution of invaders (e.g., Zengeya et al. 2013; Barbet-Massin et al. 2018; de Oliveira da Conceição et al. 2020; Stewart et al. 2022). In SDMs, geo-located observations of species occurrence are linked to environmental variables (Franklin 1995; Guisan and Zimmermann 2000).
Why the ensemble model?
Species distribution models (SDMs) are effectively used for predicting the geographic extent of species by linking information about species georeferenced and environmental parameters (Guisan et al. 2007). Along with the development of remote sensing and geographic information technology, SDM is broadly used to assess biodiversity, predict species distribution potential, and protect rare species (Ruiz-Navarro et al. 2016; Rathore et al. 2019; Zhang et al. 2019). SDMs in wide use today include Random Forest (RF), Generalized Linear Models (GLM), and Maximum Entropy (MaxEnt). However, choosing the right model for analysis and obtaining an accurate and reasonable distribution of predicted species is difficult because the principles and algorithms of each model are different (Pearson et al. 2006). To resolve this type of uncertainty, an increasing number of studies employ ensemble modeling approaches that combine predictions from different modeling techniques (Ardestani et al. 2021).
The ensemble model (EM) is an effective method to assess the potential distribution of species at a large spatial scale, and can reduce the uncertainty caused by a single model (Thuiller 2003). It is being used to predict the distribution range of various species, with good accuracy (e.g., Ardestani et al. 2021; Cheng et al. 2022).
Herein, an ensemble model was implemented to predict the potential geographic distribution of O. niloticus outside its native range, with a special reference to the inland water bodies of the southeastern Arabian Peninsula (Oman) where it has become established and is now spreading. Specifically, we sought to: (i) map the current distribution range of O. niloticus, (ii) evaluate the effect of the number and type of environmental parameters on the introduced ranges of O. niloticus, (iii) access the predicted distribution of O. niloticus across the southeastern Arabian Peninsula River systems in the Oman territory, iv) predict further spread of species in the 2050s, and (v) to provide a list of indigenous fishes that co-occurs with O. niloticus .
2. Material and methods
2.1. Specimen Data Sources
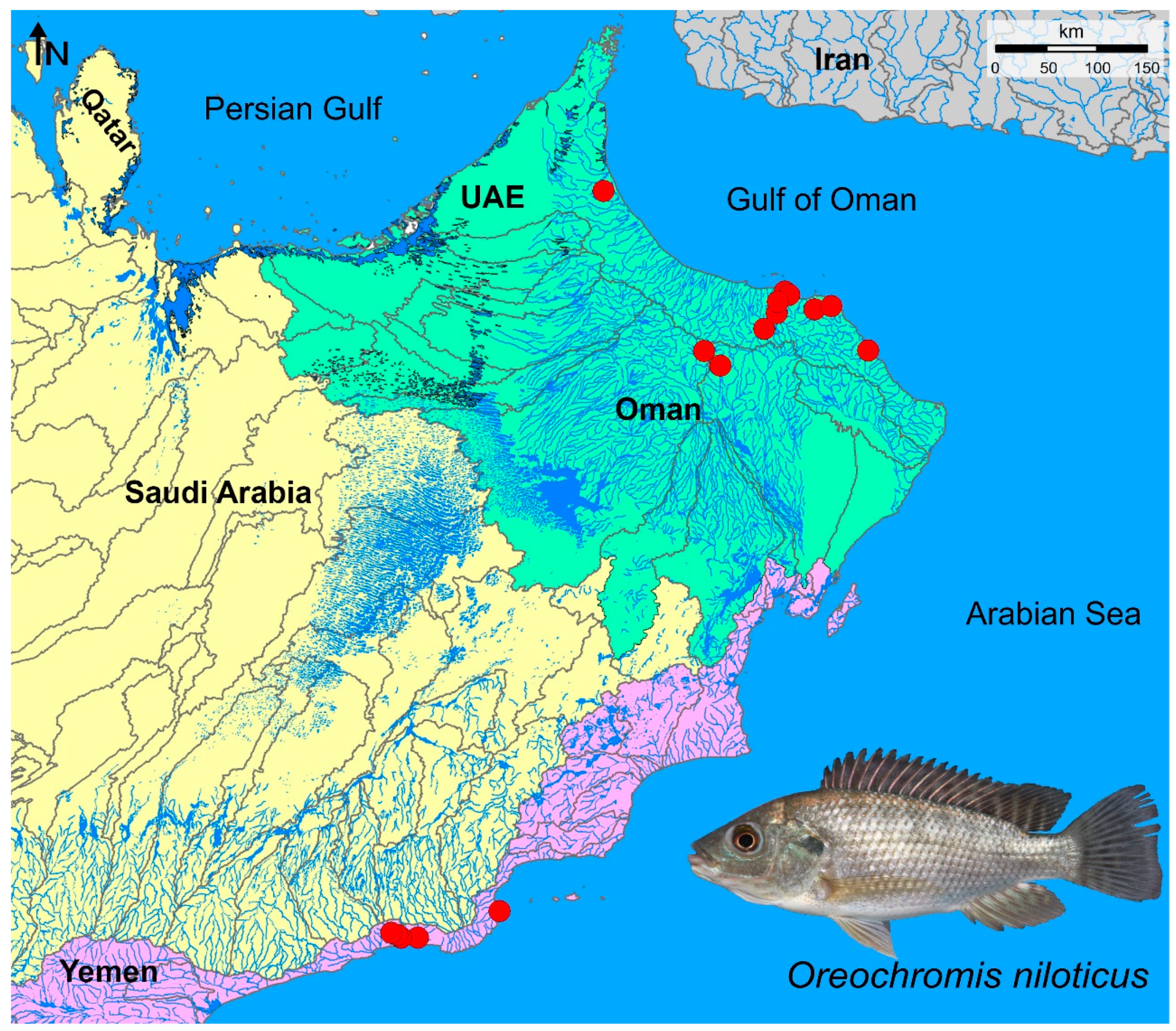
In this study, the occurrence and contemporary distribution of the invasive species Nile tilapia,
O. niloticus from entire drainage basins of the southeastern Arabian Peninsula (
Figure 1) including three main ecoregions was mapped: i) Oman Mountains, ID 443 (located in the southeastern part of the Arabian Peninsula, lying mostly within Oman and the United Arab Emirates, and it is bounded by the Persian Gulf, Strait of Hormuz, Gulf of Oman, Arabian Sea, and Rub’ al Khali Desert), ii) Southwestern Arabian Coast, ID 439 (runs along the southern and western fringes of the Arabian Peninsula, bounded by the Red Sea to the west, the Gulf of Aden to the south and the An-Nafud and Rub’ al-Khali deserts of the Arabian interior to the east and north), and iii) Arabian Interior, ID 440 (in general, the whole ecoregion includes the internal basins of the Arabian Peninsula. It is bounded to the east by the Oman Mountains ecoregion (443), the Persian Gulf, and Lower Tigris & Euphrates ecoregion (441); to the north by the Upper Tigris-Euphrates ecoregion (443); to the northwest by a small section of Coastal Levant (436), Orontes (447), and Jordan River (438) ecoregions; and to the west and south by the Southwest Arabian Coast ecoregion (439).)
This study was based on (i) available published data (Freyhof et al. 2020; Esmaeili et al. 2022), and (ii) extensive fieldworks in the three main ecoregions of the southeastern Arabian Peninsula (Oman Territory) during 2021-2022 that provided the geographic coordinates for O. niloticus distribution. During these field works, native and exotic fishes including Nile tilapia were collected using foldable shrimp and crab fishing traps, and hand nets from 89 sampling sites. To collect exotic and native fishes, several main water bodies in these three ecoregions were visited/sampled: river and stream systems (Wadis), aflaj or qanats (specific artificial irrigation channel systems), subterranean water systems, sinkholes, artificial dams, and brackish salt flats. After anesthesia, fish specimens were fixed in 10% formaldehyde or absolute alcohol and transferred to the laboratory for further identification. Fixed specimens were deposited at ZM-CBSU (Zoological Museum, Collection of Biology Department, Shiraz University, Iran). For species identification, and taxonomic nomenclature Freyhof et al. (2020), Esmaeili et al. (2022), and Esmaeili and Hamidan (2023) were followed.
2.2. Environmental Variables
The current and future bioclimatic data with a 2.5 arc-second resolution were obtained from the WorldClim database (
http://www.Worldclim.org (accessed on November 2022). To predict the spread of species through the 2050s (predicted mean for 2041–2060), we selected medium-resolution Beijing Climate Center Climate System Model version 2 (BCC-CSM2-MR) from the Coupled Model Intercomparison project phase 6 (CMIP6) for our projections. CMIP6 has higher resolution and climate sensitivity compared to CMIP5 (Petrie et al. 2021; Hamed et al. 2022), and the BCC-CSM2-MR model has a better performance compared to other global climate models (Wu et al. 2021). For the four shared socioeconomic pathways (SSP126, SSP245, SSP370, and SSP585), we chose low radiation intensity (SSP126) for the optimistic scenario and high radiation intensity (SSP 585) for the pessimistic scenario for model simulation under the future climate scenario.
To avoid the problem of model fitting due to the collinearity of climatic factors, correlation analysis was carried out. Climatic factors with |r| < 0.8 were selected. Finally, seven climatic factors were selected to build the model: mean diurnal range (mean of monthly/max temp—min temp) (Bio2), isothermally (BIO2/BIO7) (* 100) (Bio3), min temperature of coldest month (Bio6), mean temperature of wettest quarter (Bio8), precipitation of wettest month (Bio13), precipitation of driest month (Bio14), and precipitation seasonality (coefficient of variation) (Bio15).
2.3. Establishment of Single Species Distribution Model
We used 4 model algorithms in the “biomod2” package (Thuiller et al. 2016) in R 3.6.3. These model algorithms included surface range envelope (SRE), random forest (RF), generalized linear model (GLM), and maximum entropy (MaxEnt). The sample data (including occurrence data and pseudo absence points) were randomly divided into two parts: 80% of the data as the training data set, and 20% as the test data set. Each model algorithm was repeated 10 times.
2.4. Model Evaluation
We chose two evaluation metrics models embedded in the “biomod2” package to evaluate the fitting accuracy: (1) the true skill statistic (TSS), a metric that is developed from Cohen’s kappa statistics (KAPPA), and contains the advantages of KAPPA while avoiding its disadvantages (Chen et al., 2022), and (2) receiver operating characteristic (ROC) that is the most widely used index to evaluate models. The area under the ROC curve (AUC) value can indicate the precision of the model prediction. Getting higher values makes the model more predictive.
2.5. Ensemble Model (EM) Construction
To reduce the uncertainty introduced by the modeling algorithms, we implemented the two evaluation indicators of ROC and TSS to build the EM. Only single model results with a ROC value greater than 0.9 and a TSS value greater than 0.7 were retained.
3. Results
3.1. Ensemble Modeling
The algorithms with a mean ROC value greater than 0.9, and a mean TSS value greater than 0.7 were selected for ensemble modeling. This way, the SRE algorithm was removed. The values of TSS and ROC of the EM were 0.88, and 0.98 respectively, signifying more accurate predictions than all of the single models.
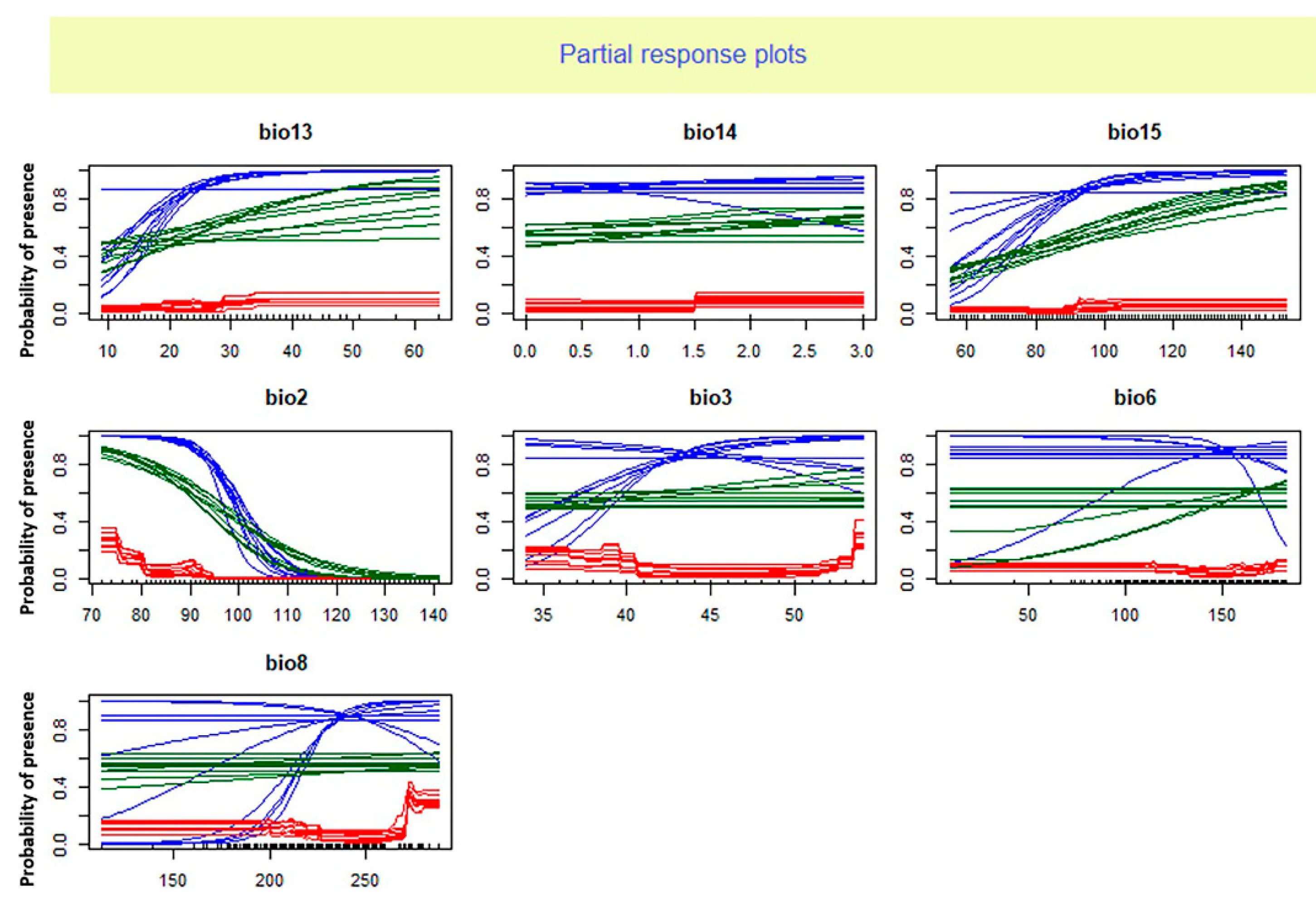
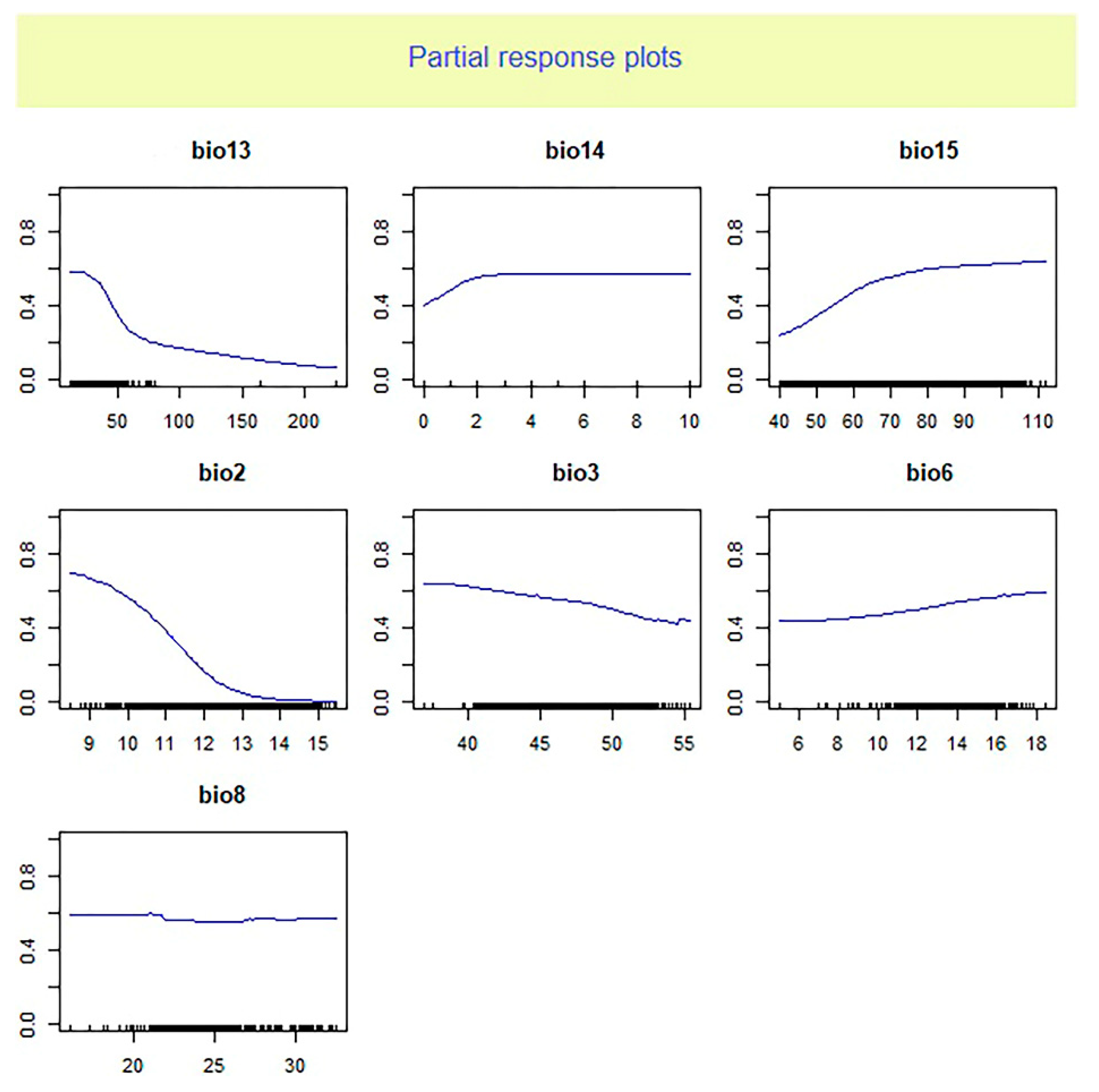
Based on our findings the mean diurnal range (BIO2) was the most significant climatic variable affecting the
O. niloticus distribution (
Table 1). The occurrence probability of
O. niloticus decreased with increasing BIO2 (
Figure 2 and
Figure 3).
3.2. Current and Future Distribution
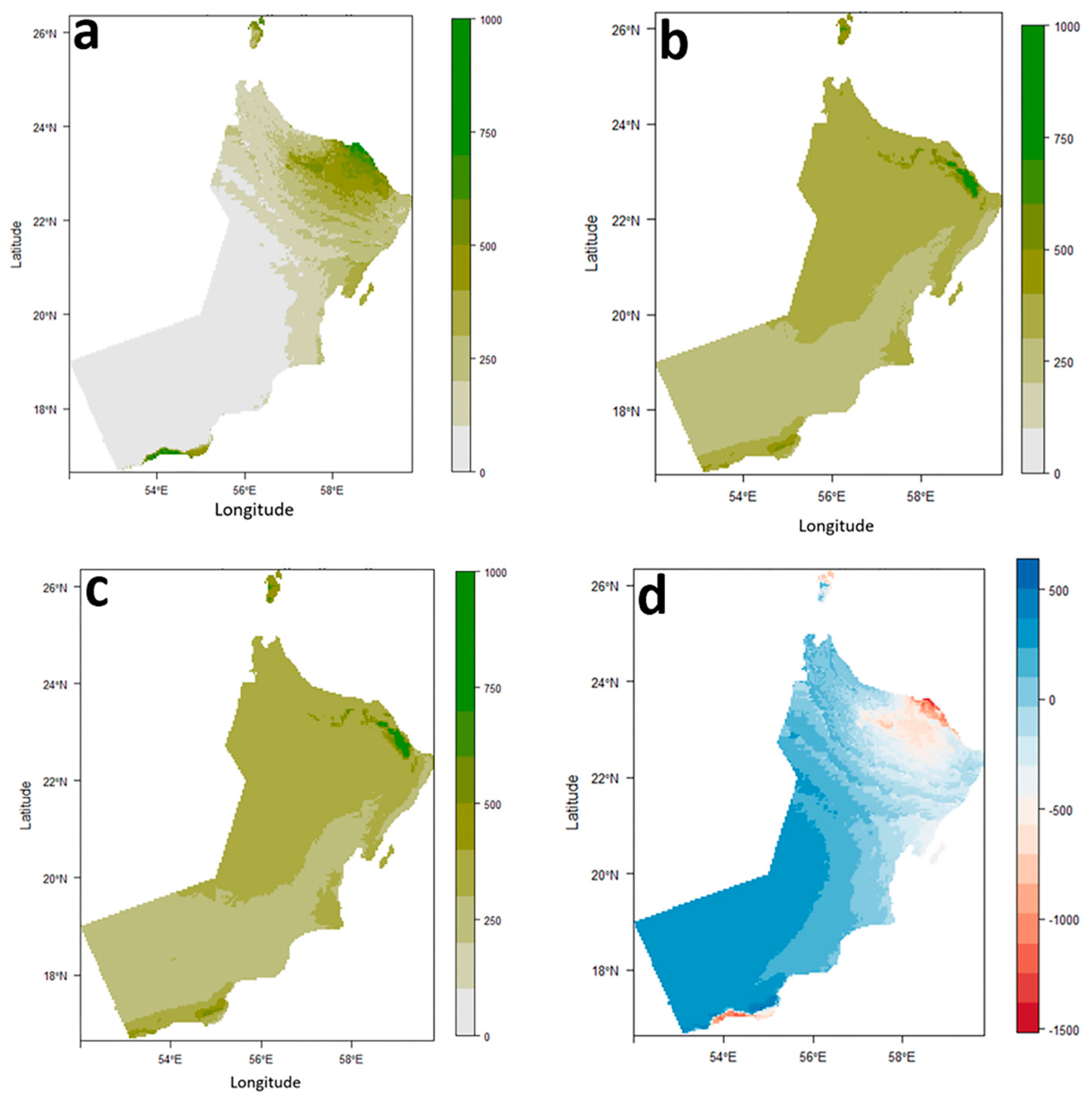
The distribution map of
O. niloticus under the current climatic conditions was accessed based on the EM prediction results (
Figure 4a,b) and the suitable distribution areas for
O. niloticus were generally distributed in the Northeast of the Arabian Peninsula.
EM results indicated that climate change had a significant impact on
O. niloticus distribution. So that highly suitable areas for this species will be reduced, while areas with low to moderate suitability increase slightly or remain unchanged. The changes in the distribution of suitable areas for
O. niloticus are not significantly different under SSP1-2.6 compared to SSP5-8.5 (
Figure 4c,d).
3.3. Native and Exotic Fish Diversity
As is shown in
Table 2, the southeastern Arabian Peninsula's fish fauna (Oman Territory) consists of 22 recognized species in 16 genera, 10 families, seven orders, and a class. The most diverse orders are Cypriniformes (two genera, seven species, 38.81%), and Gobiiformes (seven genera, seven species, 38.81%), followed by Cyprinodontiformes (two genera, three species, 13.64%), Cichliformes (two genera, two species, 9.01%), and Centrarchiformes, Gonorynchiformes, and Mugiliformes (one genus, one species, 4.54% each). A total of 20 native species (90.91%) in nine families and two exotic species (9.09%) in two families are listed here. Out of 20 native species, seven species (35%) in two families are endemic taxa that are only found in the southeastern Arabian Peninsula.
4. Discussion
4.1. Ensemble Model Evaluation
Herein, using the “Biomod2” package, four single species distribution models and EM were constructed with the latest CMIP6 climate data, and the results were assessed by ROC and TSS. According to the evaluation metrics, the SRE model performed the worst. This is in agreement with results of the other studies using multiple models to predict species distribution. Based on those studies, the predictive performance of the SRE algorithm is weaker compared to other algorithms like RF, GLM, and MAXENT (Zhao et al. 2020; Chege et al. 2022). As far as we know the research on the suitable distribution of O. niloticus has only used the single species distribution model, while the uncertainty of a single distribution model can be reduced to some extent by building an EM. As it can be found through model evaluation, in our study the EM performed better than the single species distribution models.
4.2. Dominant Climatic Factor
The analysis of relative importance of climate factors showed that the average diurnal range and precipitation seasonality are useful predictors of habitat suitability for O. niloticus. The response curve analysis indicated that the presence probability of O. niloticus decreased with increasing mean diurnal range and decreasing precipitation seasonality. Zengeya et al. (2013) found that the dominant factor affecting the distribution of O. niloticus in river systems of Africa was the minimum temperature of the coldest month. While Singh et al. (2021) reported maximum temperature in January is the main and significant variable in the probability of Nile tilapia establishment in the Ganga river system in India. These differences in the importance of environmental factors could be due to the different algorithms of different models and different ways of dealing with data as well as the differences in regions of study.
The importance of mean diurnal range and precipitation seasonality in shaping the distribution range of several species of fish has been reported in previous studies. For example, Ruiz-Navarro et al. (2016) reported the annual mean temperature and mean diurnal range of temperature as the most important climatic variables indicating the suitable habitat of Perca fluviatilis and Esox lucius in Great Britain. The study of Li et al. (2022) indicated that environmental factors related to precipitation such as precipitation of seasonality play a significant role in the establishment success of non-native fishes across the Yarlung Zangbo River Basin in the Tibetan Plateau.
The mean diurnal range is the average of the difference between the monthly maximum and minimum temperatures of days and so defines the temperature fluctuations. The importance of this variable could show the negative effects of temperature fluctuations (rather than absolute temperature) on O. niloticus. The importance of this predictor may reflect a biological reliance of O. niloticus on relatively stable environmental temperature conditions. The key effect of temperature on the distribution of O. niloticus has also been stated in studies by Zengeya et al. (2013) and Singh et al. (2021).
4.3. Distribution Pattern
Based on FishBase (Froese and Pauly 2023), Eschmeyer's Catalog of Fishes (Fricke et al. 2023), and Shuai et al. (2023), Nile tilapia has been reported from at least 114 countries. Its natural distribution includes various fresh and brackish water bodies in North and Northeast Africa from the Nile River basin southwards through the Eastern and Western Rift Valley lakes in East Africa, and westwards through the basins of Lake Chad, Niger, Benue, Volta, Gambia, and Senegal rivers (Trewevas 1983; Lind et al. 2019). But, it has been widely introduced for aquaculture elsewhere e.g., Mississippi and Florida (U.S.A.), Mexico, Honduras, Costa Rica, Brazil, Ecuador, Uruguay, Argentina, Oman, Iran, Republic of Congo, Democratic Republic of Congo, Madagascar, Malaysia, Indonesia, Philippines and southern Japan (Fricke et al. 2023; Froese and Pauly 2023). The Nile tilapia is by far the most prominent amongst tilapia species produced by aquaculture, being the most farmed tropical fish species globally (Lind et al. 2019). The world distribution range of Nile tilapia shows, worldwide increasing demand for this fish in aquaculture, the rapid expansion of its population following its introduction, and its establishment in many countries including Oman as a wild population. However, by using computational tools such as species distribution models, it is possible to predict the potential range of invasive species including Nile tilapia which usually predict new area to be occupied by the invader. Morover, modeling is an effective tool to direct management efforts to confirm establishment, direct remediation efforts, and contain further spread (Zengeya et al. 2013). Based on Lake et al. (2020) one key assumption in SDMs is that sample prevalence (the frequency of sampled sites in the total study area) accurately represents species prevalence (the frequency of species over the total study area). The same has been considered in distribution modeling of Nile tilapia in Oman, as we almost covered all the inland water bodies of this area. In the southeastern Arabian Peninsula (Oman Territory) the area predicted by the ensemble distribution model as suitable for O. niloticus is mainly distributed in the coastal area of the northeast. This area mainly coincides with the known distribution of O. niloticus represented by our records. However, there are some regions of discordance between the distribution model and known O. niloticus occurrence. For example, our model predicted high habitat suitability for O. niloticus on Masirah Island on the east coast of mainland Oman while still there is no record of this species on this Island.
Based on current results, the distribution of O. niloticus is likely to be affected by climate change, because the suitable distribution areas under various climate scenarios showed a contraction. Tolerance to temperature and physiology of Nile tilapia might be the reasons for this contraction (see below).
4.4. Nile Tilapia as an Introduced Bioinvasive Species
An invasive fish is alien or non-native species that has been introduced into sites beyond its natural distributional range and has produced self-reliant populations. The invasive fish is spreading outside its primary site of introduction, causing damage to the environment structure, economy of the involved countries, and human well-being (Kolar and Lodge 2001; Lymbery et al. 2014; Gozlan et al. 2010; Esmaeili et al. 2014; Esmaeili 2021). It involves three main processes of introduction, establishment, and invasion/biopollution (Lymbery et al. 2014; Esmaeili 2021). These three main process have already been completed for O. niloticus in the southeastern Arabian Peninsula, and thus O. niloticus is considered an invasive species here.
Based on Boudouresque and Verlaque (2002), an introduced species is defined as a species that fulfills the four following criteria (i) colonization in a new area (ii) direct or indirect impacts of anthropological activity on its distribution range, iii) geographical discontinuity between its native area (Africa) and the Arabian Peninsula (remote dispersal), and iv) its successful breeding in situ without human assistance resulting establishment of this biopollutant. Based on the above-mentioned definition, O. niloticus in southeastern Arabian Peninsula freshwater environments fulfills all four criteria: Based on our study, it has been intentionally introduced from Egypt to the area (probably into the waterbodies near Masqat for the first time to control mosquitos), where they reproduced and colonized (Esmaeili et al. 2022); its extension distribution range has been intentionally or indeliberately promoted by human activities, there is a geographical discontinuity between its native area (Africa) and the new area (southeastern Arabian Peninsula), and new generations of Nile tilapia are produced in a different new area without human support. The absence of the Nile tilapia in the list of fishes of the Arabian Peninsula (see Freyhof et al. 2020, Esmaeili et al. 2022), the presence of mature and ripe individuals in almost all the studied and sampling sites, and the absence of any Nile tilapia fish farm in the southeastern Arabian Peninsula confirm that Nile tilapia is a biological pollutant acting as a biological invader.
The successful colonization of the Nile tilapia in the southeastern Arabian Peninsula like the other parts of the world can be attributed to many of its life history characteristics: the larger size in comparison to the native fishes (attaining a marketable size of 500–800 g within 6–8 months in the fish farm), a fast growth rate, a feeding diversity strategy (consuming very diverse food items i.e. benthic algae, phytoplankton, macro-invertebrates, fishes, and eggs or young of other species), good adaptability to captive conditions, tolerance to relatively poor water quality and overcrowding, relative disease resistance, aggressive spawning behavior, high levels of parental care, and the ability to spawn multiple broods throughout the year (see Canonico et al. 2005; El-Sayed 2006; Suresh and Bhujel 2012; Rairat et al. 2022; Stauffer et al. 2022). As a tropical cichlid freshwater fish, the optimal water temperature and salinity of Nile tilapia for growth performance are between 28–32◦ and 0–8 ppt, respectively (Rairat et al. 2022). Nevertheless, it has been recommended that the Nile tilapia is also suitable for brackish water aquaculture with a salinity level of up to 15 ppt (Rairat et al. 2022). The lower and upper lethal temperatures for Nile tilapia are 11–12◦C and 42◦C, respectively (Rairat et al. 2022), whereas the upper lethal salinity varies from about 20 ppt to about 40 ppt depending on the water temperature and the rate of salinity change (i.e., direct transfer vs. gradual acclimatization) (Rairat et al. 2022). The comparatively high performance in adaptability to a broad range of environmental conditions enables Nile tilapia to establish breeding populations across different geographical sites, ranging from tropical to temperate climates, and from freshwater to brackish water. The suitable environmental condition and high performance of O. niloticus are the main reasons for the establishment of this exotic fish in the southeastern Arabian Peninsula (Oman Territory).
4.5. Nile Tilapia Vs. Indigenous Species
Biological invasions have been considered as one of the main causes of the loss of biodiversity (Vitousek et al. 1997; Mollot et al. 2017; Mooney and Hobbs 2000) because the invasive species can cause severe effects on native species and ecosystem (Catford et al. 2018) especially if they more effectively occupy the same ecological niche (Castro-Dez et al. 2004). Based on Esmaeili et al. (2022), Zarei et al. (2022) and Sayyadzadeh et al. (2023) the diversity of inland fishes of the southeastern Arabian Peninsula consists of 22 recognized species 16 genera, 10 families, seven orders, and a class including 20 native species (90.91%) in nine families and two exotic species (9.09%). Out of 20 native species, seven species (35%) in two families are endemic elements that are restricted to the Oman territory in the southeastern Arabian Peninsula only. As, O. niloticus co-occurs with several native/endemic species in its current distribution range, it can affect the indigenous fishes of the region. Currently, it is sympatric with Awaous jayakari, Glossogobius tenuiformis (Gobiidae), Aphaniops kruppi, A. stoliczkanus (Aphaniidae), Cyprinion muscatense, Garra shamal, and G. dunsirei (Cyprinidae). Although due to the recent introduction of Nile tilapia into the studied area, no comprehensive study has been conducted on different aspects of biology, ecology and genetics of this invasive species, however, there are a number of studies that have examined the adverse effects of the Nile tilapia invasion on aquatic ecosystems (e.g., Gu et al. 2015; Stauffer et al. 2022). Based on these studies, the Nile tilapia induces fishing pressure on native species, influences the growth of native fishes, reduces the income of the fishermen (Gu et al. 2015), it decreases aquatic native plant diversity and its associated fauna resulting in habitat loss, bioturbation, and nutrient recycling of which bioturbation, and nutrient recycling improves eutrophication of the waterbody (see Stauffer et al. 2022). A review of the literature reveals that in the areas where the Nile tilapia has become established, ecological effects include a decrease in abundance and extinction of native species resulting from habitat and trophic overlaps and competition for spawning, habitat destruction and water quality changes, and the introduction of diseases are the main threats affecting aquatic ecosystems (Stauffer et al. 2022). Although the effects of O. niloticus invasion on the aquatic ecosystems of the southeastern Arabian Peninsula have not been studied in detail, due to its invasion strategy, the threats might be the same as that of other parts of the world. Co-invasion of parasites along with Nile tilapia invasion also should be considered.
5. Conclusions
Based on the occurrence data and species distribution modeling, the introduced Nile tilapia i) has expanded its distribution range throughout the majority of water bodies in the southeastern Arabian Peninsula, ii) has established breeding populations in the newly occupied water bodies, and iii) it has become an invader biological pollutant. Due to its invasion strategy including its intrinsic characteristics, the Nile tilapia might act as a pest, and its threats might be the same as that of other parts of the world. Co-bioinvasion of parasites along with the Nile tilapia invasion can also induce several adverse ecological effects on the very sensitive water bodies of the southeastern Arabian Peninsula including the biodiversity of this region. Concerted conservation efforts should be implemented to keep the areas that currently harbor native/endemic fishes free of invasive species. These areas can act as ‘‘reserves’’ for the conservation of indigenous fishes. The strict prohibition on the introduction of Nile tilapia, including non-native fish stocking programs, using innovative bio-surveillance monitoring techniques like environmental DNA (eDNA) that help in the early detection of potential invaders, and public awareness are also recommended.
Author Contributions
The manuscript was written, reviewed/edited ZE and HRE. Both authors have given approval to the final version of the manuscript.
Funding
The project was supported/funded by Shiraz University (2594473081).
Institutional Review Board Statement
Materials for this study resulted from (i) available published data (Freyhof et al. 2020; Esmaeili et al. 2022), and (ii) several extensive fieldworks that provided the geographic coordinate datasets for O. niloticus distribution during 2021-2022 (deposited in the Zoological Museum-Collection of Biology Department, Shiraz University, ZM-CBSU). ethical approval is not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We would like to thank S. M. Al Jufaili (Sultan Qaboos University), A. H. Masoumi and F. Pourhosseini (Shiraz University) for helping with fish collections.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Ardestani, E.G.; Rigi, H.; Honarbakhsh, A. Predicting optimal habitats of Haloxylon persicum for ecosystem restoration using ensemble ecological niche modeling under climate change in southeast Iran. Restor. Ecol. 2021, 29, e13492. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Rome, Q.; Villemant, C.; Courchamp, F. Can species distribution models really predict the expansion of invasive species? PLoS ONE 2018, 13, e0193085. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Biological pollution in the Mediterranean Sea: invasive versus introduced macrophytes. Mar. Pollut. Bull. 2016, 44, 32–38. [Google Scholar] [CrossRef]
- Brooks, M.L.; D'antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M. . Pyke, D. Effects of invasive alien plants on fire regimes. BioScience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- Canonico, G.C.; Arthington, A.; McCrary, J.K.; Thieme, M. The effects of introduced tilapias on native biodiversity. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2005, 15, 463–483. [Google Scholar] [CrossRef]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Seedling establishment of a boreal tree species (Pinus sylvestris) at its southernmost distribution limit: consequences of being in a marginal Mediterranean habitat. J. Ecol. 2004, 92, 266–277. [Google Scholar] [CrossRef]
- Catford, J.A.; Bode, M.; Tilman, D. Introduced species that overcome life history tradeoffs can cause native extinctions. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Chege, S.; Walingo, T. Multiplexing capacity of hybrid PD-SCMA heterogeneous networks. IEEE Trans. Veh. Technol. 2022, 71, 6424–6438. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Xu, X. ; GC-LSTM: Graph convolution embedded LSTM for dynamic network link prediction. Appl. Intell. 2022, 52, 7513–7528. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, X.; Zhang, J.; Zhao, J.; Ge, Z.; Zhang, Z. Predicting the Potential Suitable Distribution of Larix principis-rupprechtii Mayr under Climate Change Scenarios. Forests 2022, 13, 1428. [Google Scholar] [CrossRef]
- Davis, J.; O'Grady, A.P.; Dale, A.; Arthington, A.H.; Gell, P.A.; Driver, P.D.; ... Specht, A. When trends intersect: The challenge of protecting freshwater ecosystems under multiple land use and hydrological intensification scenarios. Sci. Total Environ. 2015, 534, 65–78. [CrossRef]
- de Oliveira da Conceição, E.; Mantovano, T.; de Campos, R.; Rangel, T.F.; Martens, K.; Bailly, D.; Higuti, J. Mapping the observed and modelled intracontinental distribution of non-marine ostracods from South America. Hydrobiologia 2020, 847, 1663–1687. [Google Scholar] [CrossRef]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.; Knowler, D.J.; Leveque, C. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Elliott, M. Biological pollutants and biological pollution–an increasing cause for concern. Mar. Pollut. Bull. 2003, 46, 275–280. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Tilapia culture. CABI publishing. 2006.
- Esmaeili, H.R. Exotic, and Invasive Freshwater Fishes in the Tigris-Euphrates River System. In Tigris and Euphrates Rivers: Their Environment from Headwaters to Mouth, 2021; (pp. 1103-1140). Springer, Cham.
- Esmaeili, H. R. , Hamidan, N. Inland fishes of the Arabian Peninsula: Review and a revised checklist. Zootaxa 2023, 5330, 201–226. [Google Scholar] [CrossRef]
- Esmaeili, H.R.; Teimori, A.; Owfi, F.; Abbasi, K.; Brian, W.C. Alien and invasive freshwater fish species in Iran: Diversity, environmental impacts and management. Iran. J. Ichthyol. 2014, 1, 61–72. [Google Scholar]
- Esmaeili, H.R.; Al Jufaili, S.; Masoumi, A.H.; Zarei, F. Ichthyodiversity in southeastern Arabian Peninsula: Annotated checklist, taxonomy, short description and distribution of iInland fishes of Oman. Zootaxa 2022, 5134, 451–503. [Google Scholar] [CrossRef]
- Franklin, J. Predictive vegetation mapping: geographic modelling of biospatial patterns in relation to environmental gradients. Prog Phys Geogr. 1995, 19, 474–499. [Google Scholar] [CrossRef]
- Freyhof, J.Ö.R.G.; YoĞurtÇuoĞlu, B. A proposal for a new generic structure of the killifish family Aphaniidae, with the description of Aphaniops teimorii (Teleostei: Cyprinodontiformes). Zootaxa 2020, 4810, zootaxa–4810. [Google Scholar] [CrossRef]
- Froese, R., Pauly, D. FishBase. World Wide Web electronic publication. 2023, www.fishbase.org (21/08/2023).
- Gozlan, R.E.; Andreou, D.; Asaeda, T.; Beyer, K.; Bouhadad, R.; Burnard, D. . Robert Britton, J. Pan-continental invasion of Pseudorasbora parva: towards a better understanding of freshwater fish invasions. Fish Fish (Oxf) 2010, 11, 315–340. [Google Scholar] [CrossRef]
- Grammer, G.L.; Slack, W.T.; Peterson, M.S.; Dugo, M.A. Nile tilapia Oreochromis niloticus (Linnaeus, 1758) establishment in temperate Mississippi, USA: Multi-year survival confirmed by otolith ages. Aquat. Invasions. 2012, 7, 367–376. [Google Scholar] [CrossRef]
- Gu, D.E.; Ma, G.M.; Zhu, Y.J.; Xu, M.; Luo, D.; Li, Y.Y. . Hu, Y.C. The impacts of invasive Nile tilapia (Oreochromis niloticus) on the fisheries in the main rivers of Guangdong Province, China. Biochem. Syst. Ecol. 2015, 59, 1–7. [Google Scholar] [CrossRef]
- Guisan, A.; Graham, C.H.; Elith, J.; Huettmann, F. The NCEAS Species Distribution Modelling Group. Sensitivity of predictive species distribution models to change in grain size. Divers. Distrib. 2017, 13, 332–340. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol Modell 2020, 135, 147–186. [Google Scholar] [CrossRef]
- Hamed, M.M.; Nashwan, M.S.; Shahid, S.; bin Ismail, T.; Wang, X.J.; Dewan, A.; Asaduzzaman, M. Inconsistency in historical simulations and future projections of temperature and rainfall: A comparison of CMIP5 and CMIP6 models over Southeast Asia. Atmos. Res. 2022, 265, 105927. [Google Scholar] [CrossRef]
- Hamza, W.; Munawar, M. Protecting and managing the Arabian Gulf: Past, present and future. Aquat. Ecosyst. Health Manag. 2009, 12, 429–439. [Google Scholar] [CrossRef]
- Hulme, P.E. Trade, transport and trouble: managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef]
- Lake, T. A. , Briscoe Runquist, R. D., Moeller, D. A. Predicting range expansion of invasive species: Pitfalls and best practices for obtaining biologically realistic projections. Divers. Distrib. 2020, 26, 1767–1779. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; Wang, X.; Wang, M.; He, R.; Wang, M. Distribution Pattern of Fish Richness in the Yarlung Zangbo River Basin. Diversity 2022, 14, 1142. [Google Scholar] [CrossRef]
- Lind, C. E. , Agyakwah, S. K., Attipoe, F. Y., Nugent, C., Crooijmans, R. P., Toguyeni, A. Genetic diversity of Nile tilapia (Oreochromis niloticus) throughout West Africa. Sci. Rep. 2019, 9, 16767. [Google Scholar] [CrossRef] [PubMed]
- Lodge, D.M.; Williams, S.; MacIsaac, H.J.; Hayes, K.R.; Leung, B.; Reichard, S. Biological invasions: Recommendations for US policy and management. Ecol Appl. 2006, 16, 2035–2054. [Google Scholar] [CrossRef]
- Lymbery, A.J.; Morine, M.; Kanani, H.G.; Beatty, S.J.; Morgan, D.L. Co-invaders: the effects of alien parasites on native hosts. Int. J. Parasitol. Parasites Wildl. 2014, 3, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. The effects of invasive species on the decline in species richness: a global meta-analysis. In Advances in ecological research, 2017; (Vol. 56, pp. 61–83). Academic Press.
- Mooney, H.A.; Hobbs, R.J. Invasive species in a changing world. Island Press, Washington. 2000; 137pp.2000.
- Ogden, N.H.; Wilson, J.R.; Richardson, D.M.; Hui, C.; Davies, S.J.; Kumschick, S. . Pulliam, J.R. Emerging infectious diseases and biological invasions: a call for a One Health collaboration in science and management. R. Soc. Open Sci. 2019, 6, 181577. [Google Scholar] [CrossRef]
- Pearson, R.G.; Thuiller, W.; Araújo, M.B.; Martínez-Meyer, E.; Brotons, L.; McClean, C.; Miles, L.; Segurado, P.; Dawson, T.; Lees, D.C. Model-based uncertainty in species range prediction. J. Biogeogr. 2006, 33, 1704–1711. [Google Scholar] [CrossRef]
- Petrie, R.; Denvil, S.; Ames, S.; Levavasseur, G.; Fiore, S.; Allen, C.; Antonio, F.; Berger, K.; Bretonnière, P.A.; Cinquini, L. Coordinating an operational data distribution network for CMIP6 data. Geosci. Model Dev. 2021, 14, 629–644. [Google Scholar] [CrossRef]
- Rairat, T.; Liu, Y.K.; Hsu, J.C.N.; Hsieh, C.Y.; Chuchird, N.; Chou, C.C. Combined effects of temperature and salinity on the pharmacokinetics of florfenicol in Nile tilapia (Oreochromis niloticus) reared in brackish water. Front. Vet. Sci. 2022, 9, 826586. [Google Scholar] [CrossRef]
- Rathore, P.; Roy, A.; Karnatak, H. Modelling the vulnerability of Taxus wallichiana to climate change scenarios in South East Asia. Ecol. Indic. 2019, 102, 199–207. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Renault, D.; Leclerc, C.; Colleu, M.A.; Boutet, A.; Hotte, H.; Colinet, H. . Convey, P. The rising threat of climate change for arthropods from Earth's cold regions: Taxonomic rather than native status drives species sensitivity. Glob Chang Biol. 2022, 28, 5914–5927. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Hoopes, M.F.; Marchetti, M.P.; Lockwood, J.L. Progress toward understanding the ecological impacts of nonnative species. Ecol. Monogr. 2013, 83, 263–282. [Google Scholar] [CrossRef]
- Ruiz-Navarro, A.; Gillingham, P.K.; Britton, J.R. Predicting shifts in the climate space of freshwater fishes in Great Britain due to climate change. Biol. Conserv. 2016, 203, 33–42. [Google Scholar] [CrossRef]
- Santamarina, S.; Alfaro-Saiz, E.; Llamas, F.; Acedo, C. Different approaches to assess the local invasion risk on a threatened species: Opportunities of using high-resolution species distribution models by selecting the optimal model complexity. Glob. Ecol. Conserv. 2019, 20, e00767. [Google Scholar] [CrossRef]
- Saemi-Komsari, M.; Esmaeili, H.R.; Keshavarzi, B.; Abbasi, K.; Birami, F.A., Nematollahi, M.J.; ... Busquets, R. Characterization of ingested MPs and their relation with growth parameters of endemic and invasive fish from a coastal wetland. Sci. Total Environ. 2023, 860,160495. [CrossRef]
- Saunders, D.L.; Meeuwig, J.J.; Vincent, A.C.J. Freshwater protected areas: Strategies for conservation. Conserv. Biol. 2002, 16, 30–41. [Google Scholar] [CrossRef]
- Sayyadzadeh, G.; Al Jufaili, S.M.; Esmaeili, H.R. Species diversity deflation: Insight into taxonomic validity of Garra species (Teleostei: Cyprinidae) from Dhofar Region in the Arabian Peninsula using an integrated morpho-molecular approach. Zootaxa, 2023, 5230, 333–350. [Google Scholar] [CrossRef]
- Shuai, F.; Li, J. Nile Tilapia (Oreochromis niloticus Linnaeus, 1758) Invasion Caused Trophic Structure Disruptions of Fish Communities in the South China River—Pearl River. Biology 2022, 11, 1665. [Google Scholar] [CrossRef]
- Shuai, F.; Li, J.; Lek, S. Nile tilapia (Oreochromis niloticus) invasion impacts trophic position and resource use of commercially harvested piscivorous fishes in a large subtropical river. Ecol Process, 2023; 12, 22. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, S.C.; Verma, P. MaxEnt distribution modeling for predicting Oreochromis niloticus invasion into the Ganga river system, India and conservation concern of native fish biodiversity. Aquat. Ecosyst. Health Manag. 2021, 24, 43–51. [Google Scholar] [CrossRef]
- Soto, I.; Cuthbert, R.N.; Kouba, A.; Capinha, C.; Turbelin, A.; Hudgins, E.J. . Haubrock, P.J. Global economic costs of herpetofauna invasions. Sci. Rep. 2022, 12, 10829. [Google Scholar] [CrossRef]
- Stauffer Jr, J.R.; Chirwa, E.R.; Jere, W.; Konings, A.F.; Tweddle, D.; Weyl, O. Nile Tilapia, Oreochromis niloticus (Teleostei: Cichlidae): a threat to native fishes of Lake Malawi? Biol Invasions 2022, 24, 1585–1597. [Google Scholar] [CrossRef]
- Stauffer, J.R.; Chirwa, E.R.; Jere, W.; Konings, A.F.; Tweddle, D.; Weyl, O. Nile tilapia, Oreochromis niloticus (teleostei: Cichlidae): A threat to native fishes of lake malawi? Biol. Invasions 2022, 24, 1585–1597. [Google Scholar] [CrossRef]
- Stewart, B.A.; Ford, B.M.; Benson, J.A. Using species distribution modelling to identify ‘coldspots’ for conservation of freshwater fishes under a changing climate. Aquat. Conserv.: Mar. Freshw Ecosyst. 2022, 32, 576–590. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. North Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Suresh, V.; Bhujel, C. Tilapias. Aquaculture: Farming Aquatic Animals and Plants. Second Edition. Wiley-Blackwell Publishing, Chichester. 2012; 338-364 pp.
- Thuiller, W. BIOMOD-Optimizing predictions of species distributions and projecting potential future shifts under global change. Glob. Chang. Biol. 2003, 9, 1353–1362. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F.; Georges, M.D.; Thuiller, C.W. Package ‘biomod2’. Species Distrib. Model. within an ensemble Forecast. Framew. 2016, 1–135. [Google Scholar]
- Trewevas, E. Tilapiine Fishes of the Genera Sarotherodon, Oreochromis and Danakilia. 583, British Museum Natural History, 1983.
- Vitousek, P.M.; D’Antonio, C.M.; Loope, L.L.; Rejmanek, M.; Westbrooks, R. Introduced species: a significant component of human-caused global change. N. Z. J. Ecol. 1997, 21, 1–16. [Google Scholar]
- Wu, T.; Yu, R.; Lu, Y.; Jie, W.; Fang, Y.; Zhang, J.; Zhang, L.; Xin, X.; Li, L.; Wang, Z. BCC-CSM2-HR: A high-resolution version of the Beijing Climate Center Climate System Model. Geosci. Model Dev. 2021, 14, 2977–3006. [Google Scholar] [CrossRef]
- Zarei, F.; Jufaili, S.M.A.; Esmaeili, H.R. Oxyurichthys omanensis sp. nov., a new Eyebrow Goby (Teleostei: Gobiidae) from Oman. Zootaxa, 2022, 5182, 361–376. [Google Scholar] [CrossRef]
- Zengeya, T.A.; Robertson, M.P.; Booth, A.J.; Chimimba, C.T. Ecological niche modeling of the invasive potential of Nile tilapia Oreochromis niloticus in African river systems: concerns and implications for the conservation of indigenous congenerics. Biol. Invasions 2013, 15, 1507–1521. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Xu, B.; Xue, Y.; Ren, Y. How to predict biodiversity in space? An evaluation of modeling approaches in marine ecosystems. Divers. Distrib. 2019, 25, 1697–1708. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, Y.; Wei, H.; Ran, Q.; Liu, J.; Zhang, Q.; Gu, W. Potential distribution of Notopterygium incisum Ting ex H.T. Chang and its predicted responses to climate change based on a comprehensive habitat suitability model. Ecol. Evol. 2020, 10, 3004–3016. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).