Submitted:
25 August 2023
Posted:
25 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
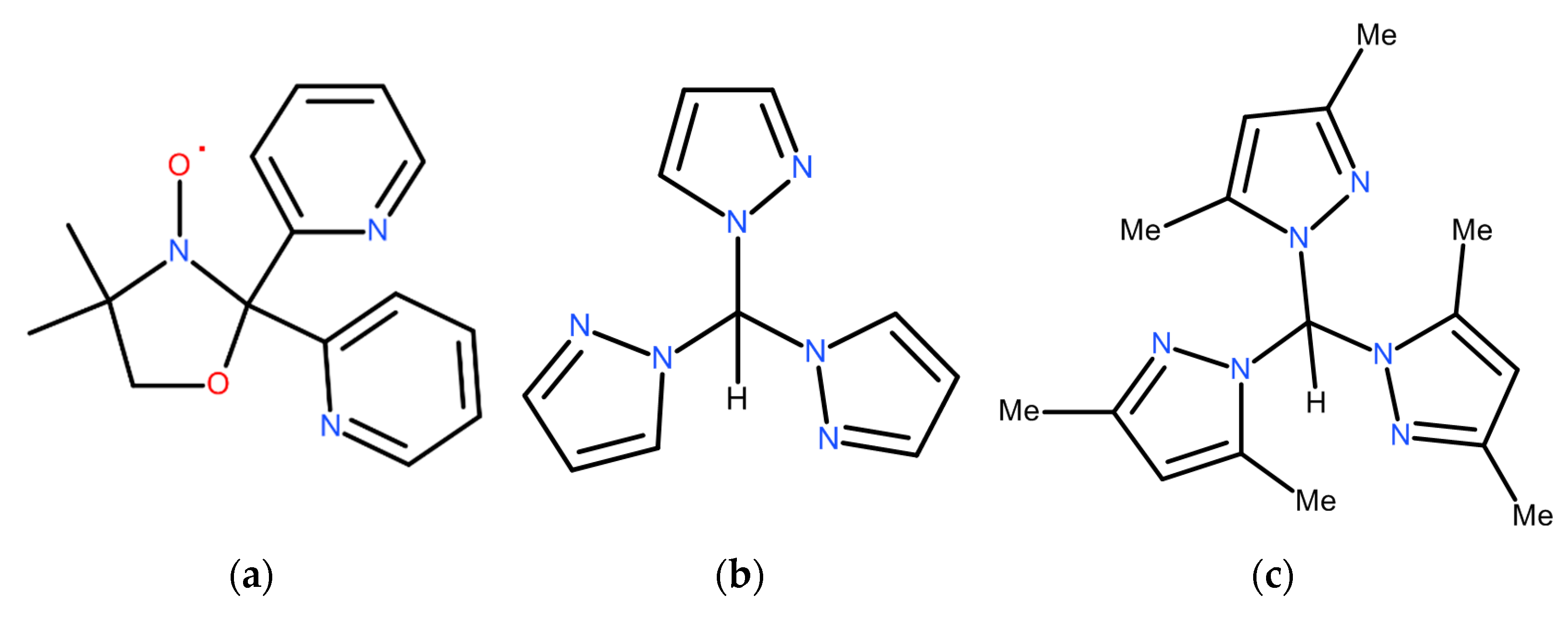
2. Short Theoretical Background
3. Results and Discussion
3.1. Synthetic Aspects
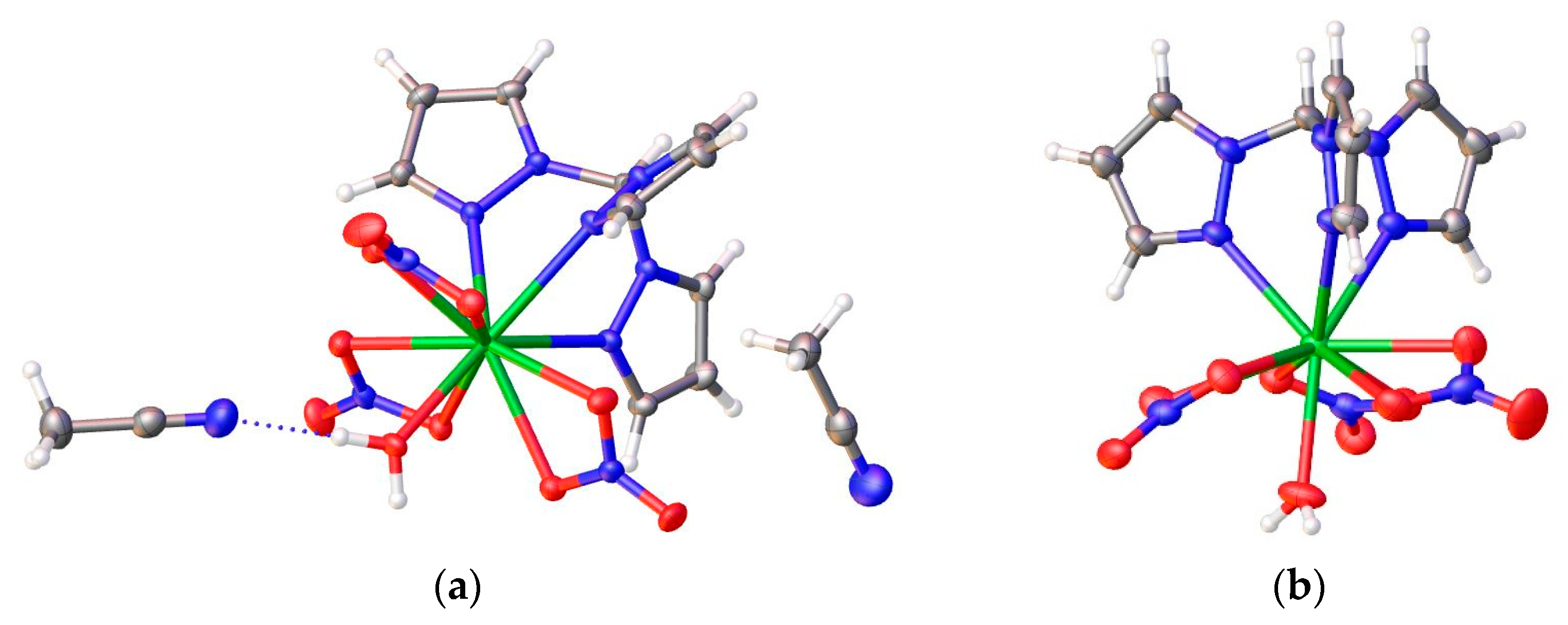
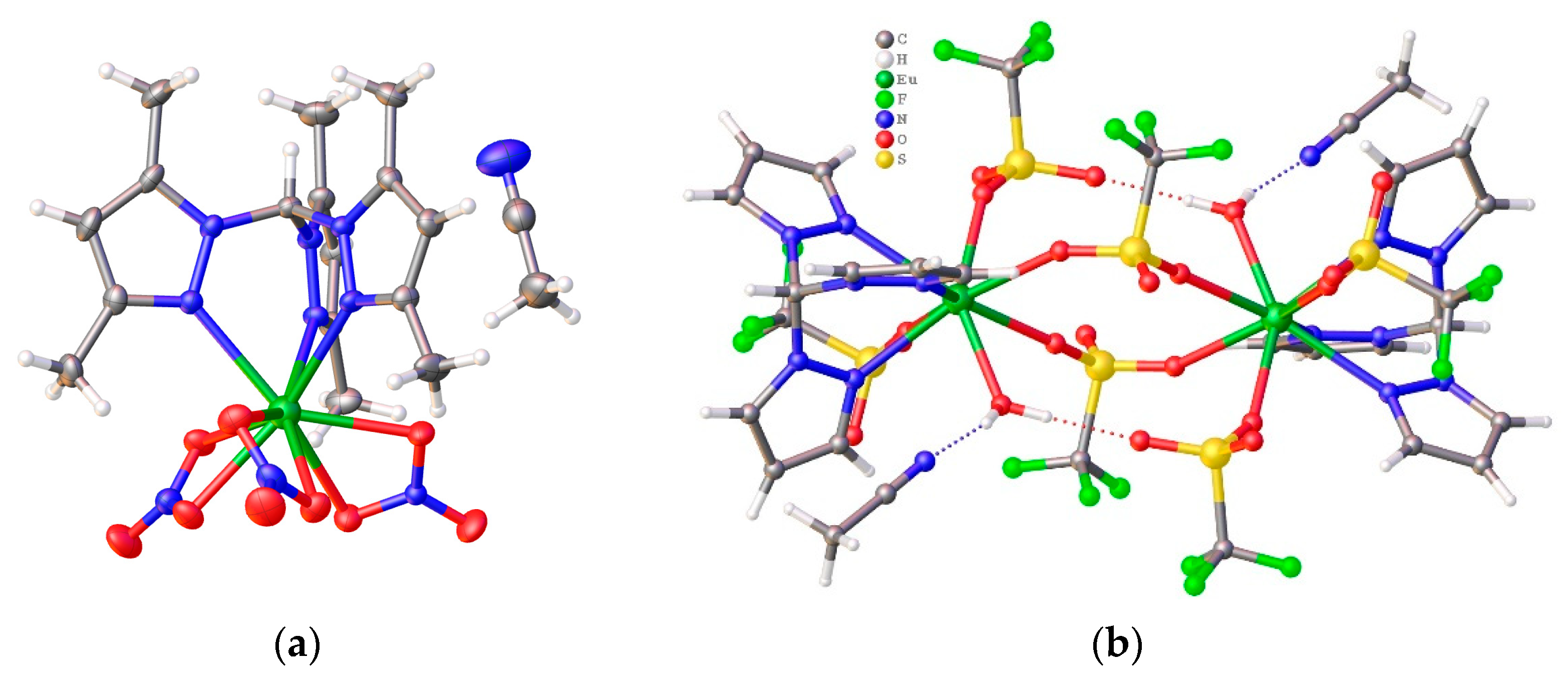
3.2. Description of the Crystal Structures 1–3
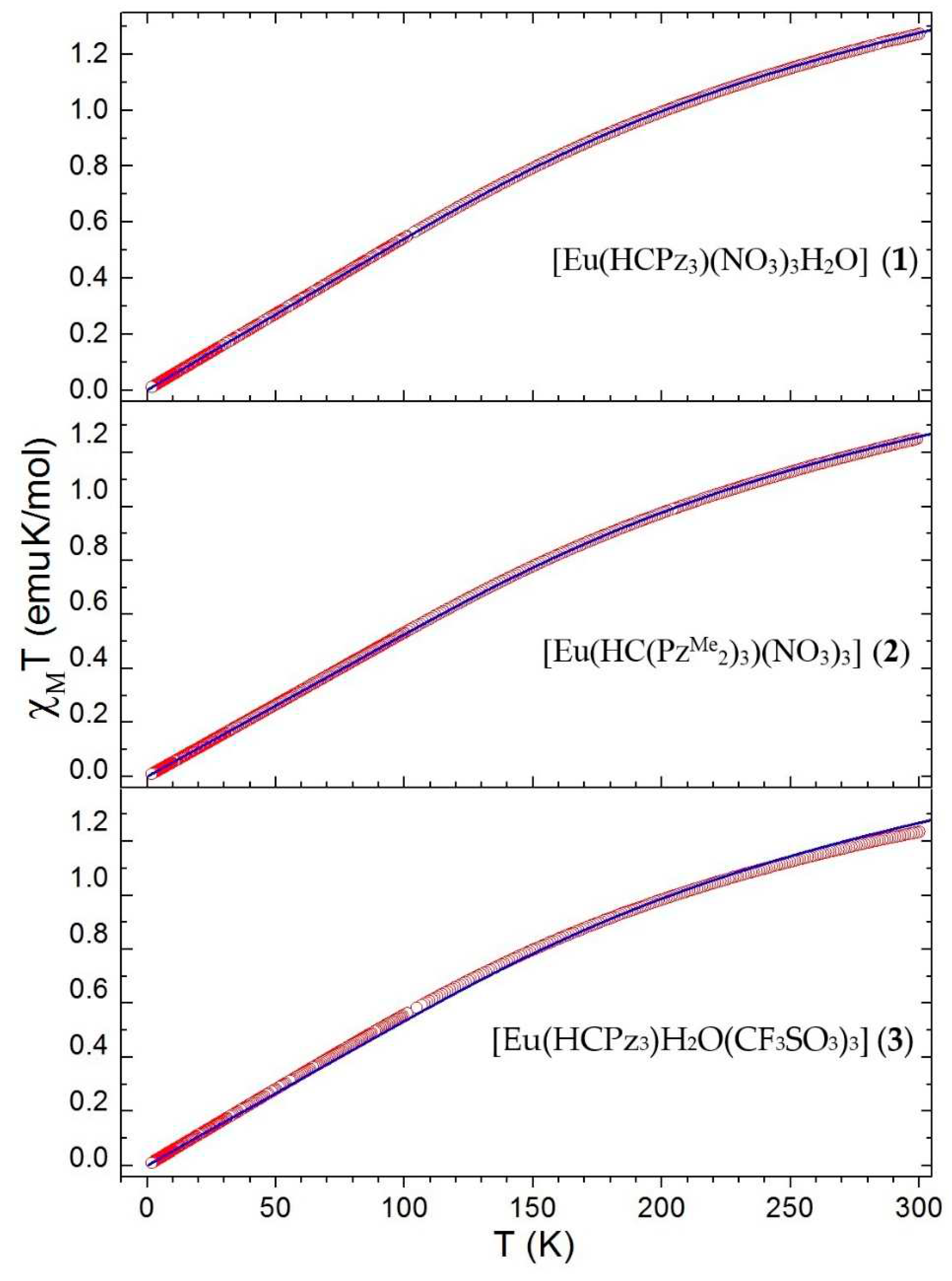
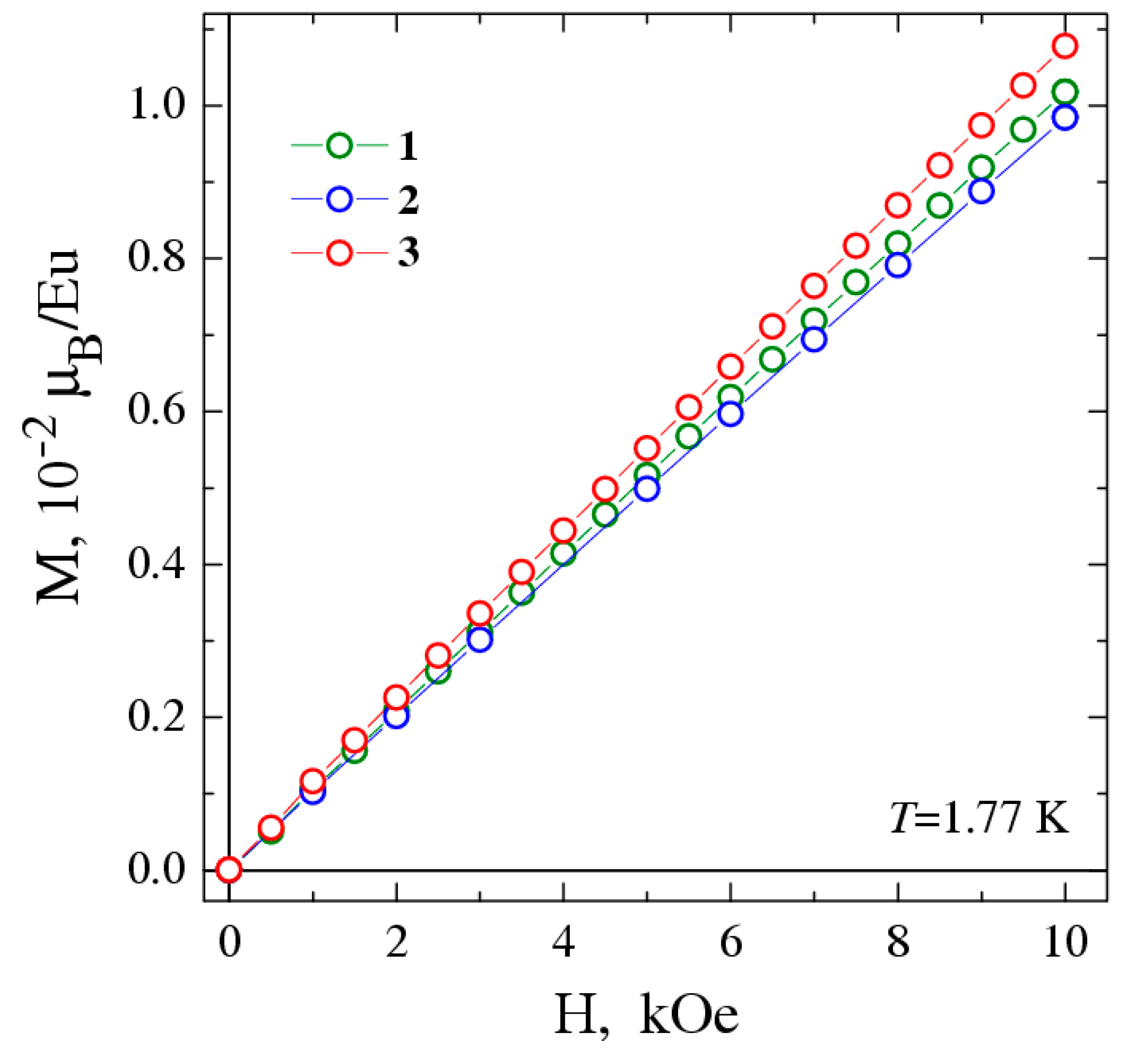
3.3. Magnetic Behavior of The Complexes
3.4. Spin-orbit Coupling Parameter Analysis
4. Experimental Section
4.1. Materials and Physical Measurements
4.2. Single-crystal XRD experiment and data refinment details
4.3. Synthesis of the Complexes
5. Conclusions and perspectives
Author Contributions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, X.; Bernot, K.; Pointillart, F.; Poneti, G.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Sessoli, R. A Luminescent and Sublimable DyIII-Based Single-Molecule Magnet. Chem. – A Eur. J. 2012, 18, 11379–11387. [Google Scholar] [CrossRef]
- Marin, R.; Brunet, G.; Murugesu, M. Shining New Light on Multifunctional Lanthanide Single-Molecule Magnets. Angew. Chemie Int. Ed. 2021, 60, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Suta, M.; Wickleder, C. Synthesis, Spectroscopic Properties and Applications of Divalent Lanthanides Apart from Eu2+. J. Lumin. 2019, 210, 210–238. [Google Scholar] [CrossRef]
- Morss, L.R.B.T.-H. on the P. and C. of R.E. Chapter 122 Comparative Thermochemical and Oxidation-Reduction Properties of Lanthanides and Actinides. In Lanthanides/Actinides: Chemistry; Elsevier, 1994; Vol. 18, pp. 239–291 ISBN 0168-1273.
- Rodrigues, L.C.V.; Hölsä, J.; Brito, H.F.; Maryško, M.; Matos, J.R.; Paturi, P.; Rodrigues, R.V.; Lastusaari, M. Magneto-Optical Studies of Valence Instability in Europium and Terbium Phosphors. J. Lumin. 2016, 170, 701–706. [Google Scholar] [CrossRef]
- Chen, J.; Carpenter, S.H.; Fetrow, T.V.; Mengell, J.; Kirk, M.L.; Tondreau, A.M. Magnetism Studies of Bis(Acyl)Phosphide-Supported Eu3+ and Eu2+ Complexes. Inorg. Chem. 2022, 61, 18466–18475. [Google Scholar] [CrossRef]
- Evans, W.J. Perspectives in Reductive Lanthanide Chemistry. Coord. Chem. Rev. 2000, 206–207, 263–283. [Google Scholar] [CrossRef]
- Dolai, M.; Ali, M.; Rajnák, C.; Titiš, J.; Boča, R. Slow Magnetic Relaxation in Cu(II)–Eu(III) and Cu(II)–La(III) Complexes. New J. Chem. 2019, 43, 12698–12701. [Google Scholar] [CrossRef]
- Wang, J.; Ruan, Z.-Y.; Li, Q.-W.; Chen, Y.-C.; Huang, G.-Z.; Liu, J.-L.; Reta, D.; Chilton, N.F.; Wang, Z.-X.; Tong, M.-L. Slow Magnetic Relaxation in a {EuCu5} Metallacrown. Dalt. Trans. 2019, 48, 1686–1692. [Google Scholar] [CrossRef]
- Perfetti, M.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Lanthanide Complexes with a Tripodal Nitroxyl Radical Showing Strong Magnetic Coupling. Inorg. Chem. 2020, 59, 16591–16598. [Google Scholar] [CrossRef]
- Rey, P.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Tripodal Oxazolidine-N-Oxyl Diradical Complexes of Dy3+ and Eu3+. Inorganics 2021, 9, 91. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Fokin, S. V; Zueva, E.M.; Tkacheva, A.O.; Romanenko, G. V; Bogomyakov, A.S.; Larionov, S. V; Popov, S.A.; Ovcharenko, V.I. Complexes of Lanthanides with Spin-Labeled Pyrazolylquinoline. Russ. Chem. Bull. 2014, 63, 1459–1464. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Hu, P.; Wang, J.; Li, L. Slow Magnetic Relaxation and Field-Induced Metamagnetism in Nitronyl Nitroxide-Dy(III) Magnetic Chains. Dalt. Trans. 2015, 44, 4560–4567. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Z.; Yin, X.; Wang, S.; Yang, S.; Zhang, J.; Geng, L.; Shi, S. Structure and Magnetic Investigation of a Series of Rare Earth Heterospin Monometallic Compounds of Nitronyl Nitroxide Radical. Zeitschrift für Anorg. und Allg. Chemie 2016, 642, 148–154. [Google Scholar] [CrossRef]
- Kahn, M.L.; Sutter, J.-P.; Golhen, S.; Guionneau, P.; Ouahab, L.; Kahn, O.; Chasseau, D. Systematic Investigation of the Nature of the Coupling between a Ln(III) Ion (Ln = Ce(III) to Dy(III)) and Its Aminoxyl Radical Ligands. Structural and Magnetic Characteristics of a Series of {Ln(Radical)2} Compounds and the Related {Ln(Nitrone)2} Derivat. J. Am. Chem. Soc. 2000, 122, 3413–3421. [Google Scholar] [CrossRef]
- Lv, X.-H.; Yang, S.-L.; Li, Y.-X.; Zhang, C.-X.; Wang, Q.-L. A Family of Lanthanide Compounds Based on Nitronyl Nitroxide Radicals: Synthesis, Structure, Magnetic and Fluorescence Properties. RSC Adv. 2017, 7, 38179–38186. [Google Scholar] [CrossRef]
- Fan, Z.; Hou, Y.-F.; Wang, S.-P.; Yang, S.-T.; Zhang, J.-J.; Shi, S.-K.; Geng, L.-N. Synthesis, Crystal Structure, and Magnetic Property of New Trispin Ln(III)-Nitronyl Nitroxide Complexes. Helv. Chim. Acta 2016, 99, 732–741. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Gao, Y.-Y.; Ma, Y.; Wang, Q.-L.; Li, L.-C.; Liao, D.-Z. Syntheses, Crystal Structures, Magnetic and Luminescence Properties of Five Novel Lanthanide Complexes of Nitronyl Nitroxide Radical. J. Solid State Chem. 2013, 202, 276–281. [Google Scholar] [CrossRef]
- Xu, J.-X.; Ma, Y.; Liao, D.; Xu, G.-F.; Tang, J.; Wang, C.; Zhou, N.; Yan, S.-P.; Cheng, P.; Li, L.-C. Four New Lanthanide−Nitronyl Nitroxide (LnIII = PrIII, SmIII, EuIII, TmIII) Complexes and a TbIII Complex Exhibiting Single-Molecule Magnet Behavior. Inorg. Chem. 2009, 48, 8890–8896. [Google Scholar] [CrossRef]
- Buchler, J.W.; De Cian, A.; Fischer, J.; Kihn-Botulinski, M.; Weiss, R. Metal Complexes with Tetrapyrrole Ligands. 46. Europium(III) Bis(Octaethylporphyrinate), a Lanthanoid Porphyrin Sandwich with Porphyrin Rings in Different Oxidation States, and Dieuropium(III) Tris(Octaethylporphyrinate). Inorg. Chem. 1988, 27, 339–345. [Google Scholar] [CrossRef]
- Klementyeva, S.V.; Gritsan, N.P.; Khusniyarov, M.M.; Witt, A.; Dmitriev, A.A.; Suturina, E.A.; Hill, N.D.D.; Roemmele, T.L.; Gamer, M.T.; Boeré, R.T.; et al. The First Lanthanide Complexes with a Redox-Active Sulfur Diimide Ligand: Synthesis and Characterization of [LnCp*2(RN=)2S], Ln=Sm, Eu, Yb; R=SiMe3. Chem. - A Eur. J. 2017, 23, 1278–1290. [Google Scholar] [CrossRef]
- Caneschi, A.; Dei, A.; Gatteschi, D.; Poussereau, S.; Sorace, L. Antiferromagnetic Coupling between Rare Earth Ions and Semiquinones in a Series of 1 ∶ 1 Complexes. Dalt. Trans. 2004, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Dei, A.; Gatteschi, D.; Pécaut, J.; Poussereau, S.; Sorace, L.; Vostrikova, K. Crystal Field and Exchange Effects in Rare Earth Semiquinone Complexes. Comptes Rendus l’Academie des Sci. - Ser. IIC Chem. 2001, 4. [Google Scholar] [CrossRef]
- Gaft, M.; Reisfeld, R.; Panczer, G. Modern Luminescence Spectroscopy of Minerals and Materials; Springer-Verlag: Berlin/Heidelberg, 2005; ISBN 3-540-21918-8. [Google Scholar]
- Machado, K.; Mukhopadhyay, S.; Videira, R.A.; Mishra, J.; Mobin, S.M.; Mishra, G.S. Polymer Encapsulated Scorpionate Eu3+ Complexes as Novel Hybrid Materials for High Performance Luminescence Applications. RSC Adv. 2015, 5, 35675–35682. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of Europium(III) Spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. On the Design of Highly Luminescent Lanthanide Complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Sorace, L.; Gatteschi, D. Electronic Structure and Magnetic Properties of Lanthanide Molecular Complexes. In Lanthanides and Actinides in Molecular Magnetism; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 1–26. [Google Scholar]
- Andruh, M.; Bakalbassis, E.; Kahn, O.; Trombe, J.C.; Porcher, P. Structure, Spectroscopic and Magnetic Properties of Rare Earth Metal(III) Derivatives with the 2-Formyl-4-Methyl-6-(N-(2-Pyridylethyl)Formimidoyl)Phenol Ligand. Inorg. Chem. 1993, 32, 1616–1622. [Google Scholar] [CrossRef]
- Huang, J.; Loriers, J.; Porcher, P.; Teste de Sagey, G.; Caro, P.; Levy-Clement, C. Crystal Field Effect and Paramagnetic Susceptibility of Na5Eu(MoO4)4 and Na5Eu(WO4)4. J. Chem. Phys. 1984, 80, 6204–6209. [Google Scholar] [CrossRef]
- Caro, P.; Porcher, P. The Paramagnetic Susceptibility of C-Type Europium Sesquioxide. J. Magn. Magn. Mater. 1986, 58, 61–66. [Google Scholar] [CrossRef]
- Sella, A.; Brown, S.E.; Steed, J.W.; Tocher, D.A. Synthesis and Solid-State Structures of Pyrazolylmethane Complexes of the Rare Earths. Inorg. Chem. 2007, 46, 1856–1864. [Google Scholar] [CrossRef]
- Chen, S.-J.; Li, Y.-Z.; Chen, X.-T.; Shi, Y.-J.; You, X.-Z. Tetrabutylammonium Tetrakis(Nitrato-κ2O,O′)[1,3,5-Tris(Pyrazolyl) Methane-κ3N]Cerate(III). Acta Crystallogr. Sect. E Struct. Reports Online 2002, 58, m753–m755. [Google Scholar] [CrossRef]
- Bagnall, K.W.; Tempest, A.C.; Takats, J.; Masino, A.P. Lanthanoid Poly(Pyrazol-1-yl) Borate Complexes. Inorg. Nucl. Chem. Lett. 1976, 12, 555–557. [Google Scholar] [CrossRef]
- Stainer, M.V.R.; Takats, J. X-Ray Crystal and Molecular Structure of Tris[Hydridotris(Pyrazol-1-yl)Borato]Ytterbium(III), Yb(HBPz3)3. Inorg. Chem. 1982, 21, 4050–4053. [Google Scholar] [CrossRef]
- Apostolidis, C.; Rebizant, J.; Kanellakopulos, B.; von Ammon, R.; Dornberger, E.; Müller, J.; Powietzka, B.; Nuber, B. Homoscorpionates (Hydridotris(1-Pyrazolyl)Borato Complexes) of the Trivalent 4fions. The Crystal and Molecular Structure of [(HB(N2C3H3)3]3LnIII, (Ln = Pr, Nd). Polyhedron 1997, 16, 1057–1068. [Google Scholar] [CrossRef]
- Apostolidis, C.; Rebizant, J.; Walter, O.; Kanellakopulos, B.; Reddmann, H.; Amberger, H.-D. Zur Elektronenstruktur Hochsymmetrischer Verbindungen Der F-Elemente. 35 [1] Kristall- Und Molekülstrukturen von Tris(Hydrotris(1-Pyrazolyl)Borato)-Lanthanid(III) (LnTp3; Ln = La, Eu) Sowie Elektronenstruktur von EuTp3. Zeitschrift für Anorg. und Allg. Chemie 2002, 628, 2013–2025. [Google Scholar] [CrossRef]
- Apostolidis, C.; Kovács, A.; Morgenstern, A.; Rebizant, J.; Walter, O. Tris-{Hydridotris(1-Pyrazolyl)Borato}lanthanide Complexes: Synthesis, Spectroscopy, Crystal Structure and Bonding Properties. Inorganics 2021, 9, 44. [Google Scholar] [CrossRef]
- Reger, D.L.; Knox, S.J.; Lindeman, J.A.; Lebioda, L. Poly(Pyrazolyl) Borate Complexes of Terbium, Samarium, and Erbium. X-Ray Crystal Structure of {[η3-HB(Pz)3]2Sm(µ-O2CPh)}2 (Pz = Pyrazolyl Ring). Inorg. Chem. 1990, 29, 416–419. [Google Scholar] [CrossRef]
- Chowdhury, T.; Evans, M.J.; Coles, M.P.; Bailey, A.G.; Peveler, W.J.; Wilson, C.; Farnaby, J.H. Reduction Chemistry Yields Stable and Soluble Divalent Lanthanide Tris(Pyrazolyl)Borate Complexes. Chem. Commun. 2023, 59, 2134–2137. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, Z.; Zhan, G.; Sun, B.; Yan, W.; Wang, C.; Wang, L.; Liu, Z.; Bian, Z.; Huang, C. Air Stable and Efficient Rare Earth Eu(II) Hydro-Tris(Pyrazolyl)Borate Complexes with Tunable Emission Colors. Inorg. Chem. Front. 2020, 7, 4593–4599. [Google Scholar] [CrossRef]
- Long, J.; Lyubov, D.M.; Mahrova, T.V.; Cherkasov, A. V.; Fukin, G.K.; Guari, Y.; Larionova, J.; Trifonov, A.A. Synthesis, Structure and Magnetic Properties of Tris(Pyrazolyl)Methane Lanthanide Complexes: Effect of the Anion on the Slow Relaxation of Magnetization. Dalt. Trans. 2018, 47, 5153–5156. [Google Scholar] [CrossRef]
- Chowdhury, T.; Horsewill, S.J.; Wilson, C.; Farnaby, J.H. Heteroleptic Lanthanide(III) Complexes: Synthetic Utility and Versatility of the Unsubstituted Bis-Scorpionate Ligand Framework. Aust. J. Chem. 2022, 75, 660–675. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, Version 2.1, Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools. Univ. Barcelona, Barcelona, Spain 2013, 2103. [Google Scholar]
- Rousset, E.; Piccardo, M.; Gable, R.W.; Massi, M.; Sorace, L.; Soncini, A.; Boskovic, C. Elucidation of LMCT Excited States for Lanthanoid Complexes: A Theoretical and Solid-State Experimental Framework. Inorg. Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Arauzo, A.; Lazarescu, A.; Shova, S.; Bartolomé, E.; Cases, R.; Luzón, J.; Bartolomé, J.; Turta, C. Structural and Magnetic Properties of Some Lanthanide (Ln = EuIII, GdIII and NdIII) Cyanoacetate Polymers: Field-Induced Slow Magnetic Relaxation in the Gd and Nd Substitutions. Dalt. Trans. 2014, 43, 12342–12356. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-F.; Wang, M.-S.; Guo, S.-P.; Zheng, F.-K.; Chen, H.-F.; Jiang, X.-M.; Liu, G.-N.; Guo, G.-C.; Huang, J.-S. Photoluminescent and Magnetic Properties of a Series of Lanthanide Coordination Polymers with 1-H-Tetrazolate-5-Formic Acid. Cryst. Growth Des. 2011, 11, 372–381. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, F.-K.; Liu, X.; Zou, W.-Q.; Guo; Lu, C. -Z.; Huang, J.-S. Crystal Structures and Magnetic and Luminescent Properties of a Series of Homodinuclear Lanthanide Complexes with 4-Cyanobenzoic Ligand. Inorg. Chem. 2006, 45, 6308–6316. [Google Scholar] [CrossRef]
- Xu, N.; Wang, C.; Shi, W.; Yan, S.-P.; Cheng, P.; Liao, D.-Z. Magnetic and Luminescent Properties of Sm, Eu, Tb, and Dy Coordination Polymers with 2-Hydroxynicotinic Acid. Eur. J. Inorg. Chem. 2011, 2011, 2387–2393. [Google Scholar] [CrossRef]
- Han, M.; Li, S.; Ma, L.; Yao, B.; Feng, S.-S.; Zhu, M. Luminescent and Magnetic Bifunctional Coordination Complex Based on a Chiral Tartaric Acid Derivative and Europium. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 1220–1227. [Google Scholar] [CrossRef]
- De Oliveira Maciel, J.W.; Lemes, M.A.; Valdo, A.K.; Rabelo, R.; Martins, F.T.; Queiroz Maia, L.J.; de Santana, R.C.; Lloret, F.; Julve, M.; Cangussu, D. Europium(III), Terbium(III), and Gadolinium(III) Oxamato-Based Coordination Polymers: Visible Luminescence and Slow Magnetic Relaxation. Inorg. Chem. 2021, 60, 6176–6190. [Google Scholar] [CrossRef]
- Vaz, R.C.A.; Esteves, I.O.; Oliveira, W.X.C.; Honorato, J.; Martins, F.T.; Marques, L.F.; dos Santos, G.L.; Freire, R.O.; Jesus, L.T.; Pedroso, E.F.; et al. Mononuclear Lanthanide(III)-Oxamate Complexes as New Photoluminescent Field-Induced Single-Molecule Magnets: Solid-State Photophysical and Magnetic Properties. Dalt. Trans. 2020, 49, 16106–16124. [Google Scholar] [CrossRef]
- Shintoyo, S.; Fujinami, T.; Matsumoto, N.; Tsuchimoto, M.; Weselski, M.; Bieńko, A.; Mrozinski, J. Synthesis, Crystal Structure, Luminescent and Magnetic Properties of Europium(III) and Terbium(III) Complexes with a Bidentate Benzoate and a Tripod N7 Ligand Containing Three Imidazole, [LnIII(H3L)Benzoate](ClO4)2·H2O·2MeOH (LnIII=EuIII and TbIII). Polyhedron 2015, 91, 28–34. [Google Scholar] [CrossRef]
- Benelli, C.; Caneschi, A.; Gatteschi, D.; Pardi, L.; Rey, P. Structure and Magnetic Properties of Linear-Chain Complexes of Rare-Earth Ions (Gadolinium, Europium) with Nitronyl Nitroxides. Inorg. Chem. 1989, 28, 275–280. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.; Xiong, C.; Xu, J.; Li, Q.; Fang, D. Three New One-Dimensional Lanthanide Complexes Bridged by Nitronyl Nitroxide Radicals: Crystal Structures and Magnetic Characterization. J. Mol. Struct. 2013, 1036, 107–110. [Google Scholar] [CrossRef]
- Mei, X.; Wang, X.; Wang, J.; Ma, Y.; Li, L.; Liao, D. Dinuclear Lanthanide Complexes Bridged by Nitronyl Nitroxide Radical Ligands with 2-Phenolate Groups: Structure and Magnetic Properties. New J. Chem. 2013, 37, 3620–3626. [Google Scholar] [CrossRef]
- Zhu, M.; Jia, L.; Li, Y.; Li, Y.; Zhang, L.; Zhang, W.; Lü, Y. Nitronyl Nitroxide Bridged 3d–4f Hetero-Tri-Spin Chains: Synthesis Strategy, Crystal Structure and Magnetic Properties. CrystEngComm 2018, 20, 420–431. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Apex3 Software Suite: Apex3, SADABS-2016/2 and SAINT 8.40a; Publisher: Bruker AXS Inc. 2017.
- Sheldrick, G.M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2 : A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
| 1 | 1a | 2 | 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eu11 | Eu21 | |||||||||
| Eu—N | 2.5810(12) | 2.5678(16) | 2.5402(19) | Eu1—N1 | 2.520(8) | Eu2—N7 | 2.533(8) | |||
| 2.5926(12) | 2.5180(17) | 2.5592(19) | Eu1—N3 | 2.537(8) | Eu2—N9 | 2.542(9) | ||||
| 2.5932(12) | 2.6072(16) | 2.5158(18) | Eu1—N5 | 2.552(8) | Eu2—N11 | 2.552(9) | ||||
| mean | 2.5889 | 2.5643 | 2.5384 | 5.36 | 5.42 | |||||
| Eu—Oanion | 2.5687(11) | 2.4909(14) | 2.4440(17) | Eu1—O1 | 2.378(6) | Eu2—O11 | 2.382(7) | |||
| 2.4846(10) | 2.7172(16) | 2.4883(17) | Eu1—O3i | 2.404(6) | Eu2—O14 | 2.520(8) | ||||
| 2.5153(11) | 2.4288(13) | 2.4718(17) | Eu1—O4 | 2.376(6) | Eu2—O17 | 2.537(8) | ||||
| 2.4655(11) | 2.4983(15) | 2.4465(17) | Eu1—O7 | 2.334(6) | Eu2—O19ii | 2.552(8) | ||||
| 2.5234(11) | 2.5306(15) | 2.4674(17) | mean | 2.373 | mean | 2.498 | ||||
| 2.5005(11) | 2.5199(15) | 2.4514(17) | ||||||||
| mean | 2.5097 | 2.5310 | 2.4616 | |||||||
| Eu—Oaqua | 2.4191(11) | 2.4237(15) | Eu1—O10 | 2.382(7) | Eu2—O20 | 2.360(8) | ||||
| Eu …. Eu | 6.151 | 6.646 | 8.931 | 6.137 | 6.073 | |||||
| Compound | χMT (emu·K/mol) | λ (cm–1)a | Rb | Reference | |
|---|---|---|---|---|---|
| 2 K | 300 K | ||||
| Free Eu3+ | 0 | 1.280 | 220–380 | [21,45] | |
| 1 | 0.0113 | 1.228 | 406 | 1.02×10–4 | this work |
| 2 | 0.0114 | 1.251 | 396 | 4.10×10–5 | this work |
| 3/Eu | 0.0120 | 1.255 | 383 | 3.26×10-4 | this work |
| [Eu2(L1)6(H2O)4]n/Eu | 0.025 | 1.325 | 343/360c | [46] | |
| [Eu2(tzf)3(H2O)6]n/Eu | 0.020 | 1.388 | 344 | 2.7×10-4 | [47] |
| [Eu2L26Pen2(H2O)2]/Eu | 404/379d | 3.1×10-4 | [48] | ||
| [EuL3(H2O)2SO4]n | 336 | 4.28×10–4 | [49] | ||
| [EuL43(CH3OH)3]n | 360 | 9.65×10–5 | [50] | ||
| [Eu2(Hpcpa)3(H2O)5]n | sap | 1.2 | 356/351c | 2.2×10–5 | [51] |
| [Eu(HLCl)4(dmso)]− | 1.37 | 348 | 4.1×10−5 | [52] | |
| [Eu(HLF)4(dmso)]− | 1.37 | 336 | 2.1×10−5 | [52] | |
| [EuL52(H2O)4](ClO4)3] | 362/370c | 7.0×10–4 | [29] | ||
| [Eu(trippBAP)3] | 363 | [6] | |||
| [EuL6PhCOO](ClO4)2 | 0.015 | 1.30 | 370/410d | [53] | |
| [Eu(Nitrone)2(NO3)3] | 1.25 | 371.5 | [15] | ||
| [Eu(HBPz3)2Trp] | 0.037 | 1.48 | [22] | ||
| [Eu(hfac)3NNe] | 0.192 | 1.946 | 332 | 2.0×10−3 | [19] |
| Eu(OEP)2–(OEP)–] | 0.208 | 1.575 | [20] | ||
| [EuIII(AP2–)(ISQ)–] | 0.252 | 1.834 | [21] | ||
| [Eu(HBPz3)2SQ] | 0.28 | 1.59 | [22] | ||
| [Eu(hfac)3NN2]SMMTb | 0.035 | 2.24 | 259 | [12] | |
| [Eu(hfac)3PhOMeNN2] | 0.11 | 1.89 | [13] | ||
| [Eu(hfac)3NN2] | 0.12 | 1.98 | 309 | [14] | |
| [EuNNtrz2(NO3)3] | 0.24 | 1.93 | [15] | ||
| [Eu(hfac)3NN2] | 0.419 | 3.09 | [16] | ||
| [Eu(hfac)3NN2] | 2.38 | 175 | [17] | ||
| [Eu(hfac)3NN2] | 2.12 | 331 | [18] | ||
| [EuRad2(OTf)3] | 0.348 | 1.78 | [11] | ||
| [Eu(hfac)3NNEt]n | 0.89 | 1.9 | [54] | ||
| [Eu(hfac)3NN]n | 1.88 | 267 | 1.47×10−4 | [55] | |
| [Eu2(acac)4PhONN2] | 0.66 | 3.47 | 326 | 2.67×10-5 | [56] |
| [NN4CuL2(Eu(hfac)3)3] | 0.38 | 5.78 | 1.8 ×10–3 | [57] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





