1. Introduction
According to the World Health Organization (WHO), more than 350 million people have already suffered from depression, and 94% of which manifest cognitive symptoms such as concentration difficulties, forgetfulness and indecisiveness, which affect their aspects of life. Moreover, it is suggested that depression is associated with a higher likelihood of unnatural death due to suicide [
1]. Even though the evident pathophysiology of depression remains largely uncharacterized, genetic factors and psychological stress events are clearly associated with major depressive disorder (MDD) [
2]. Primary stress-related hormone in human is cortisol (also corticosterone in rodents), which is regulated by the hypothalamic-pituitary-adrenal (HPA) axis. Excessive exposure to stressors leads to alteration in HPA system regulation via destruction of negative feedback resulting in hypercortisolism found in patients with mood disorder [
3,
4]. FK506 binding protein 51 (FKBP5), a co-chaperone in glucocorticoid receptor (GR) complex, has a negative effect on GR signaling. When FKBP5 is bound to GR, it increases receptor resistance that inhibits GR translocation to nucleus then negative feedback may not occur [
5,
6]. In addition, it is suggested that hypercortisolism secondarily downregulated GR expression which could be ameliorated by antidepressants [
7]. There are many evidences demonstrating that HPA-axis dysfunction could reduce neurotrophic proteins, such as brain-derived neurotrophic factor (BDNF), which contribute to neuronal cell death and alteration of neurogenesis in the hippocampus [
8]. Interestingly, the higher expression of serine/threonine-protein kinase 1 (SGK-1), another protein regulated by glucocorticoids (GCs) activities, can weaken neuroplasticity in related brain regions [
9].
Oroxylum indicum (L.) Kurz, a plant belonging to the family Bignoniaceae, has been used in a traditional medicine remedy for centuries. It is cultivated throughout Southeast and South Asian countries, and commonly known as “Trumpet tree” due to the shape of its flowers [
10]. Every part of
O. indicum, including stem barks, pods, leaves, and seeds, exerts a myriad of pharmacological activities, such as antioxidant [
11], anti-inflammatory [
12,
13] and neuroprotective [
14] activities. The constituents mostly found in
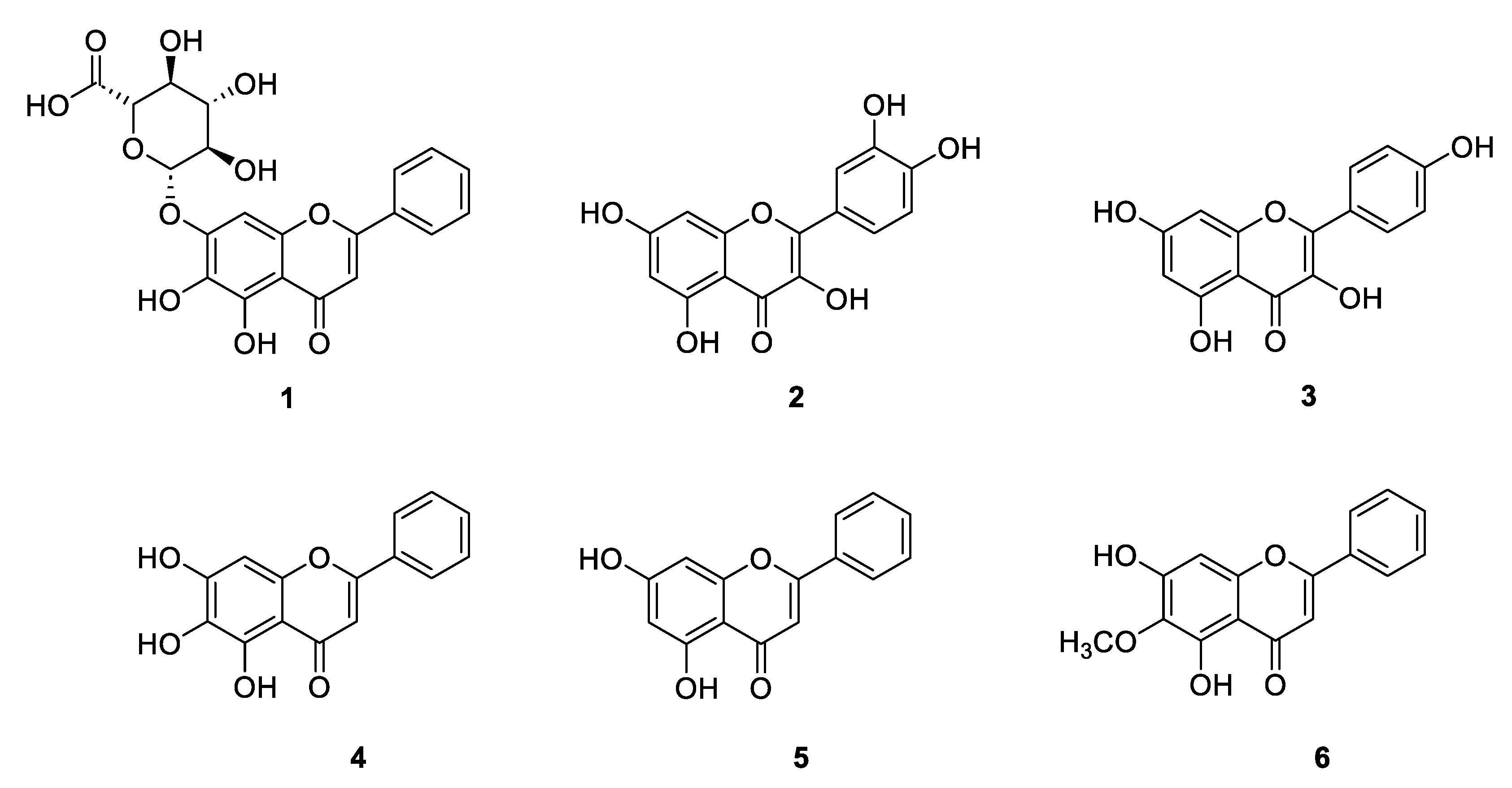
O. indicum are baicalein, baicalin (baicalein-7-O-glucoside), chrysin, and oroxylin-A [
10], however, in some cases quercetin and kaempferol were also isolated [
15] (
Figure 1).
Baicalein or 5,6,7-trihydroxyflavone presents the highest biological activity compared with other compounds [
16]. Baicalein, as well as baicalin, gains more interest in treatment of neurodegenerative diseases, such as depression or cognitive impairment [
17,
18,
19]. For this reason,
O. indicum seed, which contains baicalein and other effective flavonoids, was investigated for its antidepressant effects on UCMS-induced mouse model. Moreover, the quality control to identify the active components in the
OIS extract was also performed.
2. Materials and Methods
2.1. Preparation of the OIS extract
Seeds of O. indicum were collected in Khon Kaen province, Thailand and the plant material was identified by Dr. Prathan Leucha of the Faculty of Pharmaceutical Sciences, Khon Kaen University, Thailand. The herbarium voucher specimen was deposited at the herbarium of the Faculty of Pharmaceutical Sciences of Khon Kaen University, Thailand. Dried and powdered seeds of O. indicum (2 kg) were macerated in 95% ethanol (12 L) at room temperature for three days, and then filtered by filter paper to separate the solid residues from the ethanolic solution. The process was repeated three times and the ethanolic solutions were pooled and evaporated by a rotary evaporator at 50 °C. The crude extract (340.2 g) was then freeze-dried and stored at -20 °C throughout the experiment.
2.2. Inhibitory effect on monoamine oxidase-A (MAO-A) enzyme
The monoamine oxidase enzymes (MAOs) are involved in a depletion of monoamine neurotransmitters such as serotonin (5-HT), dopamine (DA), and norepinephrine (NE). The mechanism of antidepressant is hypothesized to be due to the inhibition of MAOs, especially the MAO-A isoform [
20]. To preliminarily screen the antidepressant effect of the
OIS extract, the recombinant human MAO-A (Sigma-Aldrich, USA) was used as the enzyme source, and kynuramine was used as a substrate to produce 4-hydroxyquinoline. The
OIS extract (250 mg) was dissolved in DMSO (1000 µL) as a stock solution (250 mg/mL), and then a serial dilution was performed. Each sample (10 µL) was mixed with kynuramine (Sigma-Aldrich, USA) (4.5 µL) and potassium phosphate buffer (234.75 µL). MAO-A enzyme (0.75 µL) was added to the mixture and kept at 37°C for 20 min, after which 2N NaOH (200 µL) and water (500 µL) were added to stop the reaction. 4-Hydroxyquinoline was measured by spectrofluorometric method at the wavelength of 310 nm for excitation and 400 nm for emission. The IC
50 values were calculated by using GraphPad Prism software. Clorgyline (Sigma-Aldrich, USA), a selective MAO-A inhibitor, was used as a positive control.
2.3. Animals
Male mice from the Institute of Cancer Research (ICR), bred by Nomura Siam International Company in Thailand, were used in the experiment. Mice were housed in transparent cages with free access to food and water under thermostatic condition at 22 ± 2 ºC with constant humidity (45% ± 2%) and a 12-h light-dark cycle (light on 06:00 am to 18:00 pm). The procedure was certified by the Animal Ethics Committee for Use and Care of Khon Kaen University (IACUC-KKU-104/64). All experiments were strictly implemented in accordance with the Guiding Principles for the Care and Use of Animals (NIH Publications No. 80–23, revised in 2011).
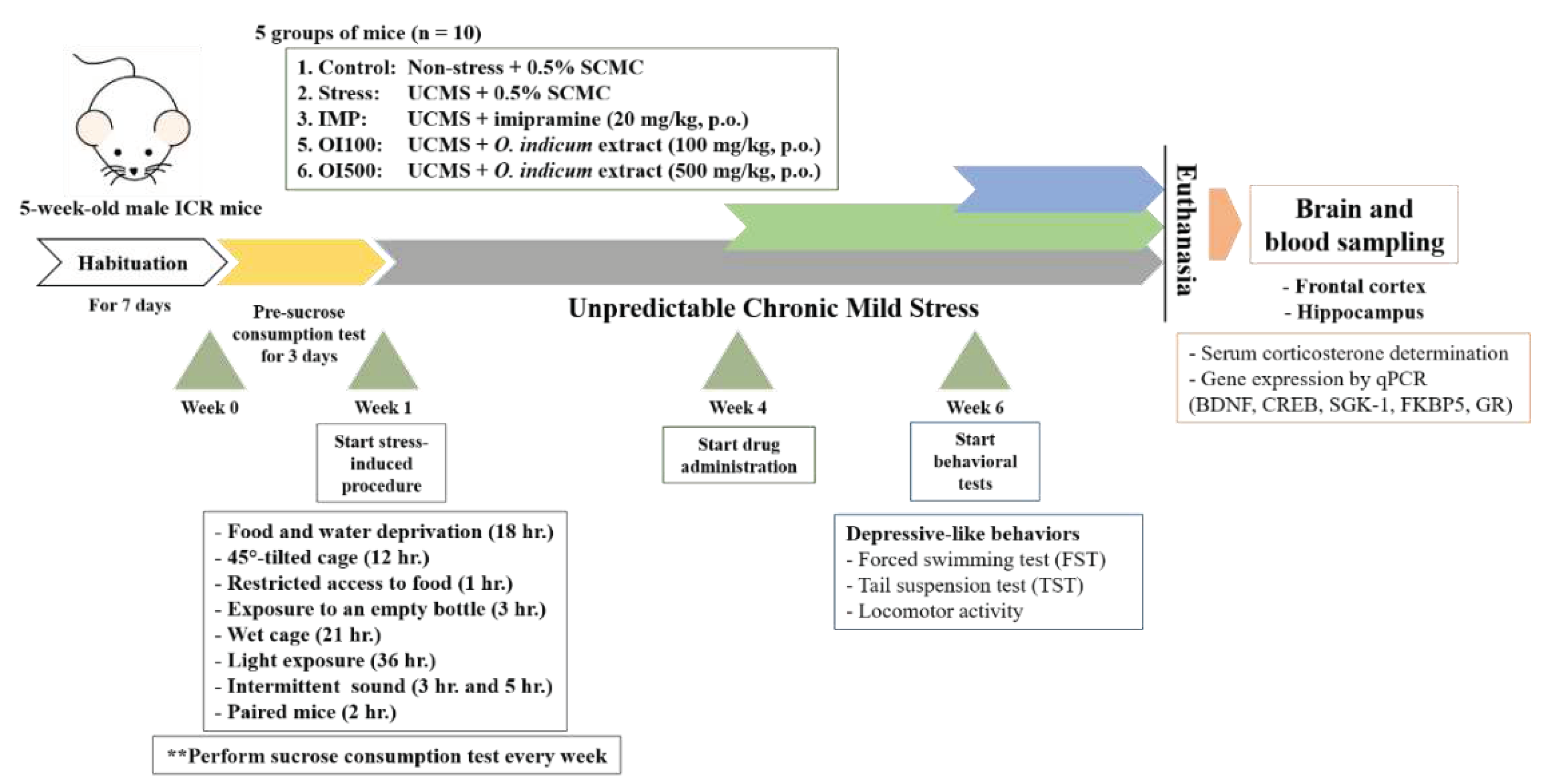
2.4. Unpredictable chronic mild stress (UCMS) paradigm
As shown in
Figure 2., mice were randomly divided into five groups; the first group was a non-stress group which was housed in a normal environment, and the others were applied to unpredictable mild stress according to the schedule over one week. The procedure was performed following the previous study [
21]. Stressors consisted of 2 groups; two periods a week, such as 45º tilted cage (12 h), access to 5 small pieces of food (1 h), exposure to empty bottle (3 h), consistent light exposure (36 h), subject to disturbed noise (3 and 5 h) while the other stressors were given once a week, such as wet cage (21 h), food and water deprivation (18 h), and paired mice from different cage (2 h). All of these stressors started at the first week and repeated throughout the experiment.
2.5. Experimental design and drug administration
After habituation, sucrose preference test (SPT), a measurement of anhedonia behavior was conducted with 2% w/v sucrose solution. All mice underwent adaptive training from day 1 to day 3, after 18 h of food and water deprivation, and each mouse was given 2% sucrose for 1 h. The amount of sucrose consumption during 1 h was recorded and calculated as the baseline of sucrose consumption in week 0. Mice were randomly divided into 5 groups (n = 10 each) as follow: (i) non-stress group which was treated with 0.5% sodium carboxymethyl cellulose (SCMC, Mumbai, India) as vehicle (0.5% SCMC, 1 mL/kg,
p.o.), (ii) UCMS-induced group which was treated with vehicle (0.5% SCMC, 1 mL/kg,
p.o.), (iii) the positive control group which was UCMS-induced group treated with imipramine hydrochloride (IMP) (Nacalai tesque, Inc., Japan) in 0.9% Normal saline solution (NSS) (IMP, 20 mg/kg,
p.o.), and (iii) the experimental groups which were the UCMS-induced groups treated with the
OIS extract at 100 and 500 mg/kg
p.o., respectively. The selected doses were calculated from an effective dose of baicalin (10 mg/kg
p.o.) [
22], which is a major constituent in
O. indicum. Daily treatments were performed for 3 weeks after day 21 at 8:00 a.m. except on behavioral testing day when mice were administered 1 h before testing. The behavioral tests were conducted in week 6. Afterward, mice were instantly euthanized by giving thiopental sodium (Anesthal
®, Jagsonpal Pharmaceutical Ltd, India) 60 mg/kg,
i.p., then blood, frontal cortex, hippocampus and other necessary organs were collected and kept at -80 ºC for neurochemical assessment.
2.6. Behavioral Studies
2.6.1. Sucrose preference test (SPT)
Anhedonia, one of the major features of depression, is widely indicated by the SPT [
23]. The test was performed once a week. Mice were individually placed in a cage for 30 min. After acclimatization, each mouse was presented to 2% sucrose solution for 1 h and the amount of sucrose intake was recorded. The depressive mice showed the reduction of sucrose consumption.
2.6.2. Tail suspension test (TST)
TST is a common behavioral paradigm used to evaluate the antidepressant activity. TST was conducted in a dark environment to limit interference. Mice were suspended upside down 30 cm above the ground with their tails attached to a black tape in a position that cannot hold on to the nearby surfaces. The despair behavior was determined by immobility time which was recorded after 2 min habituation from 6 min testing period.
2.6.3. Forced swimming test (FST)
FST is one of the most used assessments for antidepressant effect on depressive-like behavior. FST involves the instinctive behavior when mice were forced to swim in an inescapable cylinder [
24]. The mice were individually placed in a glass cylinder (20 cm in diameter, 30 cm in height) containing 15 cm height of water. In a pre-test session, mouse was forced to swim for 15 min with no observation. Twenty-four hours later (test-session), the mouse was administered a drug 1 h before the test and exposed to the same experimental condition for 5 min. No movement of any legs was considered as immobile or motionless.
2.6.4. Locomotor activity
Y-maze test was performed to emphasize that all drug administrations had no effect on locomotor function or movement of mice. The Y-maze consisted of three black arms of equal size of 38.5, 3 and 13 cm in length, width, and height, respectively. All arms were oriented at 60° angles from each other. Mouse was placed on one arm and allowed to freely explore for 5 min. After that, the area was cleaned with 70% w/v of ethanol. The total arm entries were manually recorded.
2.7. Determination of serum corticosterone level
After behavioral assessments, mice were deeply anesthetized with thiopental sodium (Anesthal®; 60 mg/kg, i.p.). Approximately half of the total blood volume was collected by cardiac puncture and kept at 20 ± 2 ºC for 24 h. Blood was centrifuged to keep only serum. To investigate the consequence of HPA-axis overactivation, corticosterone (CORT) level was measured by using corticosterone ELISA kit (Abcam, UK). 25 µL of biotinylated corticosterone protein (BCP) was added to 25 µL of mouse serum to bind with corticosterone. Then, the plate was incubated at room temperature for 2 h and washed with 200 µL of washing solution for 5 times. Then, 50 µL of streptavidin-peroxidase (SP) conjugate was pipetted into the well to attach to the BCP-corticosterone complex and the reaction was allowed to occur for 30 min. After cleaning with a washing solution, each well was added with 50 µL chromogen. After 20 min of incubation, a blue color was observed. The reaction was terminated by adding stop solution and the blue color changed to yellow color which was detected at 450 nm.
2.8. Quantitative real-time polymerase chain reaction (qPCR)
qPCR was applied to investigate the mechanism of the
OIS extract on multifactorial etiology of depression. Total RNA was isolated from the hippocampus and the frontal cortex by using TRIzol
® reagent (Invitrogen
TM, Thermo Fisher Scientific, USA), then chloroform was added for phase separation. RNA was solubilized in the aqueous phase and then precipitated by isopropanol. Nuclease-free water was used in order to dissolve the pellet. Oligo(dT) 12-18 primer, accompanied by deoxynucleotide triphosphate (dNTP), was used for complementary DNA (cDNA) synthesis with Superscript
TM III reverse transcriptase (Invitrogen
TM, Thermo Fisher Scientific, USA) by FlexCycler
2 PCR Thermal Cycler (Analytik Jena, Germany). Gene-related primer (Pacific Science, Thailand) was attached to single-stranded DNA (ssDNA) after denaturation process in the individually optimal temperature (annealing step) to start extension. During the extension, double-stranded DNA (dsDNA) was measured by non-specific fluorescent dye method, SYBR
® green supermixes (Bio-Rad, USA). The amount of fluorescence in a sample was plotted against the cycle number, and the quantitation was recorded as Ct value. The target gene expressions were expressed in fold difference relative and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene. All primers were synthesized by Macrogen (Seoul, South Korea) and were presented in
Table 1.
2.9. High performance liquid chromatography (HPLC) analysis and method validation
The OIS extract was analyzed by a reversed-phase HPLC system using a Hypersil ODS column (Agilent Technologies Inc., Santa Clara, CA, USA, 4 × 250 mm, 5 µm) with a gradient elution by 30-70% methanol and 0.2% formic acid in ultrapure water with a flow rate of 1.2 mL/min. The signal intensity was detected at 254 and 275 nm during 60 min cycle time. The standard baicalin, quercetin, kaempferol, baicalein, chrysin, and oroxylin A were used in this HPLC analysis. In order to quantify the chemical constituents, 10 mg of the OIS extract was dissolved in methanol to make a stock solution (10 mg/mL) and diluted to several appropriate solutions using mobile phase for quantification of chemical constituents. The extract solution was filtered through a 0.45 µm nylon syringe filter before injection. The amount of baicalin was determined by using standard curves (1, 2, 3, 4, 5 and 6 µg/mL) and the others (baicalein, chrysin and oroxylin A) were determined by using standard curves (2.5, 5, 7.5, 10, 12.5 and 15 µg/mL) which were prepared from a stock solution (1 mg/mL). HPLC validation are classified according to ICH guidelines (ICH Guidelines, 1996).
2.10. Statistical analysis
Non-stress group and stress-induced group were analyzed by paired Student’s t-test. One-way analysis of variance (ANOVA) was performed, followed by the Tukey test for multiple comparisons among the treatment groups. One-way repeated ANOVA was used for the assessment of sucrose intake and training for MWM in each mouse. The significant difference was determined with p < 0.05. Results are expressed as mean ± standard error of mean (S.E.M) except for in vitro studies which are expressed as mean ± standard deviation (SD). All data were analyzed by software Sigma Stat® ver. 3.5 (SYSTAT Software Inc., USA).
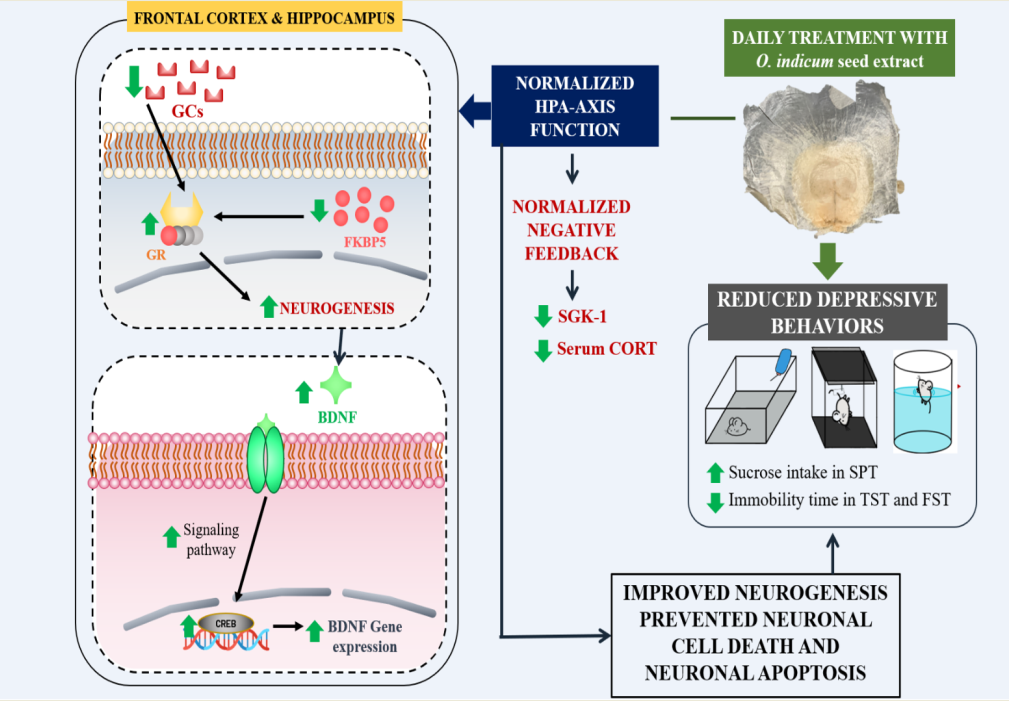
4. Discussion
MDD is prevalent among people in any ages around the world causing a serious negative impact. Although various types of antidepressants are available, approximately 10%-30% of patients show partial response along with poor quality of life or even high relapse tendency [
28]. Moreover, an early state of MDD, called “minor depression”, needs to be concerned. Minor depression shows similar symptoms to MDD and can become severe depression in one to three years if there is no diagnosis and treatment [
29]. There are strong evidences showing that alternative treatments, such as psychotherapy or herbal medicine, can have significant and beneficial effects on minor depression and prevent the onset of MDD, with lower side effects and lower costs compared with synthetic antidepressants [
30]. For this reason, the
OIS extract was investigated for its potential as herbal antidepressant. First, the
OIS extract was preliminarily screened for its inhibitory effect on MAO-A enzyme, a specific isoform of MAOs, which is associated with depression. The result showed that the
OIS extract exhibited more selectivity to the MAO-A than MAO-B inhibitory effects. This finding suggested that the inhibitory effect on MAO-A may involve in the antidepressant activity of the
OIS extract.
UCMS, a well-established study to evaluate antidepressant effect of novel substances, was used in the experiment since it does not only induce depressive-related behaviors but also causes physiological and neurological changes which are associated with clinical depression [
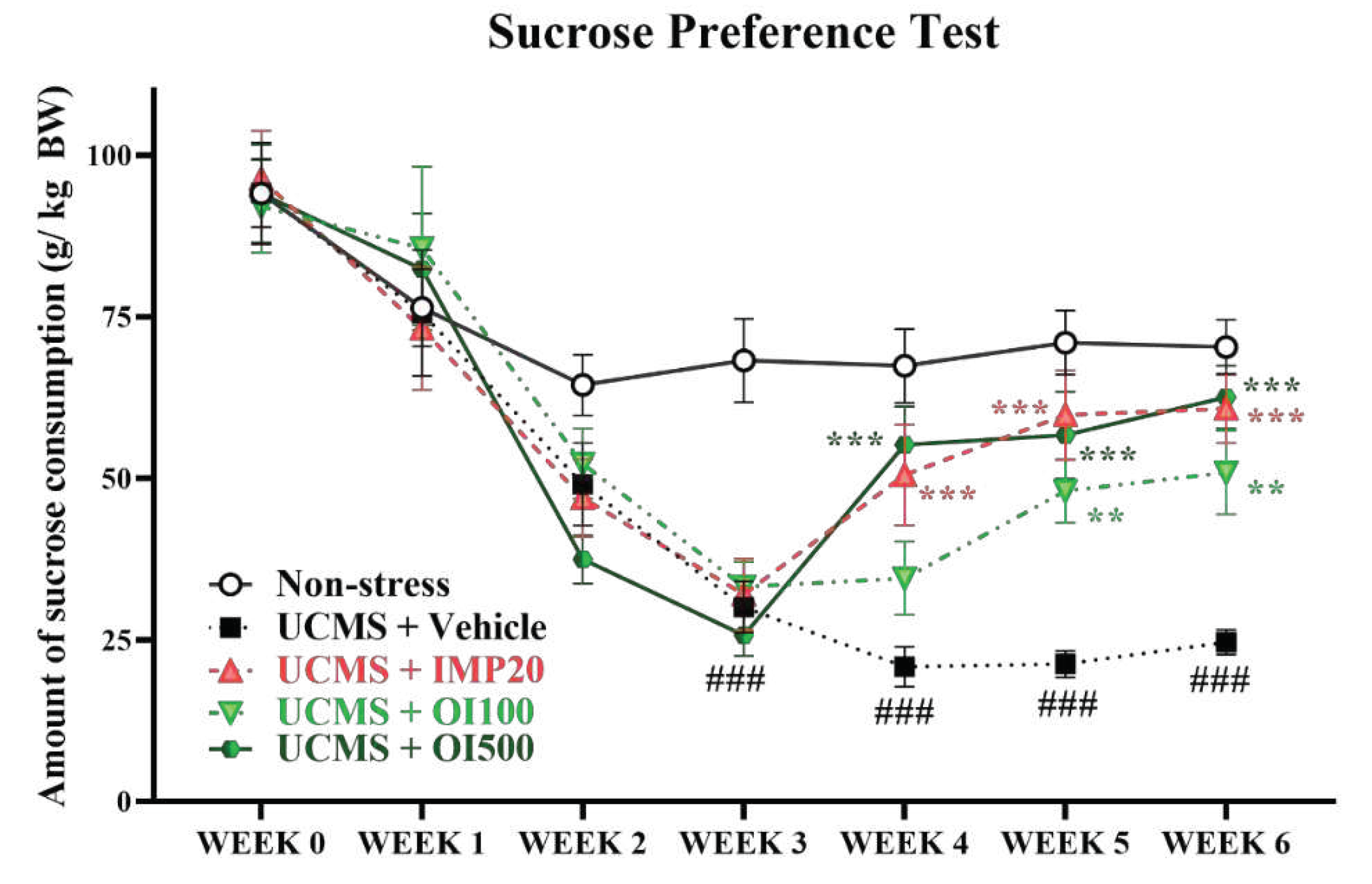
31]. UCMS-treated mice exhibited a significant reduction in sucrose consumption representing an impaired reward response or anhedonia behavior which is a core symptom of depression. Daily treatment with the
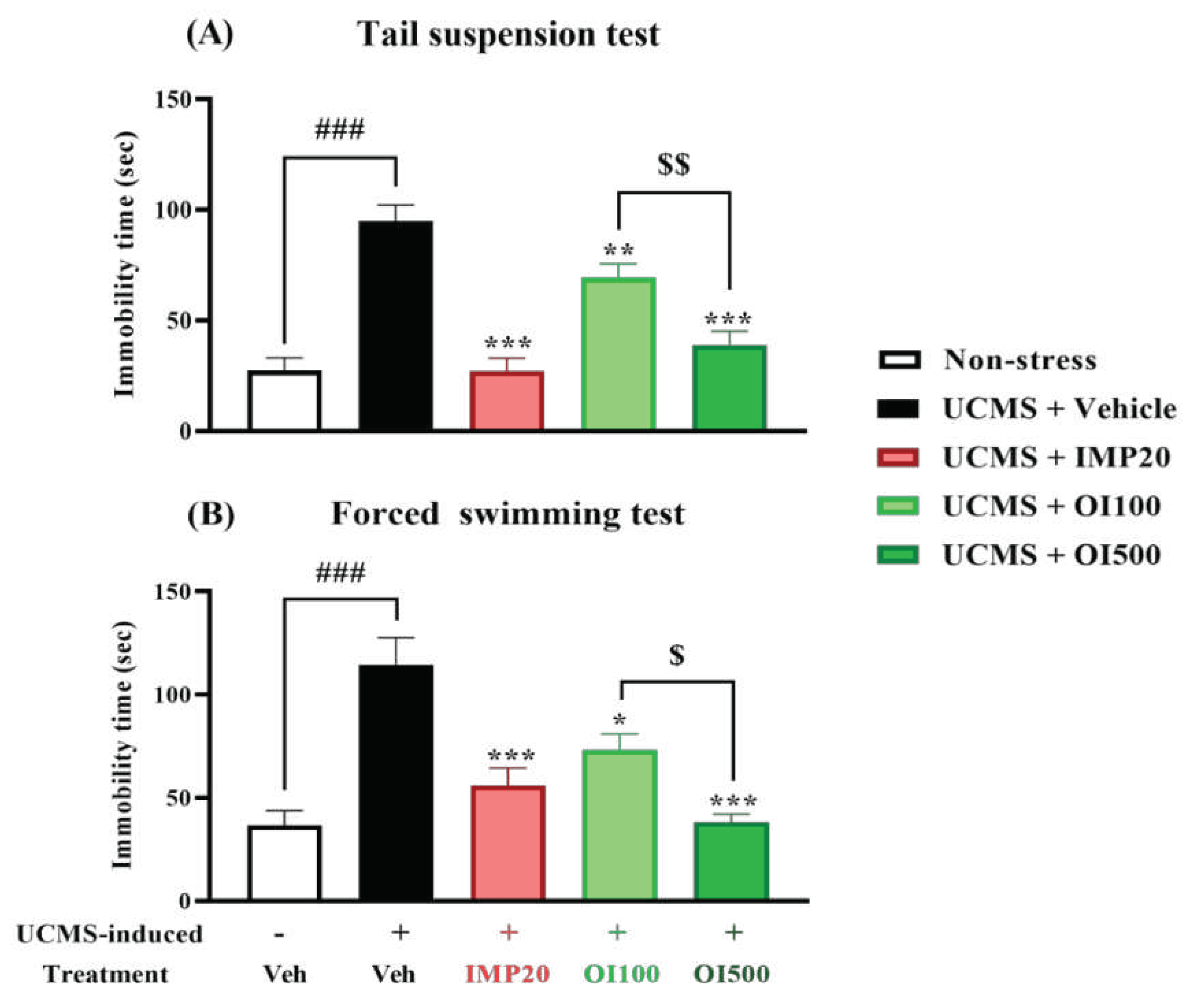
OIS extract via gastric gavage for two weeks improved sucrose preference in a similar manner to imipramine, reflecting amelioration in anhedonia. Another manifestation is a despair behavior, which was determined by FST and TST. Both tests used the same rationale, i. e. the immobility is considered as a resignation to a state of despair in which mouse learned that there was no escape from the situation. In the non-stress group, mice showed signs of struggle by trying to climb the glass cylinder or moved their legs. On the other hand, the UCMS group expressed a significant longer duration of immobility compared to the non-stress group. The results showed that the
OIS extract markedly alleviated hopeless behaviors by reducing immobility time in both tests. To ensure that the results were only from the antidepressant effect of the
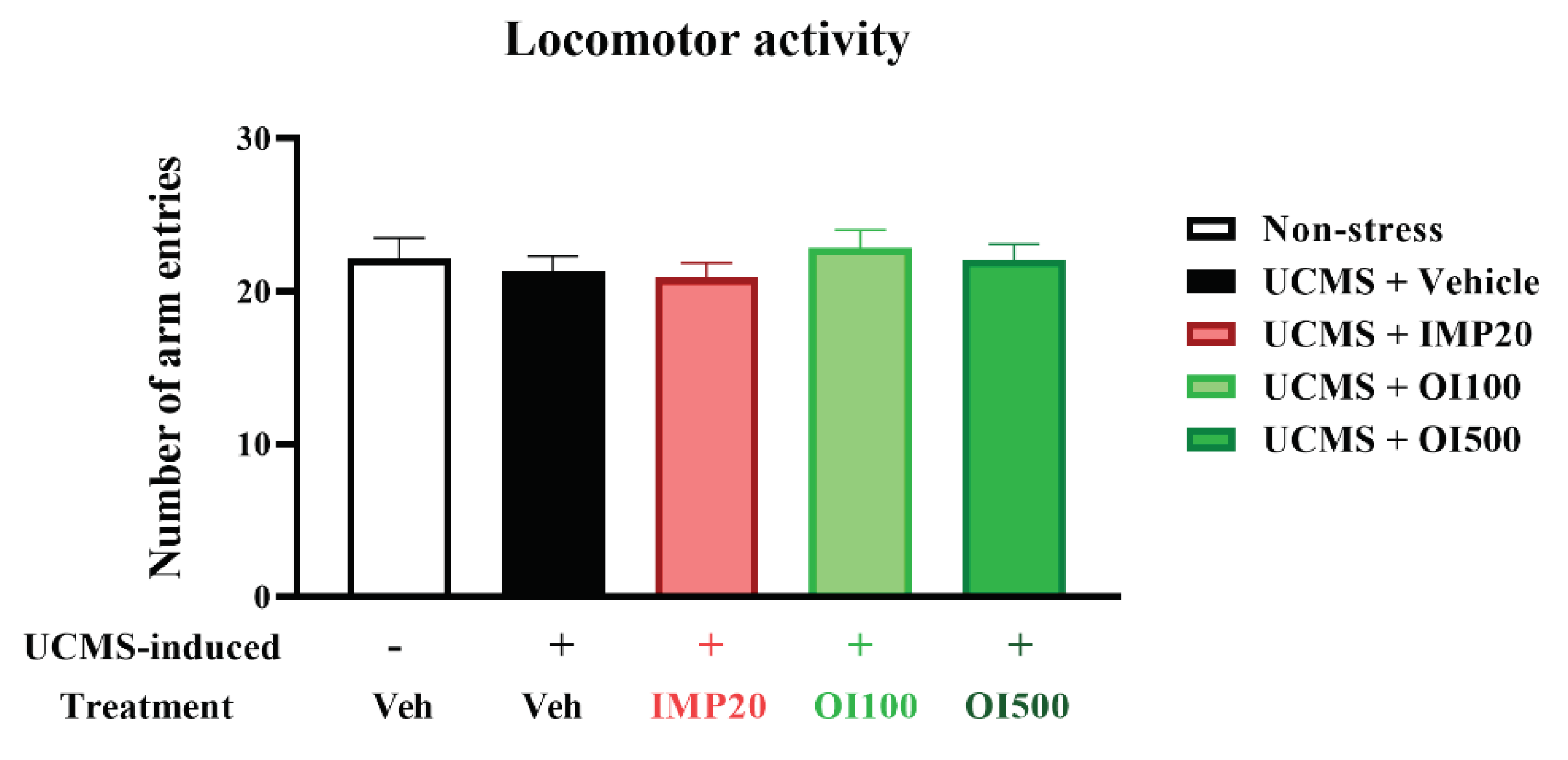
OIS extract, the Y-maze task was used to evaluate a locomotor activity. The test showed that both imipramine and the
OIS extract had no effect on the locomotor function. Taken together, the
OIS extract exhibited a potential to be an antidepressant agent in chronic stress model of depression in mouse.
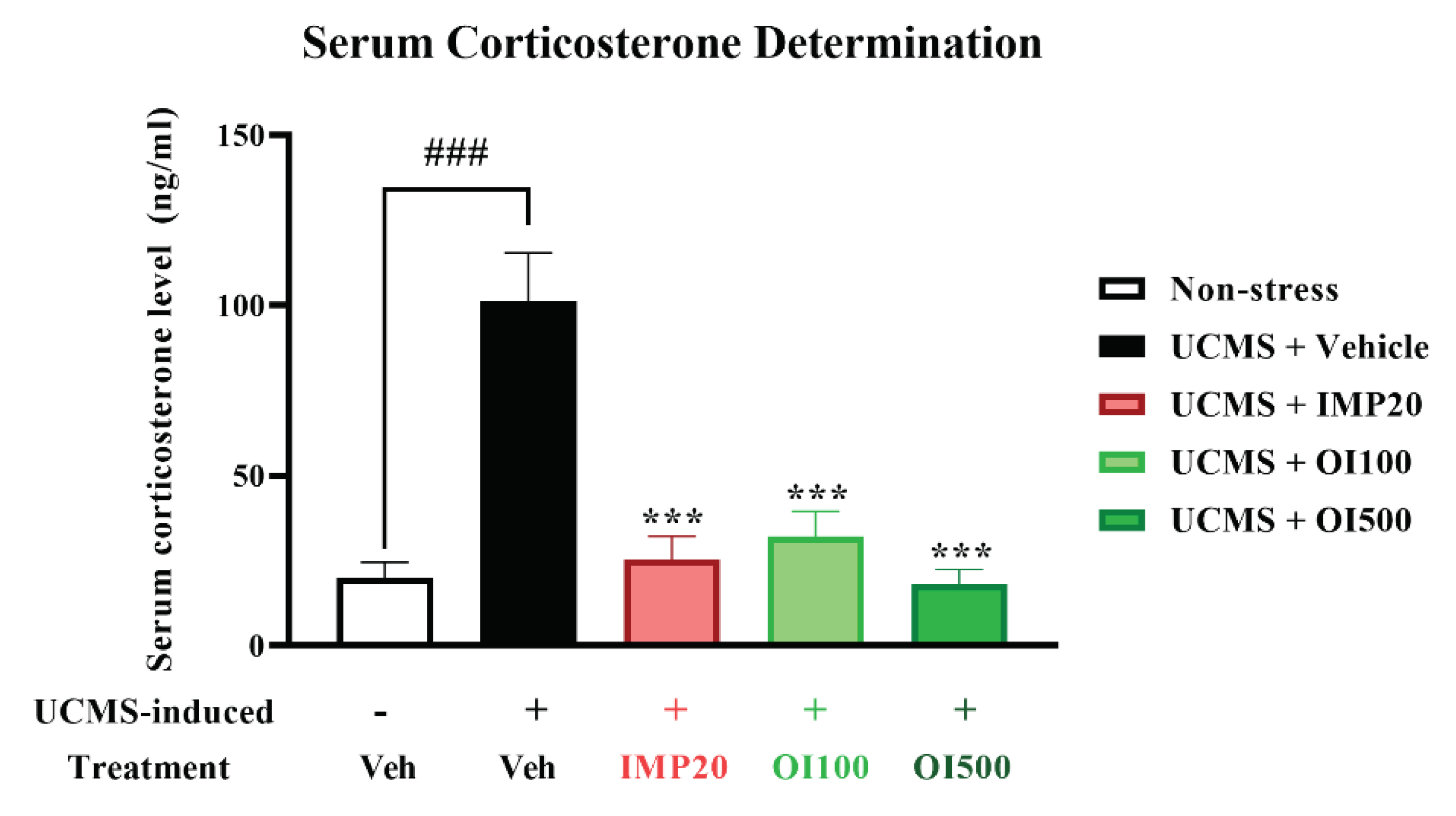
Induction by UCMS could cause physiological changes, such as hypercortisolism, which is associated with the HPA-axis hyperfunction. This condition plays an important role in the pathogenesis of depression [
3]. Serum corticosterone level was determined by ELISA to verify this hypothesis. The result showed that the serum corticosterone level was significantly increased in the stress-induced group with vehicle treatment compared to the non-stress group. Treatment with the
OIS extract attenuated the level of corticosterone similar to the effect of imipramine. In addition, mRNA expressions of gene-related HPA-axis regulation were investigated in order to confirm the mechanism of the
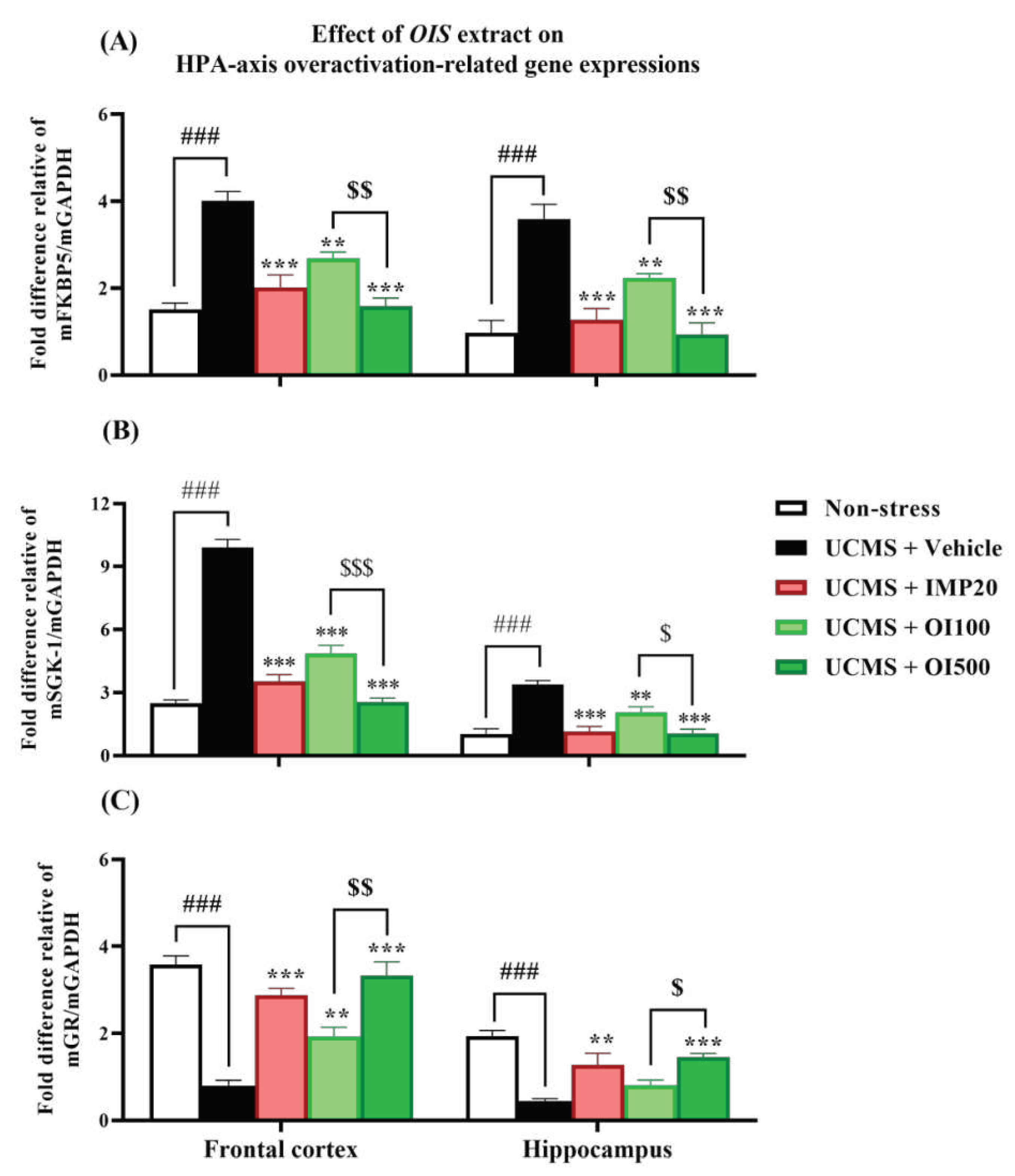
OIS extract. FKBP5, a competitive inhibitor of GCs on GR binding, exerts a negative effect on the HPA-axis regulation. FKBP5 can cause resistance to GR leading to an impaired negative feedback and resulting in a hypersecretion of GCs. Patients with depressive symptoms showed higher expression of FKPB5 but it can be reversed by a long-term antidepressant treatment [
32]. Consistently, in this study, the chronic stress-exposed group showed significantly higher expression of FKBP5 compared to the control group, especially in the depressive-related brain areas, viz. the hippocampus and the frontal cortex. Daily administration of the
OIS extract, in a dose-dependent manner, and imipramine could attenuate the elevation of FKPB5 expression in the affected brain. Moreover, it is suggested that hypercortisolism secondarily downregulated the GR expression [
7]. The reduction of available GR to bind with GCs can lead to an impairment in a negative feedback. Our finding indicated that the GR mRNA expression significantly decreased in UCMS group that could be improved after treatment with the
OIS extract. SGK-1 is one of the brain proteins, which transcriptionally regulated by GCs, also participates in a neuronal proliferation and apoptosis. GCs elevation after exposing to chronic stress can cause increment of SGK-1 in the hippocampus leading to decreased hippocampus and frontal cortex neurogenesis [
33]. Consistent with previous findings, UCMS caused an apparent increase in the SGK-1 expression and treatment with the
OIS extract at least three weeks ameliorated this abnormality. Furthermore, there is a linkage between SGK-1 and BDNF, a neurotrophin which has a leading role in the etiology of depression [
9]. Although the molecular mechanism of their interaction is still unclear, it is known that GR overactivation can suppress the BDNF signaling pathway leading to neuroplasticity impairment [
34]. BDNF and its receptor, tropomyosin kinase receptor B (TrkB), are regulators of a dendritic growth in the central nervous system (CNS). The transcription of BDNF/TrkB signaling needs a transcription factor, named cyclic adenosine monophosphate (cAMP) response element-binding protein or CREB. It is well-known that lowering CREB expression can decrease the level of BDNF [
35]. Patients with depressive state express the reduction of BDNF and CREB in the hippocampus and the neurogenesis, and neuroplasticity can be improved after treatment with antidepressant. Consequently, we have found that UCMS paradigm could impair mRNA expression of BDNF and CREB in mouse brain and treatment with the
OIS extract of the UCMS mice can improve the neurogenesis.
Lastly, phytochemical analysis of the
OIS extract by HPLC was able to identify and quantify, among five major components, the three flavonoids, including baicalin (84.22 ± 0.22 mg/g extract), baicalein (23.45 ± 0.11 mg/g extract) and chrysin (11.38 ± 0.089 mg/g extract). Many studies confirmed that all of these flavonoids could alleviate depressive symptoms with various mechanisms of action. It was proved that a high level of GC from chronic CORT injection could reduce available GR in the cytoplasm and increase GR in the nucleus, resulting in an impairment of a negative feedback [
36]. However, treatment with baicalin (40, 80, and 160 mg/kg) significantly alleviated a depressive-related manifestation in the CORT-induced mice by normalization of the GR function through SGK1- and FKBP5-mediated GR phosphorylation. In addition, baicalin also ameliorated UCMS-induced depressive-like behaviors through the hippocampal BDNF/ERK/CREB signaling pathway in male ICR mice [
37]. Interestingly, investigation of the antidepressant activity of baicalein has showed that the chronic treatment of baicalein (4 mg/kg/day,
i.p.) for 21 days also reduced the immobility time (FST and TST) in UCMS rat model. The possible mechanism of baicalein is at least partly mediated via ERK-mediated neurogenesis in the hippocampus [
38]. Moreover, chrysin at doses of 5 and 20 mg/kg could attenuate depressive behaviors by preventing the reduction of hippocampal BDNF and 5-HT due to bilateral olfactory bulbectomy in mice, and it also ameliorated the HPA-axis dysregulation, such as reduction of a serum CORT level and related regulatory hormones (CRH and ACTH) in the chronically stressed mice [
39,
40]. Taken together, the antidepressant effect of the
OIS extract could be explained by synergistic effects of its major constituents with various molecular mechanisms.
Author Contributions
Conceptualization, C.K., O.M., S.D., C.B., and Y.C. (Yaowared Chulikhit); methodology, S.D., C.K., C.B., Y.C. (Yutthana Chotritthirong); and Y.C. (Yaowared Chulikhit); validation, S.D., C.C., and O.M.; formal analysis, C.C., J.M., Y.C. (Yutthana Chotritthirong) and O.M.; investigation, C.C., C.K., J.M. and Y.C. (Yaowared Chulikhit); resources, S.D., C.B., O.M. and Y.C. (Yaowared Chulikhit); data curation, C.C., C.K., J.M. and Y.C. (Yaowared Chulikhit); writing—original draft preparation, C.C., C.K. and Y.C. (Yaowared Chulikhit); writing—review and editing, C.C., S.A., A.K. and Y.C. (Yaowared Chulikhit); visualization, A.K., and Y.C. (Yaowared Chulikhit); supervision, C.K., S.A., A.K. and Y.C. (Yaowared Chulikhit); project administration, Y.C. (Yaowared Chulikhit); funding acquisition, O.M. and Y.C. (Yaowared Chulikhit); All authors have read and agreed to the published version of the manuscript.
Figure 1.
Structures of baicalin (1), quercetin (2), kaempferol (3), baicalein (4), chrysin (5), and oroxylin A (6).
Figure 1.
Structures of baicalin (1), quercetin (2), kaempferol (3), baicalein (4), chrysin (5), and oroxylin A (6).
Figure 2.
Schematic drawing of unpredictable chronic mild stress (UCMS) procedure.
Figure 2.
Schematic drawing of unpredictable chronic mild stress (UCMS) procedure.
Figure 3.
The OIS extract (100 and 500 mg/kg) significantly improved sucrose preference which was diminished by long-term stress. SPT was performed every week to monitor anhedonia. The results were expressed as mean ± S.E.M. (n = 10 per each group). ###p<0.001 vs. the non-stress group. **p < 0.01 and ***p < 0.001 vs. the vehicle-treated UCMS group.
Figure 3.
The OIS extract (100 and 500 mg/kg) significantly improved sucrose preference which was diminished by long-term stress. SPT was performed every week to monitor anhedonia. The results were expressed as mean ± S.E.M. (n = 10 per each group). ###p<0.001 vs. the non-stress group. **p < 0.01 and ***p < 0.001 vs. the vehicle-treated UCMS group.
Figure 4.
The OIS extract (100 and 500 mg/kg) significantly decreased the immobility time which was an implication for a despair behavior in TST (panel A) and FST (panel B). Behavioral tests were performed after treatment for 3 weeks. The results were expressed as mean ± S.E.M. (n = 10 per each group). ###p<0.001 vs. the non-stress group. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. the vehicle-treated UCMS group. $p < 0.05 and $$p < 0.01 compared between the different doses of the OIS extract.
Figure 4.
The OIS extract (100 and 500 mg/kg) significantly decreased the immobility time which was an implication for a despair behavior in TST (panel A) and FST (panel B). Behavioral tests were performed after treatment for 3 weeks. The results were expressed as mean ± S.E.M. (n = 10 per each group). ###p<0.001 vs. the non-stress group. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. the vehicle-treated UCMS group. $p < 0.05 and $$p < 0.01 compared between the different doses of the OIS extract.
Figure 5.
The OIS extract (100 and 500 mg/kg) showed no effect on locomotor function in UCMS-induced mice as determined by the Y-maze test. The data were analyzed from number of arm entries and expressed as mean ± S.E.M. (n = 10 per each group).
Figure 5.
The OIS extract (100 and 500 mg/kg) showed no effect on locomotor function in UCMS-induced mice as determined by the Y-maze test. The data were analyzed from number of arm entries and expressed as mean ± S.E.M. (n = 10 per each group).
Figure 6.
The OIS extract (100 and 500 mg/kg) and imipramine (20 mg/kg) reduced the elevation of serum corticosterone level induced by the UCMS. The data are expressed as mean ± S.E.M. (n = 5). ###p<0.001 vs. the non-stress group. ***p < 0.001 vs. the vehicle-treated UCMS group.
Figure 6.
The OIS extract (100 and 500 mg/kg) and imipramine (20 mg/kg) reduced the elevation of serum corticosterone level induced by the UCMS. The data are expressed as mean ± S.E.M. (n = 5). ###p<0.001 vs. the non-stress group. ***p < 0.001 vs. the vehicle-treated UCMS group.
Figure 7.
The OIS extract (100 and 500 mg/kg) and imipramine (20 mg/kg) alleviated the impaired regulation of the HPA-axis by decreasing FKBP5 (panel A) and SGK-1 (panel B) mRNA expression, while improving GR mRNA expression (panel C) in both affected brain areas. The results are expressed as mean ± S.E.M. (n = 6). ###p<0.001 vs. the non-stress group. **p < 0.01 and ***p < 0.001 vs. the vehicle-treated UCMS group. $p < 0.05, $$p < 0.01 and $$$p < 0.001 compared between the different doses of the OIS extract.
Figure 7.
The OIS extract (100 and 500 mg/kg) and imipramine (20 mg/kg) alleviated the impaired regulation of the HPA-axis by decreasing FKBP5 (panel A) and SGK-1 (panel B) mRNA expression, while improving GR mRNA expression (panel C) in both affected brain areas. The results are expressed as mean ± S.E.M. (n = 6). ###p<0.001 vs. the non-stress group. **p < 0.01 and ***p < 0.001 vs. the vehicle-treated UCMS group. $p < 0.05, $$p < 0.01 and $$$p < 0.001 compared between the different doses of the OIS extract.
Figure 8.
The OIS extract in the dose of 500 mg/kg normalized the depletion of neurogenesis and neuroplasticity induced by chronic stress which was determined by mRNA expressions of BDNF (panel A) and CREB (panel B) in the same manner as imipramine. The results are expressed as mean ± S.E.M. (n = 6). ###p<0.001 vs. the non-stress group. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. the vehicle-treated UCMS group. $p < 0.05, $$p < 0.01 and $$$p < 0.001 compared between the different doses of the OIS extract.
Figure 8.
The OIS extract in the dose of 500 mg/kg normalized the depletion of neurogenesis and neuroplasticity induced by chronic stress which was determined by mRNA expressions of BDNF (panel A) and CREB (panel B) in the same manner as imipramine. The results are expressed as mean ± S.E.M. (n = 6). ###p<0.001 vs. the non-stress group. *p < 0.05, **p < 0.01 and ***p < 0.001 vs. the vehicle-treated UCMS group. $p < 0.05, $$p < 0.01 and $$$p < 0.001 compared between the different doses of the OIS extract.
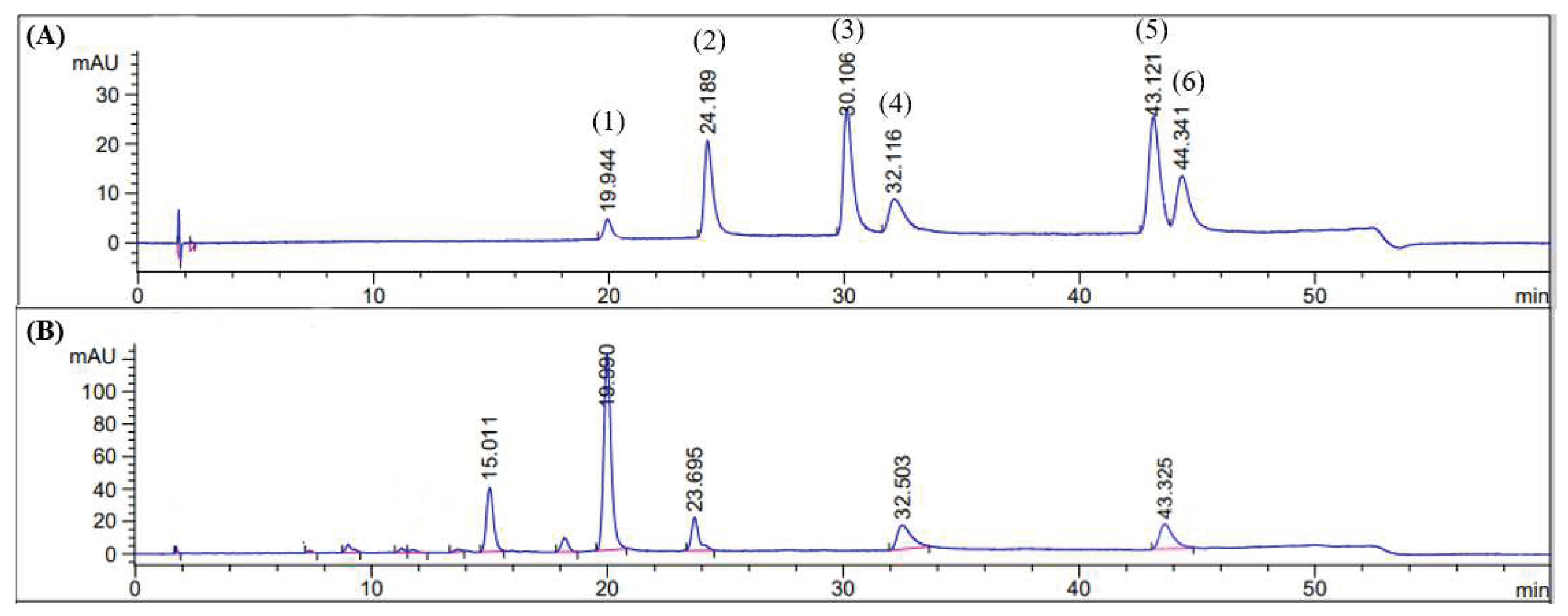
Figure 9.
Chromatogram of standard: baicalin (1), quercetin (2), kaempferol (3), baicalein (4), chrysin (5), and oroxylin A (6), respectively (panel A) and the OIS extract (panel B). Detection wavelength was 275 nm.
Figure 9.
Chromatogram of standard: baicalin (1), quercetin (2), kaempferol (3), baicalein (4), chrysin (5), and oroxylin A (6), respectively (panel A) and the OIS extract (panel B). Detection wavelength was 275 nm.
Table 1.
Summary of primer sequences used for qPCR.
Table 1.
Summary of primer sequences used for qPCR.
| Groups |
Genes |
Primer sequences |
Product length |
Reference |
| House-keeping gene |
GAPDH |
Forward: 5′-ACC ACA GTC CAT GCC ATC AC-3′
Reverse: 5′-TCC ACC ACC CTG TTG CTG TA-3′ |
452 bp |
[25] |
| Neurogenesis |
BDNF |
Forward: 5′-GAC AAG GCA ACT TGG CCT AC-3′
Reverse: 5′-CCT GTC ACA CAC GCT CAG CTC-3′ |
334 bp |
[25] |
| CREB |
Forward: 5′-TAC CCA GGG AGG AGG AAT AC-3′
Reverse: 5′-GAG GCT GCT TGA ACA ACA AC-3′ |
183 bp |
[25] |
| HPA-axis |
SGK-1 |
Forward: 5′-GGG TGC CAA GGA TGA CTT TA-3′
Reverse: 5′-CTC GGT AAA CTC GGG ATA GA-3′ |
154 bp |
[25] |
| GR |
Forward: 5′- CAC TAA TCC TCT CCA TCC TAC-3′
Reverse: 5′- AAT GTC TGC TGC CTT CTG-3′ |
479 bp |
[22] |
| |
FKBP5 |
Forward: 5′- GAA CCC AAT GCT GAG CTT ATG-3′
Reverse: 5′- ATG TAC TTG CCT CCC TTG AAG-3′ |
149 bp |
[5] |
Table 2.
Inhibitory effect of the OIS extract on MAO-A activity compared with clorgyline and deprenyl which are selective inhibitors for MAO-A and MAO-B, respectively.
Table 2.
Inhibitory effect of the OIS extract on MAO-A activity compared with clorgyline and deprenyl which are selective inhibitors for MAO-A and MAO-B, respectively.
| Extract/ Substance |
Inhibition on MAO-A enzyme |
Inhibition on MAO-B enzyme |
IC50 (µg/mL for extract
and µM for compound) |
IC50 (µg/mL for extract
and µM for compound) |
|
OIS extract |
36.07 ± 0.47 |
146.80 ± 4.94 |
| Clorgyline |
0.001201 ± 0.000014 |
20.38 ± 0.33 |
| Deprenyl |
10.49 ± 0.11 |
0.052 ± 0.009 |