Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that affects upper and lower motor neurons, resulting in muscle weakness, severe disability, and ultimately, a fatal outcome [
1,
2,
3]. The cause of ALS remains unknown for most individuals affected by the disease. Sporadic cases account for 90-95% of all cases, while genetic factors are confirmed in 5-10% [
4,
5]. The distribution of ALS is not uniform across different regions of the world. Recent data shows that the incidence varies from 0.6 to 3.8 cases per 100,000 population, with a global incidence of approximately 1.6-1.76 cases per 100,000 population [
5,
6,
7,
8]. In Asian countries, the number of cases ranges from 0.7 to 2.2, while in developed countries in Europe and North America, it is slightly higher, ranging from 1.5 to 3.8 cases per 100,000 population [
1,
3,
5,
8,
9,
10,
11]. Unfortunately, there is a lack of reliable data on the current situation of the Lithuanian population. Based on various studies, it can be estimated that the rate of occurrence in our country is between 2 to 3.5 cases per 100,000 population. The main ALS center in Lithuania, located in the Hospital of Lithuanian University of Health Sciences Kaunas Clinics, identifies approximately 20 new cases per year. ALS can occur at any age, but the incidence increases with each decade, particularly after the age of 40. The disease is most commonly diagnosed in older patients, with a peak age at the time of diagnosis of 51-66 years [
12,
13,
14]. There is a slightly higher prevalence of ALS in men than women, up to the age of 65 [
5]. In all age groups, there is a higher occurrence of ALS in men compared to women. The peak in men is 85-89 years, while for women, it is 80-84 years. According to the age-standardized ratio between women and men, it was 1.22 (1.19:1.24) in 1990 and 1.25 (1.23:1.28) in 2016 [
15]. Multiple pathophysiological mechanisms are believed to contribute to motor neuron degeneration [
16]. Due to various pathogenic factors, ALS presents with a wide range of clinical features and symptoms, making the diagnosis complex and challenging [
17]. The diagnosis of ALS is based on clinical criteria and monitoring the progression of the disease. Additional tests can help avoid diagnostic errors and provide further insights into the disease. While respiratory muscle weakness is only observed as the initial symptom in a small number of ALS patients [
18], respiratory dysfunction occurs later in the disease course and is associated with a poorer prognosis [
19]. Gradual loss of phrenic nerve (PN) function leads to weakness, paralysis, and ultimately, death of the patient [
20]. Studies using ultrasound and PN conduction can aid in assessing respiratory function and predicting patient survival [
21,
22,
23,
24].
Despite the advancements in innovative technologies and the current diagnostic capabilities in laboratories and instrumental methods, the existing diagnostic methods for amyotrophic lateral sclerosis (ALS) do not always ensure early detection, given the complexity of the diagnosis process resulting from a heterogeneous range of clinical symptoms. These factors drive the search for new and simpler diagnostic methods.
We raised the hypothesis that motor nerve size reduction in ultrasound could be one of the first signs in the diagnosis of ALS. There is still limited data and debate regarding which nerves are the most specific and sensitive to be examined by ultrasound. We hope that, due to the affected respiratory function, the phrenic nerve might be the first damaged nerve in ALS disease. PN ulltrasound and conduction studies provide hope that structural changes observed through PN ultrasound may become a diagnostic tool for determining ALS disease and evaluating respiratory function. This could enable more effective patient monitoring and care. In particular, we focused measuring the cross-sectional area (CSA) of the nerve, as it may effectively identify patients with subclinical involvement of the phrenic nerve, leading to an early diagnosis of ALS. Furthermore, the changes detected in the phrenic nerve ultrasound, such as nerve heterogeneity and altered echogenicity, may provide valuable information about the disease and its prognosis.
Materials and methods
Study Subjects and Ethical Statement
The study was conducted in accordance with the principles of the Declaration of Helsinki, and informed consent was obtained from all participants. The study protocol was approved by the Kaunas Regional Biomedical Research Ethics Committee, with bioethical permission No. BE-2-40, issued on May 17, 2022. An analysis of scientific literature, medical documentation of participants, as well as laboratory and instrumental research data was performed.
All participants were enrolled and the study was conducted at the Department of Neurology of the Lithuanian University of Health Sciences. The participants were divided into two groups: Group 1 consisted of patients with ALS (N=23), who all met the “Gold Coast” criteria (Shefner et al. 2020). Group 2 served as the control group (N=23). Each participant underwent an interview and filled out questionnaires to collect clinical and demographic data, including age, gender, height, body mass index (BMI), hip and waist circumference, duration of illness, and ALS-FRS-R score. All participants underwent a phrenic nerve ultrasound examination. In the control group, patients who matched the age and gender of the ALS patients were invited to participate. The cross-sectional area (CSA) values of the phrenic nerve in the control group were determined by measuring personal staff members and patients who were over 18 years old. Participants who had signs and symptoms of other neurodegenerative diseases, polyneuropathies, neuromuscular junction disorders, endocrine disorders, oncological diseases, or other concomitant diseases that could affect respiratory function, as well as those taking medications that could affect respiration, were excluded from the study. Both groups were matched by age, gender, height, weight, hip and waist circumference, and BMI.
The Ultrasound Examination
The ultrasound examination of the phrenic nerve was performed by two authors using a high-resolution “Philips EPIQ 7” ultrasound machine with a linear 4-18 MHz transducer (CE 0086). The transducer was placed in a transverse plane above the level of the clavicle in the neck region, at the level of the levator scapulae muscle, where the nerve attaches to the anterior scalene muscle and the neck muscles. In B-mode ultrasound images, a spindle-shaped structure with a hypoechoic appearance and a more hyperechoic periphery was visualized in the connective tissue sheath on the anterior surface of the scalene muscle, which was identified as the phrenic nerve. To ensure accurate differentiation of the nerve from vascular structures, a Doppler ultrasound mode was used during the examination. During the examination, the quantitative and qualitative characteristics of the phrenic nerve were evaluated. The texture of the nerve was assessed based on homogeneity (homogeneous or heterogeneous) and echogenicity (hypoechoic, isoechoic or hyperechoic). The cross-sectional area of the phrenic nerve was measured in a transverse plane, by tracing the hypoechogenic nerve area at the border of hyperechogenicity, using the methodology described by Walter et al. (2019). The cross-sectional area was measured three times with a 0.01 mm² error margin, and the average of the three measurements was calculated. Both examinators were blinded to the examination results of each other.
Statistical Analysis
The data analysis involved the use of both descriptive and comparative statistical analysis methods, namely Microsoft Excel and IBM SPSS v29 statistical analysis software package. Quantitative values within the sample were presented using median, minimum, and maximum values, as well as the mean of the phrenic nerve area with standard deviation. Non-parametric tests, such as the Mann-Whitney U test, were used to compare values between groups. Differences in data were analyzed using the Kruskal-Wallis test. Statistical hypotheses were tested, and significant differences and dependencies were defined as p < 0.05.
Results
A total of 46 participants were included in the study, with 23 patients in the ALS group and 23 individuals in the control group. In the ALS group, there were 9 women (39.1%) and 14 men (60.9%). The median age of the ALS group on the day of the phrenic nerve ultrasound exam was 57 years (range: 31-70). Among the women in the ALS group, the median age was 59 years (range: 31-70), and among the men, the median age was 57 years (range: 46-70). The control group consisted of 11 women (47.8%) and 12 men (52.2%), with a median age of 58 years (range: 46-65) for the entire group. There were no significant differences in age, gender, or anthropometric measures between the study groups (p > 0.05). The demographic and clinical characteristics of the study groups are presented in
Table 1.
Participants in the ALS group were categorized into four different groups based on the clinical form in which the disease initially presented. The distribution of clinical forms and sex in each group is displayed in
Table 2. Additionally, the duration of illness and ALS-FSR-R scores by gender were included in the analysis.
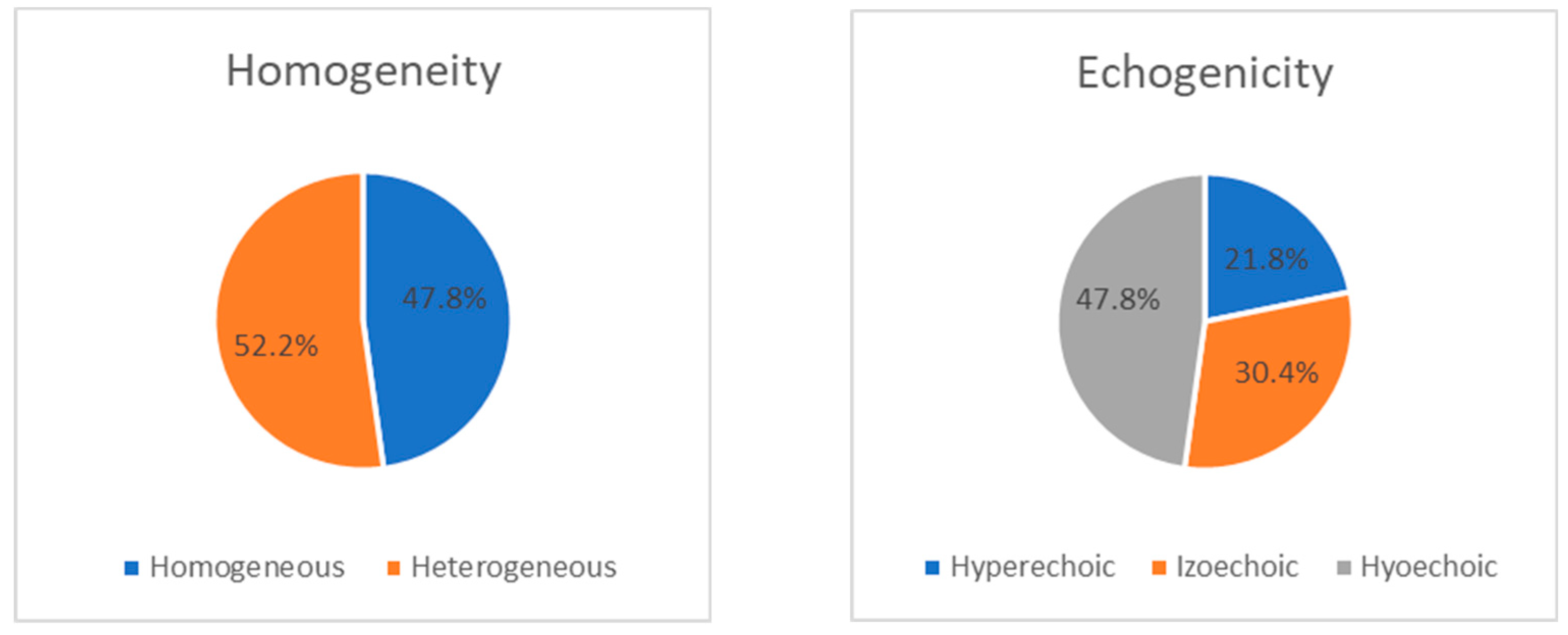
During the ultrasound examination of the phrenic nerve, heterogeneity was observed on both sides (43.5% on the right and 52.2% on the left). Among the patients, nerve heterogeneity was present on at least one side in 14 individuals (60.9%). In the remaining cases, the nerve showed a homogeneous appearance without any interruptions (13 (56.5%) on the right and 11 (47.8%) on the left). Changes in echogenicity of the phrenic nerve were detected bilaterally, but there were no significant differences in nerve echogenicity between sides. The nerve was found to be isoechoic in 8 patients (34.8%) on the right and 7 patients (30.4%) on the left, hyperechoic in 4 patients (17.4%) on the right and 5 patients (21.8%) on the left, and hypoechoic in 11 patients (47.8%) on both sides (p>0.05). The distribution of ultrasonic features, including homogeneity and echogenicity, of the right and left phrenic nerves in the ALS group is presented as a percentage in Figure 1 and
Figure 2.
All participants in the control group exhibited homogeneous structures in both the right and left phrenic nerves. Among individuals in the control group, hypoechogenic structures were more prevalent (69.6% on the right, 78.3% on the left), while isoechoic structures were less common (30.4% on the right, 21.7% on the left), and no nerves with hyperechogenic structures were observed.
Figure 1 show the distribution of ultrasonographic features (homogeneity and echogenicity) of the right and left phrenic nerves in the ALS group.
In the control group, the median cross-sectional area of the phrenic nerve was 1.16 mm² (range: 0.63-1.36) on the right side and 1.09 mm² (range: 0.63-1.20) on the left side. However, in patients with ALS, it was observed that the cross-sectional area of the phrenic nerve was significantly smaller on both the right (p < 0.001) and left (p < 0.001) sides compared to the control group. Detailed measurements are shown in
Table 3.
Discussion
Despite the advancements in innovative technologies and the current diagnostic capabilities in laboratories and instrumental methods, the existing diagnostic methods for amyotrophic lateral sclerosis (ALS) do not always ensure early detection, given the complexity of the diagnosis process resulting from a heterogeneous range of clinical symptoms These factors drive the search for new and simpler diagnostic methods. In recent decades, the hypothesis of nerve size reduction due to ongoing axonal degeneration has sparked interest in the use of peripheral nerve ultrasound for ALS diagnosis and disease monitoring [
25]. As respiratory system impairment is inevitable due to motor neuron loss in ALS, previous studies have investigated phrenic nerve conductivity, diaphragm radiology, and respiratory functionality to understand the role of the phrenic nerve in ALS [
24,
26,
27,
28,
29,
30,
31,
32,
33].
Our study revealed that the phrenic nerve is significantly smaller on both sides in ALS patients compared to (the control group arba controls) individuals without the disease (p < 0.001). Only one significant study on PN ultrasound in ALS, conducted in Japan, also showed significant results (p < 0.00001).
Comparing this study with our own, we noticed that Surat and colleagues recorded larger PN cross-sectional areas in both the control and ALS patient groups. Upon analyzing the Japanese study, we concluded that these differences could be attributed to variations in different PN evaluation sites, ultrasound machine and probe technical differences, and different researchers involved. We also evaluated and compared the ultrasound parameters of the phrenic nerve in ALS patients with individuals in a control group who were matched in age and gender. There were no significant differences in age, gender, height, waist or hip circumference, weight, or BMI between the two groups. The median age of the ALS group corresponded to the age peak of the disease reported in the scientific literature [
12,
13,
14].
In our study, there was no significant difference in the cross-sectional areas of the phrenic nerve (PN) between the right and left sides in ALS patients. This finding is consistent with our own study results, which showed a median cross-sectional area of 0.81 mm² (range: 0.58-1.07 mm²) on the right side and 0.81 mm² (range: 0.54-0.95 mm²) on the left side.The observed changes in PN cross-sectional area between the study groups, as reported by us and Surat et al., were/are supported by postmortem studies that document significant loss of large myelinated axons, predominantly in distal regions, and significant distal axonal PN atrophy [60]. Oxidative damage, which is implicated in the pathogenesis of ALS, contributes to axonal dysfunction and degradation [
34]. Oxidative stress, which affects diffuse cellular processes, can explain the diffuse degeneration of both the right and left PNs. Therefore, we believe that significantly reduced PN size in ALS patients may be one of the indicators of ongoing neurodegeneration.
There is limited data on prhrenic nerve sonography in the literature [
35,
36,
37,
38,
39]. These studies primarily focus on the nerve’s anatomical features and the quantitative analysis of its diameter or cross-sectional area. Surat et al. also measured a larger cross-sectional area of the right PN (p < 0.01) [
40]. In another small ultrasound study, Canella et al. [
35] also found a larger CSA of the right PN, although the difference between the sides was not significant. To ensure accuracy and avoid errors due to the varying experience of researchers, our ultrasound study was conducted with two independent researchers to obtain the most reliable results. In the control group, we found that the right PN was significantly larger than the left PN 1.16 (range: 0.63–1.36) mm² and 1.09 (range: 0.63–1.20) mm², respectively; p < 0.05).
Although the nerve appears as a hypoechoic structure with a hyper-echoic border peripherally on ultrasound, qualitative parameters such as homogeneity and echogenicity are not thoroughly evaluated in studies, making it unclear what the sensitivity and specificity of PN sonography are. In our study, we found a significant difference in homogeneity between the study groups (p < 0.001). However, the results are questionable, as no heterogenic nerve structures were detected in the control group during the ultrasound examination. Doubts about the results of this study are reinforced by the knowledge of morphological changes observed in peripheral and cranial nerves in healthy individuals due to age-related natural nerve degeneration [
41].
We also did not find a significant difference in nerve echogenicity (p > 0.05), which may be attributed to the small size of our study sample. These findings suggest that the observed morphological changes are non-specific to the degeneration that occurs with ALS and are not directly related to the reduction of the phrenic nerve’s cross-sectional area. Therefore, large-scale studies are necessary to obtain more accurate assessments and draw significant conclusions.
One of the objectives of this study was to identify the relationship between ultrasound changes in the phrenic nerve and clinical characteristics of ALS, such as the form of the disease, duration, and functional status. However, we did not find any significant correlations (p > 0.05). Similarly, a study conducted by Surat et al. [
40] also found no significant correlation between decreased PN cross-sectional area and any clinical variables, including disease duration, onset type, and ALS-FRS-R functional score). The authors suggest that the lack of correlation between reduced phrenic nerve CSA and disease duration may indicate changes in PN morphology in the early stages of the disease. This is consistent with evidence of distal axonal degeneration occurring before the manifestation of symptoms in animal models of motor neuron disease.
Although the evaluation of other peripheral nerves showed very small long-term nerve changes [169], researchers acknowledge that a relatively small change in nerve cross-sectional area requires a large sample size to obtain significant results and draw conclusions about the potential of ultrasound examination. However, these studies suggest that ultrasound measurements could become crucial biomarkers in the future. Due to the limited literature data and the small sample size of our ALS patient group, we cannot provide clear and definite conclusions.
This is the first study of its kind in Lithuania that attempted to link ultrasound-observed changes in the phrenic nerve to its dysfunction in patients with ALS. Therefore, future studies should aim to more thoroughly evaluate the relationship between these tests and ultrasound-observed changes in PN in our study. As mentioned earlier, the limited sample size is one of the shortcomings of this study that may have affected the reported results.
Conclusion
In this context, the results of this small study are particularly promising, as they suggest that sonographic findings could serve as a diagnostic tool for ALS. However, it is important to note that further research is necessary to validate these results and determine the full extent of its diagnostic utility. Continued investigation in this area will help refine the methodology, improve accuracy, and establish the reliability of sonographic techniques for ALS diagnosis. Such advancements can lead to a better understanding of the pathophysiology of ALS and potentially develop more effective treatments for this debilitating disease.
References
- Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013 Nov;9(11):617-28. [CrossRef]
- Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z, van den Berg LH. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 2017 Oct 5;3:17071.
- Longinetti E, Fang F. Epidemiology of amyotrophic lateral sclerosis: an update of recent literature. Curr Opin Neurol. 2019 Oct;32(5):771-776. [CrossRef]
- Byrne S, Walsh C, Lynch C, Bede P, Elamin M, Kenna K, McLaughlin R, Hardiman O. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2011 Jun;82(6):623-7. [CrossRef]
- Camacho-Soto A, Searles Nielsen S, Faust IM, Bucelli RC, Miller TM, Racette BA. Incidence of amyotrophic lateral sclerosis in older adults. Muscle Nerve. 2022 Sep;66(3):289-296. [CrossRef]
- Mehta P, Kaye W, Raymond J, Wu R, Larson T, Punjani R, Heller D, Cohen J, Peters T, Muravov O, Horton K. Prevalence of Amyotrophic Lateral Sclerosis—United States, 2014. MMWR Morb Mortal Wkly Rep. 2018 Feb 23;67(7):216-218.
- Jordan H, Rechtman L, Wagner L, Kaye WE. Amyotrophic lateral sclerosis surveillance in Baltimore and Philadelphia. Muscle Nerve. 2015 Jun;51(6):815-21. [CrossRef]
- Logroscino G, Traynor BJ, Hardiman O, Chiò A, Mitchell D, Swingler RJ, Millul A, Benn E, BeghiE; EURALS. Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2010 Apr;81(4):385-90. [CrossRef]
- 24. Marin B, Boumédiene F, Logroscino G, Couratier P, Babron MC, Leutenegger AL, Copetti M, Preux PM, Beghi E. Variation in worldwide incidence of amyotrophic lateral sclerosis: a metaanalysis. Int J Epidemiol. 2017 Feb 1;46(1):57-74. [CrossRef]
- Doi Y, Atsuta N, Sobue G, Morita M, Nakano I. Prevalence and incidence of amyotrophic lateral sclerosis in Japan. J Epidemiol. 2014;24(6):494-9.
- Barceló MA, Povedano M, Vázquez-Costa JF, Franquet Á, Solans M, Saez M. Estimation of the prevalence and incidence of motor neuron diseases in two Spanish regions: Catalonia and Valencia. Sci Rep. 2021 Mar 18;11(1):6207. [CrossRef]
- Longinetti E, Regodón Wallin A, Samuelsson K, Press R, Zachau A, Ronnevi LO, Kierkegaard M, Andersen PM, Hillert J, Fang F, Ingre C. The Swedish motor neuron disease quality registry. Amyotroph Lateral Scler Frontotemporal Degener. 2018 Nov;19(7-8):528-537. [CrossRef]
- Benjaminsen E, Alstadhaug KB, Gulsvik M, Baloch FK, Odeh F. Amyotrophic lateral sclerosis in Nordland county, Norway, 2000-2015: prevalence, incidence, and clinical features. Amyotroph Lateral Scler Frontotemporal Degener. 2018 Nov;19(7-8):522-527. [CrossRef]
- Dorst J, Chen L, Rosenbohm A, Dreyhaupt J, Hübers A, Schuster J, et al. Prognostic factors in ALS: a comparison between Germany and China. J Neurol. 2019 Jun;266(6):1516-25. [CrossRef]
- GBD 2016 Motor Neuron Disease Collaborators. Global, regional, and national burden of motor neuron diseases 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018 Dec;17(12):1083-1097.
- Bonafede R, Mariotti R. ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles. Front Cell Neurosci. 2017 Mar 21;11:80. [CrossRef]
- Al-Chalabi A, Hardiman O, Kiernan MC, Chiò A, Rix-Brooks B, van den Berg LH. Amyotrophic lateral sclerosis: moving towards a new classification system. Lancet Neurol. 2016 Oct;15(11):1182-94.
- Pinto S, Gromicho M, Oliveira Santos MO, Swash M, De Carvalho M. Respiratory onset in amyotrophic lateral sclerosis: clinical features and spreading pattern. Amyotroph Lateral Scler Frontotemporal Degener. 2023 Feb;24(1-2):40-44. [CrossRef]
- Shoesmith CL, Findlater K, Rowe A, Strong MJ. Prognosis of amyotrophic lateral sclerosis with respiratory onset. J Neurol Neurosurg Psychiatry. 2007 Jun;78(6):629-31. [CrossRef]
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC. Amyotrophic lateral sclerosis. Lancet. 2011 Mar 12;377(9769):942-55.
- Fantini R, Mandrioli J, Zona S, Antenora F, Iattoni A, Monelli M, Fini N, Tonelli R, Clini E, Marchioni A. Ultrasound assessment of diaphragmatic function in patients with amyotrophic lateral sclerosis. Respirology. 2016 Jul;21(5):932-8. [CrossRef]
- Pinto S, Alves P, Pimentel B, Swash M, de Carvalho M. Ultrasound for assessment of diaphragm in ALS. Clin Neurophysiol. 2016 Jan;127(1):892-897. [CrossRef]
- Pinto S, Alves P, Swash M, de Carvalho M. Phrenic nerve stimulation is more sensitive than ultrasound measurement of diaphragm thickness in assessing early ALS progression. Neurophysiol Clin. 2017 Feb;47(1):69-73. [CrossRef]
- Kwon S, Min JH, Cho HJ, Joo BE, Cho EB, Seok JM, Kim MJ, Kim BJ. Usefulness of phrenic latency and forced vital capacity in patients with ALS with latent respiratory dysfunction. Clin Neurophysiol. 2015 Jul;126(7):1421-6. [CrossRef]
- Hobson-Webb LD, Simmons Z. Ultrasound in the diagnosis and monitoring of amyotrophic lateral sclerosis: a review. Muscle Nerve. 2019 Aug;60(2):114-123. [CrossRef]
- Singh D, Verma R, Garg RK, Singh MK, Shukla R, Verma SK. Assessment of respiratory functions by spirometry and phrenic nerve studies in patients of amyotrophic lateral sclerosis. J Neurol Sci. 2011 Jul 15;306(1-2):76-81. [CrossRef]
- Sathyaprabha TN, Pradhan C, Nalini A, Thennarasu K, Raju TR. Pulmonary function tests and diaphragmatic compound muscle action potential in patients with sporadic amyotrophic lateral sclerosis. Acta Neurol Scand. 2010 Jun;121(6):400-5. [CrossRef]
- Paschoal IA, Villalba Wde O, Pereira MC. Chronic respiratory failure in patients with neuromuscular diseases: diagnosis and treatment. J Bras Pneumol. 2007;33(1):81-92.
- Manera U, Torrieri MC, Moglia C, Canosa A, Vasta R, Ribolla F, et al. Arterial blood gas analysis: base excess and carbonate are predictive of noninvasive ventilation adaptation and survival in 61 amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22(sup1):33-9. [CrossRef]
- Schmidt EP, Drachman DB, Wiener CM, Clawson L, Kimball R, Lechtzin N. Pulmonary predictors of survival in amyotrophic lateral sclerosis: use in clinical trial design. Muscle Nerve. 2006 Jan;33(1):127-32. [CrossRef]
- Fregonezi G, Araújo PR, Macêdo TL, Dourado Junior ME, Resqueti VR, Andrade Ade F. Monitoring respiratory muscle strength assists in early diagnosis of respiratory dysfunction as opposed to the isolated use of pulmonary function evaluation in amyotrophic lateral sclerosis. Arq Neuropsiquiatr. 2013 Mar;71(3):146-52. [CrossRef]
- Pinto S, de Carvalho M. Correlation between Forced Vital Capacity and Slow Vital Capacity for the assessment of respiratory involvement in Amyotrophic Lateral Sclerosis: a prospective study. Amyotroph Lateral Scler Frontotemporal Degener. 2017 Feb;18(1-2):86-91.
- Pinto S, Pinto A, de Carvalho M. Phrenic nerve studies predict survival in amyotrophic lateral sclerosis. Clin Neurophysiol. 2012 Dec;123(12):2454-9. [CrossRef]
- Fischer LR, Glass JD. Axonal degeneration in motor neuron disease. Neurodegener Dis. 2007;4(6):431-42. [CrossRef]
- Canella C, Demondion X, Delebarre A, Moraux A, Cotten H, Cotten A. Anatomical study of phrenic nerve using ultrasound. Eur Radiol. 2010 Mar;20(3):659-65. [CrossRef]
- Patel Z, Franz CK, Bharat A, Walter JM, Wolfe LF, Koralnik IJ, et al. Diaphragm and Phrenic Nerve Ultrasound in COVID-19 Patients and Beyond: Imaging Technique, Findings, and Clinical Applications. J Ultrasound Med. 2022 Feb;41(2):285-99.
- Nwawka OK. Ultrasound Imaging of the Brachial Plexus and Nerves About the Neck. Ultrasound Q. 2019 Jun;35(2):110-9. [CrossRef]
- Walter U, Tsiberidou P. Differential age-, gender-, and side-dependency of vagus, spinal accessory, and phrenic nerve calibers detected with precise ultrasonography measures. Muscle Nerve. 2019 Apr;59(4):486-491. [CrossRef]
- Czaplicki CD, Zhang N, Knuttinen MG, Naidu SG, Patel IJ, Kriegshauser JS. Ultrasound-Guided Phrenic Nerve Block for Lung Nodule Biopsy: Single-Center Initial Experience. Acad Radiol. 2022 Feb;29 Suppl 2:S118-S126. [CrossRef]
- Armon C. Smoking may be considered an established risk factor for sporadic ALS. Neurology. 2009 Nov 17;73(20):1693-8.
- Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000 Dec;5(4):191-208.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).