1. Introduction
Newborn bloodspot screening (NBS) is one of the most implemented population screening programs worldwide. While initially limited in the number of diseases (phenylketonuria, hypothyroidism), NBS screening programs subsequently broadened their panel of conditions screened [
1]. This was largely driven by technical improvements in diagnostics, improved knowledge on disease mechanisms and history, converging with therapeutic interventions to improve the outcome in diagnosed infants. This progress reflects the fact that screening programs are driven by the criteria of Wilson and Jungner [important health problem, natural history of the condition well understood, detectable at an early stage, earlier treatment should be beneficial, a suitable test should be available in this early stage (sensitivity/specificity), and the test should be acceptable] [
1].This expansion of NBS programs also raised awareness on shortcomings on parental education, information products and the informed consent process. Provision of information to parents has been recognized as a crucial part of sustainable NBS programs, be it that there is still lack of regulatory harmonization within Europe [
2].
Almost all European countries provide information for parents (brochures, websites). About 2/3 of the countries ask for consent, while consent for long-term storage of blood spot cards is requested in a minority (30 %) of European countries [
1]. A recent paper focused on these information products provided [
3]. In this paper, 26 European printed products were assessed on their content and knowledge, and compared to a list of eight knowledge domains (screening purpose, false positive/negative findings, uncertainties and risks, medical implications, social implications, financial implications, follow-up and support services). Despite some differences between European countries, most of these eight knowledge aspects were included in all information products, with most diversity related to handling of residual bloodspot samples [
3].
Along the same line, the script for health care providers (HCPs) involved in NBS in Flanders mentions the need to discuss the relevance of timely diagnosis, the importance of the postnatal age window (72-96 h) for screening, the practicalities related to the appointment for the screening (because of the short hospital stay after delivery), the need of verbal (concise) consent, and to make it clear that all initial screening costs are covered by the government, while costs for additional tests after screening are reimbursed by the insurance, the diseases screened for, and finally, the fact that NBS is not compulsory, but highly recommended [
4]. In Flanders, long-term storage of the blood spot charts is not part of the program, and storage is limited to one year. These topics are also discussed in leaflets (printed, and pdf) and on a specific website (aangeboren.bevolkingsonderzoek.be) to inform parents and the general public [
5].
Informed consent necessitates that the relevant person(s) have been informed on the procedure and its potential consequences in such a way that it is reasonable to assume that the information has been understood to support their decision (irrespective of the subsequent decision itself) [
6]. In the Frankova et al. paper, 12/27 countries mentioned that checks are performed to verify that information indeed reaches the targeted populations [
2]. In an attempt to get a snapshot on what mothers know on NBS, and the sources they use in Flanders (as region or country not included in European survey [
2], a pilot study was performed.
2. Materials and Methods
2.1. Perinatal health care structures in Flanders and study setting
Prenatal follow up in Flanders is mainly coordinated by obstetricians and midwives, with more limited involvement of general practitioners, and still is almost exclusively ‘hospital’ driven. In 2021, there were 63 334 (64 282 births) deliveries in Flanders. Those deliveries almost exclusively occurred in one of the 59 hospitals (‘maternities’), with about 0.8 % of deliveries elsewhere. In an attempt to evolve to a transmural care program, initiatives were taken to shorten the duration of hospitalization to 2-4 days, in part depending on the type of delivery. This shift has been facilitated by the development of midwife-driven home care programs, supported by general practitioners, obstetricians and pediatricians [
7,
8]. Consequently, a relevant portion of newborns has its NBS screening at home by midwives, and different HCPs are involved in the pregnancy and postpartum care. Within this health care framework and as a pilot study, we targeted to recruit 200 mothers who recently (maximal 1 year before the dissemination of the questionnaire) delivered in Flanders, sufficiently well understood Dutch to complete the questionnaire, were older than 18 years, and provided consent to contribute to the pilot study. We are aware that with this approach, partners of the mothers were a priori excluded. This is a deficiency, but we did this to avoid ‘dual’ reporting within the framework and limitations of this pilot study.
2.2. Questionnaire
In a first part of the questionnaire, information on characteristics and background of respondents (like age, residency, primi- or multipara, level of education, place of delivery) and on the NBS procedure (collected yes/no, location of the procedure, consent recall) were collected. The second part focused on the knowledge itself, the sources of information, and the HCPs involved. This part of the questionnaire was constructed in line with the approach described by Detmar et al. for a Dutch cohort, with some adaptations to the Flemish setting (health care organization, legal and regulatory environment, sources of information) [
6]. To respect the methodology on questionnaire design, adaptations were initially independently made by CdG and MH, with subsequent cross verification to attain consensus. In the event of absence of consensus, KA was involved. The final version was subsequently verified on face validity by these 3 authors.
2.3. Data collection and analysis
Questionnaires were distributed online (Qualtrics), using social media platforms (Facebook, personal and groups) and e-mail correspondence to nurseries. To assess the representativity of respondents, information on characteristics and background was compared to reference information on pregnancies in Flanders [
7,
9]. Data on the NBS procedure (collected yes/no, location of the procedure, consent recall) were more difficult to compare, as we could only retrieve a press release from the relevant agency that stated that 99 % of the newborns undergo NBS in Flanders [
10]. Data analysis on knowledge was based on maternal knowledge on the NBS procedure as dependent variable, independent variables were the information received (as perceived by the mother), parity (prima- versus multigravida), and the level of maternal education. We hereby hypothesized that maternal knowledge would correlate positive with parity and the level of education.
2.4. Ethics, privacy and data management
The Ethics Committee Research of KU Leuven and University Hospitals Leuven approved the study protocol (MP022668, December 5th 2022, favorable advice). The questionnaire was preceded by an information letter, describing the aims of the study and the consent to contribute to the questionnaire. Consent to contribute and store responses for analysis (anonymous, confidential) was requested before the questionnaire could be completed.
3. Results
The final version of the ‘knowledge’ part (translated version, English,
Table 1) contained 12 closed questions, and 5 multiple choice + open questions on sources of information. We have provided the Dutch version of the questionnaire in a Supplement.
Finally, based on a 5-point Likert score, respondents were requested to provided their assessment on the information transfer (clarity, appropriateness of timing, sufficiency, usefulness) process.
This questionnaire (characteristics and background, NBS procedure and knowledge part, and the general assessment of knowledge transfer process) was accessible online from February 2, 2023 until April 18, 2023 when 200 questionnaires were received.
Participants were recruited by the own Facebook profiles (CdG, MH, n = 42), specific groups within Facebook (6 groups, 709 messages, 100 participants), or nurseries (n = 79 nurseries contacted). Ninety-eight % of the participants finalized the questionnaire. To assess representativity of respondents to the Flemish pregnant and postpartum population, age, residence (postal code, provinces), parity, level of education, place of delivery (hospital, or out of hospital), collection of the NBS [yes/no, location (hospital/home)] of respondents were compared to the latest Study center Perinatal Epidemiology (SPE) 2021 annual report and STATBEL report (education) (
Table 2). Maternal age and place of delivery were similar, while there were some differences in place of residence, some overrepresentation in primigravidae, and respondents had a somewhat higher level of education compared to the reference population. Data on the NBS procedure (yes/no, place, verbal consent) were somewhat more difficult to compare with the reference population data, but also seem similar (
Table 2). NBS were collected both in the hospital, as well as at home. Mothers recalled verbal consent in 69.5 %, 12.5 % did not recall any consent request, 18 % stated that no consent has been requested.
Based on 12 questions provided, the mean level of knowledge was 7.2 (SD 2.4)/12, and 79 % of the respondents had a score ≥6. The level of knowledge was correlated positive with the level of education, without difference between primi- and multipara. An overview on the responses to the individual questions is provided in
Table 3.
The concepts of targeted screening (severe consequences, low a priori likelihood, sensitivity, carrier concept) and absence of notification in the event of normal findings are well known. In contrast, the fact that NBS is not compulsory in Flanders is only poorly known, and so is the post analysis handling of the NBS sample.
Related to the sources of information, the most relevant HCPs involved were midwifes (80.5 %) and nurses (38.5 %), while other sources were obstetricians (20 %), the leaflet (12 %), or general practitioners (1.5 %). Five % do not recall to have received any information on the NBS procedure, 5.5 % of the respondents mentioned that they had already received information on NBS during their nursing or medical training. As similar pattern was observed on the question to indicate the most relevant source(s) of information, with verbal interaction with HCPs (midwifes, 77.9 %; nurses 30.7 %; obstetricians 18.1 %) superior to the information leaflet (7.5 %). Forty-for % of the mothers reported that they received the leaflet. Of those who received the NBS leaflet, a relevant portion stated to have fully (34 %) or at least partial (87.5 %) read the leaflet.
Finally and based on a 5-point Likert score, respondents provided their general assessment on the information transfer on clarity (3.36, SD 1.22), appropriateness of timing (3.38, SD 1.46), sufficiency (3.11, SD 1.6), and its usefulness (3.35, SD 1.29). A significant positive correlation was observed between the individual respondent’s knowledge score and the Likert score.
4. Discussion
We report on what mothers know on NBS, and their sources of information in Flanders. This provides a first snapshot on the overall knowledge (mean level 7.2/12 questions) and the most relevant sources of persons involved (midwives, nurses). When the leaflet (44 %) was provided, the majority has read it at least partially. The concepts of targeted screening and absence of notification in the event of normal findings were well known. In contrast, the fact that NBS is not compulsory in Flanders was only poorly known, and so is the post analysis handling of the NBS sample (
Table 3). Finally, the overall Likert rating on knowledge transfer was reasonably good (3.3/5).
Related to the representativity and feasibility, we stress that - despite some minor differences (
Table 1) - this pilot cohort largely represents the overall population of mothers who recently gave birth in Flanders. Furthermore, the majority of the respondents finalized the questionnaire, suggested that the burden (time, type of questions) was perceived to be reasonable and relevant. Furthermore, the place of NBS (hospital/home) likely also reflects contemporary practices.
On maternal knowledge, the mean level was 7.2/12, with good to very good performance on the concepts of targeted screening (severe consequences, low a priori likelihood, sensitivity, carrier concept) and absence of any notification in the event of normal findings. In contrast, the fact that NBS is not compulsory in Flanders is only poorly known, and so is the post analysis handling of the NBS samples (destroyed after one year, but not ‘out of scope’ clinical research allowed)(
Table 3). Procedural, mothers recalled verbal consent in 69.5 %, 12.5 % do not recall any consent request, 18 % state that no consent has been requested. The results on both knowledge and consent practices are similar to somewhat better compared to other recently reported surveys on this topic [11-13]. Key HCP for this knowledge transfer are midwives and nurses, with the leaflet as supporting resource [
11,
14]
Obviously, this study has relevant limitations. Besides the pilot character and exclusive focus on mothers, this study design obviously holds the risk of recall bias (in both directions). One could also reflect on the completeness of the questionnaire, as - post hoc - not all previously mentioned eight highlighted knowledge domains (e.g. financial implications) were sufficiently well covered [
15]. Still, we feel that there is value in this pilot study beyond feasibility, as it is relevant to regularly check that information indeed reaches the targeted populations and that practices remain concordant (like relevant portion of mothers that does not recall a verbal consent request) [
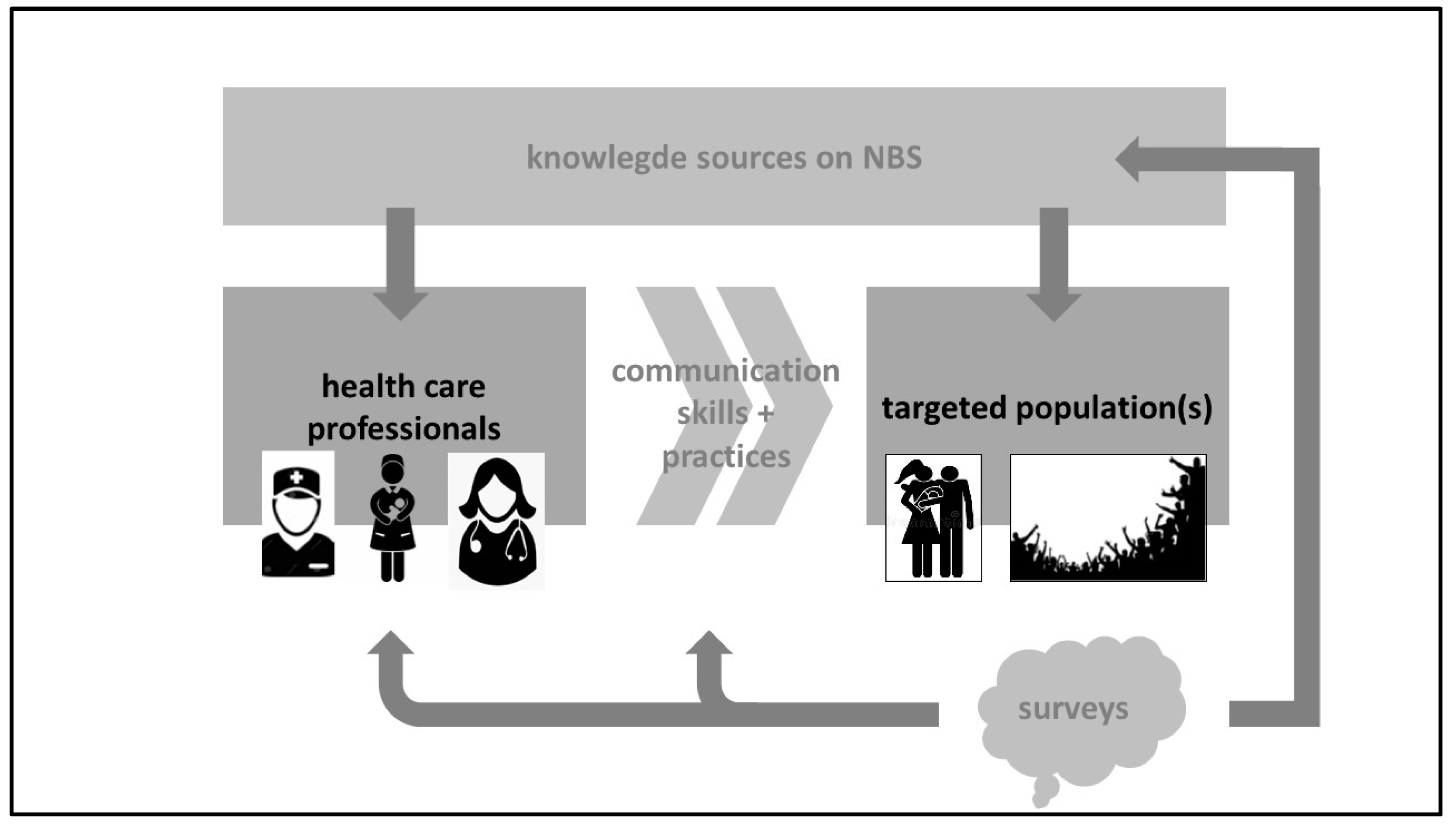
2]. We therefore suggest a quality improvement cycle towards a sustainable NBS program, with regular updated surveys as part of this strategy (
Figure 1).
Such a program should be driven by well-trained HCPs (knowledge, communication skills and practices) so that the correct, and relevant information can be provided, with access to the updated information of NBS practices, as described in the above mentioned HCP script on NBS [
4]. HCPs knowledge and skills training should focus on the relevant information to be provided, to avoid overload [
16]. Based on other research, this is preferably done during pregnancy, to be verified in early postpartum, with midwives or nurses in the lead [
6,
17]. These HCPs are also reported by the mothers as the key persons involved in knowledge transfer, in line with similar reports [
18]. The recall of verbal consent in only 69.5 % of the mothers suggests that any quality improvement program should also reinforce the verbal consent practice as part of the NBS procedure. The impact, strengths and potential weaknesses can subsequently be assessed by regular surveys, as done in this pilot. However, we do believe that there is value to co-create a next version of such questionnaire in collaboration with HCPs, the agency involved and the public. Any optimization will likely be along the 8 domains recently identified in an European survey [
15]. Similarly, a recent French qualitative study on parental information and consent also listed 5 themes (knowledge, information received, parental choice, experience of the NBS process, and parents’ perspectives and wishes) [
14]
Such a co-creation model is likely also beneficial for the knowledge sources (website, leaflet). Finally, and although this was not part of the current study, we do believe that informing the general public by websites or media is an effective additional approach to ensure sustainable NBS practices. At least, we hope this pilot study has paved the way to implement such a quality improvement program to attain a sustainable NBS program in Flanders.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Table S1: the questionnaire (in Dutch); lay summary results (in Dutch).
Author Contributions
Conceptualization, C.d.G., M.H. and K.A.; methodology, C.d.G., M.H. and K.A.; formal analysis, C.d.G., M.H. and K.A.; data curation, K.A.; writing— original draft preparation, C.d.G., M.H. and K.A.; writing—review and editing, M.R, F.E.; supervision, K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee Research of KU Leuven and University Hospitals Leuven (protocol code MP022668, favorable advice, December 5th 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The corresponding author can be contacted if additional information is requested.
Acknowledgments
We would explicitly thank all respondents who offered some of their precious time to voluntary contributed to this study. In respect for their efforts, we provided a Dutch lay summary of the most relevant findings as a supplement 2.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Loeber, J.G.; Platis, D.; Zetterström, R.H.; Almashanu, S.; Boemer, F.; Bonham, J.R.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E.; et al. Neonatal Screening in Europe Revisited: An ISNS Perspective on the Current State and Developments Since 2010. Int. J. Neonatal Screen. 2021, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Franková, V.; Driscoll, R.O.; Jansen, M.E.; Loeber, J.G.; Kozich, V.; Bonham, J.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E.; et al. Regulatory landscape of providing information on newborn screening to parents across Europe. Eur. J. Hum. Genet. 2021, 29, 67–78. [Google Scholar] [CrossRef] [PubMed]
- IJzebrink, A.; van Dijk, T.; Franková, V.; Loeber, J.G.; Kožich, V.; Henneman, L.; Jansen, M.E. Informing Parents about Newborn Screening: A European Comparison Study. Int. J. Neonatal Screen. 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Vlaanderen is Zorg. Bevolkingsonderzoek, aangeboren aandoeningen, draaiboek. Available online: https://aangeboren.bevolkingsonderzoek.be/sites/default/files/2022-11/draaiboek_vroedvrouwen_dec22.pdf (accessed on 28 July 2023).
- Vlaanderen is Zorg. Bevolkingsonderzoek, aangeboren aandoeningen. https://aangeboren.bevolkingsonderzoek.be/nl/aa/aangeboren-aandoeningen-0 (accessed on 28 July 2023).
- Detmar, S.; Hosli, E.; Dijkstra, N.; Nijsingh, N.; Rijnders, M.; Verweij, M. Information and informed consent for neonatal screening: opinions and preferences of parents. Birth 2007, 34, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Kenniscentrum, KCE. https://www.kce.fgov.be/en/publications/all-reports/organisation-of-maternity-services-in-belgium (accessed on 28 July 2023).
- Studiecentrum voor Perinatale Epidemiologie. Perinatale gezondheid in Vlaanderen. Jaar. 2021. https://zeg.paddlecms.net/sites/default/files/2022-11/SPE-Perinatale%20gezondheid%20in%20Vlaanderen-2021-FINAL.pdf (accessed on 28 July 2023).
- STATBEL. Belgium in figures. https://statbel.fgov.be/en/news/level-education-mapped-out. (accessed on 28 July 2023).
- DeMorgen. https://www.demorgen.be/nieuws/ruim-99-procent-van-baby-s-in-vlaanderen-gescreend-op-aangeboren-aandoeningen~bcd7016d/?referrer=https%3A%2F%2Fwww.google.com%2F. (accessed on 28 July 2023).
- Franková, V.; Dohnalová, A.; Pešková, K.; Hermánková, R.; O'Driscoll, R.; Ješina, P.; Kožich, V. Factors influencing parental awareness about newborn screening. Int. J. Neonatal Screen. 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Kasem, A.; Razeq, N.M.A.; Abuhammad, S.; Alkhazali, H. Mothers’ knowledge and attitudes about newborn screening in Jordan. J. Community Genet. 2022, 13, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Etchegary, H.; Nicholls, S.G.; Tessier, L.; Simmonds, C.; Potter, B.K.; Brehaut, J.C.; Pullman, D.; Hayeems, R.; Zelenietz, S.; Lamoureux, M.; et al. Consent for newborn screening: parents’ and health-care professionals’ experiences of consent in practice. Eur. J. Hum. Genet. 2016, 24, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Pinel, J.; Bellanger, A.; Jamet, C.; Moreau, C. Information and parental consent for French neonatal screening: a qualitative study on parental opinion. Int. J. Neonatal Screen. 2023, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- IJzebrink, A.; van Dijk, T.; Franková, V.; Loeber, J.G.; Kožich, V.; Henneman, L.; Jansen, M.E. Informing parents about newborn screening: a European comparison study. Int. J. Neonatal Screen. 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, T.; Kater, A.; Jansen, M.; Dondorp, W.J.; Blom, M.; Kemp, S.; Langeveld, M.; Cornel, M.C.; van der Pal, S.M.; Henneman, L. Expanding neonatal bloodspot screening: a multi-stakeholder perspective. Front. Pediatr. 2021, 9, 706394. [Google Scholar] [CrossRef]
- Davis, T.C.; Humiston, S.G.; Arnold, C.L.; Bocchini, J.A. Jr.; Bass, P.F., 3rd; Kennen, A.M.; Bocchini, A.; Kyler, P.K.; Lloyd-Puryear, M. Recommendations for effective newborn screening communication: results of focus groups with parents, providers, and experts. Pediatrics 2006, 117, S326–S340. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.G.; Southern, K.W. Parental decision-making and acceptance of newborn bloodspot screening: an exploratory study. PLoS One. 2013, 8, e79441. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).