Submitted:
25 August 2023
Posted:
28 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Antifungal peptides (AFPs)
| Antifungal peptide |
Origin | Molecular weight [Reference] | Antifungal effect |
|---|---|---|---|
| Mechanism of action: inhibition of chitin biosynthesis | |||
| Nikkomycin Z | fungi | 495.4 Da [26] | 0.5-64 mg/L (yeasts, fungi) a |
| Mechanism of action: destabilization of plasma membrane, pore formation, cell wall damage | |||
| Magainin 2 | frog | 2466.9 Da [27,28] | 6.25 µM (Saccharomyces cerevisiae), )Trichosporon beigelii, Candida albicans) a 60-100 mg/L (Penicillium digitatum; Alternaria solani; Phytophthora infestans) a |
| Halictine Hal-2 | sweat bee | 1452.85 Da [29] | 1.6-25 µM (Candida spp.) a |
| Halocidin | ascidian | 3445.1 Da [30] | Effect was not estimated |
| Polymyxin B | bacteria | 1301.6 Da [31] | 16-256 mg/L (multi-drug-resistant fungal strains) a |
| Colistin | bacteria | 1155.4 Da [31] | |
| Defensin DefMT3 | ticks | 1613.1 Da [32] | 4 µM (Fusarium culmorum; F. graminearum) b |
| Indolicidin | bovine | 1906.3 Da [33] | 12.5-50 mg/L (C. albicans) a |
| Lactoferampin B | bovine | 2389.8 Da [34] | 0.7-39 µM (C. albicans) c |
| Lactoferricin B | bovine | 3125.8 Da [34] | 0.31-400 mg/L (yeasts) a 4-32 µM (fungi) a |
| Lactoferricin H | human | 5513 Da [34] | 10 mg/L (C. albicans) a |
| Leg2 | chickpea legumin hydrolysates | 2157.6 Da [35] | 125-250 µM (S. cerevisiae, Zygosaccharomyces bailii) a |
| LL-37 | human | 4493.3 Da [36] | 4-64 µM (Candida spp.) a |
| Mechanism of action: cell/spore lysis, cell wall perturbations | |||
| Cecropin B | silkworm | 3835.7 Da [38] | 0.9 mg/L (C. albicans) a 160-320 mg/L (F. solani) a |
| Osmotin | plant | 24285.3 Da [38] | 4-25 mg/L (fungi) a |
| Stomoxyn | stable fly | 4474.2 Da [39] | 0.8-50 µM (yeasts); 0.4-7 µM (fungi) a 50-100 µM (A. fumigatus) a |
| Temporin B | frog | 1391.8 Da [40] | 1.4-4 µM (Candida spp.) a |
| Temporin G | 1457.8 Da [41] | 8-128 µM (yeasts/fungi) a | |
| Antifungal peptide | Molecular weight; [Reference] | Antifungal effect |
|---|---|---|
| Mechanism of action: inhibition of 1,3-β-d-glucan synthase | ||
| Anidulafungin | 1140.2 Da [42,43,44] | 0.06-0.25 mg/L (Candida spp.) a 0.015-32 mg/L (fungi) a |
| Caspofungin | 1093.3 Da [42,43,44] | 0.25-4 mg/L (Candida spp.) a |
| Micafungin | 1270.3 Da [42,43,44] | 0.015-4 mg/L (Candida spp.) a |
| Mechanism of action: destabilization of plasma membrane, pore formation, cell wall damage | ||
| Lf(1-11) H | 1317.5 Da [37] | > 12.5 mg/L (Candida sp.); 80-160 mg/L (F. solani) a 4.3 µM (A.fumigatus) a |
| Lfchimera (bLfcin/Lfampin) | 4422 Da [34] | 6.25 mg/L (C. parapsilosis) a |
| γ-core DefMT3 | 1611.8 Da [32] | 1-2 µM (F. culmorum; F. graminearum) b |
| Brilacidind | 936.9 Da [45] | 2.5- >80 µM (C. neoformans, C. albicans, C. auris, A. fumigatus) a |
| RcAlb-PepII | 637.77 Da [46,47] | 17-250 µM (Candida spp.) a 0.04 mg/L (Cryptococcus neoformans) a |
| Halictine Hal-2 derivatives | 1471 Da [48] | 0.5-1 µM (Candida spp., S. cerevisiae) a |
| di-K19Hc | 4115.1 Da [49] | < 4 mg/L (C. albicans); <16 mg/L (Aspergillus sp.) a |
| Pexiganan/MSI-78 | 2478.2 Da [37] | 10-80 mg/L (F. solani) a |
| PepGAT | 1044.18 Da [46,50,51] | 40-80 mg/L (Candida spp., P. digitatum) a |
| PepKAA | 1238.44 Da [46,50,51] | |
| Peptide 77-3 | 994.2 Da [52,53] | 3.5-5 mg/L (A. flavus, A. parasiticus)a |
| KK14 | 144.2 Da [54] | 6.25-100 mg/L (fungi) a |
| D4E1 | 2079.4 Da [52,53] | 7.75 µM (A. flavus); 0.60 µM (V. dahliae) b 13.02 µM (C. destructivum) b |
| PAF26 | 991.2 Da [55] | 4-6 µM (P. digitatum) a |
| Mechanism of action: production of reactive oxygen species, cell wall degradation | ||
| Octominin | 2652.2 Da [56] | 50 mg/L (C. albicans) a |
| Mo-CBP3-PepI | 893.12 Da [57] | 2.2 µM (C. albicans) c |
| Mechanism of action: Cell/spore lysis, cell wall perturbations | ||
| Osm-pepA | 3050.5 Da [58] | 40 µM (S. cerevisiae) a 20 µM (Pichia pastoris) a |
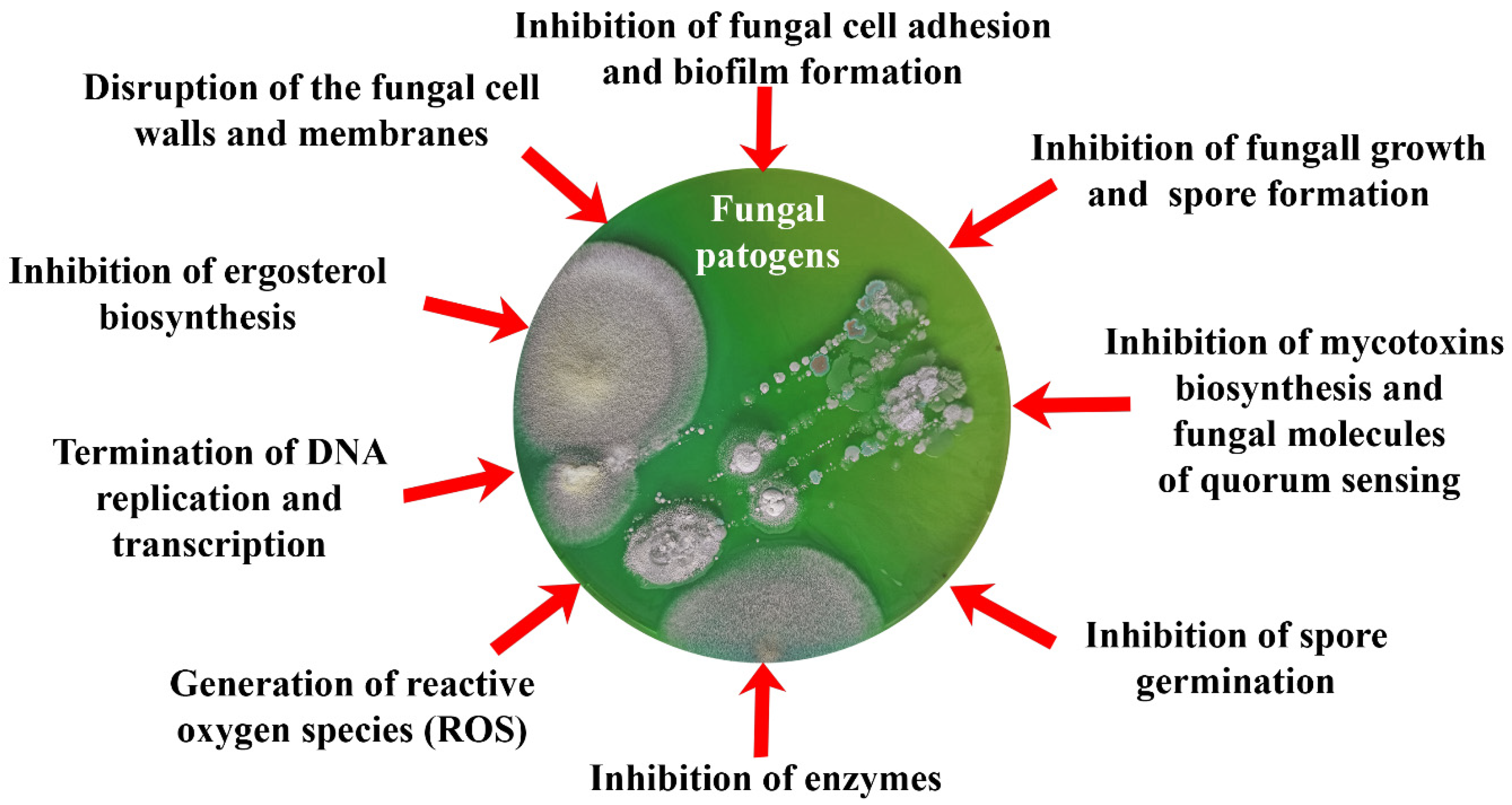
3. Biomimetics with antifungal activity of non-peptide nature
3.1. Biomimetics with a destructive effect on fungal cells
| Biomimetics as antifungals | Target of action | *Antifungal effect |
|---|---|---|
| Amphotericin B nano-aggregates [82] | Inhibition of ergosterol biosynthesis for membrane formation and provoking lysis of cells | MIC (mg/L): Candida spp. (0.125–0.5); A. fumigatus (1.0) |
| Biomimetic nanopillar Si-containing surfaces [16] | Rupture of coat and inner membrane of spores leading to cell death | 4-fold reduction of amounts of attached spores and approximately 9-fold reduction of viable conidia of Aspergillus brasisiensis on biomimetic surface |
| Methacryloyloxyethyl ester monomers with tyrosine, methionine and leucine [72] | Destruction of the cell membrane | MIC (mg/L): A. niger (0.16) |
| Synthetic peptides from predicted cysteine-rich peptides of tomato (mimicking γ-core regions of cysteine-rich peptides of Solanum lycopersicum) [83] | Charge of the derived peptides is positive, favoring interactions with the membranes of the pathogens. Inducing permeabilization and disruption of the fungal membranes | IC50 (μM): Cryptococcus neoformans (5.1-11.5); Fusarium culmorum (42.1-126.7); F. oxysporum (43.8-165.8); F. solani (47.5-138.8); F. verticillioides (99.8-152.0) |
| Itraconazole and difluorinated-curcumin containing chitosan nanoparticles in hydrogel [84] | Synergistic antifungal activity composed of increased permeation through fungal cell wall and membrane and lethal action of difluorinated curcumin | EC50 (mg/L): Trichophyton mentagrophytes (150) |
| Chitosan/polyethylene oxide (CPO) [85] |
Antifungal effect similar to voriconazole (production of intracellular ROS and increasing of permeability of plasma membrane) |
C. albicans cells: diameter of inhibition area by CPO - 25-27 mm (agar disc diffusion method); Control: inhibition by voriconazole – 27 mm |
| N-alkylated glycine oligomers (peptoids) [86] | Suppressed formation of hyphae resulted in changes in cell and organelle morphology, most dramatically in the nucleus and nucleolus, and in the number, size, and location of lipidic bodies | MIC (μM): C. albicans (3.0-13.0) |
| Metal-organic framework (Ce-MOF) with enzyme-like activity of catalase, superoxide dismutase, and peroxidase [87] | Production of ROS and inhibition of fungal growth | 40 μg/mL Ce-MOF gives 93.3–99.3% growth inhibition of Aspergillus flavus, A. niger, A. terreus, C. albicans, Rhodotorula glutinis |
| Iodine-doped carbon dots (IDCDs) with peroxidase-like activity [88] | Production of ROS | The 90% decrease in number of C. albicans CFU is observed in presence of 2.72 g/L IDCDs under visible light irradiation over 2 h in presence of 0.5 mM H2O2 |
3.2. Biomimetics with inhibition effect on fungal proteins/enzymes
| Biomimetics as antifungals | Target of action | *Antifungal effect |
|---|---|---|
| Iridoid alkaloids biomimic of camptothecin [91] | Strong inhibitory effects against mycelial growth and spore germination. Disturbing the replication and transcription of DNA by binding to topoisomerase I, inhibiting of ergosterol biosynthesis | LC50 (mg/L): F. graminearum (34.5); Rhizoctonia solani (18); Botrytis cinerea (26) |
| Sulfonyl hydrazide derivatives containing the 1,2,3,4-tetrahydroquinoline [92] | Inhibition of laccase activity | EC50 (mg/L): Sclerotinia sclerotiorum (3.32), Valsa mali (2.78) |
| 3-aryl-isoquinoline derivatives [93] | Inhibition of succinate dehydrogenase activity | EC50 (mg/L): Physalospora piricola (3.7) |
| Hymexazol glycosides [15] | Inhibition of chitinase, produced by fungi | EC50 (mg/L): Alternaria alternata (1.58) |
| N-1-(β-d-ribofuranosyl) benzimidazole derivatives [94] | Inhibition of fungal cytochrome P450 3A-dependent C14-α-demethylase which is responsible for the conversion of lanosterol to ergosterol and ergosterol biosynthesis | MIC (mg/L): A. flavus (0.8); A. niger (1.6); F. oxysporum (3.1); C. albicans (0.8) |
| pCF2Ser peptide as substrate mimetic [95] | Inhibition of Cdc14 phosphatases; formation of defective conidiation and ascospores, reducing cell virulence | Inhibitory constant (Ki) against fungal phosphatase Cdc14 homologs - 3-19 µM |
| Synthetic oxime-derivatives of resorcylate aminopyrazole [96] | Selective inhibition of chaperone Hsp90 functions | EC50 (μM): Candida neoformans (0.040); C. albicans (0.011) |
| Dextran-coated Gd-based nanoparticles (NPs) as phosphatase-like nanozyme [97] | Selective hydrolysis of the terminal high-energy phosphate bonds in ATP | 464 mg/L NPs increases ethanol yield per 17%. The characteristics of NPs: Km, Vmax, and Ea were 29.4 μM, 7.17 × 10-7 M/s, and 29.34 kJ/mol, respectively |
| Nanopillars of poly(methyl methacrylate) like cicada wing surface topography [98] | Superhydrophobic surfaces with reduced adsorption capability of proteins needed for adhesion of fungal spores | 100% removal of 105 spores of Fusarium oxysporum on the surface with antifungal activity |
3.3. Biomimetics of metabolites with effect on growth and metabolic activity of fungi
| Biomimetics as antifungals | Target of action | *Antifungal effect |
|---|---|---|
| Formyl phloroglucinol meroterpenoids [106] | Reducing of hyphae elongation and filamentation due to blocking of potential outflow of fungal substrates | MIC50 (mg/L): C. albicans (8.7), C. glabrata (13.5) |
| 4-fluorophenylalanine (FPA) [107] | Incorporation in proteins and inhibition of cell growth | Twofold decrease in growth rate of Sacharomyces serevisiae by 500 mg/L FPA |
| Phe-Ala dipeptide polymer/polyoxometalate composite [89] | Deformation of conidial heads and the appearance of indistinguishable sterigmates; smooth cell walls of hyphae become completely depressed and destroyed; spores become wrinkled | MIC (mg/L): A. niger (230) |
| 2-(2-hydroxypropyl) phenol [108] | Inhibition of respiration, causing decrease in ATP concentration and metabolic activity | EC50 (μg/mL): Rhizoctonia cerealis (1.0); Pythium aphanidermatum (20.3); V. mali (14.9); Botrytis cinerea (23.5) |
| 2- deoxyglucose [109; 110] | Violation of glycolysis and ATP biosynthesis | > 2 times increase in the doubling time of S. cerevisiae cells |
| L-pyroglutamic acid 4-chiral hydroxyl sulfonyl ester derivatives [17] | Inhibition of biosynthesis of trichothecenes | 61.6% inhibition of Fusarium graminearum growth by 100 mg/L |
| Fraxinellone [111] | Changes in lipopolysaccharide-induced DNA binding activity and reduced translation | EC50 (mg/L): Alternaria longipes (64.2); Curvularia lunata (123.3) |
| 1-amino-1-(4-imidazole)methylphosphonic acid [112] | Inhibition of various enzymes, especially proteases | MIC (mg/L): Rhodotorula mucilaginosa (1024), A. niger (5000) |
| {[(2-hydroxy-4-nitrophenyl) amino] (thiophen-3-yl) methyl} phosphonic acid (5N3TPA) and {[(2-hydroxy-4-methylphenyl) amino] (thiophen-3-yl) methyl} phosphonic acid (5M3TPA) [113] | Inhibition of fungal enzymes | 78.42% and 50% inhibition of Fusarium oxysporum and Alternaria alternata growth by 100 mg/L of 5N3TPA and 5M3TPA, respectively |
4. Combination of antifungal peptides with each other and/or with antifungal drugs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal infections in humans: the silent crisis. Microb. Cell 2020, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Bosco, S.D.M.; De Hoog; S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensn, H.E.; Martel, A.; Mignon, B.; Pasmans, F.; Pieckova, E.; Rodrigues, A.S.; Sigh, K.; Vicente, V.A.; Wibbelt, G.; Wiederhold, N.P.; Guillot, J. Fungal infections in animals: a patchwork of different situations. Med. Mycol. 2018, 56, S165–S187. [CrossRef]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi. Microbiol. Spectrum 2017, 5, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms, 2020, 8, 1930. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z. Mycotoxins in food, recent development in food analysis and future challenges; a review. Curr. Opin. Food Sci. 2021, 42, 237–247. [Google Scholar] [CrossRef]
- Jenks, J.D.; Cornely, O.A.; Chen, S.C.A.; Thompson III, G.R.; Hoenigl, M. Breakthrough invasive fungal infections: Who is at risk? Mycoses 2020, 63, 1021–1032. [Google Scholar] [CrossRef]
- Gintjee, T.J.; Donnelley, M.A.; Thompson, G.R. Aspiring antifungals: review of current antifungal pipeline developments. J. Fungi 2020, 6, 28. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E. M.; El-Maradny, Y.A.; El-Fakharany, E.M. A comprehensive insight into fungal enzymes: Structure, classification, and their role in mankind’s challenges. J. Fungi 2021, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.W.F.; Barato, M.B.; Nobre, F.S.; Polezel, D.A. , de Oliveira, T.S.; dos Santos, J.A.; Rodrigues, A.; Sette, L.D. Production of cold-adapted enzymes by filamentous fungi from King George Island, Antarctica. Polar Biol. 2018, 41, 2511–2521. [Google Scholar] [CrossRef]
- Geddes-McAlister, J.; Shapiro, R.S. New pathogens, new tricks: emerging, drug-resistant fungal pathogens and future prospects for antifungal therapeutics. Ann. N. Y. Acad. Sci. 2019, 1435, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ding, H.; Ke, W.; Wang, L. Quorum sensing in fungal species. Annu. Rev. Microbiol. 2021, 75, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal peptides as therapeutic agents. Front. Cell. Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef]
- Gao, K.; Qin, Y.; Wang, L.; Li, X.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, P. Design, synthesis, and antifungal activities of hymexazol glycosides based on a biomimetic strategy. J. Agric. Food. Chem. 2022, 70, 9520–9535. [Google Scholar] [CrossRef]
- Linklater, D.P.; Le, P.H.; Aburto-Medina, A.; Crawford, R.J.; Maclaughlin, S.; Juodkazis, S.; Ivanova, E.P. Biomimetic nanopillar silicon surfaces rupture fungal spores. Int. J. Mol. Sci. 2023, 24, 1298. [Google Scholar] [CrossRef]
- Ai, L.; Fu, S.; Li, Y.; Zuo, M.; Huang, W.; Huang, J.; Jin, Z.; Chen, Y. Natural products-based: synthesis and antifungal activity evaluation of novel L-pyroglutamic acid analogues. Front. Plant Sci. 2022, 13, 1102411. [Google Scholar] [CrossRef]
- Sharma, L.; Bisht, G.S. Synergistic effects of short peptides and antibiotics against bacterial and fungal strains. J. Pept. Sci. 2023, 29, e3446. [Google Scholar] [CrossRef]
- Aaghaz, S.; Sharma, K.; Maurya, I. K.; Rudramurthy, S.M.; Singh, S.; Kumar, V.; Tikoo, K.; Jain, R. Anticryptococcal activity and mechanistic studies of short amphipathic peptides. Arch. Pharm. 2023, 356, 2200576. [Google Scholar] [CrossRef]
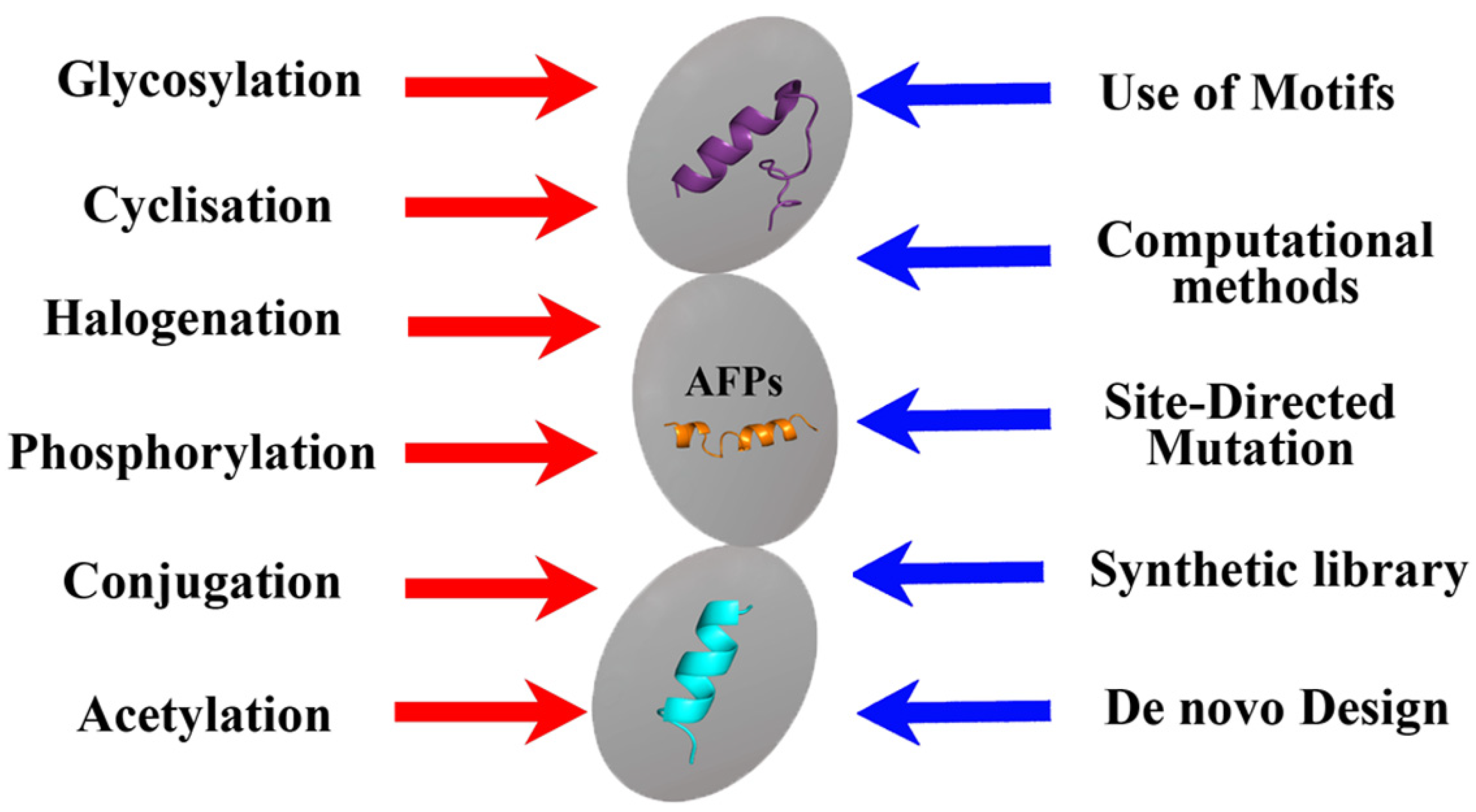
- Konakbayeva, D.; Karlsson. A.J. Strategies and opportunities for engineering antifungal peptides for therapeutic applications. Curr Opin Biotechnol. 2023, 81, 102926. [Google Scholar] [CrossRef]
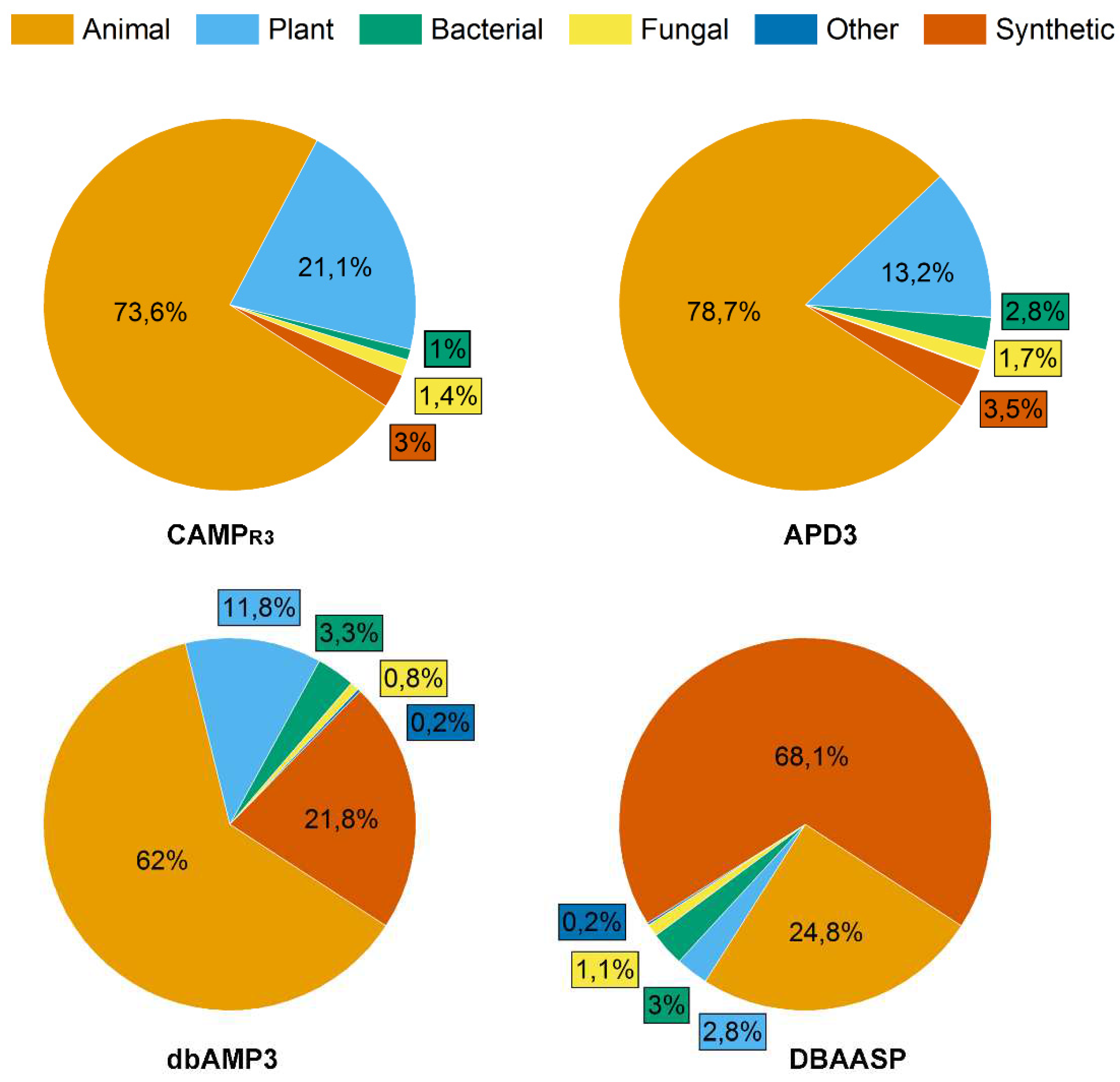
- Ramazi, S. , Mohammadi, N.; Allahverdi, A.; Khalili, E.; Abdolmaleki, P. A review on antimicrobial peptides databases and the computational tools. Database 2022, 2022, baac011. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Waghu, F.H.; Barai, R.S.; Gurung, P.; Idicula-Thomas, S. CAMPR3: a database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res. 2016, 44, D1094–D1097. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res. 2021, 49, D288–D297. [Google Scholar] [CrossRef] [PubMed]
- Jhong, J.H.; Yao, L.; Pang, Y.; Li, Z.; Chung, C.R.; Wang, R.; Li, S.; Li, W.; Luo, M.; Ma, R.; et al. dbAMP 2.0: Updated resource for antimicrobial peptides with an enhanced scanning method for genomic and proteomic data. Nucleic Acids Res. 2022, 50, D460–D470. [Google Scholar] [CrossRef]
- Bentz, M.L.; Nunnally, N.; Lockhart, S.R.; Sexton, D.J.; Berkow, E.L. Antifungal activity of nikkomycin Z against Candida auris. J. Antimicrob. Chemother. 2021, 76, 1495–1497. [Google Scholar] [CrossRef]
- Park, Y.; Lee, D.G.; Hahm, K.S. HP (2–9)-magainin 2 (1–12), a synthetic hybrid peptide, exerts its antifungal effect on Candida albicans by damaging the plasma membrane. J. Pept. Sci. 2004, 10, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Alan, A.R. Sensitivity of bacterial and fungal plant pathogens to the lytic peptides, MSI-99, magainin II, and cecropin B. Mol. Plant-Microbe Interact. 2002, 15, 701–708. [Google Scholar] [CrossRef]
- Kočendová, J.; Vaňková, E.; Volejníková, A.; Nešuta, O.; Buděšínský, M.; Socha, O.; Hájek, M.; Hadravová, R.; Čeřovský, V. Antifungal activity of analogues of antimicrobial peptides isolated from bee venoms against vulvovaginal Candida spp. FEMS Yeast Res. 2019, 19, foz013. [Google Scholar] [CrossRef]
- Jang, W.S.; Kim, K.N.; Lee, Y.S.; Nam, M.H.; Lee, I.H. Halocidin: a new antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. FEBS Lett. 2002, 521, 81–86. [Google Scholar] [CrossRef]
- Yousfi, H.; Ranque, S.; Rolain, J.M.; Bittar, F. In vitro polymyxin activity against clinical multidrug-resistant fungi. Antimicrob. Resist. Infect. Control 2019, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tonk, M.; Cabezas-Cruz, A.; Valdés, J.J.; Rego, R.O.; Grubhoffer, L.; Estrada-Peña, A.; Vilcinskas, A.; Kotsyfakis, M.; Rahnamaeian, M. Ixodes ricinus defensins attack distantly-related pathogens. Dev. Comp. Immunol. 2015, 53, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Kim, H.K.; Am Kim, S.; Park, Y.; Park, S.C.; Jang, S.H.; Hahm, K.S. Fungicidal effect of indolicidin and its interaction with phospholipid membranes. Biochem. Biophys. Res. Commun. 2003, 305, 305–310. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Carter, D.A. The antifungal activity of lactoferrin and its derived peptides: mechanisms of action and synergy with drugs against fungal pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Heymich, M.-L.; Nißl, L.; Hahn, D.; Noll, M.; Pischetsrieder, M. Antioxidative, antifungal and additive activity of the antimicrobial peptides Leg1 and Leg2 from Chickpea. Foods 2021, 10, 585. [Google Scholar] [CrossRef]
- Rather, I.A.; Sabir, J.S.M.; Asseri, A.H.; Ali, S. Antifungal activity of human cathelicidin LL-37, a membrane disrupting peptide, by triggering oxidative stress and cell cycle arrest in Candida auris. J. Fungi 2022, 8, 204. [Google Scholar] [CrossRef]
- Denardi, L.B.; Weiblen, C.; Ianiski, L.B.; Stibbe, P.C.; Santurio, J.M. Activity of MSI-78, h-Lf1-11 and cecropin B antimicrobial peptides alone and in combination with voriconazole and amphotericin B against clinical isolates of Fusarium solani. J. Med. Mycol. 2021, 31, 101119. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, C.D.T.; de Souza Lopes, J.L.; Beltramini, L.M.; de Oliveira, R.S.B.; Oliveira, J.T.A.; Ramos, M.V. Osmotin from Calotropis procera latex: new insights into structure and antifungal properties. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2501–2507. [Google Scholar] [CrossRef]
- Landon, C.; Meudal, H.; Boulanger, N.; Bulet, P.; Vovelle, F. Solution structures of stomoxyn and spinigerin, two insect antimicrobial peptides with an α-helical conformation. Biopolymers 2006, 81, 92–103. [Google Scholar] [CrossRef]
- Kakar, A.; Holzknecht, J.; Dubrac, S.; Gelmi, M.L.; Romanelli, A.; Marx, F. New perspectives in the antimicrobial activity of the amphibian temporin B: peptide analogs are effective inhibitors of Candida albicans growth. J. Fungi 2021, 7, 457. [Google Scholar] [CrossRef]
- D’Auria, F.D.; Casciaro, B.; De Angelis, M.; Marcocci, M.E.; Palamara, A.T.; Nencioni, L.; Mangoni, M.L. Antifungal activity of the frog skin peptide temporin G and its effect on Candida albicans virulence factors. Int. J. Mol. Sci. 2022, 23, 6345. [Google Scholar] [CrossRef]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on current status of echinocandins use. Antibiotics 2020, 9, 227. [Google Scholar] [CrossRef]
- Antachopoulos, C.; Meletiadis, J.; Sein, T.; Roilides, E.; Walsh, T.J. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrob. Agents Chemother. 2008, 52, 321–328. [Google Scholar] [CrossRef]
- Kovács, R.; Tóth, Z.; Locke, J.B.; Forgács, L.; Kardos, G.; Nagy, F.; Borman, A.M.; Majoros, L. Comparison of in vitro killing activity of rezafungin, anidulafungin, caspofungin, and micafungin against four Candida auris clades in RPMI-1640 in the absence and presence of human serum. Microorganisms 2021, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, T.F.; de Castro, P.A.; Bastos, R.W.; Pinzan, C.F.; Souza, P.F.; Ackloo, S.; Hossain, M.A.; Drewry, D.H.; Alkhazraji, S.; Ibrahim, A.S.; Jo, H.; Lightfoot, J.D.; Adams, E.M.; Fuller, K.K.; degrade, W.F.; Goldman, G. H. (2023). A host defense peptide mimetic, brilacidin, potentiates caspofungin antifungal activity against human pathogenic fungi. Nat. Commun. 2023, 14, 2052. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, T.K.B.; Neto, N.A.S.; Freitas, C.D.T.; Silva, A.F.B.; Bezerra, L.P.; Malveira, E.A.; Branco, L.A.C.; Mesquita, F.P.; Goldman, G.H.; Alencar, L.M.R.; Oliveira, J.T.A.; Santos-Oliveira, R.; Souza, P.F.N. Antifungal potential of synthetic peptides against Cryptococcus neoformans: mechanism of action studies reveal synthetic peptides induce membrane–pore formation, DNA degradation, and apoptosis. Pharmaceutics 2022, 14, 1678. [Google Scholar] [CrossRef]
- Dias, L.P.; Souza, P.F.; Oliveira, J.T.; Vasconcelos, I.M.; Araújo, N.M.; Tilburg, M.F.; Guedes, M.I.F.; Carneiro, R.F. , Lopes, J.L.S.; Sousa, D.O. RcAlb-PepII, a synthetic small peptide bioinspired in the 2S albumin from the seed cake of Ricinus communis, is a potent antimicrobial agent against Klebsiella pneumoniae and Candida parapsilosis. Biochim. Biophys. Acta Biomembr, 2020, 1862, 183092. [Google Scholar] [CrossRef] [PubMed]
- Kodedová, M.; Sychrová, H. Synthetic antimicrobial peptides of the halictines family disturb the membrane integrity of Candida cells. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Kim, H.K.; Lee, K.Y.; Am Kim, S.; Han, Y.S.; Lee, I.H. Antifungal activity of synthetic peptide derived from halocidin, antimicrobial peptide from the tunicate, Halocynthia aurantium. FEBS Letters 2006, 580, 1490–1496. [Google Scholar] [CrossRef]
- Souza, P.F.; Marques, L.S.; Oliveira, J.T.; Lima, P.G.; Dias, L.P.; Neto, N.A.; Lopes, F.E.S.; Sousa, J.S.; Silva, A.F.B.; Caneiro, R.F.; Lopes, J.L.S.; Ramos, M.V. , Freitas, C. D. Synthetic antimicrobial peptides: from choice of the best sequences to action mechanisms. Biochimie 2020, 175, 132–145. [Google Scholar] [CrossRef]
- Lima, P.G.; Freitas, C.D.; Oliveira, J.T.; Neto, N.A.; Amaral, J.L.; Silva, A.F.; Sousa, J.S.; Franco, O.L.; Souza, P.F.N. Synthetic antimicrobial peptides control Penicillium digitatum infection in orange fruits. Food Res. Int. 2021, 147, 110582. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.S.; Sashidhar, R.B. Antiaflatoxigenic effects of selected antifungal peptides. Peptides 2019, 115, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.M.; Raj, N.; Sashidhar, R.B. Efficacy of short-synthetic antifungal peptides on pathogenic Aspergillus flavus. Pestic. Biochem. Physiol. 2021, 174, 104810. [Google Scholar] [CrossRef]
- Thery, T.; Shwaiki, L.N.; O'Callaghan, Y.C.; O'Brien, N.M.; Arendt, E.K. (2019). Antifungal activity of a de novo synthetic peptide and derivatives against fungal food contaminants. J. Pept. Sci. 2019, 25, e3137. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; López-García, B.; Marcos, J.F. Studies on the mode of action of the antifungal hexapeptide PAF26. Antimicrob. Agents Chemother. 2006, 50, 3847–3855. [Google Scholar] [CrossRef] [PubMed]
- Nikapitiya, C.; Dananjaya, S.H.S.; Chandrarathna, H.P.S.U.; De Zoysa, M.; Whang, I. Octominin: A novel synthetic anticandidal peptide derived from defense protein of Octopus minor. Mar. Drugs 2020, 18, 56. [Google Scholar] [CrossRef]
- Lima, P.G.; Souza, P.F.N.; Freitas, C.D.T.; Oliveira, J.T.A.; Dias, L.P.; Neto, J.X.S.; Vasconcelos, I.M.; Lopes, J.L.S.; Sousa, D.O.B. Anticandidal activity of synthetic peptides: mechanism of action revealed by scanning electron and fluorescence microscopies and synergism effect with nystatin. J. Pept. Sci. 2020, 26, e3249. [Google Scholar] [CrossRef]
- Falcao, L.L.; Silva-Werneck, J.O.; Ramos, A.D.R.; Martins, N.F.; Bresso, E.; Rodrigues, M.A.; Bemquerer, M.P.; Marcellino, L.H. Antimicrobial properties of two novel peptides derived from Theobroma cacao osmotin. Peptides 2016, 79, 75–82. [Google Scholar] [CrossRef]
- Martínez-Culebras, P.V.; Gandía, M.; Garrigues, S.; Marcos, J.F.; Manzanares, P. Antifungal peptides and proteins to control toxigenic fungi and mycotoxin biosynthesis. Int. J. Mol. Sci. 2021, 22, 13261. [Google Scholar] [CrossRef]
- Struyfs, C.; Cammue. B.P.A.; Thevissen, K. Membrane-interacting antifungal peptides. Front Cell Dev Biol. 2021, 9, 649875. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, design, application and research progress in multiple fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Andrés, M.T.; Acosta-Zaldívar, M.; Fierro, J.F. Antifungal mechanism of action of lactoferrin: identification of H+-ATPase (P3A-type) as a new apoptotic-cell membrane receptor. Antimicrob. Agents Chemother. 2016, 60, 4206–4216. [Google Scholar] [CrossRef] [PubMed]
- Gruden, Š.; Poklar Ulrih, N. Diverse mechanisms of antimicrobial activities of lactoferrins, lactoferricins, and other lactoferrin-derived peptides. Int. J. Mol. Sci., 2021, 22, 11264. [Google Scholar] [CrossRef] [PubMed]
- Tóth, L.; Váradi, G.; Boros, É.; Borics, A.; Ficze, H.; Nagy, I.; Tóth, G.K.; Rákhely, G.; Marx, F.; Galgóczy, L. Biofungicidal potential of Neosartorya (Aspergillus) fischeri antifungal protein NFAP and novel synthetic - core peptides. Front. Microbiol. 2020, 11, 820. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Souza, P.F.; Vasconcelos, I.M.; Dias, L.P.; Martins, T.F.; Van Tilburg, M.F.; Guedes, M.I.F.; Sousa, D.O. Mo-CBP3-PepI, Mo-CBP3-PepII, and Mo-CBP3-PepIII are synthetic antimicrobial peptides active against human pathogens by stimulating ROS generation and increasing plasma membrane permeability. Biochimie 2019, 157, 10–21. [Google Scholar] [CrossRef]
- Bezerra, L.P.; Freitas, C.D.T.; Silva, A.F.B.; Amaral, J.L.; Neto, N.A.S.; Silva, R.G.G.; Parra, A.L.C.; Goldman, G.H.; Oliveira, J.T.A.; Mesquita, F.P.; et al. Synergistic antifungal activity of synthetic peptides and antifungal drugs against Candida albicans and C. parapsilosis biofilms. Antibiotics 2022, 11, 553. [Google Scholar] [CrossRef]
- Cardoso, M.H.; Orozco, R.Q.; Rezende, S.B.; Rodrigues, G. , Oshiro, K.G.N.; Cândido, E.S., Franco, O.L. (2020) Computer-aided design of antimicrobial peptides: Are we generating effective drug candidates? Front. Microbiol. 2020, 10, 3097. [Google Scholar] [CrossRef]
- Li, R.; Wu, J.; He, F.; Xu, Q.; Yin, K.; Li, S.; Li, W.; Wei, A.; Zhang, L.; Zhang, X.-H.; Zhang, B. Rational design, synthesis, antifungal evaluation and docking studies of antifungal peptide CGA-N12 analogues based on the target CtKRE9. Bioorg. Chem. 2023, 132, 106355. [Google Scholar] [CrossRef]
- Sharma, K.; Aaghaz, S.; Maurya, I.K.; Rudramurthy, S.M.; Singh, S.; Kumar, V.; Tikoo, K.; Jain, R. Antifungal evaluation and mechanistic investigations of membrane active short synthetic peptides-based amphiphiles. Bioorg. Chem. 2022, 127, 106002. [Google Scholar] [CrossRef]
- Thery, T.; O'Callaghan, Y.; O'Brien, N.; Arendt, E.K. Optimisation of the antifungal potency of the amidated peptide H-Orn-Orn-Trp-Trp-NH2 against food contaminants. Int. J. Food Microbiol. 2018, 265, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Datta, L.P.; Mukherjee, T.; Das, T.K. Biomimetic pH responsive amphiphilic polymers: Solution property dependent antifungal mechanism. React. Funct. Polym. 2021, 165, 104937. [Google Scholar] [CrossRef]
- Ramamourthy, G.; Park, J.; Seo, C.J.; Vogel, H.; Park, Y. Antifungal and antibiofilm activities and the mechanism of action of repeating lysine-tryptophan peptides against Candida albicans. Microorganisms 2020, 8, 758. [Google Scholar] [CrossRef] [PubMed]
- Leannec-Rialland, V.; Cabezas-Cruz, A.; Atanasova, V.; Chereau, S.; Ponts, N.; Tonk, M.; .Vivinskas, A. , Ferrer, N., Valdes, J.J.; Richard-Forget, F. Tick defensin γ-core reduces Fusarium graminearum growth and abrogates mycotoxins production with high efficiency. Sci. Rep. 2021, 11, 7962. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Aslanli, A.; Lyagin, I. Advanced situation with recombinant toxins: Diversity, production and application purposes. Int. J. Mol. Sci. 2023, 24, 4630. [Google Scholar] [CrossRef] [PubMed]
- Tevyashova, A.; Efimova, S.; Alexandrov, A.; Omelchuk, O.; Ghazy, E.; Bychkova, E.; Zatonsky, G.; Grammatikova, N.; Dezhenkova, L.; Solovieva, S.; Ostroumova, O. Semisynthetic amides of amphotericin B and nystatin A1: a comparative study of in vitro activity/toxicity ratio in relation to selectivity to ergosterol membranes. Antibiotics 2023, 12, 151. [Google Scholar] [CrossRef]
- Enache, A.C.; Cojocaru, C.; Samoila, P.; Bele, A.; Bostanaru, A.C.; Mares, M.; Harabagiu, V. Evaluation of physically and/or chemically modified chitosan hydrogels for proficient release of insoluble nystatin in simulated fluids. Gels 2022, 8, 495. [Google Scholar] [CrossRef]
- Abid, S.; Uzair, B.; Niazi, M.B.K.; Fasim, F.; Bano, S.A.; Jamil, N.; Batool, R.; Sajjad, S. Bursting the virulence traits of MDR strain of Candida albicans using sodium alginate-based microspheres containing nystatin-loaded MgO/CuO nanocomposites. Int. J. Nanomedicine 2021, 16, 1157–1174. [Google Scholar] [CrossRef]
- Nile, S.H.; Thombre, D.; Shelar, A.; Gosavi, K.; Sangshetti, J.; Zhang, W.; Sieniawska, E.; Patil, R.; Kai, G. Antifungal properties of biogenic selenium nanoparticles functionalized with nystatin for the inhibition of Candida albicans biofilm formation. Molecules 2023, 28, 1836. [CrossRef]
- El-Batal, A.I.; Nada, H.G.; El-Behery, R.R.; Gobara, M.; El-Sayyad, G.S. Nystatin-mediated bismuth oxide nano-drug synthesis using gamma rays for increasing the antimicrobial and antibiofilm activities against some pathogenic bacteria and Candida species. RSC Adv. 2020, 10, 9274–9289. [CrossRef]
- Lin, Y.; Yin, Q.; Zhuge, D.; Hu, Y.; Yang, X.; Tian, D.; Li, L.; Wang, H.; Liu, S.; Weng, C.; Zhang, X.; Wen, B.; Wang, F.; Yan, L.; Chen, M.; Wang. L.; Chen, Y. Enhanced targeting, retention, and penetration of amphotericin B Through a biomimetic strategy to treat against vulvovaginal candidiasis. Adv. Ther. 2023, 6, 2200086. [CrossRef]
- Zia, Q.; Mohammad, O.; Rauf, M.A.; Khan, W.; Zubair, S. Biomimetically engineered Amphotericin B nano-aggregates circumvent toxicity constraints and treat systemic fungal infection in experimental animals. Sci. Rep. 2017, 7, 11873. [Google Scholar] [CrossRef]
- Slezina, M.P.; Istomina, E.A.; Kulakovskaya, E.V.; Abashina, T.N.; Odintsova, T.I. Synthetic oligopeptides mimicking γ-core regions of cysteine-rich peptides of Solanum lycopersicum possess antimicrobial activity against human and plant pathogens. Curr. Issues Mol. Biol. 2021, 43, 1226–1242. [Google Scholar] [CrossRef]
- Kesharwani, P.; Fatima, M.; Singh, V.; Sheikh, A.; Almalki, W.H.; Gajbhiye, V.; Sahebkar, A. Itraconazole and difluorinated-curcumin containing chitosan nanoparticle loaded hydrogel for amelioration of onychomycosis. Biomimetics 2022, 7, 206. [Google Scholar] [CrossRef]
- Ionescu, O.M.; Iacob, A.T.; Mignon, A.; Van Vlierberghe, S.; Baican, M.; Danu, M.; Ibănescu, C.; Simionescu, N.; Profire, L. Design, preparation and in vitro characterization of biomimetic and bioactive chitosan/polyethylene oxide based nanofibers as wound dressings. Int. J. Biol. Macromol. 2021, 193, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; McDermott, G.; Wetzler, M.; Le Gros, M.A.; Myllys, M.; Knoechel, C.; Barron, A.E.; Larabell, C.A. Soft X-ray tomography of phenotypic switching and the cellular response to antifungal peptoids in Candida albicans. Proc. Natl. Acad. Sci. U.S.A., 2009, 106, 19375–19380. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; Mahmoud, G.A.E.; Sharmouk, W. A cerium-based MOFzyme with multi-enzyme-like activity for the disruption and inhibition of fungal recolonization. J. Mater. Chem. B 2020, 8, 7548–7556. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Yuan, T.; Zhu, J.; Yang, Y. Influence of the iodine content of nitrogen-and iodine-doped carbon dots as a peroxidase mimetic nanozyme exhibiting antifungal activity against C. albicans. Biochem. Eng. J. 2021, 175, 108139. [Google Scholar] [CrossRef]
- Biswas, S.; Priya Datta, L.; Kumar Das, T. Peptide core containing polymer–polyoxometalate hybrid as novel antifungal agent. J. Mol. Eng. Mater., 2022, 10, 2240004. [Google Scholar] [CrossRef]
- Ivanov, M.; Ćirić, A.; Stojković, D. Emerging antifungal targets and strategies. Int. J. Mol. Sci. 2022, 23, 2756. [Google Scholar] [CrossRef]
- Xia, Q.; Tian, H.; Li, Y.; Yu, X.; Zhang, W.; Wang, Q. Biomimetic synthesis of iridoid alkaloids as novel leads for fungicidal and insecticidal agents. J. Agric. Food Chem. 2020, 68, 12577–12584. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Su, H.; Yang, X.; Sun, T.; Lu, X.; Shi, F.; Duan, H.; Liu, X.; Ling, Y. Design, synthesis, and biological activity of novel fungicides containing a 1, 2, 3, 4-tetrahydroquinoline scaffold and acting as laccase inhibitors. J. Agric. Food Chem. 2022, 70, 1776–1787. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, R.; Chen, Y.; Yu, P.; Lan, Y.; Xu, H.; Lei, S. Design, synthesis and mechanism study of novel natural-derived isoquinoline derivatives as antifungal agents. Mol. Diverc., 2023, 27, 1011–1022. [Google Scholar] [CrossRef]
- Chaurasia, H.; Singh, V.K.; Mishra, R.; Yadav, A.K.; Ram, N.K.; Singh, P.; Singh, R.K. Molecular modelling, synthesis and antimicrobial evaluation of benzimidazole nucleoside mimetics. Bioorg. Chem. 2021, 115, 105227. [Google Scholar] [CrossRef]
- DeMarco, A.G.; Milholland, K.L.; Pendleton, A.L.; Whitney, J.J.; Zhu, P.; Wesenberg, D.T.; Nambiar, M.; Pepe, A.; Paula, S.; Chmielewski, J; Wisecaver, J.H. Conservation of Cdc14 phosphatase specificity in plant fungal pathogens: implications for antifungal development. Sci. Rep. 2020, 10, 12073. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.S.; LeBlanc, E.V.; Shekhar-Guturja, T.; Robbins, N.; Krysan, D.J.; Pizarro, J.; Whitesell, L.; Cowen, L.E.; Brown, L.E. Design and synthesis of fungal-selective resorcylate aminopyrazole Hsp90 inhibitors. J. Med. Chem. 2019, 63, 2139–2180. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Su, L.; Peng, Y.; Zhao, S.; Ye, F. Dextran-coated Gd-based ultrasmall nanoparticles as phosphatase-like nanozyme to increase ethanol yield via reduction of yeast intracellular ATP level. J. Colloid Interface Sci., 2022, 627, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, R.; Marshall, M.; Parivar, A.; Ly, V.K.; Pearlman, E.; Yee, A.F. Biomimetic nanopillared surfaces inhibit drug resistant filamentous fungal growth. ACS Appl. Bio Mater., 2019, 2, 3159–3163. [Google Scholar] [CrossRef]
- Dalefield, R. Human Pharmaceuticals. In Veterinary toxicology for Australia and New Zealand, Dalefield, R., Eds.; Elsevier: Amsterdam, Netherlands, 2017. [Google Scholar] [CrossRef]
- Millikan, L.E. Current concepts in systemic and topical therapy for superficial mycoses. Clin. Dermatol. 2010, 28, 212–216. [Google Scholar] [CrossRef]
- Parker, J.E.; Warrilow, A.G.S.; Price, C.L.; Mullins, J.G.L.; Kelly, D.E.; Kelly, S.L. Resistance to antifungals that target CYP51. J. Chem. Biol. 2014, 7, 143. [Google Scholar] [CrossRef]
- Leadbeater, A.J. Plant health management: fungicides and antibiotics. In Encyclopedia of Agriculture and Food Systems, Van Alfen, N.K., Eds; Academic Press: N-Y., USA, 2014; pp. 408–424. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Stepanov, N.; Maslova, O.; Lomakina, G.; Ugarova, N. Luminescent analysis of ATP: Modern objects and processes for sensing. Chemosensors 2022, 10, 10–493. [Google Scholar] [CrossRef]
- Batchuluun, B.; Pinkosky, S.L.; Steinberg, G.R. Lipogenesis inhibitors: therapeutic opportunities and challenges. Nat. Rev. Drug Discov., 2022, 21, 283–305. [Google Scholar] [CrossRef]
- Li, G.; Jian, T.; Liu, X.; Lv, Q.; Zhang, G.; Ling, J. Application of metabolomics in fungal research. Molecules 2022, 27, 7365. [Google Scholar] [CrossRef]
- Zhong, L.F.; Shang, Z.C.; Sun, F.J.; Zhu, P.H.; Yin, Y.; Kong, L.Y.; Yang, M.H. Anticandidal formyl phloroglucinol meroterpenoids: Biomimetic synthesis and in vitro evaluation. Bioorg. Chem., 2020, 104, 104248. [Google Scholar] [CrossRef]
- Pojitkov, A.E.; Efremenko, E.N.; Varfolomeev, S.D. Unnatural amino acids in enzymes and proteins. J. Molecul. Catal. B: Enzym. 2000, 10, 47–55. [Google Scholar] [CrossRef]
- Qu, T.; Gao, S.; Li, J.; Hao, J.J.; Ji, P. Synthesis and antifungal activity of 2-allylphenol derivatives against fungal plant pathogens. Pestic. Biochem. Physiol., 2017, 135, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Laussel, C.; Léon, S. Cellular toxicity of the metabolic inhibitor 2-deoxyglucose and associated resistance mechanisms. Biochem. Pharmacol. 2020, 182, 114213. [Google Scholar] [CrossRef] [PubMed]
- Hellemann, E.; Walker, J.L.; Lesko, M.A.; Chandrashekarappa, D.G.; Schmidt, M.C.; O’Donnell, A.F.; Durrant, J.D. Novel mutation in hexokinase 2 confers resistance to 2-deoxyglucose by altering protein dynamics. PLoS Comput. Biol. 2022, 18, e1009929. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, S.; Morken, J. Short and efficient total synthesis of fraxinellone limonoids using the stereoselective oshima−utimoto reaction. Org. Lett. 2005, 7, 5465–5468. [Google Scholar] [CrossRef]
- Serafin-Lewańczuk, M.; Brzezińska-Rodak, M.; Lubiak-Kozłowska, K.; Majewska, P.; Klimek-Ochab, M.; Olszewski, T.K.; Żymańczyk-Duda, E. Phosphonates enantiomers receiving with fungal enzymatic systems. Microb. Cell Factories 2021, 20, 81. [Google Scholar] [CrossRef]
- Tlidjane, H.; Chafai, N.; Chafaa, S.; Bensouici, C.; Benbouguerra, K. New thiophene-derived α-aminophosphonic acids: Synthesis under microwave irradiations, antioxidant and antifungal activities, DFT investigations and SARS-CoV-2 main protease inhibition. J. Mol. Struct. 2022, 1250, 131853. [Google Scholar] [CrossRef]
- Varfolomeyev, S.D.; Aliev, T.K.; Efremenko, E.N. Postgenomic chemistry: New problems and challenges. Pure Appl. Chem., 2004, 76, 1781–1798. [Google Scholar] [CrossRef]
- Maslova, O.V.; Senko, O.V.; Efremenko, E.N. Aspartic and glutamic acids polymers: preparation and applications in medicinal chemistry and pharmaceutics. Russ. Chem. Bull. 2018, 67, 614–623. [Google Scholar] [CrossRef]
- Mardirossian, M.; Rubini, M.; Adamo, M.F.A.; Scocchi, M.; Saviano, M.; Tossi, A.; Gennaro, R.; Caporale, A. Natural and synthetic halogenated amino acids—structural and bioactive features in antimicrobial peptides and peptidomimetics. Molecules 2021, 26, 7401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wolfender, J.L.; Hostettmann, K.; Xu, R.; Qin, G. Antifungal alkaloids and limonoid derivatives from Dictamnus dasycarpus. Phytochemistry 1998, 47, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, J.; Qian, Y.; Li, J.; Ji, Z. The study on fungicidal activity of Dictamnus dasycarpus. Agrochemicals 2006, 45, 739–741. [Google Scholar] [CrossRef]
- Yang, X.-J.; Dong, Q.-M.; Wang, M.-R.; Tang, J.-J. Semi-synthesis of C-ring cyclopropyl analogues of fraxinellone and their insecticidal activity against Mythimna separate walker. Molecules 2020, 25, 1109. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, S.D.; Efremenko, E.N. Organophosphorus Neurotoxins; RIOR: Moscow, Russia, 2020; p. 380. ISBN 978-5-369-02026-5. [Google Scholar] [CrossRef]
- Kirouani, I.; Hellal, A.; Haddadi, I.; Layaida, H.; Madani, A.; Madani, S.; Haroun, M.F.; Rachida, D.; Touafri, L.; Bensouici, C. Effect of the phosphonomethylene moiety on the structural, vibrational, energetic, thermodynamic and optical proprieties of ((Phenylcarbamoylmethyl-phosphonomethyl-amino)-methyl)-phosphonic acid: DFT investigation. J. Mol. Struct. 2020, 1215, 128193. [Google Scholar] [CrossRef]
- Lyagin, I.V.; Andrianova, M.S.; Efremenko, E.N. Extensive hydrolysis of phosphonates as unexpected behaviour of the known His6-organophosphorus hydrolase. Appl. Microbiol. Biotechnol. 2016, 100, 5829–5838. [Google Scholar] [CrossRef] [PubMed]
- Partida-Hanon, A.; Maestro-López, M.; Vitale, S.; Turrà, D.; Di Pietro, A.; Martínez-del-Pozo, Á.; Bruix, M. Structure of fungal α mating pheromone in membrane mimetics suggests a possible role for regulation at the water-membrane interface. Front. Microbiol., 2020, 11, 1090. [Google Scholar] [CrossRef]
- Doley, K.; Thomas, S.; Borde, M. External signal-mediated overall role of hormones/pheromones in fungi. In Fungal reproduction and growth; Sultan, S., Singh, G.K.S., Eds.; techOpen, London: United Kingdom, 2022; pp. 73–83. [Google Scholar] [CrossRef]
- Jung, S.I.; Finkel, J.S.; Solis, N.V.; Chaili, S.; Mitchell, A.P.; Yeaman, M.R.; Filler, S.G. Bcr1 functions downstream of Ssd1 to mediate antimicrobial peptide resistance in Candida albicans. Eukaryot. Cell. 2013, 12, 411–419. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers. 2018, 4, 18026. [Google Scholar] [CrossRef]
- Pimienta, D.A.; Cruz Mosquera, F.E.; Palacios Velasco, I.; Giraldo Rodas, M.; Oñate-Garzón, J.; Liscano, Y. Specific focus on antifungal peptides against azole resistant Aspergillus fumigatus: Current status, challenges, and future perspectives. J. Fungi 2023, 9, 42. [Google Scholar] [CrossRef]
- Aguiar, T.K.B.; Feitosa, R.M.; Neto, N.A.S.; Malveira, E.A.; Gomes, F.I.R.; Costa, A.C.M.; Freitas, C.D.T.; Mesquita, F.P.; Souza, P.F.N. Giving a hand: synthetic peptides boost the antifungal activity of itraconazole against Cryptococcus neoformans. Antibiotics 2023, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Tóth, L.; Poór, P.; Ördög, A.; Váradi, G.; Farkas, A.; Papp, C.; Bende, G.; . Tóth, G.K.; Rákhely, G.; Marx, F.; Galgóczy, L. The combination of Neosartorya (Aspergillus) fischeri antifungal proteins with rationally designed γ-core peptide derivatives is effective for plant and crop protection. BioControl 2022, 67, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Shen, P.; Xie, Z.; Wang, L.; Dang, X. In vitro and in vivo antifungal activity of two peptides with the same composition and different distribution. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2022, 252, 109243. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Bie, Xm.; Lv, Fx., Zhao; Lu, Zx. Antifungal activity and mechanism of fengycin in the presence and absence of commercial surfactin against Rhizopus stolonifer. J Microbiol. 2011, 49, 146–150. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento Dias, J.; de Souza Silva, C.; de Araújo, A.R.; Souza, J.M.T.; de Holanda Veloso Junior, P.H.; Cabral, W.F.; da Glória da Silva, M.; Eaton, P.; de Souza de Almeida Leite, J.R.; Nicola, A.M.; Albuquerque, P. Mechanisms of action of antimicrobial peptides ToAP2 and NDBP-5.7 against Candida albicans planktonic and biofilm cells. Sci. Rep. 2020, 10, 10327. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, E.; de Alteriis, E.; De Natale, A.; D’Alterio, A.; Siciliano, A.; Guida, M.; Lombardi, L.; Falanga, A.; Galdiero, S. , 2020. Eradication of Candida albicans persister cell biofilm by the membranotropic peptide gH625. Sci. Rep. 2020, 10, 5780. [Google Scholar] [CrossRef]
- Sharma, K.; Aaghaz, S.; Maurya, I.K.; Sharma, K.K.; Singh, S.; Rudramurthy, S.M.; Kumar, V.; Tikoo, K.; Jain, R. Synthetic amino acids-derived peptides target Cryptococcus neoformans by inducing cell membrane disruption. Bioorg. Chem. 2023, 130, 106252. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Zhu, X.; Peng, Y.; Fu, T.; Hu, C.H.; Cai, J.; Liao, G. Development of Lipo-γ-AA Peptides as potent antifungal agents. J. Med. Chem. 2022, 65, 8029–8039. [Google Scholar] [CrossRef]
- Darwish, R.M; Salama, A.H. A pilot study on ultrashort peptide with fluconazole: A promising novel anticandidal combination. Vet. World 2023, 16, 1284–1288. [Google Scholar] [CrossRef]
- Ptaszyńska, N.; Gucwa, K.; Olkiewicz, K.; Łȩgowska, A.; Okońska, J.; Ruczyński, J.; Gitlin-Domagalska, A.; Dȩbowski, D.; Milewski, S.; Rolka, K. Antibiotic-based conjugates containing antimicrobial HLopt2 peptide: design, synthesis, antimicrobial and cytotoxic activities. ACS Chem. Biol. 2019, 14, 2233–2242. [Google Scholar] [CrossRef]
- Rodríguez-López, A.D.L.; Lee, M.R.; Wang, N.B.; Dunn, K.K.; Sanchez, H.; Raman, N.; Andes, D.R.; Lynn, D.M.; Palecek, S.P. Small-molecule morphogenesis modulators enhance the ability of 14-helical β-peptides to prevent Candida albicans biofilm formation. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.E.; Payne, R.J.; Carter, D.A. Lactoferrin-derived peptide lactofungin is potently synergistic with amphotericin B. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Ptaszyńska, N.; Gucwa, K.; Olkiewicz, K.; Heldt, M.; Serocki, M.; Stupak, A.; Martynow, D.; Dębowski, D.; Gitlin-Domagalska, A.; Lica, J.; Łęgowska, A.; Sławomir Milewski, S.; Rolka, K. Conjugates of ciprofloxacin and levofloxacin with cell-penetrating peptide exhibit antifungal activity and mammalian cytotoxicity. Int. J. Mol. Sci. 2020, 21, 4696. [Google Scholar] [CrossRef] [PubMed]
- Lyagin, I.; Aslanli, A.; Domnin, M.; Stepanov, N.; Senko, O.; Maslova, O.; Efremenko, E. Metal nanomaterials and hydrolytic enzyme-based formulations for improved antifungal activity. Int. J. Mol. Sci. 2023, 24, 11359. [Google Scholar] [CrossRef]
- Efremenko, E.; Stepanov, N.; Aslanli, A.; Lyagin, I.; Senko, O.; Maslova, O. Combination of enzymes with materials to give them antimicrobial features: Modern trends and perspectives. J. Funct. Biomater. 2023, 14, 64. [Google Scholar] [CrossRef]
- Aslanli, A.; Domnin, M.; Stepanov, N.; Efremenko, E. “Universal” Antimicrobial combination of bacitracin and His6-OPH with lactonase activity, acting against various bacterial and yeast cells. Int. J. Mol. Sci. 2022, 23, 9400. [Google Scholar] [CrossRef]
- Aslanli, A.; Domnin, M.; Stepanov, N.; Efremenko, E. Synergistic antimicrobial action of lactoferrin-derived peptides and quorum quenching enzymes. Int. J. Mol. Sci. 2023, 24, 3566. [Google Scholar] [CrossRef]
- Bezerra, L.P.; Silva, A.F.; Santos-Oliveira, R.; Alencar, L.M.; Amaral, J.L.; Neto, N.A.; Silva, R.G.; Belém, M.O.; de Andrade, C.R.; Oliveira, J.T.; Freitas, C.D. Combined antibiofilm activity of synthetic peptides and antifungal drugs against Candida spp. Future Microbiol. 2022, 17, 1133–1146. [Google Scholar] [CrossRef]
- Khabbaz, H.; Karimi-Jafari, M.H.; Saboury, A.A.; BabaAli, B. Prediction of antimicrobial peptides toxicity based on their physico-chemical properties using machine learning techniques. BMC Bioinform. 2021, 22, 1–11. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Joakim Hakansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef]
- Hong, K.; Wang, L.; Johnpaul, A.; Song, Y.; Guo, L.; Xie, X.; Lv, C. ; Ma, C, Response of Saccharomyces cerevisiae var. diastaticus to nerol: Evaluation of antifungal potential by inhibitory effect and proteome analyses, Food Chem. 2023, 403, 134323. [Google Scholar] [CrossRef]
| Components of combined antifungals | Target [Reference] | Antifungal effect | |
|---|---|---|---|
| AFP as the 1st component | 2nd component | ||
| Mo-CBP3-PepI | Nystatin | C. albicans [57] | 0.13 a |
| Mo-CBP3-PepII | |||
| Mo-CBP3-PepIII | C. parapsilosis [67] | 82 % b | |
| Itraconazole | 96 % b | ||
| RcAlb-PepIII | C. neoformans [128] | 84.1 % b | |
| MSI-78 | Voriconazole | Fusarium solani [37] | 0.34 a |
| hLf(1-11) | 0.21 a | ||
| Cecropin B | 0.17 a | ||
| MSI-78 | Amphotericin B | 0.37 a | |
| hLf(1-11) | 0.31 a | ||
| Cecropin B | 0.28 a | ||
| Brilacidin (non-peptide mimetic of host defense peptides) | Caspofungin |
Aspergillus fumigatus [45] |
0.39 a |
| Voriconazole | 1.0 a | ||
| Geldanamycin | 0.64 a | ||
| Neosartorya fischeri AFPs (NFAP) | NFAP2 | Botrytis cinerea, Cladosporium herbarum [129] | 1.25 a |
| NFAP | γNFAP-opt | 0.28 - 1.50 a | |
| NFAP2 | γNFAP-opt | 0.31-1.5 a | |
| P256 and P256 | Amphotericin B | C. albicans [130] | 0.28 a |
| Fengycin | Surfactin | Rhizopus solonifer [131] | 5 a |
| ToAP2 | NDBP-5.7 | C. albicans [132] | 0.75 a |
| ToAP2 | Amphotericin B | 0.18 a | |
| NDBP-5.7 | 0.18 a | ||
| gH625M | C. albicans; [133] | 0.5-0.8 a | |
| L-His(2-adamantyl)-L-Trp-L-His(2-phenyl)-OMe | C. neoformans [19] | 0.28 a | |
| l-Trp-l-His(1-biphenyl)-NHBzl | C. neoformans [134] | 0.28 a | |
| Fluconazole | 1.04 a | ||
| l-His[1-(4-n-butylphenyl)]-l-Trp-l-His[1-(4-n-butylphenyl)]-NHBzl | Amphotericin B | 0.31 a | |
| Fluconazole | 0.75 a | ||
| DP-23 peptoid | A. flavus, A. niger [18] | 0.16-0.38 a | |
| SPO peptoid | |||
| γ-AA peptide MW5 | C. albicans [135] | ≤0.5 a | |
| ToAP2 | C. albicans [132] | 0.5 a | |
| NDBP-5.7 | 0.56 a | ||
| KW-23 | C. albicans [136] | 0.37-0.60 a | |
| HLopt2 (mimic of human lactoferrin) | Candida spp. [137] | 2-125 mg/L c | |
| gH625M | C. albicans [133] | 0.30 a | |
| Flucytosine | 0.20 a | ||
| 14-helical β-peptide | Isoamyl alcohol | C. albicans [138] | 4 mg/L d |
| Lactofungin | Amphotericin B |
C. albicans, C. glabrata, C. neoformans, C. deuterogattii [139] |
0.16-0.28 a |
| TP10-NH2 (analog of transporan 10) | Ciprofloxacin or Levofloxacin | Candida spp. [140] | 6.3-100 μM c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





