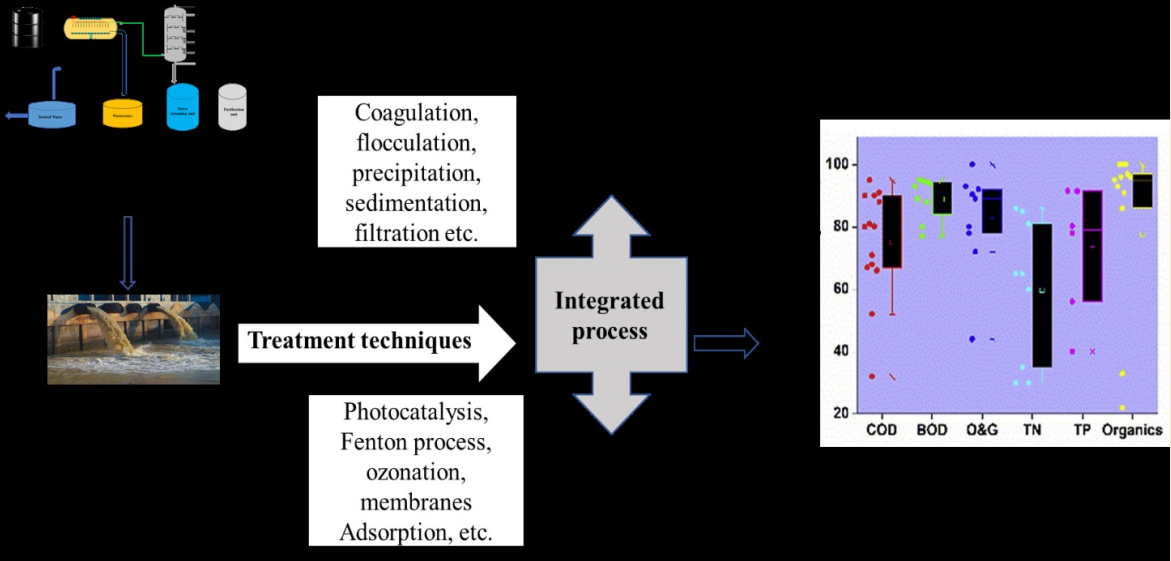
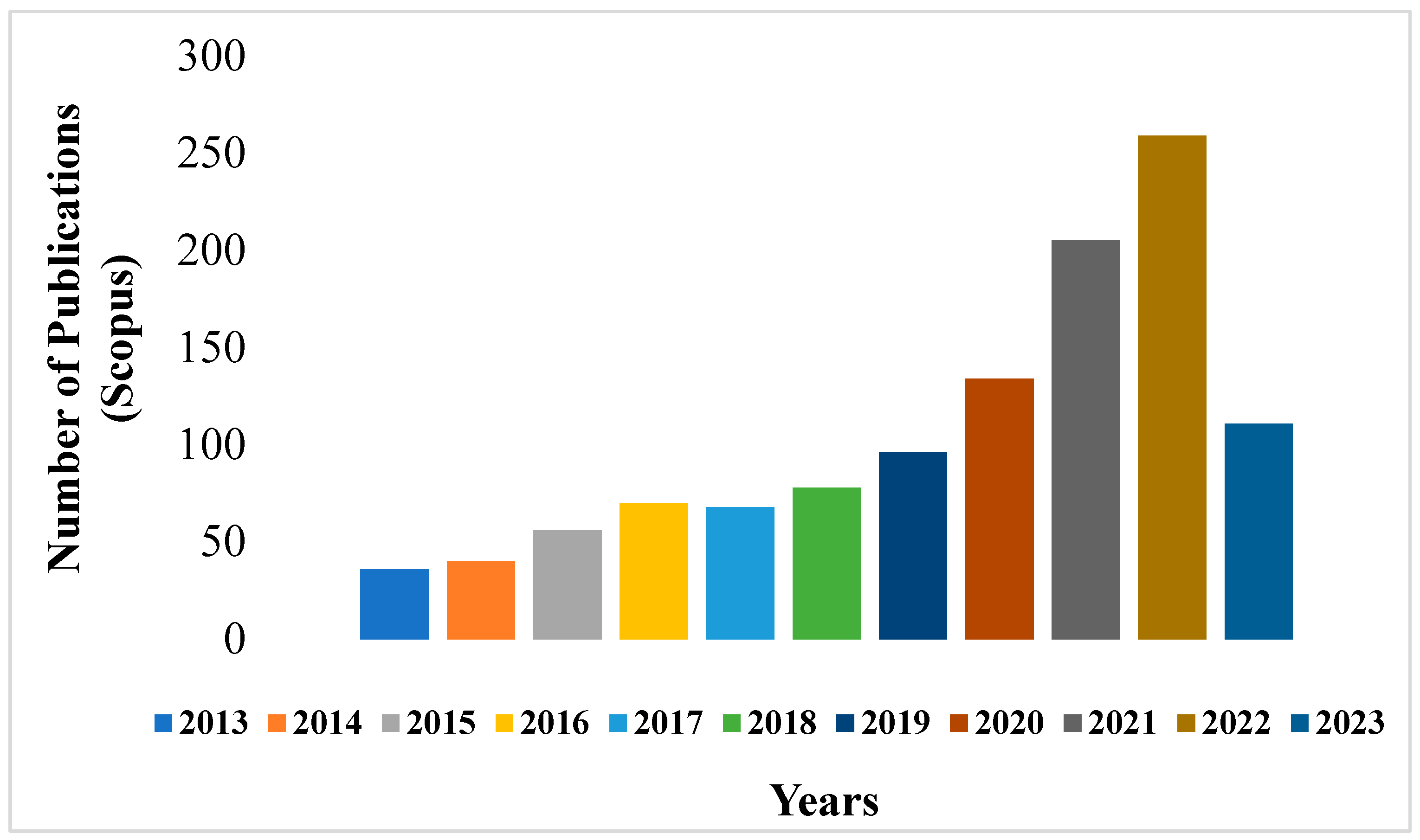1. Introduction
Water is one of the most valuable natural resources of the world and alongside air and soil, they support our environmental ecosystem. It is a vital resource for a variety of human activities from domestic to industrial applications and also provides the living environment for marine biodiversity. Despite this integral support, global industrialization and advancement in technology are associated with the generation of different types of wastewater into our environmental ecosystems. On another hand, the demand for petroleum resources has continued to rise in many parts of the world as a means to increase the economy. This process of petroleum production and refining is also associated with the generation of large volumes of wastewater which contains hazardous pollutants that some of which are highly toxic even at low concentrations (Salem and Thiemann 2022; Al-Khalid and El-Naas 2018). The impact of the environmental pollution caused by petroleum refinery wastewater (PRWW) is widely manifested in different areas including affecting aquatic life, destructing natural land for agricultural production and contaminating groundwater resources (Yu et al., 2017). As a result of this, almost all countries nowadays have environmental regulating agencies and departments which are concerned with the aspect of protecting the environmental ecosystem with the United Nations Environment Programme (UNEP) as the leading environmental authority (Zinicovscaia and Cepoi 2016). But on the other hand, the treatment of petroleum refinery wastewater (PRWW) is also becoming a growing challenge in the petroleum industry due to its complex and dynamic nature. Different treatment techniques including; physical, chemical, biological, or hybrid processes have been employed for the treatment of PRWW in the literature. However, many of these processes have their distinct advantages and disadvantages in terms of efficiency, energy requirements and treatment cost. In light of this, more research work is necessary to explore the most appropriate treatment techniques that are cost-effective as well as environmentally friendly. Therefore, the review article aims to critically provide a fundamental review of the existing knowledge on the conventional, advanced as well as integrated or hybrid treatment techniques of petroleum wastewater reported in the literature and highlight some of the basic challenges or limitations of each technique as well as a discussion for the prospects.
The methodology of the review is set on the search for the findings from recent studies on the conventional and advanced as well the integrated techniques which have been applied to degrade the different pollutants from petroleum refinery wastewater. However, a few articles published from 2012–2015 were also considered as they presented crucial data. The major source of these articles was from the Scopus databases obtained using the search keywords such as “petroleum”, “refinery”, “wastewater pollutants”, “advanced techniques”, “integrated”,” hybrid” and “review”. To determine the range of the available research evidence and identify the literature gap on this theme, a search on the published literature reviews database conducted using the Scopus database for 10 years, between 2013 and May 2023 returned approximately 1,153 papers. About 259 review papers were published in 2022 alone and already 111 reviews were published in the current year 2023 (
Figure 1). The search to find the most effective treatment method in the changing phase of petroleum wastewater has significantly derived much interest leading to more publications within these years. There have been several comprehensive reviews such as (Elmobarak, et al. 2021; Mokif, et al. 2022; Yu, et 2017; Kulkarni, & Goswami, 2015; Aljuboury, et al. 2017; Jain, et al. 2020; Adetunji, & Olaniran, 2021; Kulkarni, 2016; Rahi, et al. 2021; Abbassi & Livingstone, 2018; Asaithambi, et al. 2021 and Thorat & Sonwani, 2022). However, most of these reviews do not provide a combined critical report of both the conventional and recent advanced as well as integrated techniques used for the treatment of refinery wastewater. Hence, this review attempts to fill this gap with a focus on research mostly undertaken from 2015-2023 periods. The data collection involved several steps, starting from the description of the petroleum industry and the refining process, characterization of the petroleum wastewater and determination of its composition. This is followed by a discussion of the conventional techniques and then the reported advanced and integrated treatment methods. Finally, critical conclusion notes and recommendations for future research prospects were presented.
Figure 1.
Number of published review papers on the treatment of petroleum wastewater on the Scopus database from 2013-2023.
Figure 1.
Number of published review papers on the treatment of petroleum wastewater on the Scopus database from 2013-2023.
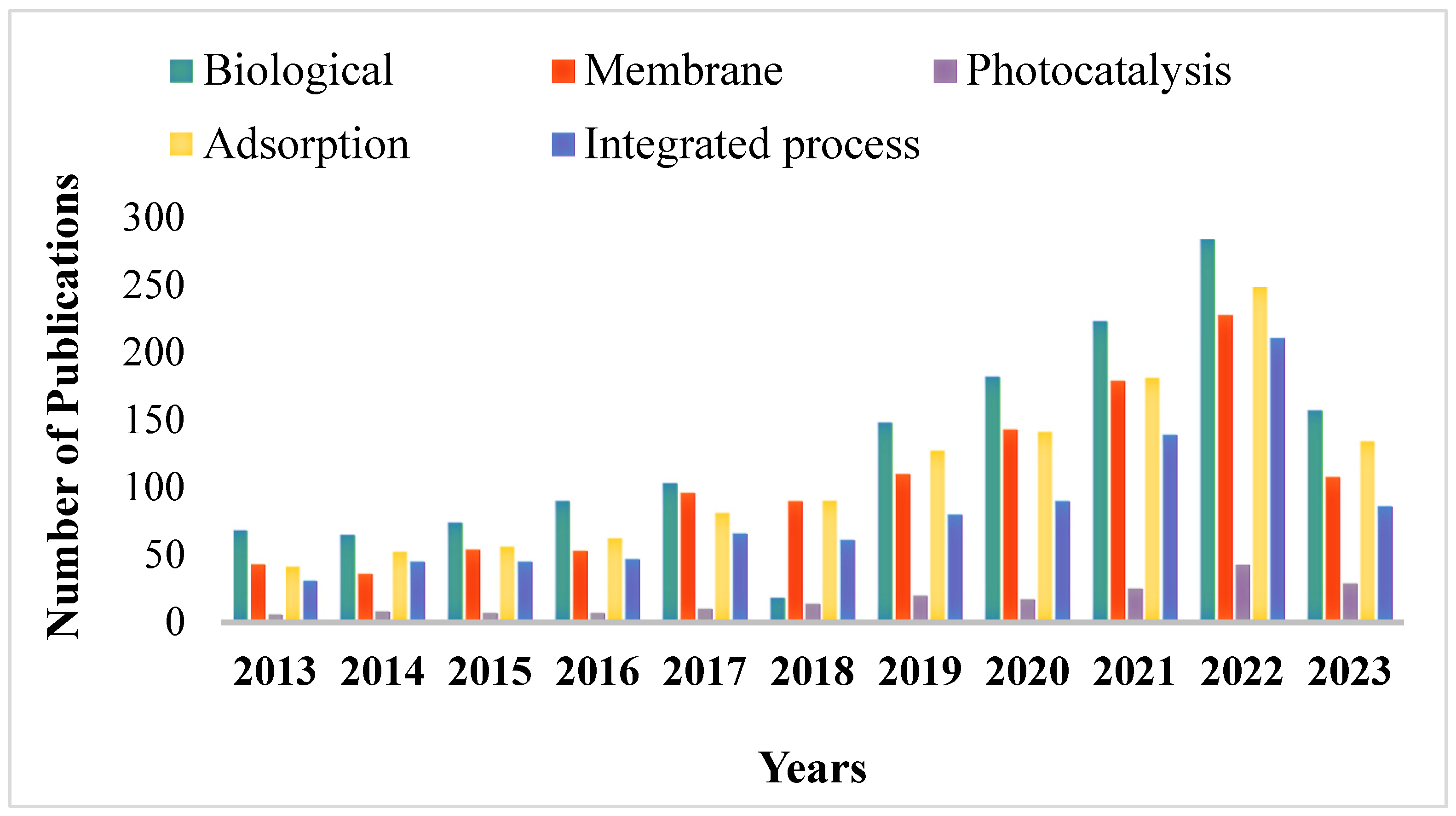
Figure 2.
Number of research articles on the treatment of petroleum wastewater based on different treatment techniques on the Scopus database from 2013-2023.
Figure 2.
Number of research articles on the treatment of petroleum wastewater based on different treatment techniques on the Scopus database from 2013-2023.
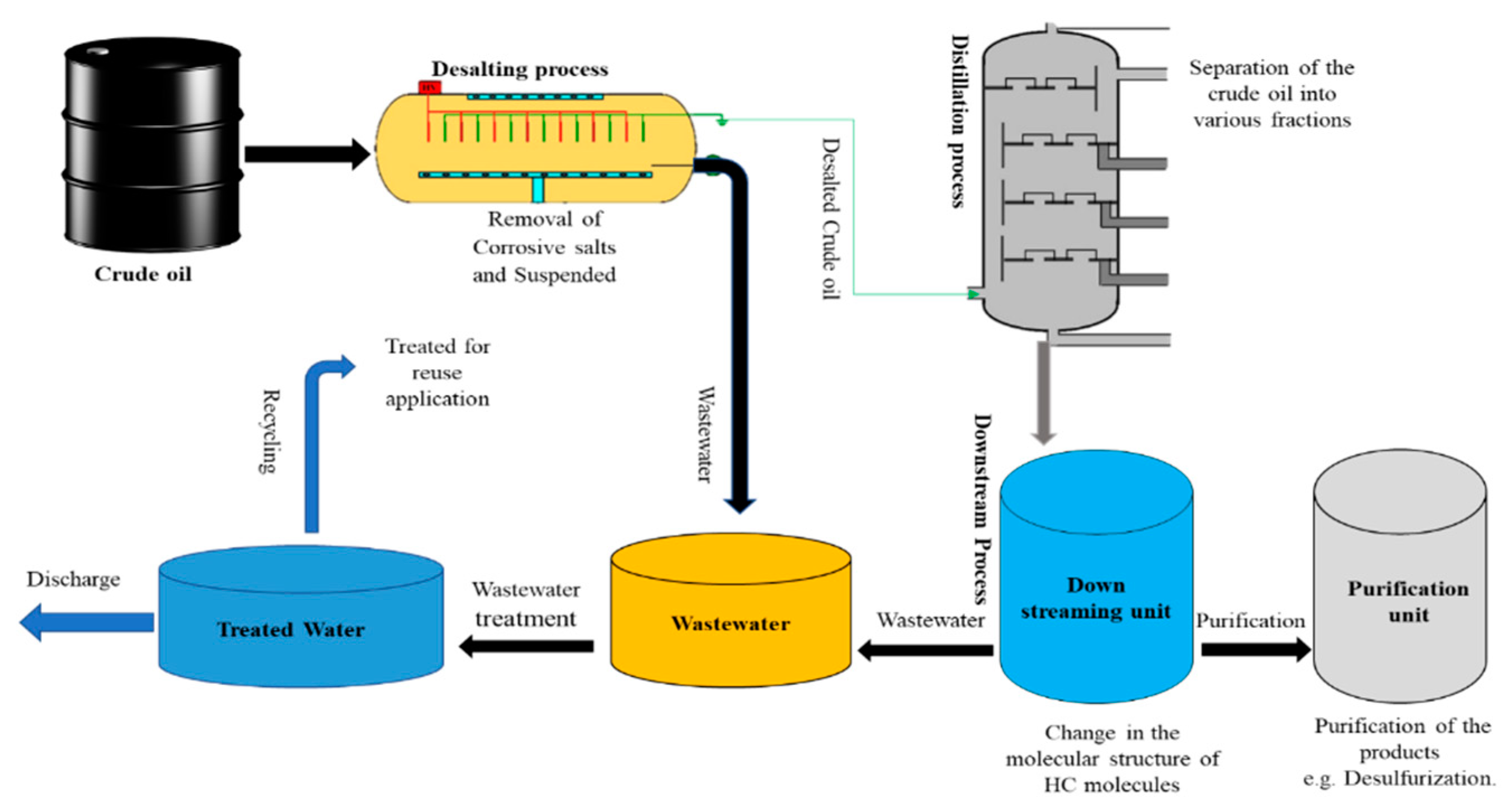
Petroleum refineries are complex industrial systems which are designed to refine crude oil after an exploration into various desired products through distillation, cracking and reforming processes. This categorised the nature of the refining process into three main categories which are separation, conversion and chemical treatment processes (Pinzón-Espinosa 2018). Meanwhile, petroleum is the term used for the unprocessed oil that comes out of the ground source rock during the drilling process and is also called crude oil. It is the fossil fuel that is naturally made from the decaying of plants and animals millions of years ago (Kalair, et al. 2021). During the distillation stage which is also the first refining process, crude oil is separated into its various fractions based on their boiling point temperature to obtain different usable products including solvents, gaseous fuels, gasoline, lubricating oils, grease, waxes and asphalts (Handogo, et al. 2022). A large amount of water is required for the various processes of refining the crude oil including distillation, desalting, hydro heating and cooling. Consequently, these processes generate wastewater which is channelled into a sewer system in most modern petroleum refineries (Rahi et. al. 2021). Meanwhile, the specific industrial operations of every refinery depend on the crude oil type and the choice of refined products. For this basic reason, almost all petroleum oil refineries are unique in their operations and hence, distinctive from one another (IPIECA, 2010). However, water is a basic requirement for the success of many of the industrial operations in every oil refinery. It is used in various applications including cooling, steam generation, crude desalting, distillation as well as hydrocarbon/chemical processing (Al Zarooni and Elshorbagy 2006; Whale et al. 2022). Hence, petroleum refineries generate a large volume of effluent which is characterized by a composition of large quantities of crude oil products, suspended solids, polyaromatic hydrocarbons (PAHs), sulphides, ammonia, phenols, metals and their derivatives (Mustapha, 2018; Diya'uddeen et al., 2011).
Figure 3.
Simplified crude oil refining process and generation of the PRWW.
Figure 3.
Simplified crude oil refining process and generation of the PRWW.
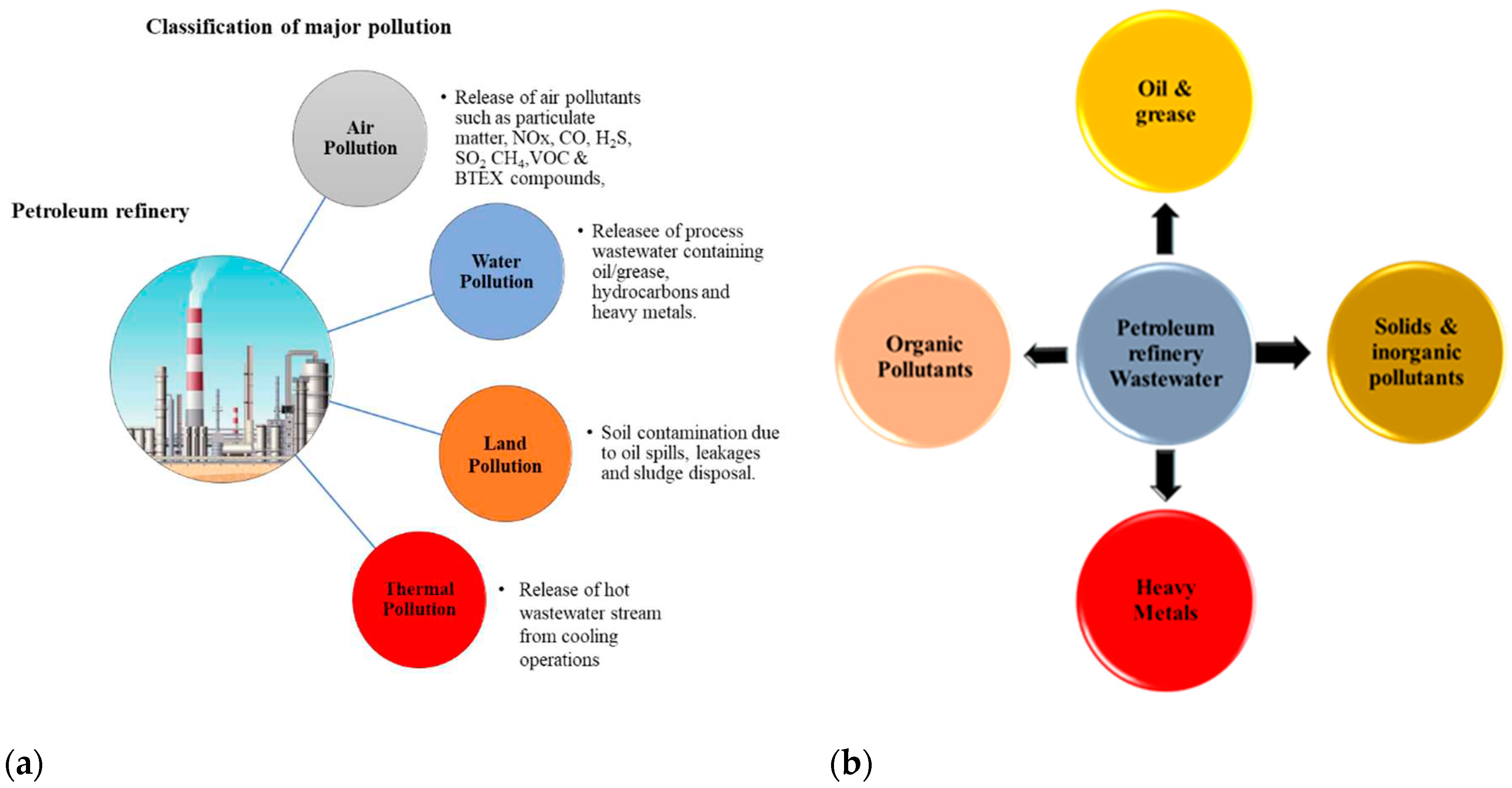
The activities of petroleum refineries usually have the potential to contribute to the contamination of our environmental ecosystem. As a gigantic refining industry, there is associated potential pollution which affects the qualities of the atmosphere, water and land where the industry makes its operations. Toxic air pollutants such as particulate matter (PM), carbon monoxide (CO), nitrogen oxides (NOx), Sulfide (SO2), hydrogen Sulfide (H2S), methane (CH4), BTEX, volatile organic compounds can be released during the petroleum refining process and constitute the major atmospheric pollutants (Polvara, et al. 2021). Soil contamination during the petroleum refining process is usually less significant compared to air and water pollution. However, post-refining practices may eventually lead to oil spills and leakages from pipelines and trucks during the distribution stage of refined petroleum products. The most significant post-refining soil contamination from the petroleum refinery industry comes from the disposal of wastewater sludge in landfills (Islam 2015). The petroleum refining industry generates a large amount of effluents that when released without appropriate treatment can lead to chronic effects on living organisms. It contains a high concentration of toxic recalcitrant substances originating from the composition of the refining crude oil and hence a serious cause of pollution to the receiving environmental ecosystem (Pal, et al. 2016). Studies have shown that the pollutants that are most responsible for living tissue toxicity are; ammonia, Sulfide, cyanide, phenols and hydrocarbons (Daflon, 2015). The contaminants are usually toxic and a major threat to the environment and human health due to their solubility and long accumulation period in living tissues and environmental substrates (Rahi, et al. (2021; Al-Khalid and El-Naas, 2018;) For example, compounds like phenols which is highly soluble in water can be detected from few concentrations up to 7000 mg/L from a PRWW composition. Furthermore, under favourable reaction conditions, phenols can undergo various reactions such as chlorination and methylation thereby producing more toxic recalcitrants including Chlorophenols and cresols (Al-Khalid and El-Naas 2018). Wang et al., (2015) also reported that Naphthenic acids (NAs) are toxic organic contaminants which can pose a serious challenge in the treatment of PRWW using biological processes. They are usually a key indicator for toxicity from the refining process of heavy crude oils accounting for about 15% of the COD concentration and a major recalcitrant for biodegradation. (Misiti, et al. 2013). Similarly, long-period of exposure to phenols and hydrocarbon compounds such as benzene, toluene, ethyl benzene and xylenes (BTEX) can cause leukaemia and tumours in multiple organs especially the lungs and vascular system infections (Ishak, et al. 2012). Marine species such as fishes and all other aquatic living organisms are also affected by petroleum aromatic hydrocarbons (PAHs) leading to their DNA and cardiac systems damage as well as oxidative stress (Pal, et al. 2016). Similarly, the general marine biodiversity can also be affected by thermal pollution as a result of the disposal of hot wastewater effluent from cooling operations which can increase the temperature of the receiving body of water. Lattanzio (2016) also reported that PRWW contaminants can also end up polluting the groundwater aquifers in cases where some petroleum refining industries adopt deep wells injection practices for the disposal of their wastewater. Subsequently, the contaminants from this wastewater infiltrate into the groundwater aquifers over time. Furthermore, PRWW also contains concentrations of different toxic heavy metals which can create cumulative and adverse effects on the biological systems of living species. Cancer, skin rashes, asthma, weight loss and among other symptoms can be observed in human health as a result of exposure to an elevated concentration of heavy metals (Barakat 2011). Meanwhile, due to the highly complex and dynamic nature of the PRWW pollutants, it is usually difficult to understand their complete chemistry and link their toxicity to the receiving environmental ecosystem (Pinzón-Espinosa and Kanda 2020). Therefore, there is a need for toxicity identification and evaluation (TIE) studies in case of pollution due to PRWW to identify the agents responsible for toxicity and evaluate an effective mitigation and management strategy (Daflon, 2025).
Figure 4.
(a) Major environmental pollution from the petroleum refining industry. (b) Major classification of PRWW contaminants.
Figure 4.
(a) Major environmental pollution from the petroleum refining industry. (b) Major classification of PRWW contaminants.
Since water is a vital and necessary component of the petroleum refinery industry as it is required in various applications, the generation of oily wastewater is always an inevitable aspect of the industry value chain (Whale et al. 2022). Petroleum refinery wastewater (PRWW) effluents are the aqueous form of the waste generated from the crude oil refining process (Bastos, et al. 2021). And the characteristics of the PRWW effluents are very much dependent on the type of crude oil being processed, the refinery plant configuration and the operation procedures (Diya'uddeen et al., 2011; Nacheva, 2011). Hence, it is composed of a diverse range of toxic compounds, such as oil and grease, phenols, sulphides and ammonia, constituting the major source of environmental pollution. Obotey Ezugbe, (2020) reported that for every single barrel of crude oil being processed it generates approximately about 10 barrels of petroleum wastewater. Furthermore, several literature data have indicated that about 1.6 times the volume of refined crude oil is generated as wastewater (Ali, A.M et al. 2017; Al-Khalid and El-Naas, 2018; Rahi, Jaeel and Abbas, 2021). The current global output for PRWW is about 33.5 Mbps from the existing 85 Mbps of crude oil production. And this global output is expected to increase by about 32% by 2030 (Rahi, Jaeel and Abbas, 2021). Precisely, a minimum of about 60-90 gallons of water (approximately 246–341 L) is reported to be used in other to process one barrel of crude oil (El-Naas, Alhaija and l-Zuhair, 2014). These reported data indicate that there is a huge amount of PRWW effluents from the petroleum industry continually being produced and discharged into the world’s main water bodies. As a result, an effective approach must be developed to discharge regulatory requirements and for recycling purposes. Petroleum refinery wastewater (PRWW) is usually characterized by a high level of BOD and COD, suspended and dissolved solids, floating and emulsified oils, metal derivatives, Sulfides, as well as various organic and inorganic contaminants. The wastewater effluents are also usually rich in aromatic organic compounds such as; phenols and polyaromatic hydrocarbons (PAHs), (Aziz and Sabbar (2013); Al Zarooni and Elshorbagy (2006)). The transport fate of these contaminants when discharged into the environment also depends on the conditions and hydrodynamics of the receiving water and the environmental ecosystem (Wake, 2005). Hence, periodic monitoring and evaluation of the quality of the receiving environment for PRWW are required to protect living organisms from the toxicity of these pollutants. Hence, while there is a need for highly efficient and economically vibrant treatment technologies, PRWW effluents on the other hand are considered the main cause of environmental pollution (Beni, et al. 2023).
2. Characterization of Petroleum Refinery Wastewater
The composition of a typical PRWW usually depends on the crude oil qualities as well as the complexity and process configuration of the petroleum refining industry. Petroleum crude oil is a complex mixture of hydrocarbon compounds of different carbon chains and other toxic organics such as phenols, they usually constitute the major organic pollutants in petroleum wastewater (Al-Khalid and El-Naas, 2018). According to Diya’uddeen, et al. (2011) and Whale, et al. (2022), the most significant contaminants that are of environmental concern from PRWW generally include; oil, Phenols, suspended solids, metals, ammonia, dissolved minerals and substances which are responsible for oxygen level depletion as a measure of the biological oxygen demand (BOD) and chemical oxygen demand (COD). Generally, the pollutants from PRWW can be classified into; a) oil and grease b) organic pollutants (which includes the hydrocarbons, organic compounds and all other BOD contaminants) c) inorganic pollutants (including; ammonia, nitrogen, phosphorus, chlorides and other inorganic salts) and d) Heavy metals (Aljuboury et al., 2017; Tengrui
et al., 2007). Therefore, PRWW treatment facilities are usually designed to be capable of the removal of both organic and inorganic contaminants. A typical PRWW effluent is usually characterized by high BOD and COD as a result of the overall distribution of the aliphatic and aromatic hydrocarbons, grease and emulsified oils, ammonia, cyanides and other inorganic substances from the crude oil composition. These contaminants constitute the major environmental pollution as a result of PRWW disposal. It is usually rich in hydrocarbons of three main classes which include; a) The Paraffins comprising low-chain carbon atoms such as Methane (CH4), Ethane (C2H6) and Propane (C3H8); Secondly; b) The Naphthene such as dimethyl cyclopentane and cyclohexane; and c) The aromatic compounds comprising of the benzene compounds and its derivatives (Bayona, et al. 2015). The aromatics are the unsaturated hydrocarbon containing at least one or more benzene ring and their derivatives generally referred to as BTEX (Benzene, Toluene, Ethyl Benzene and Xylene) which are mostly toxic in nature to both human and aquatic species (Tang et al. 2009; Bayona, et al. 2015). Hence, a typical PRWW effluent is usually characterized by high BOD and COD as a result of the overall distribution of the aliphatic and aromatic hydrocarbons, grease and emulsified oils, ammonia, cyanides and other inorganic substances from the crude oil composition (Pinzón-Espinosa, 2018; Al Zarooni and Elshorbagy, 2006). Average reported values for BOD and COD were up to 400 mg/l and 600 mg/l respectively (Al-Khalid and El-Naas, 2018; Pinzón-Espinosa, 2018; Aziz and Sabbar, 2013). Rahi, et al. (2021) reported about 150–250 mg/l, 300–600 mg/l, and 20–200 mg /l for BOD, COD and oils concentrations in desalted PRWW effluent respectively. They further revealed that the level of oil concentration can reach up to 5000 mg/l in the effluents from the bottom of tanks with about 1-100mg/l benzene concentration. Similarly, Elmobarak, et al. (2021) reported a COD concentration of 1200 mg/l in their review of the treatment of PRWW. However, El-Naas, Alhaija and l-Zuhair, (2014) have also reported a COD value in the range of 3600–5300 mg/l. Aljuboury et al., (2017) reported an average concentration of 20 mg/l for Sulphide concentration. A review summary of the characterization of a typical PRWW effluent presented in
Table 2 from different regions of the world shows that concentrations of the pollutants vary significantly from one location to the other. Different level of COD concentration was reported with the lowest being 112 mg/L from Brazil up to 74,800 mg/L from Doha, Qatar. Similarly, the concentrations of heavy metals reported from the literature also varies significantly where some metals like Cadmium is not even detected in some case. The variations in concentrations of the effluent qualities must be attributed to the dynamic complexity of the wastewater which is also related to the difference in the crude oil composition as well as the process configuration of the petroleum refining industry. Overall, these studies have proved the heterogenicity nature of the PRWW effluent. Therefore, advancement in the development of new techniques which would allow the complete identification and proper treatment of emerging contaminants is a major aspect concerning this theme. Due to its high environmental impact, discharge limits for the concentration of the various parameters are always established in every petroleum refining industry for policy compliance (Tetteh and Rathilal 2020). Different review papers and research articles on PRWW including (Diya’uddeen et al. 2011; Aljoubory & Senthilkumar 2014; Radelyuk et al. 2019; Qaderi and Abdolalian 2022; and Eldos, et al. 2022) have reported the discharge standard of the PRWW effluent quality. Based on the reviews, the average effluent qualities reported for pH, BOD, COD, Oil/grease, phenols and total organic carbon (TOC) are 6-9, < 20 mg/l, < 200 mg/l, < 10 mg/l, < 0.25 mg/l, and < 75 mg/l respectively.
Table 1: Characteristics of typical petroleum refinery wastewater reported from the literature.
Table 2.
Concentrations of some heavy metal concentration from typical refinery wastewater.
Table 2.
Concentrations of some heavy metal concentration from typical refinery wastewater.
|
.
|
Parameters |
|
| Location of PRWW |
pH |
BOD (mg/L) |
COD (mg/L) |
TSS (mg/L) |
TDS (mg/L) |
TOC (mg/L) |
NH3 (mg/L) |
Phenols (mg/L) |
Sulphides (mg/L) |
Oil & grease (mg/l) |
Reference |
| Kurdistan region-Iraq. |
7.74 |
155 |
485 |
600 |
800 |
- |
13.7 |
3.5 |
- |
17.36 |
Aziz and Fakhrey (2016). |
| Guangdong China |
---- -- |
1198 |
2554 |
- |
- |
610.93 |
81.2 |
- |
- |
-- |
Dai, et al. (2020) |
| Isfahan, Iran |
6.7 |
174 |
450 |
150 |
- |
119 |
- |
- |
- |
870 |
Saber, et al. (2014) |
| Japan |
8.3–8.9 |
- |
3600–5300 |
30–40 |
3.8–6.2 |
- |
|
11–14 |
- |
- |
El-Naas, et al. (2014) |
| Rawalpindi, Pakistan. |
9.2 |
- |
970 |
42.3 |
1,220 |
- |
|
|
- |
- |
Ul haq, et al. (2020) |
| Nigeria |
7.2 |
107.3 |
232.7 |
86.2 |
276 |
- |
0.7 |
0.17 |
- |
2.9 |
Mustapha, (2018) |
| Not reported |
8.0 |
718 |
1494 |
75 |
- |
-- |
- |
70 |
142 |
- |
Jafarineja (2017). |
| India |
8.0 |
195 |
480 |
315 |
- |
- |
- |
13.8 |
16.8 |
94 |
Ibrahim et al. (2013) |
| Doha, Qatar |
8.3–8.7 |
- |
3970–4745 |
30–40 |
3800–6200 |
|
|
8–10 |
- |
- |
El-Naas, et al. (2016) |
| Mathura, India |
7.82 |
- |
310 |
- |
1910 |
-- |
-- |
- |
- |
- |
Khatoon & Malik, (2021) |
| Republic of Iraq |
8.2 |
23 |
- |
31 |
- |
- |
0.81 |
20.7 |
- |
- |
Aziz and Sabbar (2013) |
| Qatar |
7.8 |
44,300 |
74,800 |
2010 |
41,600 |
5490 |
- |
- |
- |
- |
Eldos, et al. (2022) |
| Arzew, Algeria |
7.3 |
- |
330 |
253.3 |
-- |
391 |
9.5 |
- |
- |
- |
Ghezali, et al. (2022) |
| Sines, Portugal |
7.2 |
- |
1179 |
- |
74 |
-- |
- |
257 |
0.18 |
217 |
Bastos, et al. (2021) |
| Niger Delta, Nigeria |
8.0 |
138 |
350 |
60 |
2100 |
- |
- |
7.35 |
- |
14.75 |
Nkwocha, et al. (2013) |
| Brazil |
8.0 |
8.6 |
112 |
|
930 |
- |
0.7 |
|
- |
- |
Daflon, et al. (2015) |
| Heavy Metals |
|
| Cadmium |
Chromium |
Copper |
Lead |
Manganese |
Iron |
Zinc |
Arsenic |
Mercury |
Nickel |
Reference |
| <0.005 –0.2 |
0.02–1.1 |
<0.002–1.5 |
<0.004–175 |
– |
<0.1–100 |
0.01–35 |
0.01–35 |
<0.001–0.002 |
– |
Elmobarak, et al. (2021) |
| – |
<0.01 |
<0.01 |
0.04 |
0.58 |
5.14 |
0.75 |
<0.4 |
<0.15 |
0.02 |
Khatoon and Malik, (2021) |
| 0.045 |
0.022 |
– |
0.03 |
– |
– |
– |
– |
– |
0.176 |
Hashemi, et al. (2018) |
| ND |
– |
– |
0.0135 |
– |
0.253 |
0.33 |
– |
– |
– |
Wokoma and Edori (2017) |
| – |
1.225 |
0.005 |
0.47 |
– |
– |
0.45 |
– |
– |
– |
Olayebi and Adebayo (2017) |
| < 0.001 |
0.06 |
– |
– |
0.149 |
2.535 |
1.133 |
– |
– |
– |
Igbagara and Ntekim (2021) |
| 0.031 |
2.33 |
0.86 |
2.06 |
– |
2.28 |
7.56 |
|
|
1.03 |
Ghezali, et al. (2022a) |
| 0.054 |
0.025 |
|
0.031 |
– |
0.775 |
0.75 |
– |
– |
0.188 |
Stanley, et al. (2017) |
| 0.026 |
0.04 |
0.03 |
0.01 |
– |
0.88 |
0.03 |
|
– |
– |
Ghezali, et al. (2022b) |
| 5.93 |
– |
– |
– |
– |
– |
– |
2.78 |
1.05264 |
– |
Ugboma, et al. (2020) |
3. Treatment of Petroleum Refinery Wastewater
For the fact that petroleum wastewater contains toxic contaminants which are a source of a major threat to the environmental ecosystem, hence it is necessary to receive the appropriate treatment before disposal and to meet the regulatory requirement. Given this, there are various treatment techniques reported in the literature for the treatment of PRWW (Petrowiki, 2018). However, while some already established technologies are efficient in terms of their treatment, cost and energy requirement, others are associated with high energy and maintenance costs and hence as such are not environmentally friendly. Therefore, efficiency assessment in terms of energy requirements, flexibility to treat various contaminants, and level of waste generation as a by-product at the end of the treatment process is very critical in the development and application of any treatment technology (Amakiri et al. 2022). Generally, the treatment of PRWW has two main stages; The pre-treatment stage; is used to reduce the contaminants loads such as oil, grease, and suspended solids. Secondly, the degradation of the pollutants to an acceptable discharge limit (Diya’uddeen, et al. (2011; Aljuboury et al., 2017; Al-Khalid and El-Naas, 2018). Some of the reported treatment techniques in the literature include; biological processes (Tong, et al. 2013; Wang, et al. 2016; Vendramel, et al. 2015; El-Naas 2014; Wang, et al. 2021), coagulation process (Dehghani, 2016; Singh, et al. 2020; Zueva, et al. 2020), adsorption process (El-Naas 2014), membrane processes (Ratman, et al. 2020; Estrada-Arriaga, et al. 2016; Razavi, et al. 2015; Hashimi, et al. 2018 and Kusworo, et al. 2021), chemical oxidation (Nogueira, et al. 2016; Rubio-Clemente), advanced oxidation process (AOPS) (Coelho, et al. 2006; Chen, et al. 2015; Ebrahiem, et al. 2017 Zhang, et al. 2006). In most cases, the determination of the treatment efficiency of these techniques focuses on the efficiency in the removal of the BOD, COD, oils & grease, phenols, Sulphates, TOC as well as concentration of heavy metals. Based on this, advanced oxidation processes such as Fenton-oxidation and photocatalysis are nowadays receiving more attention due to their high capability in the degradation of recalcitrant petroleum contaminants (Aljuboury et al., 2017). Many advances in treatment technologies have been achieved in recent years which can be attributed to advancements in the application of technology in the area of material sciences and dynamic approach to the treatment of the phase of modern and complex pollutants (Abuhasel, et al. 2021). In this paper, a review of the works previously reported using conventional methods as well as advanced and integrated treatment techniques would be discussed.
Figure 5.
Composition of petroleum refinery wastewater and major classification of treatment techniques.
Figure 5.
Composition of petroleum refinery wastewater and major classification of treatment techniques.
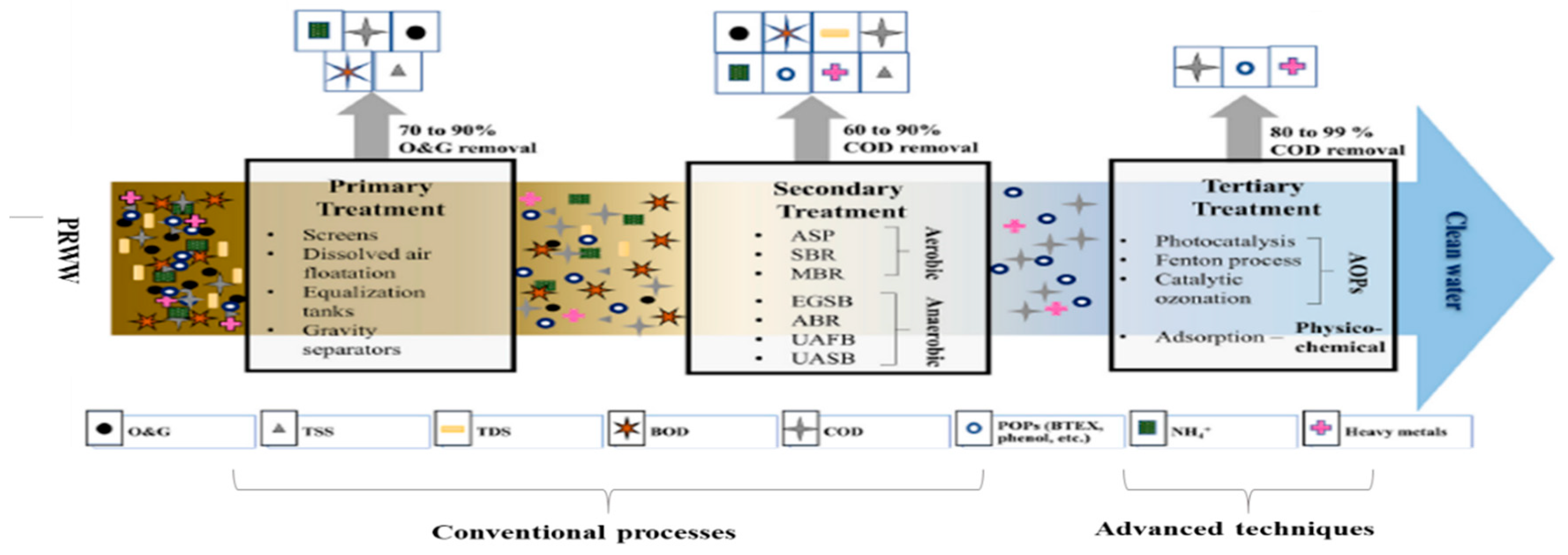
3.1. Conventional Treatment Techniques
Petroleum wastewater effluent can be treated using either conventional, advanced or integrated treatment processes. The conventional treatment techniques include a sequence of mechanical and physicochemical processes, followed by biological treatment of usually activated sludge treatment units. The sequence of the conventional treatment techniques is divided into four; (a) Pre-treatment (b) Primary treatment (c) Secondary treatment (c) Tertiary treatment. Although, the pre-treatment stage is sometimes regarded as part of the primary treatment where the majority of the suspended solids are separated and removed with the help of gravity, sedimentation and filtration processes (Radelyuk, et al. 2019). Conventional techniques have been widely used since the beginning of the 20th century and mainly consist of a combination of physical, chemical, and biological processes. According to Yu, et al. (2017), conventional techniques for the treatment of PRWW include; flotation, coagulation, biological treatment and membrane separation technology. However, these techniques are usually associated with various limitations including, low efficiency, high capital operating cost as well as low sensitivity to emerging complex organic contaminants (Varjani, et al. 2019). Toxic recalcitrant pollutants from hydrocarbon source such as Naphthenic acids (NAs) usually remains a considerable challenge in the treatment of PRWW using biological processes. Furthermore, due to the low efficiency and operational limitations of the conventional techniques, it makes it necessary to adopt more robust advanced treatment systems. The advanced treatment systems which include; advanced oxidation processes, (such as photocatalysis, Fenton-oxidation, and electrochemical processes) have been reported to provide more efficient treatment and less production of waste by-products that may also require further treatment. Alternatively, the use of an integrated or hybrid system which combines two or more advanced processes is also nowadays receiving more attention to provide the most effective treatment for the removal of oil and other hazardous pollutants from petroleum wastewater (Adetunji and Olaniran 2021).
Figure 6.
General overview of the PRWW treatment techniques. (Jain, et al. 2020).
Figure 6.
General overview of the PRWW treatment techniques. (Jain, et al. 2020).
3.1.1. Physicochemical Processes
The physicochemical processes are a set of techniques that combines both physical and chemical properties of the PRWW in the removal of pollutants. The physical part may include the use of filtration, floatation, adsorption and sedimentation while the chemical part includes precipitation and coagulation processes (Aljuboury et al. 2017)
3.1.2. Flotation Sedimentation and Filtration
The physical PRWW treatment processes are the sets of techniques such as screening, floatation, sedimentation and gravity separation that do not require the application of biological or chemical changes during the treatment processes (Aljuboury et al., 2017). They usually constitute the primary treatment processes to reduce the waste load before proceeding to the secondary treatment units (Ahmad et al. 2016). A typical example is the course screening, sedimentation, floatation, and filtration in the removal of large particles, sediments as well as fine grains from the PRWW (Shuokr and Sazan, 2021). The sedimentation process is used for the separation of water and oil due to the density difference between the oil and water. Hence, a significant density difference is required to provide an optimum separation. Oil and water sedimentation can be mechanically achieved using separators such as the API separator which operates on the principle of specific gravity difference to allow the settlement of heavy oil and pollutants (Varjani, et al. 2020). Diffused air flotation (DAF) is achieved by introducing fine air bubbles to enhance the formation of a scum layer between the oil and the water for easy separation. The technique is achieved by introducing air under pressure which would bring the pollutants to rise to the top surface. High levels of total suspended solids, colloids as well as some immiscible liquids are significantly reduced during this stage as they can reduce treatment efficiency and even cause damage to treatment facilities (Renault et al., 2009). Abuhasel, et al. (2021), DAF techniques enhanced by nanobubbles systems were applied along with surfactants in other to reduce the surface tension of the oil concentration. About 90% oil separation efficiency was reported using this system more than the traditional DAF system. Floatation and gravity separation were usually used as the first stage separation process to remove floating and dispersed oil efficiently. However, they are not efficient in terms of the separation of emulsified oil (Abuhasel, et al. 2021; Le et al. 2013). Li, et al. (2013) reported that Wang (2007) applied diffused floatation process to a sedimentation tank of PRWW with an influent oil concentration of 3000-14000 mg/l. An average effluent oil concentration of 300 mg/l with a minimum value of 97 mg/l was achieved using the process. Wang et al. (2015) reported that most conventional physicochemical techniques especially floatation and sedimentation processes do not yield more than 16% to 24% efficiency in the removal of organic aromatic pollutants. Furthermore, high maintenance costs and increased energy consumption were also among the major disadvantages of the DAF system.
3.1.3. Coagulation/Flocculation
The use of coagulants to form flocs as a result of coagulation is also another physicochemical treatment process for the removal of pollutants from PRWW. Iwuozor (2019) reported that coagulants are generally polyelectrolytes or synthetic organic polymers with high molecular which form multi-charged poly nuclear complexes in solution that makes flocs and settle easily. Coagulation processes were reported to be effective to treat heavy metals and high-level concentrations of organic pollutants. Hassan et al., (2012) reported that coagulation/flocculation are the most popular techniques for the removal of pollutants related to turbidity, colour, and TSS. However, it is an inappropriate technology for the complete treatment of organic pollutants, but it is an efficient conventional treatment process used before membrane and biological processes to eliminate or reduce the level of non-biodegradable organic pollutants (Aljuboury et al. 2017). The coagulation process is also reported to remove dissolved and emulsified oils. It is also greatly influenced by pH, coagulant dose and settling time (Sukmana et al. 2021). Aluminium and iron salt coagulants such as aluminium sulfate (alum), ferrous sulfate and ferric chloride were among the most widely used coagulants (Lal and Garg 2019). The function of the coagulant is to promote the agglomeration or accumulation of the wastewater particle by reducing the surface charges of the electrostatic particles. Different investigations have indicated the ability of the coagulation process in the treatment of PRWW. Zueva et al. (2020) reported research conducted to prove the petroleum wastewater treatment capabilities of Ca (OH)2 and Al2(SO4)3. Under optimum conditions, the removal efficiencies of turbidity, total hydrocarbons and COD were 100%, 90% and 70% respectively.
Table 3.
Petroleum refinery wastewater treatment by coagulation.
Table 3.
Petroleum refinery wastewater treatment by coagulation.
| |
Experimental conditions |
Reference |
| Coagulant |
pH |
Dosage |
Tempt. (°C) |
Time (Min) |
Pollutants removed |
Removal efficiency (%) |
|
| Ca (OH)2 and Al2(SO4)3 |
7.3 |
0.43 mg/L. |
NR |
NR |
Turbidity |
100 |
Zueva et al. (2020) |
| |
|
|
|
|
TOC |
90 |
|
| |
|
|
|
|
COD |
70 |
|
| CuSO4+FeCl3 |
7.1 |
0.20 g/L |
NR |
NR |
COD |
76.77 |
Singh and Kumar (2020) |
| |
|
|
|
|
Turbidity |
89.47 |
|
| |
|
|
|
|
TDS |
94.16 |
|
| |
|
|
|
|
Colour |
95.29 |
|
| Land snail shells (LSS) |
6 |
0.1 g/L |
NR |
30 |
Turbidity |
90 |
Ovuoraye, et al. (2022) |
| Ca (OH)2-based coagulant |
|
|
|
|
Turbidity |
95.1 |
Benouis et al. (2020) |
| |
|
|
|
|
Hydrocarbons |
90.4 |
|
3.1.4. Adsorption Using Conventional Adsorbents
The adsorption process is both a conventional and advanced treatment technique depending upon the adsorbent material. Conventional adsorbent materials such as activated carbon, zeolites and silica are been used for a long time in the treatment of PRWW. These are called conventional adsorbent materials. Nowadays, adsorption techniques (using both conventional and non-conventional adsorbents) are one of the most commonly studied techniques for most industrial wastewater treatment due to their simplicity and lower treatment cost (Cai, et al. 2019). Additionally, besides its effectiveness and economic advantage, the adsorption technique is sometimes a reversible process where adsorbents can be regenerated simply through an appropriate desorption process (Gkika, et al. 2022). The adsorption process can occur between a combination of systems such as; solid-liquid, solid-gas, liquid-gas, and liquid-liquid (Sukmana et al. 2021). Parameters affecting adsorption process efficiency include; pH, temperature, contact time and adsorbent porosity and dose. The pH factor affects the ability of the hydrogen ions in the solution and their interaction with the functional groups and the metal ions (Afroze et al. 2016).
Adsorbents with high porosity tend to have a high surface area as well as high adsorption capacity (Ballav, et al. 2018). Activated carbons, polymeric organic resins (such as ion exchange resins) and inorganic adsorption materials such as zeolites, silica gel, as well as activated alumina, were all classified as conventional adsorbents. Meanwhile, industrial or agricultural by-products such as rice husk and sawdust were categorized as non-conventional adsorbents (Crini, et al. 2019). Various adsorbent materials derived from agricultural or industrial by-products originating from natural material or modified biopolymers were reported to be used for the removal of heavy metals (Barakat 2011). Mahmoud et al. (2013) have reported the application of activated carbon, natural clay and sawdust for the treatment of petroleum wastewater from Kuwait Gulf Oil Company (KGOC). The sorption capacities reported are; 15.52 mg/g, 16.23 mg/g and 12.91 mg/g for the activated carbon, natural clay and sawdust respectively at 100 min. equilibrium time. Jun et al. (2020) investigated the adsorption potential and efficiency of palm kernel shells (PKS) as biomass integrated with iron oxide and zeolite. The analysis revealed that the optimized PKS can remove colour (83.1%) and COD (67.2%) within a contact time of 30 minutes. Similarly, Kassob and Abbar (2022) also investigated the COD removal efficiency of an activated carbon fixed-bed column operated at a batch recirculation mode using petroleum wastewater from Iraq's Al-Diwaniyah petroleum refinery plant. At an optimal condition and pH of 5.7, 80% activated carbon parking and 73 adsorption time, 96.70% COD removal efficiency was recorded.
Table 4.
Petroleum refinery wastewater treatment by adsorption.
Table 4.
Petroleum refinery wastewater treatment by adsorption.
| sssss |
Experimental conditions |
|
|
| Adsorbent |
pH |
Dosage |
Tempt. (°C) |
Time (Min) |
Pollutants removed |
Removal efficiency (%) |
Reference |
| Activated carbon (AC), natural clay (NC) and sawdust (SD) |
7 |
NC 18.96 mg/g,
AC 16.25 mg/g & SD 14.11 mg/g. |
NR |
100 |
Colour |
83.1 |
Mahmoud et al. (2013) |
| |
|
|
|
|
COD |
67.2 |
|
| Activated carbon fixed-bed column |
5.7 |
80% Parking |
25±2 |
73 |
COD |
96.7 |
Kassob and Abbar (2022) |
| Synthesised nanorods ZnO/SiO2 via the sol–gel |
|
|
|
|
Pb2+
|
85.06 |
Shaba, et al. (2022) |
| |
|
|
|
|
Cd2+
|
84.12 |
|
| Functionalized mesoporous material with amine groups (NH2-MCM-41) |
7 |
0.4 g/L |
|
50 |
PAHs |
85.7 |
Kalash and Albayati (2021) |
| ZnO/Fe3O4 nanocomposite |
NR |
0.08 g |
30 |
900 |
Cu2+
|
92.99% |
Shaba et al. (2023). |
| |
|
|
|
|
Cr6+
|
77.60% |
|
3.1.5. Membrane Processes
Membrane technology has been in existence since around the 18th century for the treatment of wastewater. It is a physicochemical treatment technology that is gaining more acceptance and is also efficient in the treatment of organic matter. Membranes are used as a selective barrier in the separation of two phases through semi-permeable pore space by restriction of movement between components (Obotey Ezugbe, 2020). According to Obotey Ezugbe, (2020) and Aljuboury et al., (2017), membranes can be generally classified into two main types; organic membranes (usually made from organic polymers) and inorganic membranes (made from silica, metals, zeolites, or ceramics). Depending on their pore sizes, membranes can be used for microfiltration ultrafiltration or nanofiltration (Barakat 2011; Moslehyani, et al. 2015). A high level of concentrate generation which subsequently leads to membrane fouling is the major drawback in the membrane treatment systems (Chun, et al. 2017). Ratman et al. (2020) reported the application of a Polyether sulfone (PES) membrane consisting of zinc oxide (ZnO) nanoparticles followed by UV irradiation for the pre-treatment of RWW. The result showed pre-treatment enhanced rejections up to 18.6%, 16.7%, and 87.1%, for total dissolved solids, chemical oxygen demand, and Ammonia respectively. Hashimi et al. (2018) used micellar-enhanced ultrafiltration (MEUF) for the treatment of heavy metals at Kermanshah Oil Refinery and the result showed 96%, 95%, 92% and 86% removal efficiency for nickel, lead, cadmium and chromium respectively. Sodium Dodecyl Sulfate (SDS) was used as a surfactant and added to the effluent to enhance complex formation that can trap the heavy metals. Kusworo, et al (2021) utilised Polysulfone (PSf) membrane with improved efficiency using zinc oxide (ZnO) nanoparticles and reported rejection values of 70.21% for TDS and 74.68% for COD. Similarly, Kusworo, et al. (2022) have reported a study using a Polysulfone-Nano TiO2 Hybrid membrane coupled with an ozonation process as a pre-treatment for the removal of TDS, COD, and phenols. The ozonation process enhanced the membrane permeate capacity by up to 96% and improved the pollutant removal efficiency by up to 77%. They further reported that the ozonation process also reduces the fouling of the membrane and increases surface resistance by up to 21%. Although, ozonation itself is a costly process, utilising a hybrid process that can reduce the concentration of pollutants before membrane filtration can enhance membrane efficiency and reduce fouling which is a major problem with membrane applications.
Table 5.
Petroleum refinery wastewater treatment by membrane process.
Table 5.
Petroleum refinery wastewater treatment by membrane process.
| No. |
Membrane |
Pollutants removed |
Removal Efficiency (%) |
Reference |
| 1 |
Polyether sulfone (PES) membrane consisting of zinc oxide (ZnO) nanoparticles |
TDS |
18.6 |
Ratman et al. (2020) |
| |
|
COD |
16.7 |
|
| |
|
Ammonia |
87.1 |
|
| 2 |
Micellar-enhanced ultrafiltration (MEUF) |
Nickel |
96 |
Hashimi et al. (2018) |
| |
|
Lead |
95 |
|
| |
|
Cadmium |
92 |
|
| |
|
Chromium |
86 |
|
| 3 |
Polysulfone zinc oxide (ZnO) nanoparticles to PSf membrane |
TDS |
70.21 |
Kusworo, et al (2021) |
| 4 |
|
COD |
74.68 |
|
| |
Polysulfone-Nano TiO2 Hybrid Membrane |
TDS |
77% |
Kusworo, et al. (2022) |
| |
|
COD |
77.2 |
|
| |
|
Phenols |
78.5 |
|
| |
|
|
|
|
3.2. Chemical Processes
3.2.1. Chemical Precipitation & Ion Exchange
Chemical processes utilize the application of chemical reactions in the treatment of wastewater contaminants. Neutralization, ozonation, ion exchange and oxidation processes were among the most widely used chemical processes in the treatment of RWW (Aziz and Fakhrey 2016). Neutralization consists of the use of an acid or base such as lime to adjust the pH level (Aljuboury et al. 2017). Generally, chemical precipitation is one of the most widely used conventional treatment processes for the removal of heavy metal concentrations from inorganic effluents (Barakat, 2011). In the precipitation process, heavy metal ions from the PRWW react with suitable chemicals to form insoluble participates which can be further separated by a sedimentation or filtration process (Zinicovscaia and Cepoi 2016). It is relatively a simple and less costly technique which can be used for the removal of metals and sulphides. The coagulation precipitation method is broadly used with the help of chemical precipitants such as Ca (OH)2 and NaOH (Qasem, et al 2021). Alnakeeb and Rasheed (2021) have reported the application of BaCl2 and Al (OH)3 in the treatment of PRWW from Al-Doura Refinery in Iraq. High sulphate removal efficiency was obtained with BaCl2 over Al (OH)3 and concluded that aluminium hydroxide is unsuitable for PRWW with neutral pH and low sulphate concentrations. Meanwhile, Barium salts are highly insoluble and hence making them an excellent precipitant for sulphate ions. Altaş and Büyükgüngör (2008), also reported the use of Ca (OH)2 as precipitant together with Fe2+ ions and obtained (96–99%) and (50–80%) removal efficiencies for Sulfide and COD respectively. Alternatively, precipitation can also be achieved using sodium or calcium carbonates in which classical carbonates are formed. Habte et al. (2020) investigated the Removal of Cadmium and Lead via carbonation of aqueous Ca (OH)2 derived from eggshell and found the results to be efficient for obtaining very low concentrations of the heavy metals. About 99.99% and 99.63% treatment efficiency for Cd2+ and Pb2+ were obtained at an optimum condition of 3 g/L dosages of Ca (OH)2, the initial metal concentration of 100 mg/L and the CO2 flow rate of 1 L/min. The study has provided evidence for the application of Ca (OH)2 derived from an eggshell for the treatment of heavy metals. Furthermore, the impact of carbonation to enhance calcium hydroxide-based precipitation can be an attractive method for the capture and utilization of CO2 as a greenhouse gas. However, the formation of large sludge and the effect of pH is the main disadvantage of the precipitation process (Park et al. 2014). Furthermore, precipitation performance is also mostly affected by a high-level concentration of chlorides from the wastewater. High chlorine concentration usually favours the formation of hydroxo salts precipitates instead of the typical heavy metal hydroxides (Stec, et al. 2020). From their review of about 185 articles from 1988–2010, Fu and Wang (2011) stated that ion exchange, adsorption and membrane filtration were the most widely studied methods for the treatment of heavy metals. The potential recovery of the metal, higher selectivity, and lower sludge production are among the main advantages of the ion exchange technique. The main principle of the technique is the exchange of ions in a chemically equivalent amount between a resin (usually a solid) and an electrolytic solution (Zinicovscaia and Cepoi 2016). Generally, ion-exchange resins are applied in the isolation of rare metals, regeneration of metal wastes desalination as well as softening process (Sillanpää and Shestakova 2017). The resin materials can be natural such as inorganic zeolites or synthetically produced organic resins (Qasem, et al 2021).
Table 6.
Treatment of petroleum refinery wastewater by precipitation.
Table 6.
Treatment of petroleum refinery wastewater by precipitation.
| |
Experimental conditions |
|
| Precipitant |
pH |
Dosage |
Tempt. (°C) |
Time (Min) |
Pollutants removed |
Removal efficiency (%) |
Reference |
| BaCl2 and Al (OH)3 |
7 |
0.36 g/L |
NR |
15 |
Sulphate ion |
|
Alnakeeb and Rasheed (2021) |
| Ca (OH)2 and Fe2+ ions |
5 |
40 mg/L |
NR |
NR |
Sulfide |
97.5 |
Altaş and Büyükgüngör (2008) |
| |
|
|
|
|
COD |
65 |
|
| Ca (OH)2 derived from eggshell |
NR |
3 g/L |
NR |
NR |
Cd 2+
|
99.99 |
Habte et al. (2020) |
| |
|
|
|
|
Pb 2+
|
99.63 |
|
3.3. Biological Processes
The biological processes utilize the use of the microbial activity of living organisms such as bacteria to decompose or degrade organic contaminants. The four major groups of biological treatment processes are aerobic, anaerobic, anoxic (the process by which nitrate is converted biologically into nitrogen gas in the absence of oxygen), or a combination of the three. The principal applications for these processes are removing carbonaceous organic matter (measured in BOD, COD, or TOC), nitrification, denitrification, or stabilization (Roy and Saha 2021). There are various biological processes which have been reported to be effective in treating PRWW among which the activated sludge process is the most widely used (Elmobarak et al. 2021). However, there is little removal efficiency of petroleum hydrocarbons and large sludge production, but at least up to 60%-90% COD removal efficiency was observed in many biological treatments of PRWW (Jain et al. 2020). Based on the requirement, the general application of biological treatments can be grouped into two main classes; aerobic and anaerobic methods where the latter is widely used due to its simplicity and high efficiency (Aljuboury et al. 2017). Furthermore, anaerobic digestion produces methane gas as renewable energy and requires less space and generates lower sludge than the aerobic process. Pretreatment processes such as flotation, flocculation, sedimentation, and filtration are usually applied in the treatment of PRWW to eliminate free oil and gross solids as well as increase biodegradability (Ghimire and Wang 2018). Different reactor systems of the aerobic process have been reported to treat PRWW including; the traditional activated sludge (ASP), contact stabilization active sludge, membrane bioreactor (MB), biological aerated filter (BAF), moving bed biofilm reactor (MBBR), sequence batch reactor (SBR) etc. Bioreactors adopted for PRWW are generally categorised into suspended growth, attached growth or hybrid processes advanced treatment (Pal et al. 2016). Shuokr and Sazan (2021) have reported that high COD removal efficiency of up to 78% and 94% for total organic carbon and oil degradation were achieved using aerobic biological treatment. Rasheed and Muthukumar (2010) preserved a PRWW sample with an initial COD of 40000 mg/l and a pH of 5.4 under a deep freeze for a limited number of days before undertaking biological treatment using sequencing batch bioreactor (SBR) with sonication pre-treatment for 30 minutes. The investigation revealed that there is a significant decrease in COD with an increase in time. An industrial scale granular sludge bed bioreactor and aerobic activated sludge treatment (EGSB-BR) were developed by Liang et al. (2019) to RWW and the overall COD and petrochemical removal efficiencies of the plant are 85.6 % and 81.5 %, respectively. El-Naas et al. (2016) investigated a three-step pilot plant process consisting of biological treatment in a spouted bed bioreactor (SBBR) unit for the treatment of highly contaminated RWW in which they achieved 96% COD removal and nearly 100% Phenols degradation. Vendramel (2015) utilized the capability of an aerobic submerged fixed-bed reactor (ASFBR) to treat a high organic strength RWW and found COD, dissolved organic carbon and TSS removal efficiencies at 91%, 90% and 92%, respectively. About 90% reduction in the ammonium level was also obtained.
Table 7.
Treatment of petroleum refinery wastewater by biological process.
Table 7.
Treatment of petroleum refinery wastewater by biological process.
| No. |
Biological process/reactor |
COD (%) |
TOC (%) |
Phenols (%) |
TSS (%) |
Reference |
| 1. |
Aerobic biological treatment |
78 |
94 |
|
|
Shuokr and Sazan (2021) |
| 2. |
Granular sludge bed bioreactor and aerobic-activated sludge treatment (GSB-BR) |
85.6 |
|
|
|
Liang et al. (2019) |
| 3. |
Spouted bed bioreactor (SBBR) |
96 |
|
|
|
El-Naas et al. (2016) |
| |
|
|
|
100 |
|
|
| 4. |
Aerobic submerged fixed-bed reactor (ASFBR) |
91 |
|
|
92 |
Vendramel (2015) |
3.4. Advanced Treatment Processes
The problem of low treatment efficiency and high operational costs and among others in most conventional treatment processes have led to the need to adopt more advanced treatment technologies. Alternatively, the use of a hybrid system which combines two or more processes is sometimes effective for the removal of oil and other hazardous pollutants from petroleum wastewater (Adetunji and Olaniran 2021).
3.4.1. Adsorption Using Modified Adsorbents
With the advancement in the field of material science and the need for an effective and low-cost adsorbent, many natural and synthetic materials have been tested for the adsorption of pollutants from wastewater of different industrial effluents. Although the selection of an appropriate adsorbent with a suitable property is also indispensable to obtaining the maximum adsorption capacity, the adsorption technique is often seen as the best choice in the treatment of different types of wastewater among the available treatment options. This is for the fact that it is regarded as the most simple and fitting treatment technique (Vikrant & Kim 2019). Furthermore, the adsorption technique is also believed to be the optimal method for crude oil spill clean-up because of its relatively low cost and high effectiveness. Various oil hydrophobic adsorbents exist, such as natural sorbents, organic polymers (synthetic), and mineral materials (inorganic) are nowadays generated from a variety of sources that can be used to treat oily PRWW (Sabir, 2015). For example, Abdeen & Moustafa (2016) have reported their study for the adsorption of crude oil from wastewater on a crosslinked poly (vinyl alcohol) hydrogel (HPVA) and its foam (HPVAF). The macroporous adsorbent of HPVAF was prepared by adding CaCO3 and epichlorohydrin which act as the pore-forming agent and crosslinker, respectively. The adsorption ability of the two materials was assessed using the gravimetric method where the HPVAF carrier demonstrated an improvement in hydrocarbon trapping than the HPVA. The crude oil removal ability of the HPVAF was approximately 82% at a pH of 3. Meanwhile, the removal percentage is higher at pH 3 and 9 compared with pH 7. This study confirms the potential ability of using HPVA and HPVAF films as crude oil adsorbents from oily PRWW especially in an open marine environment. It also proved the good ability of calcium carbonate as a pore-forming agent in the preparation of hydrogel adsorbents. However, there is a need for low or high pH concentrations for the effective use of the HPVA and HPVAF hydrogel films. Similarly, Li, et al. (2022) also reported the use of hydrogel composite produced by freezing–thawing process using chitosan, polyvinyl alcohol, and carbon black as the raw materials and used for oil/water separation. The prepared hydrogel displayed oil repellence and water affinity properties and when submerged in oil was able to separate oil/water mixtures efficiently. After 25 oil–water separation cycles, the hydrogel-coated filter still had a separation efficiency of over 98%. Furthermore, they also reported that due to its super hydrophilicity and active functional groups, it was able to effectively absorb dye molecules dissolved in water. Li, et al. (2021) similarly reported the synthesis of a highly hydrophobic and self-recoverable hydrogel sponge prepared from cellulose nanofibrils (CNFs), N-alkylated chitosan (NCS), and poly (vinyl alcohol) (PVA) for oil/water separation. The interconnected microstructure CNF/NCS/PVA hydrogel was found to have 96% porosity. The hydrogel sponge effectively separates oil/water mixtures and water-in-oil emulsions with high separation efficiency and good stability in various acidic, saline and mechanical conditions. They further maintained that it can absorb various organic solvents with an absorption capacity of about 19.05–51.08 times its original weight. Similarly, Xue, et al. (2019) have also conducted a study to separate oil/water mixture in a highly acidic, alkaline, and salty condition using a porous calcium alginate/Ag nanoparticle (Ca-ALG/Ag) hydrogel film with super hydrophilic and underwater superoleophobic properties which is fabricated through an eco-friendly process. The synthesis of the Ca-ALG hydrogel film was conducted by combining ionic cross-linking of Ca+ ions and soluble NaCl salt-template method and also incorporating the Ag nanopar ticles into the alginate matrix by a simple reduction process. NaCl crystals were used as templates and sifted on the ALG solution films which can be easily removed by water. The ALG/NaCl composites were quickly immersed in CaCl2 solution and sonicated at the same time. The NaCl crystals pierced through the ALG film and dissolved in water gradually and generating a macro-pore structure. They finally reported that the oil/water separation efficiency of the Ca-ALG/Ag hydrogel film was above 98%. Polyvinyl alcohol and formaldehyde hydrogel composite sponges (PVF/PVF) were also synthesised from a study conducted by Zheng, et al. (2023) for the treatment of oily wastewater. Although the prepared hydrogels sponge shows almost 100% oil removal efficiency, it could effectively only remove oil emulsion under the action of gravity with a maximum flux of 2.9 × 105 L m-2h−1 bar−1. The hydrogel has displayed excellent reusability after use and recovered simply by washing. Tai, et al. (2022) reported the development of a superhydrophobic composite aerogel-prepared leached carbon black waste (LCBW) obtained from industrial waste and polyvinyl alcohol (PVA) via conventional freeze-casting and followed by a surface coating. The synthesised PVA/LCBW aerogel was used as a selective adsorbent for different oils and organic solvents and showed an adsorption capacity of about 35 times its original weight. It can also be reused repeatedly and recovered easily through a simple drying process. The maximum removal efficiency was obtained from a PVA/LCBW combination ratio of 1 and 0.5 wt % PVA. This corresponds to the highest water contact angle of 156.7 ± 2.9°. Regarding oil/water emulsion separation using porous materials such as hydrogels, the two most important key points for consideration are 1) proper average pore size and 2) the wettability of the adsorbent. This important property simply describes the level of hydrophilicity and hydrophobicity of the adsorbent material. The superoleophobicity and wettability of the hydrogel adsorbents protect them from fouling by oils, thus making them better performance in the removal of oil concentrations and reuse of materials (Zhang, et al. 2020). Sha, et al. (2021) developed a polyvinyl alcohol-formaldehyde (PVA-PVF) sponges with harmonious pore size through a crosslinking reaction of polyvinyl alcohol (PVA) in the polyvinyl alcohol-formaldehyde (PVF) and under acidic condition. They further stated that the use of PVA containing chitosan, diatomite and sodium alginate (SA) can effectively decrease the average pore size of PVF from approximately 75 μm to 23 μm along with a few hundred nanometres pore channels while maintaining porosity above 73.4%. The oil/water emulsion separation efficiency can reach up to 97.40% with a high-water flux of 2.40 × 104 L m−2 h−1 bar−1.
Although there are many non-conventional adsorbent materials such as those developed from biopolymers or hydrogels that have been used in the application of wastewater treatment, only a few studies were reported using biopolymers and hydrogels in the treatment of real PRRW samples. Moreover, most of the reported studies were conducted on a small laboratory scale. Hence, there is a need for further research on this to theme understand the suitability of these materials for PRWW treatment in practical applications.
3.4.2. Electrochemical Technology
Electrochemical technology is nowadays a promising treatment technology for the removal of organic pollutants from PRWW using the application of electric currents supplied to electrodes. This technology can occur in the forms of electrocoagulation, electro-floatation electro-oxidation, electro-Fenton electrodialysis, electrodeposition, and electrode ionization etc. (Treviño-Reséndez 2021; Khalifa et al. 2022). Adetunji and Olaniran (2021) reported that electrochemical technologies are usually affected by operating conditions such as; current density, pH, electrode materials, temperature and concentration and structure of phenols. For the fact that no chemical addition is needed and less waste generation, electrochemical processes were considered green technologies which are simple to operate and combine with other technologies (Khalifa et al. 2022). The average estimated time for an electrochemical treatment process to treat PRWW is about 5-6 hours. However, most of the studies conducted on electrochemical treatment were lab-scale technologies with only a few evaluated at a pilot scale. Hence, there is a need for more efforts to determine the applicability of the prototype technology of the system to establish its viability (Ibrahim et al. 2022). Furthermore, there is no universally accepted electrochemical treatment technology for the treatment of highly contaminated RWW, but hybrid application with other treatment processes (such as biological and physicochemical processes) may prove the required efficiency and more work is needed in this direction (Treviño-Reséndez 2021; Ibrahim et al. 2022)
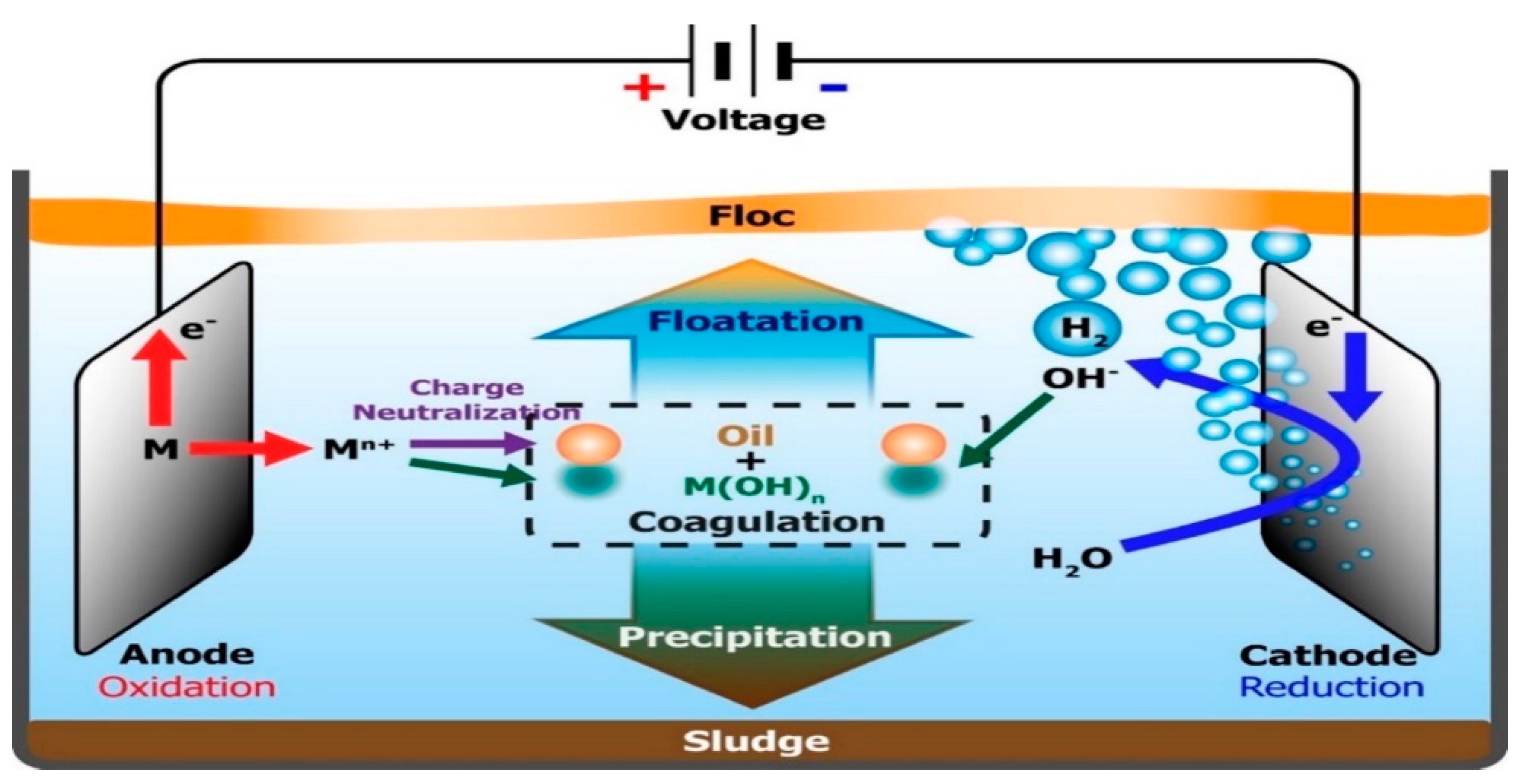
Figure 7.
Electrochemical cell showing the different electrochemical processes. (Khalifa et al. 2022). DC: Direct current. EF: Electro-flotation. EC: Electrocoagulation EO: Electrooxidation. A: Aluminium. EP: electrophoresis.
Figure 7.
Electrochemical cell showing the different electrochemical processes. (Khalifa et al. 2022). DC: Direct current. EF: Electro-flotation. EC: Electrocoagulation EO: Electrooxidation. A: Aluminium. EP: electrophoresis.
Electro Floatation (EF)
This is an advanced and enhanced form air floatation process which carries floating pollutants to the surface by buoyancy and gas bubbles (usually oxygen and hydrogen gases) produced as a result of the electrolysis of water (Khalifa et al. 2022). Unlike the conventional DAF which depends on the solubility of oxygen and nitrogen in the wastewater, in electro floatation oxygen and hydrogen gas bubbles were formed at the surface of the anode and cathode respectively. Although it can be used as a separate process, it is usually combined with coagulation, flocculation, or both to remove pollutants by skimming (Mickova 2015). The efficiency of the EF process is dependent on the current density, pH of solution and temperature. Furthermore, it differs from conventional air floatation in that it provides uniform and finely dispersed gas bubbles and requires little space and less operation cost (Adetunji and Olaniran 2021). While the choice of the electrode material is very vital for a successful implementation of EF, Titanium-based inert anodes in the form of dimensional stable anodes (DAS) are the most dominantly used anodes (Mohtashami and Shang 2019). Alam and Shang (2017) studied the treatment of synthetic oil sand tailing using a batch cell electro-flotation reactor made up of stainless steel mesh cathode and Ti-IrO2 mesh anode. At an optimum current density of 150 A/m2, about 90% oil flotation efficiency was achieved.
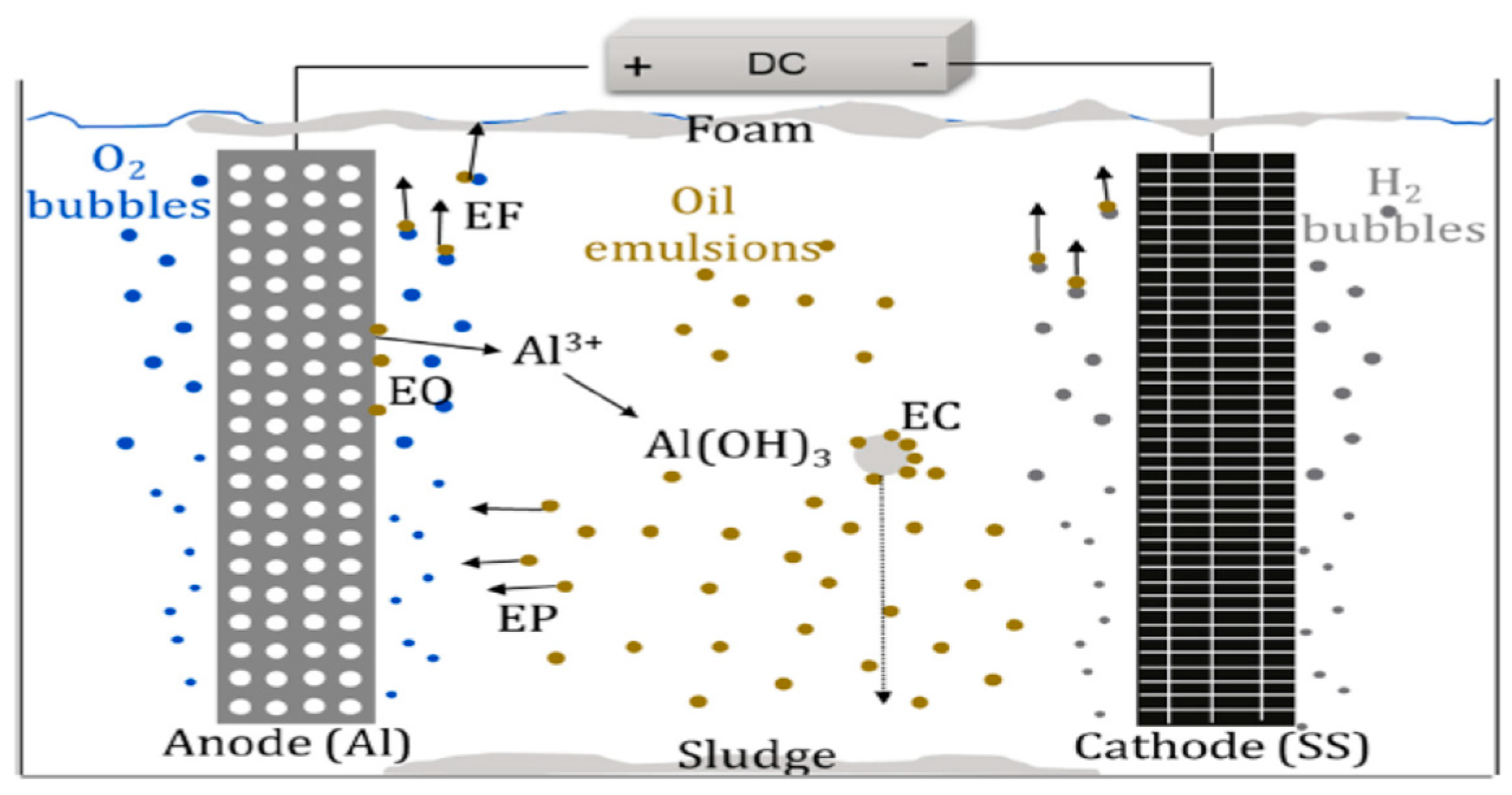
Electrocoagulation (EC)
Electrocoagulation is one of the most prominent electrochemical processes employed for the treatment of oily wastewater such as PRWW and is efficient for the removal of a colloidal immiscible form of pollutants of less than 10 micrometres (Khalifa et al. 2022). This technology involves an in-situ release of appropriate coagulant (such as aluminium or iron species) from a metal electrode with the application of an electric current leading to the electrolytic dissolution of metal ions. The process would result in a simultaneous formation of hydroxyl ions and hydrogen gas production while the coagulant aggregate and precipitates suspended solids (Adetunji and Olaniran 2021). Among the advantages of this technology are simple and automated operation, lower sludge volume and no chemical requirement except for pH control (Merma et al. 2020). Akkaya (2022) reported the use of aluminium and iron cathode electrodes from scrap metals disposed by different industries for the electrocoagulation process of PRWW under an optimum condition of 6.30 pH, current density of 22 mA/cm2 and exposure time of 39 minutes. The process obtained COD and phenol removal efficiencies of 91.18% and 91.46%, respectively. The three-step pilot plant process reported by El-Naas et al. (2016) consisting of an electro-coagulation unit has resulted in the best performance to enhance COD and suspended solids removal. The plant achieved a 96% reduction of COD and a 100% reduction of phenol and cresols concentrations. El-Ashtoukhy et al. (2013), also utilised a fixed-bed electrochemical reactor for the electrocoagulation of phenolic compounds in a real RWW sample. They reported 100 per cent phenol removal efficiency for 3 mg/l in two hours. Gousmi et al. (2016), similarly reported the application of iron and aluminium electrodes in an electrolytic reactor to determine the treatment efficiencies of COD and turbidity from synthetic PRWW. The process revealed 83.52% and 99.94% removal efficiencies for COD and turbidity respectively. The major drawback of this system especially when used separately often yields a lower efficiency in a high concentration of oily wastewater. Thus, it is commonly combined in an integrated process with other methods. Furthermore, it involves the application of electrochemical cells, where electrodes are dipped into oily wastewater, and determine their potential current difference being applied (Elmobarak, 2021).
Figure 8.
Mechanism of electrocoagulation process for oil removal. (An, et al. 2017).
Figure 8.
Mechanism of electrocoagulation process for oil removal. (An, et al. 2017).
Electrooxidation (EO)
This is an advanced form of chemical oxidation process which involves the generation of the oxidants that oxidize the pollutants through the application of electric current (Adetunji and Olaniran 2021). The EO process is sometimes considered as part of the AOPs from a broad perspective but only the oxidation process here occurs on the surface of the anode electrode as opposed to direct oxidation in the latter (Khalifa et al. 2022). The efficiency of the EO process is affected by operating conditions such as the current density and electrode activity as well as pollutants diffusion rate (Adetunji and Olaniran 2021). Ibrahim et al. (2013) reported an electrochemical oxidation process for the treatment of RWW effluent with optimized conditions of 30 mA/cm2 current density, pH 8, supporting electrolyte 2g/l, and operation time of 120 minutes. Ruthenium oxide-coated Titanium and stainless steel served as the anode and cathode respectively. were estimated. FTIR analysis was conducted to determine the removal of pollutants by electrooxidation degradation and 92% COD removal efficiency was estimated. The efficacy of lead oxide reinforced on tantalum (Ta/PbO2) and boron-doped diamond (BDD) anodes contained in an electrolytic batch cell were determined by Gargouri et al. (2014) for the treatment of oily wastewater. At different current densities of 30, 50 and 100 mA/cm2 COD removal efficiency of 85% and 96% were obtained after 11 and 7 hours respectively.
Table 8.
Treatment of petroleum refinery wastewater by an electrochemical process.
Table 8.
Treatment of petroleum refinery wastewater by an electrochemical process.
| |
|
Removal Efficiency |
|
| Electrodes/reactor |
Process |
COD (%) |
Phenols (%) |
Oil (%) |
Reference |
| Porous graphite electrodes. |
EFen |
95.9 |
|
|
Fahim and Abbar (2020) |
| Electrochemical reactor with Ti-IrO2 mesh anode |
EF |
|
|
90 |
Alam and Shang (2017) |
| Aluminium and iron cathode electrodes from scrap metals |
EC |
91.18 |
91.46 |
|
Akkaya (2022) |
| Fixed-bed electrochemical reactor |
EC |
|
100 |
|
El-Ashtoukhy et al. (2013) |
| Aluminium electrodes in an electrolytic reactor |
EC |
83.5 |
|
|
Gousmi et al. (2016), |
| Ruthenium oxide-coated Titanium and stainless steel |
EO |
92 |
|
|
Ibrahim et al. (2013) |
| Lead oxide reinforced on tantalum (Ta/PbO2) and boron-doped diamond (BDD) anodes |
EO |
96 |
|
|
Gargouri et al. (2014) |
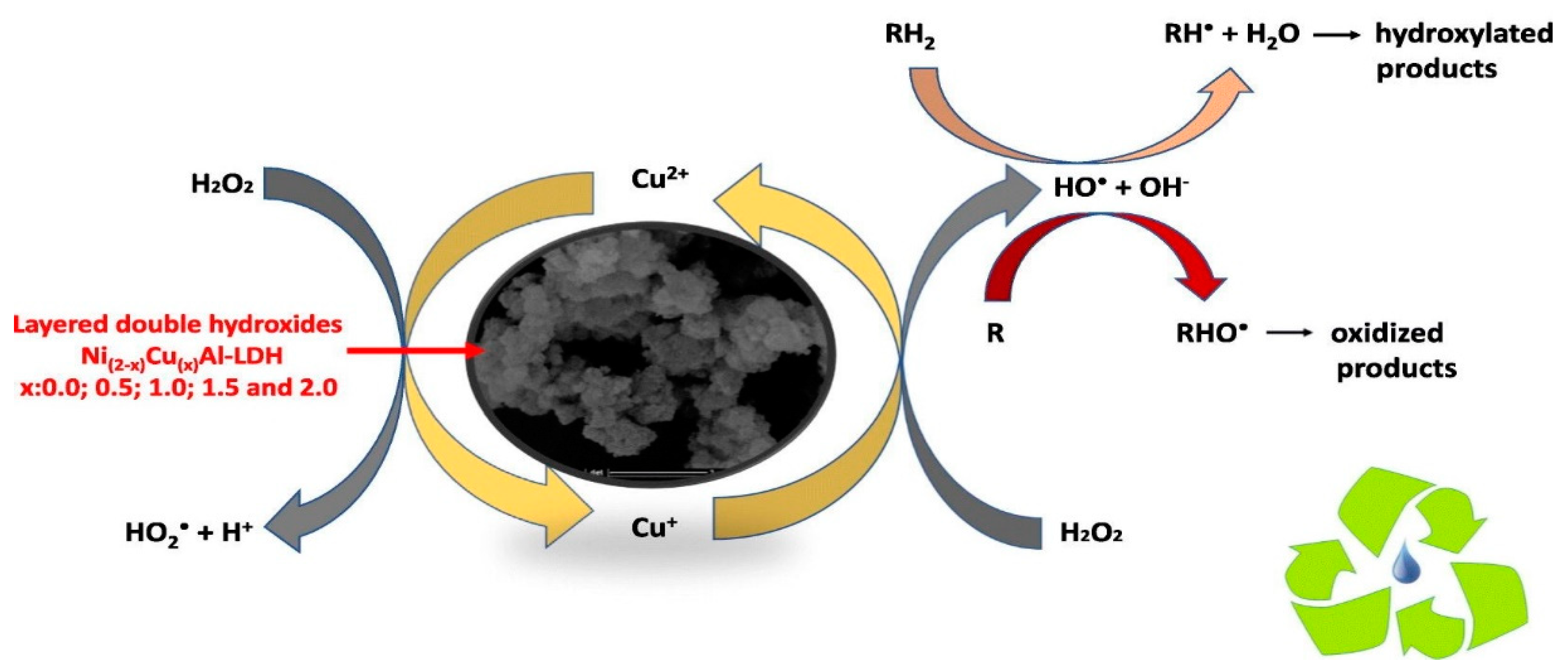
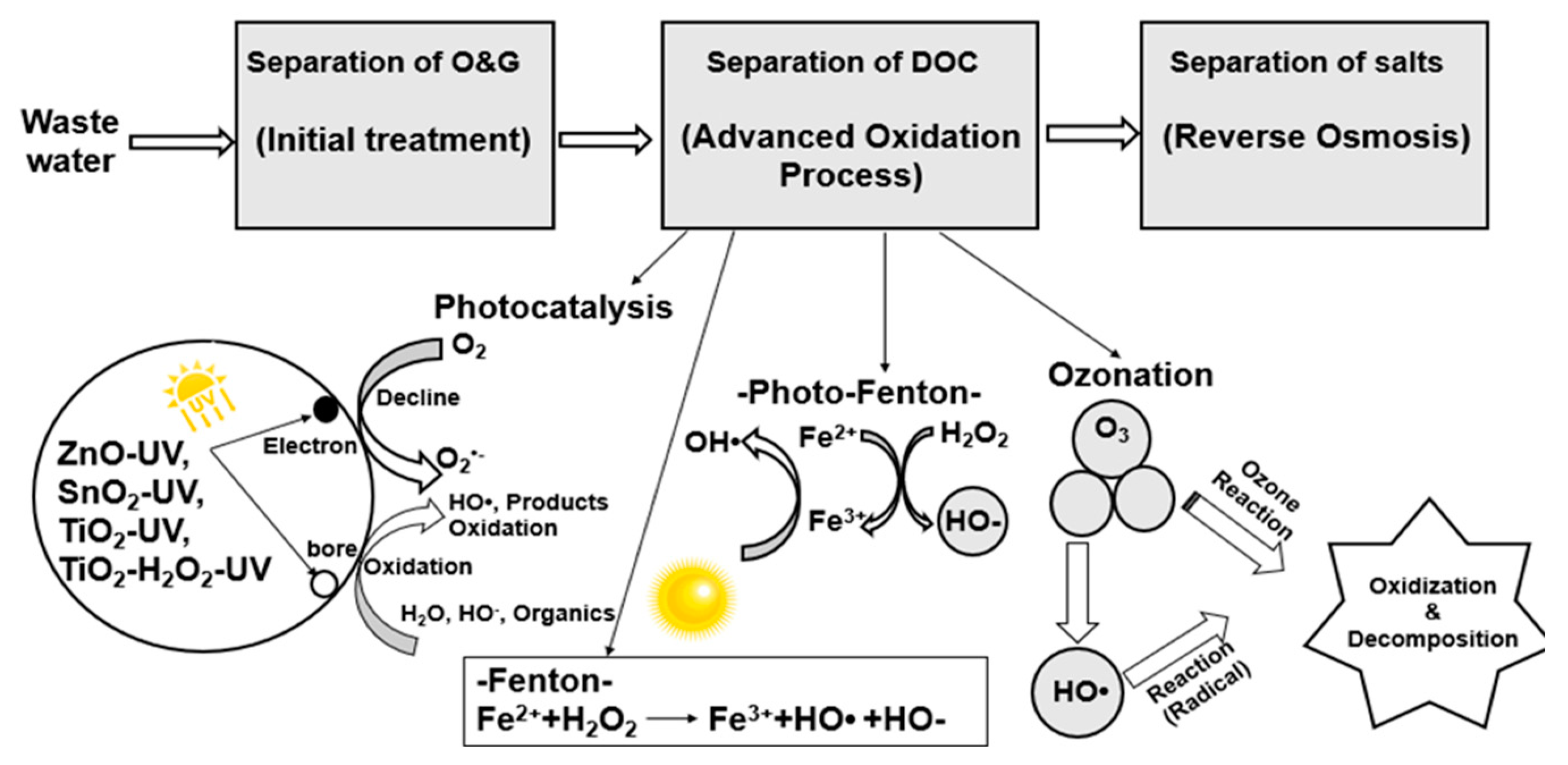
3.4.3. Advanced Oxidation Processes
The chemical oxidation techniques are a set of treatment processes which can be broadly classified into two types; conventional chemical treatments and advanced oxidation processes (Almomani, et al. 2016). Advanced oxidation processes are highly efficient techniques used in the treatment of different types of wastewater including petroleum industry wastewater, toxic effluents from pharmaceutical industries wastewaters, etc. In previous years, several works have been reported in the literature been conducted to examine the efficiency of the advanced oxidation processes in the treatment of different wastewaters containing recalcitrant and toxic pollutants (Elmobarak et al. 2021). Advanced oxidation processes (AOPs) are a category of chemical treatment methods that produce free hydroxyl radical groups with strong oxidant potential and are capable of degrading contaminants. The most commonly employed AOPs in the treatment of RPWW include; Fenton and Photo-Fenton oxidation reaction processes, electrochemical oxidation, ozonation processes (O3), as well as heterogeneous photocatalytic oxidation (Elmobarak et al. 2021; Khalifa et al. 2022). The AOPs are nowadays gaining more attention as they are environmentally friendly techniques with less generation of hazardous by-products and have shown high treatment efficiencies in the removal of organic compounds even at low concentrations (Tetteh et al. 2020). The treatment capability is attributed to the strong hydroxyl radical (-OH) which has strong reactivity towards organic compounds and colour degradation potential (Palaniandy and Feroz 2019). Based on this, AOPs have been reported as an efficient treatment technology for the reduction of COD, odour, colour other specific pollutants as well as sludge treatment. It can also be used in combination with biological treatment processes as a non-selective integrated chemical oxidant with high efficiency in removing toxic organic compounds such as phenols. Wang et al. (2019) also reported that AOPs are usually rapid processes with high treatment efficiency and little residual production but on the other hand associated with high energy requirements. Azizah and Widiasa (2018) investigated the application of H2O2/UV and H2O2/UV/O3 configurations for the treatment of PRWW with high phenol concentration. High phenol degradation of about 93.75% was achieved using H2O2/UV/O3 configuration with 1000 ppm concentration of H2O2 after 120 minutes. Several studies have also shown more than 90% COD and Phenol removal efficiencies from the application of the H2O2-based advanced oxidation process. Similarly, de Oliveira, et al. (2020) have reported their study in which they synthesised TiO2 nanoparticles assisted by microwave, from titanium tetrachloride and water, and used it as a catalyst subjected to photodegradation under UV-C irradiation using promising UF-Permeate from a Membrane Bioreactor. TOC and total nitrogen (TN) removal efficiencies were 32 % and 67 %, respectively, under a pH of 10 and catalyst concentration of 100 mg /L in a reaction time of 90 minutes. Furthermore, the catalyst shows stability after 4 different cycles of application and the data obtained is promising to prove the capability of the catalyst in the removal of recalcitrant organic pollutants of the UF-Permeate from a Membrane Bioreactor which can also reduce fouling in downstream polishing processes. Most of the reviews (Elmobarak et al. 2021; Palaniandy and Feroz 2019; Adetunji and Olaniran 2021; Aljuboury et al. 2017; Khalifa et al. 2022) have shown best treatment experimental results obtained using the AOPs than conventional methods.
Fenton-Oxidation
Generally, among AOPs, the Fenton technology is very attractive due to their simplicity, high performance, low cost and the lack of toxicity of the Fenton's reagents which are the ferrous ion and hydrogen peroxide (He & Zhou 2017). The Fenton process (FP) is based on a redox reaction between a chemical mixture of hydrogen peroxide (H2O2) and ferric ions Fe2+ founded by Henry John Horstman Fenton in 1894 which has a strong oxidizing potential in an acid medium (Raji and Mirbagheri 2021). The Fenton reagent is a strong oxidant of hydroxyl radicals formed by a reaction between the ferrous ion (Fe2+) and the hydrogen peroxide (H2O2). The hydroxyl radical is capable of the degradation of toxic and non-biodegradable pollutants by direct or indirect anodic oxidation (Giri and Golder 2014; Adetunji and Olaniran 2021). The OH- radicals are extremely strong reactive oxidizers with an oxidation potential of approximately, Eθ = 2.8 V and they are generally non-selective towards organic pollutants in wastewater (Cardoso, et al. 2021). There are two types of Fenton reactions; the standard Fenton reaction which is formed as a result of a reaction between ferrous iron (Fe+2) ions and hydrogen peroxide (H2O2) as well as the Fenton-like which is formed by a reaction of (Fe+3) ions and hydrogen peroxide (Palaniandy and Feroz 2019). Fenton reaction conducted under light intensity such as UV or sunlight which generates more hydroxyl radicals is called Photo-Fenton. While normally the ratio between the iron ions and the peroxide which is [Fe2+] / [H2O2] is 1:2. However, the study reported by Wang et al. (2019) suggested a ratio of 1:5 for a greater rate of degradation. The Fenton-oxidation technique has been widely investigated in the treatment of different types of wastewater effluents including textiles (Nidheesh, & Gandhimathi 2015) and pharmaceuticals (Quang, et al. 2022). Fenton's reaction process has yielded excellent results as an advanced oxidation process to reduce organic pollutants from PRWW and has been reviewed by many researchers in the literature. However, the Fenton process's general limitations include the problem of adding the H2O2 and its low utilization and mineralization efficiencies (Bello, et al. 2019). The review reported by Elmobarak et al. (2021) summarised that the major drawbacks of the Fenton and the Photo-Fenton processes include their requirement for a very low pH value of usually less than 2 as well as the need for the elimination of the iron ions after the reaction process. Additionally, the potentiality of the -OH radical degradation tends to reduce with a rise in the pH value. At a very low pH, there would be a creation of Fe (II) (H2O)2+ which can react with the hydrogen peroxide (H2O2) leading to reduced generation of the hydroxyl radicals. Shokria et al. (2019) have reported a study using FeCl3 and hydrogen peroxide on Box-Behnken design to decrease the COD of petrochemical wastewater. At a pH value of 5.63, maximum COD removal efficiencies of 72.06% and 74.9% were obtained at an operation condition of [Fe3+] = 1.76 mM and [H2O2] = 17.86 mM. Other contaminants including the BOD TOC and TDS were also decreased considerably. Similarly, Tony et al. (2012) have investigated the capabilities of using Fenton's reagent (Fe2+/H2O2) and Photo-Fenton's reagent (Fe2+/H2O2/UV) for the treatment of oil refinery wastewater from Whitegate refinery, County Cork, Ireland. At an optimized condition of pH 3, H2O2 (400 mg/L) and Fe2+ (40 mg/L), the photo-Fenton treatment achieved approximately 50% COD removal efficiency. Similarly, the report of a study conducted by Hassan et al. (2020) using Fenton reagent (Fe2+/H2O2) for the treatment of refinery wastewater achieved 86% and 97% COD and total petroleum hydrocarbons removal efficiencies at a pH of 3.5 and reaction time of 60 minutes. A Heterogeneous Fenton-like degradation technique of organic pollutants from PRWW by copper-type layered double hydroxides to degrade aromatic and aliphatic organic compounds was reported by Radji, et al. (2022). From their study, they synthesized Ni(2-x) Cu(x)Al-LDH layered ternary double hydroxides as a catalyst with a series of x ratios: 0.0; 0.5; 1.5; and 2.0. Their findings on the oxidation reaction showed that catalytic activity varied inversely with the Ni2+/Cu2+ ratio and activity was maximum for x:2.0 where the catalyst can remove about 74.8% of TOC, and the aromatic compounds. Hence, they conclude that Cu+ is catalytically active and increases the TOC reduction in this case.
Figure 9.
Heterogeneous Fenton-like degradation of PRWW pollutants using synthesized Ni(2-x)Cu(x)Al-LDH layered ternary double hydroxides catalyst. (Radji, et al. 2022).
Figure 9.
Heterogeneous Fenton-like degradation of PRWW pollutants using synthesized Ni(2-x)Cu(x)Al-LDH layered ternary double hydroxides catalyst. (Radji, et al. 2022).
Electro-Fenton Process
This is a novel oxidation process which employs the electrochemical process and generation of TiO2 oxidants by the Fenton-oxidation process. This technique has been studied for the removal of COD, BOD, total petroleum hydrocarbons (TPH), Phenols and other recalcitrant compounds that are not easily degraded in conventional treatment plants (Kulkarni and Goswami 2015). Fahim and Abbar (2020) have reported a study of treating Al-Dewaniya petroleum refinery plant wastewater in Iraq by electro-Fenton process using porous graphite electrodes as anode and cathode materials. They used a tubular type electrochemical reactor with a cylindrical cathode made from porous graphite and concentric porous graphite rode which acts as an anode. At a current density of 25 A/cm2, and operation time of 45 minutes with no addition of NaCl, the removal efficiency of COD was found to be 95.9% with an energy consumption of 8.921kWh/kg per COD. The outcome of the experimental work has demonstrated the capability of the graphite–graphite Electro-Fenton system as an effective technique in the removal of COD from petroleum wastewater. Divyapriya & Nidheesh (2020) also reported from their review that the use of graphene-based electrodes in the electro-Fenton technique is usually considered to be a promising and cleaner method to produce the reactive oxygen species that can mineralize organic contaminants rapidly. They also added due to its catalytic activity, stability, and reusability, Graphene derivatives have been used to immobilize various heterogeneous Fenton catalysts. The application of a photovoltaic cell electro-Fenton oxidation has been reported by Atiyah et al. (2020) for the treatment of refinery wastewater. During the treatment process, hydrogen peroxide dosage, the electrolysis time, and current density including the rate of energy consumption and cost were examined for the efficiency in the removal of total organic carbon. The optimum operational conditions include current (0.5-2 mA), H2O2 concentration (10-50 ppm) and electrolysis time (10-30 min). Under these conditions, about 98% removal efficiency of the organic content was achieved at 39.67 kWh/m3 of energy. However, only a few studies related to the use of the electro-Fenton process in the treatment of real petroleum refinery wastewater have been reported. Model wastewater consisting of demineralised water and phenol (0.5 g/l) has been used for the removal of the phenol from a study reported by Procházka, et al. (2019). In their experiment, they used iron sulphate dosed into the solution (m = 0.261 g) acting as the source of Fe2+ ions which constitute the iron anode and the cathode is made of titanium in other to electrochemically enhanced the reaction. A current density of 408.16 A/m2 and a pH of 3 were employed and subsequently dosed hydrogen peroxide provided free hydroxyl radicals and started the reaction with concurrently added iron ions while the hydrogen peroxide dosage is determined in the process. The process shows excellent performance in the reduction of the COD until the postponement of the hydrogen peroxide addition. The major advantage of this process is the indirect addition of the Fe+2 in the solid phase which eliminates the use of the Fe in the form of its solution such as iron sulphate or iron chloride salts. However, besides the requirement for the addition of hydrogen peroxide catalyst, the major drawback associated with the electro-Fenton process which can prevent its industrial application or commercialization is its energy consumption to support the electrochemical process. Hence, there is a need for further research applications to determine the appropriateness as well as the efficiency of the treatment technique in the degradation of PRWW contaminants.
Photocatalysis
Photocatalysis is nowadays regarded as one of the most advanced as well as an environmental-friendly technique for the total degradation of organic contaminants in various forms of wastewater (Rashid et al. 2015). The term photo-catalysis is a powerful chemical technology process which converts solar energy to chemical energy for the synthesis of highly functionalized complex molecules in the form of radicals (Yang and Wang 2018; Candish, 2021; Khan, 2022). Meanwhile, a photo-catalyst is often defined as a material such as titanium oxide (TiO2) and transition metal oxides which can decompose harmful substances under the effect of the sun lights containing UV rays (Sakka, 2013). The process occurs by the excitation of pairs of electrons in the valence band by a UV which causes them to absorb higher energy than their gap band energy which then causes a simultaneous production of a hole in the valence band (h+) and an electron (e-) in the conduction band. Furthermore, the (h+) and (e-) species would then react with oxygen or water molecules to produce peroxide or hydroxyl radicals which are capable to degrade or decompose organic compounds (Tetteh et al. 2020; Lau et al. 2018; Sakka, 2013). Depending on the specific characteristics of the semiconductor, the photolytic activity in photocatalysis is firstly initiated with the absorption of energy in the form of photons which has an energy equal to or more than the band gap exhibited by the semiconductor such as TiO2 (Abeish 2015). This absorption creates a hole which induces the excitation of an electron to the conduction band while a positive hole is created in the valence band. This hole would in turn generates highly reactive hydroxyl radicals with high reduction-oxidation potentials such as •O2−, H2O2, and •O2 that can play an important role in the photocatalytic reaction mechanism (Subramaniam et al. 2019). Photocatalysis has been employed as a more advanced practical and efficient process in the treatment of wastewater to degrade organic contaminants (Khan, 2022; Lau et al. 2020; Park, et al. 2022). In achieving this, pore volume, pore structure, crystalline sizes, light intensity as well as specific surface area are the important parameters which determine the excellent performance of photocatalysts. Abeish (2015) further noted that important operational parameters affecting the degradation of organic pollutants from RWW using photocatalysis include; temperature, pH, photocatalyst loading, wavelength and light intensity, initial pollutant concentration as well as TOC and COD concentrations. For example, a laboratory study reported by Pardeshi and Patil (2008) revealed that the degradation of phenol is more effective under solar light intensity than artificial visible light irradiation. In the photocatalytic degradation of phenols and their chlorophenol and nitrophenol derivatives, the hydroxyl radicals usually attack cyclic carbon atoms as the main reaction site leading to the formation of various oxidation intermediates. The intermediates are then eventually converted to acetylene, maleic acid, carbon monoxide and carbon dioxide (Ren et al. 2021). The CO2 produced during the photocatalytic process can be trapped for other uses in other to prevent further environmental pollution (Qaderi and Abdolalian 2022).
A nano-catalyst usually possesses high surface area and density (Yadav 2020) which gives it more photocatalytic activity and applicability in wastewater treatment (Al-Mamun et al. 2019). For example, Titanium dioxide (TiO₂)-based photocatalyst is the most widely used in wastewater treatment due to their high oxidizing ability of organic compounds, cost-effectiveness, nontoxicity, and environmentally friendly (Anucha, 2022; Lau et al. 2020; Stasinakis 2008). Dang et al (2016) reported that the most important semiconductor catalyst widely employed for photocatalytic degradation of phenols in wastewater treatment includes; ZnO, CdS, TiO2, GaP, ZnS and Fe2O3. Meanwhile, Park et al. (2022) also reported that the most widely studied and developed pollutant removal photocatalysts are titanium dioxide (TiO2) and transition metal oxides. However, the fast recombination of charge carriers is one of the major limitations in the photocatalytic performance associated with TiO2 (Nasr et al. 2018). Graphene, which is a Carbon-based material has been also tested and demonstrated high potential applicability for general pollutant removal. The UV light interaction with a photocatalyst works within a wavelength range of 280-400nm and 400-700nm for the visible light range (Lau et. Al. 2018). UV light intensity and initial concentration are very influential factors which affect the performance of photocatalytic degradation. Moreover, the efficiency of photocatalysis can be enhanced through the combination of photocatalysts with oxidizing agents such as H2O2 (Kane et al. 2022).
Properties of Photocatalyst
Photocatalysts are usually employed either in the form of powders or thin films based on the requirements and scope of application (Baradaran and Ghodsi 2021). Nano forms of photocatalyst are better to fast reaction rates than the bulk form due to their small size and high surface area (Tahir 2020). However, using nanoparticles for wastewater treatment and pollutant degradation also has limitations related to their fast recombination losses as well as inadequate utilization of the solar spectrum (Das and Dhara 2021). Estrada-Flores et al. (2020) reported a study on the relationship between morphology, porosity, and the photocatalytic activity of the anatase phase of TiO2 synthesized by a modified sol-gel using a different ionic surfactant. The result of the experiments shows that the specific surface area of the anatase photocatalyst increases with an increase in pore sizes and that sodium dodecyl sulfate (SDS) modified anatase has the lowest band gap value of 2.97eV and highest specific surface area of 138.72m2/g as well as highest photocatalytic activity. Other important photocatalysts reported to have been used in the treatment of PRWW and their corresponding band gaps include; Fe2O3 (2.2 eV), α-FeO3 (3.1 eV), SnO2 (3.5 eV), Si (1.1 eV), SrTiO3 (3.4 eV), ZnS (3.2 eV) and WO3 (2.7 eV).
Treatment using Photocatalysis
Process simplicity and ease of operation as well as the potential of using sunlight energy make photocatalysis to be an attractive prospect for the degradation of organic pollutants in petroleum refinery wastewater (Ren et al. 2021). The degradation of organic contaminants from PRWW by Photocatalysis has been widely investigated using various forms of photocatalyst at varying conditions. In this work, we review the recent studies conducted in the photocatalytic removal of organic contaminants from RWW and hope to provide prospects for the development of complex structured photocatalysts. Ghasemi et al. (2016) reported the treatment of PRWW by photocatalytic degradation using TiO2/Fe-ZSM-5 photocatalyst with as-synthesized Fe-ZSM-5 zeolite produced via the sol-gel method with a specific surface area of 304.6 m2 /g and 29.28% loaded TiO2. About 80% COD removal was achieved at a pH level of 4, photocatalyst concentration of 2.1 g/l, and 45 °C UV exposure temperature through 240 minutes. Although high COD removal efficiency is achieved, the synthesis of ZSM-5 zeolite catalyst is associated with high production cost via complex processes influenced by the effect of time and temperature (Widayat and Annisa 2017). The study reported by Ul haq et al. (2020) using Fe2O3, MnO2, TiO2 and ZnO for the photocatalytic oxidative degradation of hydrocarbon pollutants from PRWW of Attock Oil refinery in Pakistan shows that the highest photocatalytic degradation was exhibited by TiO2 conversion for benzene, toluene, phenol, and naphthalene at 92, 98.8, 91.5, and 93% respectively. The reaction condition includes; a 100 mg/L catalyst dose at a pH of 3 and temperature of 30°C through a 90-minute reaction time. Moreover, 93.2% COD removal efficiency is also recorded. Complete photodegradation of the parent hydrocarbons is observed using the UV/TiO2 system. This study has proved a reference for the photocatalytic degradation ability of the TiO2 photocatalyst and its low pH requirement and high COD efficiency from RWW.
Similarly, Aljuboury et al. (2016) have reported the results of an investigation of using combined solar photocatalyst titanium oxide/zinc oxide (TiO2/ZnO) with aeration processes. The Maximum removal efficiencies for TOC and COD were achieved at 99.3% and 76%, respectively. Optimum operating conditions included; 0.5 g/L each for TiO2 and ZnO dosage, pH 6.8, air flow of 4.3 L/min and reaction time of 170 minutes. Fernandes et al. (2020) have reported the synergistic effects of using O3, H2O2 and O3/H2O2 as an external oxidant with TiO2 under UV light intensity were evaluated for the reduction of COD and BOD as well as degradation of Volatile Organic Carbons (VOCs). After 280 minutes of the treatment process, 38 and 32% COD and BOD reduction was observed and up to 84% degradation of the total VOCs. Furthermore, Sulfide ions concentration was completely depleted in the first 30 minutes of the experimentation. However, for an industrial practice implementation, there is a need to scale up a pilot scale of the process in a real-case scenario. Titanium oxide and a Silver doped nanoparticle synthesized as TiO2/Ag photocatalyst fixed on lightweight concrete plates were used in a study reported by Delnavaz and Bos' hagh (2021) on a real oil refinery effluent. Investigation of the effects of pH and mass loading on the system efficiency shows that the highest removal efficiencies were at the ranges of 4.5 was 15 g/m2 respectively. The COD removal efficiencies recorded after 8 hours under the sunlight and using the UV-A lamps for TiO2/Ag photocatalysts were 51.8% and 76.3%, respectively. Hence based on this experiment, the synthetic TiO2/Ag photocatalysts are capable of treating COD from real RWW both under sunlight and UV light intensities.
Photo-catalysts treating efficiencies of TiO
2 and zeolite for the removal of COD and SO
2− from PRWW were compared using a photo-catalytic system by Tetteh et al. (2020a). The operating conditions of the system include; a catalyst dosage of 0.5–1.5g/L, a mixing rate of 30–90 rpm and an 18W UV light. At a reaction time of 15–45 minutes, the results of the reaction show almost the same efficiency of 92% for zeolite and 91% for TiO
2. Oil removal efficiency by Photocatalysis has been examined by Mohammed et al. (2021) using ZnO photocatalyst under solar light and determined the effects of catalyst loading, pH and initial oil concentration. The outcome of the experimentation shows 75% oil reduction and that optimum catalyst concentration is found with a 3g/l dosage of ZnO and a pH of 10. The oil degradation rate decreased with increasing oil concentration which might be due to an increased level of turbidity as a result of the oil suspension which in turn decreased the permeation of the solar-light intensity. Hence, based on this, it can be ascertained that zinc oxide catalyst has been found very proper at a high pH level. Similarly, the Phenols degradation capacity of ZnO nanorods (NRs) grown on a glass substrate has been investigated by Daher et al. (2019) which reported about 90% phenolic degradation under 254 nm UV light energy within 10 hours of irradiation time. Local South African oil refinery wastewater was treated by Tetteh et al. (2020b) via photocatalytic degradation using TiO
2 Degussa P25 catalyst comprising 80% anatase, and 20% rutile. A batch-aerated photocatalytic reactor was used at different levels of operational variables including; TiO
2 dosage (2–8 g/L), reaction time (30–90 minutes) as well as airflow (0.768–1.48 L/min). The optimum condition for Phenol removal up to 76% was achieved at 8 g/L TiO
2 dosage of, 90 minutes reaction time and 1.225 L/min aeration flow rate. The Photocatalytic degradation of phenols experiment conducted by Ramachandran et al. (2021) using 0.2 g/l of TiO
2 as photocatalyst and employing 8W UV lamps revealed that COD concentration is completely reduced after 5 hours of reaction time. However, despite the efficiency of the UV lamps, they also reported that solar-supported photocatalysis is better considering the implications of time, space and cost. Aljuboury and Shaik (2021) have reported a study conducted to investigate COD and TOC removal efficiencies from real RWW using TiO
2/ZnO/ air/Solar and TiO
2/ZnO/Fenton/Solar processes. About 74% of COD and 99% of TOC removal efficiencies were achieved after 180 minutes under an optimal condition of 54 g/L and 50 g/L ZnO and TiO
2 dosages respectively. The report further revealed that the solar photocatalyst of TiO
2 /ZnO/Fenton is most efficient at neutral pH and hence no pH level adjustment during the treatment process. Although TiO
2/ZnO/ air process is costly it is found to be more efficient in a situation where the pH level is greater than 7. It can be deduced from this study and those previously reported studies that acidic and alkaline conditions of the PRWW determination are very significant in the choice of an appropriate photocatalyst and its application. A review summary of the treatment of petroleum wastewater using photocatalysis techniques is presented in
Table 9.
3.4.4. Combined H2O2/UV Advanced Oxidation Process
The use of hydrogen peroxide as an oxidant in combination with potential sources of photon energy which can separate it to produce HO- radicals has been reported to be more successful to generate the hydroxyl radical that can degrade organic pollutants (Cardoso, et al. 2021). Using UV radiation of wavelengths>300 nm, hydrogen peroxide (H2O2) can decompose and generate HO- radicals (Elmobarak, et al. 2021). Different researchers have reported that H2O2 pollution degradation continues steadily up to its highest efficiency after which it would start to decrease. This sudden decrease has been proven to be a result of the generating hydrogel radical start to react with the additional H2O2 (Huang, et al. 2020). However, different advantages can be mentioned from the application of the H2O2/UV oxidation process, for example, there is no requirement for the removal of the hydroxyl radical after the treatment process and it is completely soluble in water (Dhivakar & Rajan 2018). Elmobarak et al. (2021) reported that the optimum operating pH of using the H2O2/UV oxidation process should be small usually pH < 4 in other to avoid the impact of ionic radicals such as carbonate and bicarbonate ions.
Figure 10.
Summary of the advanced oxidation processes in the treatment of petroleum wastewater (Elmobarak, et al. 2021).
Figure 10.
Summary of the advanced oxidation processes in the treatment of petroleum wastewater (Elmobarak, et al. 2021).
Table 9.
Treatment of PRWW using photocatalysis technique.
Table 9.
Treatment of PRWW using photocatalysis technique.
| |
Experimental conditions |
Efficiency (%) |
|
| Photocatalyst |
Light |
pH |
Dosage |
Tempt. (°C) |
Time (Min) |
COD |
Phenols |
Oil |
Reference |
| TiO2/Fe-ZSM-5 |
UV |
4 |
2.1 g/L |
45 |
240 |
80 |
|
|
Ghasemi et al. (2016) |
| TiO2 |
UV |
3 |
100 mg/L |
30°C |
90 |
93.1 |
98.8 |
|
Ul haq et al. (2020) |
| TiO2/ZnO |
Solar |
6.8 |
0.5 g/L |
NR |
170 |
76 |
|
|
Aljuboury et al. (2016) |
| TiO2 with synergistic effects of O3, H2O2 and O3/H2O2 |
UV |
NR |
|
280 |
|
38 |
|
|
Fernandes et al. (2020) |
| TiO2/Ag |
Solar/UV |
4.5 |
NR |
NR |
NR |
51.8/76.3 |
|
|
Delnavaz and Bos'hagh (2021) |
| Zeolite and TiO2. |
UV |
NR |
0.5–1.5g/L |
|
|
92/91 |
|
|
Tetteh et al. (2020a). |
| ZnO |
Solar |
10 |
3g/L |
NR |
NR |
|
|
75 |
Mohammed et al. (2021) |
| TiO2 Degussa P25 (80% anatase & 20% rutile) |
UV |
NR |
8 g/L |
|
|
|
76 |
|
Tetteh et al. (2020b). |
| TiO2
|
UV |
NR |
0.2 g/L |
NR |
300 |
100 |
|
|
Ramachandran et al. (2021) |
| TiO2/ZnO |
UV |
7 |
54 g/L & 50 g/L |
|
|
74 |
|
|
Aljuboury and Shaik (2021) |
| ZnO nanorods (NRs) |
UV |
NR |
NR |
NR |
600 |
|
90 |
|
Daher et al. (2019) |
3.5. Integrated Treatment Processes (ITP)
Each of the existing conventional and advanced treatment techniques has specific limitations in terms of their efficiency and is sometimes associated with various demerits for the treatment of PRWW. Based on this there is always a key interest to design and create a novel procedure that overcomes limitations such as operational costs, treatment efficiency, and generation of a secondary pollutant. Refinery wastewater treatment challenges can sometimes be addressed through an integrated or combined treatment process which can yield the benefits required in terms of environmental, operational and cost-effectiveness. For example, a combination of AOPs techniques integrated with conventional methods used for the treatment of different contaminated industrial wastewater has been confirmed to be more efficient. However, the selection for the combination and development of an integrated treatment process requires a good understanding of the wastewater properties, cost determination, as well as the requirements of environmental policies (Elmobarak, et al. 2021).
Figure 11.
The basic considerations in the selection of an appropriate treatment technique.
Figure 11.
The basic considerations in the selection of an appropriate treatment technique.
In most cases, the development of an integrated treatment process is initiated from a combination of two or more conventional and advanced methods. Hence, the configurations of such a combination can be a two-step, three-step or even multiple treatment processes. This might comprise at least one conventional and one advanced, two conventional and one advanced, or even two advanced processes. For example, an advanced oxidation process integrated with biological methods or other processes increases the efficiency of oxidation degradation as well as the separation of contaminants. The biological process can provide the needed decomposition of the residual oil and degradation etc (Jafarinejad & Jiang 2019). The integration or combination of biological methods with membrane-based AOPs techniques was also found to be an efficient process for the treatment of petroleum wastewater. However, in the development of a biological and chemical integrated process, the determination of the individual biological activity and chemical oxidation efficiencies is important for finding the optimal operating conditions for the combined process. This task involves a profound knowledge of the operational conditions for both the biological and chemical processes. Therefore, several analytical parameters such as the TOC (and/or COD) concentration must be monitored during each step of the treatment stage (Oller, et al. 2011). The study reported by (Obuebite & Okwonna 2023) for the treatment of PRWW from Port Harcourt Refining Company in Nigeria applied an integrated treatment approach using a biological aerated lagoon system and a UV light degradation process. With a pH of 7.84, the efficiency of the process yielded BOD 0.65 mg/L, COD 1.87 mg/L, TDS 69.96 ppm and TSS 14.82 mg/L. Ul Haq, et al. (2023) carried out integrated photocatalytic oxidation and adsorption processes for the treatment of PRWW using a TiO2/Activated Carbon hybrid material. The hybrid adsorbent/catalyst was prepared by the impregnation of the TiO2 over the activated carbon. Under the optimized reaction conditions of pH 3, temperature 300C, and 1000 mg TiO2/AC per 500 mL of the sample and a contact time of 50 min, the integrated photocatalytic oxidation-adsorption process achieved a net percentage removal of benzene, toluene, aniline, and naphthalene concentrations of 91% from model HCs solutions. Applying the same process under the same conditions for the treatment of real PRWW using TiO2 and AC caused a 95% decrease in chemical oxygen demand (COD) but at a longer contact time of 105 min and 90 min, respectively using higher adsorbent and catalyst doses. Hence, the integrated photocatalytic oxidation and adsorption techniques using the hybrid TiO2/AC showed a better advantage over the individual adsorption and photocatalytic oxidation processes using the individual AC and TiO2. Using a membrane and biological bioreactor has also been popular in the degradation of PRWW. Razazi, et al. (2015) reported the treatment of real PRWW using a hollow fibre membrane bioreactor (HF-MBR). The bioreactor included an ultrafiltration membrane (UF) and the HF-MBR was run for 160 days. The results of the process indicated an excellent average elimination of the COD, BOD5, TSS, volatile suspended solids (VSS) and turbidity at 82%, 89%, 98%, 99%, and 98%, respectively. However, the efficiency of the process was found to be excellent but the problem of long duration time for the biological degradation is mostly a common limitation associated with biological processes using bioreactors. The study reported by El-Naas et al. (2014) utilized a three-step integrated process consisting of an electrocoagulation cell (EC), a spouted bed bioreactor (SBBR) and an adsorption column. The reactor contains Pseudonymous putida immobilized in polyvinyl alcohol gel while the adsorption column was packed with granular activated carbon adsorbent produced from agricultural waste. The process was able to achieve a reduction in the concentration of COD, phenol and cresols by 97%, 100% and 100%, respectively. They reported that the process can handle highly contaminated refinery wastewater with a relatively wide range of operating conditions. Similarly, Wang et al. (2021) have reported the treatment of PRWW using a multistage-enhanced biochemical process. The technique comprises different units of biological aerated filter (ICBAF), hydrolysis acidification (HA), two anaerobic–aerobic (A/O) units, a membrane biological reactor (MBR), and ozone-activated carbon (O3-AC) units. Hence, the integrated system is made up of biological, chemical, and adsorption processes. They reported that the overall efficiency of the system has achieved 94% removal of the COD, BOD5, ammonia nitrogen (NH4+-N) and phosphorus. The ICBAF unit accounts for 54.6% of COD removal and 83.6% of BOD5 removal, and the two A/O units account for 33.3% of COD removal and 9.4% of BOD5 removal efficiencies. Conventional biological systems such as biological aerated filters (BAF), and membrane bioreactors are usually environmentally friendly but they are often inefficient to remove total pollutants from PRWW that has high COD concentration above 2000mg/L (Wu, et al. 2016). Keramati & Ayati, (2019). Have reported their study for the treatment of petroleum wastewater using a combination of electrocoagulation and photocatalytic process. ZnO nanoparticles immobilized on a concrete surface were used as the photocatalyst to evaluate the efficiency of the system for COD removal. The system's efficiencies were first determined individually before integrating them and evaluating the optimum operating conditions. At a COD concentration of 900 m/L the optimum condition of the EC process was 20 mA/cm2 current density, 8.5 pH and 0.5 g/L NaCl concentration a COD removal rate were 94% was obtained after 60 min. For the photocatalytic process, they used a COD concentration of 600 mg/L at optimum conditions of 80 g/m2 ZnO concentration, pH of 5 and 32 W irradiation power. The COD removal efficiency was 76% after 300 minutes. Thereafter, they implemented the integrated EC and photocatalytic system using an initial COD concentration of 1000 mg/L where a COD removal efficiency of 47% was achieved after 8.5 min using the EC process; after that, the effluent entered the concrete photoreactor for 120 min, which lead to 85% of COD reduction and final COD concentration reached was 75 mg/L. Similarly, Ratman, et al. (2021) have conducted a study to explore the application of an advanced membrane process integrated with ozonation as a preliminary treatment. They used a synthesized Polysulfone PSf-TiO2 membrane and a constant ozonation dose of 3000 mg/h at different times and temperature combinations. They noticed a longer ozonation time significantly improved the removal of pollutants. However, an increase in temperature does not significantly affects COD, phenol and TDS, removal efficiencies but only ammonia removal up to 82%. The use of the ozonation process also enhanced the permeate flux of the membrane by up to 96% and improved pollutant removal efficiency by up to 77%. Mokhtari, et al. (2021) have also reported the application of an innovative method of biological process coupled with a sand filter column for the treatment of Iranian petroleum refinery wastewater as a hybrid process. It is a simple integrated process where the sand filter column was used in the last part of the treatment process. The biological system consisted of four fully immersed vertical rotating bioreactors (RBCs) with the sand column filter placed at the end. Overall treatment efficiencies recorded for COD, TSS, oil, ammonia (NH3), and turbidity were 94%, 90%, 88%, 93%, and 92%, respectively. These results have also confirmed the effectiveness of the integrated system in achieving high removal efficiencies in the treatment of PRWW.
4. Summary and Future Research Perspectives
This review presents an overview of the recent application of conventional and advanced technologies for the treatment of petroleum refinery wastewater. Environmental pollution due to oil refinery wastewater is a global phenomenon that attracts serious attention due to its hazardous effects on the ecosystem. Nowadays, the need to meet environmental discharge limit regulations for petroleum oil refinery wastewater is usually a challenge to petroleum refinery industries. This is due to the dynamic complexity of the Petroleum refinery wastewater. Hence, over the years conventional and advanced treatment technologies such as adsorption, membrane filtration, chemical precipitation and biological systems have been designed to address this challenge. The appropriate selection of the treatment technology mostly depends on the wastewater composition, operational costs, efficiency, and its end environmental impact. While some already established technologies are mature in their applications, some others lack to meet the requirements of environmental regulations and are also associated with high energy and maintenance costs. The new techniques such as adsorption with modified adsorbents, photocatalysis, and other advanced oxidation processes have demonstrated a considerable efficiency in the treatment of refinery wastewater. For example, it is evident from the literature that advanced treatment techniques such as the AOPs and photocatalysis have been found as one to be the most effective techniques to reduce COD, remove oil and grease as well as phenol degradation. Additionally, adsorption techniques using the non-conventional adsorbents such as hydrogel were also found to be effective in the treatment of synthetic petroleum wastewater. However, it is important to note that the use of single treatment technique does not usually achieve the required full efficiency. The application of an integrated or hybrid process is nowadays taking much interest to design and create a novel procedure that overcomes limitations such as operational costs, treatment efficiency, and generation of a secondary pollutant. However, little research work is been reported to explore the application of hybrid techniques using modified adsorbents such as hydrogels on real petroleum refinery wastewater while almost all reported studies were limited to laboratory scale test. This is because the synthetic petroleum refinery wastewater sample might tend to have different characterization to a synthetic sample. Therefore, more research work would be required to be conducted on the real wastewater sample. This could perhaps lead to the development of an innovative treatment techniques for the petroleum industry which in turn would promote water sustainability. Hence, the recommendations put forward for future direction in improving the treatment of oily petroleum refinery wastewater are as follows;
1. More research work is needed on the applicability and utilization of hydrogels and biopolymers, natural geomaterials as well as agricultural by-products as non-conventional adsorbents in the treatment of real petroleum wastewater.
2. The use of hybrid treatment techniques which combines two or more different treatment methods tend to increase the advantage to maximize the treatment efficiency of various techniques and reduce their limitations.
3. Application of pre-treatment processes such as the physical separation methods using coagulation, filtration, and membranes to reduce oil concentration as well as TSS may tend to improve the efficiency of many advanced as well as integrated processes.
4. There is need to address issues limiting the application of most advanced and integrated techniques most importantly related to high energy requirements and operational costs.
5. Since of the reported advanced and hybrid treatment techniques were tested only based on a laboratory scale using synthetic samples, hence there is a need to apply such methods on a real industrial scale to confirm their applicability and efficiency.
References
- Abdeen, Z.; Moustafa, Y. M. M. (2016). Treatment of oily wastewater by using porous PVA hydrogels as oil adsorbent. Journal of Dispersion Science and Technology, 37, 799-805. [CrossRef]
- Abeish, A.B.M.S. 2015. Enhanced photocatalytic degradation of biorefractory pollutants in petroleum refinery wastewater (Doctoral dissertation, Curtin University).
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. 2021, Oily Wastewater Treatment: Overview of Conventional and Modern Methods, Challenges, and Future Opportunities. Water 13, 980. [CrossRef]
- Adetunji, A.I.; Olaniran, A.O., 2021. Treatment of industrial oily wastewater by advanced technologies: a review. Applied Water Science, 11, 1-19. [CrossRef]
- Afroze, S.; Sen, T.K. and Ang, H.M., 2016. Adsorption removal of zinc (II) from aqueous phase by raw and base modified Eucalyptus sheathiana bark: Kinetics, mechanism and equilibrium study. Process Safety and Environmental Protection, 102, 336-352. [CrossRef]
- Ahmad T, Ahmad K, Alam M (2016) Sustainable management of water treatment sludge through 3‘R’ concept. J.
- Akkaya, G.K., 2022. Treatment of petroleum wastewater by electrocoagulation using scrap perforated (Fe-anode) and plate (Al and Fe-cathode) metals: Optimization of operating parameters by RSM. Chemical Engineering Research and Design, 187, 261-275. [CrossRef]
- Al Zarooni, M. and Elshorbagy, W., 2006. Characterization and assessment of Al Ruwais refinery wastewater. Journal of hazardous materials, 136, 398-405. [CrossRef]
- Alam, R. and Shang, J.Q., 2017. Removal of bitumen from mature oil sands tailings slurries by electro-flotation. Journal of Water Process Engineering, 15, 116-123. [CrossRef]
- Ali, A.M., Abu-Hassan, M.A., Ibrahim, R.R., Zaini, M.A., Abdulkarim, B.I., Hussein, A.S., Su, S.M. and Halim, M.A.I.M., 2017. Characterization of petroleum sludge from refinery industry biological wastewater treatment unit.
- Aljuboury, D.A.D.A.; Shaik, F. Optimization of the petroleum wastewater treatment process using TiO2/Zn photocatalyst. South African Journal of Chemical Engineering 2021, 38, 61–69. [Google Scholar] [CrossRef]
- Aljuboury, D.A.D.A.; Palaniandy, P.; Abdul Aziz, H.B.; Feroz, S.A.D.A. Treatment of petroleum wastewater by conventional and new technologies-A review. Glob. Nest J 2017, 19, 439–452. [Google Scholar]
- Aljuboury, D.A.D.A.; Palaniandy, P.; Abdul Aziz, H.B.; Feroz, S.; Abu Amr, S.S. Evaluating photo-degradation of COD and TOC in petroleum refinery wastewater by using TiO2/ZnO photo-catalyst. Water Science and Technology 2016, 74, 1312–1325. [Google Scholar] [CrossRef] [PubMed]
- Aljuboury, D.D.A.; Senthilkumar, R. Phenol degradation of industrial wastewater by photocatalysis. J. Innov. Eng. 2014, 2, 1–10. [Google Scholar]
- Al-Khalid, T. and El-Naas, M.H., 2018. Organic contaminants in refinery wastewater: characterization and novel approaches for biotreatment. Recent Insights Pet. Sci. Eng, 371. [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. Journal of Environmental Chemical Engineering 2019, 7, 103248. [Google Scholar] [CrossRef]
- Almomani, F. A., Shawaqfah, M., Bhosale, R. R.; Kumar, A. (2016). Removal of emerging pharmaceuticals from wastewater by ozone-based advanced oxidation processes. Environmental Progress & Sustainable Energy, 35, 982-995. [CrossRef]
- Alnakeeb, A. and Rasheed, R.M., 2021. Chemical Precipitation method for Sulphate Removal from Treated Wastewater of Al-Doura Refinery. Engineering and Technology Journal, 39, 338-354. [CrossRef]
- Altaş, L. and Büyükgüngör, H., 2008. Sulfide removal in petroleum refinery wastewater by chemical precipitation. Journal of hazardous materials, 153(1-2), 462-469. [CrossRef]
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of Petroleum oilfield produced water treatment technologies. Chemosphere 2022, 134064. [Google Scholar] [CrossRef]
- Anucha, C.B., Altin, I., Bacaksiz, E. and Stathopoulos, V.N., 2022. Titanium Dioxide (TiO₂)-Based Photocatalyst Materials Activity Enhancement for Contaminants of Emerging Concern (CECs) Degradation: In the Light of Modification Strategies. Chemical Engineering Journal Advances, p.100262. [CrossRef]
- Atiyah, A. S., Al-Samawi, A. A. A.; Hassan, A. A. (2020, May). Photovoltaic cell electro-Fenton oxidation for treatment oily wastewater. In AIP Conference Proceedings (Vol. 2235, No. 1, p. 020009). AIP Publishing LLC. [CrossRef]
- Aziz, N.M.; Sabbar, A.A. Physiochemical Properties of Basrah oil refinery discharges and its potential effects on Shatt Al-Basrah Canal. Marsh Bulletin 2013, 8, 39–57. [Google Scholar]
- Aziz, S.Q.; Fakhrey, E.S.A. The effect of kawergosk oil refinery wastewater on surrounding water resources. Zanco-Journal of Pure and Applied Sciences Salahaddin University-Erbil 2016, 28, 656–667. [Google Scholar]
- Azizah, A.N. and Widiasa, I.N., 2018. Advanced oxidation processes (AOPs) for refinery wastewater treatment contains high phenol concentration. In MATEC Web of Conferences (Vol. 156, p. 03012). EDP Sciences. [CrossRef]
- Bachmann, R. T., Johnson, A. C.; Edyvean, R. G. (2014). Biotechnology in the petroleum industry: an overview. International Biodeterioration & Biodegradation, 86, 225-237. [CrossRef]
- Ballav, N., Das, R., Giri, S., Muliwa, A.M., Pillay, K. and Maity, A., 2018. L-cysteine doped polypyrrole (PPy@ L-Cyst): a super adsorbent for the rapid removal of Hg+ 2 and efficient catalytic activity of the spent adsorbent for reuse. Chemical Engineering Journal, 345, 621-630. [CrossRef]
- Baradaran, M. and Ghodsi, F.E., 2021. Investigation of the properties of oxide-based multilayer thin films and their use in the photocatalytic applications. In Chemical Solution Synthesis for Materials Design and Thin Film Device Applications ( 697-715). Elsevier. [CrossRef]
- Barakat, M.A., 2011. New trends in removing heavy metals from industrial wastewater. Arabian journal of chemistry, 4, 361-377. [CrossRef]
- Barakat, M.A., Tseng, J.M. and Huang, C.P., 2005. Hydrogen peroxide-assisted photocatalytic oxidation of phenolic compounds. Applied Catalysis B: Environmental, 59(1-2), 99-104. [CrossRef]
- Bastos, P. D., Santos, M. A., Carvalho, P. J., Velizarov, S.; Crespo, J. G. (2021). Pilot scale reverse osmosis refinery wastewater treatment–a techno-economical and sustainability assessment. Environmental Science: Water Research & Technology, 7, 549-561. [CrossRef]
- Bayona, J.M., Domínguez, C. and Albaigés, J., 2015. Analytical developments for oil spill fingerprinting. Trends in Environmental Analytical Chemistry, 5, 26-34. [CrossRef]
- Beni, A. A., Adel, M. S. S., Zaeimdar, M., Ghadi, A., Hassani, V., Jalalvandi, K.; Abdollahi, S. A. (2023). Petroleum Wastewater Treatment. [CrossRef]
- Cai, L., Zhang, Y., Zhou, Y., Zhang, X., Ji, L., Song, W., Zhang, H. and Liu, J., 2019. Effective adsorption of diesel oil by crab-shell-derived biochar nanomaterials. Materials, 12, p.236. [CrossRef]
- Candish, L., Collins, K.D., Cook, G.C., Douglas, J.J., Gómez-Suárez, A., Jolit, A. and Keess, S., 2021. Photocatalysis in the life science industry. Chemical Reviews, 122, 2907-2980. [CrossRef]
- Cardoso, I. M., Cardoso, R. M.; da Silva, J. C. E. (2021). Advanced oxidation processes coupled with nanomaterials for water treatment. Nanomaterials, 11, 2045. [CrossRef]
- Chen, C., Yoza, B.A., Wang, Y., Wang, P., Li, Q.X., Guo, S. and Yan, G., 2015. Catalytic ozonation of petroleum refinery wastewater utilizing Mn-Fe-Cu/Al2O3 catalyst. Environmental Science and Pollution Research, 22, 5552-5562. [CrossRef]
- Chun, Y., Mulcahy, D., Zou, L. and Kim, I.S., 2017. A short review of membrane fouling in forward osmosis processes. Membranes, 7, p.30. [CrossRef]
- Coelho, A.; Castro, A.V.; Dezotti, M.; Sant’Anna Jr, G.L. Treatment of petroleum refinery sourwater by advanced oxidation processes. Journal of hazardous materials 2006, 137, 178–184. [Google Scholar] [CrossRef]
- Crini, G., Lichtfouse, E., Wilson, L.D. and Morin-Crini, N., 2019. Conventional and non-conventional adsorbents for wastewater treatment. Environmental Chemistry Letters, 17, 195-213. [CrossRef]
- Daflon, S. D. A., Guerra, I. L., Reynier, M. V., Botta, C. R.; Campos, J. C. (2015). Toxicity identification and evaluation of a refinery wastewater from Brazil (Phase I). Ecotoxicology and Environmental Contamination, 10, 41-45. [CrossRef]
- Daher, E.; Francis, W.H. (2019). Photocatalytic degradation of phenolic effluents in petroleum refineries. The International Journal of E-Learning and Educational Technologies in the Digital Media.
- Dai, X.; Chen, C.; Chen, Y.; Guo, S. Comprehensive Evaluation of a Full-Scale Combined Biological Process for the Treatment of Petroleum Refinery Wastewater using GC-MS and PCR-DGGE Techniques. Int. J. Electrochem. Sci. 2020, 15, 2013–2026. [Google Scholar] [CrossRef]
- Dang, T.T.T., Le, S.T.T., Channei, D., Khanitchaidecha, W. and Nakaruk, A., 2016. Photodegradation mechanisms of phenol in the photocatalytic process. Research on Chemical Intermediates, 42, 5961-5974. [CrossRef]
- Das, S. and Dhara, S. eds., 2021. Chemical solution synthesis for materials design and thin film device applications. Elsevier. [CrossRef]
- de Oliveira, C. P. M., Viana, M. M.; Amaral, M. C. S. (2020). Coupling photocatalytic degradation using a green TiO2 catalyst to membrane bioreactor for petroleum refinery wastewater reclamation. Journal of Water Process Engineering, 34, 101093. [CrossRef]
- Dehghani, M. and Alizadeh, M.H., 2016. The effects of the natural coagulant Moringa oleifera and alum in wastewater treatment at the Bandar Abbas Oil Refinery. Environmental Health Engineering and Management Journal, 3, 225-230. [CrossRef]
- Delnavaz, M.; Bos' hagh, M.A. Photocatalytic treatment of real oil refinery wastewater using TiO2/Ag-doped nanoparticles. Sharif Journal of Civil Engineering 2021, 37, 121–129. [Google Scholar]
- Dhivakar, V.; Rajan, T. BTEX compounds removal from waste water by using UV&UV/H2O2 process. Int J Recent Eng Sci 2018, 5, 22–25. [Google Scholar]
- Divyapriya, G.; Nidheesh, P. V. (2020). Importance of graphene in the electro-Fenton process. ACS omega, 5, 4725-4732. [CrossRef]
- Diya’uddeen, B.H., Daud, W.M.A.W. and Aziz, A.A., 2011. Treatment technologies for petroleum refinery effluents: A review. Process safety and environmental protection, 89, 95-105. [CrossRef]
- Ebrahiem, E.E., Al-Maghrabi, M.N. and Mobarki, A.R., 2017. Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arabian Journal of Chemistry, 10, S1674-S1679. [CrossRef]
- El-Ashtoukhy, E.S.Z., El-Taweel, Y.A., Abdelwahab, O. and Nassef, E.M., 2013. Treatment of petrochemical wastewater containing phenolic compounds by electrocoagulation using a fixed bed electrochemical reactor. Int. J. Electrochem. Sci, 8, 1534-1550. [CrossRef]
- Eldos, H.I., Khan, M., Zouari, N., Saeed, S. and Al-Ghouti, M.A., 2022. Characterization and assessment of process water from oil and gas production: A case study of process wastewater in Qatar. Case Studies in Chemical and Environmental Engineering, p.100210. [CrossRef]
- Elmobarak, W.F., Hameed, B.H., Almomani, F. and Abdullah, A.Z., 2021. A review on the treatment of petroleum refinery wastewater using advanced oxidation processes. Catalysts, 11, p.782. [CrossRef]
- El-Naas MH, Alhaija MA, Al-Zuhair S., 2014 Evaluation of a three-step process for the treatment of petroleum refinery wastewater. Journal of Environmental Chemical Engineering.; 2:56-62. [CrossRef]
- El-Naas, M.H., Surkatti, R. and Al-Zuhair, S., 2016. Petroleum refinery wastewater treatment: A pilot scale study. Journal of Water Process Engineering, 14, 71-76. [CrossRef]
- Estrada-Arriaga, E.B., Zepeda-Aviles, J.A. and García-Sánchez, L., 2016. Post-treatment of real oil refinery effluent with high concentrations of phenols using photo-ferrioxalate and Fenton’s reactions with membrane process step. Chemical Engineering Journal, 285, 508-516. [CrossRef]
- Estrada-Flores, S.; Martínez-Luévanos, A.; Perez-Berumen, C.M.; García-Cerda, L.A.; Flores-Guia, T.E. Relationship between morphology, porosity, and the photocatalytic activity of TiO2 obtained by sol–gel method assisted with ionic and nonionic surfactants. boletín de la sociedad española de cerámica y vidrio 2020, 59, 209–218. [Google Scholar] [CrossRef]
- Fahim, A.S. and Abbar, A.H., 2020. Treatment of petroleum refinery wastewater by electro-Fenton process using porous graphite electrodes. Egyptian Journal of Chemistry, 63, 4805-4819. [CrossRef]
- Fernandes, A., Makoś, P., Wang, Z. and Boczkaj, G., 2020. Synergistic effect of TiO2 photocatalytic advanced oxidation processes in the treatment of refinery effluents. Chemical Engineering Journal, 391, p.123488. [CrossRef]
- Fu, F. and Wang, Q., 2011. Removal of heavy metal ions from wastewaters: a review. Journal of environmental management, 92, 407-418. [CrossRef]
- Ghasemi, Z.; Younesi, H.; Zinatizadeh, A.A. Preparation, characterization and photocatalytic application of TiO2/Fe-ZSM-5 nanocomposite for the treatment of petroleum refinery wastewater: Optimization of process parameters by response surface methodology. Chemosphere 2016, 159, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Ghezali, K., Bentahar, N., Barsan, N., Nedeff, V. and Moșneguțu, E., 2022a. Potential of Canna indica in Vertical Flow Constructed Wetlands for Heavy Metals and Nitrogen Removal from Algiers Refinery Wastewater. Sustainability, 14, p.4394. [CrossRef]
- Ghezali, K.; Tahar, N.B.; Otmanine, G.; Guettaf, N. Environmental assessment and compliance of wastewater from arzew oil refinery for future development and reuse. Journal of Environmental Treatment Techniques 2022, 10, 18–23. [Google Scholar]
- Ghimire, N. and Wang, S., 2018. Biological treatment of petrochemical wastewater. Petroleum Chemicals-Recent Insight, 55-74. [CrossRef]
- Giri, A.S.; Golder, A.K. Fenton, photo-fenton, H2O2 photolysis, and TiO2 photocatalysis for dipyrone oxidation: drug removal, mineralization, biodegradability, and degradation mechanism. Industrial & Engineering Chemistry Research 2014, 53, 1351–1358. [Google Scholar]
- Gkika, D. A., Mitrpoulos, A. C.; Kyzas, G. Z. (2022). Why reuse spent adsorbents? The latest challenges and limitations. Science of the Total Environment, 153612. [CrossRef]
- Gousmi, N., Sahmi, A., Li, H.Z., Poncin, S., Djebbar, R. and Bensadok, K., 2016. Purification and detoxification of petroleum refinery wastewater by electrocoagulation process. Environmental technology, 37, 2348-235. [CrossRef]
- Habte, L., Shiferaw, N., Thriveni, T., Mulatu, D., Lee, M.H., Jung, S.H. and Ahn, J.W., 2020. Removal of Cd (II) and Pb (II) from wastewater via carbonation of aqueous Ca (OH) 2 derived from eggshell. Process Safety and Environmental Protection, 141, 278-287. [CrossRef]
- Hamal, D.B. and Klabunde, K.J., 2007. Synthesis, characterization, and visible light activity of new nanoparticle photocatalysts based on silver, carbon, and sulfur-doped TiO2. Journal of Colloid and Interface Science, 311, 514-522. [CrossRef]
- Handogo, R.; Prasetyo, F.; Sanjaya, S. P.; Anugraha, R. P. Preliminary design of mini oil refinery plant. Journal of Advanced Research in Fluid Mechanics and Thermal Sciences 2022, 92, 39–50. [Google Scholar] [CrossRef]
- Haneef, T., Mustafa, M.R.U., Rasool, K., Ho, Y.C. and Mohamed Kutty, S.R. (2020) Removal of Polycyclic Aromatic Hydrocarbons in a Heterogeneous Fenton Like Oxidation System Using Nanoscale Zero-Valent Iron as a Catalyst. Water, 12, Article 2430. [CrossRef]
- Hashemi, F.; Hashemi, H.; Dehghani, M.; Hoseini, M. Removal of heavy metals from oil refinery effluent by micellar-enhanced ultrafiltration (MEUF). Journal of Health Sciences & Surveillance System 2018, 6, 123–129. [Google Scholar]
- Hassan, A.K., Hassan, M.M.A. and Hasan, A.F., 2020, November. Treatment of Iraqi Petroleum Refinery Wastewater by Advanced Oxidation Processes. In Journal of Physics: Conference Series (Vol. 1660, No. 1, p. 012071). IOP Publishing. [CrossRef]
- He, H.; Zhou, Z. (2017). Electro-Fenton process for water and wastewater treatment. Critical Reviews in Environmental Science and Technology, 47, 2100-2131. [CrossRef]
- Hu, Y., Song, X., Jiang, S. and Wei, C., 2015. Enhanced photocatalytic activity of Pt-doped TiO2 for NOx oxidation both under UV and visible light irradiation: A synergistic effect of lattice Pt4+ and surface PtO. Chemical Engineering Journal, 274, 102-112. [CrossRef]
- Huang, Y., Kong, M., Coffin, S., Cochran, K. H., Westerman, D. C., Schlenk, D., ... & Dionysiou, D. D. (2020). Degradation of contaminants of emerging concern by UV/H2O2 for water reuse: Kinetics, mechanisms, and cytotoxicity analysis. Water Research, 174, 115587. [CrossRef]
- Ibrahim, D. S., Lathalakshmi, M., Muthukrishnaraj, A.; Balasubramanian, N. (2013). An alternative treatment process for upgrade of petroleum refinery wastewater using electrocoagulation. Petroleum Science, 10, 421-430. [CrossRef]
- Ibrahim, D.S.; Devi, P.S.; Balasubramanian, N. Electrochemical oxidation treatment of petroleum refinery effluent. Int. J. Sci. Eng. Res. 2013, 4, 1–5. [Google Scholar]
- Ibrahim, M.H., Banerjee, A. and El-Naas, M.H., 2022. Treatment of petroleum industry wastewater: current practices and perspectives. In Petroleum Industry Wastewater ( 1-6). Elsevier. [CrossRef]
- Igbagara, P.W.; Ntekim, J. Determination of heavy metals concentration in waste water of Warri Refining and Petrochemicals Company (WRPC). Tropical Journal of Science and Technology 2021, 2, 29–37. [Google Scholar]
- IPIECA, 2010. Petroleum refining water/wastewater use and management. IPIECA Operations Best Practice Series.
- Ishak, S., Malakahmad, A.; Isa, M. H. (2012). Refinery wastewater biological treatment: A short review.
- Islam, B. Petroleum sludge, its treatment and disposal: A review. Int. J. Chem. Sci. 2015, 13, 1584–1602. [Google Scholar]
- Jafarinejad, S.; Jiang, S. C. (2019). Current technologies and future directions for treating petroleum refineries and petrochemical plants (PRPP) wastewaters. Journal of Environmental Chemical Engineering, 7, 103326. [CrossRef]
- Jafarinejad, S. Activated sludge combined with powdered activated carbon (PACT process) for the petroleum industry wastewater treatment: a review. Chem. Int. 2017, 3, 368. [Google Scholar]
- Jain, M., Majumder, A., Ghosal, P.S. and Gupta, A.K., 2020. A review on treatment of petroleum refinery and petrochemical plant wastewater: a special emphasis on constructed wetlands. Journal of Environmental Management, 272, p.111057. [CrossRef]
- Jun, K.C., Raman, A.A.A. and Buthiyappan, A., 2020. Treatment of oil refinery effluent using bio-adsorbent developed from activated palm kernel shell and zeolite. RSC advances, 10, 24079-24094. [CrossRef]
- Kalair, A., Abas, N., Saleem, M. S., Kalair, A. R.; Khan, N. (2021). Role of energy storage systems in energy transition from fossil fuels to renewables. Energy Storage, 3, e135. [CrossRef]
- Kalash, K. R.; Albayati, T. M. (2021). Remediation of oil refinery wastewater implementing functionalized mesoporous materials MCM-41 in batch and continuous adsorption process. Desalin. Water Treat, 220, 130-141. [CrossRef]
- Kane, A., Assadi, A. A., El Jery, A., Badawi, A. K., Kenfoud, H., Baaloudj, O.; Assadi, A. A. (2022). Advanced photocatalytic treatment of wastewater using immobilized titanium dioxide as a photocatalyst in a pilot-scale reactor: process intensification. Materials, 15, 4547. [CrossRef]
- Kassob, A.N.; Abbar, A.H. Treatment of petroleum refinery wastewater by adsorption using activated carbon fixed bed column with batch recirculation mode. Al-Qadisiyah Journal for Engineering Sciences 2022, 15. [Google Scholar] [CrossRef]
- Keramati, M.; Ayati, B. (2019). Petroleum wastewater treatment using a combination of electrocoagulation and photocatalytic process with immobilized ZnO nanoparticles on concrete surface. Process Safety and Environmental Protection, 126, 356-365. [CrossRef]
- Khalifa, O., Banat, F. and Hasan, S.W., 2022. Electrochemical treatment of petroleum wastewater: standalone and integrated processes. In Petroleum Industry Wastewater ( 171-183). Elsevier. [CrossRef]
- Khan, M.M. Nanostructured materials for visible light photocatalysis. Micro and Nano Technologies 2022, 185–195. [Google Scholar]
- Khatoon, K. and Malik, A., 2021. Cyto-genotoxic potential of petroleum refinery wastewater mixed with domestic sewage used for irrigation of food crops in the vicinity of an oil refinery. Heliyon, 7, p.e08116. [CrossRef]
- Kulkarni, S.J.; Goswami, A.K. A review on wastewater treatment for petroleum industries and refineries. Int. J. Innov. Res. Sci. Eng. Technol。 2015, 1, 280–283. [Google Scholar]
- Kusworo, T. D., Kumoro, A. C., Nabilah, Y., Rasendriya, A., Utomo, D. P.; Hasbullah, H. (2022). Advanced method for clean water recovery from batik wastewater via sequential adsorption, ozonation and photocatalytic membrane PVDF-TiO2/rGO processes. Journal of Environmental Chemical Engineering, 10, 108708. Journal of Environmental Chemical Engineering. [CrossRef]
- Kusworo, T.D., Dalanta, F., Aryanti, N. and Othman, N.H., 2021. Intensifying separation and antifouling performance of PSf membrane incorporated by GO and ZnO nanoparticles for petroleum refinery wastewater treatment. Journal of Water Process Engineering, 41, p.102030. [CrossRef]
- Lal, K. and Garg, A., 2019. Effectiveness of synthesized aluminum and iron based inorganic polymer coagulants for pulping wastewater treatment. Journal of Environmental Chemical Engineering, 7, p.103204. [CrossRef]
- Lattanzio, R. K. (2016). An Overview of Air Quality Issues in Natural Gas Systems. Congressional Research Service, the Library of Congress.
- Lau, W.J., Ismail, A.F., Isloor, A.M. and Al-Ahmed, A. eds., 2018. Advanced Nanomaterials for Membrane Synthesis and its Applications. Elsevier. [CrossRef]
- Le, T.V.; Imai, T., Higuchi, T., Yamamoto, K., Sekine, M.; Doi, R., Vo, H.T. and Wei, J. 2013. Performance of tiny microbubbles enhanced with “normal cyclone bubbles” in separation of fine oil-in-water emulsions. Chem. Eng. Sci., (94) 1–6. [CrossRef]
- Li, F., Miao, G., Gao, Z., Xu, T., Zhu, X., Miao, X., ... & Li, X. (2022). A versatile hydrogel platform for oil/water separation, dye adsorption, and wastewater purification. Cellulose, 29, 4427-4438. [CrossRef]
- Li, M., Liu, H., Liu, J., Pei, Y., Zheng, X., Tang, K.; Wang, F. (2021). Hydrophobic and self-recoverable cellulose nanofibrils/N-alkylated chitosan/poly (vinyl alcohol) sponge for selective and versatile oil/water separation. International Journal of Biological Macromolecules, 192, 169-179. [CrossRef]
- Liang, J., Mai, W., Tang, J. and Wei, Y., 2019. Highly effective treatment of petrochemical wastewater by a super-sized industrial scale plant with expanded granular sludge bed bioreactor and aerobic activated sludge. Chemical Engineering Journal, 360, 15-23. [CrossRef]
- Mahmoud, Mohamed & Ebrahiem, Ebrahiem. Treatment of Oily Wastewater Produced from Refinery Processes Using Adsorption Technique. Minia Journal of Engineering and Technology 2013, 32, 88–101.
- Merma, A.G., Santos, B.F., Rego, A.S., Hacha, R.R. and Torem, M.L., 2020. Treatment of oily wastewater from mining industry using electrocoagulation: fundamentals and process optimization. Journal of Materials Research and Technology, 9, 15164-15176. [CrossRef]
- Mickova, I.L. Advanced electrochemical technologies in wastewater treatment. Part II: Electro-flocculation and electro-flotation. American Academic Scientific Research Journal for Engineering, Technology, and Sciences 2015, 14, 273–294. [Google Scholar]
- Misiti, T., Tezel, U.; Pavlostathis, S. G. (2013). Fate and effect of naphthenic acids on oil refinery activated sludge wastewater treatment systems. Water research, 47, 449-460. [CrossRef]
- Mohammed, S. A.; Al-Azawiey, S. S.; Ali, A. H. Treatment of Organic Compounds Resulting from Oil Refineries under Solar Light and Reuse it for Industrial Purpose. Muthanna Journal of Engineering and Technology 2021, 9, 20–24. [Google Scholar] [CrossRef]
- Mohtashami, R. and Shang, J.Q., 2019. Electroflotation for treatment of industrial wastewaters: a focused review. Environmental processes, 6, 325-353. [CrossRef]
- Mokhtari, H. A., Mirbagheri, S. A.; Dehkordi, N. R. (2021). Performance, evaluation, and modeling of an integrated petroleum refinery wastewater treatment system using multi-layer perceptron neural networks. Desalin Water Treat, 212, 31-42. [CrossRef]
- Moslehyani, A.; Ismail, F.; Othman, M.H.D.; Matsuura, T. Design and performance study of hybrid photocatalytic reactor-PVDF/MWCNT nanocomposite membrane system for treatment of petroleum refinery wastewater. Des. 2015, 363, 99–111. [Google Scholar] [CrossRef]
- Mustapha, H. I. (2018). Treatment of petroleum refinery wastewater with constructed wetlands. CRC Press.
- Nasiri, Masoud, Jafari, Iman, 2017. ’Produced water from oil-gas plants: a short review on challenges and opportunities. Period. Polytech. - Chem. Eng. 61, 73–81. [CrossRef]
- Nasr, M., Eid, C., Habchi, R., Miele, P. and Bechelany, M., 2018. Recent progress on titanium dioxide nanomaterials for photocatalytic applications. ChemSusChem, 11, 3023-3047. [CrossRef]
- Nidheesh, P. V.; Gandhimathi, R. (2015). Textile wastewater treatment by electro-Fenton process in batch and continuous modes. Journal of Hazardous, Toxic, and Radioactive Waste, 19, 04014038. [CrossRef]
- Nkwocha, A. C.; Ekeke, I. C.; Kamen, F. L.; Oghome, P. I. Performance Evaluation of Petroleum Refinery Wastewater Treatment Plant. International Journal of Science and Engineering Investigations 2013, 2. [Google Scholar]
- Nogueira, A.A., Bassin, J.P., Cerqueira, A.C. and Dezotti, M., 2016. Integration of biofiltration and advanced oxidation processes for tertiary treatment of an oil refinery wastewater aiming at water reuse. Environmental Science and Pollution Research, 23, 9730-9741. [CrossRef]
- Obotey Ezugbe, E. and Rathilal, S., 2020. Membrane technologies in wastewater treatment: a review. Membranes, 10, p.89. [CrossRef]
- Obuebite, A. A.; Okwonna, O. O. (2023). Refinery effluent water treatment: An integrated approach. [CrossRef]
- Olayebi, O.O. and Adebayo, A.T., 2017. Removal of Heavy Metals from Petroleum Refinery Effluent Using Coconut Shell Effluent Using Coconut Shell-Based Activated Based Activated Carbon.
- Oller, I., Malato, S.; Sánchez-Pérez, J. (2011). Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Science of the total environment, 409, 4141-4166. [CrossRef]
- Pal, S., Banat, F., Almansoori, A. and Abu Haija, M., 2016. Review of technologies for biotreatment of refinery wastewaters: progress, challenges and future opportunities. Environmental Technology Reviews, 5, 12-38. [CrossRef]
- Palaniandy, P. and Feroz, S., 2019. Advanced Oxidation Processes (AOPs) to Treat the Petroleum Wastewater. In Advanced Oxidation Processes (AOPs) in Water and Wastewater Treatment ( 99-122). IGI Global.
- Pardeshi, S.K. and Patil, A.B., 2008. A simple route for photocatalytic degradation of phenol in aqueous zinc oxide suspension using solar energy. Solar Energy, 82, 700-705. [CrossRef]
- Park, J.H.; Choi, G.J.; Kim, S.H. Effects of pH and slow mixing conditions on heavy metal hydroxide precipitation. Journal of the Korea Organic Resources Recycling Association 2014, 22, 50–56. [Google Scholar]
- Park, S.J., Yang, J.H., Joo, M.H. and Sohn, Y., 2022. Current status, research gaps, and future scope for nanomaterials toward visible light photocatalysis. In Nanostructured Materials for Visible Light Photocatalysis ( 569-608). Elsevier.
- Petrowiki, 2018. ’Produced oilfield water, 15/7/2019. Available online: https://petrowiki.org/Produced _oilfield_water.
- Pinzón-Espinosa, A.; Kanda, R. (2020). Naphthenic acids are key contributors to toxicity of heavy oil refining effluents. Science of the Total Environment, 729, 138119. [CrossRef]
- Pinzón-Espinosa, A., 2018. Unravelling the chemistry behind the toxicity of oil refining effluents: from characterisation to treatment (Doctoral dissertation, Brunel University London).
- Polvara, E., Roveda, L., Invernizzi, M., Capelli, L.; Sironi, S. (2021). Estimation of Emission Factors for Hazardous Air Pollutants from Petroleum Refineries. Atmosphere, 12, 1531. [CrossRef]
- Procházka, P., Tutter, Š.; Ditl, P. (2019). Electrochemically Enhanced Fenton’s Reaction. CHEMICAL ENGINEERING, 74.
- Qaderi, F. and Abdolalian, S., 2022. Treatment of petroleum wastewater using solar power-based photocatalysis. In Petroleum Industry Wastewater ( 161-170). Elsevier. [CrossRef]
- Qasem, N.A., Mohammed, R.H. and Lawal, D.U., 2021. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water, 4, 1-15. [CrossRef]
- Quang, H. H. P., Dinh, N. T., Thi, T. N. T., Bao, L. T. N., Yuvakkumar, R.; Nguyen, V. H. (2022). Fe2+, Fe3+, Co2+ as highly efficient cocatalysts in the homogeneous electro-Fenton process for enhanced treatment of real pharmaceutical wastewater. Journal of Water Process Engineering, 46, 102635. [CrossRef]
- Radelyuk, I., Tussupova, K., Zhapargazinova, K., Yelubay, M.; Persson, M. (2019). Pitfalls of wastewater treatment in oil refinery enterprises in Kazakhstan—a system Approach. Sustainability, 11, 1618. [CrossRef]
- Radji, G., Bettahar, N., Bahmani, A., Boukhetache, I.; Contreras, S. (2022). Heterogeneous Fenton-like degradation of organic pollutants in petroleum refinery wastewater by copper-type layered double hydroxides. Journal of Water Process Engineering, 50, 103305. [CrossRef]
- Rahi, M.N., Jaeel, A.J. and Abbas, A.J., 2021, February. Treatment of petroleum refinery effluents and wastewater in Iraq: A mini review. In IOP Conference Series: Materials Science and Engineering (Vol. 1058, No. 1, p. 012072). IOP Publishing. [CrossRef]
- Raji, M. and Mirbagheri, S.A., 2021. A global trend of Fenton-based AOPs focused on wastewater treatment: a bibliometric and visualization analysis. Water Practice and Technology, 16, 19-34. [CrossRef]
- Ramachandran, B.; Sushma, G.; Rani, P.Y. Phenol Waste Water Treatment Using Solar Photo Catalytic Treatment in Industry. Turkish Journal of Computer and Mathematics Education (TURCOMAT) 2021, 12, 826–834. [Google Scholar]
- Rasheed, Q.J. and Muthukumar, K., 2010. ‘Treatment of Petrochemical Wastewater using Sequencing Batch Reactor. Proceedings of the “Recent Trends in Engineering & Education” (RTEE), NITTTR, Kolkata, 28-29.
- Rashid, J.; Barakat, M.A.; Ruzmanova, Y.; Chianese, A. Fe3O4/SiO2/TiO2 nanoparticles for photocatalytic degradation of 2-chlorophenol in simulated wastewater. Environmental Science and Pollution Research 2015, 22, 3149–3157. [Google Scholar] [CrossRef] [PubMed]
- Ratman, I., Kusworo, T. D.; Utomo, D. P. (2021). Petroleum Refinery Wastewater Treatment Using a Polysulfone-Nano TiO2 Hybrid Membrane Coupled with an Ozonation Process as a Pre-Treatment. Journal of Membrane Science and Research, 7, 141-151. [CrossRef]
- Ratman, I., Kusworo, T.D., Utomo, D.P., Azizah, D.A. and Ayodyasena, W.A., 2020. Petroleum refinery wastewater treatment using three steps modified nanohybrid membrane coupled with ozonation as integrated pre-treatment. Journal of Environmental Chemical Engineering, 8, p.103978. [CrossRef]
- Razavi, S.M.R. and Miri, T., 2015. A real petroleum refinery wastewater treatment using hollow fiber membrane bioreactor (HF-MBR). Journal of Water Process Engineering, 8, 136-141. [CrossRef]
- Ren, G., Han, H., Wang, Y., Liu, S., Zhao, J., Meng, X. and Li, Z., 2021. Recent advances of photocatalytic application in water treatment: a review. Nanomaterials, 11, p.1804. [CrossRef]
- Renault F., Sancey B., Badot P.-M. and Crini G. (2009), Chitosan for coagulation/flocculation processes, an eco-friendly approach, Eur. Polym. J., 45, 1337-1348. [CrossRef]
- Rubio-Clemente, A., Chica, E. and Peñuela, G.A., 2015. Petrochemical wastewater treatment by photo-Fenton process. Water, Air, & Soil Pollution, 226, 1-18. [CrossRef]
- Saber, A., Hasheminejad, H., Taebi, A. and Ghaffari, G., 2014. Optimization of Fenton-based treatment of petroleum refinery wastewater with scrap iron using response surface methodology. Applied Water Science, 4, 283-290. [CrossRef]
- Sabir, S. (2015). Approach of cost-effective adsorbents for oil removal from oily water. Critical Reviews in Environmental Science and Technology, 45, 1916-1945. [CrossRef]
- Sahoo, C., Gupta, A.K. and Sasidharan Pillai, I.M., 2012. Photocatalytic degradation of methylene blue dye from aqueous solution using silver ion-doped TiO2 and its application to the degradation of real textile wastewater. Journal of Environmental Science and Health, Part A, 47, 1428-1438. [CrossRef]
- Sakka, S., 2013. Handbook of Advanced Ceramics: Chapter 11.1. 2. Sol–Gel Process and Applications. Elsevier Inc. Chapters.
- Saleh, T.A., 2021. Polymer Hybrid Materials and Nanocomposites: Fundamentals and Applications. William Andre.
- Salem, F.; Thiemann, T. Produced Water from Oil and Gas Exploration—Problems, Solutions and Opportunities. Journal of Water Resource and Protection 2022, 14, 142–185. [Google Scholar] [CrossRef]
- Shaba, E. Y., Tijani, J. O., Jacob, J. O.; Suleiman, M. A. T. (2022). Simultaneous removal of Cu (II) and Cr (VI) ions from petroleum refinery wastewater using ZnO/Fe3O4 nanocomposite. Journal of Environmental Science and Health, Part A, 1-22. [CrossRef]
- Shuokr, Q.A. and Sazan, M.A., 2021. Characteristics, treatment techniques, and operational limitations for refinery wastewater. Recycling and Sustainable Development, 14, 19-30. [CrossRef]
- Shymanovska, V.V., Khalyavka, T.A., Manuilov, E.V., Gavrilko, T.A., Aho, A., Naumov, V.V. and Shcherban, N.D., 2022. Effect of surface doping of TiO2 powders with Fe ions on the structural, optical and photocatalytic properties of anatase and rutile. Journal of Physics and Chemistry of Solids, 160, p.110308. [CrossRef]
- Sillanpää, M.; Shestakova, M. Emerging and combined electrochemical methods. Electrochemical water treatment methods: Fundamentals, methods and full scale Applications 2017, 131–225. [Google Scholar]
- Singh, B. and Kumar, P., 2020. Pre-treatment of petroleum refinery wastewater by coagulation and flocculation using mixed coagulant: Optimization of process parameters using response surface methodology (RSM). Journal of water process engineering, 36, p.101317. [CrossRef]
- Stanley, H.O., Ihennacho, C.M. and Stanley, C.N., 2017. Bioremoval of heavy metals from effluent of Port Harcourt Refinery using Pluerotus ostreatus. Journal of Petroleum and Environmental Biotechnology, 7, p.324. [CrossRef]
- Stasinakis, A.S. Use of selected advanced oxidation processes (AOPs) for wastewater treatment–a mini review. Global NEST Journal 2008, 10, 376–385. [Google Scholar]
- Stec, M., Jagustyn, B., Słowik, K., Ściążko, M.; Iluk, T. (2020). Influence of high chloride concentration on pH control in hydroxide precipitation of heavy metals. Journal of Sustainable Metallurgy, 6, 239-249. [CrossRef]
- Stefanakis, A.I. and Thullner, M., 2016. Fate of phenolic compounds in constructed wetlands treating contaminated water. In Phytoremediation ( 311-325). Springer, Cham. [CrossRef]
- Subramaniam, M.N., Goh, P.S., Lau, W.J., Ng, B.C. and Ismail, A.F., 2019. Development of nanomaterial-based photocatalytic membrane for organic pollutants removal. In Advanced nanomaterials for membrane synthesis and its applications ( 45-67). Elsevier. [CrossRef]
- Sukmana, H., Bellahsen, N., Pantoja, F. and Hodur, C., 2021. Adsorption and coagulation in wastewater treatment–Review. Progress in Agricultural Engineering Sciences, 17, 49-68. [CrossRef]
- Tahir, M.B., Sohaib, M., Sagir, M. and Rafique, M., 2020. Role of nanotechnology in photocatalysis. Reference Module in Materials Science and Materials Engineering.
- Tai, M. H., Mohan, B. C., Yao, Z.; Wang, C. H. (2022). Superhydrophobic leached carbon Black/Poly (vinyl) alcohol aerogel for selective removal of oils and organic compounds from water. Chemosphere, 286, 131520. [CrossRef]
- Tang, X.; Eke, P. E.; Scholz, M.; Huang, S., Processes impacting on benzene removal in Vertical-flow constructed wetlands. Bioresource technology 2009, 100 (1), 227-234. 1. [CrossRef]
- Tetteh, E. K.; Rathilal, S. (2020). Evaluation of different polymeric coagulants for the treatment of oil refinery wastewater. Cogent Engineering, 7, 1785756. [CrossRef]
- Tetteh, E.K.; Obotey Ezugbe, E.; Rathilal, S.; Asante-Sackey, D. Removal of COD and SO42− from oil refinery wastewater using a photo-catalytic system—comparing tio2 and zeolite efficiencies. Water 2020, 12, 214. [Google Scholar] [CrossRef]
- Tetteh, E.K., Rathilal, S. and Naidoo, D.B., 2020b. Photocatalytic degradation of oily waste and phenol from a local South Africa oil refinery wastewater using response methodology. Scientific reports, 10, 1-12. [CrossRef]
- Tong, K., Zhang, Y., Liu, G., Ye, Z. and Chu, P.K., 2013. Treatment of heavy oil wastewater by a conventional activated sludge process coupled with an immobilized biological filter. International Biodeterioration & Biodegradation, 84, 65-71. [CrossRef]
- Tony, M.A., Purcell, P.J. and Zhao, Y., 2012. Oil refinery wastewater treatment using physicochemical, Fenton and Photo-Fenton oxidation processes. Journal of Environmental Science and Health, Part A, 47, 435-440. [CrossRef]
- Treviño-Reséndez, J., Medel, A. and Meas, Y., 2021. Electrochemical technologies for treating petroleum industry wastewater. Current Opinion in Electrochemistry, 27, p.100690. [CrossRef]
- Ugboma, C.J., Sampson, T. and Mbonu, N.E., 2020. Bioremediation of heavy metals from artisanal crude oil refinery (kpo-fire) impacted soil using Bacillus flexus and Pseudomonas aeruginosa in Ngie community, Degema Local Government Area, Rivers State, Nigeria. Journal of Applied Sciences and Environmental Management, 24, 2049-2054. [CrossRef]
- Ul Haq, I., Ahmad, W., Ahmad, I. and Yaseen, M., 2020. Photocatalytic oxidative degradation of hydrocarbon pollutants in refinery wastewater using TiO2 as catalyst. Water Environment Research, 92, 2086-2094. [CrossRef]
- Varjani S., Joshi R., Srivastava, V.K., Ngo H. H. and Guo W., 2020. Treatment of Wastewater from Petroleum Industry: Current Practices and Perspectives. Environmental Science and Pollution Research, 27 (22), pp 27172-27180. [CrossRef]
- Vendramel, S.; Bassin, J.P.; Dezotti, M.; Sant'Anna Jr, G.L. Treatment of petroleum refinery wastewater containing heavily polluting substances in an aerobic submerged fixed-bed reactor. Environmental Technology 2015, 36, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, K.; Kim, K. H. (2019). Nanomaterials for the adsorptive treatment of Hg (II) ions from water. Chemical Engineering Journal, 358, 264-282. [CrossRef]
- Wang, B.; Yi, W.; Yingxin, G.; Guomao, Z.; Min, Y.; Song, W.; Jianying, H. Occurrences and behaviors of Naphthenic Acids in a petroleum refinery wastewater treatment plant. Environ. Sci. Technol. 2015, 49, 5796–5804. [Google Scholar] [CrossRef]
- Wang, B., Zhang, H., Wang, F., Xiong, X., Tian, K., Sun, Y. and Yu, T., 2019. Application of heterogeneous catalytic ozonation for refractory organics in wastewater. Catalysts, 9, p.241. [CrossRef]
- Wang, C., Chen, Z., Li, Y., Feng, K., Peng, Z., Zhu, Y.; Yang, X. (2021). Refinery wastewater treatment via a multistage enhanced biochemical process. Scientific Reports, 11, 10282. [CrossRef]
- Wang, Y., Wang, Q., Li, M., Yang, Y., He, W., Yan, G. and Guo, S., 2016. An alternative anaerobic treatment process for treatment of heavy oil refinery wastewater containing polar organics. Biochemical Engineering Journal, 105, 44-51. [CrossRef]
- Whale, G.F., Hjort, M., Di Paolo, C., Redman, A.D., Postma, J.F., Legradi, J. and Leonards, P.E.G., 2022. Assessment of oil refinery wastewater and effluent integrating bioassays, mechanistic modelling and bioavailability evaluation. Chemosphere, 287, p.132146. [CrossRef]
- Widayat, W. and Annisa, A.N., 2017, July. Synthesis and characterization of ZSM-5 catalyst at different temperatures. In IOP Conference Series: Materials Science and Engineering (Vol. 214, No. 1, p. 012032). IOP Publishing. [CrossRef]
- Wokoma, O.A.F.; Edori, O.S. Heavy metals content of an oily wastewater effluent from an oil firm at the point of discharge. Int. J. Chem. Pharm. Technol. 2017, 2, 154–161. [Google Scholar]
- Wu, C., Zhou, Y., Sun, Q., Fu, L., Xi, H., Yu, Y.; Yu, R. (2016). Appling hydrolysis acidification-anoxic–oxic process in the treatment of petrochemical wastewater: from bench scale reactor to full scale wastewater treatment plant. Journal of Hazardous Materials, 309, 185-191. [CrossRef]
- Xu, J.C., Shi, Y.L., Huang, J.E., Wang, B. and Li, H.L., 2004. Doping metal ions only onto the catalyst surface. Journal of Molecular Catalysis A: Chemical, 219, 351-355. [CrossRef]
- Xue, Z., Xing, X., Zhu, S., Zhang, W., Luan, L., Niu, Y., ... & Tao, Q. (2019). Underwater superoleophobic porous hydrogel film prepared by soluble salt-template method for oil/water separation in complex environments. Desalin Water Treat, 164, 151-161. [CrossRef]
- Yadav, A., 2020. Biological and physicochemical combination processes. In Nanomaterials for Air Remediation ( 361-372). Elsevier. [CrossRef]
- Yang, X. and Wang, D., 2018. Photocatalysis: from fundamental principles to materials and applications. ACS Applied Energy Materials, 1, 6657-6693. [CrossRef]
- Yu, L., Han, M. and He, F., 2017. A review of treating oily wastewater. Arabian journal of chemistry, 10, S1913-S1922. [CrossRef]
- Zhang, E., Li, W., Gao, Y., Lei, C., Huang, H., Yang, J., ... & Li, D. (2020). High-capacity reusable chitosan absorbent with a hydrogel-coated/aerogel-core structure and superhydrophilicity under oil for water removal from oil. ACS Applied Bio Materials, 3, 5872-5879. [CrossRef]
- Zhang, Y., Quan, X., Chen, S., Zhao, Y. and Yang, F., 2006. Microwave assisted catalytic wet air oxidation of H-acid in aqueous solution under the atmospheric pressure using activated carbon as catalyst. Journal of hazardous materials, 137, 534-540. [CrossRef]
- Zhao, Y., Nakamura, R., Kamiya, K., Nakanishi, S. and Hashimoto, K., 2013. Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nature communications, 4, 1-7. [CrossRef]
- Zheng, R., Wang, B., Shi, K., Sha, D.; Ji, X. (2023). Polyvinyl alcohol formaldehyde three-dimensional composite sponges with hierarchical pore structure for W/O emulsion separation. Applied Surface Science, 611, 155754. [CrossRef]
- Zinicovscaia, I. and Cepoi, L. eds., 2016. Cyanobacteria for bioremediation of wastewaters ( 1-124). Basel, Switzerland: Springer International Publishing.
- Zueva, S., Corradini, V., Ruduka, E. and Veglio, F., 2020. Treatment of petroleum refinery wastewater by physicochemical methods. In E3S Web of Conferences (Vol. 161, p. 01042). EDP Sciences. 292 Cleaner Prod 124:1-13. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).